Abstract
Objective
Cobalt and chromium (CoCr) ions from metal implants are released into the joint due to biotribocorrosion, inducing apoptosis and altering gene expression in various cell types. Here, we asked whether CoCr ions concentration-dependently changed viability, transcriptional activity, and inflammatory response in human articular chondrocytes.
Design
Human articular chondrocytes were exposed to Co (1.02-16.33 ppm) and Cr (0.42-6.66 ppm) ions and cell viability and early/late apoptosis (annexin V and 7-AAD) were assessed in 2-dimensional cell cultures using the XTT assay and flow cytometry, respectively. Changes in chondrocyte morphology were assessed using transmitted light microscopy. The effects of CoCr ions on transcriptional activity of chondrocytes were evaluated by quantitative polymerase chain reaction (qPCR). The inflammatory responses were determined by measuring the levels of released pro-inflammatory cytokines (interleukin-1β [IL-1β], IL-6, IL-8, and tumor necrosis factor–α [TNF-α]).
Results
CoCr ions concentration-dependently reduced metabolic activity and induced early and late apoptosis after 24 hours in culture. After 72 hours, the majority of chondrocytes (>90%) were apoptotic at the highest concentrations of CoCr ions (16.33/6/66 ppm). SOX9 expression was concentration-dependently enhanced, whereas expression of COL2A1 linearly decreased after 24 hours. IL-8 release was enhanced proportionally to CoCr ions levels, whereas IL-1β, IL-6, and TNF-α levels were not affected by the treatments.
Conclusions
CoCr ions showed concentration- and time-dependent effects on articular chondrocytes. Fractions of apoptotic articular chondrocytes were proportional to CoCr ion concentrations. In addition, metabolic activity and expression of chondrocyte-specific genes were decreased by CoCr ions. Furthermore, exposure to CoCr ions caused a release of pro-inflammatory cytokines.
Keywords: chondrocytes, metal ions, gene expression, tribocorrosion
Introduction
Cobalt-chromium-molybdenum (CoCrMo) alloys are commonly used in orthopedic implants, for example, in knee arthroplasty and partial surface replacement.1,2
Unicompartmental knee arthroplasty (UKA) is a treatment option for medial and lateral tibiofemoral osteoarthritis. UKA is a less invasive operation than total knee arthroplasty; it allows shorter recovery time and may provide less postoperative pain. However, the cumulative revision rate for UKA is significantly higher than that for total knee arthroplasty, and it has not improved over the last decades. 3 Secondary to aseptic loosening, revision surgery because of osteoarthritis progression is necessary in up to 27% of cases.3-5 The mechanisms of this progression and subsequent breakdown of the contralateral compartment remain poorly understood.6-8 Despite appropriate patient selection, preexisting cartilage damage, and alignment overcorrection, 5 biotribocorrosion, and its byproducts might also play a role in the degeneration of the contralateral compartment.
Biotribocorrosion is a surface degradation mechanism that combines tribology and corrosion in a biological environment. CoCr implants undergo biotribocorrosion, 9 and produce metal ions in low quantities even in contact with a much softer counterpart like articular cartilage. 10 We recently demonstrated increased biotribocorrosion with higher loads and sliding velocities. Co and Cr ions might be released into synovial fluid in low quantities after partial surface replacement or UKA. 11 Furthermore, Co and Cr containing compounds are deposited on the surface of articular cartilage. 9
Wear and corrosion of orthopedic implants are important causes of failure in joint arthroplasty. 12 Metal-on-metal bearings have been associated with local soft tissue inflammation.12,13 This local adverse tissue reaction and subsequent loosening of orthopedic implants have been under intense research scrutiny. 14 Therefore, in-depth studies regarding the toxicity mechanisms of independent metal particles in different cells (e.g., macrophages and osteoblasts) have been performed.15,16 Many studies have investigated effects of wear debris of CoCrMo alloys on various cells.17-19 Scientific evidence regarding both local and systemic toxicity of these metal particles and ions has been obtained from in vitro and in vivo studies.16,20 Co and Cr are biologically reactive metals with various adverse effects. 21 In vitro studies examining cytotoxic effects of Co and Cr ions, wear debris, and particles have indicated that corrosion is a vital aspect of the mechanism that causes the release of Co ions and the inflammatory response caused by macrophages before cell death and apoptosis.22,23
Nevertheless, there is a paucity of studies of the effects of metal ions on chondrocytes. Wear debris, including metal ions and particles, are phagocytosed by chondrocytes and alter biosynthetic activity. 24 CoCr ions at high concentrations kill chondrocytes via the nitric oxide pathway and promote the degradation of healthy cartilage. 25 However, the exact effects of metal ions on the viability and gene expression levels in chondrocytes remain poorly understood.
The primary objective of this study was to investigate the response of chondrocytes to CoCr ions in a 2-dimensional (2D) cell culture. By exposing human osteoarthritic chondrocytes to CoCr ions at different concentrations, we sought to identify effects of Co and Cr on transcriptional activity and viability. The secondary objective of this study was to determine whether there is a critical metal ion concentration that diminishes chondrocyte survival.
Material and Methods
Production of Metal Ion Solution
For determining the effects of CoCr ions on osteoarthritic chondrocytes, an electrolyte solution was prepared by transpassive dissolution of a CoCrMo cylinder as previously described. 9 To obtain different concentrations of CoCr ions for cell culture experiments, the prepared solution underwent serial 1:2 dilutions. The final composition of 1 mL of cell culture medium for the incubation of the chondrocytes was 733.33 µL of the medium (supplemented with antibiotics and antimycotic), 100 µL fetal calf serum (FCS; to obtain 10% FCS) and 166.67 µL electrolyte solution (containing metal ions). Thus, 5 different CoCr ions concentrations were produced (1.02/0.42 ppm; 2.04/0.84 ppm; 4.08/1.67 ppm; 8.17/3.33 ppm, and 16.33/6.66 ppm Co/Cr). For control purposes, phosphate-buffered saline (PBS; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was added to the culture medium instead of the electrolyte solution.
Isolation and Cultivation of Human Osteoarthritic Chondrocytes
Human articular cartilage was retrieved from 3 osteoarthritis patients who underwent total knee arthroplasty at an average age of 54 years. Duration of symptoms ranged from 2 to 5 years, and all patients had received intra-articular infiltration of the knee. Informed consent was obtained in all cases, and the study was approved by the Regional Ethical Committee (GS4-EK-4/199-2016).
For chondrocyte isolation, articular cartilage was minced into 2 mm3 pieces prior to enzymatic digestion with Liberase TM (0.2 WU/ml, Roche Diagnostics GmbH, Mannheim, Germany) in the medium (GIBCO DMEM/F-12 GlutaMAX-I, Life Technologies, Carlsbad, CA, USA) with antibiotics (penicillin 200 U/mL; streptomycin 0.2 mg/mL) and an antimycotic (amphotericin B 2.5 µg/mL; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) under permanent agitation for 18 to 22 hours at 37°C. The resulting chondrocyte suspension was passed through a cell strainer with 40 µm pores (BD Biosciences, Franklin Lakes, NJ, USA) to remove undigested debris. Cells were washed with PBS, centrifuged (10 minutes, 500 × g, room temperature) and resuspended in the medium. Viability was determined by staining with trypan blue (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), and cells were counted using a hemocytometer.
The isolated cells were seeded in growth medium supplemented with antibiotics and antimycotic, 10% FCS (GIBCO, Life Technologies, Carlsbad, CA, USA), and 0.05 mg/mL ascorbic acid (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) in 175 cm2 culture flasks (Nunc, Rochester, NY, USA) at a density of 1 × 104 cells/cm2 and cultivated at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium was changed every 2 to 3 days until 80% confluence. After expansion, the cells were harvested by the use of accutase (1.5 mL/flask; Sigma-Aldrich Chemie GmbH, Steinheim, Germany), counted, and seeded in 25 cm2 flasks (3.75 × 105 cells per flask) for microscopy, flow cytometry, gene expression, and determination of cytokines in the supernatant, and in 24-well plates (2 × 104 cells per well) for measuring metabolic activity of the chondrocytes. Chondrocytes were incubated for 3 days before culture medium was replaced with the final composition medium, FCS, and CoCr ions as mentioned above. The analysis was carried out after 24 and 72 hours.
Metabolic Activity: Cell Viability
Metabolic activity of the chondrocytes was measured by using an XTT-based ex vivo toxicology assay kit according to the manufacturer’s instructions (Cell Proliferation Kit II [XTT], Roche Diagnostics, Basel, Switzerland). Briefly, XTT solution (490 µL of XTT reagent and 10 µL of activation reagent) was added to each of the 24 wells with culture medium followed by a 4-hour incubation period at 37°C in the atmosphere of 95% air and 5% CO2. After incubation, the absorbance was measured at 492 nm and background wavelength of 690 nm in triplicate in a 24-well plate using a multimode microplate reader (SynergyTM 2, Winooski, Vermont, USA) with Gen 5 software. Culture medium that contained just CoCr ions at different concentrations but no cells was used as blank control.
Flow Cytometry
To analyze the distribution of live and apoptotic cells following the exposure to metal ions at different concentrations, the chondrocytes were detached by the use of accutase after 24 and 72 hours. Cells were counted, and half of the cell suspension (0.5 mL) from each condition was used for flow cytometry, whereas the other half was used for RNA isolation. Cell suspension used for flow cytometry was centrifuged (439 × g; 10 minutes), the supernatant was discarded, and the cell pellet was resuspended in 200 µL of 1X annexin V binding buffer. Staining with 7-AAD (late apoptosis, dead cells) and annexin V (early apoptosis) phycoerythrin (PE) was performed using a PE Annexin V Apoptosis Detection Kit I according to the manufacturer’s instructions (BD Biosciences, Franklin Lakes, NJ, USA). Flow cytometry analysis was performed using a Gallios flow cytometer (Beckman Coulter, Brea, CA, USA) equipped with 405 nm, 488 nm, and 638 nm lasers and Kaluza Analysis Software (Beckman Coulter, Brea, CA, USA), which was used for measurements and data analysis.
Transmitted Light Microscopy
Cell morphology of monolayer cell cultures grown in the presence of different concentrations of CoCr ions was studied after 24 and 72 hours. Images were captured using an EVOS FLoid Cell Imaging Station (Life Technologies, Carlsbad, CA, USA).
Gene Expression
Total RNA isolation and Reverse Transcription
For isolation of RNA from osteoarthritic chondrocytes incubated with different concentrations of CoCr ions, half of the cell suspension of detached and counted cells (see Flow Cytometry subsection) was used and centrifuged to pellet the cells. The cell pellets were resuspended in 200 µL of PBS before total RNA was extracted using a High Pure RNA Isolation Kit (Roche Diagnostics, Basel, Switzerland) in accordance with the manufacturer’s protocol. Isolated RNA was stored at −80°C until cDNA synthesis.
To synthesize complementary DNA (cDNA) from messenger RNA (mRNA), a Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Basel, Switzerland) was used. Additionally, RNA from bacteriophage MS2 was added to stabilize isolated RNA during cDNA synthesis.
Quantitative Polymerase Chain Reaction
Gene expression analysis using quantitative polymerase chain reaction (qPCR) was performed with FastStart TaqMan Probe Master (Roche Diagnostics, Basel, Switzerland) and gene-specific primers (Eurofins MWG Synthesis GmbH, Ebersberg, Germany) in triplicate on a LightCycler 96 instrument (Roche Diagnostics, Basel, Switzerland). Expression levels of 4 genes—collagen type 2 (COL2A1), aggrecan core protein 1 (ACAN), versican (VCAN), and transcription factor SOX 9 (SOX9)—were analyzed, whereas the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as housekeeping gene expression control. The selection of probes and primers was made by using the Universal Probe Library System and by applying in silico PCR (Roche Diagnostics, Basel, Switzerland). The primer-dependent optimal annealing temperature was determined experimentally. qPCR was carried out with the following steps: an initial denaturation step at 95°C for 30 seconds, an annealing step from 55°C to 63°C optimized for the respective primers ( Table 1 ) for 30 seconds, and a polymerization step at 72°C for 15 seconds. Fluorescence measurement data were relatively quantified without efficiency correction by the R = 2−ΔCt [Mean target − Mean reference] method. 26
Table 1.
Sequences of Primers Used in Quantitative Polymerase Chain Reaction.
| Gene | Sequence (3′–5′) | |
|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | Sense | ctctgctcctcctgttcgac |
| (GAPDH) | Antisense | acgaccaaatccgttgactc |
| Aggrecan core protein 1 | Sense | cctccccttcacgtgtaaaa |
| (ACAN) | Antisense | gctccgcttctgtagtctgc |
| Collagen type II, alpha 1 | Sense | gtgtcagggccaggatgt |
| (COL2A1) | Antisense | tcccagtgtcacagacacagat |
| Versican | Sense | gcacctgtgtgccaggata |
| (VCAN) | Antisense | cagggattagagtgacattcatca |
| Transcription factor SOX-9 | Sense | tacccgcacttgcacaac |
| (SOX9) | Antisense | tctcgctctcgttcagaagtc |
Cytokine Quantification
Stored supernatants from osteoarthritic chondrocytes cultivated for 24 and 72 hours with CoCr ions were analyzed for the levels of cytokines (interleukin-1β [IL-1β], IL-6, tumor necrosis factor–α [TNF-α], IL-8) using the Bio-Plex Pro Assay and a Bio-Plex 200 analyzer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). In this cytokine multiplex assay, antibodies are covalently coupled to magnetic beads with a unique fluorescence dye. Thus, the concentrations of each analyte can be determined. For analysis, values below the lower limit of detection for each analyte were recorded as the lower limit of quantification (LLOQ). Analyzed cytokines had a LLOQ of 3.2 pg/mL for IL-1β, 2.3 pg/mL for IL-6, 1.9 pg/mL for IL-8, and 5.8 pg/mL for TNF-α. The volume of every sample supernatant was measured to quantify proteins.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism Software (GraphPad Prism Software Inc., San Diego, CA, USA). Data are expressed as mean ± standard deviation. The statistical analysis was carried out by using two-way analysis of variance (ANOVA). Post hoc multiple comparisons were performed followed by the Šidák test for correction of multiple comparisons. All statistical analyses were conducted with a significance level of α = 0.05 (P < 0.05). Statistical significance of differences is indicated in the figures as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Metabolic Activity: Cell Viability
Cell viability decreased proportionally to CoCr ions concentration after 24 and 72 hours ( Fig. 1 ). After 24 hours, cell viability in the presence of CoCr ions at the highest concentration was significantly lower than in control group (P < 0.05). After 72 hours, the inverted relationship between chondrocyte viability and CoCr ions concentration was almost linear. The cells had significantly lower metabolic activity at the 2 highest concentrations compared to activity values in all other conditions and in control group. Cell proliferation from 24 to 72 hours, assessed by the increase in cell numbers, occurred only in the presence of CoCr ions at the 3 lower concentrations and in control group (PBS). In contrast, no cell proliferation from 24 to 72 hours was observed at the 2 highest CoCr concentrations (8.17/3.33 and 16.33/6.66 ppm).
Figure 1.

Metabolic activity (XTT reading) of chondrocytes exposed to different concentrations of CoCr ions after 24 and 72 hours. The single, double, and triple asterisks indicate P < 0.05, P < 0.01, and P < 0.001, respectively. If no standard deviation is shown, results are not significant.
Flow Cytometry
Additionally, cell viability was determined using flow cytometry to identify early/late apoptotic and live cells ( Fig. 2 ). After 24 hours, an increase in the percentages of early (6.38%) and late apoptotic (13.24%) cells was seen only at the highest CoCr ions concentration (16.33/6.66 ppm). After 72 hours, increased numbers of apoptotic cells were seen at the 2 highest CoCr ions concentrations. Whereas in the presence of the second highest concentration (8.17/3.33 ppm CoCr) only 8.67% and 5.25% of chondrocytes showed signs of late and early apoptosis, respectively, the majority of cells incubated with the highest concentration were late apoptotic (68.76%) or in early apoptosis (23.17%), with just a small number of live cells (8.07%). In contrast, live cells predominated (97.70%) in control group and in the groups exposed to lower CoCr ions concentrations (1.02/0.41 ppm CoCr and 2.04/0.84 ppm CoCr), with low numbers of apoptotic cells (early apoptotic 0.97%; late apoptotic 1.33%) observed after both 24 and 72 hours. Chondrocytes incubated with an intermediate concentration (4.08/1.67 ppm CoCr) showed a slightly higher number of apoptotic cells (3.73%) after 72 hours compared with the numbers of apoptotic cells in control group and in the groups exposed to lower CoCr ions concentrations.
Figure 2.
Distribution of late apoptotic (staining for 7-AAD), early apoptotic (annexin V) and living chondrocytes exposed to different concentrations of CoCr ions after 24 hours (left) and 72 hours (right), measured using flow cytometry.
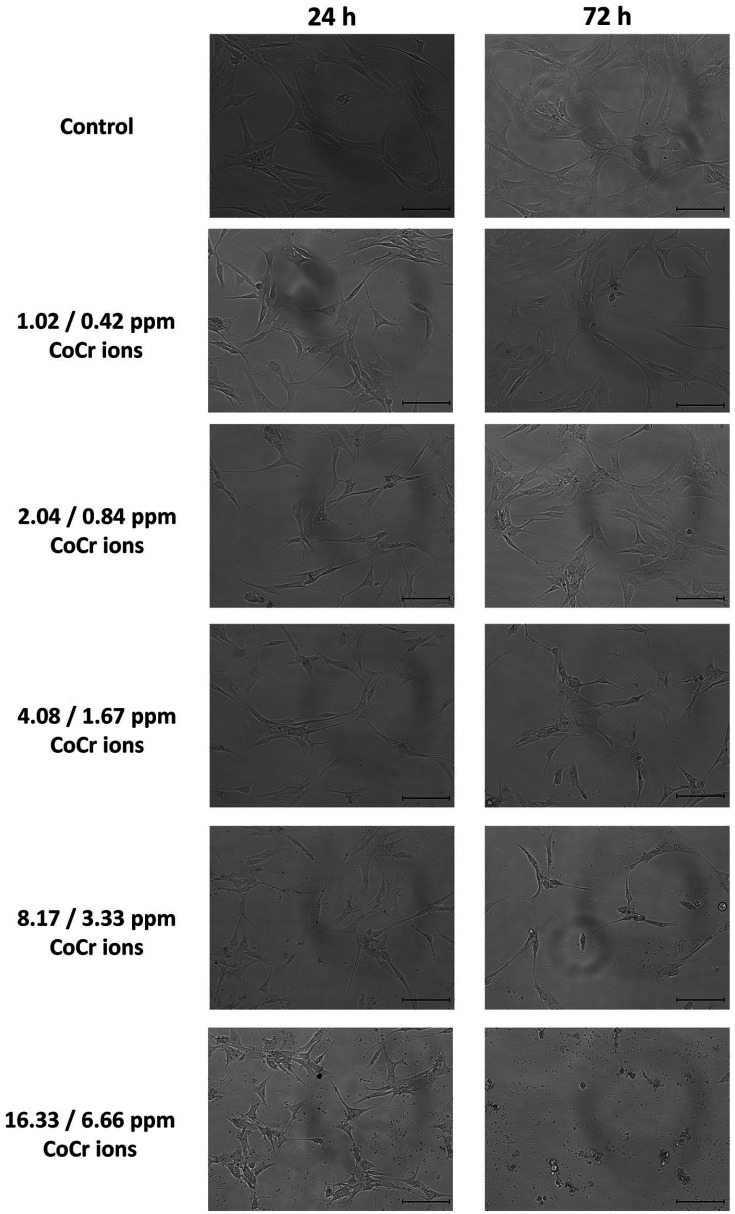
Microscopic Images
Figure 3 shows representative microscopic images of articular chondrocytes treated with CoCr ions at different concentrations after 24 and 72 hours of incubation. In each test group, the cells in cell culture flasks showed a homogenous distribution. After 24 hours, osteoarthritic chondrocytes in control group showed elongated fibrochondrocytic appearance, that is, their typical morphology in 2D culture. When cells were exposed to CoCr ions at the lowest concentration, their morphology was comparable to that of cells in control group, whereas cells treated with CoCr ions at 8.17/3.33 ppm to 16.33/6.66 ppm seemed to start undergoing apoptosis as cellular protrusions became thin and cells were shrinking. After 72 hours, cells cultivated with PBS (control group) or with CoCr ions at low concentrations (1.02/0.42 ppm and 2.04/0.84 ppm) showed a higher density compared with that after 24 hours due to proliferation. In the presence of 4.08/1.67 ppm CoCr ions, cell proliferation likely stopped, as cell density did not increase after 72 hours. Furthermore, protrusions were further thinned compared with their appearance after 24 hours. Osteoarthritic chondrocytes incubated with CoCr ions at 2 highest concentrations (8.17/3.33 ppm and 16.33/6.66 ppm) for 72 hours showed most pronounced phenotypic changes. Fewer cells were observed and cells started to undergo apoptosis or had been already dead as they were shrinking and detaching from the surface.
Figure 3.
Representative microscopic images of articular chondrocytes treated with different concentrations of metal ions after 24 hours (left) and 72 hours (right) of incubation; scale bar 100 µm.
Expression of Cartilage-Specific Genes
Quantitative PCR results demonstrated altered gene expression profiles in osteoarthritic chondrocytes exposed to different concentrations of CoCr ions ( Fig. 4 ). After 24 hours, COL2A1 gene expression levels nominally negatively correlated with CoCr ions concentrations except for the relationship at the highest CoCr ions concentration, at which COL2A1 level did not decrease further. Significant differences between any of the 6 groups were only seen between the control group and the 2 highest concentrations ( Fig. 4A ). After 72 hours, the expression level of COL2A1 decreased in all experimental groups. The lowest expression level of COL2A1 after 72 hours was observed in the presence of 8.17/3.33 ppm CoCr ions. From 24 to 72 hours, the difference in COL2A1 expression level was statistically significant between cells of the control group. The expression levels of mRNA encoding transcription factor SOX9 was nominally higher in cells treated with intermediate CoCr ions concentrations (4.08/1.67 ppm and 8.17/3.33 ppm) after 24 hours ( Fig. 4B ) but the effect did not reach statistical significance. SOX9 mRNA expression levels remained high after 72 hours in both groups but statistically significant differences occured only for the group exposed to 8.17/3.33 ppm CoCr ions in comparison with the highest concentration. From 24 to 72 hours the 4.08/1.67 ppm and 16.33/6.66 ppm reached significant difference, while the concentration with 8.17/3.33 ppm CoCr ions stayed on the same level. Control group and low concentrations were not significant different. Expression levels of the genes encoding the proteoglycans aggrecan (ACAN, Fig. 4C ) and versican (VCAN, Fig. 4D ) were lower in all treatment groups compared with those in control group, both after 24 and 72 hours. VCAN expression levels concentration-dependently decreased after 24 and 72 hours. However, the difference was statistically significant only after 72 hours between the control group and all used concentrations.
Figure 4.
Gene expression levels in chondrocytes exposed to different concentrations of CoCr ions after 24 and 72 hours of incubation: (A) collagen type 2, (B) transcription factor SOX9, and the proteoglycans aggrecan (C) and versican (D). The expression levels were normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The single, double, and triple asterisks indicate P < 0.05, P < 0.01, and P < 0.001, respectively.
Cytokine Release
The relationships between the levels of pro-inflammatory cytokines IL-6 and IL-8 in the supernatant of cultured cells and concentrations of CoCr ions are displayed in Figure 5 . IL-8 level was directly proportional to the CoCr ions concentration up to 8.17/3.33 ppm CoCr ions point ( Fig. 5B ). However, at the highest CoCr ions concentration (16.33/6.66 ppm), the amount of released IL-8 was lower than at 8.17/3.33 ppm CoCr ions after 24 and 72 hours. Differences between any groups were not statistically significant. The amount of released IL-6 in the supernatant remained similar at all but the highest CoCr ions concentration after 24 and 72 hours ( Fig. 5A ). At 16.33/6.66 ppm CoCr ions, IL-6 levels were lower than in other groups at both time points. Differences between any groups did not reach statistical significance. Measured values for IL-1β and TNF-α were below the lower limit of quantification.
Figure 5.
Cytokine release in the supernatant (A, IL-8; B, IL-6) of chondrocytes exposed to different concentrations of CoCr ions after 24 and 72 hours. If no standard deviation is shown, results are not significant.
Discussion
This study aimed to evaluate the effect of CoCr ions at different concentrations on human osteoarthritic chondrocytes and their biological properties in vitro. For this, we examined cell viability and proliferation along with morphological changes and propensity to undergo apoptosis. Furthermore, the influence of CoCr ions on the expression of cartilage-specific genes and released cytokines in the supernatant was analyzed. Our results suggest that metal ions, which are typical causative agents of biotribocorrosion, impair metabolic and synthesis activity as well as inflammatory mediator release in articular chondrocytes.
Numerous studies have investigated the effects of metal ions on various cells and organs. Initially, such studies focused on the adverse local tissue reaction that followed metal-on-metal (MoM) hip arthroplasty. 17 Accordingly, mainly osteoblasts and macrophages were studied.15,19 As elevated blood levels of metal ions were detected in patients after MoM arthroplasty, systemic toxicity and effects on various cells were examined.18,27 However, how metal ions affect articular chondrocytes has not been properly addressed. A thorough insight into biological responses of chondrocytes to the exposure to CoCr ions might be relevant for better understanding of the consequences of partial joint and surface replacement failures.
In UKA and in partial surface replacement, the progression of osteoarthritis often leads to high revision or failure rates in the contralateral compartment of the knee joint.6,7,28 Factors to be considered for circumventing these issues are proper indication and correct implantation. 1 In a systematic review of almost 4000 failures, van der List et al. 29 showed that the most common failure modes after medial UKA were aseptic loosening (36%) and osteoarthritis progression (20%). Interestingly, aseptic loosening was more prevalent early after surgery (<5), whereas osteoarthritis progression caused mid- and long-term failure (5-10 years, >10 years). 29 Furthermore, one of the causative factors for osteoarthritis progression could be implant biotribocorrosion that might degrade articular cartilage. Biotribocorrosion itself is a phenomenon that describes the synergy between mechanical wear and corrosion in a biocorrosive environment (e.g., synovial joint). The mechanical damage imparted to the passive layer during sliding contact results in depassivation and release of metal ions by wear accelerated corrosion.30,31 Elevated levels of metal ions after total hip and knee arthroplasty 32 are commonly reported, whereas little information is available regarding metal ion release post-UKA and partial surface replacements. Recently, our study group reported the occurrence of biotribocorrosion in CoCr implants articulating against articular cartilage, 9 which was enhanced with frictional loading. 10
Considering the fact that CoCr ions release could be a crucial factor in progressive degeneration of articular cartilage, human osteoarthritic chondrocytes were incubated with Co (1.02-16.33 ppm) and Cr (0.42-6.66 ppm) ions at different concentrations. As metal ion levels in the joint after UKA have not been reported, CoCr ions concentrations tested were set according to the findings in MoM bearings. 33 Lass et al. 34 reported median levels of cobalt and chromium in patients with MoM total hip arthroplasty after a minimum of 18 years of 81.9 ppm (Co) and 54.0 ppm (Cr). Naturally, lower levels of metal ions are expected after partial joint replacement. Furthermore, preliminary tests showed that osteoarthritic chondrocytes underwent apoptosis or cell death when exposed to a concentration higher than 16.33 ppm of Co and 6.66 ppm of Cr. Molybdenum was not added, as molybdenum levels do not increase after implantation of CoCrMo implants.35,36 In the present study, we used these established toxic levels as the highest concentrations and diluted serial concentrations to study the effects on gene expression and metabolic activity. CoCrMo implants release not only Co2+ and Cr3+ ions but also Cr6+ ions, which may all induce cell apoptosis or damage surrounding tissues. 37 Therefore, the levels of Co and Cr in the synovial fluid may be, respectively, 300- and 1,000-fold higher than in the serum. 33 Likewise, metal ion levels in the synovial fluid, 33 serum,38,39 and synovial membrane 33 are different. Furthermore, methods for determining metal ion levels vary between studies and possible differences need to be considered.
CoCr ions concentration-dependently decreased metabolic activity of the chondrocytes in an almost linear fashion at both 24 and 72 hours. These findings indicated that CoCr ions caused arrest of cell proliferation, as nontreated control group exhibited higher metabolic activity over the culture period than treatment groups. Comparable results have been reported in human osteoblast-like cells incubated with a maximum of 0.1 ppm Cr ions. 16 Furthermore, Fleury et al. 40 cultured osteoblasts in the presence of CoCr ion concentrations close to those used by our experimental setup (0 -10 ppm) and found a time- and concentration-dependent decrease in metabolic activity. In our study, this time-dependent effect up to 72 hours in culture has been confirmed for osteoarthritic chondrocytes. Nevertheless, differences between different cell types or cell lines may occur.
Flow cytometry analysis confirmed an increase in apoptotic and dead cells from 24 to 72 hours at the 2 highest CoCr concentrations compared with the numbers of such cells after treatments with 3 lower concentrations. After 72 hours of incubation, more than 90% of all chondrocytes treated with the highest level of CoCr ions were apoptotic. It has to be emphasized that these findings in 2D cell culture may differ in explant cultures or in vivo. Furthermore, such critical concentrations could be expected at the earliest years after UKA or focal metal resurfacing, as deposition and accumulation of Co and Cr in the cartilage matrix will occur slowly. This contrasted with published reports, in which cytotoxic effect on osteoblast-like cells has been demonstrated only at an earlier time point than later culture stages 16 or has not been observed at all. 41 These discrepancies could be attributed to the fact that those studies examined effects of lower concentrations of CoCr ions (0.05-5.9 ppm) and shorter culture period (24-48 hours). Several studies that investigated cytotoxic effects of Co on osteoblasts and macrophages used concentrations (9.42-21.8 ppm) comparable to those used in our study, whereas Cr concentrations (24.95-145.57 ppm) were much higher than in our experiments.20,40,42 These methodological differences make a direct comparison of published data and our present results difficult. However, incubation with Co or Cr separately or in combination decreased cell counts even at lower concentrations. 16 Furthermore, Co ions at a range of 1 to 10 ppm have been shown to inhibit cell proliferation and induce apoptosis in lymphocytes.18,43
After 24 and 72 hours, no morphological changes were observed at the 2 lowest CoCr concentrations, however at 4.08 ppm Co and 1.67 Cr, thinner cell protrusions were seen already at 24 hours and more elongated protrusions—after 72 hours. At the latter time point, cell shrinkage occurred followed by the arrest of cell proliferation as denoted by cessation of the metabolic activity. At the highest concentration of CoCr ions (16.33/6.66 ppm), all cells contracted, and the formation of apoptotic corpuscles was visible. 18 These observations are in line with the results of flow cytometry experiments that showed an increased number of late apoptotic cells at 72 hours.
Expression levels of cartilage-specific genes encoding anabolic markers were dysregulated proportionally to CoCr ion concentrations. COL2A1 expression tended to decrease after 24 hours only at higher CoCr ions concentrations, whereas after 72 hours, lower expression was observed at all CoCr ion concentrations tested. Expression levels of aggrecan and versican exhibited a similar trend, whereas versican was expressed at a significantly higher level in the control group compared to its expression in the presence of metal ions at all concentrations. In contrast, a study by Kurdziel et al., 24 where human chondrocytes were treated with CoCrMo particles, a dramatic increase in COL2A1 expression was observed over 10 days. Furthermore, the authors reported that wear debris from CoCrMo decreased expression levels of genes encoding the synthesis of proteoglycans in human chondrocytes during culturing. 24 This trend was in accordance with the results of our study. Expression of SOX9 gene encoding a transcription factor of type II collagen gene at CoCr concentrations of 4.08/1.67 ppm and 8.17/3.33 ppm ions group tended to be increased after 24 hours, but the effect did not reach statistical significance. After 72 h, SOX9 gene expression significantly decreased at CoCr concentrations of 4.08/1.67 ppm and 16.33/6.66 ppm in comparison to 24 hours expression values, whereas in the presence of 8.17/3.33 ppm CoCr for 72 hours, SOX9 gene expression was nearly at its highest level. At all other concentrations, SOX9 expression level was similar to that in control. Contrary to the effects observed by us in chondrocytes, gene expression in osteoblasts treated with CoCr ions has been reported to be unaffected. 44
In addition to the analysis of the transcriptional activity of chondrocytes, the release of pro-inflammatory cytokines, crucial markers of osteoarthritis progression,45,46 was investigated. The release of the cytokines IL-6 and IL-8 into the supernatant was altered by the exposure to CoCr ions, whereas levels of TNF-α and IL-1β were below the detection limit. IL-6 levels did not increase at lower CoCr ions concentrations compared to those in control group, but decreased at the highest concentration. However, differences did not reach statistical significance after 24 or 72 hours. Similar observations have been reported in peripheral blood mononuclear cells. 41 The release of IL-8, a chemokine relevant for the ability of early stress response to attract neutrophils and monocytes, 47 was generally directly proportional to CoCr ion concentrations. However, at the highest concentration, IL-8 level was nominally lower than at the preceding lower concentration of 8.17/3.33 ppm CoCr ions. The pattern of the concentration-response relationship for IL-6 and IL-8 supports flow cytometry data that revealed extensive apoptosis of chondrocytes at the highest CoCr ions concentration. An increase in IL-8 levels similar to that observed in our study was also shown in endothelial and epithelial cells incubated with Co and Cr ions.47,48 Several studies have demonstrated a pro-inflammatory effect caused by the exposure to metal ions. In these studies, CoCrMo ions at a concentration range from 4 to 5.9 ppm led to increased secretion of pro-inflammatory cytokines and matrix metalloproteinases in human macrophages20,49-51 as well as triggered a chronic inflammatory response in T-cells. 52
Limitations of our present study included culturing only osteoarthritic chondrocytes that were pre-exposed to inflammatory environment in vivo, whereas a control group of healthy chondrocytes would be also desirable. Furthermore, our study investigated the response of chondrocytes in a 2D culture, which is nevertheless the most widely used cell culture model. However, the results might be different in a 3D setup or in vivo. In future studies, cartilage tissue (e.g., osteochondral grafts) could be used in a dynamic environment to better simulate in vivo conditions. Furthermore, additional time points past 72 hours of cultivation could be beneficial to see if lower concentrations would already be toxic for the chondrocytes over a longer time or if they might adapt to the changed conditions. Longer follow-up studies with lower concentrations are necessary to detect toxic effects on cartilage and confirm whether Co and Cr ions play a role in the failure mechanism after partial joint replacement.
Conclusion
CoCr ions had a time- and concentration-dependent effect on human osteoarthritic chondrocytes grown in a monolayer culture. CoCr ions concentration-dependently impaired metabolic activity and cell proliferation. Furthermore, at higher concentrations, signs of early and late apoptosis were observed in chondrocytes during 3-day culture. Complex effects of CoCr ions were revealed on gene expression, with higher concentrations generally decreasing expression of cartilage-specific anabolic genes compared with control level. The critical concentrations of Co and Cr for the induction of apoptosis in chondrocytes appeared to be approximately 10 and 5 ppm, respectively.
This study suggests a potential biotribocorrosive effect of metal implant components. However, further research is required to gain additional knowledge in both basic science and clinical settings. More experiments, especially in 3D cultures, are needed to better understand important signaling pathways and interactions between chondrocytes and metal ions.
The present study suggests an adverse effect of metal ions on the metabolism of articular chondrocytes. Correspondingly, wear and corrosion after UKA and partial surface replacement should be minimized to prevent degeneration of the preserved articular cartilage.
Footnotes
Acknowledgments and Funding: We thank Daniela Kern for assistance and continuous support with cell culture and flow cytometry experiments. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NÖ Forschungs- und Bildungsges.m.b.H. (NFB) and the provincial government of Lower Austria through the Life Science Calls (Project ID: LSC14-015) and has been carried out within Danube-University Krems—University for Continuing Education in cooperation with the Austrian Excellence Center for Tribology (AC2T research GmbH) and Landesklinikum Baden-Mödling that supported this study. The authors also gratefully acknowledge funds for parts of this work from the Austrian COMET-Program (project XTribology, no. 849109) via the Austrian Research Promotion Agency (FFG) and the Province of Lower Austria, Vorarlberg, and Vienna.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The present study was approved by the Regional Ethical Committee (GS4-EK-4/199-2016).
Informed Consent: All patients provided written informed consent for the use of articular cartilage remnants and chondrocytes.
Trial Registration: Not applicable.
ORCID iDs: Christoph Bauer  https://orcid.org/0000-0002-5142-3834
https://orcid.org/0000-0002-5142-3834
Manel Rodríguez Ripoll  https://orcid.org/0000-0001-9024-9587
https://orcid.org/0000-0001-9024-9587
Stefan Nehrer  https://orcid.org/0000-0001-8008-2226
https://orcid.org/0000-0001-8008-2226
References
- 1. Lorbach O, Pape D, Mosser P, Kohn D, Anagnostakos K. Die mediale monokondyläre Kniegelenkprothese. Der Orthopäde. 2014;43(10):875-82. [DOI] [PubMed] [Google Scholar]
- 2. van Bergen CJA, van Eekeren ICM, Reilingh ML, Sierevelt IN, van Dijk CN. Treatment of osteochondral defects of the talus with a metal resurfacing inlay implant after failed previous surgery. Bone Joint J. 2013;95-B(12):1650-5. [DOI] [PubMed] [Google Scholar]
- 3. Robertsson O, Ranstam J, Sundberg M, W-Dahl A, Lidgren L. The Swedish Knee Arthroplasty Register: a review. Bone Joint Res. 2014;3:217-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kristensen PW, Holm HA, Varnum C. Up to 10-year follow-up of the Oxford medial partial knee arthroplasty—695 cases from a single institution. J Arthroplasty. 2013;28(9 Suppl):195-8. [DOI] [PubMed] [Google Scholar]
- 5. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesæter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89(3):519-25. [DOI] [PubMed] [Google Scholar]
- 6. Argenson JNA, Parratte S, Bertani A, Flecher X, Aubaniac JM. Long-term results with a lateral unicondylar replacement. Clin Orthop Relat Res. 2008;466(11):2686-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernandez NM, Petis SM, Hanssen AD, Sierra RJ, Abdel MP, Pagnano MW. Infection after unicompartmental knee arthroplasty: a high risk of subsequent complications. Clin Orthop Relat Res. 2019;477(1):70-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohr G, Martin J, Clarius M. Revision after unicompartmental knee arthroplasty [in German]. Orthopade. 2014;43(10):883-90. [DOI] [PubMed] [Google Scholar]
- 9. Stojanović B, Bauer C, Stotter C, Klestil T, Nehrer S, Franek F, et al. Tribocorrosion of a CoCrMo alloy sliding against articular cartilage and impact of metal ion release on chondrocytes. Acta Biomater. 2019;94:597-609. [DOI] [PubMed] [Google Scholar]
- 10. Stotter C, Stojanovic B, Bauer C, Ripoll MR, Franek F, Klestil T, et al. Effects of loading conditions on articular cartilage in a metal-on-cartilage pairing. J Orthop Res. Epub 2019 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espallargas N, Torres C, Muñoz AI. A metal ion release study of CoCrMo exposed to corrosion and tribocorrosion conditions in simulated body fluids. Wear. 2015;332-333:669-78. [Google Scholar]
- 12. Berry DJ, Abdel MP, Callaghan JJ; Members of the Clinical Research Group. What are the current clinical issues in wear and tribocorrosion? Clin Orthop Relat Res. 2014;472(12):3659-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pelt CE, Erickson J, Clarke I, Donaldson T, Layfield L, Peters CL. Histologic, serologic, and tribologic findings in failed metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2013;95(21):e163. [DOI] [PubMed] [Google Scholar]
- 14. Ebramzadeh E, Campbell P, Tan TL, Nelson SD, Sangiorgio SN. Can wear explain the histological variation around metal-on-metal total hips? Clin Orthop Relat Res. 2014;473(2_suppl):487-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. VanOs R, Lildhar LL, Lehoux EA, Beaulé PE, Catelas I. In vitro macrophage response to nanometer-size chromium oxide particles. J Biomed Mater Res B Appl Biomater. 2014;102(1):149-59. [DOI] [PubMed] [Google Scholar]
- 16. Zijlstra WP, Bulstra SK, van Raay JJAM, van Leeuwen BM, Kuijer R. Cobalt and chromium ions reduce human osteoblast-like cell activity in vitro, reduce the OPG to RANKL ratio, and induce oxidative stress. J Orthop Res. 2012;30(5):740-7. [DOI] [PubMed] [Google Scholar]
- 17. Delaunay C, Petit I, Learmonth ID, Oger P, Vendittoli PA. Metal-on-metal bearings total hip arthroplasty: The cobalt and chromium ions release concern. Orthop Traumatol Surg Res. 2010;96:894-904. [DOI] [PubMed] [Google Scholar]
- 18. Huk OL, Catelas I, Mwale F, Antoniou J, Zukor DJ, Petit A. Induction of apoptosis and necrosis by metal ions in vitro. J Arthroplasty. 2004;19(8 suppl 3)84-7. [DOI] [PubMed] [Google Scholar]
- 19. Andrews RE, Shah KM, Wilkinson JM, Gartland A. Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-on-metal hip replacement on human osteoblasts and osteoclasts: implications for skeletal health. Bone. 2011;49(4):717-23. [DOI] [PubMed] [Google Scholar]
- 20. Catelas I, Petit A, Vali H, Fragiskatos C, Meilleur R, Zukor DJ, et al. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials. 2005;26(15):2441-53. [DOI] [PubMed] [Google Scholar]
- 21. Ferguson GM, Watanabe S, Georgescu HI, Evans CH. The synovial production of collagenase and chondrocyte activating factors in response to cobalt. J Orthop Res. 1988;6(4):525-30. [DOI] [PubMed] [Google Scholar]
- 22. Maloney WJ, Castro F, Schurman DJ, Smith RL. Effects of metallic debris on adult bovine articular chondrocyte metabolism in vitro. J Appl Biomater. 1994;5(2_suppl):109-15. [Google Scholar]
- 23. Czarnek K, Terpilowska S, Siwicki AK. Selected aspects of the action of cobalt ions in the human body. Cent Eur J Immunol. 2015;40(2_suppl):236-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurdziel MD, Salisbury M, Kaplan L, Maerz T, Baker KC. Exposure of articular chondrocytes to wear particles induces phagocytosis, differential inflammatory gene expression, and reduced proliferation. J Mater Sci Mater Med. 2017;28(7):106. [DOI] [PubMed] [Google Scholar]
- 25. Lonu P, Srinivas A, Overstreet M, Hungerford M, Frondoza CG. editors. Cell death induced by prosthetic metal ions in human chondrocytes. 7th World Biomaterials Congress; 2004 May 17-22; Sydney, Australia. [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402-8. [DOI] [PubMed] [Google Scholar]
- 27. Roesems G, Hoet PHM, Dinsdale D, Demedts M, Nemery B. In vitro cytotoxicity of various forms of cobalt for rat alveolar macrophages and type II pneumocytes. Toxicol Appl Pharmacol. 2000;162(1):2-9. [DOI] [PubMed] [Google Scholar]
- 28. Malahias M-A, Chytas D, Thorey F. The clinical outcome of the different HemiCAP and UniCAP knee implants: a systematic and comprehensive review. Orthop Rev (Pavia). 2018;10(2_suppl):7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplast. 2016;31:1061-21. [DOI] [PubMed] [Google Scholar]
- 30. Mischler S, Muñoz AI. Wear of CoCrMo alloys used in metal-on-metal hip joints: a tribocorrosion appraisal. Wear. 2013;297:1081-94. [Google Scholar]
- 31. Mischler S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: a comparative evaluation. Tribol Int. 2008;41:573-83. [Google Scholar]
- 32. Van Der Straeten C, Banica T, De Smet A, Van Onsem S, Sys G. Metal ion measurements from total knee arthroplasty. Bone Jt J Orthop Proc Suppl. 2017;99-B(Suppl 2):69. [Google Scholar]
- 33. De Smet K, De Haan R, Calistri A, Campbell PA, Ebramzadeh E, Pattyn C, et al. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am. 2008;90(Suppl 4):202-8. [DOI] [PubMed] [Google Scholar]
- 34. Lass R, Grübl A, Kolb A, Stelzeneder D, Pilger A, Kubista B, et al. Comparison of synovial fluid, urine, and serum ion levels in metal-on-metal total hip arthroplasty at a minimum follow-up of 18 years. J Orthop Res. 2014;32:1234-40. [DOI] [PubMed] [Google Scholar]
- 35. Witzleb WC, Ziegler J, Krummenauer F, Neumeister V, Guenther KP. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop. 2006;77(5):697-705. [DOI] [PubMed] [Google Scholar]
- 36. Antoniou J, Zukor DJ, Mwale F, Minarik W, Petit A, Huk OL. Metal ion levels in the blood of patients after hip resurfacing: a comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg Am. 2008;90(Suppl 3):142-8. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Yan Y, Su Y, Qiao L. Release of metal ions from nano CoCrMo wear debris generated from tribo-corrosion processes in artificial hip implants. J Mech Behav Biomed Mater. 2017;68:124-33. [DOI] [PubMed] [Google Scholar]
- 38. Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Elevated serum cobalt with metal-on-metal articulating surfaces. J Bone Joint Surg Br. 1997;79(2_suppl):316-21. [DOI] [PubMed] [Google Scholar]
- 39. Jacobs JJ, Skipor AK, Doom PF, Campbell P, Schmalzried TP, Black J, et al. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop Relat Res. 1996;(329 Suppl):S256-63. [DOI] [PubMed] [Google Scholar]
- 40. Fleury C, Petit A, Mwale F, Antoniou J, Zukor DJ, Tabrizian M, et al. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: morphology, cytotoxicity, and oxidative stress. Biomaterials. 2006;27(18):3351-60. [DOI] [PubMed] [Google Scholar]
- 41. Mabilleau G, Gill R, Sabokbar A. Cobalt and chromium ions affect human osteoclast and human osteoblast physiology in vitro. Open Access Sci Rep. 2012;1(3):1-6. Available from: http://opus.bath.ac.uk/34871/ [Google Scholar]
- 42. Anissian L, Stark A, Dahlstrand H, Granberg B, Good V, Bucht E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop Scand. 2002;73(3):369-74. [DOI] [PubMed] [Google Scholar]
- 43. Akbar M, Brewer JM, Grant MH. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J Immuno-toxicol. 2011;8(2_suppl):140-9. [DOI] [PubMed] [Google Scholar]
- 44. Drynda A, Drynda S, Kekow J, Lohmann CH, Bertrand J. Differential effect of cobalt and chromium ions as well as CoCr particles on the expression of osteogenic markers and osteoblast function. Int J Mol Sci. 2018;19(10):E3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42. [DOI] [PubMed] [Google Scholar]
- 46. Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016;85:81-90. [DOI] [PubMed] [Google Scholar]
- 47. Devitt BM, Queally JM, Vioreanu M, Butler JS, Murray D, Doran PP, et al. Cobalt ions induce chemokine secretion in a variety of systemic cell lines. Acta Orthop. 2010;81(6):756-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ninomiya JT, Kuzma SA, Schnettler TJ, Krolikowski JG, Struve JA, Weihrauch D. Metal ions activate vascular endothelial cells and increase lymphocyte chemotaxis and binding. J Orthop Res. 2013;31(9):1484-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caicedo MS, Pennekamp PH, McAllister K, Jacobs JJ, Hallab NJ. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J Biomed Mater Res A. 2010;93(4):1312-21. [DOI] [PubMed] [Google Scholar]
- 50. Samelko L, Caicedo MS, Lim SJ, Della-Valle C, Jacobs J, Hallab NJ. Cobalt-alloy implant debris induce HIF-1α hypoxia associated responses: a mechanism for metal-specific orthopedic implant failure. PLoS One. 2013;8(6):e67127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trindade MCD, Lind M, Sun D, Schurman DJ, Goodman SB, Smith RL. In vitro reaction to orthopaedic biomaterials by macrophages and lymphocytes isolated from patients undergoing revision surgery. Biomaterials. 2001;22(3):253-9. [DOI] [PubMed] [Google Scholar]
- 52. Pearson MJ, Williams RL, Floyd H, Bodansky D, Grover LM, Davis ET, et al. The effects of cobalt-chromium-molybdenum wear debris in vitro on serum cytokine profiles and T cell repertoire. Biomaterials. 2015;67:232-9. [DOI] [PubMed] [Google Scholar]