Abstract
Objective
To evaluate if 3 peptides derived from the cartilage oligomeric matrix protein (COMP), which wounded zones of cartilage secrete into synovial fluid, possess biological activity and might therefore be involved in the regulation of specific aspects of joint regeneration.
Methods
The 3 peptides were produced by chemical synthesis and then tested in vitro for known functions of the COMP C-terminal domain from which they derive, and which are involved in osteoarthritis: transforming growth factor-β (TGF-β) signaling, vascular homeostasis, and inflammation. Results. None of the peptides affected the gene expression of COMP in osteochondral progenitor cells (P > 0.05). We observed no effects on the vascularization potential of endothelial cells (P > 0.05). In cultured synovium explants, no differences on the expression of catabolic enzymes or proinflammatory cytokines were found when peptides were added (P > 0.05).
Discussion and Conclusions
The 3 peptides tested do not regulate TGF-β signaling, angiogenesis and vascular tube formation, or synovial inflammation in vitro and therefore most likely do not play a major role in the disease process.
Keywords: cartilage oligomeric matrix protein, matrikine, synovium, endothelial cells, transforming growth factor-β
Introduction
Osteoarthritis (OA) is the most prevalent chronic degenerative joint disease. It affects all tissues of the joint, including the cartilage, synovium, vascular system, and subchondral bone. 1 During OA, the cartilage matrix experiences degradative changes, resulting in the release of proteins and their fragments into the synovial fluid. OA can be diagnosed by detecting these degradation products in synovial fluid and serum, as biomarkers of the disease stage. For this reason, extensive research has focused on looking for more specific and reliable biomarkers associated with OA, in particular novel neoepitopes produced during proteolysis. 2 However, little is known about possible biochemical functions that these fragments may still play during the onset of the disease and its progression. Bioactive fragments of extracellular matrix proteins, known as matrikines and matricryptins, are produced by limited proteolysis and can regulate a wide variety of cellular functions such as cell adhesion, migration, proliferation, angiogenesis, or apoptosis. 3 Multiple matricellular proteins give rise to matricryptins. For example, the matricryptin PEX derives from the C-terminal domain of the matrix metalloproteinase-2 (MMP-2) and inhibits MMP-2 enzymatic activity in a negative feedback loop, blocking in turn angiogenesis. 4 Other matricryptins can promote catabolic and proinflammatory responses, such as the aggrecan 32-mer fragment does in cartilage 5 or fibronectin fragments do on monocytes and cartilage.6,7
After joint injury and in early stages of osteoarthritis, increased amounts of cartilage oligomeric matrix protein (COMP) fragments are released into synovial fluid. 8 COMP, which belongs to the family of thrombospondins, is currently being used as a biomarker of joint destruction, 9 as its concentration correlates with both the severity of the disease as well as with the number of affected joints. An abstract published by Calamia et al. 10 in 2016 identified 3 specific peptides derived from COMP that wounded zones of cartilage release into synovial fluid. The 3 peptides reported originate from the multifunctional C-terminal domain of COMP, which plays diverse roles in cartilage homeostasis, 11 inflammation, 12 and transforming growth factor-β (TGF-β) signaling.13,14 Previous research found that full-length COMP was unable to modify the expression of proinflammatory markers in cartilage, 15 unlike its binding partner MATN3 and its fragments. 16 However, we hypothesized that those COMP fragments released by cartilage could have an OA-relevant biological function.
In this work, we asked if any of those 3 peptides is involved in responses associated to osteoarthritic joint disease; for example, by affecting vascularization, by modulating downstream TGF-β signaling or by altering the expression of synovial catabolic enzymes.
Methods
Peptides
Three peptides were produced by chemical synthesis (Peptide2.0, Chantilly, VA, USA), with the sequences 631AEPGIQLKAV (peptide 1) 642SSTGPGEQLRNA (peptide 2) and 553VLNQGREIVQT (peptide 3), reconstituted in dimethyl sulfoxide (DMSO) and stored at −80 °C. Phosphate buffered saline (PBS) was used to adjust the concentration and equivalent DMSO dilutions were used as controls on the experiments. To screen for the possible functions of the peptides, we selected a single concentration of 100 nM for most of the assays. We based our choice on the maximum amount of peptides that 10 μg/mL of the parent protein COMP would release if COMP was completely cleaved, as COMP demonstrates bioactive effects in vitro at this concentration17,18 and COMP is present in this concentration range in human serum. 19
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza, and expanded in endothelial growth media (EGM-2), containing 2% fetal bovine serum (FBS), 5 ng/mL epidermal growth factor (EGF), 10 ng/ml basic fibroblast growth factor (bFGF), 20 ng/mL insulin-like growth factor (IGF), 0.5 ng/mL vascular endothelial growth factor (VEGF) 165, 1 µg/mL l-ascorbic acid 2-phosphate, 22.5 µg/mL heparin, and 0.2 µg/mL hydrocortisone (all from Promocell via Bioconnect, Huissen, the Netherlands). Peptide testing was carried on endothelial basal media (EBM-2), which did not contain growth factors or supplements (Lonza, Geleen, the Netherlands).
Osteochondroprogenitor cells were isolated from leftover iliac crest bone chip material obtained from 4 pediatric patients undergoing alveolar bone graft surgery (following parental consent and approval of medical ethics committee of Erasmus MC: MEC-2014-16; age 9-13 years). Cells were expanded in α-minimum essential medium (αMEM; Gibco, Bleiswijk, the Netherlands) containing 10% FBS (Gibco, Bleiswijk, the Netherlands) and supplemented with 50 µg/mL gentamycin (Gibco, Bleiswijk, the Netherlands), 1.5 µg/mL amphotericin B (Gibco, Bleiswijk, the Netherlands), 25 µg/mL l-ascorbic acid 2-phosphate (Sigma, Zwijndrecht, the Netherlands), and 1 ng/mL FGF-2 (Bio-Rad via Bioconnect, Huissen, the Netherlands) in a humidified atmosphere at 37 °C and 5% CO2. Cells from passages 4 and 5 were used. For the following cell stimulation experiments, peptides were incorporated to αMEM supplemented with 50 µg/mL gentamycin, 1.5 µg/mL amphotericin B, and 1.25 mg/mL bovine serum albumin (BSA; product code 1002759876, Sigma, Zwijndrecht, the Netherlands). For those experiments involving cell stimulation with the peptides in combination with TGF-β, a dose of 0.1 ng/mL of TGF-β3 (R&D, Abingdon, UK) was added to the stimulation media. This dose of TGF-β3 was first determined experimentally and corresponded to the half-effective dose to upregulate the expression of COMP in the osteochondroprogenitor cells (data not shown), which corresponded to a molar ratio of TGF-β3:peptide of 1:25.
Migration Assay
Migration was assessed with modified Boyden chambers (polyethylene terephthalate cell culture inserts with 8 µm pore size) (Corning, Durham, NC, USA). In brief, 5 × 104 HUVECs were seeded on the upper insert membrane in EBM-2 and allowed to migrate toward the lower chamber containing EBM-2 and the peptides for 10 hours at 37 °C. In order to ensure the ability of the cells to migrate, EGM-2 was loaded in parallel as a positive control and the basal medium EBM-2 was used as a negative control. Then, the cells on the membrane were fixed with 4% formalin and cells on the upper surface were removed with a cotton swab, followed by 5-minute DAPI (4′,6-diamidino-2-phenylindole) staining. Migrated cells on each membrane were imaged with a fluorescence microscope (Zeiss Axiovert 200M) in 5 random fields of 1.51 mm2 each, automatically counted using ImageJ software, and the average counts per membrane expressed as cells/mm2.
Proliferation Assay
The number of HUVECs that proliferated after 24 hours was investigated with the EdU cell proliferation kit (Baseclik, Neuried, Germany). First, 7,500 cells/cm2 were seeded in EGM-2 and allowed to attach overnight. Then, cell cycles were synchronized by substituting the media with EBM-2 supplemented with 1.25 mg/mL BSA for 8 hours. Next, cells were stimulated with the peptides in combination with EdU 10 µM in EBM-2. EBM-2 alone was used as a negative control for proliferation, and EGM-2 was used as a positive control. After 24 hours, cells were fixed with 4% formalin and stained according to the manufacturer’s kit protocol. Finally, positive cells were imaged with a fluorescence microscope (Zeiss Axiovert 200M) in 5 random fields of 1.51 mm2 each. Total nuclei (DAPI) and EdU+ nuclei were automatically counted using ImageJ software. Percentage of proliferated cells per field was calculated as the number of EdU+ nuclei divided by total nuclei. Then, total proliferation per membrane was calculated as the average proliferation of its 5 fields.
Tube Formation Assay
Fifty microliters of Geltrex LDEV-Free Reduced Growth Factor Basement Membrane Matrix (Fischer Scientific, Landsmeer, the Netherlands) were allowed to polymerize for 30 minutes at 37 °C on a 96-well plate. Then, HUVECs were resuspended in EBM-2 containing the peptides seeded at a density of 2 × 104 cells per cm2 and incubated at 37 °C. EGM-2 and EBM-2 were used as positive and negative controls, respectively. After 24 hours, 5 random images of the tubes formed (1.9 mm2 each) were taken using an inverted microscope. Automatic measurements of the tubes were performed with the Angiogenesis Analyzer plugin for ImageJ to determine the average number of nodes per condition.
Viability Assay
Osteochondroprogenitor cellular viability in presence of the peptides was assessed with the colorimetric MTT assay. This assay relies on the metabolic reduction of the tetrazolium dye MTT to formazan, which has a purple color. A total of 24,000 cells/cm2 from one single donor were seeded in expansion media. Next day, medium was replaced by αMEM supplemented with 50 µg/mL gentamycin, 1.5 µg/mL amphotericin B, 1.25 mg/mL BSA, and with different peptides concentrations up to 1 µM for 24 hours. MTT was added into the media at a final concentration of 0.9 mM during the 3 last hours. After a PBS wash, precipitated colorant was extracted with absolute ethanol. Absorbance was measured on a spectrophotometer (VersaMax, Molecular Devices), as A570 − A670. Finally, viability was calculated as absorbance relative to the untreated condition.
Synovial Explant Culture
Synovial explants were obtained from leftover material from 4 patients undergoing total knee replacement surgery, both male and female and ranging from 67 to 77 years old, at the hospitals of Erasmus MC, Rotterdam and Reinier de Graaf Gasthuis, Delft (the Netherlands). The patients gave implicit consent as stated by guidelines of the Federation of Biomedical Scientific Societies (www.federa.org) and with approval of the local ethical committee at Erasmus MC (MEC-2004-322). Explants were cut in pieces of similar size and washed with saline solution (0.90% w/v of NaCl). Then, they were cultured for 24 hours in low-glucose DMEM (Dulbecco’s modified Eagle medium) containing 1:100 v/v insulin-transferrin-selenium (ITS+; Corning, Amsterdam, the Netherlands), 50 µg/mL gentamycin, and 1.5 µg/mL amphotericin B. Peptides were supplemented twice during the following 48 hours. Explants were snap frozen and stored at −80 °C for further analyses.
Gene Expression
Frozen samples were microdismembrated and total RNA was isolated using the RNeasy Plus Micro kit (Qiagen-Benelux, Venlo, the Netherlands). Three hundred nanograms RNA were reverse transcribed to cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Bleiswijk, the Netherlands). mRNA expression was measured for COMP, B2M, TNFA, IL6, and UBC with qPCR Mastermix Plus for SYBR Green I (Eurogentec, Nederland B.V., Maastricht, the Netherlands). For ADAMTS4, ADAMTS5, GAPDH, MMP1, MMP3, and MMP13, TaqMan Master Mix (ABI, Branchburg, NJ, USA) was used. Primer sequences were as follows:
SYBR probes.
COMP fw: 5′-CCCCAATGAAAAGGACAACTGC-3′; rv: 5′-GTCCTTTTGGTCGTCGTTCTTC-3′
B2M fw: 5′-TGCTCGCGCTACTCTCTCTTT-3′; rv: 5′-TCTGCTGGATGACGTGAGTAAAC-3′
TNFA fw: 5′-GCCGCATCGCCGTCTCCTAC-3′; rv: 5′-GCCGCATCGCCGTCTCCTAC-3′
IL6 fw: 5′-TCGAGCCCACCGGGAACGAA-3′; rv: 5′-GCAGGGAGGGCAGCAGGCAA-3′
UBC fw: 5′-ATTTGGGTCGCGGTTCTTG-3′; rv: 5′-TGCCTTGACATTCTCGATGGT-3′
TaqMan probes.
ADAMTS4 fw: 5′-CAAGGTCCCATGTGCAACGT-3′; rv: 5′-CATCTGCCACCACCAGTGTCT-3′;
probe: FAM-5′-CCGAAGAGCCAAGCGCTTTGCTTC-3′-TAMRA
ADAMTS5 fw: 5′-TGTCCTGCCAGCGGATGT-3′; rv: 5′-ACGGAATTACTGTACGGCCTACA-3′;
probe: FAM-5′-TTCTCCAAAGGTGACCGATGGCACTG-3′-TAMRA
GAPDH fw: 5′- ATGGGGAAGGTGAAGGTCG-3′; rv: 5′-TAAAAGCAGCCCTGGTGACC-3′;
probe: FAM-5′-CGCCCAATACGACCAAATCCGTTGAC-3′-TAMRA
MMP1 fw: 5′-CTCAATTTCACTTCTGTTTTCTG-3′; rv: 5′-CATCTCTGTCGGCAAATTCGT-3′;
probe: FAM-5′-CACAACTGCCAAATGGGCTTGAAGC-3′-TAMRA
MMP3 fw: 5′-TTTTGGCCATCTCTTCCTTCA-3′; rv: 5′-TGTGGATGCCTCTTGGGTATC-3′;
probe: FAM-5′-AACTTCATATGCGGCATCCACGCC-3′-TAMRA
MMP13 fw: 5′-AAGGAGCATGGCGACTTCT-3′; rv: 5′-TGGCCCAGGAGGAAAAGC-3′;
probe: FAM-5′-CCCTCTGGCCTGCGGCTCA-3′-TAMRA
Data were analyzed using the Livak method (2−ΔΔCT). For the set of experiments of osteochondroprogenitor cells, the reference gene used was GAPDH. For the set of experiments using synovial explants, normalization was based on the BestKeeper Index (BKI) using the genes GAPDH, UBC, and B2M. Gene expression was expressed relative to the untreated condition.
Statistical Analysis
Differences between treatments were assessed with 1-way analysis of variance, 2-sided with Dunnett’s post hoc test. For those experiments involving multiple donor samples, data were analyzed using the linear mix model with Bonferroni correction. In both cases, normality of the data was assumed. Statistics were performed with IBM SPSS Statistics (version 25.0.0.1 for Windows, IBM Corp., Armonk, NY, USA), and graphs were created using GraphPad Prism (version 6.1 for Windows, GraphPad Software, La Jolla, CA, USA).
Results
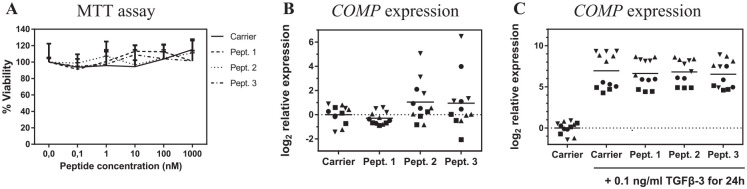
None of the Peptides Were Able to Modify the Gene Expression of COMP
TGF-β can trigger the expression of COMP, 20 and the resulting COMP protein can further enhance TGF-β activity in a positive feedback loop. Because the 3 peptides derive from the COMP C-terminal domain, which is responsible for the binding to TGF-β, 13 we hypothesized that the peptides could have a modulatory activity over this COMP-TGF-β-COMP feedback loop. Therefore, we investigated whether any of the peptides could modulate the expression of their parental gene COMP. For this, we used bone marrow osteochondroprogenitor stem cells, which have a moderate ability to differentiate and to repair damaged articular cartilage by depositing fibrocartilage. 21 After confirming that the peptides did not affect cellular viability ( Fig. 1A ), we evaluated whether any of the peptides could upregulate the expression of COMP at the mRNA level. Consequently, cells were stimulated with the peptides at 100 nM and COMP expression was assessed by qPCR. However, no significant differences were found in COMP expression levels ( Fig. 1B ). Then, we asked if any of the peptides could modulate COMP production when TGF-β signaling was triggered. Accordingly, COMP expression was measured after 24 hours of peptide stimulation in presence of TGF-β3. Despite COMP was being upregulated by TGF-β in a donor-dependent manner, no differences in COMP expression were found in the presence or absence of the peptides.
Figure 1.
Effects of the 3 peptides on bone marrow osteochondroprogenitor cell viability and COMP expression. (A) Viability assay. Cells were incubated for 24 hours in combination with the peptides, then viability was quantified by the MTT assay where the signal was normalized to the untreated cells (n = 1 donor by quadruplicate) and each peptide concentration was compared with the equivalent carrier concentration; bars represent SD. (B, C) Gene expression of COMP (n = 4 donors by triplicate) was measured by qPCR (quantitative polymerase chain reaction) after 24 hours on incubation with the peptides at 100 nM (B) or 100 nM peptides plus 0.1 ng/mL TGF-β3 (C). In both cases, all treated conditions were normalized to GAPDH, and relative to the dimethyl sulfoxide (DMSO) only condition; each donor is represented by a different symbol. Grand mean is represented.
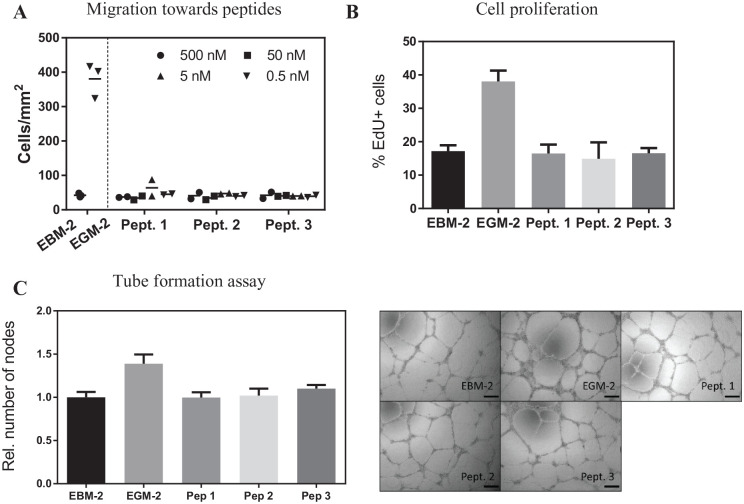
None of the Peptides Affected Vascular Tube Formation In Vitro
During osteoarthritis, increased vascular remodeling is seen in the synovium 22 and in the cartilage, which is normally avascular in healthy adult joints. 23 In order to study if the peptides would be capable of modulating vascularization, we performed different in vitro assays. First, we allowed endothelial cells (HUVECs) to migrate in a modified Boyden chamber assay with the peptides, ranging in concentrations from 0.5 to 500 nM, and we observed that none of the peptides were chemokinetic for endothelial cells at the concentrations tested ( Fig. 2A ). Next, we asked if the peptides could stimulate endothelial cell proliferation, as it is one of the common responses to angiogenic stimuli. For this purpose, cells were stimulated for 24 hours in a media containing the nucleoside analogue EdU, which labeled the replicating cells. The peptides did not significantly increase the number of proliferating cells ( Fig. 2B ). Last, we performed a tube formation assay with HUVEC on a basement membrane coated plate stimulated with the peptides for 24 hours. In this case, peptides did not influence network formation ( Fig. 2C ).
Figure 2.
Assays to test the peptides influence on vascularization potential by endothelial cells (HUVECs). (A) Migration assay. Cells were seeded on a microporous membrane and allowed to migrate towards a compartment with different concentrations of each of the peptides (n = 2/concentration). (B) Proliferation assay. Cells were incubated for 8 hours in basal medium followed by a 24-hour exposure to 100 nM peptides in basal medium (n = 3 replicates). (C) Cells were seeded on GeltrexTM in combination with the peptides at 100 nM and allowed to form a tubular network for 24 hours. Then, nodes formed were quantified (n = 3 replicates). Scale bar = 200 µm. In all experiments, both basal and growth media contained the same concentration of vehicle (dimethyl sulfoxide [DMSO]), and growth media was used as a positive control. EBM-2, endothelial basal media 2; EGM-2, endothelial growth media 2. −(EBM-2), +(EGM-2). The bars represent the mean (A) and the mean ± SD (B, C). All samples were compared with the untreated condition.
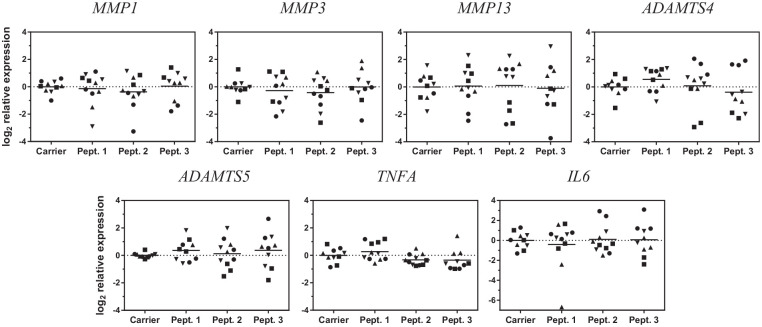
None of the Peptides Altered Gene Expression of Synovial Catabolic Enzymes or Inflammatory Mediators
As the peptides were found in synovial fluid, we asked if they could trigger an inflammatory response on the synovium. For this, synovial explants from patients undergoing total knee replacement surgery were cultured with 100 nM of the peptides or with the carrier (DMSO) for 48 h, and gene expression of MMP1, -3, -13 and ADAMTS-4 and -5—proteases known to degrade extracellular matrix in osteoarthritis—was analyzed. Gene expression of none of the proteases analyzed was affected by the presence of the peptides ( Fig. 3 ). IL6 and TNFA, which are potent regulators of catabolic processes in chondrocytes and synovial cells, were also unaffected by exposure to the peptides.
Figure 3.
Effect of the peptides on gene expression of proteolytic enzymes in synovium. Synovial explants were treated for 48 hours with the peptides and gene expression of different proteolytic enzymes was measured by quantitative polymerase chain reaction (qPCR). Each gene expression measurement was first normalised to the BestKeeper Index (BKI). Each condition is relative to the carrier control, to which is compared; each donor is represented by a different symbol (n = 4 donors in triplicate, one of them in duplicate) and grand mean is represented.
Discussion and Conclusions
Our work has focused on the search for novel bioactive fragments of COMP, one of the main extracellular matrix proteins of the cartilage which is often used as a biomarker for OA. For this, we tested if any of the three COMP-derived peptides reported by Calamia et al. 10 in 2016 could affect specific aspects related to OA. We concluded that those 3 peptides are unlikely to be involved in the expression of COMP, vascularization, or synovial expression of extracellular matrix proteases at the concentrations and time frames studied.
Identification of novel matrikines and matricryptins is challenging, as their functions can be similar, opposite, or completely different to their parent protein. During OA, increased amounts of COMP are released 24 and increased concentrations of monomeric forms of COMP as well as smaller fragments appear as a result of proteolytic activity. 25 In a recent study, we showed that in the absence of its oligomerization domain, which is required to form pentameric structures, COMP’s angiogenic-related functions disappear. 26 Matrikines and matricryptins, however, have been shown to have different biological functions to the protein from which they are derived, leading us to assess the effects of these identified peptides on processes crucial for the onset and development of OA.
The 3 peptides derive from COMP’s C-terminal domain: 631AEPGIQLKAV (peptide 1), 642SSTGPGEQLRNA (peptide 2), and 553VLNQGREIVQT (peptide 3) and are well conserved across species. Peptides 1 and 2 are completely identical among mammals, while 558R in peptide 3 is exclusive for primates instead of the 558M found among other mammals. Interestingly, peptides with similar sequences can be potentially released from other proteins of the thrombospondin family, due to their high similarities. Although it is not clear which are the proteases responsible to produce these peptides, they might be related to OA-relevant proteases. ADAMTS-4 could be responsible for the production of 553V cleavage on peptide 3. 27 These peptides are not likely to be involved in PSACH/MED, as none of the closest COMP mutations fall on the peptides 529T, 585T, and 718R.28-30 The 3 peptides span sequences where secondary structures initiate: beta sheets for peptides 1 and 3 and alpha helix for peptide 2. However, it is possible that the proteolytic events that release the peptides from the protein results on a peptide’s secondary structure different to the one in the parent protein. Our experiments were designed based on the known functions of the protein domain where the peptides are derived from. The main limitations for this study were that we could not account for the possible interactions between the peptides and other factors. In our screening approach, we assumed that cellular responses would be proportional to the peptide concentration, and that our selected single dose would be the highest possible within the physiological range. We cannot exclude, however, that other doses or experimental durations (though optimized previously) could have had an effect on the processes. Also, we could not account for the possibility that the synovial explants used may have already encountered the peptides in vivo, which could have led to some level of desensitization. In addition, we observed a high variability between donors and between explants of the same donor. This could be explained by the different degrees of inflammation and synovial damage of the patients, which could turn a patient unresponsive to our testing stimuli. In order to reduce interindividual variability, it would be advisable for future experiments to seek for sources of healthy synovium. Last, because matrikines and matricryptins may play different roles to their parent proteins, we cannot exclude their involvement on other processes in OA than those analyzed. Following this research, Calamia et al. 31 identified more peptides derived from COMP, in parallel to multiple peptides derived from other matricellular proteins. For future preselection of candidates, it might be advisable to prioritize those matrikines and matricryptins either known or strongly suspected to be biomarkers of diseases, in particular if containing certain motifs such as RGD or known interaction sites for growth factors. The reason is that, because diseased individuals possess those molecules in concentrations different than healthy individuals, those molecules are more likely to be actively involved into the pathogenesis of the disease, either by playing detrimental roles or by stimulating compensatory mechanisms.
Footnotes
Authors’ Note: The present study was performed at the Erasmus MC, University Medical Center Rotterdam, the Netherlands. Data supporting the findings of this study are available within the article. Raw data were generated at Erasmus MC. Raw data are available on request from the corresponding author.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project has received funding from the European Union’s Horizon 2020 research and innovation program under Marie Sklodowska-Curie grant agreement no. 721432.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Osteochondroprogenitor cells were isolated after approval of medical ethics committee of Erasmus MC (MEC-2014-16). Synovial explants were obtained from patients with approval of the local ethical committee at Erasmus MC (MEC-2004-322).
Informed Consent: Parental consent was obtained in case of pediatric patients. Patients gave implicit consent as stated by guidelines of the Federation of Biomedical Scientific Societies (www.federa.org).
Trial Registration: Not applicable.
ORCID iDs: Enrique Andrés Sastre  https://orcid.org/0000-0003-2041-6014
https://orcid.org/0000-0003-2041-6014
Frank Zaucke  https://orcid.org/0000-0002-7680-9354
https://orcid.org/0000-0002-7680-9354
References
- 1. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 2. Skioldebrand E, Ekman S, Mattsson Hulten L, Svala E, Bjorkman K, Lindahl A, et al. Cartilage oligomeric matrix protein neoepitope in the synovial fluid of horses with acute lameness: a new biomarker for the early stages of osteoarthritis. Equine Vet J. 2017;49(5):662-7. doi: 10.1111/evj.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol. 2014;23(7):457-63. doi: 10.1111/exd.12435 [DOI] [PubMed] [Google Scholar]
- 4. Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of Angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92(3):391-400. doi: 10.1016/s0092-8674(00)80931-9 [DOI] [PubMed] [Google Scholar]
- 5. Lees S, Golub SB, Last K, Zeng W, Jackson DC, Sutton P, et al. Bioactivity in an aggrecan 32-mer fragment is mediated via toll-like receptor 2. Arthritis Rheumatol. 2015;67(5):1240-9. doi: 10.1002/art.39063 [DOI] [PubMed] [Google Scholar]
- 6. López-Moratalla N, del Mar Calonge M, López-Zabalza MJ, Pérez-Mediavilla LA, Subirá ML, Santiago E. Activation of human lymphomononuclear cells by peptides derived from extracellular matrix proteins. Biochim Biophys Acta. 1995;1265(2-3):181-8. doi: 10.1016/0167-4889(94)00199-o [DOI] [PubMed] [Google Scholar]
- 7. Stanton H, Ung L, Fosang AJ. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem J. 2002;364(Pt 1):181-90. doi: 10.1042/bj3640181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lohmander LS, Saxne T, Heinegard DK. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann Rheum Dis. 1994;53(1):8-13. doi: 10.1136/ard.53.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorenzo P, Aspberg A, Saxne T, Onnerfjord P. Quantification of cartilage oligomeric matrix protein (COMP) and a COMP neoepitope in synovial fluid of patients with different joint disorders by novel automated assays. Osteoarthritis Cartilage. 2017;25(9):1436-42. doi: 10.1016/j.joca.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 10. Calamia V, Fernández-Puente P, Lourido L, Camacho M, González L, Blanco FJ, et al. Development of a multiplexed assay for the targeted analysis of cartilage endogenous peptides in synovial fluid and serum from osteoarthritis patients [Abstract]. Osteoarthritis Cartilage. 2016;24(Suppl 1):S160. doi: 10.1016/j.joca.2016.01.315 [DOI] [Google Scholar]
- 11. Di Cesare PE, Chen FS, Moergelin M, Carlson CS, Leslie MP, Perris R, et al. Matrix–matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002; 21(5):461-70. doi: 10.1016/s0945-053x(02)00015-x [DOI] [PubMed] [Google Scholar]
- 12. Fu Y, Gao C, Liang Y, Wang M, Huang Y, Ma W, et al. Shift of macrophage phenotype due to cartilage oligomeric matrix protein deficiency drives atherosclerotic calcification. Circ Res. 2016;119(2_suppl):261-76. doi: 10.1161/CIRCRESAHA.115.308021 [DOI] [PubMed] [Google Scholar]
- 13. Haudenschild DR, Hong E, Yik JH, Chromy B, Morgelin M, Snow KD, et al. Enhanced activity of transforming growth factor beta1 (TGF-β1) bound to cartilage oligomeric matrix protein. J Biol Chem. 2011;286(50):43250-8. doi: 10.1074/jbc.M111.234716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du Y, Wang Y, Wang L, Liu B, Tian Q, Liu CJ, et al. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ Res. 2011;108(8):917-28. doi: 10.1161/CIRCRESAHA.110.234328 [DOI] [PubMed] [Google Scholar]
- 15. Ruthard J, Kamper M, Renno JH, Kuhn G, Hillebrand U, Hollriegl S, et al. COMP does not directly modify the expression of genes involved in cartilage homeostasis in contrast to several other cartilage matrix proteins. Connect Tissue Res. 2014;55(5-6):348-56. doi: 10.3109/03008207.2014.951440 [DOI] [PubMed] [Google Scholar]
- 16. Klatt AR, Klinger G, Paul-Klausch B, Kuhn G, Renno JH, Wagener R, et al. Matrilin-3 activates the expression of osteoarthritis-associated genes in primary human chondrocytes. FEBS Lett. 2009;583(22):3611-7. doi: 10.1016/j.febslet.2009.10.035 [DOI] [PubMed] [Google Scholar]
- 17. Ma B, Yao F, Xie N, Mao C, Liu F, Gong Z, et al. Cartilage oligomeric matrix protein is a novel notch ligand driving embryonic stem cell differentiation towards the smooth muscle lineage. J Mol Cell Cardiol. 2018;121:69-80. doi: 10.1016/j.yjmcc.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 18. Geng H, Nandakumar KS, Xiong L, Jie R, Dong J, Holmdahl R. Incomplete B cell tolerance to cartilage oligomeric matrix protein in mice. Arthritis Rheum. 2013;65(9):2301-9. doi: 10.1002/art.38046 [DOI] [PubMed] [Google Scholar]
- 19. Saxne T, Heinegard D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31(9):583-91. doi: 10.1093/rheumatology/31.9.583 [DOI] [PubMed] [Google Scholar]
- 20. Li H, Haudenschild DR, Posey KL, Hecht JT, Di Cesare PE, Yik JH. Comparative analysis with collagen type II distinguishes cartilage oligomeric matrix protein as a primary TGFβ-responsive gene. Osteoarthritis Cartilage. 2011;19(10): 1246-53. doi: 10.1016/j.joca.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orth P, Gao L, Madry H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):670-706. doi: 10.1007/s00167-019-05359-9 [DOI] [PubMed] [Google Scholar]
- 22. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390-8. doi: 10.1038/nrrheum.2012.80 [DOI] [PubMed] [Google Scholar]
- 23. Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Franses RE, Mapp PI, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49(10):1852-61. doi: 10.1093/rheumatology/keq188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams FM, Spector TD. Biomarkers in osteoarthritis. Arthritis Res Ther. 2008;10(1):101. doi: 10.1186/ar2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahrman E, Lorenzo P, Holmgren K, Grodzinsky AJ, Dahlberg LE, Saxne T, et al. Novel cartilage oligomeric matrix protein (COMP) neoepitopes identified in synovial fluids from patients with joint diseases using affinity chromatography and mass spectrometry. J Biol Chem. 2014;289(30):20908-16. doi: 10.1074/jbc.M114.554683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andrés Sastre E, Zaucke F, Witte-Bouma J, Maly K, van Osch GJVM, Farrell E. Cartilage oligomeric matrix protein inhibits angiogenesis in vitro, depending upon its oligomerisation state [Abstract]. In: Proceedings of Tissue Engineering and Regenerative Medicine International Society (TERMIS) European Chapter Meeting; May 27-31, 2019; Rhodes, Greece; p. 988. https://termis.org/eu2019/. [Google Scholar]
- 27. Zhen EY, Brittain IJ, Laska DA, Mitchell PG, Sumer EU, Karsdal MA, et al. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008;58(8):2420-31. doi: 10.1002/art.23654 [DOI] [PubMed] [Google Scholar]
- 28. Kennedy J, Jackson G, Ramsden S, Taylor J, Newman W, Wright MJ, et al. COMP mutation screening as an aid for the clinical diagnosis and counselling of patients with a suspected diagnosis of pseudoachondroplasia or multiple epiphyseal dysplasia. Eur J Hum Genet. 2005;13(5):547-55. doi: 10.1038/sj.ejhg.5201374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Briggs MD, Mortier GR, Cole WG, King LM, Golik SS, Bonaventure J, et al. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am J Hum Genet. 1998;62(2_suppl):311-9. doi: 10.1086/301713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jakkula E, Lohiniva J, Capone A, Bonafe L, Marti M, Schuster V, et al. A recurrent R718W mutation in COMP results in multiple epiphyseal dysplasia with mild myopathy: clinical and pathogenetic overlap with collagen IX mutations. J Med Genet. 2003;40(12):942-8. doi: 10.1136/jmg.40.12.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandez-Puente P, Gonzalez-Rodriguez L, Calamia V, Picchi F, Lourido L, Camacho-Encina M, et al. Analysis of endogenous peptides released from osteoarthritic cartilage unravels novel pathogenic markers. Mol Cell Proteomics. 2019;18(10):2018-28. doi: 10.1074/mcp.RA119.001554 [DOI] [PMC free article] [PubMed] [Google Scholar]