Abstract
Numerous reports support the possible occurrence of acute disseminated encephalomyelitis (ADEM) following COVID-19. Herein, we report a case of ADEM in a 53-year-old man 2 weeks after SARS-CoV-2 infection. We reviewed the reports of adult cases of ADEM and its variant acute necrotizing hemorrhagic leukoencephalitis (ANHLE) to check for possible prognostic factors and clinical/epidemiological peculiarities. We performed a descriptive analysis of clinical and cerebrospinal fluid data. Ordinal logistic regressions were performed to check the effect of clinical variables and treatments on ADEM/ANHLE outcomes. We also compared ADEM and ANHLE patients. We identified a total of 20 ADEM (9 females, median age 53.5 years) and 23 ANHLE (11 females, median age 55 years). Encephalopathy was present in 80% of ADEM and 91.3% of ANHLE patients. We found that the absence of encephalopathy predicts a better clinical outcome in ADEM (OR 0.027, 95% CI 0.001–0.611, p = 0.023), also when correcting for the other variables (OR 0.032, 95% CI 0.001–0.995, p = 0.05). Conversely, we identified no significant prognostic factor in ANHLE patients. ANHLE patients showed a trend towards a worse clinical outcome (lower proportion of good/complete recovery, 4.5% vs 16.7%) and higher mortality (36.4% vs 11.1%) as compared to ADEM. Compared to pre-pandemic ADEM, we observed a higher median age of people with post-COVID-19 ADEM and ANHLE, a shorter interval between infection and neurological symptoms, and a worse prognosis both in terms of high morbidity and mortality. Despite being affected by the retrospective nature of the study, these observations provide new insights into ADEM/ANHLE following SARS-CoV-2 infection.
Keywords: COVID-19, ADEM, ANHLE, Acute disseminated encephalomyelitis, Acute necrotizing hemorrhagic leukoencephalitis, Encephalopathy, SARS-CoV-2
Introduction
The ongoing COVID-19 pandemic has extensively shown the multisystemic impact of viral infections. Among the threats posed by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), ample literature supports the possible occurrence of neurological complications [1]. Besides neurovascular diseases, a constantly increasing number of reports regards neuro-inflammatory disorders following COVID-19. Guillan–Barré syndrome, cytotoxic lesion of corpus callosum, acute disseminated encephalomyelitis (ADEM), and its variant acute necrotizing hemorrhagic leukoencephalitis (ANHLE) have all been reported throughout the COVID-19 pandemic [1]. ADEM is an autoimmune demyelinating disease of the central nervous system (CNS), preferentially occurring in childhood, but affecting also adults [2]. It is typically characterized by an acute, monophasic course with multifocal neurological signs and symptoms. While encephalopathy is a required feature for ADEM diagnosis in children, in adults it has been reported in only 20–56% of cases [3–5]. In adults, ADEM is associated with a previous infection in 50–75% of cases, with a lag period ranging from [4–6] few days to 2 months. Concerning prognosis, a complete recovery has been reported in 10–46% of the adult patients [3, 5, 7] with mortality ranging from 4 to 12% [3, 5, 8].
We recently faced the challenge of diagnosing and managing ADEM occurred in the context of COVID-19 disease. Herein, we report the case of a 53-year-old man who developed ADEM following SARS-CoV-2 infection. Furthermore, we reviewed the current literature concerning ADEM and ANHLE adult cases likely triggered by SARS-CoV-2, checking for possible prognostic factors and differences between ADEM and ANHLE.
Case report
A 53-year-old male patient developed a febrile episode with mild respiratory symptoms. A nasal swab tested positive for SARS-CoV-2 RNA. Two weeks later, he presented with subacute bilateral blindness. He was admitted to a COVID-19 department of our hospital, since a nasal swab was still positive for SARS-CoV-2 RNA. The patient deserved non-invasive oxygen therapy because of mild hypoxia. Head CT showed a mild hypodensity in the right occipital lobe, while a CT-angiography was unrevealing. In the following days, the patient developed a subacute encephalopathy characterized by fluctuations in consciousness level, spatial and temporal disorientation associated with severe dysarthria, ophthalmoplegia, left hemiparesis, four limbs ataxia, and left upper limb dystonia associated with facial and left arm stereotypic movement disorder. Brain MRI showed the presence of supra- and infratentorial bilateral hyperintense white matter lesions (Fig. 1A–D), with incomplete gadolinium enhancement (Fig. 1F–I). Spinal cord MRI revealed a dorsal enhancing lesion (Fig. 1E, J). A lumbar puncture was performed. CSF analysis showed 1 cell/µl with a mild increase in protein concentration (74 mg/dl). Oligoclonal bands (OCB) were negative. PCR performed on CSF were negative for neurotropic viruses (VZV, HSV 1–2, EBV, CMV, enterovirus), including SARS-CoV-2. Anti-MOG anti-AQP4 both tested negatives. Anti-Hu, anti-Yo, anti-Ri, anti-Tr, anti-CV2, anti-Ma proteins, anti-amphiphysin, and anti-GAD also tested negative. According to the clinical picture and the radiological findings, along with the unrevealing CSF analysis, the patient was diagnosed with ADEM. High-dose intravenous methylprednisolone was administered (1 g for 7 days followed by intravenous tapering for a total of 10.5 g), together with intravenous immunoglobulins (IVIG) (2 g/kg in 5 days), obtaining a stabilization of the neurological picture followed by a mild recovery. The clinical course was complicated by recurrent infections (C. Albicans and E. faecium sepsis, K. Pneumonia urinary infection) and by spontaneous retroperitoneal hemorrhage. A follow-up MRI performed 1 month later documented a stable lesion load, with a persistent enhancement of part of the lesions. 5-day IVIG therapy was then again administered. Three weeks later, a follow-up MRI showed a significant reduction of gadolinium enhancement. Further 3 days of IVMP were administered. At the moment of the present report, the patient only recovered partially, with neurological examination showing bilateral blindness, partial time and space disorientation, moderate dysarthria, echolalia, four limbs and truncal ataxia with an inability to walk.
Fig. 1.
Brain and spinal cord MRI. Brain MRI showing supra- and infratentorial bilateral FLAIR-hyperintense white matter lesions suggestive of ADEM (A–D). In (E) spinal cord MRI documenting a T2-hyperintense dorsal lesion. After gadolinium administration, brain (F–I) and spinal cord (J) are characterized by incomplete contrast enhancement
The patient and his wife provided informed consent for this report.
Review
Methods
We performed a literature review, searching on PubMed the papers concerning the occurrence of ADEM or ANHLE in patients with SARS-CoV-2 infection, published in the English language from the beginning of the pandemic to early June 2021. Concerning ANHLE, all the studies describing “acute necrotizing hemorrhagic leukoencephalitis”, “acute necrotizing encephalopathy”, and “acute hemorrhagic encephalomyelitis” and “acute hemorrhagic leukoencephalitis” were considered. We used the following search strategy: [ADEM OR acute disseminated encephalomyelitis OR acute necrotizing hemorrhagic leukoencephalitis OR ANHLE OR acute necrotizing encephalopathy OR ANE OR acute hemorrhagic encephalomyelitis OR AHEM OR acute hemorrhagic leukoencephalitis OR AHLE OR acute necrotizing leukoencephalitis OR ANLE] AND [COVID-19 OR SARS-CoV-2 OR COVID OR coronavirus disease]. The search identified 254 papers. Article titles and abstracts were screened by the authors of the present paper, including all the works potentially relevant for the search topic. Among the 254 papers screened, 48 were identified as relevant and underwent a full-text assessment to check eligibility according to the inclusion criteria of the present review. First, as previously published by other authors, we included patients whose case descriptions provided clinical and radiological details sufficient for ADEM or ANHLE diagnosis [9]. ADEM was defined as the acute onset of multifocal neurological signs/symptoms supported by white matter lesions suggestive of demyelination at central nervous system MRI [9]. ANHLE was defined by the evidence of micro- and/or macro-hemorrhage at brain MRI along with a hyperacute onset of the disease [9]. The studies were then selected according to the following inclusion criteria: adult patients (i.e., ≥ 18 years); SARS-CoV-2 infection confirmed with RT-PCR or serum antibody test; availability of clinical and radiological data; fulfillment of criteria for a probable association between SARS-CoV-2 infection and ADEM/ANHLE (neurological symptoms within 6 weeks from SARS-CoV-2 infection; either a PCR test positive for SARS-CoV-2 RNA or serology suggestive for SARS-CoV-2 acute infections; no evidence of other causes) [1]. Studies describing pathological or MRI findings without a clinical picture suggestive of ADEM or ANHLE were not included in the analyses. Following the application of the inclusion criteria, we retained 32 papers (14 concerning ADEM, 18 ANHLE) reporting a total of 43 cases (20 ADEM, 23 ANHLE) (Fig. 2).
Fig. 2.
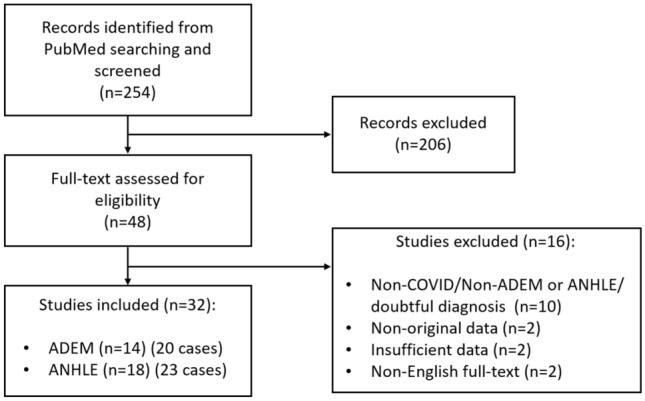
Search strategy flow chart
We classified the patients according to the severity of COVID-19 infection and the clinical outcome of the neurological disease. COVID-19 severity was classified, adapting previous classification [10] into: asymptomatic, mild, severe, and critical. Asymptomatic were patients with no respiratory symptoms. Mild disease included patients without pneumonia or with mild pneumonia; patients with dyspnea and hypoxia who required oxygen therapy were defined as severe; critical disease was defined by the need for mechanical ventilation and/or the occurrence of shock and/or multiorgan failure. The clinical outcome of the neurological disease was categorized as follows: complete recovery, good recovery, partial/poor recovery, no recovery, death. Complete recovery was defined by a normal neurological examination at follow-up; good recovery by the persistence of mild neurological signs/symptoms, likely not affecting the activity of daily living; partial/poor recovery was defined by the persistence of significant neurological disability; no recovery when the neurological picture did not improve over time.
Analyses were performed with SPSS statistical 26.0 (IBM SPSS, NY, USA). Univariate and multivariate ordinal logistic regressions were performed to check the effect of different variables on the clinical outcome of ADEM and ANHLE patients.
Results
ADEM
According to the inclusion criteria, we identified 14 studies reporting a total of 20 patients who developed ADEM following SARS-CoV-2 infection [10–23]. The infection was confirmed by a nasal swab positive for SARS-CoV-2 RNA (RT-PCR) in all but one patient [22]. In this case, the infection was documented with a positive SARS-CoV-2 serology [22].
Patients with ADEM had a median age of 53.5 years (range 21–70, IQR 40–58.25) with males accounting for the 55% of the total (Table 1). In 15% of patients, the SARS-CoV-2 infection was asymptomatic, while mild symptoms were observed in 25%. The 60% of patients developing ADEM had a critical course of COVID-19 infection. The main reason requiring hospitalization was neurological for the 40% of patients, while the remaining were hospitalized because of the respiratory clinical course. The median time between SARS-CoV-2 documented infection and the development of neurological symptoms was 15.5 days (IQR 9.5–20.5 days). Encephalopathy was present in 80% of the patients. In 50% of the cases, a neurological disorder was suspected following consciousness impairment despite withdrawal from sedation. Spinal cord MRI was performed in 10 out of 20 patients, revealing an incidence of spinal cord demyelinating lesions of 70%. Cerebrospinal fluid (CSF) was collected in 19 patients. In 17 of those, oligoclonal bands (OCB) were measured, obtaining only two positive results (11.8%). The median number of CSF nucleated cells was 3/µl (IQR 1.25–6/µl) and median CSF proteins were 48.85 mg/dl (IQR 35.25–57.5 mg/dl). CSF PCR analysis for SARS-CoV-2 was reported in 15 cases, with only two positive results [12, 14] (one of those was uncertain [14]). Anti-MOG and anti-AQP4 antibodies were, respectively, tested in 5 and 6 patients with all negative results. The treatment approach was reported in 18 cases, varying among the different reports. In two cases (11.1%) no specific treatment was administered [10, 18]. Three patients (16.7%) were treated with low dosage steroid therapy [19, 20, 23]. In four cases (22.2%), high dose IVMP pulse therapy was administered followed by steroid oral tapering [10, 11]. In five patients (27.8%), IVMP was followed by the administration of intravenous immunoglobulin (IVIG) [12–14, 20]. One patient received IVIG alone [15] and another one was treated with 5-day plasmapheresis (PLEX) [22]. Finally, two patients were treated with rituximab (11.1%), one following IVMP [17] and the other following IVMP and PLEX treatment [21]. The clinical outcome was reported in 18 cases. One patient showed complete recovery from the neurological symptoms and signs (5.6%) [10]. A good clinical recovery was observed in two patients (11.1%) [12, 17], while partial/poor recovery was observed in ten (55.6%) [10, 11, 13, 14, 18, 20, 22] and no recovery in three patients (16.7%) [15, 20, 21]. Two patients died (11.1%) [18, 19].
Table 1.
Clinical and CSF data of ADEM and ANHLE patients
| ADEM | ANHLE | |
|---|---|---|
| Number of patients (n) | 20 | 23 |
| Female (%) | 45% | 47.8% |
| Age (years) | 53.5 ± 40–58.25 | 55 ± 46.75–58.25 |
| Severity of COVID-19 infection (%) | ||
| Asymptomatic | 15% | 0% |
| Mild | 25% | 34.8% |
| Severe | 0% | 8.7% |
| Critical | 60% | 56.5% |
| Main reason for hospitalization: neurological | 40% | 52.2% |
| Lag time between infection and neurological disease (days) | 15.5 ± 9.5–20.5 | 9 ± 2–5–20.75 |
| Presence of encephalopathy | 80% | 91.3% |
| Presenting with difficult awakening from sedation | 50% | 30.4% |
| Presence of spinal cord lesion | 70% (n = 10) | Na |
| CSF | ||
| OCB presence | 11.8% (n = 17) | 33.3% (n = 3) |
| Cells/ΜL | 3 ± 1.25–6 (n = 19) | 4 ± 3–5 (n = 18) |
| Protein (mg/dl) | 48.85 ± 35.25–57.5 (n = 19) | 230 ± 80–5–605.5 (n = 18) |
| Anti-MOG | 0% (n = 5) | 0% (n = 1) |
| Anti-AQP4 | 0% (n = 6) | 0% (n = 1) |
| Neurological clinical outcome: | n = 18 | n = 22 |
| Complete recovery | 5.6% | 0% |
| Good recovery | 11.1% | 4.5% |
| Partial/poor recovery | 55.6% | 59.1% |
| No recovery | 16.7% | 0% |
| Death | 11.1% | 36.4% |
Data are expressed as percentage or median ± interquartile range. Sample size of the analyses are specified in brackets when the specific variable was not available for all the reported patients.
ADEM acute disseminated encephalomyelitis, ANHLE acute necrotizing hemorrhagic leukoencephalitis, CSF cerebrospinal fluid, na not applicable, OCB oligoclonal bands
Univariate ordinal logistic regressions were performed to check the effect of age, sex, the severity of COVID-19 symptoms, main reason for hospitalization (respiratory vs neurological), the presence of encephalopathy, OCB status, the presence of spinal cord lesions, and the treatment on the clinical outcome of patients with ADEM. We found that the absence of encephalopathy was associated with a lower risk of a worse clinical outcome, OR 0.027 (95% CI 0.001–0.611), Wald χ2 (1) = 5.153, p = 0.023. On the opposite, no effect was observed for the other variables. We then performed a multivariate ordinal logistic regression including encephalopathy, the reason for hospitalization, and the COVID-19 severity. In this model, the absence of encephalopathy was the only significant predictor of clinical outcome (OR 0.032 (95% CI 0.001–0.995), Wald χ2 (1) = 3.854, p = 0.05).
ANHLE
According to the inclusion criteria we identified in the literature 18 studies reporting the occurrence of ANHLE in 23 patients following SARS-CoV-2 infection [10, 24–40]. The infection was confirmed with a nasal swab positive for SARS-CoV-2 RNA (RT-PCR) in all but one patient [10].
The median age of patients with ANHLE was 55 years (range 33–77, IQR 46.75–58.25) with males representing 52.2% of the patients (Table 1). In all the reported cases. The SARS-CoV-2 infection was symptomatic. 34.8% of patients had mild symptoms, while a severe course was observed in 8.7% and critical disease in 56.5%. The main reason for hospitalization was neurological in 52.2% of the patients. The median time between SARS-CoV-2 infection and the onset of neurological symptoms was 9 days (IQR 2–5–20.75 days). 91.3% of the patients presented with encephalopathy. An altered consciousness state after the end of sedation arose the suspicion of a CNS neurological disorder in 30.4% of the cases [10, 24, 28, 30, 39]. In one case ANHLE was associated with AIDP [10]. CSF was collected in 18 patients. OCB were measured in three patients with one positive result [28]. The median number of CSF nucleated cells was 4/µl (IQR 3–5/µl), and median CSF protein concentration was 230 mg/dl (IQR 80–5–605.5 mg/dl). CSF PCR analysis for SARS-CoV-2 was reported in 13 cases with one positive result [25]. Anti-MOG and anti-AQP4 antibodies were tested in one patient with negative results [27]. The treatment strategy was reported in all cases. Six patients (26.1%) received no specific treatment [10, 28, 30, 35, 38, 39]. Three patients (13%) received low-dose steroid treatment [10, 30, 31]. Six patients (26.1%) were treated with IVMP pulse therapy [10, 32, 33, 36, 37, 40] and six others (26.1%) with IVMP followed by IVIG [10, 24, 26, 27, 34]. One patient was treated with IVIG and PLEX [25], another with IVIG alone [29]. The clinical outcome was reported for 22 patients. No patient showed a complete recovery. Good clinical recovery was observed in one patient (4.5%) [39], while partial/poor recovery was reported in 13 cases (59.1%) [10, 24–26, 28, 33–35, 38, 40]. Eight patients died (36.4%) [10, 27, 30–32, 36, 37]. In one case the diagnosis was pathologically confirmed [10].
Univariate ordinal logistic regressions were performed to check the effect of age, sex, the severity of COVID-19 symptoms, main reason for hospitalization (respiratory vs neurological), the presence of encephalopathy, and the treatment on the clinical outcome of patients with ANHLE. We found that female patients have an increased risk for a worse outcome, OR 8.035 (95% CI 1.132–57.132), Wald χ2 (1) = 4.343, p = 0.037. No effect was observed for the other tested variables. The effect of gender on the clinical outcome, however, did not survive to a multivariate ordinal logistic regression including the main reason for hospitalization and sex (p = 0.095).
ADEM vs ANHLE
We then compared ADEM and ANHLE patients to check for any possible differences in clinical and CSF characteristics. Mann–Whitney showed no difference concerning age, the time between SARS-CoV-2 infection and neurological disease, CSF nucleated cells. We run Chi-square test of homogeneity with no differences between groups in terms of gender, the incidence of encephalopathy, the main reason for hospitalization, COVID-19 disease severity. We found a trend toward significance for the clinical outcome (p = 0.091), with ANHLE group having a higher incidence of fatal course (36.4% vs 11.1%) and a lower proportion of good or complete recovery (4.5% vs 16.7%).
Discussion
Our report confirms the possible occurrence of ADEM following SARS-CoV-2 infections in adult patients. We reviewed the literature available at the moment of the present report, addressing clinical and CSF findings. We also checked for possible prognostic factors, comparing ADEM and ANHLE patients.
Previous studies have reviewed the cases of ADEM [9, 41, 42] and ANHLE9 following COVID-19. However, those studies did not focus on possible disease prognostic factors nor the clinical differences between ADEM and ANHLE. Clinical [43] and radiological [43, 44] prognostic factors have been previously proposed for the risk of multiphasic ADEM, both for children and adult pre-pandemic patients [43, 44]. Conversely, to date, no factor predicting the outcome of the disease was identified. In the present study, we observed that the absence of encephalopathy was associated with a lower risk of a worse clinical outcome in ADEM patients, both in univariate and multivariate analyses. On the opposite, we failed to identified valuable prognostic factors for ANHLE patients. We found that encephalopathy was present in 80% of ADEM and 91.3% of ANHLE patients. This incidence appears to be higher as compared to what was previously reported in pre-pandemic ADEM patients (20–56%) [3–5]. Furthermore, in 50% of ADEM patients and 30.4% of ANHLE patients, difficult awakening from sedation was the presenting neurological symptoms, suggesting the need to promptly investigate encephalopathy in COVID-19 patients.
Despite the growing number of ADEM and ANHLE reports in people with SARS-CoV-2 infection, the development of these neurological complications is very rare. At the time of the present report, the global number of SARS-CoV-2 infections counted from the beginning of the pandemic exceeded 175 million (WHO COVID-19 weekly epidemiological update, edition 44, published 15 June 2021, available at https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---15-june-2021). Against the ample number of SARS-CoV-2 infections, we identified only 43 reported patients who developed ADEM or ANHLE in the context of COVID-19, with a prevalence of about 2.4 cases per 10,000,000 COVID-19 patients. This prevalence is much lower than what is expected in the general population. In a previous study published before the pandemic, the prevalence of ADEM was 3.3 per 100,000 population [45]. This 100-fold difference may be at least partially explained by an under-reporting of ADEM/ANHLE cases in COVID-19. Conversely, one could argue that SARS-CoV-2 may be a less effective trigger for ADEM, as compared to other viral infections. While the prevalence of ANHLE is not established, it is usually considered a rare variant of ADEM [46]. Interestingly, we identified a higher number of ANHLE reports as compared to ADEM. As previously highlighted by Manzano et al., other significant epidemiological differences exist between pre-pandemic ADEM and post-COVID-19 ADEM [9]. We observed a higher median age of people with ADEM (55 years vs 33–41 years [3, 5, 44, 47, 48]) and ANHLE (55 years vs 38 years [46]), a shorter lag time between infection and neurological symptoms [47] and a worse prognosis both in terms of high morbidity and mortality [3, 5, 7, 8] and the absence of anti-MOG antibodies in ADEM patients. When comparing ADEM and ANHLE, we observed a higher mortality and worse prognosis trend in ANHLE patients, consistently with previous studies [46].
Our findings should be read in light of the study limitations. These include the small sample size, and the retrospective collection of published reports, which are highly variable in terms of data reporting and quality. Moreover, our analyses concerning prognosis were based on a retrospective categorization of clinical outcomes that may have biased the study. In addition, follow-up periods were mostly short and not clearly specified in most of the studies, being another possible source of bias. Other study limitations are the low number of anti-MOG and anti-AQP testing and the possible under-reporting of ADEM/ANHLE cases.
Despite these limitations, the present study, suggests encephalopathy as a possible prognostic factor in post-COVID-19 ADEM and highlights the possible epidemiological differences between pre-pandemic and post-COVID-19 ADEM in adults. Future prospective multi-center studies are needed to shed light on this rare but yet possible complication of SARS-CoV-2 infection.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declarations
Conflicts of interest
The authors declare no conflicting interests concerning the present study.
Informed consent
The patient and his wife provided informed consent for this report.
References
- 1.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pohl D, Alper G, Van Haren K, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 Supplement 2):S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz S, Mohr A, Knauth M, et al. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56(10):1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 4.Koelman DLH, Chahin S, Mar SS, et al. Acute disseminated encephalomyelitis in 228 patients: a retrospective, multicenter US study. Neurology. 2016;86(22):2085–2093. doi: 10.1212/WNL.0000000000002723. [DOI] [PubMed] [Google Scholar]
- 5.Ketelslegers IA, Visser IER, Neuteboom RF, et al. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler. 2011;17(4):441–448. doi: 10.1177/1352458510390068. [DOI] [PubMed] [Google Scholar]
- 6.Noorbakhsh F, Johnson RT, Emery D, Power C. Acute disseminated encephalomyelitis: clinical and pathogenesis features. Neurol Clin. 2008;26(3):759–780. doi: 10.1016/j.ncl.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Höllinger P, Sturzenegger M, Mathis J, et al. Acute disseminated encephalomyelitis in adults: a reappraisal of clinical, CSF, EEG, and MRI findings. J Neurol. 2002;249(3):320–329. doi: 10.1007/s004150200012. [DOI] [PubMed] [Google Scholar]
- 8.Marchioni E, Marinou-Aktipi K, Uggetti C, et al. Effectiveness of intravenous immunoglobulin treatment in adult patients with steroid-resistant monophasic or recurrent acute disseminated encephalomyelitis. J Neurol. 2002;249(1):100–104. doi: 10.1007/pl00007836. [DOI] [PubMed] [Google Scholar]
- 9.Manzano GS, McEntire CRS, Martinez-Lage M, et al. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID-19: systematic review and meta-synthesis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):1080. doi: 10.1212/NXI.0000000000001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley L, Zeicu C, Whitton L, Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. 2020;13(12):239597. doi: 10.1136/bcr-2020-239597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novi G, Rossi T, Pedemonte E, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons T, Banks S, Bae C, et al. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267(10):2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utukuri PS, Bautista A, Lignelli A, Moonis G. Possible acute disseminated encephalomyelitis related to severe acute respiratory syndrome coronavirus 2 infection. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umapathi T, Quek WMJ, Yen JM, et al. Encephalopathy in COVID-19 patients; viral, parainfectious, or both? eNurologicalSci. 2020;21:100275. doi: 10.1016/j.ensci.2020.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assunção FB, Fragoso DC, Donoso Scoppetta TLP, Martins Maia AC. COVID-19-associated acute disseminated encephalomyelitis-like disease. AJNR Am J Neuroradiol. 2021;42(4):E21–E23. doi: 10.3174/ajnr.A6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahmirzaei S, Naser MA. Association of COVID-19 and acute disseminated encephalomyelitis (ADEM) in the absence of pulmonary involvement. Autoimmun Rev. 2021;20(3):102753. doi: 10.1016/j.autrev.2021.102753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes CCB, Brucki SMD, Passos Neto CEB, et al. Acute disseminated encephalomyelitis in COVID-19: presentation of two cases and review of the literature. Arq Neuro-Psiquiatr. 2020;78(12):805–810. doi: 10.1590/0004-282X20200186. [DOI] [PubMed] [Google Scholar]
- 19.Abdi S, Ghorbani A, Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416:117001. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCuddy M, Kelkar P, Zhao Y, Wicklund D. Acute demyelinating encephalomyelitis (ADEM) in COVID-19 infection: a case series. Neurol India. 2020;68(5):1192–1195. doi: 10.4103/0028-3886.299174. [DOI] [PubMed] [Google Scholar]
- 21.Fitouchi S, Heger B, Kremer L, et al. A case of acute disseminate encephalomyelitis after SARS-CoV-2 related acute respiratory distress syndrome. J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoghi A, Ramezani M, Roozbeh M, et al. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Sclerosis Relat Disord. 2020;44:102324. doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brun G, Hak J-F, Coze S, et al. COVID-19—White matter and globus pallidum lesions: demyelination or small-vessel vasculitis? Neurol Neuroimmunol Neuroinflamm. 2020;7(4):777. doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong MH, Chan YFZ, Liu J, et al. A rare case of acute Hemorrhagic leukoencephalitis in a COVID-19 patient. J Neurolo Sci. 2020;416:117035. doi: 10.1016/j.jns.2020.117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virhammar J, Kumlien E, Fällmar D, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445–449. doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delamarre L, Gollion C, Grouteau G, et al. COVID-19–associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J Neurol Neurosurg Psychiatry. 2020;91(9):1004–1006. doi: 10.1136/jnnp-2020-323678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh R, Dubey S, Finsterer J, et al. SARS-CoV-2-Associated Acute Hemorrhagic, Necrotizing Encephalitis (AHNE) Presenting with Cognitive Impairment in a 44-Year-Old Woman without Comorbidities: A Case Report [Internet]. Am J Case Rep 2020; 21[cited 2021 Jun 19] Available from: https://www.amjcaserep.com/abstract/index/idArt/925641. [DOI] [PMC free article] [PubMed]
- 28.Haqiqi A, Samuels TL, Lamb FJ, et al. Acute haemorrhagic leukoencephalitis (Hurst disease) in severe COVID- 19 infection. Brain Behav Immun Health. 2021;12:100208. doi: 10.1016/j.bbih.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullaguri N, Sivakumar S, Battineni A, et al. COVID-19 Related Acute Hemorrhagic Necrotizing Encephalitis: A Report of Two Cases and Literature Review [Internet]. Cureus 2021;[cited 2021 Jun 27] Available from: https://www.cureus.com/articles/50440-covid-19-related-acute-hemorrhagic-necrotizing-encephalitis-a-report-of-two-cases-and-literature-review. [DOI] [PMC free article] [PubMed]
- 31.Dixon L, Varley J, Gontsarova A, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkady A, Rabinstein AA. Acute necrotizing encephalopathy and myocarditis in a young patient with COVID-19. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e801. [Google Scholar]
- 33.Ciolac D, Crivorucica I, Zota E, et al. Extensive cerebellar involvement and cognitive impairment in COVID-19-associated acute necrotizing encephalopathy. Ther Adv Neurol Disord. 2021;14:175628642098517. doi: 10.1177/1756286420985175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bansal P, Fory EK, Malik S, Memon AB. Clinical course of a patient with radiographically described acute necrotizing encephalopathy. Radiology. 2020;297(2):E278–E280. doi: 10.1148/radiol.2020203132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krett JD, Jewett GAE, Elton-Lacasse C, et al. Hemorrhagic encephalopathy associated with COVID-19. J Neuroimmunol. 2020;346:577326. doi: 10.1016/j.jneuroim.2020.577326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varadan B, Shankar A, Rajakumar A, et al. Acute hemorrhagic leukoencephalitis in a COVID-19 patient—a case report with literature review. Neuroradiology. 2021;63(5):653–661. doi: 10.1007/s00234-021-02667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handa R, Nanda S, Prasad A, et al. Covid-19-associated acute haemorrhagic leukoencephalomyelitis. Neurol Sci. 2020;41(11):3023–3026. doi: 10.1007/s10072-020-04703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalil A, Baker CS, Johnston RB, et al. Acute hemorrhagic encephalitis related to COVID-19. Neurol Clin Pract. 2021;11(2):e147–e151. doi: 10.1212/CPJ.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karapanayiotides T, Geka E, Prassopoulos P, et al. Concentric demyelination pattern in COVID-19-associated acute haemorrhagic leukoencephalitis: a lurking catastrophe? Brain. 2020;143(12):e100–e100. doi: 10.1093/brain/awaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green C, Morrison H, Smith P, et al. Teaching neuroimages: COVID-19 associated acute disseminated encephalomyelitis with corpus callosal hemorrhage. Neurology. 2020 doi: 10.1212/WNL.0000000000011001. [DOI] [PubMed] [Google Scholar]
- 41.Zelada-Ríos L, Pacheco-Barrios K, Galecio-Castillo M, et al. Acute disseminated encephalomyelitis and COVID-19: a systematic synthesis of worldwide cases. J Neuroimmunol. 2021;359:577674. doi: 10.1016/j.jneuroim.2021.577674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Wang Y, Huo L, et al. SARS-CoV-2-associated acute disseminated encephalomyelitis: a systematic review of the literature [Internet] J Neurol. 2021 doi: 10.1007/s00415-021-10771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikaeloff Y, Caridade G, Husson B, et al. Acute disseminated encephalomyelitis cohort study: prognostic factors for relapse. Eur J Paediatr Neurol. 2007;11(2):90–95. doi: 10.1016/j.ejpn.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Koelman DLH, Benkeser DC, Klein JP, Mateen FJ. Acute disseminated encephalomyelitis: prognostic value of early follow-up brain MRI. J Neurol. 2017;264(8):1754–1762. doi: 10.1007/s00415-017-8563-3. [DOI] [PubMed] [Google Scholar]
- 45.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166–177. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grzonka P, Scholz MC, De Marchis GM, et al. Acute hemorrhagic leukoencephalitis: a case and systematic review of the literature. Front Neurol. 2020;11:899. doi: 10.3389/fneur.2020.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Seze J, Debouverie M, Zephir H, et al. Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol. 2007;64(10):1426–1432. doi: 10.1001/archneur.64.10.1426. [DOI] [PubMed] [Google Scholar]
- 48.Sonneville R, Demeret S, Klein I, et al. Acute disseminated encephalomyelitisin the intensive care unit:clinical features and outcome of 20 adults. Intensive Care Med. 2008;34(3):528–532. doi: 10.1007/s00134-007-0926-2. [DOI] [PubMed] [Google Scholar]



