Abstract
Cancer tissues are not just simple masses of malignant cells, but rather complex and heterogeneous collections of cellular and even non-cellular components, such as endothelial cells, stromal cells, immune cells, and collagens, referred to as tumor microenvironment (TME). These multiple players in the TME develop dynamic interactions with each other, which determines the characteristics of the tumor. Platelets are the smallest cells in the bloodstream and primarily regulate blood coagulation and hemostasis. Notably, cancer patients often show thrombocytosis, a status of an increased platelet number in the bloodstream, as well as the platelet infiltration into the tumor stroma, which contributes to cancer promotion and progression. Thus, platelets function as one of the important stromal components in the TME, emerging as a promising chemotherapeutic target. However, the use of traditional antiplatelet agents, such as aspirin, has limitations mainly due to increased bleeding complications. This requires to implement new strategies to target platelets for anti-cancer effects. In oral squamous cell carcinoma (OSCC) patients, both high platelet counts and low tumor-stromal ratio (high stroma) are strongly correlated with increased metastasis and poor prognosis. OSCC tends to invade adjacent tissues and bones and spread to the lymph nodes for distant metastasis, which is a huge hurdle for OSCC treatment in spite of relatively easy access for visual examination of precancerous lesions in the oral cavity. Therefore, locoregional control of the primary tumor is crucial for OSCC treatment. Similar to thrombocytosis, higher expression of podoplanin (PDPN) has been suggested as a predictive marker for higher frequency of lymph node metastasis of OSCC. Cumulative evidence supports that platelets can directly interact with PDPN-expressing cancer cells via C-type lectin-like receptor 2 (CLEC2), contributing to cancer cell invasion and metastasis. Thus, the platelet CLEC2-PDPN axis could be a pinpoint target to inhibit interaction between platelets and OSCC, avoiding undesirable side effects. Here, we will review the role of platelets in cancer, particularly focusing on CLEC2-PDPN interaction, and will assess their potentials as therapeutic targets for OSCC treatment.
Keywords: platelets, tumor cell-induced platelet aggregation (TCIPA), CLEC2, PDPN, ezrin/radixin/moesin (ERM), oral cancer
Introduction
Oral squamous cell carcinoma (OSCC) is the most prevalent type of head and neck malignancies that occur in oral cavity, salivary gland, pharynx, larynx, nasal cavity, thyroid, and bone (1). Unlike the other types of cancers, OSCC usually arises from the body part that is easily accessible for visual examinations. Despite this advantage in detection of precancerous lesions, most of the OSCC patients are not diagnosed until the advanced stages with metastasis, which is attributed to low overall survival rates (2). Oral mucosa contains a connective tissue enriched with type I collagen that is synthesized by stromal cells (3). Desmoplasia, a status of the excessive growth of the stromal tissue, is closely associated with OSCC (4, 5). In OSCC patients, stroma-rich tumors are more aggressive and metastatic than stroma-poor tumors, finally contributing to the poor survival rates (6, 7). The activated tumor stroma can supply a variety of growth factors and cytokines that induces cancer cell proliferation as well as extracellular matrix (ECM) remodeling (5, 8). In support of the tumor stroma, OSCC cells tend to invade adjacent tissues, such as bones, and spread to the lymph nodes (9). This locoregional characteristic of OSCC is the primary cause of treatment failure (10). Thus, how to control the local and distal metastasis is crucial for successful treatment and better prognosis in OSCC patients.
Platelets, the smallest cells in blood circulation, play a major role in blood coagulation and hemostasis (11–13). In addition to their primary physiological functions, platelets are profoundly involved in cancer promotion and progression (14, 15). Recently, it has been reported that platelets can infiltrate into the tumor stroma in colorectal and pancreatic cancer patients (16–18). As a part of the tumor stromal components, platelets crosstalk with cancer cells either directly or indirectly, promoting invasion and metastasis (19–21). For the physical interaction, C-type lectin-like receptor 2 (CLEC2) and podoplanin (PDPN) are suggested as the key molecular links expressed in platelets and tumors, respectively (22). Moreover, cancer cells activate and educate platelets, thus the bilateral interaction between platelets and cancer can further promote tumorigenesis, creating a positive feedback loop (23). Notably, OSCC patients often show increased platelet counts, which is strongly associated with poor prognosis (24, 25). Thus, platelets are emerging as an important target for chemotherapy in OSCC patients.
Aspirin, a representative antiplatelet agent, is well known to protect against carcinogenesis (26–28). Aspirin irreversibly inhibits both cyclooxygenase-1 (COX-1) and COX-2, reducing synthesis of prostaglandins and thromboxanes responsible for inflammation and platelet aggregation (27). Despite its chemopreventive effect, a daily use of low-dose aspirin frequently causes adverse complications, primarily increased bleeding risk (29, 30). Thus, instead of using traditional antiplatelet agents, the pinpoint targeting of the platelet-tumor cell interaction would be a more precise and effective strategy for OSCC treatment, avoiding undesirable harmful effects. In this regards, we will highlight the role of platelets in carcinogenesis and OSCC, particularly focusing on the physical interaction between platelets and tumors via the CLEC2-PDPN axis.
Roles of Platelets in Cancer
Thrombocytosis in Cancer Patients
Platelets are anucleated cells originated from megakaryocytes in the bone marrow and abundant in healthy individual 150,000~400,000 per microliter of blood (11–13). In spite of lack of genomic DNA, platelets release plenty of granular ingredients, such as platelet-derived growth factor (PDGF), transforming growth factor β (TGFβ), stromal cell-derived factor-1 (SDF-1), and serotonin, which contributes to signal transduction in nearby cells (31). Cancer is often associated with thrombocytosis, a status of an abnormal elevation of platelet counts, which shows a positive correlation with worse outcomes in many types of cancers (24, 32–34). High platelet counts are involved with development of venous thromboembolism (VTE) in cancer patients, the second leading cause of cancer death (35–38). Besides an increased risk of VTE, thrombocytosis is associated with cancer mortality by accelerating tumor promotion and progression as well (39–41). In mice bearing tumors, platelet transfusion induced the blood platelet counts as well as tumor growth, while reducing the survival rates (37, 42). Thus, the platelet counts have long been considered as a valuable prognostic marker in cancer patients.
It has been reported that inflammatory cytokines, such as interleukin-6 (IL-6), are highly associated with thrombocytosis in cancer patients (33, 43). IL-6 can stimulate platelet production through inducing thrombopoietin (33, 44). In murine colitis model, colitis-induced wild type (WT) mice showed thrombocytosis and platelet aggregation, which were absent in IL-6-deficient mice (45). Moreover, neutralization of IL-6 led to reduction of platelet counts and tumor growth in the mouse ovarian cancer model (33). Thus, IL-6 inhibitors might be utilized to mitigate cancer-associated thrombocytosis (46). However, anti-IL-6 treatments need meticulous assessment, regarding that IL-6 pleiotropically functions in immune system (47, 48).
Platelets as a Part of Stromal Components in Tumor Microenvironment
Tumor tissues are not just simple masses of malignant cells, but rather complex and heterogeneous collections of cellular and even non-cellular components, referred to as tumor microenvironment (TME) (49). The multiple players in the TME develop dynamic interactions with each other, which determines the characteristics of the tumor (50). The non-cellular parts of the TME comprise primarily the ECM, a three-dimensional scaffold that contains collagens, proteoglycans, and fibronectins (51). The acellular ECM is crucial for providing mechanical (structural) and biochemical (nutritional) supports to cellular components in the TME (52). The cellular players in the TME can be largely divided into stromal cells and tumor-infiltrating immune cells. The tumor stroma is a heterogeneous population of distinct types of cells, including fibroblasts and endothelial cells (53). Among them, cancer-associated fibroblasts (CAFs) are the most abundant type of the stromal cells in TME that display enhanced expression of the signature proteins, including α-smooth muscle actin and PDGF receptors (54). Moreover, the TME contains a broad spectrum of immune cells, such as tumor-associated macrophages, tumor-associated neutrophils, and regulatory T cells. Notably, the infiltration of platelets into the tumor stroma has been observed in cancer patients (18, 55, 56), along with increased blood platelet counts (24, 32–34). Tumor-infiltrating platelets can interact with other stromal players of TME, contributing to tumor promotion and progression (57). Miyashita et al. have found that CAFs were surrounded by platelets in almost half of the pancreatic cancer patients (58). Platelet-derived factors, like TGFβ, PDGF, and SDF-1, can stimulate recruitment and activation of CAFs in the TME (59–62). Platelets also accommodate various angiogenesis regulators, which can turn on local angiogenesis in the TME (63). Depletion of tumor-infiltrating platelets showed impaired tumor blood vessel structures in mice (64). Moreover, it has been reported that fusion between platelets and endothelial cells promotes cancer metastasis by facilitating adhesion of tumor and endothelial cells (65). In consistent, the intratumoral accumulation of platelets are related to tumor progression (18, 55, 56). These investigations support that platelets function as a crucial stromal component in the TME through vigorous interplay with other members.
Platelets in Cancer Invasion and Metastasis
Metastasis is a multi-step process, including local invasion, intravasation, and colonization at the distal sites (66). Invading cancer cells undergo dramatic alterations in their morphology and phenotypes, such as epithelial-to-mesenchymal transition (EMT), which is accompanied by remodeling of the ECM (66). As a poor prognostic indicator, thrombocytosis is associated with lymph node metastasis and invasion in cancer patients (24, 67). In consistent, platelet transfusion significantly enhanced metastasis of cancer cells in the murine experimental models (15, 68). However, platelet decoys bound to tumor cells as effectively as normal intact platelets and inhibited thrombosis and metastatic tumor formation, further supporting the role of platelets in metastasis (69). Of note, platelets are frequently detected at the invasive front where both EMT and ECM remodeling occur actively (70). Platelets contain about 40% of TGFβ found in the peripheral blood plasma, which plays a crucial role in cancer cell invasion (71). Co-culture with platelets remarkably enhanced invasiveness and EMT process of cancer cells in a TGFβ-dependent manner (72, 73). Platelet-specific Tgfb1-deficient mice showed reduction in tumor growth and platelet extravasation, compared to WT mice (74). Moreover, various types of matrix metalloproteinases (MMPs) responsible for ECM degradation are stored in the resting platelets and released upon stimulation, such as cancer cell-induced aggregation (35, 75, 76). Platelets upregulate production of MMPs in cancer cells as well as fibroblasts, accelerating invasion of cancer cells (77–79). These data suggest that platelets can change TME through their releasates, such as TGFβ and MMPs, conferring cancer cells invasive capability and metastatic potential. In addition, direct contact with platelets can promote invasion and metastasis of cancer cells in vitro and in vivo (19, 80).
Platelets can promote metastasis through interaction with other cells in the bloodstream as well, like in the TME. Platelets rapidly adhere to circulating cancer cells in the blood, protecting tumors from immune surveillance (41, 81). Natural killer (NK) and CD8 T cells are cytotoxic lymphocytes that play a central role in cancer immunosurveillance (82). Once tumors are coated by platelets, platelets inhibit NK cell-mediated antitumor activity through downregulating tumor cell NK2D expression by TGFβ and inducing pseudoexpression of immunomodulating molecules, such as MHC I and GITR (83–85). Moreover, platelet-derived factors, such as TGFβ and programmed death-ligand 1 (PD-L1), suppressed the cytotoxic antitumor T cell immunity in the mouse cancer models (86–88). Taken together, these data suggest that platelets facilitate tumor immune escape by surrounding cancer cells in the bloodstream, thus, the platelet-camouflaged cancer cells safely migrate to the metastatic sites. Of note, co-incubation with platelets protected cancer cells against anoikis, implying that platelets enhance anchorage-independent survival of circulating tumor cells in the bloodstream (15).
Platelets as a Potential Target for OSCC Treatment
Similar to other types of cancer patients, increased platelet counts are significantly correlated with poor prognosis in OSCC patients (25, 89). Based on the analysis of relationship between platelet counts and disease progression in a total of 253 OSCC patients, thrombocytosis was associated with lymph node metastasis as well as distant metastasis (90). Along with metastasis, advanced OSCC often shows invasion into the facial bones, due to close anatomical relationship (91, 92). The bone invasion causes severe pains, greatly lowering the quality of life and the survival rates in OSCC patients (91, 93). Notably, platelet aggregation plays a critical role in tumor-associated bone destruction (94). In line with that, the pharmacological inhibition of platelet aggregation reduced bone metastasis in the murine cancer model (95). Platelet-secreted lysophosphatidic acid is thought to be one of the primary mediators in platelet-promoted bone invasion and metastasis (96, 97). Taken together, platelets can facilitate bone invasion through direct contact with tumor as well as their releasates. In OSCC, bone destruction and invasion are closely related to TGFβ signaling pathway (98, 99). Considering that platelets store most of the plasma TGFβ, it is plausible that platelets aggravate invasion of OSCC, and thus further pre-clinical and clinical investigations will shed light on a noble would be novel strategies for OSCC treatment.
Interaction Between Platelets and Cancer: CLEC2-PDPN-ERM Axis
PDPN in Cancer and Platelet Aggregation
PDPN is a type I transmembrane glycoprotein expressed in kidney podocytes, skeletal muscles, lungs, hearts, myofibroblasts, osteoblasts, mesothelial cells, and lymphatic endothelial cells (100). PDPN knockout mice die shortly after birth due to an impaired respiratory system (101). These mice also show defects in the lymphatic vasculature, disorganization of spleen, and lack of lymph nodes (102, 103). PDPN is thus an important regulator in the normal organogenesis and development processes.
Upregulation of PDPN has been observed in a variety of human cancers, including brain cancer, breast cancer, lung cancer, and mesothelioma, which is associated with poor prognosis (104–107). In athymic nude mice, injection of PDPN-overexpressing cancer cells generated bigger tumors, while silencing of PDPN suppressed tumor growth (108). Moreover, PDPN-high tumors exhibited increased peritumoral lymphangiogenesis, invasiveness, migratory ability, and metastasis, implying a pro-tumorigenic role of PDPN (109–112). Notably, it has been reported that PDPN expression is elevated at the leading edge of tumor tissues, which promotes cell surface extension and cell motility in keratinocytes (111, 113). In the two-stage skin carcinogenesis model, epidermal ablation of PDPN reduced tumor growth and invasion (109). Overall, these data suggest that PDPN confers cancer cells survival benefits, promoting tumor growth, invasion, and metastasis.
Interestingly, PDPN-overexpressing cancer cells evoke platelet aggregation, also known as tumor cell-induced platelet aggregation (TCIPA) (108, 114). PDPN-positive human glioblastoma Gli16 cells were able to markedly induce platelet aggregation, whereas not detected by PDPN-negative cells (115). In tumor-bearing mouse models, either ablating PDPN gene or blocking PDPN by monoclonal antibody (mAb) injection effectively suppressed platelet aggregation, supporting that PDPN is crucial for TCIPA formation (116, 117). The PDPN-mediated TCIPA was strongly associated with an increased incidence of VTE in cancer patients (115, 118). Moreover, PDPN overexpression is also involved in TCIPA-induced tumor promotion and progression. The platelet-tumor aggregates are readily arrested in the microvasculature, facilitating tumor metastasis (20). PDPN neutralization significantly inhibited TCIPA occurrence, tumor growth, and metastasis in nude mice injected with human melanoma or lung cancer cell lines (108, 116, 119). Moreover, platelet-derived TGFβ upregulated PDPN expression in human bladder cancer cells, which induced EMT process and cancer cell invasion (120). Taken together, PDPN is considered as a ‘pinpoint’ that interconnects between tumor and platelets, regulating VTE as well as tumor progression.
Platelet CLEC2-PDPN Axis: A Pinpoint of Platelet-Tumor Cell Interaction
PDPN consists of an extracellular domain, a transmembrane domain, and a cytoplasmic domain (121). The extracellular domain of PDPN carries four platelet aggregation-stimulating (PLAG) domains with a plenty of potential O-glycosylation sites, crucial for interaction with platelets (121). The PLAG domain of PDPN has been reported to bind to CLEC2 that is abundantly expressed on the surface of platelets (122). Interestingly, CLEC2-deficient mice phenocopy PDPN-knockout mice, like prenatal lethality and impaired lymphatic vasculature (123). Either platelet-specific deletion of CLEC2 or inhibition of PDPN was associated with reduced thrombosis in a murine deep vein thrombosis model of inferior vena cava stenosis (124). Similarly, cancer cell lines with high endogenous PDPN expression levels, such as LN319 and Colon-26, showed induced platelet aggregation, which was attenuated by pre-incubation with an anti-CLEC2 antibody (125). Tsukiji et al. have found that cobalt hematoporphyrin (Co-HP) directly binds to PDPN-binding sites of CLEC2, functioning as an inhibitor of the CLEC2-PDPN axis (126). Both Co-HP administration and CLEC2 neutralization significantly inhibited CLEC2-dependent platelet aggregation in tumor-bearing mice (126, 127). Taken together, these data support that PDPN is interdependent with CLEC2, thus, the platelet CLEC2-PDPN axis is crucial for platelet-tumor cell interaction ( Figure 1 ).
Figure 1.
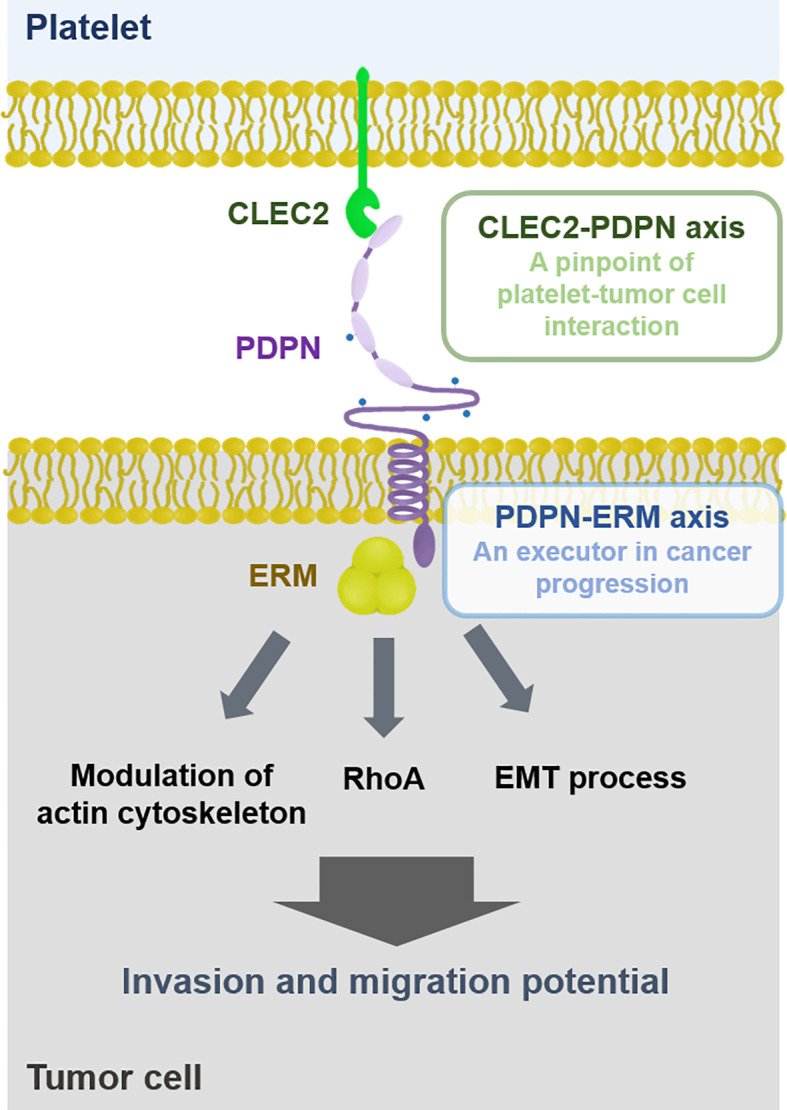
Interaction between platelet and tumor cell. Platelets can physically interact with tumor cells via the CLEC2-PDPN axis. PDPN is associated with ERM proteins that promote cancer cell migration and invasion through modulating actin cytoskeleton, RhoA, and EMT process. Thus, the CLEC2-PDPN-ERM axis is a crucial target for chemotherapy.
In conjunction with TCIPA formation, the platelet CLEC2-PDPN axis mediates cancer promotion and progression. In mice inoculated with PDPN-expressing B16F10 melanoma cells, CLEC2 depletion by anti-CLEC2 mAb 2A2B10 injection reduced plasma levels of inflammatory cytokines and lung metastasis, resulting in prolonged survival compared to control mice (127). Treatment with a CLEC2 inhibitor Co-HP suppressed lung metastasis of PDPN-expressing melanoma cells, but not that of PDPN-negative lung cancer cells (126). In platelet-depleted mice, platelet transfusion induced much more lung colonization as well as bone metastasis of PDPN-expressing osteosarcoma cells, while CLEC2 mAb injection reduced lung colonization (68). Likewise, injection of PDPN mAb (MS-1) remarkably suppressed platelet aggregation as well as lung metastasis in the murine cancer metastasis model (128). Therefore, the platelet CLEC2-PDPN axis is considered as a pinpoint for platelet-tumor interaction that promotes tumor progression ( Figure 1 ). It has been demonstrated that CLEC2 deficiency is not significantly related to bleeding tendency (123, 129). In this regard, the platelet CLEC2-PDPN axis could be a promising target to inhibit TCIPA-induced tumor progression without bleeding risk, a major complication of the traditional antiplatelet agents.
PDPN-ERM Axis: An Executor in Cancer Progression
PDPN has a short cytoplasmic tail associated with ezrin/radixin/moesin (ERM) proteins that primarily bridge between plasma membrane proteins and F-actin filaments of the cytoskeleton (100, 130). It is well documented that cells and tissues utilize this ERM crosslink system to maintain the architectures necessary for their own biological functions (131). In particular, ERM proteins are crucial regulators for epithelial morphogenesis and integrity, mitosis, cell polarity, and cell adhesion (132, 133). Among the ERM protein members, ezrin-null mice displayed much more severe phenotypes compared to moesin- or radixin-deficient mice (134). Ezrin-deficient mice showed defects in intestinal villus morphogenesis and epithelial cell organization (135). In addition, ERM proteins regulate the cell-cell and cell-matrix interactions, particularly in cancer cells (136). Thus, PDPN is engaged in cell adhesion, migration, and invasion through association with the ERM proteins, as illustrated in Figure 1 (113, 133).
PDPN expression is upregulated peculiarly in the growing edge of tumors and commonly co-localized with ERM proteins (100, 106). Similar to PDPN, overexpression of ERM proteins has been detected in various types of cancers: ezrin overexpression in breast, hepatocellular, colon, ovarian, and pancreatic cancers (137–141); radixin overexpression in pancreatic cancer with lymph node metastasis (142); moesin overexpression in skin cancer, colorectal carcinoma, endometrial adenocarcinoma, and glioma (143–146). Moreover, upregulation of ERM proteins is associated with poor prognosis in cancer patients (140, 147–150). In athymic nude mice, intracranial injection of moesin-overexpressing glioblastoma cells significantly reduced the survival rates compared to the control group (146). Moreover, ERM proteins were frequently mislocalized during tumor progression, from plasma membrane to cytoplasm (136). Thus, dysregulation of ERM proteins takes part in cancer promotion and progression, possibly in an interdependent manner with PDPN.
It has been reported that PDPN mediated TCIPA-induced EMT process in human cancer cell lines (120). In non-cancerous experimental settings, PDPN can bind to ERM proteins through its cytoplasmic domain, promoting the EMT process as well as cell migration (130, 151). Silencing of radixin, one of the ERM protein members, suppressed the EMT process as well as migration and invasion in human gastric carcinoma SGC-7901 cells (152). Moreover, PDPN can induce migration ability in cancer cells that bypass the EMT process via filopodia formation (106). Instead of the EMT process, PDPN recruits ERM proteins to modulate the actin cytoskeleton in a RhoA-dependent manner, consequently promoting cancer cell migration and invasion. Taken together, the PDPN-ERM axis can promote migratory capability and invasiveness of tumor cells, through either EMT process or cytoskeletal rearrangement.
It has been reported that the CLEC2-PDPN axis can regulate cell contractility and migration through activation of ERM proteins in non-cancerous settings (153–155). In this regard, it is plausible that the PDPN-ERM axis could be recruited by tumors bound to platelets via CLEC2-PDPN interaction, conferring cancer cells metastatic potentials ( Figure 1 ). Further investigation is necessary to clarify the role of the platelet CLEC2-PDPN-ERM axis in cancer progression.
Platelet CLEC2-PDPN and PDPN-ERM Axes in OSCC
According to the Cancer Genome Atlas analysis, head and neck cancer patients present much higher PDPN expression levels compared to other types of cancer patients. While PDPN expression is rarely detected in normal oral epithelial cells, OSCC patients show upregulation of PDPN in tumors, which contributed to poor prognosis (156–159). In the xenograft mouse model, PDPN-overexpressing OSCC cells promoted tumor growth and intratumoral platelet accumulation, implying that PDPN mediates TCIPA formation in OSCC (160). Similar to high platelet counts (90), elevated PDPN expression was often found at the invasive front and correlated with lymph node metastasis in OSCC patients (156, 161). In line with that, silencing of PDPN gene expression attenuated migration and invasion in human OSCC cell lines (160, 162–164). Considering that platelet CLEC2 is crucial for PDPN-dependent TCIPA formation, the platelet CLEC2-PDPN axis would be a feasible target for successful local control in OSCC patients.
In OSCC patients, overexpression of ezrin and moesin has been detected in advanced staged tumors and significantly associated with worse overall survival rates (149, 164, 165). Kobayashi et al. have reported that cytoplasmic expression of moesin shows a strong correlation with lymph node metastasis in OSCC patients (166). Of note, PDPN expression was positively related to ezrin expression, particularly in the cytoplasm of the odontogenic tumors (167). Moreover, this co-expression between PDPN and ezrin was frequently detected in the invasive front and possibly involved with lymph node metastasis in the lip cancer (168). These data suggest that the PDPN-ERM axis may contribute to increased metastatic potential in OSCC. In consistent, PDPN has been reported to enhance cell motility and invasiveness through interaction with ERM binding partners, such as membrane type 1 MMP, Cdc42, and CD44, in humans OSCC cell lines (162, 164). These data suggest that ERM proteins function as an intracellular executor of the CLEC2-PDPN axis in invasion and metastasis of OSCC.
Targeting Platelet-Tumor Interaction for Chemotherapy
Aspirin
Considering pro-tumorigenic activities of platelets, antiplatelet agents could be promising chemotherapeutics, as shown in Table 1 . A classical antithrombotic drug aspirin has been used for chemoprevention. The meta-analysis and retrospective cohort study showed that a regular use of aspirin is associated with reduced risk of cancers in liver, stomach, colorectum, lung, pancreas, and oesophagus (26, 169). In head and neck cancer patients, evaluation of aspirin as a chemopreventive agent is still controversial. A hospital-based case control study revealed that aspirin use can reduce head and neck cancer risk (170), whereas the other investigations demonstrated that there was no significant correlation between aspirin intake and head and neck cancer (171, 172). Moreover, the risk of gastrointestinal bleeding could limit the use of aspirin for cancer prevention and/or treatment (29, 30).
Table 1.
Strategies to target platelet-tumor interaction for chemotherapy.
| Agent | TCIPA | Cancer risk/metastasis | Bleeding | References |
|---|---|---|---|---|
| Classical antiplatelet drug | ||||
| Aspirin | Inhibit TCIPA in vitro and in vivo | Inhibit metastasis in vivo | Increased gastrointestinal bleeding | (26, 29, 30, 169–175) |
| Cancer preventive effect in human subjects (controversial in head and neck cancer) | ||||
| Reduce metastasis in cancer patients | ||||
| P2Y12 receptor antagonism | ||||
| Clopidogrel | Inhibit TCIPA in mice | Inhibit tumor metastasis in mice | A long-term use can increase bleeding risk | (176–182) |
| No impact on cancer motility in human colorectal, breast, and prostate cancer patients | ||||
| Ticagrelor | Inhibit TCIPA | Increase cancer risks in human | More major bleeding compared to clopidogrel in patients with acute coronary syndrome | (180, 183–185) |
| GPVI antagonism | ||||
| Anti-GPVI mAb (JAQ1) | Inhibit TCIPA | Inhibit cancer cell extravasation in vitro | No impact on bleeding time | (129, 186–188) |
| Inhibit metastasis in mice | ||||
| Induce intratumoral hemorrhage and accumulation of co-administrated anticancer drugs in mice | ||||
| Revacept | Inhibit TCIPA in mice and human | Inhibit EMT marker expression in vitro | No impact on bleeding time in mice and human | (189–191) |
| Targeting CLEC2-PDPN axis | ||||
| Anti-CLEC2 mAb (2A2B10 and INU1) | Inhibit intratumoral thrombus formation in mice | Inhibit metastasis in mice | No impact on bleeding time | (68, 127, 129) |
| Anti-PDPN mAb (NZ-1, MS-1, and SZ-168) | Inhibit platelet aggregation in mice | Inhibit metastasis in mice | (119, 128, 192–194) | |
| Inhibit VET in mice | ||||
| 2CP | Inhibit TCIPA in mice | Inhibit metastasis in mice | No impact on bleeding time | (195) |
| Co-HP | Inhibit platelet aggregation | Inhibit metastasis in mice | No impact on bleeding time | (126) |
| Inhibit VET in mice | ||||
| Polysaccharide extracted from Artemisia argyi leaves | Inhibit TCIPA | (196) | ||
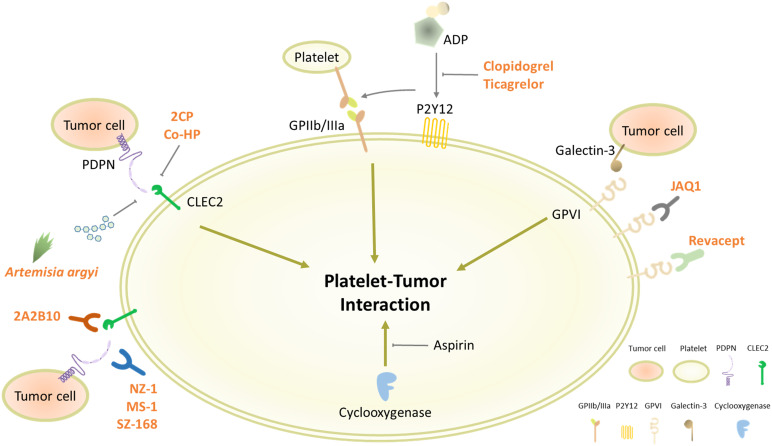
Platelet P2Y12 Receptor Antagonists
Platelet P2Y12 receptor is involved in ADP-stimulated activation of glycoprotein IIb/IIIa (GPIIb/IIIa) responsible for platelet aggregation (197). It has been reported that GPIIb/IIIa mediates platelet-tumor interaction and cancer metastasis (198–200). In conjunction with GPIIb/IIIa, stimulation of P2Y12 receptor can promote platelet-tumor crosstalk and cancer metastasis ( Figure 2 ), suggesting P2Y12 receptor antagonists as anticancer drugs (73, 201). Clopidogrel, the most widely used P2Y12 receptor antagonist, markedly inhibited tumor growth in mouse ovarian and liver cancer models (176, 177). Another P2Y12 inhibitor ticagrelor suppressed proliferation of ovarian cancer cells in vivo and in vitro, which was not detected in absence of platelets (176). Moreover, treatment with ticagrelor attenuated TCIPA formation and cancer metastasis in the murine experimental models (178–180). These pre-clinical data suggest platelet P2Y12 receptor as a target for cancer treatment by controlling platelet-tumor aggregation. However, a population-based cohort study showed that the use of clopidogrel has no huge impact on cancer mortality in colorectal, breast, and prostate cancer patients (181). Even worse, the clinical trial-based analyses revealed that ticagrelor increased cancer risks (183, 184). In another patient-level meta-analysis of randomized clinical trials, a long-term intake of clopidogrel was associated with bleeding risk and hemorrhage (182). Overall, the use of P2Y12 receptor antagonists for chemotherapy is controversial, in spite of the compelling pre-clinical evidence.
Figure 2.
Platelet receptors involved in platelet-tumor interaction. Platelets contain various types of receptors on the cell surface for diverse physiological functions, including cell adhesion and aggregation. Some of the surface molecules, such as CLEC2, P2Y12, and GPVI, can promote the interaction between platelets and cancer cells, which could be plausible targets for blocking TCIPA formation.
Platelet GPVI Antagonism
GPVI is the major platelet-activating receptor exclusively expressed on platelets and megakaryocytes (202). GPVI-null mice showed lack of thrombus formation and defective platelet activation without severe bleeding tendency (203, 204). Moreover, these GPVI-deficient mice developed less metastatic tumors by injection of lung cancer or melanoma cells than WT mice (205). Notably, platelet GPVI can bind to galectin-3 on tumor cells, provoking platelet-tumor cell interaction and metastasis ( Figure 2 ) (186, 189). These pre-clinical data suggest that GPVI antagonism is a conceivable strategy to block TCIPA-mediated tumor progression without adverse effects. In line with this notion, platelets preincubated with an anti-GPVI antibody (JAQ1) were less able to form aggregates with human breast cancer cells and eventually reduced cancer cell extravasation in the transendothelial migration assay (187). Moreover, treatment with JAQ1 reduced tumor metastasis in the murine lung metastasis models, further supporting antitumor effects of GPVI antagonism via blocking TCIPA formation (186). Interestingly, JAQ1 Fab2 fragment induced intratumoral hemorrhage that led to accumulation of co-administrated chemotherapeutics without systemic bleeding complications, thus allowing to maximize anticancer effects (188). Revacept, a competitive GPVI inhibitor comprising a soluble Fc fusion protein, decreased platelet-tumor interaction and metastatic potential in vitro (189). In atherosclerotic mice and healthy human subjects, Revacept reduced platelet aggregation with no impact on bleeding times (190, 191). Based on this drug safety assurance, the antitumor efficacy of GPVI antagonists must be further evaluated in human cancer patients.
Targeting Platelet CLEC2-PDPN Axis
As described in Figure 1 , the platelet CLEC2-PDPN axis is emerging as a pinpoint to control the platelet-tumor interaction and subsequent tumor progression. In order to disconnect the platelet CLEC2-PDPN axis, diverse approaches have been made, including mAbs against CLEC2 or PDPN and pharmacological inhibitors. PDPN mAbs, such as NZ-1 and MS-1, can bind to the PLAG domain of PDPN and neutralize interaction with platelet CLEC2 ( Figure 2 ) (192, 193). These PDPN mAbs specifically inhibited PDPN-mediated platelet aggregation and cancer metastasis in the murine experimental models (128, 192, 193). Moreover, anti-PDPN antibody SZ-168 reduced the incidence of VTE in mice (194). Similar to PDPN mAbs, anti-CLEC2 antibody 2A2B10 suppressed intratumoral thrombus formation as well as metastasis in mice (68, 127). These investigations suggest that mAbs neutralizing either CLEC2 or PDPN specifically inhibit platelet-tumor interaction and tumor metastasis. Although the influence of CLEC2 deficiency on bleeding is conflicting in CLEC2-null mice, CLEC2 mAb-treated mice had no sign of prolonged bleeding compared to control mice (123, 129, 206, 207). Overall, CLEC2 neutralization seems not to affect bleeding time profoundly.
In addition to neutralizing antibodies, pharmacological inhibitors display potent inhibitory effects on the CLEC2-PDPN axis. Chang et al. have newly synthesized a non-cytotoxic 5-nitrobenzoate compound 2CP that specifically inhibits the CLEC2-PDPN interaction (195). 2CP selectively blocked PDPN-induced TCIPA formation and lung metastasis in the xenograft model, whereas bleeding time was not affected by 2CP (195). Co-HP can directly bind to CLEC2 at PDPN-binding sites and potently block CLEC2-PDPN interaction (126). Co-HP injection significantly reduced tumor metastasis and the incidence of VTE in mice, but not affecting the bleeding time (126). Moreover, a bioactive polysaccharide extracted from Artemisia argyi leaves inhibited CLEC2-PDPN interaction and PDPN-dependent TCIPA formation (196).
Taken together, inhibition of the platelet CLEC2-PDPN axis is a promising chemotherapeutic strategy by suppressing TCIPA formation and metastasis ( Table 1 ). In particular, targeting the CLEC2-PDPN axis seems to be a relatively safer approach to block platelet-tumor interaction without severe adverse effects, such as increased bleeding risk. Further clinical studies are needed to validate their anti-thrombotic and anti-metastatic effects in human subjects. Although targeting the CLEC2-PDPN axis is relatively harmless, it still requires caution to be clinically applied, since CLEC2- or PDPN-deficient mice showed abnormal lymphatic vessel formation (123).
Conclusion
Despite advances in surgical techniques and therapeutic strategies including radiotherapy and immunotherapy, the survival rate of OSCC has not been improved for the past decade due to failure of local control of primary tumor (2, 208). Currently, platelets are well recognized as a stromal member of the TME and an important prognostic index in OSCC patients (25, 57, 89). In particular, platelets directly interact with cancer cells via CLEC2-PDPN binding, fortifying metastatic potentials of cancer cells. Regarding that PDPN is the only known endogenous ligand for CLEC2, the platelet CLEC2-PDPN axis is a pinpoint target to control TCIPA formation-mediated metastasis without undesirable complications. Thus, blockade of the CLEC2-PDPN axis could be a prospective strategy for successful local control and improvement of survival in OSCC patients, which merits further pre-clinical and clinical investigations.
Author Contributions
N-YS contributed to study conception. S-YP, B-OH, ESC, XZ, SKL, H-JA, K-SC, W-YC, and N-YS performed literature review and analysis and revised the manuscript. N-YS, S-YP, and B-OH drafted the manuscript, figures, and tables. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Research Foundation of Korea (NRF) Grants funded by the Korean Government (grant numbers NRF-2020R1C1C1003338 and NRF-2016R1A5A2008630 to N-YS) and by the Yonsei University Research Fund of 2021 (Yonsei Signature Research Cluster Program 2021-22-0017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Chow LQM. Head and Neck Cancer. N Engl J Med (2020) 382(1):60–72. doi: 10.1056/NEJMra1715715 [DOI] [PubMed] [Google Scholar]
- 2. Bugshan A, Farooq I. Oral Squamous Cell Carcinoma: Metastasis, Potentially Associated Malignant Disorders, Etiology and Recent Advancements in Diagnosis. F1000Res (2020) 9:229. doi: 10.12688/f1000research.22941.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nikoloudaki G, Creber K, Hamilton DW. Wound Healing and Fibrosis: A Contrasting Role for Periostin in Skin and the Oral Mucosa. Am J Physiol Cell Physiol (2020) 318(6):C1065–77. doi: 10.1152/ajpcell.00035.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. An YZ, Cho E, Ling J, Zhang X. The Axin2-Snail Axis Promotes Bone Invasion by Activating Cancer-Associated Fibroblasts in Oral Squamous Cell Carcinoma. BMC Cancer (2020) 20(1):987. doi: 10.1186/s12885-020-07495-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zainab H, Sultana A, Shaimaa . Stromal Desmoplasia as a Possible Prognostic Indicator in Different Grades of Oral Squamous Cell Carcinoma. J Oral Maxillofac Pathol (2019) 23(3):338–43. doi: 10.4103/jomfp.JOMFP_136_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dourado MR, Miwa KYM, Hamada GB, Paranaiba LMR, Sawazaki-Calone I, Domingueti CB, et al. Prognostication for Oral Squamous Cell Carcinoma Patients Based on the Tumour-Stroma Ratio and Tumour Budding. Histopathology (2020) 76(6):906–18. doi: 10.1111/his.14070 [DOI] [PubMed] [Google Scholar]
- 7. Huang S, Cai H, Song F, Zhu Y, Hou C, Hou J. Tumor-Stroma Ratio is a Crucial Histological Predictor of Occult Cervical Lymph Node Metastasis and Survival in Early-Stage (Ct1/2N0) Oral Squamous Cell Carcinoma. Int J Oral Maxillofac Surg (2021). doi: 10.1016/j.ijom.2021.06.011 [DOI] [PubMed] [Google Scholar]
- 8. Vucicevic Boras V, Fucic A, Virag M, Gabric D, Blivajs I, Tomasovic-Loncaric C, et al. Significance of Stroma in Biology of Oral Squamous Cell Carcinoma. Tumori (2018) 104(1):9–14. doi: 10.5301/tj.5000673 [DOI] [PubMed] [Google Scholar]
- 9. Siriwardena S, Tsunematsu T, Qi G, Ishimaru N, Kudo Y. Invasion-Related Factors as Potential Diagnostic and Therapeutic Targets in Oral Squamous Cell Carcinoma-A Review. Int J Mol Sci (2018) 19(5):1462. doi: 10.3390/ijms19051462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caldeira PC, Soto AML, de Aguiar MCF, Martins CC. Tumor Depth of Invasion and Prognosis of Early-Stage Oral Squamous Cell Carcinoma: A Meta-Analysis. Oral Dis (2020) 26(7):1357–65. doi: 10.1111/odi.13194 [DOI] [PubMed] [Google Scholar]
- 11. Gremmel T, Frelinger AL, 3rd, Michelson AD. Platelet Physiology. Semin Thromb Hemost (2016) 42(3):191–204. doi: 10.1055/s-0035-1564835 [DOI] [PubMed] [Google Scholar]
- 12. Holinstat M. Normal Platelet Function. Cancer Metastasis Rev (2017) 36(2):195–8. doi: 10.1007/s10555-017-9677-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Machlus KR, Italiano JE., Jr. The Incredible Journey: From Megakaryocyte Development to Platelet Formation. J Cell Biol (2013) 201(6):785–96. doi: 10.1083/jcb.201304054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gay LJ, Felding-Habermann B. Platelets Alter Tumor Cell Attributes to Propel Metastasis: Programming in Transit. Cancer Cell (2011) 20(5):553–4. doi: 10.1016/j.ccr.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 15. Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, et al. Platelets Reduce Anoikis and Promote Metastasis by Activating YAP1 Signaling. Nat Commun (2017) 8(1):310. doi: 10.1038/s41467-017-00411-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miao Y, Xu Z, Feng W, Zheng M, Xu Z, Gao H, et al. Platelet Infiltration Predicts Survival in Postsurgical Colorectal Cancer Patients. Int J Cancer (2021). doi: 10.1002/ijc.33816 [DOI] [PubMed] [Google Scholar]
- 17. Xu SS, Xu HX, Wang WQ, Li S, Li H, Li TJ, et al. Tumor-Infiltrating Platelets Predict Postoperative Recurrence and Survival in Resectable Pancreatic Neuroendocrine Tumor. World J Gastroenterol (2019) 25(41):6248–57. doi: 10.3748/wjg.v25.i41.6248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang SR, Yao L, Wang WQ, Xu JZ, Xu HX, Jin W, et al. Tumor-Infiltrating Platelets Predict Postsurgical Survival in Patients With Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol (2018) 25(13):3984–93. doi: 10.1245/s10434-018-6727-8 [DOI] [PubMed] [Google Scholar]
- 19. Labelle M, Begum S, Hynes RO. Direct Signaling Between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell (2011) 20(5):576–90. doi: 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Labelle M, Begum S, Hynes RO. Platelets Guide the Formation of Early Metastatic Niches. Proc Natl Acad Sci USA (2014) 111(30):E3053–61. doi: 10.1073/pnas.1411082111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang S, Li Z, Xu R. Human Cancer and Platelet Interaction, a Potential Therapeutic Target. Int J Mol Sci (2018) 19(4):1246. doi: 10.3390/ijms19041246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and Podoplanin in Cancer Metastasis. Thromb Res (2012) 129(Suppl 1):S30–7. doi: 10.1016/S0049-3848(12)70013-0 [DOI] [PubMed] [Google Scholar]
- 23. Asghar S, Parvaiz F, Manzoor S. Multifaceted Role of Cancer Educated Platelets in Survival of Cancer Cells. Thromb Res (2019) 177:42–50. doi: 10.1016/j.thromres.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 24. Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor Prognosis Associated With Thrombocytosis in Patients With Gastric Cancer. Ann Surg Oncol (2002) 9(3):287–91. doi: 10.1007/BF02573067 [DOI] [PubMed] [Google Scholar]
- 25. Rachidi S, Wallace K, Day TA, Alberg AJ, Li Z. Lower Circulating Platelet Counts and Antiplatelet Therapy Independently Predict Better Outcomes in Patients With Head and Neck Squamous Cell Carcinoma. J Hematol Oncol (2014) 7:65. doi: 10.1186/s13045-014-0065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsoi KKF, Ho JMW, Chan FCH, Sung JJY. Long-Term Use of Low-Dose Aspirin for Cancer Prevention: A 10-Year Population Cohort Study in Hong Kong. Int J Cancer (2019) 145(1):267–73. doi: 10.1002/ijc.32083 [DOI] [PubMed] [Google Scholar]
- 27. Thun MJ, Jacobs EJ, Patrono C. The Role of Aspirin in Cancer Prevention. Nat Rev Clin Oncol (2012) 9(5):259–67. doi: 10.1038/nrclinonc.2011.199 [DOI] [PubMed] [Google Scholar]
- 28. Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, et al. Associations Between Aspirin Use and the Risk of Cancers: A Meta-Analysis of Observational Studies. BMC Cancer (2018) 18(1):288. doi: 10.1186/s12885-018-4156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cea Soriano L, Vora P, Soriano-Gabarro M, Garcia Rodriguez LA. The Effect of Low-Dose Aspirin on Colorectal Cancer Prevention and Gastrointestinal Bleeding According to Bodyweight and Body Mass Index: Analysis of UK Primary Care Data. Int J Cardiol (2019) 297:135–9. doi: 10.1016/j.ijcard.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 30. Garcia Rodriguez LA, Martin-Perez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding Risk With Long-Term Low-Dose Aspirin: A Systematic Review of Observational Studies. PloS One (2016) 11(8):e0160046. doi: 10.1371/journal.pone.0160046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flaumenhaft R. Molecular Basis of Platelet Granule Secretion. Arterioscler Thromb Vasc Biol (2003) 23(7):1152–60. doi: 10.1161/01.ATV.0000075965.88456.48 [DOI] [PubMed] [Google Scholar]
- 32. Maraz A, Furak J, Varga Z, Kahan Z, Tiszlavicz L, Hideghety K. Thrombocytosis Has a Negative Prognostic Value in Lung Cancer. Anticancer Res (2013) 33(4):1725–9. [PubMed] [Google Scholar]
- 33. Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N Engl J Med (2012) 366(7):610–8. doi: 10.1056/NEJMoa1110352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki K, Aiura K, Kitagou M, Hoshimoto S, Takahashi S, Ueda M, et al. Platelets Counts Closely Correlate With the Disease-Free Survival Interval of Pancreatic Cancer Patients. Hepatogastroenterology (2004) 51(57):847–53. [PubMed] [Google Scholar]
- 35. Jurasz P, Sawicki G, Duszyk M, Sawicka J, Miranda C, Mayers I, et al. Matrix Metalloproteinase 2 in Tumor Cell-Induced Platelet Aggregation: Regulation by Nitric Oxide. Cancer Res (2001) 61(1):376–82. [PubMed] [Google Scholar]
- 36. Tsuruo T, Fujita N. Platelet Aggregation in the Formation of Tumor Metastasis. Proc Jpn Acad Ser B Phys Biol Sci (2008) 84(6):189–98. doi: 10.2183/pjab.84.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haemmerle M, Bottsford-Miller J, Pradeep S, Taylor ML, Choi HJ, Hansen JM, et al. FAK Regulates Platelet Extravasation and Tumor Growth After Antiangiogenic Therapy Withdrawal. J Clin Invest (2016) 126(5):1885–96. doi: 10.1172/JCI85086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khorana AA. Venous Thromboembolism and Prognosis in Cancer. Thromb Res (2010) 125(6):490–3. doi: 10.1016/j.thromres.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gu D, Szallasi A. Thrombocytosis Portends Adverse Prognosis in Colorectal Cancer: A Meta-Analysis of 5,619 Patients in 16 Individual Studies. Anticancer Res (2017) 37(9):4717–26. doi: 10.21873/anticanres.11878 [DOI] [PubMed] [Google Scholar]
- 40. Bensalah K, Leray E, Fergelot P, Rioux-Leclercq N, Tostain J, Guille F, et al. Prognostic Value of Thrombocytosis in Renal Cell Carcinoma. J Urol (2006) 175(3 Pt 1):859–63. doi: 10.1016/S0022-5347(05)00526-4 [DOI] [PubMed] [Google Scholar]
- 41. Gay LJ, Felding-Habermann B. Contribution of Platelets to Tumour Metastasis. Nat Rev Cancer (2011) 11(2):123–34. doi: 10.1038/nrc3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan L, Liu X. Platelets are Associated With Xenograft Tumor Growth and the Clinical Malignancy of Ovarian Cancer Through an Angiogenesis-Dependent Mechanism. Mol Med Rep (2015) 11(4):2449–58. doi: 10.3892/mmr.2014.3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alexandrakis MG, Passam FH, Moschandrea IA, Christophoridou AV, Pappa CA, Coulocheri SA, et al. Levels of Serum Cytokines and Acute Phase Proteins in Patients With Essential and Cancer-Related Thrombocytosis. Am J Clin Oncol (2003) 26(2):135–40. doi: 10.1097/00000421-200304000-00007 [DOI] [PubMed] [Google Scholar]
- 44. Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 Stimulates Thrombopoiesis Through Thrombopoietin: Role in Inflammatory Thrombocytosis. Blood (2001) 98(9):2720–5. doi: 10.1182/blood.v98.9.2720 [DOI] [PubMed] [Google Scholar]
- 45. Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Yan LS, Zhang S, et al. Interleukin-6 Mediates the Platelet Abnormalities and Thrombogenesis Associated With Experimental Colitis. Am J Pathol (2013) 183(1):173–81. doi: 10.1016/j.ajpath.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a Therapeutic Target. Clin Cancer Res (2015) 21(6):1248–57. doi: 10.1158/1078-0432.CCR-14-2291 [DOI] [PubMed] [Google Scholar]
- 47. Ho LJ, Luo SF, Lai JH. Biological Effects of Interleukin-6: Clinical Applications in Autoimmune Diseases and Cancers. Biochem Pharmacol (2015) 97(1):16–26. doi: 10.1016/j.bcp.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 48. Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C, et al. Trans-Presentation of IL-6 by Dendritic Cells Is Required for the Priming of Pathogenic TH17 Cells. Nat Immunol (2017) 18(1):74–85. doi: 10.1038/ni.3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of Tumor Microenvironment in Tumorigenesis. J Cancer (2017) 8(5):761–73. doi: 10.7150/jca.17648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun Signal (2020) 18(1):59. doi: 10.1186/s12964-020-0530-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The Extracellular Matrix: Tools and Insights for the “Omics” Era. Matrix Biol (2016) 49:10–24. doi: 10.1016/j.matbio.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat Commun (2020) 11(1):5120. doi: 10.1038/s41467-020-18794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. doi: 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of Fibroblast Heterogeneity in the Tumor Microenvironment. Cancer Biol Ther (2006) 5(12):1640–6. doi: 10.4161/cbt.5.12.3354 [DOI] [PubMed] [Google Scholar]
- 55. Qi C, Li B, Guo S, Wei B, Shao C, Li J, et al. P-Selectin-Mediated Adhesion Between Platelets and Tumor Cells Promotes Intestinal Tumorigenesis in Apc(Min/+) Mice. Int J Biol Sci (2015) 11(6):679–87. doi: 10.7150/ijbs.11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu H, He A, Liu A, Tong W, Cao D. Evaluation of the Prognostic Role of Platelet-Lymphocyte Ratio in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Int Immunopharmacol (2019) 77:105957. doi: 10.1016/j.intimp.2019.105957 [DOI] [PubMed] [Google Scholar]
- 57. Yan M, Jurasz P. The Role of Platelets in the Tumor Microenvironment: From Solid Tumors to Leukemia. Biochim Biophys Acta (2016) 1863(3):392–400. doi: 10.1016/j.bbamcr.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 58. Miyashita T, Tajima H, Gabata R, Okazaki M, Shimbashi H, Ohbatake Y, et al. Impact of Extravasated Platelet Activation and Podoplanin-Positive Cancer-Associated Fibroblasts in Pancreatic Cancer Stroma. Anticancer Res (2019) 39(10):5565–72. doi: 10.21873/anticanres.13750 [DOI] [PubMed] [Google Scholar]
- 59. Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-Beta and Stromal Cell-Derived Factor-1 (SDF-1) Signaling Drives the Evolution of Tumor-Promoting Mammary Stromal Myofibroblasts. Proc Natl Acad Sci USA (2010) 107(46):20009–14. doi: 10.1073/pnas.1013805107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, et al. Dependency of Colorectal Cancer on a TGF-Beta-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell (2012) 22(5):571–84. doi: 10.1016/j.ccr.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, et al. Paracrine Signaling by Platelet-Derived Growth Factor-CC Promotes Tumor Growth by Recruitment of Cancer-Associated Fibroblasts. Cancer Res (2009) 69(1):369–78. doi: 10.1158/0008-5472.CAN-08-2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-Associated Fibroblasts Drive the Progression of Metastasis Through Both Paracrine and Mechanical Pressure on Cancer Tissue. Mol Cancer Res (2012) 10(11):1403–18. doi: 10.1158/1541-7786.MCR-12-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan M, Lesyk G, Radziwon-Balicka A, Jurasz P. Pharmacological Regulation of Platelet Factors That Influence Tumor Angiogenesis. Semin Oncol (2014) 41(3):370–7. doi: 10.1053/j.seminoncol.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 64. Li R, Ren M, Chen N, Luo M, Deng X, Xia J, et al. Presence of Intratumoral Platelets is Associated With Tumor Vessel Structure and Metastasis. BMC Cancer (2014) 14:167. doi: 10.1186/1471-2407-14-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang N, Zhang WJ, Cai HQ, Liu HL, Peng L, Li CH, et al. Platelet Adhesion and Fusion to Endothelial Cell Facilitate the Metastasis of Tumor Cell in Hypoxia-Reoxygenation Condition. Clin Exp Metastasis (2011) 28(1):1–12. doi: 10.1007/s10585-010-9353-9 [DOI] [PubMed] [Google Scholar]
- 66. van Zijl F, Krupitza G, Mikulits W. Initial Steps of Metastasis: Cell Invasion and Endothelial Transmigration. Mutat Res (2011) 728(1-2):23–34. doi: 10.1016/j.mrrev.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bai YY, Du L, Jing L, Tian T, Liang X, Jiao M, et al. Clinicopathological and Prognostic Significance of Pretreatment Thrombocytosis in Patients With Endometrial Cancer: A Meta-Analysis. Cancer Manag Res (2019) 11:4283–95. doi: 10.2147/CMAR.S186535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ichikawa J, Ando T, Kawasaki T, Sasaki T, Shirai T, Tsukiji N, et al. Role of Platelet C-Type Lectin-Like Receptor 2 in Promoting Lung Metastasis in Osteosarcoma. J Bone Miner Res (2020) 35(9):1738–50. doi: 10.1002/jbmr.4045 [DOI] [PubMed] [Google Scholar]
- 69. Papa AL, Jiang A, Korin N, Chen MB, Langan ET, Waterhouse A, et al. Platelet Decoys Inhibit Thrombosis and Prevent Metastatic Tumor Formation in Preclinical Models. Sci Transl Med (2019) 11(479):eaau5898. doi: 10.1126/scitranslmed.aau5898 [DOI] [PubMed] [Google Scholar]
- 70. Miyashita T, Tajima H, Makino I, Nakagawara H, Kitagawa H, Fushida S, et al. Metastasis-Promoting Role of Extravasated Platelet Activation in Tumor. J Surg Res (2015) 193(1):289–94. doi: 10.1016/j.jss.2014.07.037 [DOI] [PubMed] [Google Scholar]
- 71. Karolczak K, Watala C. Blood Platelets as an Important But Underrated Circulating Source of TGFbeta. Int J Mol Sci (2021) 22(9):4492. doi: 10.3390/ijms22094492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guo Y, Cui W, Pei Y, Xu D. Platelets Promote Invasion and Induce Epithelial to Mesenchymal Transition in Ovarian Cancer Cells by TGF-Beta Signaling Pathway. Gynecol Oncol (2019) 153(3):639–50. doi: 10.1016/j.ygyno.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 73. Wang Y, Sun Y, Li D, Zhang L, Wang K, Zuo Y, et al. Platelet P2Y12 is Involved in Murine Pulmonary Metastasis. PloS One (2013) 8(11):e80780. doi: 10.1371/journal.pone.0080780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hu Q, Hisamatsu T, Haemmerle M, Cho MS, Pradeep S, Rupaimoole R, et al. Role of Platelet-Derived Tgfbeta1 in the Progression of Ovarian Cancer. Clin Cancer Res (2017) 23(18):5611–21. doi: 10.1158/1078-0432.CCR-16-3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kazes I, Elalamy I, Sraer JD, Hatmi M, Nguyen G. Platelet Release of Trimolecular Complex Components MT1-MMP/TIMP2/MMP2: Involvement in MMP2 Activation and Platelet Aggregation. Blood (2000) 96(9):3064–9. doi: 10.1182/blood.V96.9.3064 [DOI] [PubMed] [Google Scholar]
- 76. Falcinelli E, Giannini S, Boschetti E, Gresele P. Platelets Release Active Matrix Metalloproteinase-2 In Vivo in Humans at a Site of Vascular Injury: Lack of Inhibition by Aspirin. Br J Haematol (2007) 138(2):221–30. doi: 10.1111/j.1365-2141.2007.06632.x [DOI] [PubMed] [Google Scholar]
- 77. Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles Derived From Activated Platelets Induce Metastasis and Angiogenesis in Lung Cancer. Int J Cancer (2005) 113(5):752–60. doi: 10.1002/ijc.20657 [DOI] [PubMed] [Google Scholar]
- 78. Dashevsky O, Varon D, Brill A. Platelet-Derived Microparticles Promote Invasiveness of Prostate Cancer Cells via Upregulation of MMP-2 Production. Int J Cancer (2009) 124(8):1773–7. doi: 10.1002/ijc.24016 [DOI] [PubMed] [Google Scholar]
- 79. Shin MK, Lee JW, Kim YI, Kim YO, Seok H, Kim NI. The Effects of Platelet-Rich Clot Releasate on the Expression of MMP-1 and Type I Collagen in Human Adult Dermal Fibroblasts: PRP Is a Stronger MMP-1 Stimulator. Mol Biol Rep (2014) 41(1):3–8. doi: 10.1007/s11033-013-2718-9 [DOI] [PubMed] [Google Scholar]
- 80. Zuo XX, Yang Y, Zhang Y, Zhang ZG, Wang XF, Shi YG. Platelets Promote Breast Cancer Cell MCF-7 Metastasis by Direct Interaction: Surface Integrin Alpha2beta1-Contacting-Mediated Activation of Wnt-Beta-Catenin Pathway. Cell Commun Signal (2019) 17(1):142. doi: 10.1186/s12964-019-0464-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Semple JW, Italiano JE, Jr, Freedman J. Platelets and the Immune Continuum. Nat Rev Immunol (2011) 11(4):264–74. doi: 10.1038/nri2956 [DOI] [PubMed] [Google Scholar]
- 82. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat Immunol (2002) 3(11):991–8. doi: 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 83. Kopp HG, Placke T, Salih HR. Platelet-Derived Transforming Growth Factor-Beta Down-Regulates NKG2D Thereby Inhibiting Natural Killer Cell Antitumor Reactivity. Cancer Res (2009) 69(19):7775–83. doi: 10.1158/0008-5472.CAN-09-2123 [DOI] [PubMed] [Google Scholar]
- 84. Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, et al. Platelet-Derived MHC Class I Confers a Pseudonormal Phenotype to Cancer Cells That Subverts the Antitumor Reactivity of Natural Killer Immune Cells. Cancer Res (2012) 72(2):440–8. doi: 10.1158/0008-5472.CAN-11-1872 [DOI] [PubMed] [Google Scholar]
- 85. Placke T, Salih HR, Kopp HG. GITR Ligand Provided by Thrombopoietic Cells Inhibits NK Cell Antitumor Activity. J Immunol (2012) 189(1):154–60. doi: 10.4049/jimmunol.1103194 [DOI] [PubMed] [Google Scholar]
- 86. Rachidi S, Metelli A, Riesenberg B, Wu BX, Nelson MH, Wallace C, et al. Platelets Subvert T Cell Immunity Against Cancer via GARP-TGFbeta Axis. Sci Immunol (2017) 2(11):eaai7911. doi: 10.1126/sciimmunol.aai7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zaslavsky AB, Adams MP, Cao X, Maj T, Choi JE, Stangl-Kremser J, et al. Platelet PD-L1 Suppresses Anti-Cancer Immune Cell Activity in PD-L1 Negative Tumors. Sci Rep (2020) 10(1):19296. doi: 10.1038/s41598-020-76351-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Riesenberg BP, Ansa-Addo EA, Gutierrez J, Timmers CD, Liu B, Li Z. Cutting Edge: Targeting Thrombocytes to Rewire Anticancer Immunity in the Tumor Microenvironment and Potentiate Efficacy of PD-1 Blockade. J Immunol (2019) 203(5):1105–10. doi: 10.4049/jimmunol.1900594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Furlan C, Steffan A, Polesel J, Trovo M, Gobitti C, Vaccher E, et al. Lower Platelet Counts and Antiplatelet Therapy Independently Predict Better Outcomes in Patients With Head and Neck Squamous Cell Carcinoma: A Retrospective Analysis. biomark Res (2015) 3:25. doi: 10.1186/s40364-015-0051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lu CC, Chang KW, Chou FC, Cheng CY, Liu CJ. Association of Pretreatment Thrombocytosis With Disease Progression and Survival in Oral Squamous Cell Carcinoma. Oral Oncol (2007) 43(3):283–8. doi: 10.1016/j.oraloncology.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 91. Brown JS, Lowe D, Kalavrezos N, D’Souza J, Magennis P, Woolgar J. Patterns of Invasion and Routes of Tumor Entry Into the Mandible by Oral Squamous Cell Carcinoma. Head Neck (2002) 24(4):370–83. doi: 10.1002/hed.10062 [DOI] [PubMed] [Google Scholar]
- 92. Jimi E, Furuta H, Matsuo K, Tominaga K, Takahashi T, Nakanishi O. The Cellular and Molecular Mechanisms of Bone Invasion by Oral Squamous Cell Carcinoma. Oral Dis (2011) 17(5):462–8. doi: 10.1111/j.1601-0825.2010.01781.x [DOI] [PubMed] [Google Scholar]
- 93. Shaw RJ, Brown JS, Woolgar JA, Lowe D, Rogers SN, Vaughan ED. The Influence of the Pattern of Mandibular Invasion on Recurrence and Survival in Oral Squamous Cell Carcinoma. Head Neck (2004) 26(10):861–9. doi: 10.1002/hed.20036 [DOI] [PubMed] [Google Scholar]
- 94. Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, et al. Platelet and Osteoclast Beta3 Integrins Are Critical for Bone Metastasis. Proc Natl Acad Sci USA (2003) 100(24):14205–10. doi: 10.1073/pnas.2234372100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Uluckan O, Eagleton MC, Floyd DH, Morgan EA, Hirbe AC, Kramer M, et al. APT102, A Novel Adpase, Cooperates With Aspirin to Disrupt Bone Metastasis in Mice. J Cell Biochem (2008) 104(4):1311–23. doi: 10.1002/jcb.21709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. Platelet-Derived Lysophosphatidic Acid Supports the Progression of Osteolytic Bone Metastases in Breast Cancer. J Clin Invest (2004) 114(12):1714–25. doi: 10.1172/JCI22123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ward Y, Lake R, Faraji F, Sperger J, Martin P, Gilliard C, et al. Platelets Promote Metastasis via Binding Tumor CD97 Leading to Bidirectional Signaling That Coordinates Transendothelial Migration. Cell Rep (2018) 23(3):808–22. doi: 10.1016/j.celrep.2018.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Goda T, Shimo T, Yoshihama Y, Hassan NM, Ibaragi S, Kurio N, et al. Bone Destruction by Invading Oral Squamous Carcinoma Cells Mediated by the Transforming Growth Factor-Beta Signalling Pathway. Anticancer Res (2010) 30(7):2615–23. [PubMed] [Google Scholar]
- 99. Son SH, Park J, Jung MJ, Lee SK, Kim H, Kim KR, et al. Transforming Growth Factor-Beta-Regulated Fractalkine as a Marker of Erosive Bone Invasion in Oral Squamous Cell Carcinoma. Eur J Oral Sci (2021) 129(1):e12750. doi: 10.1111/eos.12750 [DOI] [PubMed] [Google Scholar]
- 100. Astarita JL, Acton SE, Turley SJ. Podoplanin: Emerging Functions in Development, the Immune System, and Cancer. Front Immunol (2012) 3:283. doi: 10.3389/fimmu.2012.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC. T1alpha, a Lung Type I Cell Differentiation Gene, is Required for Normal Lung Cell Proliferation and Alveolus Formation at Birth. Dev Biol (2003) 256(1):61–72. doi: 10.1016/s0012-1606(02)00098-2 [DOI] [PubMed] [Google Scholar]
- 102. Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, et al. T1alpha/podoplanin Deficiency Disrupts Normal Lymphatic Vasculature Formation and Causes Lymphedema. EMBO J (2003) 22(14):3546–56. doi: 10.1093/emboj/cdg342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, et al. Th17 Cells Induce Ectopic Lymphoid Follicles in Central Nervous System Tissue Inflammation. Immunity (2011) 35(6):986–96. doi: 10.1016/j.immuni.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shibahara J, Kashima T, Kikuchi Y, Kunita A, Fukayama M. Podoplanin is Expressed in Subsets of Tumors of the Central Nervous System. Virchows Arch (2006) 448(4):493–9. doi: 10.1007/s00428-005-0133-x [DOI] [PubMed] [Google Scholar]
- 105. Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo T, Osawa M. Enhanced Expression of Aggrus (T1alpha/podoplanin), a Platelet-Aggregation-Inducing Factor in Lung Squamous Cell Carcinoma. Tumour Biol (2005) 26(4):195–200. doi: 10.1159/000086952 [DOI] [PubMed] [Google Scholar]
- 106. Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor Invasion in the Absence of Epithelial-Mesenchymal Transition: Podoplanin-Mediated Remodeling of the Actin Cytoskeleton. Cancer Cell (2006) 9(4):261–72. doi: 10.1016/j.ccr.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 107. Kimura N, Kimura I. Podoplanin as a Marker for Mesothelioma. Pathol Int (2005) 55(2):83–6. doi: 10.1111/j.1440-1827.2005.01791.x [DOI] [PubMed] [Google Scholar]
- 108. Miyata K, Takemoto A, Okumura S, Nishio M, Fujita N. Podoplanin Enhances Lung Cancer Cell Growth In Vivo by Inducing Platelet Aggregation. Sci Rep (2017) 7(1):4059. doi: 10.1038/s41598-017-04324-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sesartic M, Ikenberg K, Yoon SY, Detmar M. Keratinocyte-Expressed Podoplanin is Dispensable for Multi-Step Skin Carcinogenesis. Cells (2020) 9(6):1542. doi: 10.3390/cells9061542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Grau SJ, Trillsch F, Tonn JC, Goldbrunner RH, Noessner E, Nelson PJ, et al. Podoplanin Increases Migration and Angiogenesis in Malignant Glioma. Int J Clin Exp Pathol (2015) 8(7):8663–70. [PMC free article] [PubMed] [Google Scholar]
- 111. Scholl FG, Gamallo C, Quintanilla M. Ectopic Expression of PA2.26 Antigen in Epidermal Keratinocytes Leads to Destabilization of Adherens Junctions and Malignant Progression. Lab Invest (2000) 80(11):1749–59. doi: 10.1038/labinvest.3780185 [DOI] [PubMed] [Google Scholar]
- 112. Sikorska J, Gawel D, Domek H, Rudzinska M, Czarnocka B. Podoplanin (PDPN) Affects the Invasiveness of Thyroid Carcinoma Cells by Inducing Ezrin, Radixin and Moesin (E/R/M) Phosphorylation in Association With Matrix Metalloproteinases. BMC Cancer (2019) 19(1):85. doi: 10.1186/s12885-018-5239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wicki A, Christofori G. The Potential Role of Podoplanin in Tumour Invasion. Br J Cancer (2007) 96(1):1–5. doi: 10.1038/sj.bjc.6603518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mir Seyed Nazari P, Riedl J, Pabinger I, Ay C. The Role of Podoplanin in Cancer-Associated Thrombosis. Thromb Res (2018) 164(Suppl 1):S34–9. doi: 10.1016/j.thromres.2018.01.020 [DOI] [PubMed] [Google Scholar]
- 115. Riedl J, Preusser M, Nazari PM, Posch F, Panzer S, Marosi C, et al. Podoplanin Expression in Primary Brain Tumors Induces Platelet Aggregation and Increases Risk of Venous Thromboembolism. Blood (2017) 129(13):1831–9. doi: 10.1182/blood-2016-06-720714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sugimoto Y, Watanabe M, Oh-hara T, Sato S, Isoe T, Tsuruo T. Suppression of Experimental Lung Colonization of a Metastatic Variant of Murine Colon Adenocarcinoma 26 by a Monoclonal Antibody 8F11 Inhibiting Tumor Cell-Induced Platelet Aggregation. Cancer Res (1991) 51(3):921–5. [PubMed] [Google Scholar]
- 117. Costa B, Eisemann T, Strelau J, Spaan I, Korshunov A, Liu HK, et al. Intratumoral Platelet Aggregate Formation in a Murine Preclinical Glioma Model Depends on Podoplanin Expression on Tumor Cells. Blood Adv (2019) 3(7):1092–102. doi: 10.1182/bloodadvances.2018015966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Campello E, Ilich A, Simioni P, Key NS. The Relationship Between Pancreatic Cancer and Hypercoagulability: A Comprehensive Review on Epidemiological and Biological Issues. Br J Cancer (2019) 121(5):359–71. doi: 10.1038/s41416-019-0510-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Xu M, Wang X, Pan Y, Zhao X, Yan B, Ruan C, et al. Blocking Podoplanin Suppresses Growth and Pulmonary Metastasis of Human Malignant Melanoma. BMC Cancer (2019) 19(1):599. doi: 10.1186/s12885-019-5808-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Takemoto A, Okitaka M, Takagi S, Takami M, Sato S, Nishio M, et al. A Critical Role of Platelet TGF-Beta Release in Podoplanin-Mediated Tumour Invasion and Metastasis. Sci Rep (2017) 7:42186. doi: 10.1038/srep42186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Takemoto A, Miyata K, Fujita N. Platelet-Activating Factor Podoplanin: From Discovery to Drug Development. Cancer Metastasis Rev (2017) 36(2):225–34. doi: 10.1007/s10555-017-9672-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Suzuki-Inoue K. Roles of the CLEC-2-Podoplanin Interaction in Tumor Progression. Platelets (2018) 4:1–7. doi: 10.1080/09537104.2018.1478401 [DOI] [PubMed] [Google Scholar]
- 123. Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, et al. Essential In Vivo Roles of the C-Type Lectin Receptor CLEC-2: Embryonic/Neonatal Lethality of CLEC-2-Deficient Mice by Blood/Lymphatic Misconnections and Impaired Thrombus Formation of CLEC-2-Deficient Platelets. J Biol Chem (2010) 285(32):24494–507. doi: 10.1074/jbc.M110.130575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Payne H, Ponomaryov T, Watson SP, Brill A. Mice With a Deficiency in CLEC-2 Are Protected Against Deep Vein Thrombosis. Blood (2017) 129(14):2013–20. doi: 10.1182/blood-2016-09-742999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the Snake Toxin Receptor CLEC-2, in Podoplanin-Mediated Platelet Activation, by Cancer Cells. J Biol Chem (2007) 282(36):25993–6001. doi: 10.1074/jbc.M702327200 [DOI] [PubMed] [Google Scholar]
- 126. Tsukiji N, Osada M, Sasaki T, Shirai T, Satoh K, Inoue O, et al. Cobalt Hematoporphyrin Inhibits CLEC-2-Podoplanin Interaction, Tumor Metastasis, and Arterial/Venous Thrombosis in Mice. Blood Adv (2018) 2(17):2214–25. doi: 10.1182/bloodadvances.2018016261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, et al. C-Type Lectin-Like Receptor 2 Promotes Hematogenous Tumor Metastasis and Prothrombotic State in Tumor-Bearing Mice. J Thromb Haemost (2017) 15(3):513–25. doi: 10.1111/jth.13604 [DOI] [PubMed] [Google Scholar]
- 128. Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, et al. Platelets Promote Tumor Growth and Metastasis via Direct Interaction Between Aggrus/podoplanin and CLEC-2. PloS One (2013) 8(8):e73609. doi: 10.1371/journal.pone.0073609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bender M, May F, Lorenz V, Thielmann I, Hagedorn I, Finney BA, et al. Combined In Vivo Depletion of Glycoprotein VI and C-Type Lectin-Like Receptor 2 Severely Compromises Hemostasis and Abrogates Arterial Thrombosis in Mice. Arterioscler Thromb Vasc Biol (2013) 33(5):926–34. doi: 10.1161/ATVBAHA.112.300672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Martin-Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M. Podoplanin Binds ERM Proteins to Activate RhoA and Promote Epithelial-Mesenchymal Transition. J Cell Sci (2006) 119(Pt 21):4541–53. doi: 10.1242/jcs.03218 [DOI] [PubMed] [Google Scholar]
- 131. Louvet-Vallee S. ERM Proteins: From Cellular Architecture to Cell Signaling. Biol Cell (2000) 92(5):305–16. doi: 10.1016/s0248-4900(00)01078-9 [DOI] [PubMed] [Google Scholar]
- 132. Fievet B, Louvard D, Arpin M. ERM Proteins in Epithelial Cell Organization and Functions. Biochim Biophys Acta (2007) 1773(5):653–60. doi: 10.1016/j.bbamcr.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 133. Fehon RG, McClatchey AI, Bretscher A. Organizing the Cell Cortex: The Role of ERM Proteins. Nat Rev Mol Cell Biol (2010) 11(4):276–87. doi: 10.1038/nrm2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kawaguchi K, Yoshida S, Hatano R, Asano S. Pathophysiological Roles of Ezrin/Radixin/Moesin Proteins. Biol Pharm Bull (2017) 40(4):381–90. doi: 10.1248/bpb.b16-01011 [DOI] [PubMed] [Google Scholar]
- 135. Saotome I, Curto M, McClatchey AI. Ezrin is Essential for Epithelial Organization and Villus Morphogenesis in the Developing Intestine. Dev Cell (2004) 6(6):855–64. doi: 10.1016/j.devcel.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 136. Clucas J, Valderrama F. ERM Proteins in Cancer Progression. J Cell Sci (2014) 127(Pt 2):267–75. doi: 10.1242/jcs.133108 [DOI] [PubMed] [Google Scholar]
- 137. Horwitz V, Davidson B, Stern D, Trope CG, Tavor Re’em T, Reich R. Ezrin Is Associated With Disease Progression in Ovarian Carcinoma. PloS One (2016) 11(9):e0162502. doi: 10.1371/journal.pone.0162502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Li N, Kong J, Lin Z, Yang Y, Jin T, Xu M, et al. Ezrin Promotes Breast Cancer Progression by Modulating AKT Signals. Br J Cancer (2019) 120(7):703–13. doi: 10.1038/s41416-019-0383-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pan D, Wang S, Ye H, Xu S, Ye G. Ezrin Expression in the Primary Hepatocellular Carcinoma Patients and Associated With Clinical, Pathological Characteristics. J Cancer Res Ther (2016) 12(Supplement):C291–4. doi: 10.4103/0973-1482.200761 [DOI] [PubMed] [Google Scholar]
- 140. Piao J, Liu S, Xu Y, Wang C, Lin Z, Qin Y, et al. Ezrin Protein Overexpression Predicts the Poor Prognosis of Pancreatic Ductal Adenocarcinomas. Exp Mol Pathol (2015) 98(1):1–6. doi: 10.1016/j.yexmp.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 141. Wang Y, Yang Y, Wang X, Jin T, Zhu G, Lin Z. Ezrin as a Prognostic Indicator Regulates Colon Adenocarinoma Progression Through Glycolysis. J Gastroenterol Hepatol (2020) 36(3):710–20. doi: 10.1111/jgh.15195 [DOI] [PubMed] [Google Scholar]
- 142. Cui Y, Wu J, Zong M, Song G, Jia Q, Jiang J, et al. Proteomic Profiling in Pancreatic Cancer With and Without Lymph Node Metastasis. Int J Cancer (2009) 124(7):1614–21. doi: 10.1002/ijc.24163 [DOI] [PubMed] [Google Scholar]
- 143. Ichikawa T, Masumoto J, Kaneko M, Saida T, Sagara J, Taniguchi S. Expression of Moesin and Its Associated Molecule CD44 in Epithelial Skin Tumors. J Cutan Pathol (1998) 25(5):237–43. doi: 10.1111/j.1600-0560.1998.tb01727.x [DOI] [PubMed] [Google Scholar]
- 144. Kim CY, Jung WY, Lee HJ, Kim HK, Kim A, Shin BK. Proteomic Analysis Reveals Overexpression of Moesin and Cytokeratin 17 Proteins in Colorectal Carcinoma. Oncol Rep (2012) 27(3):608–20. doi: 10.3892/or.2011.1545 [DOI] [PubMed] [Google Scholar]
- 145. Mhawech-Fauceglia P, Wang D, Lele S, Frederick PJ, Pejovic T, Liu S. Claudin7 and Moesin in Endometrial Adenocarcinoma; A Retrospective Study of 265 Patients. BMC Res Notes (2012) 5:65. doi: 10.1186/1756-0500-5-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhu X, Morales FC, Agarwal NK, Dogruluk T, Gagea M, Georgescu MM. Moesin is a Glioma Progression Marker That Induces Proliferation and Wnt/beta-Catenin Pathway Activation via Interaction With CD44. Cancer Res (2013) 73(3):1142–55. doi: 10.1158/0008-5472.CAN-12-1040 [DOI] [PubMed] [Google Scholar]
- 147. Makitie T, Carpen O, Vaheri A, Kivela T. Ezrin as a Prognostic Indicator and Its Relationship to Tumor Characteristics in Uveal Malignant Melanoma. Invest Ophthalmol Vis Sci (2001) 42(11):2442–9. [PubMed] [Google Scholar]
- 148. Arumugam P, Partelli S, Coleman SJ, Cataldo I, Beghelli S, Bassi C, et al. Ezrin Expression Is an Independent Prognostic Factor in Gastro-Intestinal Cancers. J Gastrointest Surg (2013) 17(12):2082–91. doi: 10.1007/s11605-013-2384-1 [DOI] [PubMed] [Google Scholar]
- 149. Barros FBA, Assao A, Garcia NG, Nonogaki S, Carvalho AL, Soares FA, et al. Moesin Expression by Tumor Cells Is an Unfavorable Prognostic Biomarker for Oral Cancer. BMC Cancer (2018) 18(1):53. doi: 10.1186/s12885-017-3914-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kong J, Li Y, Liu S, Jin H, Shang Y, Quan C, et al. High Expression of Ezrin Predicts Poor Prognosis in Uterine Cervical Cancer. BMC Cancer (2013) 13:520. doi: 10.1186/1471-2407-13-520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fernandez-Munoz B, Yurrita MM, Martin-Villar E, Carrasco-Ramirez P, Megias D, Renart J, et al. The Transmembrane Domain of Podoplanin is Required for Its Association With Lipid Rafts and the Induction of Epithelial-Mesenchymal Transition. Int J Biochem Cell Biol (2011) 43(6):886–96. doi: 10.1016/j.biocel.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 152. Zhu YW, Yan JK, Li JJ, Ou YM, Yang Q. Knockdown of Radixin Suppresses Gastric Cancer Metastasis In Vitro by Up-Regulation of E-Cadherin via NF-Kappab/Snail Pathway. Cell Physiol Biochem (2016) 39(6):2509–21. doi: 10.1159/000452518 [DOI] [PubMed] [Google Scholar]
- 153. Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, et al. Podoplanin-Rich Stromal Networks Induce Dendritic Cell Motility via Activation of the C-Type Lectin Receptor CLEC-2. Immunity (2012) 37(2):276–89. doi: 10.1016/j.immuni.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR, Nieves-Bonilla JM, et al. The CLEC-2-Podoplanin Axis Controls the Contractility of Fibroblastic Reticular Cells and Lymph Node Microarchitecture. Nat Immunol (2015) 16(1):75–84. doi: 10.1038/ni.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bourne JH, Beristain-Covarrubias N, Zuidscherwoude M, Campos J, Di Y, Garlick E, et al. CLEC-2 Prevents Accumulation and Retention of Inflammatory Macrophages During Murine Peritonitis. Front Immunol (2021) 12:693974. doi: 10.3389/fimmu.2021.693974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, et al. Overexpression of Podoplanin in Oral Cancer and Its Association With Poor Clinical Outcome. Cancer (2006) 107(3):563–9. doi: 10.1002/cncr.22061 [DOI] [PubMed] [Google Scholar]
- 157. Retzbach EP, Sheehan SA, Nevel EM, Batra A, Phi T, Nguyen ATP, et al. Podoplanin Emerges as a Functionally Relevant Oral Cancer Biomarker and Therapeutic Target. Oral Oncol (2018) 78:126–36. doi: 10.1016/j.oraloncology.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 158. Kreppel M, Drebber U, Wedemeyer I, Eich HT, Backhaus T, Zoller JE, et al. Podoplanin Expression Predicts Prognosis in Patients With Oral Squamous Cell Carcinoma Treated With Neoadjuvant Radiochemotherapy. Oral Oncol (2011) 47(9):873–8. doi: 10.1016/j.oraloncology.2011.06.508 [DOI] [PubMed] [Google Scholar]
- 159. Kawaguchi H, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, Feng L, et al. Podoplanin: A Novel Marker for Oral Cancer Risk in Patients With Oral Premalignancy. J Clin Oncol (2008) 26(3):354–60. doi: 10.1200/JCO.2007.13.4072 [DOI] [PubMed] [Google Scholar]
- 160. Lee HY, Yu NY, Lee SH, Tsai HJ, Wu CC, Cheng JC, et al. Podoplanin Promotes Cancer-Associated Thrombosis and Contributes to the Unfavorable Overall Survival in an Ectopic Xenograft Mouse Model of Oral Cancer. BioMed J (2020) 43(2):146–62. doi: 10.1016/j.bj.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Huber GF, Fritzsche FR, Zullig L, Storz M, Graf N, Haerle SK, et al. Podoplanin Expression Correlates With Sentinel Lymph Node Metastasis in Early Squamous Cell Carcinomas of the Oral Cavity and Oropharynx. Int J Cancer (2011) 129(6):1404–9. doi: 10.1002/ijc.25795 [DOI] [PubMed] [Google Scholar]
- 162. Tsuneki M, Yamazaki M, Maruyama S, Cheng J, Saku T. Podoplanin-Mediated Cell Adhesion Through Extracellular Matrix in Oral Squamous Cell Carcinoma. Lab Invest (2013) 93(8):921–32. doi: 10.1038/labinvest.2013.86 [DOI] [PubMed] [Google Scholar]
- 163. Mei Y, Zhang P, Zuo H, Clark D, Xia R, Li J, et al. Ebp1 Activates Podoplanin Expression and Contributes to Oral Tumorigenesis. Oncogene (2014) 33(29):3839–50. doi: 10.1038/onc.2013.354 [DOI] [PubMed] [Google Scholar]
- 164. Li YY, Zhou CX, Gao Y. Podoplanin Promotes the Invasion of Oral Squamous Cell Carcinoma in Coordination With MT1-MMP and Rho GTPases. Am J Cancer Res (2015) 5(2):514–29. [PMC free article] [PubMed] [Google Scholar]
- 165. Safi AF, Nickenig HJ, Rothamel D, Zirk M, Thiele O, Grandoch A, et al. Expression of Ezrin in Oral Squamous Cell Carcinoma: Prognostic Impact and Clinicopathological Correlations. J Craniomaxillofac Surg (2015) 43(9):1899–905. doi: 10.1016/j.jcms.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 166. Kobayashi H, Sagara J, Kurita H, Morifuji M, Ohishi M, Kurashina K, et al. Clinical Significance of Cellular Distribution of Moesin in Patients With Oral Squamous Cell Carcinoma. Clin Cancer Res (2004) 10(2):572–80. doi: 10.1158/1078-0432.ccr-1323-03 [DOI] [PubMed] [Google Scholar]
- 167. Costa YF, Tjioe KC, Nonogaki S, Soares FA, Lauris JR, Oliveira DT. Are Podoplanin and Ezrin Involved in the Invasion Process of the Ameloblastomas? Eur J Histochem (2015) 59(1):2451. doi: 10.4081/ejh.2015.2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Assao A, Nonogaki S, Lauris JRP, Carvalho AL, Pinto CAL, Soares FA, et al. Podoplanin, Ezrin, and Rho-A Proteins may Have Joint Participation in Tumor Invasion of Lip Cancer. Clin Oral Investig (2017) 21(5):1647–57. doi: 10.1007/s00784-016-1956-3 [DOI] [PubMed] [Google Scholar]
- 169. Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and Cancer Risk: A Quantitative Review to 2011. Ann Oncol (2012) 23(6):1403–15. doi: 10.1093/annonc/mds113 [DOI] [PubMed] [Google Scholar]
- 170. Jayaprakash V, Rigual NR, Moysich KB, Loree TR, Nasca MA, Menezes RJ, et al. Chemoprevention of Head and Neck Cancer With Aspirin: A Case-Control Study. Arch Otolaryngol Head Neck Surg (2006) 132(11):1231–6. doi: 10.1001/archotol.132.11.1231 [DOI] [PubMed] [Google Scholar]
- 171. de la Cour CD, Verdoodt F, Aalborg GL, von Buchwald C, Friis S, Dehlendorff C, et al. Low-Dose Aspirin Use and Risk of Head and Neck Cancer-A Danish Nationwide Case-Control Study. Br J Clin Pharmacol (2021) 87(3):1561–7. doi: 10.1111/bcp.14502 [DOI] [PubMed] [Google Scholar]
- 172. Wilson JC, Anderson LA, Murray LJ, Hughes CM. Non-Steroidal Anti-Inflammatory Drug and Aspirin Use and the Risk of Head and Neck Cancer: A Systematic Review. Cancer Causes Control (2011) 22(5):803–10. doi: 10.1007/s10552-011-9751-6 [DOI] [PubMed] [Google Scholar]
- 173. Cooke NM, Spillane CD, Sheils O, O’Leary J, Kenny D. Aspirin and P2Y12 Inhibition Attenuate Platelet-Induced Ovarian Cancer Cell Invasion. BMC Cancer (2015) 15:627. doi: 10.1186/s12885-015-1634-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Guillem-Llobat P, Dovizio M, Bruno A, Ricciotti E, Cufino V, Sacco A, et al. Aspirin Prevents Colorectal Cancer Metastasis in Mice by Splitting the Crosstalk Between Platelets and Tumor Cells. Oncotarget (2016) 7(22):32462–77. doi: 10.18632/oncotarget.8655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Algra AM, Rothwell PM. Effects of Regular Aspirin on Long-Term Cancer Incidence and Metastasis: A Systematic Comparison of Evidence From Observational Studies Versus Randomised Trials. Lancet Oncol (2012) 13(5):518–27. doi: 10.1016/S1470-2045(12)70112-2 [DOI] [PubMed] [Google Scholar]
- 176. Cho MS, Noh K, Haemmerle M, Li D, Park H, Hu Q, et al. Role of ADP Receptors on Platelets in the Growth of Ovarian Cancer. Blood (2017) 130(10):1235–42. doi: 10.1182/blood-2017-02-769893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Pavlovic N, Kopsida M, Gerwins P, Heindryckx F. Activated Platelets Contribute to the Progression of Hepatocellular Carcinoma by Altering the Tumor Environment. Life Sci (2021) 277:119612. doi: 10.1016/j.lfs.2021.119612 [DOI] [PubMed] [Google Scholar]
- 178. Gareau AJ, Brien C, Gebremeskel S, Liwski RS, Johnston B, Bezuhly M. Ticagrelor Inhibits Platelet-Tumor Cell Interactions and Metastasis in Human and Murine Breast Cancer. Clin Exp Metastasis (2018) 35(1-2):25–35. doi: 10.1007/s10585-018-9874-1 [DOI] [PubMed] [Google Scholar]
- 179. Geranpayehvaghei M, Shi Q, Zhao B, Li S, Xu J, Taleb M, et al. Targeting Delivery of Platelets Inhibitor to Prevent Tumor Metastasis. Bioconjug Chem (2019) 30(9):2349–57. doi: 10.1021/acs.bioconjchem.9b00457 [DOI] [PubMed] [Google Scholar]
- 180. Gebremeskel S, LeVatte T, Liwski RS, Johnston B, Bezuhly M. The Reversible P2Y12 Inhibitor Ticagrelor Inhibits Metastasis and Improves Survival in Mouse Models of Cancer. Int J Cancer (2015) 136(1):234–40. doi: 10.1002/ijc.28947 [DOI] [PubMed] [Google Scholar]
- 181. Hicks BM, Murray LJ, Hughes C, Cardwell CR. Clopidogrel Use and Cancer-Specific Mortality: A Population-Based Cohort Study of Colorectal, Breast and Prostate Cancer Patients. Pharmacoepidemiol Drug Saf (2015) 24(8):830–40. doi: 10.1002/pds.3807 [DOI] [PubMed] [Google Scholar]
- 182. Elmariah S, Doros G, Benavente OR, Bhatt DL, Connolly SJ, Yusuf S, et al. Impact of Clopidogrel Therapy on Mortality and Cancer in Patients With Cardiovascular and Cerebrovascular Disease: A Patient-Level Meta-Analysis. Circ Cardiovasc Interv (2018) 11(1):e005795. doi: 10.1161/CIRCINTERVENTIONS.117.005795 [DOI] [PubMed] [Google Scholar]
- 183. Serebruany VL, Cherepanov V, Cabrera-Fuentes HA, Kim MH. Solid Cancers After Antiplatelet Therapy: Confirmations, Controversies, and Challenges. Thromb Haemost (2015) 114(6):1104–12. doi: 10.1160/TH15-01-0077 [DOI] [PubMed] [Google Scholar]
- 184. Serebruany VL, Tomek A, Kim MH. Survival After Solid Cancers in Antithrombotic Trials. Am J Cardiol (2015) 116(6):969–72. doi: 10.1016/j.amjcard.2015.06.026 [DOI] [PubMed] [Google Scholar]
- 185. Turgeon RD, Koshman SL, Youngson E, Har B, Wilton SB, James MT, et al. Association of Ticagrelor vs Clopidogrel With Major Adverse Coronary Events in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. JAMA Intern Med (2020) 180(3):420–8. doi: 10.1001/jamainternmed.2019.6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, et al. Platelet Glycoprotein VI Promotes Metastasis Through Interaction With Cancer Cell-Derived Galectin-3. Blood (2020) 135(14):1146–60. doi: 10.1182/blood.2019002649 [DOI] [PubMed] [Google Scholar]
- 187. Xiong Y, Liu L, Xia Y, Wang J, Xi W, Bai Q, et al. High CLEC-2 Expression Associates With Unfavorable Postoperative Prognosis of Patients With Clear Cell Renal Cell Carcinoma. Oncotarget (2016) 7(39):63661–8. doi: 10.18632/oncotarget.11606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Volz J, Mammadova-Bach E, Gil-Pulido J, Nandigama R, Remer K, Sorokin L, et al. Inhibition of Platelet GPVI Induces Intratumor Hemorrhage and Increases Efficacy of Chemotherapy in Mice. Blood (2019) 133(25):2696–706. doi: 10.1182/blood.2018877043 [DOI] [PubMed] [Google Scholar]
- 189. Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Munch G, et al. Pharmacological Inhibition of Platelet-Tumor Cell Cross-Talk Prevents Platelet-Induced Overexpression of Cyclooxygenase-2 in HT29 Human Colon Carcinoma Cells. Mol Pharmacol (2013) 84(1):25–40. doi: 10.1124/mol.113.084988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ungerer M, Li Z, Baumgartner C, Goebel S, Vogelmann J, Holthoff HP, et al. The GPVI-Fc Fusion Protein Revacept Reduces Thrombus Formation and Improves Vascular Dysfunction in Atherosclerosis Without Any Impact on Bleeding Times. PloS One (2013) 8(8):e71193. doi: 10.1371/journal.pone.0071193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ungerer M, Rosport K, Bultmann A, Piechatzek R, Uhland K, Schlieper P, et al. Novel Antiplatelet Drug Revacept (Dimeric Glycoprotein VI-Fc) Specifically and Efficiently Inhibited Collagen-Induced Platelet Aggregation Without Affecting General Hemostasis in Humans. Circulation (2011) 123(17):1891–9. doi: 10.1161/CIRCULATIONAHA.110.980623 [DOI] [PubMed] [Google Scholar]
- 192. Kato Y, Kaneko MK, Kuno A, Uchiyama N, Amano K, Chiba Y, et al. Inhibition of Tumor Cell-Induced Platelet Aggregation Using a Novel Anti-Podoplanin Antibody Reacting With its Platelet-Aggregation-Stimulating Domain. Biochem Biophys Res Commun (2006) 349(4):1301–7. doi: 10.1016/j.bbrc.2006.08.171 [DOI] [PubMed] [Google Scholar]
- 193. Sekiguchi T, Takemoto A, Takagi S, Takatori K, Sato S, Takami M, et al. Targeting a Novel Domain in Podoplanin for Inhibiting Platelet-Mediated Tumor Metastasis. Oncotarget (2016) 7(4):3934–46. doi: 10.18632/oncotarget.6598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wang X, Liu B, Xu M, Jiang Y, Zhou J, Yang J, et al. Blocking Podoplanin Inhibits Platelet Activation and Decreases Cancer-Associated Venous Thrombosis. Thromb Res (2021) 200:72–80. doi: 10.1016/j.thromres.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 195. Chang YW, Hsieh PW, Chang YT, Lu MH, Huang TF, Chong KY, et al. Identification of a Novel Platelet Antagonist That Binds to CLEC-2 and Suppresses Podoplanin-Induced Platelet Aggregation and Cancer Metastasis. Oncotarget (2015) 6(40):42733–48. doi: 10.18632/oncotarget.5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Tseng CP, Huang YL, Chang YW, Liao HR, Chen YL, Hsieh PW. Polysaccharide-Containing Fraction From Artemisia Argyi Inhibits Tumor Cell-Induced Platelet Aggregation by Blocking Interaction of Podoplanin With C-Type Lectin-Like Receptor 2. J Food Drug Anal (2020) 28(1):115–23. doi: 10.1016/j.jfda.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 197. Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 Platelet Inhibition in Clinical Practice. J Thromb Thrombolysis (2012) 33(2):143–53. doi: 10.1007/s11239-011-0667-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhao F, Li L, Guan L, Yang H, Wu C, Liu Y. Roles for GP IIb/IIIa and Alphavbeta3 Integrins in MDA-MB-231 Cell Invasion and Shear Flow-Induced Cancer Cell Mechanotransduction. Cancer Lett (2014) 344(1):62–73. doi: 10.1016/j.canlet.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 199. McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized Platelets Support Human Colon Carcinoma Cell Tethering, Rolling, and Firm Adhesion Under Dynamic Flow Conditions. Blood (2000) 96(5):1789–97. doi: 10.1182/blood.V96.5.1789.h8001789_1789_1797 [DOI] [PubMed] [Google Scholar]
- 200. Zhang C, Liu Y, Gao Y, Shen J, Zheng S, Wei M, et al. Modified Heparins Inhibit Integrin Alpha(IIb)beta(3) Mediated Adhesion of Melanoma Cells to Platelets In Vitro and In Vivo . Int J Cancer (2009) 125(9):2058–65. doi: 10.1002/ijc.24561 [DOI] [PubMed] [Google Scholar]
- 201. Ballerini P, Dovizio M, Bruno A, Tacconelli S, Patrignani P. P2Y12 Receptors in Tumorigenesis and Metastasis. Front Pharmacol (2018) 9:66. doi: 10.3389/fphar.2018.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Dutting S, Bender M, Nieswandt B. Platelet GPVI: A Target for Antithrombotic Therapy? Trends Pharmacol Sci (2012) 33(11):583–90. doi: 10.1016/j.tips.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 203. Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, et al. The Contribution of Glycoprotein VI to Stable Platelet Adhesion and Thrombus Formation Illustrated by Targeted Gene Deletion. Blood (2003) 102(5):1701–7. doi: 10.1182/blood-2003-03-0717 [DOI] [PubMed] [Google Scholar]
- 204. Konstantinides S, Ware J, Marchese P, Almus-Jacobs F, Loskutoff DJ, Ruggeri ZM. Distinct Antithrombotic Consequences of Platelet Glycoprotein Ibalpha and VI Deficiency in a Mouse Model of Arterial Thrombosis. J Thromb Haemost (2006) 4(9):2014–21. doi: 10.1111/j.1538-7836.2006.02086.x [DOI] [PubMed] [Google Scholar]
- 205. Jain S, Russell S, Ware J. Platelet Glycoprotein VI Facilitates Experimental Lung Metastasis in Syngenic Mouse Models. J Thromb Haemost (2009) 7(10):1713–7. doi: 10.1111/j.1538-7836.2009.03559.x [DOI] [PubMed] [Google Scholar]
- 206. Hughes CE, Navarro-Nunez L, Finney BA, Mourao-Sa D, Pollitt AY, Watson SP. CLEC-2 Is Not Required for Platelet Aggregation at Arteriolar Shear. J Thromb Haemost (2010) 8(10):2328–32. doi: 10.1111/j.1538-7836.2010.04006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. May F, Hagedorn I, Pleines I, Bender M, Vogtle T, Eble J, et al. CLEC-2 Is an Essential Platelet-Activating Receptor in Hemostasis and Thrombosis. Blood (2009) 114(16):3464–72. doi: 10.1182/blood-2009-05-222273 [DOI] [PubMed] [Google Scholar]
- 208. Saka-Herran C, Jane-Salas E, Mari-Roig A, Estrugo-Devesa A, Lopez-Lopez J. Time-to-Treatment in Oral Cancer: Causes and Implications for Survival. Cancers (Basel) (2021) 13(6):1321. doi: 10.3390/cancers13061321 [DOI] [PMC free article] [PubMed] [Google Scholar]