Abstract
Objective
The aims of the study were to assess the anti-inflammatory properties of platelet-rich plasma (PRP) and investigate its regenerative potential in osteoarthritic (OA) human chondrocytes. We hypothesized that PRP can modulate the inflammatory response and stimulate cartilage regeneration.
Design
Primary human chondrocytes from OA knees were treated with manually prepared PRP, after which cell migration and proliferation were assessed. Next, tumor necrosis factor-α–stimulated chondrocytes were treated with a range of concentrations of PRP. Expression of genes involved in inflammation and chondrogenesis was determined by real-time polymerase chain reaction. In addition, chondrocytes were cultured in PRP gels and fibrin gels consisting of increasing concentrations of PRP. The production of cartilage extracellular matrix (ECM) was assessed. Deposition and release of glycosaminoglycans (GAG) and collagen was quantitatively determined and visualized by (immuno)histochemistry. Proliferation was assessed by quantitative measurement of DNA.
Results
Both migration and the inflammatory response were altered by PRP, while proliferation was stimulated. Expression of chondrogenic markers COL2A1 and ACAN was downregulated by PRP, independent of PRP concentration. Chondrocytes cultured in PRP gel for 28 days proliferated significantly more when compared with chondrocytes cultured in fibrin gels. This effect was dose dependent. Significantly less GAGs and collagen were produced by chondrocytes cultured in PRP gels when compared with fibrin gels. This was qualitatively confirmed by histology.
Conclusions
PRP stimulated chondrocyte proliferation, but not migration. Also, production of cartilage ECM was strongly downregulated by PRP. Furthermore, PRP did not act anti-inflammatory on chondrocytes in an in vitro inflammation model.
Keywords: platelet-rich plasma, chondrocyte, inflammation, regeneration, PRP gel
Introduction
Platelet-rich plasma (PRP) is a biological, blood-derived product. It can be produced from a patient’s own blood for autologous applications. Because of the high concentration of blood platelets, PRP contains a rich cocktail of growth factors. 1 Its applications in the field of orthopedics have been described to a large extent, both in basic and clinical research. In clinical practice, PRP is mostly administered by intra-articular injection for the treatment of osteoarthritis (OA) in various joints,2,3 tendinopathies, 4 meniscal damage, 5 or osteochondral lesions. 6 In general, clinical studies report on (short term) positive clinical outcomes, characterized by a decrease in pain and improvement of function.7,8 The effects of PRP are generally attributed to the cocktail of growth factors, combined with anti-inflammatory mediators present in the PRP. Injection of PRP in OA could potentially stimulate regeneration of the cartilage by suppressing the pro-inflammatory environment in the joint and stimulate an anabolic state. Overall, clinical results are presented in a range from highly positive to highly negative or even considering the presence of a substantial placebo effect.9-12 In basic science, in vitro studies report on enhancement of chondrogenic differentiation of mesenchymal stromal cells (MSCs), 13 and matrix production by chondrocytes stimulated with PRP. 14 In addition, enhancement of proliferation by PRP has been described for a range of intra-articular cell types, such as meniscal cells, 15 articular chondrocytes,16-18 tenocytes, 19 and osteoblasts. 20
Moreover, PRP is ideally suited for making a gel rich in platelets by mimicking physiological blood coagulation. By activation of the fibrinogen in PRP by either centrifugation of non-anticoagulated PRP or addition of thrombin and calcium to anticoagulated PRP, a 3-dimensional (3D) platelet-rich fibrin clot can be created. This allows encapsulation of primary chondrocytes, comparable to what can be done with commercially available fibrin glue. This system has the potential to be used for tissue engineering applications, or as a biomaterial to, for example, treat focal cartilage defects in a clinical setting.21,22
Considering the controversy around the use of PRP and variety in reported clinical results, the objective of this study was to study several potential working mechanisms of PRP with primary OA chondrocytes in vitro in a controlled setting. We hypothesized that PRP would have an anti-inflammatory, and migration-stimulating effect on OA chondrocytes and would be effective in stimulating matrix synthesis by OA chondrocytes in a 3D PRP gel environment.
Methods
Platelet-Rich Plasma Preparation and Characterization
Blood was obtained from healthy donors through the Mini Donor Service of the University Medical Center Utrecht approved by the medical ethics committee. All donors provided written informed consent in accordance with the Declaration of Helsinki and were healthy, and free from antiplatelet and nonsteroidal anti-inflammatory drugs. Blood was collected in sterile 9-mL tubes containing 1 mL 3.2% (w/v) sodium citrate. To make PRP, the blood was centrifuged at 130 × g for 15 minutes, after which the plasma layer was transferred to a new tube. The plasma was then centrifuged at 250 × g for 15 minutes and pelleted platelets were resuspended in one-third of the supernatant platelet-poor plasma (PPP). Platelet count was measured using a CELL-DYN Emerald Hematology Analyzer (Abbott) (n = 9). For further experiments, platelet count was standardized at 400 × 109/L by dilution with phosphate buffered saline free from Ca2+ and Mg2+ (PBS0). The concentrations of transforming growth factor-β1 (TGF-β1), platelet-derived growth factor-AB (PDGF-AB), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF) were determined using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (all Duoset ELISA kit, R&D Systems) (n = 5).
Chondrocyte Isolation
Human osteoarthritic articular cartilage was obtained from redundant material after total knee arthroplasty surgery. The anonymous collection of this material is approved by the local medical ethics committee (University Medical Center Utrecht).23,24 Cartilage was separated from the bone, washed with PBS0, and cut into 2-mm pieces using a scalpel. To minimize intra- and interdonor variability, all cartilage was pooled per donor for cell isolation. Chondrocytes were extracted by exposing the cartilage pieces to 0.15% (w/v) collagenase II (CLS-2, Worthington, Lakewood, NJ) in Dulbecco’s modified Eagle medium (DMEM, Gibco, Life Technologies) supplemented with 10% (v/v) fetal bovine serum (FBS, Biowest), and penicillin and streptomycin (100 U/mL and 100 µg/mL; 1%) overnight at 37°C on a moving platform. Isolated cells were expanded at 37°C and 5% CO2 using chondrocyte expansion medium, consisting of DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Chondrocytes were used at passage 2, cells from different donors were kept separate throughout the experiments.
Proliferation and Migration Assay
Proliferation and migration of chondrocytes on addition of PRP into the culture medium was assessed using a micro-wound assay.25-27 Chondrocytes (n = 2, age range 48-64 years) were seeded in monolayers in 24-well plates using expansion medium. On confluence, a micro-wound was created across each well using a sterile 200 µL pipette tip. Monolayers were washed using PBS0, and treated with redifferentiation medium (DMEM supplemented with 2% [v/v] human serum albumin [HSA; Sanquin Blood Supply Foundation], 2% insulin-transferrin selenium [ITS]-X [Gibco], 0.2 mM l-ascorbic acid 2-phosphate [ASAP; Sigma-Aldrich], 100 U/mL penicillin, and 100 µg/mL streptomycin) with respectively 0%, 2%, 5%, 10%, or 20% (v/v) PRP. All PRP-containing media were supplemented with 3.3 U/mL heparin (Sigma-Aldrich). All medium was supplemented with 10 μM 5-ethylnyl-2′-deoxyuridine (EdU; Click-iT EdU Alexa Fluor 488 Imaging Kit; Invitrogen). After 48 hours of incubation at 37°C and 5% CO2, monolayers were washed with PBS0 and EdU was detected according to the manufacturer’s instructions. Nuclei were counterstained with Hoechst. Photographs were taken using an inverted fluorescent microscope (IX53; Olympus). Total migrated cells and proliferated cells in the micro-wound area were quantified using ImageJ software via color thresholding and the “analyze particles” function.
In Vitro Inflammation Assay
Chondrocytes (n = 6, age range = 48-74 years) were seeded in monolayers at 100,000 cells/cm2 in chondrocyte expansion medium. After 24 hours of preincubation, monolayers were treated with 10 ng/mL recombinant human tumor necrosis factor-α (TNF-α, R&D Systems) and redifferentiation medium with, respectively, 0%, 2%, 5%, 10%, and 20% (v/v) PRP. Cells were incubated for 48 hours at 37°C and 5% CO2 before gene expression analysis.
Regeneration Assay: Gels with Variable PRP Concentration
Cells (n = 2 donors, age range = 53-79 years) were cultured in fibrin gels containing increasing concentrations of PRP. For this purpose, PRP was mixed with 1:15 in PBS0 diluted fibrinogen component (Baxter) in which cells were resuspended. The commercial fibrinogen was diluted to match the concentrations within the full range of conditions. 28 Gels were made by injecting 50 µL of the mixture into a 96-well plate well and adding 50 µL 1:50 in PBS0 diluted thrombin component (Baxter) and incubated for 15 minutes at 37°C. This resulted in gels containing 0.25 × 106 cells per construct with, respectively, 20%, 40%, 50%, 60%, and 80% PRP. Gels were cultured in 24-well plates for 28 days in redifferentiation medium. Medium was changed twice per week and saved for biochemical analysis.
Regeneration Assay: PRP versus Fibrin Gels
Chondrocytes were equally divided over the PRP and fibrin gel group. Fibrin gels were made as stated in the previous section by mixing 50 µL of each diluted component. PRP gels were made by pipetting 60 µL PRP into a 96-well plate well, adding 20 µL CaCl2 (500 mM in 0.9% NaCl) and 20 µL 1:50 in PBS0 diluted thrombin solution (Baxter), and incubation for 15 minutes at 37°C. This resulted in gels containing 0.25 × 106 chondrocytes (n = 3 donors, age range = 56-79 years). Gels were cultured in 24-well plates for 28 days using redifferentiation medium. Medium was changed twice per week and all medium was stored for biochemical analysis. Control fibrin gels were made by combining 50 µL 1:15 diluted fibrinogen component and 50 µL 1:15 diluted thrombin component (Baxter).
Real-Time Polymerase Chain Reaction
Total RNA was isolated from monolayers and gels using TRIzol (Invitrogen) according to the manufacturer’s instruction. Total RNA (200-500 ng) was reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time polymerase chain reactions (PCRs) were performed in a LightCycler 96 (Roche Diagnostics) using iTaq Universal SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instructions. Quantification was performed relative to the levels of the housekeeping gene 18S. The primer sequences are listed in Supplementary Table S1.
Biochemical Analysis
Gels were digested in a papain digestion buffer (250 µg/mL papain; Sigma-Aldrich, 0.2 M NaH2PO4, 0.1 M ethylenediaminetetraacetic acid [EDTA], 0.01 M cysteine, pH 6) at 60°C overnight. Glycosaminoglycan (GAG) content was measured using a dimethylmethylene blue (DMMB; pH 3) assay with chondroitin-6-sulfate (Sigma-Aldrich) as a standard. Absorbance ratio of 525/595 nm was measured using a spectrophotometer. DNA content in the digests was quantified using a Quant-iT PicoGreen dsDNA assay (Invitrogen) according to the manufacturer’s instruction. Collagen content was determined by measuring the hydroxyproline content 29 . Digested samples were lyophilized and hydrolyzed in 4 M NaOH (Sigma-Aldrich) in Milli-Q water at 108°C overnight. The next day, samples were neutralized using 1.4 M citric acid (Sigma-Aldrich) in Milli-Q water, after which 50 mM freshly prepared Chloramin-T (Merck) in oxidation buffer was added. Samples were incubated under agitation for 20 minutes, after which 1.1 M freshly prepared dimethylaminobenzoaldehyde (Merck) in 25% (w/v) perchloric acid (Merck) in 2-propanol (Sigma-Aldrich) was added. After incubation for 20 minutes at 60°C, samples were cooled and absorbance at 570 nm was measured using hydroxyproline (Merck) as a standard.
Histological Analysis
Gels were fixed using 3.7% formalin, dehydrated through graded alcohol steps, immersed in xylene, and embedded in paraffin. Sections of 5 µm were cut and deparaffinized and rehydrated before staining. Proteoglycans were stained using 0.125% (w/v) safranin-O (Merck; counterstained with 0.4% fast green [Sigma-Aldrich] and Weigert’s hematoxylin [Clin-Tech]). Type I and II collagen deposition was visualized by immunohistochemistry. Sections were blocked with 0.3% H2O2, followed by antigen retrieval with pronase (1 mg/mL; Sigma-Aldrich) for 30 minutes at 37°C and hyaluronidase (10 mg/mL; Sigma-Aldrich) for 30 minutes at 37°C. Sections were blocked using bovine serum albumin (BSA; 5% [w/v] in PBS) for 30 minutes, followed by overnight incubation at 4°C with the primary antibody for either type I collagen (EPR7785, BioConnect; 1:400 in PBS/BSA 5%) or type II collagen (II-II6B3; DHSB, 1:100 in PBS/BSA 5%). Sections were washed, then incubated with an HRP-conjugated anti-rabbit or anti-mouse secondary antibody for 1 hour at room temperature, after which the staining was developed using 3,3′-diaminobenzidine (DAB, Sigma-Aldrich). Sections were counterstained using Mayer’s hematoxylin (Klinipath).
Statistical Analysis
Data were analyzed using the GraphPad Prism 7.0 software package (GraphPad Software). Comparisons between groups were performed by paired and unpaired 2-sided Student t tests and 1-way analysis of variance (ANOVA) with a Tukey post hoc test. A P value of <0.05 was considered statistically significant.
Results
Manually Prepared Platelet-Rich Plasma Is Depleted from Leukocytes and Rich in Growth Factors
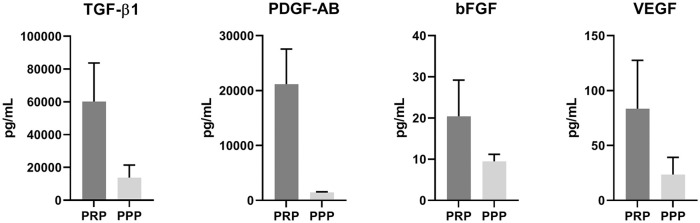
PRP was prepared from citrated blood of nine donors. Cell and platelet counts were measured and revealed a significant decrease in the number of leukocytes (0.3 ± 0.1 × 109/L vs. 5.5 ± 1.4 × 109/L, P < 0.001) and erythrocytes (0.02 ± 0.01 × 1012/L vs. 4.0 ± 0.4 × 1012/L, P < 0.001) in all PRP samples compared with whole blood samples of the same donors. Platelet count was significantly increased after PRP preparation (458 ± 245 × 109/L vs. 207 ± 74 × 109/L, P = 0.006). Increase in platelet count varied from 1.0- to 3.0-fold ( Table 1 ). Quantification of growth factors by ELISA revealed manually prepared PRP contains high amounts of TGF-β1 (60234 ± 23499 pg/mL) and PDGF-AB (21163 ± 6399 pg/mL) and lower concentrations of bFGF (20.4 ± 8.8 pg/mL) and VEGF (83.5 ± 44.1 pg/mL) ( Fig. 1 ).
Table 1.
Cell Count in Manually Prepared Platelet-Rich Plasma (PRP). a
| Whole blood | PRP | P | |
|---|---|---|---|
| Leukocytes (×109/L) | 5.5 ± 1.4 | 0.3 ± 0.1 | <0.001 |
| Erythrocytes (×1012/L) | 4.0 ± 0.4 | 0.02 ± 0.01 | <0.001 |
| Platelets (×109/L) | 207 ± 74 | 458 ± 245 | 0.006 |
Leukocyte, erythrocyte, and platelet concentrations in whole blood and PRP after the double centrifugation protocol. Data are shown as mean ± SD.
Figure 1.
Growth factor concentrations in manually prepared platelet-rich plasma. Quantification of growth factors TGF-β1, PDGF-AB, bFGF, and VEGF in platelet-rich plasma (PRP) and platelet-poor plasma (PPP) of healthy human donors (n = 5), measured by enzyme-linked immunosorbent assay (ELISA). TGF-β1, transforming growth factor-β1; PDGF-AB, platelet-derived growth factor-AB; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor.
Platelet-Rich Plasma Stimulates Chondrocyte Proliferation, but Not Migration
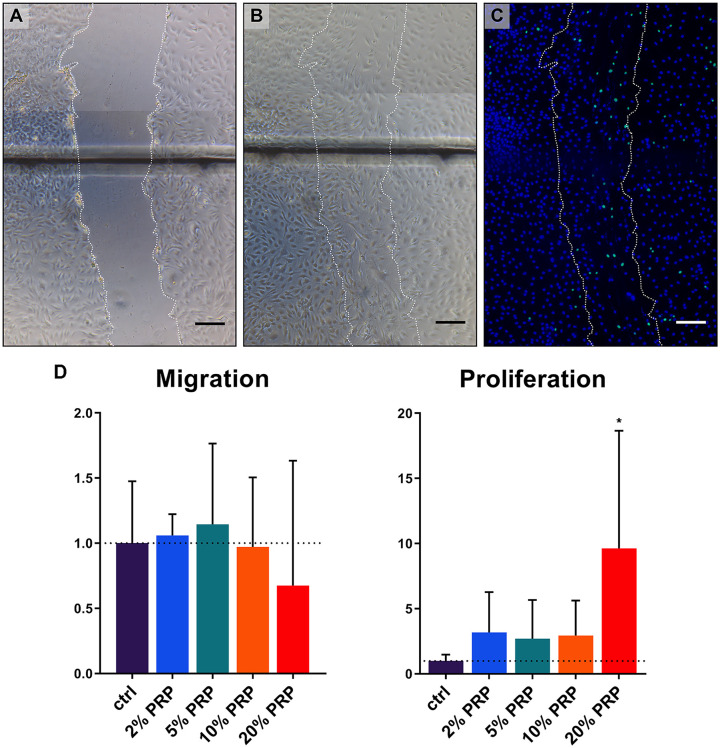
Migration of chondrocytes into a micro-wound created in a monolayer using different concentrations of PRP was compared after 48 hours ( Fig. 2A-C ). Quantification of cells migrated into the micro-wound area revealed no differences in groups containing PRP versus control group. On the contrary, proliferation of chondrocytes was significantly increased when micro-wounds were treated with 20% PRP ( Fig. 2D ).
Figure 2.
Migration and proliferation of chondrocytes into a micro-wound. Representative brightfield microscopy photographs of a micro-wound created in a chondrocyte monolayer at T0h (A), T48h (B), and fluorescent microscopy photograph at T48h (C). Scale bar = 200 µm. Migration and proliferation of chondrocytes into a micro-wound as quantified by amount of cells relative to the amounts in the control group (D). Migrated cells were quantified using Hoechst staining, proliferated cells by 5-ethylnyl-2′-deoxyuridine (EdU). Data are shown as mean ± SD. *P < 0.05.
Platelet-Rich Plasma Does Not Inhibit an Inflammatory Response in Chondrocytes
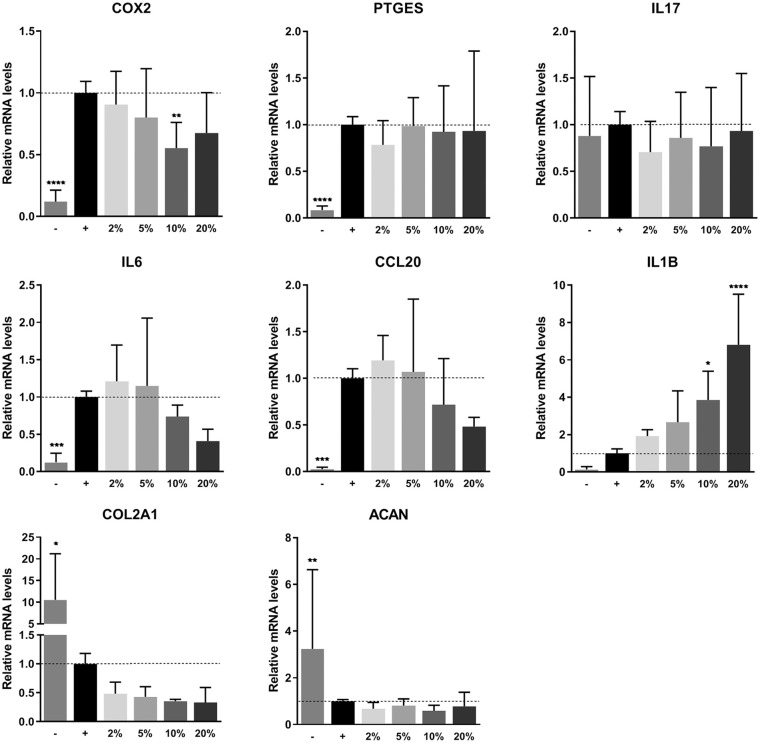
PRP was not able to inhibit the inflammatory response of chondrocytes after stimulation with TNF-α. Expression of OA-associated inflammation markers cyclooxygenase-2 (COX2, also known as prostaglandin-endoperoxide synthase 2 [PTGS2]), prostaglandin E synthase (PTGES), and interleukin 17 (IL17) was significantly upregulated when chondrocytes were treated with TNF-α. Addition of PRP to the medium was ineffective to reduce gene expression of these markers. Expression of interleukin 6 (IL6) and C-C motif chemokine ligand 20 (CCL20), other important players in OA, seemed to be downregulated dose dependently. However, this decrease was not found to be statistically significant. Expression of interleukin 1Β (IL1Β) was upregulated in a dose-dependent manner on addition of PRP. Two of the most important markers for chondrogenesis, collagen type II alpha 1 chain (COL2A1) and aggrecan (ACAN), were significantly downregulated in inflamed chondrocytes. Addition of PRP to these monolayers could not recover this gene expression ( Fig. 3 ).
Figure 3.
Gene expression on inflammation. Gene expression of chondrocyte monolayers as measured by real-time polymerase chain reaction. Monolayers were stimulated with 10 ng/mL TNF-α and PRP for 48 hours. Gene expression was normalized for 18S. Data are presented as fold changes relative to the TNF-α stimulated control group and shown as mean ± SD. ***P < 0.001, ****P < 0.0001. PRP, platelet-rich plasma; TNF-α, tumor necrosis factor-α; COX2, cyclooxygenase 2; PTGES, prostaglandin E synthase; IL17, interleukin 17; IL6, interleukin 6; CCL20, C-C motif chemokine ligand 20; IL1B, interleukin 1B; COL2A1, collagen type II alpha 1 chain; ACAN, aggrecan.
No Dose-Dependent Regenerative Effect in Gels with Variable Platelet-Rich Plasma Concentration
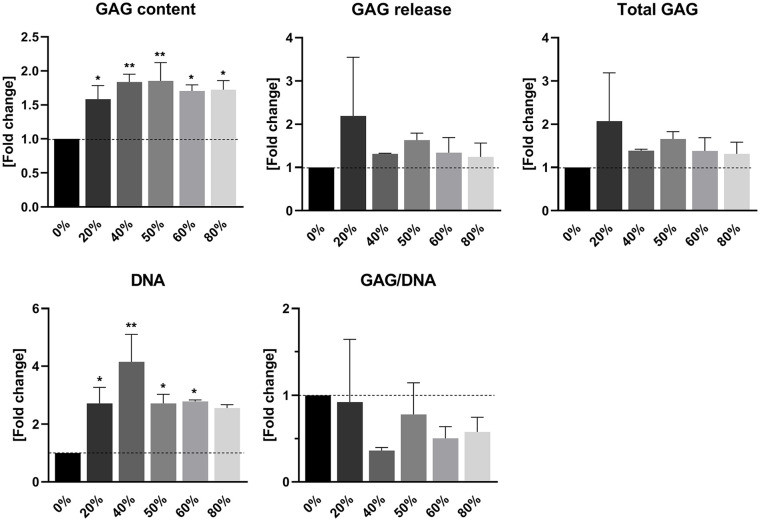
Biochemical analysis of PRP-enriched fibrin gels revealed a stimulatory effect of PRP on the production of GAGs deposited within the gels by chondrocytes. This pattern was not found in GAG release from these gels. Similar to an increase in absolute GAG content, the amount of DNA in PRP-enriched gels was also elevated. The production of GAGs corrected for the amount of DNA in the gels was significantly decreased in PRP-containing gels ( Fig. 4 ).
Figure 4.
Cartilage matrix production in fibrin gel enriched with platelet-rich plasma (PRP). Quantification of glycosaminoglycan (GAG) and DNA contents in PRP-enriched fibrin gels by chondrocytes. Release of GAGs into the culture medium was additionally quantified. Data are presented as mean ± SD and as fold changes relative to the control group devoid of PRP. *P < 0.05, **P < 0.01.
Inhibition of Chondrogenic Potential of Chondrocytes in Platelet-Rich Plasma Gels versus Fibrin Gels
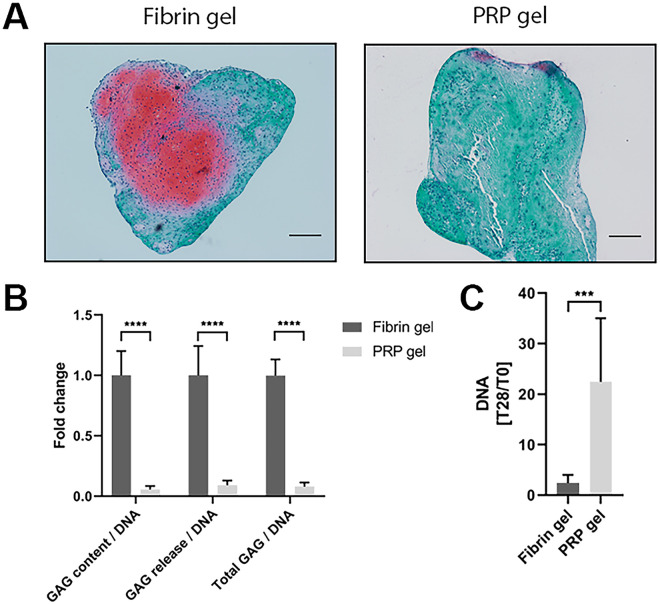
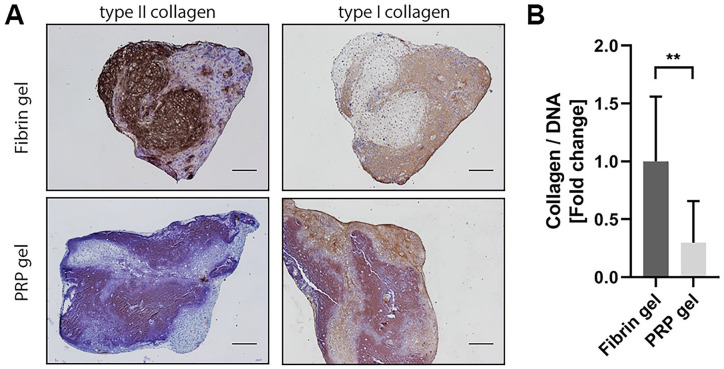
To assess the capacity of PRP to be used as a stand-alone biomaterial for cartilage tissue engineering, a PRP gel was made in which chondrocytes were encapsulated. Safranin-O staining revealed considerable amounts of GAGs produced by chondrocytes encapsulated in commercial fibrin gel. Morphology of chondrocytes in PRP gel was similar to that of cells cultured in fibrin gel. The chondrocytes exhibited a round morphology and seem to reside in lacunae, typical for hyaline cartilage (Supplementary Figure S1). Production of GAGs in PRP-based gel was absent ( Fig. 5A ). Biochemical quantification of the gels confirmed a significantly lower relative production of GAGs in PRP gels, as seen both in GAG content as well as in GAG release into the culture medium ( Fig. 5B ). Quantification of the amount of DNA in the gels revealed a significant increase in DNA in PRP gels over the culture period of 28 days when compared with fibrin gels ( Fig. 5C ). When looking at the production of collagen in these gels, a similar pattern was seen. No positive staining for type II collagen, a major component of hyaline cartilage, could be observed in PRP gels, while this was observed in fibrin gels. No distinguishable differences were found in the production of type I collagen, an indicator for fibrocartilage ( Fig. 6A ). In addition, total collagen content was significantly lower in PRP gels compared with fibrin gels ( Fig. 6B ).
Figure 5.
Proteoglycan production and proliferation in platelet-rich plasma (PRP) gel. Proteoglycans were visualized in PRP gels as well as control fibrin gels by safranin-O staining. Scale bar = 200 µm (A). Quantification of data presented in A. Production of glycosaminoglycans (GAGs) was normalized for DNA content and presented as fold change to the fibrin gel control group. Data are shown as mean ± SD. ****P < 0.0001 (B). Increase in DNA content in fibrin and PRP gels over the culture period of 28 days. Data are depicted as mean ± SD. ***P < 0.001 (C).
Figure 6.
Collagen production in platelet-rich plasma (PRP) gel. Visualization of type II and type I collagen production in fibrin and PRP gels by immunohistochemistry. Scale bar = 200 µm (A). Total collagen was quantified and normalized for DNA content in the gels. Data are presented as fold change to the fibrin gel group and shown as mean ± SD. **P < 0.01 (B).
Discussion
PRP is an appealing product that is widely applied to treat orthopedic conditions. Positive outcomes are attributed to the high concentrations of growth factors and anti-inflammatory components in PRP. Nonetheless, other studies report on negative results and consensus has not been reached. Proper understanding of the mechanism of action of PRP in the osteoarthritic joint is desired. The current study attempted to elucidate the effects of PRP specifically on chondrocytes in an in vitro controlled setting.
In this study, we used manually prepared, pooled human PRP which was deprived of leukocytes. It therefore can be defined as leukocyte-poor (LP-)PRP. LP-PRP generally contains a smaller amount of platelets30,31 compared with leukocyte-rich (LR-)PRP, which results in a lower concentration of growth factors.31,32 Substantial evidence showing lower concentrations of growth factors can negatively influence outcome in OA does not exist. For that reason, it cannot be concluded whether a certain preparation method produces qualitatively better PRP for the treatment of OA. There is a great lack of randomized controlled trials directly comparing LR- and LP-PRP. Just one study made a direct comparison in a clinical setting 33 comparing single spin and double spin PRP. Double spin PRP was shown to have a 1.4 times increase in leukocyte compared with whole blood. The authors reported on initially more pain and swelling in the group that received LR-PRP, but no difference in long-term outcome. In vitro evidence shows a more pro-inflammatory environment when LR-PRP is used. 34
We found that our PRP stimulated proliferation of OA chondrocytes in vitro both in a 2D as well as in a 3D setting. The general opinion is that PRP and PRP derivatives like platelet lysate stimulate proliferation of a variety of cell types. Many studies looked into the effects of PRP on MSCs derived from various sources,35-38 where all studies report on increased proliferation when PRP was compared with conventional culture medium. Likewise, studies describing cell types derived from other tissues in the knee also report on increased proliferation.16,35,39-41 It is important to highlight our study found increased proliferation only in the case where 20% PRP was added to the culture medium. Growth factor concentrations in this experimental condition are most likely much higher than when PRP is used in a clinical setting for intra-articular injections. Although stimulation of migration by PRP has been previously reported on in MSCs and other chondroprogenitor cells,35,42-44 we did not confirm this for OA chondrocytes in our micro-wound setup. It was expected that the high concentrations of chemoattractants in PRP could have promoted migration similar to a report on cell outgrowth from pieces of cartilage cultured in a gel containing PRP. 45
Another key finding of the current study is that our PRP was unable to inhibit the inflammatory response in OA chondrocytes in an in vitro inflammation model. Some of the most important markers expressed by OA chondrocytes in OA are COX2 and PTGES. Both are involved in the synthesis of prostaglandin E, an important pro-inflammatory marker in OA.46,47 We found that PRP had an inconsistent effect on the gene expression of COX2. The elevated expression of PTGES in inflamed chondrocytes could neither be reduced by PRP. We also showed that PRP was unable to downregulate the expression of cytokine IL6 and chemokine CCL20, both involved in the progression of OA.48,49 Both TNF-α and PRP were unable to alter the expression of IL17. Interestingly, the increased expression of IL1Β on stimulation of the cells with TNF-α was not downregulated by PRP. On the contrary, IL1Β was increasingly expressed in a dose-dependent manner on treatment with PRP. The striking increase in gene expression of IL1Β might be caused by positive feedback loops induced through production of other cytokines.48,50 This is contradictory to previously found anti-inflammatory properties attributed to PRP. 51 The in vitro system presented in this study only included cartilage cells, making a translation to the clinical situation difficult. Other tissues, like the synovium, have been shown to be a very important participant in the inflammatory response in OA. The step toward animal models and clinical studies is necessary to shed light on the effects of PRP on inflammation of the whole joint environment.
In this study, we additionally investigated the potential of PRP-incorporation in a commercial fibrin gel to stimulate cartilage regeneration. We report that incorporation of PRP leads to an increase in GAG content, independent of the concentration of PRP added. At the same time, an increase in the amount of DNA was found on addition of PRP into the gel, suggesting either an increase in proliferation or a reduction in cell death as compared with the control gels consisting of fibrin alone. Yet, the relative GAG production in PRP-supplemented gels greatly decreased, suggesting a strong inhibition of GAG formation by the cells in these gels. Our study is the first to incorporate different concentrations of PRP into a fibrin gel seeded with human OA chondrocytes. Other tissue engineering approaches where PRP is incorporated in other types of hydrogels, like an in situ photo-crosslinkable hydrogel 52 and an injectable PRP-containing gel 53 report on a chondrogenic effect of PRP, although both studies mentioned use MSCs.
To the best of our knowledge, our study is one of the first assessing cartilage matrix production of human OA chondrocytes in a hydrogel consisting purely of PRP. Despite a high concentration of TGF-β1 in our PRP, we found an evident inhibition of cartilage-like matrix formation, while desirable production of GAGs and type II collagen was seen in commercial fibrin gel. The biochemical data was supported by a clear absence of positive histological staining for safranin-O and type II collagen, where fibrin gels were evidently positive for both stainings. While in an unaffected joint TGF-β maintains a healthy chondrocyte phenotype, 54 a high concentration of TGF-β in the OA knee joint can accelerate cartilage damage. 55 As PRP continuously releases growth factors, including TGF-β, for at least 6 days, 56 the situation in the PRP gel presented in the current study would correspond to an ongoing diseased state. In vivo, this can lead to progression of OA, synovial fibrosis, and development of osteophytes. 57
Again, we observed an evident increase in cell proliferation in chondrocytes cultured in the presence of high concentrations of PRP. The only study carrying out a similar approach as we did, also shows an inferior effect of PRP when compared with fibrin gel. 21 A minimal amount of studies have looked into the behavior of cells of other species in a PRP gel.58,59 While both studies report an increase in proliferation of the cells, their results on cartilage matrix production contradict. What specifically happens to the chondrocytes in PRP gel remains inconclusive. Although we see a similar cell morphology in fibrin and PRP gel, their capacity of forming cartilage-like matrix is evidently impaired. In addition, it should be noted that all experiments in the current study were performed with chondrocytes isolated from redundant material from OA donors. The conclusions are therefore limited to the cell origin and interpretation and extrapolation to other cell types or disease states should therefore be handled with care. Evidently, more and extensive research is needed to determine whether PRP is a suitable candidate to be used as a novel autologous scaffold for cartilage tissue engineering.
Discrepancies between studies remain a major problem in PRP research. Preparation methods vary greatly, and the majority of studies fail to report on characterization of their PRP. 60 Variations in growth factor content and concentrations, as well as the presence of leukocytes may alter outcomes drastically. Also, inconsistencies remain between in vitro studies and clinical outcomes when PRP is used for the treatment of OA. It is crucial to consider the complexity of the articular joint and the tissues involved in the progression of OA. This illustrates the importance to move toward more complex culture systems, preferably using tissue explants in coculture to have a closer resemblance of the human joint.
In conclusion, the data presented here show absence of an anti-inflammatory effect of PRP on OA chondrocytes. Besides, PRP fails to stimulate OA chondrocytes into producing hyaline cartilage matrix in vitro. In addition, a pronounced stimulation of chondrocyte proliferation was seen both in cell expansion as well as in 3D cultures.
Supplemental Material
Supplemental material, Manuscript_platelet-rich_plasma_Table_S1 for Platelet-Rich Plasma Does Not Inhibit Inflammation or Promote Regeneration in Human Osteoarthritic Chondrocytes In Vitro Despite Increased Proliferation by Margot Rikkers, Koen Dijkstra, Bastiaan F. Terhaard, Jon Admiraal, Riccardo Levato, Jos Malda and Lucienne A. Vonk in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the partners of Regenerative Medicine Crossing Borders (RegMed XB), a public-private partnership that uses regenerative medicine strategies to cure common chronic diseases. This collaboration project is financed by the Dutch Ministry of Economic Affairs by means of the Public-Private Partnership Allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships. Financial support by the Dutch Arthritis Association is also gratefully acknowledged by all authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Blood was obtained from healthy donors through the Mini Donor Service of the University Medical Center Utrecht approved by the medical ethics committee.
Informed Consent: All blood donors provided written informed consent in accordance with the Declaration of Helsinki.
Trial Registration: Not applicable.
ORCID iDs: Margot Rikkers  https://orcid.org/0000-0002-4453-5184
https://orcid.org/0000-0002-4453-5184
Jon Admiraal  https://orcid.org/0000-0002-5964-0753
https://orcid.org/0000-0002-5964-0753
References
- 1. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15. doi: 10.1160/TH03-07-0440 [DOI] [PubMed] [Google Scholar]
- 2. Loibl M, Lang S, Dendl LM, Nerlich M, Angele P, Gehmert S, et al. Leukocyte-reduced platelet-rich plasma treatment of basal thumb arthritis: a pilot study. Biomed Res Int. 2016;2016:9262909. doi: 10.1155/2016/9262909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2_suppl):356-64. doi: 10.1177/0363546512471299 [DOI] [PubMed] [Google Scholar]
- 4. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2_suppl):245-51. doi: 10.1177/0363546506294078 [DOI] [PubMed] [Google Scholar]
- 5. Zhang H, Chen S, Qiu M, Zhou A, Yan W, Zhang J. Lateral meniscus allograft transplantation with platelet-rich plasma injections: a minimum two-year follow-up study. Knee. 2018;25(4):568-76. doi: 10.1016/j.knee.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 6. Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534-41. doi: 10.1177/0363546511431238 [DOI] [PubMed] [Google Scholar]
- 7. Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY, Wang Y, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627-37. doi: 10.1016/j.joca.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 8. Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2_suppl):347-54. doi: 10.1177/0363546518814532 [DOI] [PubMed] [Google Scholar]
- 9. Martínez-Martínez A, Ruiz-Santiago F, García-Espinosa J. Platelet-rich plasma: myth or reality? Radiologia. 2018;60(6):465-75. doi: 10.1016/j.rx.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 10. Lee KS, Wilson JJ, Rabago DP, Baer GS, Jacobson JA, Borrero CG. Musculoskeletal applications of platelet-rich plasma: fad or future? Am J Roentgenol. 2011;196(3):628-36. doi: 10.2214/AJR.10.5975 [DOI] [PubMed] [Google Scholar]
- 11. Filardo G, Kon E. PRP: product rich in placebo? Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3702-3. doi: 10.1007/s00167-015-3778-2 [DOI] [PubMed] [Google Scholar]
- 12. Gato-Calvo L, Magalhaes J, Ruiz-Romero C, Blanco FJ, Burguera EF. Platelet-rich plasma in osteoarthritis treatment: review of current evidence. Ther Adv Chronic Dis. 2019;10:2040622319825567. doi: 10.1177/2040622319825567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, et al. et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431-5. doi: 10.1089/ten.tec.2008.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pereira C, Scaranari M, Benelli R, Strada P, Reis RL, Cancedda R, et al. Dual effect of platelet lysate on human articular cartilage: a maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng Part A. 2013;19(11-12):1476-88. doi: 10.1089/ten.tea.2012.0225 [DOI] [PubMed] [Google Scholar]
- 15. Gonzales VK, De Mulder ELW, De Boer T, Hannink G, Van Tienen TG, Van Heerde WL, et al. Platelet-rich plasma can replace fetal bovine serum in human meniscus cell cultures. Tissue Eng Part C Methods. 2013;19(11):892-9. doi: 10.1089/ten.tec.2013.0009 [DOI] [PubMed] [Google Scholar]
- 16. Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJMA, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-80. doi: 10.1016/j.joca.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 17. Petrera M, De Croos JN, Iu J, Hurtig M, Kandel RA, Theodoropoulos JS. Supplementation with platelet-rich plasma improves the in vitro formation of tissue-engineered cartilage with enhanced mechanical properties. Arthroscopy. 2013;29(10):1685-92. doi: 10.1016/j.arthro.2013.07.259 [DOI] [PubMed] [Google Scholar]
- 18. Rikkers M, Levato R, Malda J, Vonk LA. Importance of timing of platelet lysate-supplementation in expanding or redifferentiating human chondrocytes for chondrogenesis. Front Bioeng Biotechnol. 2020;8:804. doi: 10.3389/fbioe.2020.00804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anitua E, Andía I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23(2_suppl):281-6. doi: 10.1016/j.orthres.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 20. Uggeri J, Belletti S, Guizzardi S, Poli T, Cantarelli S, Scandroglio R, et al. Dose-dependent effects of platelet gel releasate on activities of human osteoblasts. J Periodontol. 2007;78(10):1985-91. doi: 10.1902/jop.2007.070116 [DOI] [PubMed] [Google Scholar]
- 21. Ahmed TAE, Giulivi A, Griffith M, Hincke M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Eng Part A. 2011;17(3-4):323-35. doi: 10.1089/ten.tea.2009.0773 [DOI] [PubMed] [Google Scholar]
- 22. de Windt TS, Vonk LA, Slaper-Cortenbach ICM, Nizak R, van Rijen MHP, Saris DBF. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients. Stem Cells. 2017;35(8):1984-93. doi: 10.1002/stem.2657 [DOI] [PubMed] [Google Scholar]
- 23. Federa Coreon. Code good use of human body material 2011. Ordering the new good use code [in Dutch]. Accessed September 9, 2020. https://www.federa.org/code-goed-gebruik-van-lichaamsmateriaal-2011
- 24. Van Diest PJ. No consent should be needed for using leftover body material for scientific purposes. BMJ. 2002;325:648-51. [PubMed] [Google Scholar]
- 25. Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM. et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol. 2019;7:107. doi: 10.3389/fcell.2019.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2_suppl):329-33. doi: 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- 27. Ali Khaghani S, Denyer MCT, Youseffi M. Effect of transforming growth factor-β1 in biological regulation of primary chondrocyte. Am J Biomed Eng. 2012;2(1):1-8. doi: 10.5923/j.ajbe.20120201.01 [DOI] [Google Scholar]
- 28. Castillo TN, Pouliot MA, Hyeon Joo Kim, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2_suppl):266-71. doi: 10.1177/0363546510387517 [DOI] [PubMed] [Google Scholar]
- 29. Neuman R, Logan M. The determination of hydroxyproline. J Biol Chem. 1950;184:299-306. [PubMed] [Google Scholar]
- 30. Mazzocca AD, McCarthy MBR, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-16. doi: 10.2106/JBJS.K.00430 [DOI] [PubMed] [Google Scholar]
- 31. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-40. doi: 10.1177/0363546511417792 [DOI] [PubMed] [Google Scholar]
- 32. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502-08. doi: 10.1097/01.PRS.0000138251.07040.51 [DOI] [PubMed] [Google Scholar]
- 33. Filardo G, Kon E, Ruiz MTP, Vaccaro F, Guitaldi R, Di Martino A, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sport Traumatol Arthrosc. 2012;20(10):2082-91. doi: 10.1007/s00167-011-1837-x [DOI] [PubMed] [Google Scholar]
- 34. Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS One. 2015;10(3):e0121713. doi: 10.1371/journal.pone.0121713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hildner F, Eder MMJ, Hofer K, Aberl J, van Griensven M, Gabriel C, et al. Human platelet lysate successfully promotes proliferation and subsequent chondrogenic differentiation of adipose-derived stem cells: a comparison with articular chondrocytes. J Tissue Eng Regen Med. 2016;9:808-18. doi: 10.1002/term.1649 [DOI] [PubMed] [Google Scholar]
- 36. Cowper M, Frazier T, Wu X, Curley J, Ma M, Mohiuddin O, et al. Human platelet lysate as a functional substitute for fetal bovine serum in the culture of human adipose derived stromal/stem cells. Cells. 2019;8(7):724. doi: 10.3390/cells8070724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2_suppl):228-36. doi: 10.1002/jcp.20391 [DOI] [PubMed] [Google Scholar]
- 38. Neubauer M, Kuten O, Stotter C, Kramer K, De Luna A, Muellner T, et al. The effect of blood-derived products on the chondrogenic and osteogenic differentiation potential of adipose-derived mesenchymal stem cells originated from three different locations. Stem Cells Int. 2019;2019:1358267. doi: 10.1155/2019/1358267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakata R, Mcnary SM, Miyatake K, Lee CA, Van Den Bogaerde JM, Marder RA, et al. Stimulation of the superficial zone protein and lubrication in the articular cartilage by human platelet-rich plasma. Am J Sports Med. 2015;43(6):1467-73. doi: 10.1177/0363546515575023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gaissmaier C, Fritz J, Krackhardt T, Flesch I, Aicher WK, Ashammakhi N. Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials. 2005;26(14):1953-60. doi: 10.1016/j.biomaterials.2004.06.031 [DOI] [PubMed] [Google Scholar]
- 41. Gilbertie JM, Long JM, Schubert AG, Berglund AK, Schaer TP, Schnabel LV. Pooled platelet-rich plasma lysate therapy increases synoviocyte proliferation and hyaluronic acid production while protecting chondrocytes from synoviocyte-derived inflammatory mediators. Front Vet Sci. 2018;5:150. doi: 10.3389/fvets.2018.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oprea WE, Karp JM, Hosseini MM, Davies JE. Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac Surg. 2003;14(3):292-300. doi: 10.1097/00001665-200305000-00006 [DOI] [PubMed] [Google Scholar]
- 43. Krüger JP, Hondke S, Endres M, Pruss A, Siclari A, Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30(6):845-52. doi: 10.1002/jor.22005 [DOI] [PubMed] [Google Scholar]
- 44. Kreuz PC, Krüger JP, Metzlaff S, Freymann U, Endres M, Pruss A, et al. Platelet-rich plasma preparation types show impact on chondrogenic differentiation, migration, and proliferation of human subchondral mesenchymal progenitor cells. Arthroscopy. 2015;31(10):1951-61. doi: 10.1016/j.arthro.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 45. Vinod E, Vinod Francis D, Manickam Amirtham S, Sathishkumar S, Boopalan PRJVC. Allogeneic platelet rich plasma serves as a scaffold for articular cartilage derived chondroprogenitors. Tissue Cell. 2019;56:107-13. doi: 10.1016/j.tice.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 46. Alaaeddine N, Di Battista JA, Pelletier J-P, Kiansa K, Cloutier J-M, Martel-Pelletier J. Inhibition of tumor necrosis factor α-induced prostaglandin E2 production by the antiinflammatory cytokines interleukin-4, interleukin-10, and interleukin-13 in osteoarthritic synovial fibroblasts: distinct targeting in the signaling pathways. Arthritis Rheum. 1999;42(4):710-8. doi: [DOI] [PubMed] [Google Scholar]
- 47. Fan HW, Liu GY, Zhao CF, Li XF, Yang XY. Differential expression of COX-2 in osteoarthritis and rheumatoid arthritis. Genet Mol Res. 2015;14(4):12872-9. doi: 10.4238/2015.October.21.7 [DOI] [PubMed] [Google Scholar]
- 48. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan J, Li Y, Ding L, Bin, Liu GY, Zheng XF, Xue W, et al. Relationship between serum and synovial fluid CCL20 concentrations with disease severity in primary knee osteoarthritis. J Musculoskelet Neuronal Interact. 2019;19(3):326-32. [PMC free article] [PubMed] [Google Scholar]
- 50. Messerschmidt L, Fischer S, Wiedemann P, Bringmann A, Hollborn M. Osmotic induction of cyclooxygenase-2 in RPE cells: Stimulation of inflammasome activation. Mol Vis. 2019;25:329-44. [PMC free article] [PubMed] [Google Scholar]
- 51. Moussa M, Lajeunesse D, Hilal G, El Atat O, Haykal G, Serhal R, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146-56. doi: 10.1016/j.yexcr.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 52. Liu X, Yang Y, Niu X, Lin Q, Zhao B, Wang Y, et al. An in situ photocrosslinkable platelet rich plasma—complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017;62:179-87. doi: 10.1016/j.actbio.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 53. Jooybar E, Abdekhodaie MJ, Alvi M, Mousavi A, Karperien M, Dijkstra PJ. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019;83:233-44. doi: 10.1016/j.actbio.2018.10.031 [DOI] [PubMed] [Google Scholar]
- 54. Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153(1):35-46. doi: 10.1083/jcb.153.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Plaas A, Velasco J, Gorski DJ, Li J, Cole A, Christopherson K, et al. The relationship between fibrogenic TGFβ1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthritis Cartilage. 2011;19(9):1081-90. doi: 10.1016/j.joca.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 56. Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25(18):4489-502. doi: 10.1016/j.biomaterials.2003.11.013 [DOI] [PubMed] [Google Scholar]
- 57. Blaney Davidson EN, Vitters EL, Van Der Kraan PM, Van Den Berg WB. Expression of transforming growth factor-β (TGFβ) and the TGFβ signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65(11):1414-21. doi: 10.1136/ard.2005.045971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drengk A, Zapf A, Stürmer EK, Stürmer KM, Frosch KH. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. 2008;189(5):317-26. doi: 10.1159/000151290 [DOI] [PubMed] [Google Scholar]
- 59. Xie A, Nie L, Shen G, Cui Z, Xu P, Ge H, et al. The application of autologous platelet-rich plasma gel in cartilage regeneration. Mol Med Rep. 2014;10(3):1642-8. doi: 10.3892/mmr.2014.2358 [DOI] [PubMed] [Google Scholar]
- 60. Fice MP, Miller JC, Christian R, Hannon CP, Smyth N, Murawski CD, et al. The role of platelet-rich plasma in cartilage pathology: an updated systematic review of the basic science evidence. Arthroscopy. 2019;35(3):961-76.e3. doi: 10.1016/j.arthro.2018.10.125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Manuscript_platelet-rich_plasma_Table_S1 for Platelet-Rich Plasma Does Not Inhibit Inflammation or Promote Regeneration in Human Osteoarthritic Chondrocytes In Vitro Despite Increased Proliferation by Margot Rikkers, Koen Dijkstra, Bastiaan F. Terhaard, Jon Admiraal, Riccardo Levato, Jos Malda and Lucienne A. Vonk in CARTILAGE