Abstract
Measles infection, caused by the “Rubeola” virus is a highly contagious disease with outrageously fatal consequences. Initiating with a variety of symptoms including fever, cough, conjunctivitis, and runny nose, it can lead to more severe sequelae including sub-acute sclerosing pan-encephalitis which is a potentially fatal and serious complication of measles. The lackluster vaccination processes in underdeveloped areas of the world due to suboptimal immunization programs, scarce resources, and insufficient political constancy still leads to increased cases of measles and its complications. A variety of management programs including the use of several medications have been introduced according to the literature in order to counter this dreadful disease. In this review article, we focus on assessment of the previous literature and discussing the possible treatment modalities of this currently irremediable disease.
Keywords: measles, panencephalitis, rubeola, immunization, anti-viral drugs, immune modulators
Introduction
The Rubeola virus is the causative agent for a highly infectious disease known as Measles, which can spread by both direct contact and the air droplets. Historically, within the United States of America (USA) only, measles spread at a rate of 3 to 4 million cases per year. 1 Due to a high mortality rate, it caused 2.6 million deaths per year throughout the world in the pre-vaccination era. 2 Since vaccination was introduced in the 1960s, infection rates have reduced substantially but this decrease has not been worldwide due to a lackluster vaccination campaign owing to suboptimal immunization programs, scarce resources, and insufficient political constancy amongst a host of other factors.
A typical acute febrile measles infection leads to fever, cough, conjunctivitis, coryza, and a distinguishing rash which can lead to complications in multiple organ systems such as pneumonia, otitis media, and the worst of all—encephalitis. Sub-acute Sclerosing Pan-encephalitis, abbreviated as SSPE, is a slow onset disease that affects younger population, most commonly manifesting in children that contract the measles infection before the age of 5. 3 SSPE has a debilitating progression that most often turns fatal. It is known to be caused by a mutation of measles that was first found from the brains of SSPE patients in 1969.4,5 Documented history for SSPE stretches as far back as 1935 when Dawson first identified the pathology in 2 children giving the disease its original name of “Dawson’s encephalitis.”6,7 Children in regions with high human immunodeficiency virus (HIV) positivity and incomplete vaccination coverage are the ones most at risk for developing this progressively deteriorating condition. 8 Clinical manifestation occurs approximately after a decade from the initial measles infection with a clinical window lasting 1 to 3 years but can also lead to a rapid death known as fulminant SSPE.9-11 In a review by Garg et al 3 diagnosis is confirmed on “Demonstration of elevated measles antibody titers in cerebrospinal fluid (CSF).”
Vaccination can prevent measles through vaccines such as combined measles-mumps-rubella (MMR). The currently available 2-dose strategy provides more than 98% protection against measles virus infection. 12 Even though current scientific evidences indicate that the MMR vaccine does not cause serious or permanent neurologic disorders, a substantial amount of controversy is still present in the lay press with emphasis on the safety of immunization.13,14 Despite the current advances in healthcare, currently there is no specific treatment regimen and protocol for this disease. All patients become bedridden as the disease progresses and the given care is mostly supportive. 15
Epidemiology
Currently, measles infections remain as large burden worldwide; especially in the resource-limited regions that are unable to carry out vaccination programs. The World Health Organization (WHO) reported close to 150,000 cases globally of measles infections in year 2020. Asia and Africa account for most of the cases with the incidence in Africa reaching over 115,000 cases in year 2020. In Table 1 we showed the total number of reported cases of measles globally. 16
Table 1.
Total Number of Reported Cases of Measles Globally in 2019 to 2020.
| Country | Number of reported measles cases | Year reported |
|---|---|---|
| Angola | 1085 | 2020 |
| Austria | 25 | 2020 |
| Bangladesh | 2410 | 2020 |
| Brazil | 20 901 | 2019 |
| Central African Republic | 3433 | 2020 |
| Canada | 1 | 2020 |
| China | 867 | 2020 |
| Democratic People’s republic of Korea | 0 | 2020 |
| Democratic Republic of Congo | 82 290 a | 2020 |
| Denmark | 4 | 2020 |
| Equatorial Guinea | 53 | 2020 |
| Ethiopia | 1952 | 2020 |
| France | 2637 | 2019 |
| Georgia | 20 | 2020 |
| Guinea | 506 | 2020 |
| Haiti | 0 | 2020 |
| India | 5604 | 2020 |
| Indonesia | 524 | 2020 |
| Italy | 103 | 2020 |
| Japan | 12 | 2020 |
| Kazakhstan | 3270 | 2020 |
| Kenya | 597 | 2020 |
| Lebanon | 15 | 2020 |
| Lithuania | 2 | 2020 |
| Madagascar | 777 b | 2020 |
| Malaysia | 478 | 2020 |
| Mexico | 196 | 2020 |
| Nepal | 388 | 2020 |
| New Zealand | 9 | 2020 |
| Nigeria | 8877 c | 2020 |
| Occupied Palestinian territory | 1001 | 2020 |
| Pakistan | 2747 | 2020 |
| Philippines d | 3832 | 2020 |
| Qatar | 3 | 2020 |
| Russian Federation | 1212 | 2020 |
| Rwanda | 108 | 2020 |
| Saudi Arabia | 35 | 2020 |
| Senegal | 212 | 2020 |
| Somalia | 2531 | 2020 |
| Thailand | 5412 | 2019 |
| Tunisia | 20 e | 2020 |
| Ukraine | 264 f | 2020 |
| United Kingdom | 95 | 2020 |
| United States of America | 1275 | 2020 |
| Uzbekistan | 4103 | 2020 |
| Vietnam | 846 | 2020 |
| Yemen | 298 | 2020 |
| Zambia | 237 | 2020 |
| Zimbabwe | 3 | 2020 |
Democratic Republic of Congo reported 333,017 measles cases in 2019.
Madagascar reported 213,231 measles cases in 2019.
Nigeria has 28,094 measles cases in 2019.
Philippines had 48,525 measles cases in 2019.
Tunisia had 4,669 measles cases in 2019.
Ukraine had 57,282 measles cases in 2019.
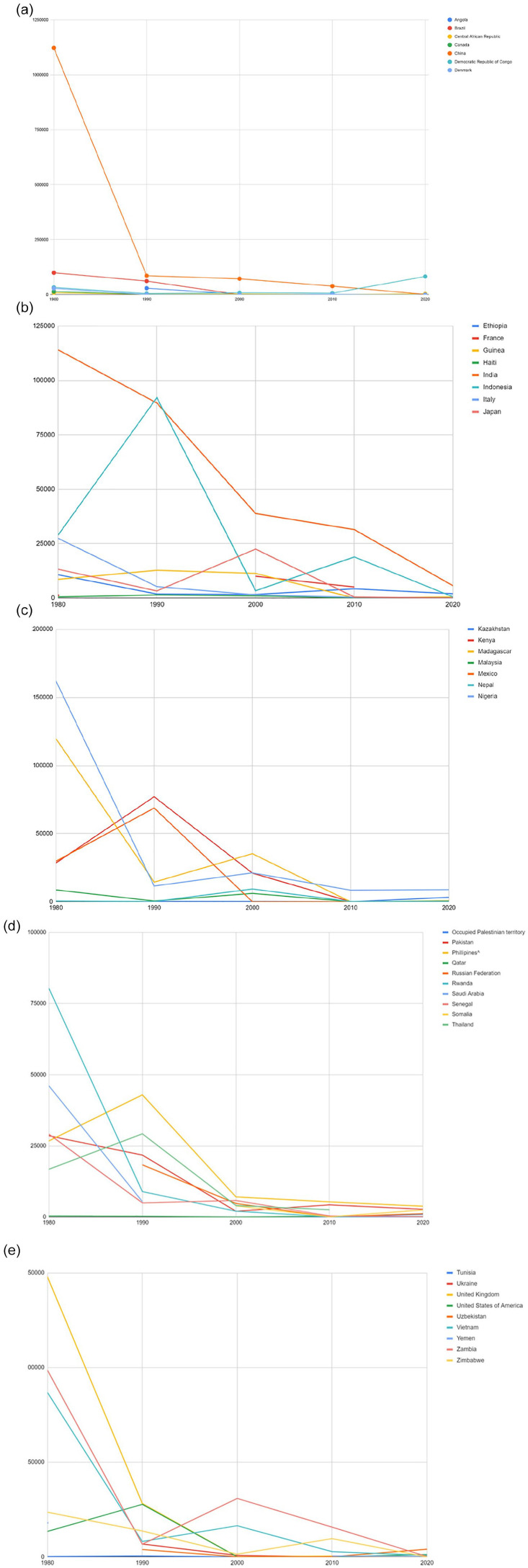
Trends in measles infections have fluctuated greatly. Most developed countries have encountered fewer cases over the decades while developing countries have had limited success in preventing epidemics. Figure 1a to e all represent trends over 5 decades for multiple countries across the world.
Figure 1.
(a-e) Cases of measles infection over 5 decades for multiple countries across the world. Note the downward trend of the cases due to immunization and awareness. Most developed countries have encountered fewer cases over the decades while developing countries have had limited success in preventing epidemics.
The incidence of SSPE on the other hand is much less tracked and documented. A WHO report states that the global incidence of SSPE is about 4 to 11 cases per 100,000 measles cases’ risk of developing SSPE to approximately 18 per 100,000 measles cases. Incidence is quite raised, up to “27.9 patients per 100,000 cases of measles for Lower- and Middle-Income Countries (LMICs).” 17 Papua New Guinea, Turkey, Pakistan, and India seem to be the top contributors to global SSPE cases. 3 Within 1992 and 2001, 114 confirmed cases were registered from Northern India in 1 tertiary center. 18 Four hundred fifty-eight identified SSPE cases were registered over 10 years (1996-2005), in a different North Indian tertiary care center. 19 Between 2000 and 2012, 43 cases of SSPE were confirmed from Karachi, Pakistan. 20 Developing countries are more prone to SSPE due to a variety of factors including high population density, poverty, low parental education, and older mothers. 22
In the western hemisphere and other developed countries, SSPE incidence is low due to successful vaccination campaigns eradicating measles in most areas. Most SSPE cases here can be attributed to measles outbreaks. A population study in Germany found a ratio of 1:1700 to 1:3300 for children likely to develop SSPE that contracted measles before an age of 5. However, the probability is 1.7 folds for children infected by measles under 3 years old compared to under 5. 23 Furthermore, if measles was contracted before 5 years of age the ratio was reported as 1:1367. While if there was a measles infection in infancy (<1 year of age), it was 1:609, a study from California shows. 9
Treatment
SSPE is a severe life-threatening illness making up one of the causes behind a higher rate of mortality worldwide. Although there are several medications for treating this disease, only quite a few have good results. There is no current permanent cure for SSPE and no surgical treatment as well. As the disease results in death in almost every single case, the treatment is mostly symptomatic and palliative. 24 Death commonly ensues within 4 years however it can be prolonged to an extent using medications. 25
Anti-Virals
As of today, no antiviral therapies which are clinically beneficial are available for the treatment of measles. Yet, a few case reports suggest that aerosolized or intravenous ribavirin might prove to be beneficial in a complicated course of the disease. 26 |The first antiviral agent used to treat SSPE is Isoprinosine (inosine pranobex). It brings about its anti-viral effects by stimulating immunity. Since it is expensive it is not widely available where the disease is prevalent. 3 Treating with Isoprinosine can lead to occasional nausea and raised serum as well as urinary uric acid. However, there are no side effects reported. 15 In a study, patients were treated with Isoprinosine, demonstrated an increase in life span for over 2 years. 3 Isoprinosine is also known to prolong survival of patients suffering from SSPE, the 8-year survival rate wars 61% in patients who were given Isoprinosine as compared to only 8% in patients who were not administered Isoprinosine. 21 Within a trial not comprising of a concurrent placebo control group, 35% of subjects who were administered Isoprinosine were stabilized or their condition was substantially better at a much higher rate than a rate of only 5% to 10%. 27
Several studies that evaluated the use of Isoprinosine in curing SSPE and their results are mentioned in Table 2.
Table 2.
Results of Studies3,21,24,27 That Were Conducted to Evaluate the Effects of Isoprinosine on Patients Having SSPE.
| Study name | Author (s) | Location | Study type | Result (s) |
|---|---|---|---|---|
| Subacute sclerosing panencephalitis 3 | Ravindra Kumar Garg, Anita Mahadevan, Hardeep Singh Malhotra Imran Rizvi, Neeraj Kumar, Ravi Uniyal | India | Review | Increased survival for over 2 years in treatment with Isoprinosine |
| Subacute sclerosing panencephalitis (SSPE) the story of a vanishing disease 24 | Natan Gadoth | Israel | Review | Long-term administration of Isoprinosine is safe and lacks significant adverse side effects |
| Advances in Antiviral Therapy for Subacute Sclerosing Panencephalitis 21 | Koichi Hashimoto, Mitsuaki Hosoya | Japan | Review | The 8-year survival rate of patients who received Isoprinosine was 61% as compared to a survival rate of only 8% seen in patients who did not receive this treatment |
| Measles, mumps, rubella, and human parvovirus B19 infections and neurologic disease 27 | James F Bale Jr | USA | Review | 35% of Isoprinosine-treated subjects stabilized or improved at a rate substantially higher than the historical remission rates of 5% to 10% |
Lamivudine and Ribavirin are nucleoside analogs also used for treating SSPE. Ribavirin possesses inhibitory properties against RNA viruses. Commonly it is used together with interferon-α and Isoprinosine through intraventricular, intravenous, or oral routes in doses of 40 to 60 mg/kg/day. 3 The usual oral dose of lamivudine is 10 mg/kg/day. 15 Ribavirin is known to provide clinical improvement, although partially. By maintaining CSF levels through a continuous subcutaneous infusion, Ribavirin is known to provide clinical benefit. 25 Results have shown that through the intraventricular mode of administration of Ribavirin, it is highly beneficial to treat SSPE as it keeps up a constantly high level of the drug in the CSF.
Thus, Intraventricular Ribavirin therapy is a possible treatment modality for RNA viruses causing encephalitis. 28 However, a study has shown that the oral administration of Ribavirin does not affect SSPE patients, mainly because the concentration required to deliver a therapeutic effect were not reached within the CSF. 21 In a limited number of random trials a little beneficial effect is shown when used together with Thymic Humoral Factor, Amantadine, steroids, and plasmapheresis intraventricular IFN and anti-CD 20 antibodies. 24 CSF measles antibody titers in SSPE patients are lowered by Ribavirin, decreasing neurological symptoms without any adverse effects. This effect is more noted when the drug is given in combination with IFN-α. The progression of SSPE is halted when Ribavirin and IFN-α are continuously administered intravenously via a subcutaneous infusion pump with combination or oral Inosine pranobex. Moreover, Remdesivir and subsequent active nucleosides can travel to the brain and hence possibly inhibit MeV (Measles morbillivirus) variants adapted by the CNS (Central nervous system) seen in MIBE (Measles inclusion body encephalitis) and SSPE cases. 29 Table 3 shows results of various studies conducted to evaluate the effect of ribavirin in curing SSPE.
Table 3.
Results of Various Studies25,26,28 Conducted to Evaluate the Effect of Ribavirin in Curing Cases of SSPE.
| Study name | Author (s) | Location | Study type | Result (s) |
|---|---|---|---|---|
| Subacute sclerosing panencephalitis: clinical phenotype, epidemiology, and preventive interventions 25 | Mohammed Mekki, Brian Eley, Diana Hardie, Jo M Wilmshurst | South Africa | Review | Ribavirin is associated with partial clinical improvement. Clinical benefit is brought through the maintenance of CSF levels with a subcutaneous continuous infusion mode of delivery. |
| Pharmacokinetics and effects of ribavirin following intraventricular administration for treatment of subacute sclerosing panencephalitis 28 | Mitsuaki Hosoya, Shuichi Mori, Akemi Tomoda, Kenji Mori, Yukio Sawaishi, Hiroshi Kimura, Shiro Shigeta, Hitoshi Suzuki | Japan | Clinical Trial | Intraventricular administration of ribavirin is effective against SSPE when CSF ribavirin concentration is maintained at a high level |
| Measles 26 | Paul A. Rota, William J Moss, Makoto Takeda, Rik L de Swart, Kimberly M Thompson, James L Goodson | USA | Review | Aerosolized or intravenous ribavirin provides some benefit in severe disease |
Immunomodulators
Interferon-α is known to have an immunomodulatory effect against a good number of illnesses. Interferon-β is extracted from fibroblasts that have been infected by the virus and by recombinant deoxyribonucleic acid (DNA) technology for clinical use. The Parenteral treatment of interferon-β treatment is typically combined with oral inosiplex. 3 The best route for delivery is through intraventricular route into the cerebrospinal fluid circulation because it spreads poorly through the blood-brain barrier. The typical dose is from 100,000 to 1,000,000 U/m2 given via the intraventricular route 2 to 5 times a week or daily. Intraventricular interferon-alpha is the only treatment protocol that affects interleukin-1 receptor antagonist levels within the cerebrospinal fluid. However, more research is required in the future that can document the patient data more effectively. 30 Although it is effective for treatment, IFN-α has some notable side effects, including chemical meningitis and loss of appetite. Moreover, interferonopathy as an impact on the central nervous system has also been reported. Intraventricular IFN and oral IP have not shown adverse effects, but long-term treatments carry the risk of upper and lower motor neuron toxicity developing, IFN α-induced encephalopathy and meningitis. 21 Oral Isoprinosine combined with Intrathecal IFN-α is the most common treatment for SSPE nowadays. 29 However, in a study where intrathecal IFN- in combination with Isoprinosine was administered and considered to be of benefit by most, however due to the presence of severe side effects to intrathecal administration was a huge downfall for this mode of treatment. 24 IFN-β has also shown similar benefits, especially when delivered with Isoprinosine subcutaneously 3 times weekly. 25 Currently, intravenous immunoglobulin and steroids are not a part of the routine treatment for SSPE. 15
Despite a wide range of medication being used in SSPE, with ribavirin, lamivudine, inosine, pranobex, and interferon (α) being the most frequently used in routine clinical practice. 15 Yet, a great majority of patients succumb to the disease within 5 years of onset. 27 There are multiple studies done using the medications mentioned above in combination with each other and isolation as well. In a case-control study involving children who were given a combination of oral lamivudine (10 mg/kg/day) and oral Isoprinosine (100 mg/kg/day), subcutaneous a-IFN 2a (10 mU/m2/5 times weekly), for 6 months, and a control group who were not given antiviral therapy (n = 13 children), the remission rate was 36.8% compared to 0% in the control. 25 Another study suggests that Isoprinosine together with intraventricular interferon or when given in isolation as well did not have an impact on the prognosis in long-term follow-ups. 10
Symptomatic Treatment
Anti-convulsants have been used to control myoclonic jerks. These included, Clobazam, Carbamazepine, and Levetiracetam. Carbamazepine was used the most, however it did not affect neurological deterioration. 3 Antiepileptic drugs such as clonazepam, sodium valproate, and Benzodiazepines can improve the myoclonus. Baclofen can also be given as an anti-spastic. 25 Children co-infected with SSPE and HIV are challenging to treat as Antiretroviral drugs and non-nucleoside reverse transcriptase inhibitors might cause virological failure when they combine with carbamazepine. Hence, “they require careful monitoring of carbamazepine levels and HIV viral titers.” 25
Alternative Treatment
Interventions with drugs such as intravenous immunoglobulin, plasmapheresis, and corticosteroids, amantadine, and cimetidine have yielded varying results. Antineoplastic AS2-1 has failed to alter the natural course of SSPE in an impactful manner. 31 Also, in another study; a tentative treatment with Antineoplastic AS2-1 was carried out among 16 patients having subacute sclerosing panencephalitis (SSPE). Even though progression was observed of the disturbances of higher nervous functions, there was less improvement in motor functions. The treatment did not affect anti-measles antibodies levels, MRI results, EEG, and anti-measles. None side effects were observed. 32 Adding on, a study found that, corticosteroids, intravenous immunoglobulin, amantadine, Interferon (including intraventricular therapy with interferon-a2b) also do not improve the course of SSPE. 27 Some individuals who received 2 vaccination doses after first infection developed SSPE, implying that the vaccine may not work as a therapeutic treatment or prevent encephalitis in this circumstance. 29
Supportive Care and Management
In uncomplicated cases of measles, therapies are mostly focused on supportive needs. They include “antipyretics, antitussives, hydration, and/or environmental controls (eg, humidification).”25,26 In order to the suppress myoclonic jerks and/or epileptic seizures, most patients require supportive treatment in addition to antiviral medications. Clobazam, Carbamazepine, and levetiracetam, are the drugs administered by majority centers, while on the other hand topiramate, oxcarbazepine phenobarbital, and lamotrigine, are applied less frequently. 33
A neurokinin-1 receptor antagonist, Aprepitant is approved for the treatment of chemotherapy-induced nausea. The drug was safe and well-tolerated in its first clinical trial, in patients with subacute sclerosing panencephalitis. Null clinical effect was demonstrated. Slight improvement in EEG findings can be a reason to conduct trials for longer periods as EEG changes are known to precede clinical findings in subacute sclerosing panencephalitis. 34
Future Treatments
Fusion inhibitor peptides (compound AS-48), that can combine to the viral fusion proteins are being developed as future treatment modalities. They suppress membrane fusion mediated by hyperfusogenic measles virus by binding to the site that bridges the stalk and the head of the “F” protein, where substitutions in an amino acid within the virus isolate. However, these forms of treatment need to be closely evaluated before becoming a part of the routine management of SSPE.25,26 A few fusion/entry inhibitors and RNA synthesis are known to have compounds with anti-SSPE or anti-measles activity. In vitro, the anti-measles virus compound 16,677 and its analogs show significant anti-measles virus activity. Molecules created from neutralizing anti-bodies, such as single-strand variable fragments that attack the H protein, could be a potential treatment for SSPE. Favipiravir, an RNA-Polymerase inhibitor, could potentially be exploited for the treatment of SSPE in the future. 21 Interferon-Stimulating Genes (ISGs) and a variety of other therapy methods have ben tried to treat SSPE, but their efficacy has to be confirmed because it appears to be case-dependent. Table 4 shows results of various studies related to the future treatments of SSPE including fusion inhibitor peptides, RNA polymerase inhibitors, and Neutralizing antibody-derived molecules.
Table 4.
Results of Various Studies3,21 Related to the Future Treatments of SSPE Including Fusion Inhibitor Peptides, RNA Polymerase Inhibitors and Neutralizing Antibody-Derived Molecules.
| Study name | Author (s) | Location | Study type | Result (s) |
|---|---|---|---|---|
| Subacute sclerosing panencephalitis 3 | Ravindra Kumar Garg, Anita Mahadevan, Hardeep Singh Malhotra Imran Rizvi, Neeraj Kumar, Ravi Uniyal | India | Review | Fusion inhibitor peptides (compound AS-48) that bind to the viral fusion proteins are being developed as potential therapeutic agents |
| Advances in antiviral therapy for subacute sclerosing panencephalitis 21 | Koichi Hashimoto, Mitsuaki Hosoya | Japan | Review | An RNA-Polymerase inhibitor, Favipiravir could also be exploited for the treatment of SSPE in the future |
| Advances in antiviral therapy for subacute sclerosing panencephalitis 21 | Koichi Hashimoto, Mitsuaki Hosoya | Japan | Review | Neutralizing antibody-derived molecules such as single-strand variable fragments that target the H protein are possible candidates for the treatment of SSPE |
Conclusion
Through our review we were able to elaborate the sequalae of a preventable infection that is, measles, although in many part of the world measles has been eradicated largely due to vaccination but developing countries such as Pakistan, India, Bangladesh, etc. where healthcare is still sub-optimal complications like SSPE are more frequent and fatal, Since majority of research activities are concentrated in HMICs, there is a lack of research on conditions like SSPE and thus they remain far highly morbid and mortality conditions. Through our review we identified both the current and future treatment options of SSPE while discussing the presentation and geographic distribution of this fatal neurological manifestation of measles.
Acknowledgments
We would also like to acknowledge and thank Dr Hassan Abdullah Shakeel, MBBS from Nishtar Medical University Pakistan, for his contribution in this review article research project and for his valuable comments that greatly improved the manuscript.
Footnotes
Author Contribution: Shahzeb Ali Memon and Syed Shabbir Afzal: Wrote the initial draft of the manuscript, did a detailed literature review; Alaa Tukruna and Asma Tasnim Khan: Wrote the discussion and edited the manuscript, along with the final review and proofreading of the manuscript; Sameer Saleem Tebha: Researched the epidemiology with statistics and drafted the figures; Zain Ali Zaidi: Did detailed research on current and experimental future treatments and wrote the conclusion. All authors approved the final version for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval and Informed Consent: Ethical approval and/or informed consent was not required since the study did not enroll any human or animal subjects considering this was a review article.
ORCID iDs: Syed Shabbir Afzal  https://orcid.org/0000-0002-0753-5492
https://orcid.org/0000-0002-0753-5492
Sameer Saleem Tebha  https://orcid.org/0000-0001-8480-6148
https://orcid.org/0000-0001-8480-6148
References
- 1. CDC. History of measles. Centers for Disease Control and Prevention. Published November 5, 2020. Accessed June 2021. https://www.cdc.gov/measles/about/history.html [Google Scholar]
- 2. World Health Organization. Measles. 2019. Accessed June 2021. https://www.who.int/news-room/fact-sheets/detail/measles
- 3. Garg RK, Mahadevan A, Malhotra HS, Rizvi I, Kumar N, Uniyal R. Subacute sclerosing panencephalitis. Rev Med Virol. 2019;29:e2058. doi: 10.1002/rmv.2058 [DOI] [PubMed] [Google Scholar]
- 4. Horta-Barbosa L, Fuccillo DA, London WT, Jabbour JT, Zeman W, Sever JL. Isolation of measles virus from brain cell cultures of two patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1969;132:272-277. doi: 10.3181/00379727-132-34196 [DOI] [PubMed] [Google Scholar]
- 5. Payne FE, Baublis JV, Itabashi HH. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969; 281:585-589. doi: 10.1056/NEJM196909112811103 [DOI] [PubMed] [Google Scholar]
- 6. Dawson JR. Cellular inclusions in cerebral lesions of lethargic encephalitis. Am J Pathol. 1933;9:7-16.3. [PMC free article] [PubMed] [Google Scholar]
- 7. Dawson JR. Cellular inclusions in cerebral lesions of epidemic encephalitis. Arch Neurol Psychiatry. 1934;31:685-700. [Google Scholar]
- 8. Kija E, Ndondo A, Spittal G, Hardie DR, Eley B, Wilmshurst JM. Subacute sclerosing panencephalitis in South African children following the measles outbreak between. 2009;2011:713-718. doi: 10.7196/SAMJnew.7788 [DOI] [PubMed] [Google Scholar]
- 9. Wendorf KA, Winter K, Zipprich J, et al. Subacute sclerosing panencephalitis: the devastating measles complication that might be more common than previously estimated. Clin Infect Dis. 2017;65:226-232. doi: 10.1093/cid/cix302 [DOI] [PubMed] [Google Scholar]
- 10. Prashanth LK, Taly AB, Ravi V, Sinha S, Arunodaya GR. Adult onset subacute sclerosing panencephalitis: clinical profile of 39 patients from a tertiary care centre. J Neurol Neurosurg Psychiatry. 2006;77:630-633. doi: 10.1136/jnnp.2005.085829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. PeBenito R, Naqvi SH, Arca MM, Schubert R. Fulminating subacute sclerosing panencephalitis: case report and literature review. Clin Pediatr. 1997;36:149-154. doi: 10.1177/000992289703600306 [DOI] [PubMed] [Google Scholar]
- 12. Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin a treatment. Internet J Epidemiol. 2010;39(Suppl 1):i48-i55. doi: 10.1093/ije/dyq021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Institute of Medicine (US). Immunization Safety Review Committee: Immunization Safety Review: Vaccines and Autism. National Academies Press; 2004. [PubMed] [Google Scholar]
- 14. CDC. MMR vaccine safety studies. Published June 8, 2020. https://www.cdc.gov/vaccinesafety/vaccines/mmr/mmr-studies.html.
- 15. Kannan L, Garg SK, Arya R, Sankar MJ, Anand V. Treatments for subacute sclerosing panencephalitis. Cochrane Database Syst Rev. 2013;12:CD010867. doi: 10.1002/14651858.CD010867 [DOI] [Google Scholar]
- 16. World Health Organization. Measles – number of reported cases. Accessed July 29, 2021. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/measles—number-of-reported-cases
- 17. World Health Organization. Accessed June 2021. http://www.who.int/vaccine_safety/committee/reports/wer8102.pdf?ua=1
- 18. Mishra B, Kakkar N, Ratho RK, Singhi P, Prabhakar S. Changing trend of SSPE over a period of ten years. Indian J Public Health. 2005;49:235-237. [PubMed] [Google Scholar]
- 19. Sonia M, Lalit D, Shobha B, et al. Subacute sclerosing panencephalitis in a tertiary care centre in post measles vaccination era. J Commun Disord. 2009;41:161-167. [PubMed] [Google Scholar]
- 20. Ibrahim SH, Amjad N, Saleem AF, Chand P, Rafique A, Humayun KN. The upsurge of SSPE—a reflection of national measles immunization status in Pakistan. J Trop Pediatr. 2014;60:449-453. doi: 10.1093/tropej/fmu050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hashimoto K, Hosoya M. Advances in antiviral therapy for subacute sclerosing panencephalitis. Molecules. 2021;26:427-2021. doi: 10.3390/molecules26020427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zilber N, Kahana E. Environmental risk factors for subacute sclerosing panencephalitis (SSPE). Acta Neurol Scand. 1998;98:49-54. doi: 10.1111/j.1600-0404.1998.tb07377.x [DOI] [PubMed] [Google Scholar]
- 23. Schönberger K, Ludwig MS, Wildner M, Weissbrich B. Epidemiology of subacute sclerosing panencephalitis (SSPE) in Germany from 2003 to 2009: a risk estimation. PLoS One. 2013;8:e68909. doi: 10.1371/journal.pone.0068909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gadoth N. Subacute sclerosing panencephalitis (SSPE) the story of a vanishing disease. Brain Dev. 2012;34:705-711. doi: 10.1016/j.braindev.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 25. Mekki M, Eley B, Hardie D, Wilmshurst JM. Subacute sclerosing panencephalitis: clinical phenotype, epidemiology, and preventive interventions. Dev Med Child Neurol. 2019;61:1139-1144. doi: 10.1111/dmcn.14166 [DOI] [PubMed] [Google Scholar]
- 26. Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. Measles. Nat Rev Dis Primers. 2016;2:16049. doi: 10.1038/nrdp.2016.49 [DOI] [PubMed] [Google Scholar]
- 27. Bale JF., Jr. Measles, mumps, rubella, and human parvovirus B19 infections and neurologic disease. Handb Clin Neurol. 2014;121:1345-1353. doi: 10.1016/B978-0-7020-4088-7.00091-2 [DOI] [PubMed] [Google Scholar]
- 28. Hosoya M, Mori S, Tomoda A, et al. Pharmacokinetics and effects of ribavirin following intraventricular administration for treatment of subacute sclerosing panencephalitis. Antimicrob Agents Chemother. 2004;48:4631-4635. doi: 10.1128/AAC.48.12.4631-4635.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferren M, Horvat B, Mathieu C. Measles encephalitis: towards new therapeutics. Viruses. 2019;11:1017-2019. doi: 10.3390/v11111017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haspolat S, Anlar B, Köse G, Coskun M, Yegin O. Interleukin-1beta, interleukin-1 receptor antagonist levels in patients with subacute sclerosing panencephalitis and the effects of different treatment protocols. J Child Neurol. 2001;16:417-420. doi: 10.1177/088307380101600606 [DOI] [PubMed] [Google Scholar]
- 31. Sobczyk W, Piłkowska E, Iwińska-Buksowicz B. Ocena zastosowania antyneoplastonu AS2-1 w podostrym stwardniajacym zapaleniu mózgu [The evaluation of the use of antineoplaston AS2-1 treatment in subacute sclerosing panencephalitis]. Neurol Neurochir Pol. 1999;33:797-805. [PubMed] [Google Scholar]
- 32. Sobczyk W, Piłkowska E, Iwińska-Buksowicz B, Jakubowska T. Ocena bezpośrednich (wczesnych) wyników leczenia antyneoplastonem AS2-1 u chorych z podostrym stwardniajacym zapaleniem mózgu (SSPE) [Assessment of early treatment results with antineoplaston AS2-1 in subacute sclerosing panencephalitis]. Neurol Neurochir Pol. 1997;31:1147-1156. [PubMed] [Google Scholar]
- 33. Häusler M, Aksoy A, Alber M, et al. A multinational survey on actual diagnostics and treatment of subacute sclerosing panencephalitis. Neuropediatrics. 2015;46: 377-384. doi: 10.1055/s-0035-1564618 [DOI] [PubMed] [Google Scholar]
- 34. Oncel I, Sancar M, Konuskan B, et al. Aprepitant in the treatment of subacute sclerosing panencephalitis: a randomized, double-blind, placebo-controlled study. Pediatr Neurol. 2020;110:59-63. doi: 10.1016/j.pediatrneurol.2020.05.009 [DOI] [PubMed] [Google Scholar]