Abstract
Background:
The aging of liver transplant (LT) recipients, the weighting of the MELD score, and the increased prevalence of NASH has led to an increased number of older LT recipients with pre-LT chronic kidney disease (CKD). There are limited data on the impact of increased recipient age on postsimultaneous liver kidney (SLK) transplant outcomes among patients with CKD, leading some centers to employ subjective age cutoffs for potential SLK recipients.
Methods:
We evaluated UNOS data of adult SLK recipients from 2/27/2002–12/31/2018, restricted to recipients with ≥90 days of waiting time and CKD (eGFR persistently <60 mL/min/1.73m2 for ≥90 days using the MDRD-4 equation). We fit mixed-effects Cox regression models (center as random effect) to evaluate the association of recipient age and patient survival.
Results:
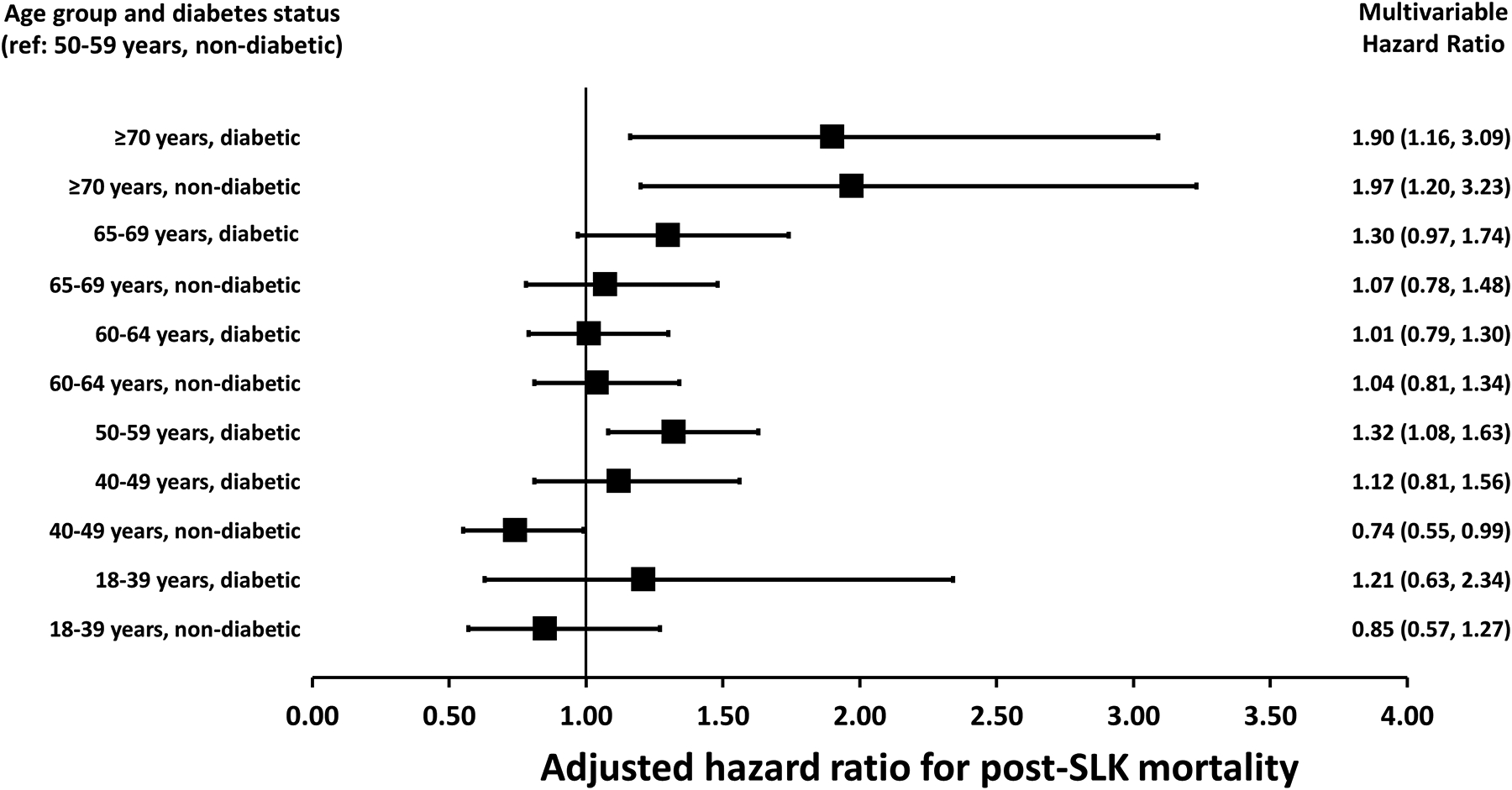
Among 3146 SLK recipients with CKD, nearly two-thirds were 50–64 years of age, while 465 (14.8%) and 93 (3.0%) were 65–69 years and ≥70 years, respectively. Compared to nondiabetic SLK recipients aged 50–59 years, SLK recipients ≥70 years of age without diabetes (HR: 1.97, 95% CI: 1.20–3.23; p=0.007) and with diabetes (HR: 1.90, 95% CI: 1.16–3.09; p=0.01) had higher mortality compared to the reference group. In absolute terms, SLK recipients ≥70 years of age had 25% lower patient survival at 5 years compared to recipients aged 40–49 years.
Conclusions:
Although careful selection is required of any SLK recipient, especially those with increased comorbidities, there are no objective data to justify a specific age cutoff <70 years among potential SLK recipients with CKD.
Introduction
Liver transplant (LT) recipients in the US are getting older reflecting in part aging of hepatitis C virus (HCV)-infected baby boomers as well as an increased number of older LT recipients with nonalcoholic steatohepatitis (NASH) and alcoholic liver disease (ALD).1–9 In addition recipient age restrictions have progressively eased at most centers. According to Organ Procurement and Transplantation (OPTN)/United Network for Organ Sharing (UNOS) data, the median age of LT recipients has increased from 50 years in 2002 to 57 in 2018. The weighting of the Model for End-Stage Liver Disease (MELD) score10–12 and the increased prevalence of NASH as an indication for LT2–9 has led to an increased number of LT recipients having pre-LT chronic kidney disease (CKD). Despite changes in allocation policies for patients evaluated for a simultaneous liver-kidney (SLK) transplant, the persistent organ scarcity and ethical imperative to optimize utilization of the scarce resource of donor organs continues to lead to debates about which candidates are best suited for LT alone, SLK, or no transplant at all.
Several studies have evaluated the impact of recipient age on post-LT outcomes. Despite consistent data demonstrating an increased mortality among older LT recipients (especially those >70 years), the survival benefit is not significantly different after accounting for the increased risk of pre-LT mortality in older patients.1,13–15 In contrast to the effect of increasing age on LT-alone recipients, the impact of recipient age on outcomes among SLK recipients has received less attention. A 2008 paper suggested that SLK outcomes were worse in SLK recipients ≥65 years of age, while a secondary analysis in a 2016 manuscript suggested 70 years might be a more appropriate cutoff.16,17 Both studies had important limitations because: a) age was modeled using a binary cutoff of 65; and b) all SLK recipients were included, even those without CKD who would not be eligible for an SLK under current policies (limiting the internal and external validity).16,17
Concerns about increasing recipient age compromising SLK outcomes led to recommendations from a Consensus Conference that, “Given the unfavorable outcomes in older liver candidates on dialysis, selection of such patients for SLK warrants careful consideration.”18 No specific age cutoff was proposed, but the report suggested that a cutoff of 65 or 70 might be appropriate, despite limited empirical data. Given the knowledge gaps in this area, we sought to identify a cohort of SLK recipients with well-defined pretransplant CKD in order to: 1) evaluate the association between recipient age and patient survival among SLK recipients with CKD and; 2) determine whether there is an age threshold at which restricting SLK may be justified based on the concept of utility.
Materials and Methods
Study subjects
We analyzed OPTN/UNOS data to identify adult SLK recipients ≥18 years of age who were transplanted between February 27, 2002 and December 31, 2018.19,20 The goal of this study was to specifically evaluate SLK outcomes only among patients with CKD, a more homogeneous cohort that is increasing in prevalence due to the NASH epidemic, rather than those with sustained acute kidney injury that might be due to hepatorenal syndrome (a potentially reversible condition) or less reversible diseases such as acute tubular necrosis. Furthermore, this restriction allowed us to focus largely (although not exclusively) on a group of patients who by and large meet the current SLK criteria for patients with CKD.
To create a more homogeneous cohort with CKD, we restricted our analysis to SLK recipients with: 1) ≥90 days of pretransplant waiting time (the minimum time period to define CKD); and 2) an estimated glomerular filtration rate (eGFR) that was <60 mL/min/1.73m2 at every time point during the 90-day pretransplant period. We calculated the eGFR using the Modification of Diet in Renal Disease (MDRD)-4 equation that is based on the serum creatinine, race, age, and gender. This formula was used given its routine application in clinical practice to determine whether a patient is eligible for an SLK based on CKD criteria, and the available data in the OPTN/UNOS database (lack of BUN data prevents the ability to calculate the MDRD-6 equation).
Study outcomes and variables
The primary study outcome was posttransplant patient survival, based on coding in the OPTN/UNOS datasets, and additionally available death date data in the dataset. We included covariates potentially associated with posttransplant patient survival, pertinent to SLK among older recipients, including: age, race/ethnicity, sex, etiology of liver disease, diabetes (binary yes/no), prior transplant, laboratory values at transplant (eGFR, bilirubin, INR), calculated MELD score, allocation MELD score (accounting for MELD exception points), receipt of exception points, functional status (Karnofsky score), pretransplant dialysis (week prior to transplant, and chronic dialysis defined as dialysis at listing and transplant), body mass index, donor kidney donor risk index (KDRI), and donor age. Posttransplant length of stay was modeled as a secondary outcome.
Statistical analysis
Baseline clinical and demographic data were compared based on recipient age using previously evaluated and/or clinically interpretable cutpoints.16,17 Continuous variables were compared using the Kruskall-Wallis test given the nonnormal distribution of the data, and categorical variables were compared using chi-square tests.
Patient survival was thmodeled using Cox regression models in order to evaluate the time to death for SLK recipients. Given differences in transplant center decision-making about which SLK recipients to waitlist (especially before the current SLK policy was enacted),21,22 and baseline differences in center outcomes,23 transplant center was modeled as a random effect to account for correlated outcomes among centers (using the mestreg function in STATA 15). Recipient age was modeled as either a continuous or categorical covariate (based on previously published cutpoints) in multivariable models, and included the recipient age variable in the final multivariable model that had the best model fit (based on the lowest Akaike Information Criterion [AIC]). The upper age cutoff was capped at 70 given: a) the small number of recipients >70 years of age which limited applicability of the results; and b) the similar point estimates for the hazard of mortality among all recipients ≥70 years of age. We included other relevant covariates in univariable models, and considered those with a p<0.1 in univariable models for inclusion in the final multivariable model. In the final model, we retained variables with a p<0.05. We a priori evaluated the potential interaction of recipient age (categorical variable) and diabetes with a p<0.1 as the significance threshold for the interaction term given that increased age is associated with increased diabetes duration, and therefore an increase burden of diabetes related comorbidities (eg, cardiovascular disease) that could impact posttransplant mortality. In a secondary analysis, we fit age as a categorical variable, whereby each 1 year age increment was a distinct category. This allowed us to compare the point estimate of the multivariable adjusted hazard ratio for every 1-year age group to a reference point. A recipient age of 65 was selected as the reference as this was the lower age limit suggested as a possible age cutoff for SLKs from prior studies, while it also allowed us to compare the upper limit of 70 from prior studies to a reference of 65.16–18 In a secondary analysis, we compared the unadjusted posttransplant survival of patients with CKD who received an SLK to recipients who received a liver transplant alone to frame the potential survival benefit of an SLK.
All analyses were performed using STATA 15.0. This study was deemed exempt by the Institutional Review Board at the University of Pennsylvania.
Results
During the study period, there were a total of 7581 SLK recipients. Of this total, 4435 were excluded— 4156 had <90 days of waiting time and could not be classified as having pretransplant CKD, and an additional 279 had ≥90 days waiting time but did not have CKD based on having a pretransplant eGFR≥60mL/min/1.73m2 in the 90-day pretransplant period.
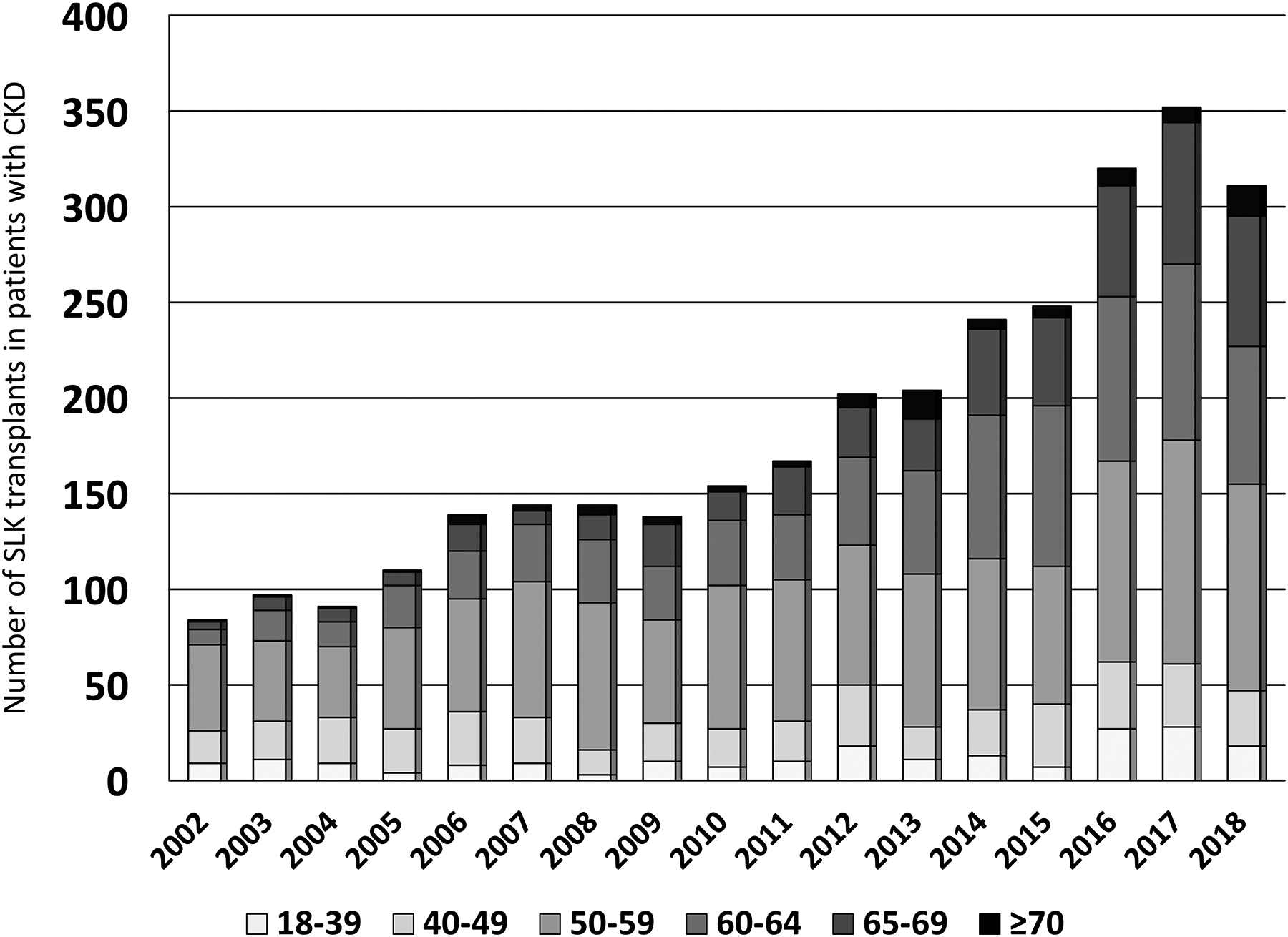
Among the 3146 included SLK recipients with pretransplant CKD, nearly two-thirds were 50–64 years of age, while 465 (14.8%) and 93 (3.0%) were 65–69 years and ≥70 years, respectively. This distribution was similar to the SLK cohort with <90 days of waiting time (or with ≥90 days but did not meet CKD criteria): 13.0% and 2.6% were 65–69 years and ≥70 years, respectively. SLK recipients in the non-CKD cohort were more likely to be white race (55.8% vs 44.2%; p=0.02), male gender (55.6% vs 44.5%; p=0.08), have a significantly higher calculated MELD score (median: 32 vs 25; p<0.001). The percentage of SLK recipients with CKD who had an eGFR <30 mL/min/1.73m2 at transplant (the threshold required under the current SLK policy) differed significantly by age group (p=0.002) and was highest in the youngest age group: a) 18–39: 96.5%; b) 40–49: 88.6%; c) 50–59: 91.7%; d) 60–64: 90.4%; e) 65–69: 87.1%; and f) ≥70: 88.2%. The number of SLK recipients increased each calendar year until 2017 (year new SLK allocation policy was implemented), with the number of SLK recipients with CKD increasing the most (in absolute and relative terms) among those aged 60–69 years (Figure 1A).
Figure 1 (2 panels):
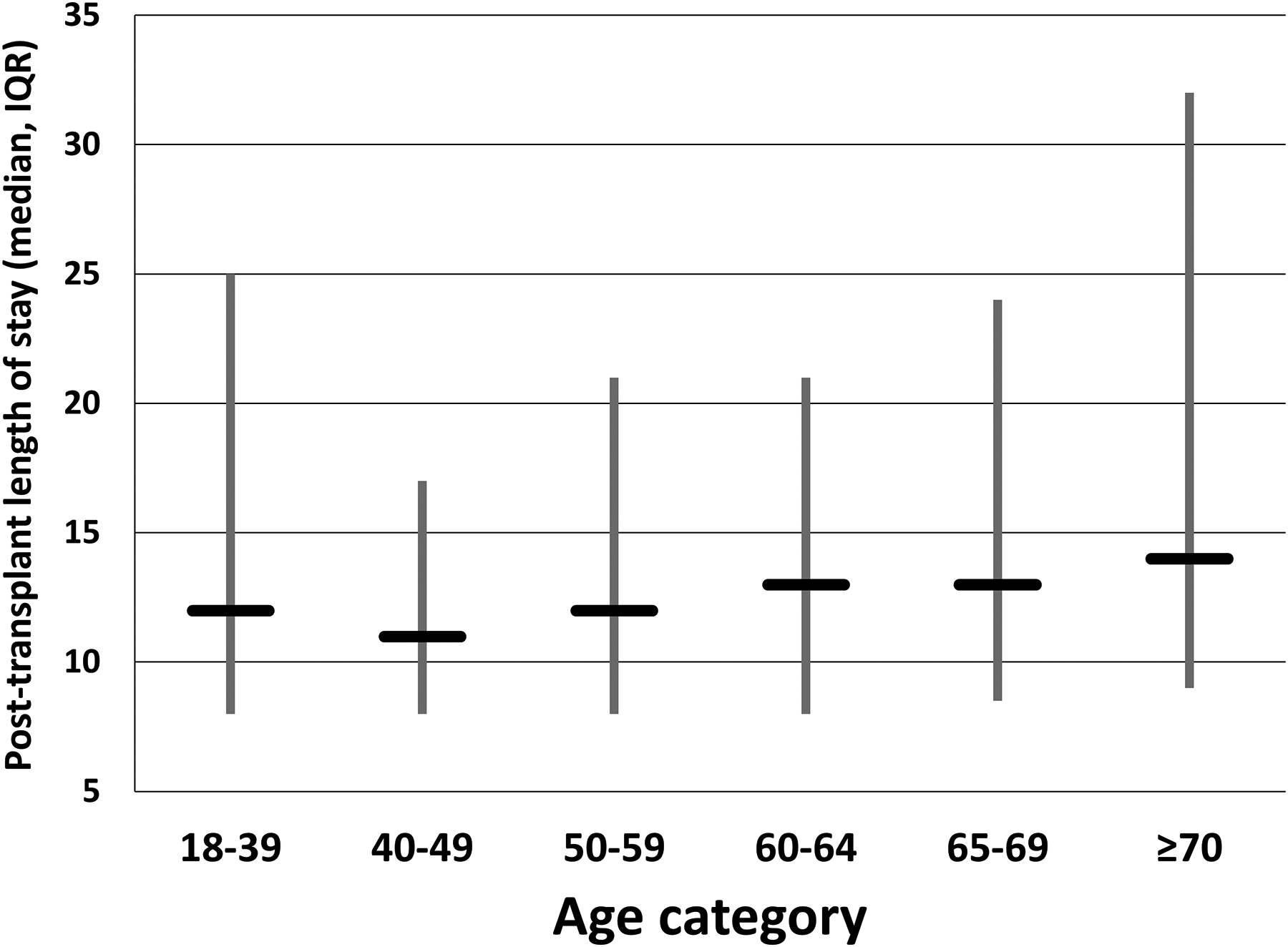
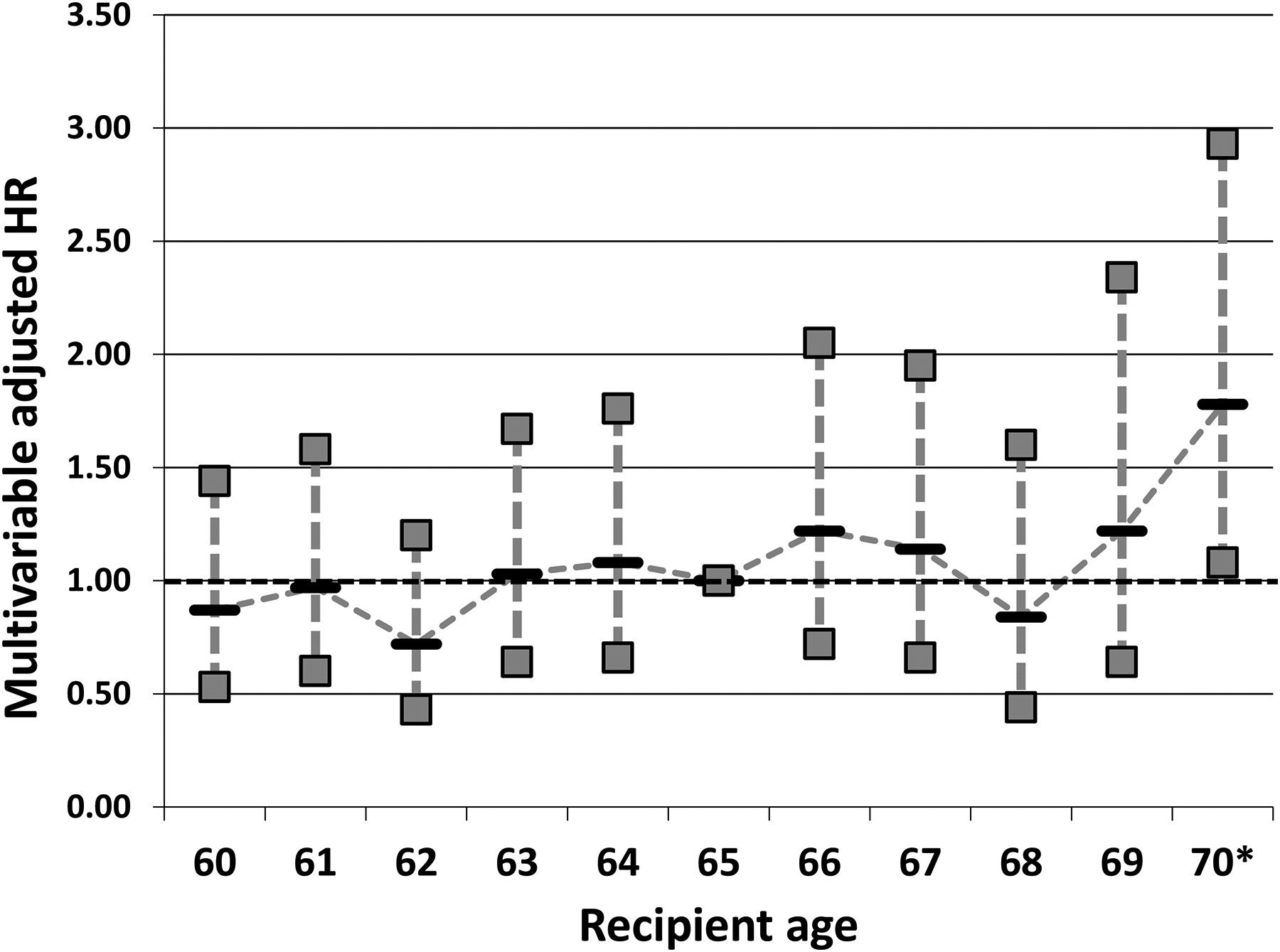
Figure 1A: Age distribution of SLK recipients with CKD by calendar year from 2002–2018
Figure 1B: Maximum age of SLK recipients with CKD per center from 2002–2018 among center performing >20 SLKs in patients with CKD
Compared to SLK recipients aged 50–64 years, the most notable differences among SLK recipients with CKD 65–69 and ≥70 years of age were that they were more likely to be of white race, have NASH/cryptogenic cirrhosis as the etiology of their liver disease, and less likely to be on dialysis prior to transplant (Table 1). The unadjusted 5-year survival of SLK recipients ≥70 years of age with CKD was 57.8% (95% CI: 43.6–69.6%), as compared to 68.8% (95% CI: 55.4–78.0%) in SLK recipients ≥70 years of age without CKD.
Table 1:
Characteristics of adult SLK recipients with CKD prior to transplant from 2/27/02–12/31/2018
| Variable* | 18–39 N=202 |
40–49 N=413 |
50–59 N=1,221 |
60–64 N=752 |
65–69 N=465 |
70+ N=93 |
P-value |
|---|---|---|---|---|---|---|---|
| Male gender | 102 (50.5) | 264 (63.9) | 781 (64.0) | 481 (64.0) | 290 (62.4) | 57 (61.3) | 0.012 |
| Race/ethnicity | |||||||
| White | 145 (71.8) | 238 (57.6) | 699 (57.2) | 462 (61.4) | 319 (68.6) | 74 (79.6) | <0.001 |
| Black | 21 (10.4) | 62 (15.0) | 213 (17.4) | 118 (15.7) | 58 (12.5) | 2 (2.2) | |
| Hispanic | 28 (13.9) | 77 (18.6) | 242 (19.8) | 130 (17.3) | 65 (14.0) | 12 (12.9) | |
| Asian | 7 (3.5) | 22 (5.3) | 49 (4.0) | 29 (3.9) | 16 (3.4) | 4 (4.3) | |
| Other | 1 (0.5) | 14 (3.4) | 18 (1.5) | 13 (1.7) | 7 (1.5) | 1 (1.1) | |
| Etiology of liver disease | <0.001 | ||||||
| NASH/cryptogenic | 10 (5.0) | 55 (13.3) | 192 (15.7) | 197 (26.2) | 176 (37.8) | 45 (48.4) | |
| HCV | 23 (11.4) | 132 (32.0) | 575 (47.1) | 320 (42.6) | 136 (29.2) | 21 (22.6) | |
| Alcohol | 22 (10.9) | 92 (22.3) | 205 (16.8) | 106 (14.1) | 73 (15.7) | 9 (9.7) | |
| Autoimmune | 24 (11.9) | 27 (6.5) | 54 (4.4) | 41 (5.5) | 26 (5.6) | 13 (14.0) | |
| HBV | 6 (3.0) | 14 (3.4) | 38 (3.1) | 15 (2.0) | 7 (1.5) | 1 (1.1) | |
| PCLD/PCKD | 8 (4.0) | 30 (7.3) | 102 (8.4) | 40 (5.3) | 24 (5.2) | 0 (0.0) | |
| Other | 109 (54.0) | 63 (15.3) | 55 (4.5) | 33 (4.4) | 23 (4.9) | 4 (4.3) | |
| Diabetes | 27 (13.4) | 128 (31.0) | 536 (43.9) | 406 (54.0) | 252 (54.2) | 53 (57.0) | <0.001 |
| eGFR<30mL/min at transplant | 195 (96.5) | 366 (88.6) | 1,119 (91.7) | 680 (90.4) | 405 (87.1) | 82 (88.2) | 0.002 |
| Dialysis week prior to LT | 155 (77.1) | 277 (67.1) | 788 (64.6) | 476 (63.3) | 259 (55.7) | 45 (48.4) | <0.001 |
| Chronic dialysis | 97 (48.0) | 188 (45.5) | 509 (41.7) | 299 (39.8) | 142 (30.5) | 26 (28.0) | <0.001 |
| Days on chronic dialysis | 655 (330–1,311) | 564 (219–1,164) | 460 (174–1,068) | 439 (136–1,237) | 495 (195–915) | 169 (49–661) | <0.001 |
| Prior transplant | 30 (14.9) | 31 (7.5) | 90 (7.4) | 46 (6.1) | 18 (3.9) | 4 (4.3) | <0.001 |
| BMI | 23.0 (20.7, 27.9) | 26.0 (23.0, 30.2) | 26.5 (23.5, 30.7) | 26.7 (23.5, 30.8) | 26.6 (23.6, 30.4) | 26.9 (23.5, 31.5) | <0.001 |
| Bilirubin | 1.1 (0.5, 4.0) | 1.5 (0.7, 4.0) | 1.6 (0.8, 3.9) | 1.6 (0.8, 4.0) | 1.6 (0.8, 3.4) | 2.2 (1.1, 5.4) | 0.002 |
| INR | 1.2 (1.1, 1.6) | 1.4 (1.2, 1.8) | 1.4 (1.2, 1.8) | 1.4 (1.2, 1.9) | 1.4 (1.2, 1.8) | 1.5 (1.3, 1.9) | <0.001 |
| MELD exception type | <0.001 | ||||||
| No exception | 133 (65.8) | 327 (79.2) | 974 (79.8) | 574 (76.3) | 343 (73.8) | 75 (80.7) | |
| HCC exception | 2 (1.0) | 13 (3.2) | 112 (9.2) | 106 (14.1) | 73 (15.7) | 15 (16.1) | |
| Non-HCC exception‡ | 67 (33.2) | 71 (17.7) | 135 (11.1) | 72 (9.6) | 49 (10.5) | 3 (3.2) | |
| Laboratory MELD score | 24 (21–30) | 24 (21–31) | 24 (21–30) | 25 (21–31) | 24 (21–30) | 26 (21–33) | 0.57 |
| Allocation MELD score | 30 (25–35) | 27 (23–32) | 27 (23–33) | 28 (24–33) | 28 (24–33) | 29 (24–34) | <0.001 |
| Functional status** | 0.002 | ||||||
| No impairment | 49 (24.3) | 85 (20.6) | 201 (16.5) | 116 (15.4) | 86 (18.5) | 15 (16.1) | |
| Mild impairment | 77 (38.1) | 180 (43.6) | 493 (40.4) | 339 (45.1) | 199 (42.8) | 39 (41.9) | |
| Moderate to severe impairment | 58 (28.7) | 127 (30.8) | 458 (37.5) | 266 (35.4) | 165 (35.5) | 38 (40.9) | |
| Missing | 18 (8.9) | 21 (5.1) | 69 (5.7) | 31 (4.1) | 15 (3.2) | 1 (1.1) | |
| Donor KDRI | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.4) | <0.001 |
| Donor KDPI | 0.27 (0.12, 0.45) | 0.31 (0.13, 0.51) | 0.33 (0.15, 0.57) | 0.32 (0.14, 0.56) | 0.37 (0.17, 0.59) | 0.39 (0.19, 0.65) | 0.002 |
| Donor LDRI | 1.22 (1.09, 1.40) | 1.24 (1.10, 1.45) | 1.26 (1.11, 1.51) | 1.27 (1.10, 1.51) | 1.30 (1.14, 1.52) | 1.28 (1.11, 1.54) | 0.049 |
| Donor age | 28.0 (20.0, 41.0) | 35.0 (23.0, 46.0) | 35.0 (24.0, 48.0) | 33.0 (24.0, 46.5) | 35.0 (24.0, 50.0) | 39.0 (26.0, 49.0) | <0.001 |
Abbreviations: NASH=nonalcoholic steatohepatitis; HCV=hepatitis C virus; HBV=hepatitis B virus; PCLD=polycystic liver disease; PCKD=polycystic kidney disease; LT=liver transplant; BMI=body mass index; KDRI=kidney donor risk index; KDPI=kidney donor profile index; LDRI=liver donor risk index
Categorical data as: Number (%); continuous data reported as: median (IQR)
Primary sclerosing cholangitis, primary biliary cholangitis, or autoimmune hepatitis. Includes exceptions for symptoms of liver disease (eg, refractory ascites), polycystic liver disease, and metabolic conditions.
Functional status based on Karnofsky score data in the OPTN/UNOS database; no impairment: 80–100%; mild impairment: 50–70%; moderate to severe impairment: 10–40%
Based on OPTN/UNOS coding, the use of induction immunosuppression among SLK recipients differed by age group (p=0.01 for chi-square test comparing use of induction immunosuppression by age category). SLK recipients ≥70 years of age were significantly less likely to receive depleting immunosuppression (12.9% vs 17.0–22.8% in the other age groups). The maintenance immunosuppression regimen at discharge did not differ among the different age groups (p=0.70).
The rates of DGF were lowest in the ≥70 year age group (11.5%), compared to 26.3%, 19.3%, 19.3%, 24.6%, and 20.7% in the 18–39, 40–49, 50–59, 60–64, and 65–69 year age groups, respectively (p=0.01).
Variability in center practices
During the study, there were 60 centers that had performed at least 20 SLKs among patients meeting inclusion criteria based on having CKD. Among those 60 centers, 28 (46.7%) only performed SLKs in patients <70 years of age (data not available on whether these centers declined to list or perform SLKs in patients ≥70 years of age), while only 4 (6.7%) had a maximum age of their oldest SLK recipient ≤65 (Figure 1B).
Multivariable models
In multivariable mixed effects logistic regression models, there was a significant interaction between increasing recipient age and diabetes on post-SLK mortality (p=0.001). After accounting for this interaction, along with key donor and recipient variables, there were significant among center differences in posttransplant survival (likelihood ratio test for model including random effects p=0.004). Several covariates were significantly associated with posttransplant survival, with higher mortality: 1) being on dialysis immediately prior to transplant; b) lower serum albumin at transplant (higher albumin associated with lower mortality); c) being hospitalized or in the intensive care unit (compared to being transplanted from home); d) prior liver transplant; and e) transplantation from a donor with a higher KDRI (Table 2). Key clinical covariates that define increased severity of illness (eg, bilirubin, INR, ventilator status, Karnofsky score) or could be associated with survival (eg, use and type of inductin therapy) were not retained in the final multivariable model as they were not significantly associated with the outcome (p>0.1; Table 2). DGF was associated with increased mortality (HR: 1.49, 95% CI: 1.25–1.78) but was not included in the final model as it is in the causal pathway of mortality, rather than a confounder/covariate known pretransplant that should be adjusted for.
Table 2-.
Multivariable mixed-effects Cox regression model evaluating factors associated with posttransplant survival of adult SLK recipients with CKD*
| Variable | Multivariable HR | P-value |
|---|---|---|
| Dialysis in week prior to transplant | 1.28 (1.11–1.48) | 0.001 |
| Serum albumin at transplant | 0.81 (0.74–0.89) | <0.001 |
| Etiology of liver disease | ||
| HCV | Reference | <0.001 |
| NASH/cryptogenic | 0.79 (0.65–0.95) | |
| Alcohol | 0.73 (0.59–0.90) | |
| Autoimmune‡ | 0.88 (0.66–1.16) | |
| HBV | 0.47 (0.28–0.78) | |
| PCLD/PCKD | 0.38 (0.25–0.60) | |
| Other | 0.85 (0.63–1.13) | |
| Location at transplant | 0.04 | |
| Home | Reference | |
| Hospitalized, not ICU | 1.21 (1.01–1.45) | |
| ICU | 1.36 (1.10–1.68) | |
| Prior liver transplant | 1.31 (1.02–1.66) | 0.03 |
| KDRI | 1.42 (1.07–1.87) | 0.02 |
| Donor age | 1.01 (0.99–1.02) | 0.06 |
Abbreviations: HCV=hepatitis C virus; NASH=nonalcoholic steatohepatitis; HBV=hepatitis B virus; PCLD=polycystic liver disease; PCKD=polycystic kidney disease; ICU=intensive care unit; KDRI=kidney donor risk index
Model also included age, diabetes, and interaction of age and diabetes, shown in Figure 2. Other variables tested for inclusion but were found not to be significant in univariable or multivariable models (p>0.1) included: pretransplant ventilation, donation after cardiac death donor, recipient bilirubin at transplant, recipient INR at transplant, recipient estimated glomerular filtration rate at transplant, recipient gender, recipient race/ethnicity, recipient BMI, exception type, functional status, use of chronic dialysis, liver DRI, kidney cold ischemia time, and use and type of induction therapy. Data for model with age as a categorical variable shown because of similar model fit using fitting age as continuous vs categorical.
Primary sclerosing cholangitis, primary biliary cholangitis, or autoimmune hepatitis
Results of the multivariable model for recipient age and diabetes are presented for Figure 2 in order to highlight the interaction of recipient age and diabetes (the hazard ratio for mortality among recipients of the same age group differed based on diabetes status but to a different degree based on the recipient age). Compared to SLK recipients without diabetes aged 50–59 years, nondiabetics aged 40–49 years had a significantly lower risk of mortality (HR: 0.74, 95% CI: 0.55–0.99; p=0.04), while SLK recipients ≥70 years of age without diabetes (HR: 1.97, 95% CI: 1.20–3.23; p=0.007) and with diabetes (HR: 1.90, 95% CI: 1.16–3.09; p=0.01) both had higher mortality compared to the reference group, but did not differ from one another based on their diabetes status (Figure 2). When the study cohort was split into those transplanted before or after August 10, 2017 (the date of implementation of the current SLK policy), the HR for the oldest age groups was unchanged in the cohort transplanted prior to the current policy. However, for the cohort transplanted under the new policy, the point estimate for the HR increased, although it did not meet statistical significant due to smaller sample sizes: a) adjusted HR for nondiabetics ≥70 years of age: 2.44 (95% CI: 0.21–28.86); b) adjusted HR for diabetics ≥70 years of age: 2.97 (95% CI: 0.47–18.70).
Figure 2:
Multivariable hazard ratios for recipient age categories
The cause of death among patients who died did not differ among the different age categories (p=0.30; Supplemental Figure 1 aggregated together by age group). Notably, cardiovascular and malignancy as causes of death did not differ significantly in the different age groups. Patients with HCC were more likely to have malignancy as their cause of death (23.2% of deaths in HCC patients due to malignancy vs 7.4% in non-HCC patients; p<0.001). When stratified into those with vs without HCC, non-HCC patients ≥70 years of age were numerically (but not statistically) more likely to have malignancy as their cause of death (p=0.36), while HCC patients ≥70 years of age were numerically (but not statistically) less likely to have malignancy as their cause of death (p=0.16).
In absolute terms, the differences in post-SLK survival increased over time when comparing the cohort with the best survival (nondiabetics ages 40–49 years) to and the lowest (nondiabetics ≥70 years of age, although survival was nearly identical in diabetics ≥70 years of age; Figure 3A). At 1-year posttransplant, the absolute difference in adjusted survival was 10.3%, which increased over time: 19.9% at 3 years, 25.0% at 5 years, to 31.5% at 10 years. The 5-year adjusted survival exceeded 60% for all age groups except those ≥70 years of age (54.6% for nondiabetics; 55.8% for diabetics). In Kaplan-Meier analyses, the unadjusted survival of transplant recipients with CKD in every age group was higher in those who received an SLK except for those ≥70, where the unadjusted 5-year survival was lower in SLK recipients (Figure 3B).
Figure 3 (2 panels):
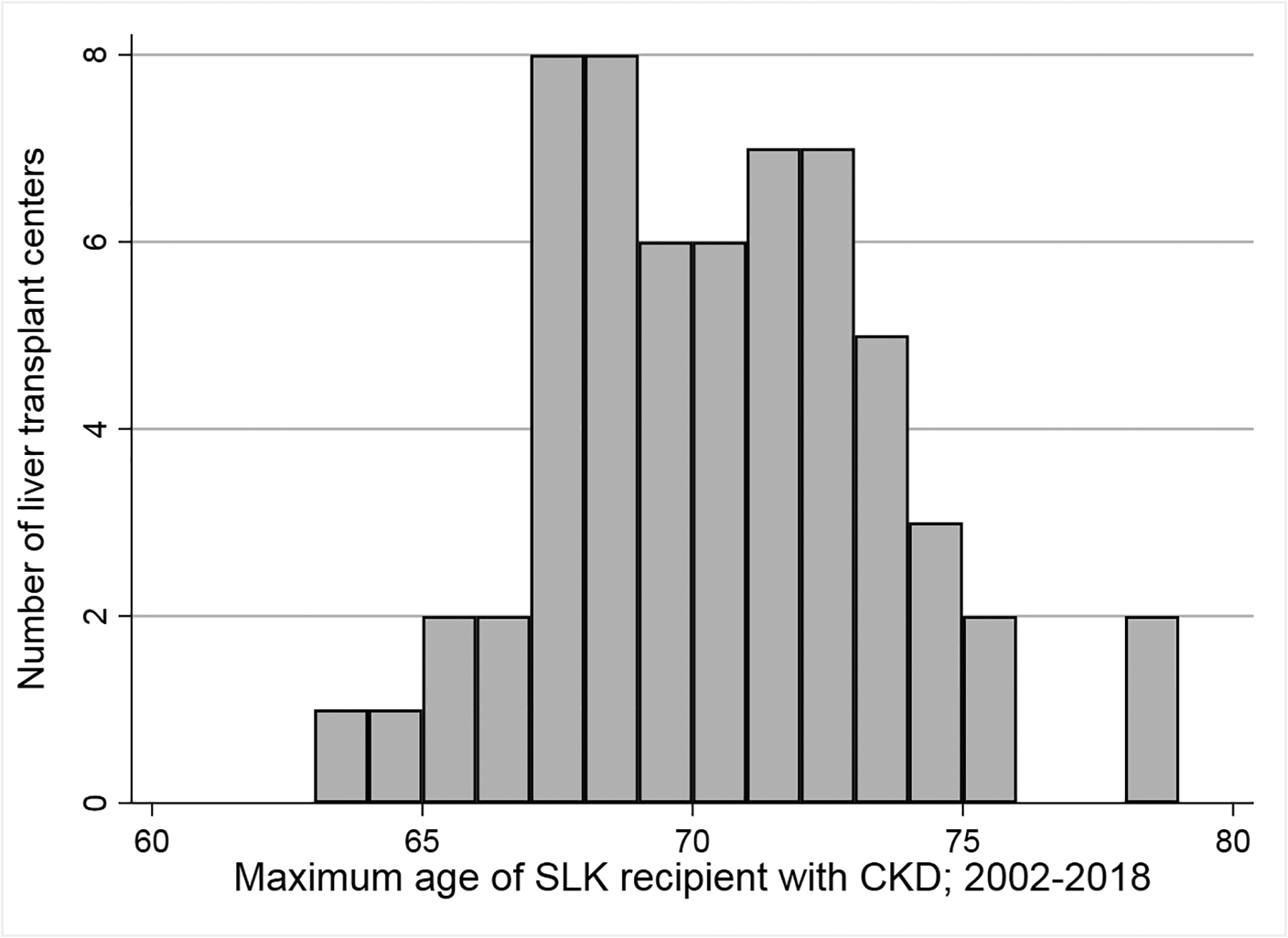
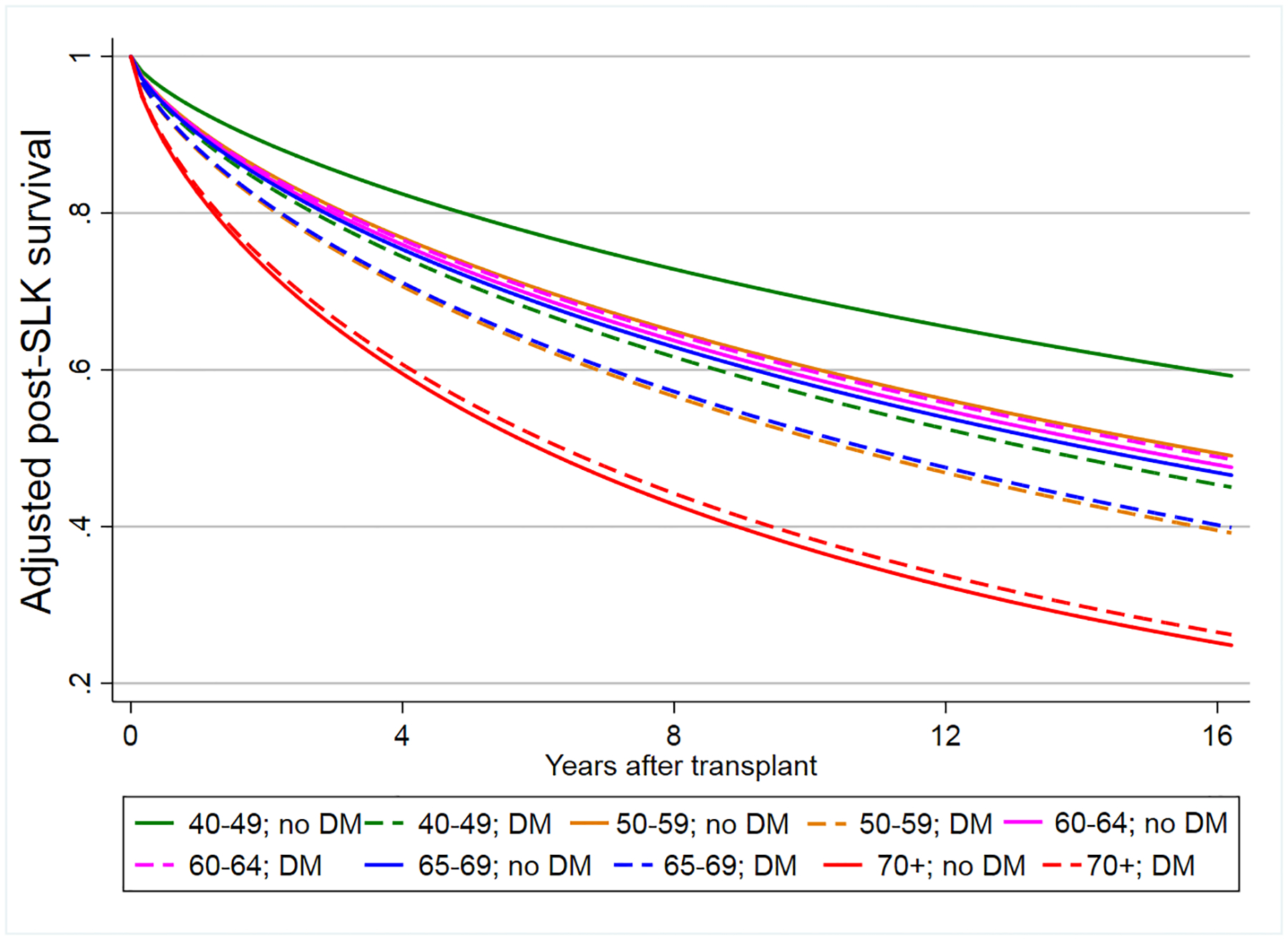
Figure 3A: Adjusted survival curve stratified by recipient age and diabetes
Figure 3B: Unadjusted posttransplant survival for recipients with CKD based on receipt of a liver transplant alone versus simultaneuous liver-kidney transplant from 2002–2018
Abbreviations: LTA=liver-transplant alone; SLK=simultaneous liver-kidney transplant
Secondary analyses
In a secondary analyses that modeled age as a categorical variable (each category represented each 1-year interval of recipient age), the risk of increased post-SLK mortality was only significant in recipients ≥70 years of age. Compared to a reference age group of 65, SLK recipients ≥70 years had a greater than 75% increased risk of posttransplant mortality (HR: 1.78, 95% CI: 1.08–2.93; p=0.02; cohorts not stratified by diabetes status given similar hazards of mortality for recipients ≥70 years with or without diabetes; Figure 4).
Figure 4:
Multivariable hazard ratio per 1-year age increments among SLK recipients ≥60 years of age
Posttransplant length of stay was significantly different based on recipient age, and was longest for SLK recipients ≥70 years of age (Kruskall-Wallis test p-value<0.001; Figure 5). Among SLK recipients ≥70 years of age, 25 (26.9%) had a posttransplant length of stay that exceeded 30 days, significantly higher than the 4 other age groups (p<0.001) which ranged from 10.0% (40–49 year age group) to 18.5% (18–39 year age group).
Figure 5:
Posttransplant length of stay for SLK recipient by receipint age category
Although the sample size limited statistical testing, the unadjusted survival was numerically higher for SLK recipients <65 years of age who had acute on chronic kidney injury (mean eGFR ≥30 mL/min/1.73m2 during the 90-day pretransplant period) compared to those on chronic dialysis, but did not differ numerically among SLK recipients ≥65 years of age.
Discussion
In this analysis of SLK recipients with pre-LT CKD between 2002 and 2018, we found that increasing recipient age was only associated with increased mortality among recipients ≥70 years of age. Although the posttransplant survival of diabetic SLK recipients ages 50–59 and 65–69 was numerically worse than nondiabetic recipients ages 50–59, this did not meet statistical significance. The number of SLK recipients with pretransplant CKD that could be identified based on available data in the OPTN/UNOS database was small (<100 patients).Thus the number of SLK these high-risk SLK recipients is small relative to the entire pool of SLK recipients. However, these data are important nonetheless for several reasons. First, the clinical characteristics of the SLK recipients ≥70 years of age (eg, MELD score, Karnofsky score) surprisingly did not differ significantly from the younger groups, which suggests that this oldest cohort was likely highly selected, yet still had significantly worse survival. Second, the lack of significantly increased survival in SLK recipients ages 65–69, especially among nondiabetics who had survival curves that mirrored those of nondiabetics ages 60–64, would suggest that an upper age cutoff of 65 for SLK recipients, as suggested in the SLK Consensus Conference paper, would not be supported by empirical data.18 Third, the worse survival in the oldest cohort was not limited to early posttransplant mortality that would be attributable solely to operative-related mortality, but rather grew over time. This would suggest that a safety net protocol for eldely patients with CKD (eg, only allowing a kidney after liver rather than SLK after a certain period of time) may not mitigate the worse outcomes in this group. And even though patients in the oldest age group received kidneys with the relatively ‘worst’ kidney quality, as measured by the KDRI/KDPI, which independently was associated with increased mortality, this fact did not explain the worse survival in the oldest age group as their increased mortality persistent in multivariable models that adjusted for KDRI.
Despite multiple publications evaluated the impact of recipient age on post-LT outcomes, there are limited data on post-LT outcomes among SLK recipients. The 2 published studies evaluating outcomes among older SLK recipients provided conflicting data. Early data from the MELD era suggested that SLK outcomes were worse in SLK recipients ≥65 years of age, while a secondary analysis of a manuscript published in 2016 showed that a more appropriate age cutoff to define increased SLK risks was 70.16,17 Our study had important differences from these studies. First, we restricted our analyses to SLK recipients with at least 90 days of pretransplant waiting time in order to create a well-defined cohort with CKD (which by definition requires renal function data for at least 3 months). Although this may have limited the external validity, it ensured internal validity to phenotyped cohort with CKD. Secondly, the 2 previous studies used age 65 as a binary cutoff, and the age cutoff of 70 was only explored in a secondary analysis. Third, until recently there were no policies limiting access to an SLK, and thus patients may have received an SLK in the absence of CKD or prolonged acute kidney injury. This therefore limited the ability of these studies to address the question of recipient age among SLK recipients with CKD. It is for these reasons our study provides new data.
Even though there had been limited objective data on SLK outcomes among older transplant recipients, an SLK Consensus Conference recommended careful selection of older SLK recipients, without a specific age cutoff.18 Although our data should not necessarily be used as an absolute cutoff to restrict transplant, it should raise caution when evaluating potential SLK recipients aged 70 years or older. Furthermore, given the organ scarcity and the ethical imperative to maximize utilization of a scarce resource, these data should be taken into account given the loss of potential life-years in the setting of an SLK, which our data would suggest is magnified among older recipients.24 And although comparing SLK versus liver-alone recipients was not the focus of our analyses, the data suggests the survival benefit of an SLK was not seen among the oldest SLK cohort. Conversely, the lack of significantly worse survival in SLK recipients <70 may call into question center policies that restrict SLK to recipients ages 65–69 years of age. In 2017, the UNOS SLK allocation policy changed in order to provide minimal criteria needed for a patient to be eligible to receive an SLK. These policy importantly does not state that such a patient must get an SLK, but rather in order to receive one, he/she must meet the specific criteria. The decision whether to perform a liver transplant alone, with consideration of a ‘safety net’ kidney if renal dysfunction persists, is left to the individual transplant enter. At the patient level, centers must consider whether a kidney transplant will benefit the patient, or whether there is the potential for recovery, in spite of the SLK criteria. On the population level, the goal is to minimize the number of unnecessary SLKs when renal recovery is possible, especially given the shortage of transplantable kidneys. Our data can help to inform such decisions among older recipients, when the decision may be that the expected posttransplant survival is too low to justify a dual organ transplant (and maybe even a liver alone in the setting of CKD). And although the older SLK recipients did not die from causes of death that might be more attributable to renal disease (eg, cardiovascular disease), this may be due to greater scrutiny on these patients. Nevertheless, regardless of the cause of death, the higher mortality overall is of concern, especially as there is no apparent pattern that could be used to better identify lower-risk older patients.
This study had limitations. We restricted our cohort to those with CKD, and in doing so, excluded half of SLK recipients who had <90 days of waiting time. However, this ensured internal validity, and addressed the specific question of SLK among recipients with CKD. Also, out inclusion/exclusion criteria allowed us to restrict our analyses to patients who by and large would meet the current SLK criteria for those with CKD, despite these patients being transplanted prior to the recent changes in SLK allocation. Second, we used the MDRD-4 equation to calculate eGFR, which may overestimate renal function in patients with cirrhosis. This is unlikely to be substantial as only a small number of SLK recipients with ≥90 days of waiting time were excluded based on our eGFR criteria. Third, we could not account for other predictors of survival such as frailty and could only on the Karnofsky score, or other medical factors that are not as well coded in OPTN/UNOS data (eg, hypertension requiring multiple medications). This may have prevented us from identifying older SLK recipients with better outcomes in the absence of frailty, and/or identifying other risk factors that can help to prognosticate outcomes in elderly SLK recipients. Fourth, we could not evaluate patients who were turned down for SLK, and/or evaluate data on time interval between the liver and kidney transplant (eg, liver transplant followed by a period of recovery of several hours prior to the kidney transplant). Lastly, while we could evaluate certain comorbidities (eg, presence/absence of diabetes), we could not evaluate the severity of comorbidities or other medical conditions that might impact survival (eg, coronary artery disease). It is therefore possible that the older patients were more highly selected. If this were the case, then the worse survival in the oldest recipients is even more noteworthy when compared to younger patients who may have had a greater burden of other medical conditions.
In conclusion, this study demonstrates significantly worse posttransplant survival in SLK recipients ≥70 years of age. These data should help to inform clinical decisions to transplant older SLK recipients, while at the same time, should provide a level of reassurance that patients ages 65–69 years of age do not have significantly worse outcomes. Further studies are needed to evaluate additional clinical data that can help guide decision-making in making decisions about SLK in older patients.
Supplementary Material
Funding
NIH R01 DK120561 (David Goldberg)
Financial support:
Dr. Golderg was funded by NIH DK-R01-120561
Abbreviations page
- LT
Liver transplant
- HCV
Hepatitis C virus
- NASH
Nonalcoholic steatohepatitis
- ALD
Alcoholic liver disease
- OPTN
Organ Procurement and Transplantation
- UNOS
United Network for Organ Sharing
- MELD
Model for End-Stage Liver Disease
- CKD
Chronic kidney disease
- SLK
Simultaneous liver-kidney
- MDRD
Modification of Diet in Renal Disease
- AIC
Akaike Information Criterion
Footnotes
Disclosure
The authors declare no conflicts of interest
Potential conflict of interest: Nothing to report
References
- 1.Su F, Yu L, Berry K, et al. Aging of liver transplant registrants and recipients: trends and impact on waitlist outcomes, post-transplantation outcomes, and transplant-related survival benefit. Gastroenterology. 2016;150(2):441–453.e6; quiz e16. [DOI] [PubMed] [Google Scholar]
- 2.Cholankeril G, Wong RJ, Hu M, et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci. 2017;62(10):2915–2922. [DOI] [PubMed] [Google Scholar]
- 3.Golabi P, Bush H, Stepanova M, et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: data from the scientific registry of transplant recipients (SRTR): 1994 to 2016. Medicine (Baltimore). 2018;97(31):e11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113(11):1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddique O, Joseph-Talreja M, Yoo ER, et al. Rising rate of liver transplantation in the baby boomer generation with non-alcoholic steatohepatitis in the United States. J Clin Transl Hepatol. 2017;5(3):193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. [DOI] [PubMed] [Google Scholar]
- 8.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–2195. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755.e3. [DOI] [PubMed] [Google Scholar]
- 10.da Silva Machado AG, de Medeiros Fleck A Jr, Marroni C, et al. Impact of MELD score implementation on liver allocation: experience at a Brazilian center. Ann Hepatol. 2013;12(3):440–447. [PubMed] [Google Scholar]
- 11.Sharma P, Schaubel DE, Goodrich NP, et al. Serum sodium and survival benefit of liver transplantation. Liver Transpl. 2015;21(3):308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P, Schaubel DE, Guidinger MK, et al. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11(11):2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz J, Thiesset H, Box T, et al. Liver transplantation in septuagenarians receiving model for end-stage liver disease exception points for hepatocellular carcinoma: the national experience. Liver Transpl. 2012;18(11):1395–1396. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz JJ, Pappas L, Thiesset HF, et al. Liver transplantation in septuagenarians receiving model for end-stage liver disease exception points for hepatocellular carcinoma: the national experience. Liver Transpl. 2012;18(4):423–433. [DOI] [PubMed] [Google Scholar]
- 15.Sharma M, Ahmed A, Wong RJ. Significantly higher mortality following liver transplantation among patients aged 70 years and older. Prog Transplant. 2017;27(3):225–231. [DOI] [PubMed] [Google Scholar]
- 16.Croome KP, Lee DD, Burns JM, et al. Simultaneous liver and kidney transplantation in elderly patients: outcomes and validation of a clinical risk score for patient selection. Ann Hepatol. 2016;15(6):870–880. [DOI] [PubMed] [Google Scholar]
- 17.Dellon ES, Galanko JA, Medapalli RK, et al. Impact of dialysis and older age on survival after liver transplantation. Am J Transplant. 2006;6(9):2183–2190. [DOI] [PubMed] [Google Scholar]
- 18.Eason JD, Gonwa TA, Davis CL, et al. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK). Am J Transplant. 2008;8(11):2243–2251. [DOI] [PubMed] [Google Scholar]
- 19.Cullaro G, Hirose R, Lai JC. Changes in simultaneous liver-kidney transplant allocation policy may impact postliver transplant outcomes. Transplantation. 2019;103(5):959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lum EL, Cárdenas A, Martin P, et al. Current status of simultaneous liver-kidney transplantation in the United States. Liver Transpl. 2019;25(5):797–806. [DOI] [PubMed] [Google Scholar]
- 21.Volk ML, Biggins SW, Huang MA, et al. Decision making in liver transplant selection committees: a multicenter study. Ann Intern Med. 2011;155(8):503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadim MK, Davis CL, Sung R, et al. Simultaneous liver-kidney transplantation: a survey of US transplant centers. Am J Transplant. 2012;12(11):3119–3127. [DOI] [PubMed] [Google Scholar]
- 23.Asrani SK, Kim WR, Edwards EB, et al. Impact of the center on graft failure after liver transplantation. Liver Transpl. 2013;19(9):957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhury RA, Reese PP, Goldberg DS, et al. A paired kidney analysis of multiorgan transplantation: implications for allograft survival. Transplantation. 2017;101(2):368–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


