Abstract
Neuropathic injury is accompanied by chronic inflammation contributing to the onset and maintenance of pain after an initial insult. In addition to their roles in promoting immune cell activation, inflammatory mediators like secretory phospholipase A2 (sPLA2) modulate nociceptive and excitatory neuronal signaling during the initiation of pain through hydrolytic activity. Despite having a known role in glial activation and cytokine release, it is unknown if sPLA2 contributes to the maintenance of painful neuropathy and spinal hyperexcitability later after neural injury. Using a well-established model of painful nerve root compression, this study investigated if inhibiting spinal sPLA2 7 days after painful injury modulates the behavioral sensitivity and/or spinal dorsal horn excitability that is typically evident. The effects of sPLA2 inhibition on altered spinal glutamatergic signaling was also probed by measuring spinal intracellular glutamate levels and spinal glutamate transporter (GLAST and GLT1) and receptor (mGluR5, GluR1, and NR1) expression. Spinal sPLA2 inhibition at day 7 abolishes behavioral sensitivity, reduces both evoked and spontaneous neuronal firing in the spinal cord, and restores the distribution of neuronal phenotypes to those of control conditions. Inhibiting spinal sPLA2 also increases intracellular glutamate concentrations and restores spinal expression of GLAST, GLT1, mGluR5, and GluR1 to uninjured expression with no effect on NR1. These findings establish a role for spinal sPLA2 in maintaining pain and central sensitization after neural injury and suggest this may be via exacerbating glutamate excitotoxicity in the spinal cord.
Keywords: Neuropathic pain, nerve root injury, neuroinflammation, secretory phospholipase A2, spinal electrophysiology, excitotoxicity
Introduction
Neuropathic pain from neural tissue damage occurs in approximately 10% of the US population, 1 with the cervical dorsal nerve roots especially vulnerable to trauma.2–5 Nerve root compression is associated with robust neuroimmune activation cascades including increased production of inflammatory mediators and spinal glial activation that can initiate and maintain pain.6–14 Although increased inflammatory responses13–16 alter spinal neuronal hyperexcitability,11,14,17–21 much is still unknown about how inflammatory mediators promote chronic pain states.
Among the robust inflammatory mediators involved in spinal neuroimmune activation is the family of phospholipase A2 enzymes22,23 and, in particular, the cytosolic phospholipase A2 (cPLA2) and secretory phospholipase A2 (sPLA2) isoforms that contribute to the production of downstream inflammatory proteins like prostaglandins and leukotrienes.24–30 Both enzymatic isoforms hydrolyze the sn-2 ester bond of glycerophospholipids, releasing free fatty acids, like arachidonic acid (AA) and lysophospholipids, that act as secondary signaling molecules to enhance spinal nociceptive cascades.25,27 Despite being constitutively active, increased sPLA2 and cPLA2 expression occurs concurrent with immune activation early after neural injury.29,30 Secretory PLA2 is upregulated as early as 4 h after spinal cord injury. 23 Its direct spinal application in naive rats induces behavioral sensitivity as soon as within 1 h and microglial activation within 5 h, 31 suggesting that early increases in spinal sPLA2 may initiate pain and inflammation. In fact, inhibiting spinal sPLA2 immediately after painful root trauma prevents the onset of pain and spinal hyperexcitability. 21 Although spinal sPLA2 expression remains elevated 7 days after a painful root compression,13,32 it is unknown if inhibiting spinal sPLA2 at that late time, when spinal hyperexcitability and glial activation are already established32–34 can attenuate existing pain.
Elevated phospholipases generate bioactive lipid mediators that can further exacerbate spinal inflammation and potentiate nociceptive signaling.35–39 AA concentrations increase in pain states, including preferentially only in the dorsal horn ipsilateral to neural injury. 40 Moreover, inhibiting AA production by the cPLA2 inhibitor, AACOCF3, after chronic sciatic nerve constriction prevents the onset of pain. 25 Together, these findings suggest that inhibiting generation of bioactive lipids could provide pain relief; however, since both the sPLA2 and cPLA2 isoforms hydrolyze phospholipids, with cPLA2 implicated for primary AA production,25,41,42 it is unknown if inhibiting either or both isoforms attenuates neuropathic pain. 42 The extent of cross-talk between isoforms has only been probed in cultured mammalian renal mesangial cells and findings suggest sPLA2 may regulate cPLA2 generation of AA in a protein kinase C- and extracellular signaling kinases 1/2 (ERK1/2)-dependent manner. 43 Despite this relationship between isoforms, whether spinal sPLA2 regulates cPLA2 activity in persistent pain states is unknown.
Lipid mediators directly and indirectly contribute to neuronal hyperexcitability,17–20,44,45 including enhancing spinal glutamate excitotoxicity by increasing receptor activity and inhibiting glutamate uptake in glia.46–49 Increases in spinal AA concentration competitively bind to the glutamate-binding pocket of the transporter proteins glutamate aspartate transporter 1 (GLAST1) and glutamate transporter-1 (GLT1), reducing glutamate uptake with glutamate spillover into the extracellular synaptic space.49–52 Unlike glutamate transporters, AA and other lipid mediators have not been shown to directly bind to metabotropic or ionotropic glutamate receptors; therefore, altered expression of the metabotropic glutamate receptor 5 (mGluR5), N-methly-d-aspartate receptor 1 (NR1) or glutamate receptor GluR153,54 following AA or lipid mediator application may be due to indirect activation via spinal glial glutamate uptake disruption. Both decreased spinal GLT1 and increased spinal mGluR5 accompany pain and spinal neuronal hyperexcitability at day 7 after root injury.9,33 The concurrent increase in lipid mediator production through increased sPLA213,32 and hydrolytic activity may further contribute to this altered spinal glutamatergic signaling. Although immediate inhibition of spinal sPLA2 reduces spinal mGLUR5 transcript levels, but does not alter glutamate transporter expression 1 day after a painful nerve root injury, 21 it is unknown if inhibiting spinal sPLA2 later after painful injury restores altered spinal glutamate signaling or reduces pain and/or hyperexcitability.
Since sPLA2 modulates pain, neuronal hyperexcitability,22,55 and the inflammatory cascades implicated in central sensitization after neuropathic injury,23,24,31,56 this study investigated if inhibiting spinal sPLA2 with thioetheramide-PC (TEA-PC) at day 7 after a painful root compression in the rat reduces existing behavioral sensitivity and/or spinal hyperexcitability14,33,57 via altered spinal glutamatergic signaling. Although sPLA2 expression increases at 1 day after painful root compression and remains elevated through day 7,13,32 spinal sPLA2 activity persistence has not been measured. Therefore, spinal sPLA2 activity was first measured at day 7 after a painful root compression to establish its relevance as a target.
Although immediate intrathecal TEA-PC administration prevents increased spinal sPLA2 activity at day 1, 21 the duration of active inhibition is not known nor are the effects when given after spinal neuroimmune responses and pain are established (at day 7).13,34,58 Furthermore, the effects of spinal sPLA2 inhibition by TEA-PC at that dose on the catalytic activity of the cPLA2 isoform and glutamate signaling that is involved in establishing pain and spinal hyperexcitability59-62 have not been measured for later times. Understanding if and/or how spinal sPLA2 inhibition mediates these processes that maintain pain later after neuropathic injury may provide useful insights into how to effectively treat neuropathic pain once established.
Materials and methods
Surgical procedures and inhibitor treatment
All procedures were approved by our institution’s Institutional Animal Care and Use Committee and performed under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. 63 Spinal sPLA2 activity at day 7 after a painful nerve root compression was first characterized using two groups of rats (377 ± 21g) undergoing either a painful nerve root compression (NRC; n = 4) or a sham surgery (sham; n = 3) to serve as surgical controls. Under inhalation anesthesia (4% induction, 2% for maintenance) male Holtzman rats underwent a unilateral C7 nerve root compression (NRC) that induces behavioral sensitivity.21,32,33,57,64 Briefly, following a midline incision between the C2 and T2 vertebrae, a C6/C7 hemilaminectomy and partial facetectomy were performed on the right side to expose the C7 right dorsal nerve root. A 10-gf microvascular clip (World Precision Instruments; Sarasota, FL) was inserted through a small incision in the dura and placed around the nerve root to apply compression (NRC) for 15 min.14,21,32,33 Rats undergoing a sham surgery received identical surgical procedures with the nerve root exposed but not compressed. After each procedure, surgical wounds were closed using 3–0 polyester sutures and surgical staples and monitored while rats recovered in room air.
To assess the effectiveness of spinal sPLA2 inhibition against established neuropathic pain, in a second set of rats (452 ± 38 g) separate groups received an intrathecal injection of either the sPLA2 inhibitor TEA-PC (NRC+sPLA 2 inhibitor n = 8) or a vehicle injection of phosphate buffered saline (PBS) (NRC+vehicle n = 8) 7 days after a painful nerve root compression. Sham procedures were also included to control for the effects of surgery and intrathecal administration; that additional group of rats received a sham surgery in which the nerve root was exposed but not compressed, and an intrathecal injection of PBS was administered at day 7 following surgery (sham+vehicle n = 4).
Rats underwent either a painful nerve root compression or sham exposure and on post-operative day 7, rats were randomly assigned to receive an intrathecal injection of either TEA-PC or PBS. All drug solutions were administered via syringe in the intrathecal space between L4 and L5 via a lumbar puncture to the rats under anesthesia.14,33 The treatment group (NRC+sPLA 2 inhibitor n = 8) received a single 40–60 µL injection administered slowly over 1–2 min of the sPLA2 inhibitor TEA-PC (0.25 mg/mL) dissolved in PBS. In the vehicle treatment group (NRC+vehicle n = 8), rats received a 40–60 µL injection of PBS, corresponding to the weight-matched volume of the inhibitor administration. The same vehicle treatment was administered to rats that underwent sham procedures (sham+vehicle n = 4).
Behavioral sensitivity measurements
To assess if inhibiting spinal sPLA2 at day 7 after a painful nerve root compression attenuates established behavioral sensitivity, mechanical hyperalgesia in response to mechanical stimulation was measured in the forepaw ipsilateral to the injury. 65 Behavioral sensitivity was measured prior to any surgical procedures on day 0 (baseline) and daily thereafter through day 14. During testing, the plantar surface of the forepaw was stimulated by a series of weighted von Frey filaments of increasing strengths (1.4–26 g) to identify the withdrawal threshold to forepaw stimulation.14,21,66 An observer, blinded to each group, applied each filament five times before advancing to the next highest strength and if two consecutive filaments both induced a withdrawal response exhibited by licking or lifting of the paw, the lower strength filament was recorded as the withdrawal threshold. On each assessment day, rats were acclimated to the testing environment and tester for at least 15 min, followed by three rounds of testing with 10 min of rest between each round. Paw withdrawal thresholds were averaged across rounds for each rat on each day. Differences in the ipsilateral forepaw thresholds between groups were compared using a two-way repeated measures ANOVA with post-hoc Tukey’s test.
Spinal phospholipase activity rate assay
In the first set of rats, spinal cord tissue was harvested at day 7 (NRC n = 4; sham n = 3) to evaluate sPLA2 activity after injury. In the second set of rats (NRC+sPLA 2 inhibitor n = 8; NRC+vehicle n = 8; sham+PBS n = 4), spinal cord was assayed at day 14 to evaluate whether the sPLA2 inhibitor stopped sPLA2 cleavage activity. Spinal sPLA2 activity was measured in the ipsilateral C6 spinal cord using a sPLA2 Activity Kit (Cayman; Ann Arbor, MI) as previously described. 21 Rats were transcardially perfused with 250 mL of chilled PBS. Spinal tissue was then homogenized in 50 mM HEPES buffer, pH 7.4 and centrifuged at 10,000g for 15 min at 4°C. Supernatant was removed and incubated with both cPLA2 and iPLA2 inhibitors to isolate sPLA2 activity, and then incubated with the substrate arachidonoyl thio-PC, at room temperature as per assay instructions. In the same set of rats (NRC+sPLA 2 inhibitor n = 8; NRC+vehicle n = 8; sham+PBS n = 4), spinal cPLA2 activity was assessed at day 14 using a cPLA2 Activity Kit (Cayman; Ann Arbor, MI). 67 Similar to the sPLA2 activity assay, homogenized samples were centrifuged at 10,000g and the supernatant was incubated with sPLA2 and iPLA2 inhibitors to isolate cPLA2 activity before incubation with the arachidonoyl thio-PC substrate at room temperature for 1 h in assay buffer.
For both sPLA2 and cPLA2 activity assays, fluorescent measurements were made every minute over 10 min using a fluorescent plate reader (Tecan InfinitePro; Mannedorf, Switzerland). The reaction rate for each of sPLA2 and cPLA2 activity for each sample was separately calculated by measuring absorbance readings over time after subtracting absorbances from non-enzymatic controls. Rates of sPLA2 activity on day 7 were compared separately between NRC and sham groups using a Student’s t-test. For spinal tissues on day 14, sPLA2 and cPLA2 activity rates were separately compared between inhibitor- and vehicle-treated groups using a one-way ANOVA (group) with post-hoc Tukey’s test.
Spinal intracellular glutamate measurements
Intracellular glutamate was also assayed in the ipsilateral C6 spinal cord tissue of rats in the inhibitor- (NRC+sPLA 2 inhibitor n = 8) and PBS-treated (NRC+vehicle n = 8; sham+PBS n = 4) groups using the tissues homogenized for the phospholipase A2 activity assays at day 14. Using a Glutamate Glo Assay Kit (Promega; Madison, WI) as described, 59 homogenized spinal tissue was further permeabilized (1% Triton X-100, 500 mM NaCl, 5 mM HEPES, pH 7.4) and combined with a luciferin detection solution consisting of glutamate hydrogenase, NAD, reductase, and the reductase substrate for 1 h. 59 Luminescence readings were then collected on a fluorescent plate reader (Tecan InfinitePro; Mannedorf, Switzerland) with the extent of luminescence corresponding to the concentration of glutamate in the sample. Relative luminescence in spinal tissue samples was compared across the inhibitor-treated (NRC+sPLA 2 inhibitor n = 8) and PBS-treated (NRC+vehicle n = 8; sham+PBS n = 4) groups using a one-way ANOVA (group) with Tukey’s post-hoc test.
Spinal electrophysiological recordings
On day 14 before the tissue harvest, electrophysiological recordings of the spinal neurons were acquired from the C6–C7 dorsal horn on the side ipsilateral to the surgery from rats in the inhibitor-treated (NRC+sPLA 2 inhibitor n = 8) and PBS-treated (NRC+vehicle n = 8; sham+PBS n = 4) groups. For electrophysiology recordings, rats received an intraperitoneal (i.p.) injection of sodium pentobarbital (45 mg/kg) and anesthesia was maintained with supplementary doses (5–10 mg/kg i.p.) for the duration of the recording procedures.33,68,69 Rats also received a tracheotomy and were connected to a mechanical ventilator (40–50 cycles/min) and CO2 monitor (CWE, Inc.; Ardmore, PA). Prior to recording, the spinal cord was exposed and the dura around the C6/C7 spinal levels was removed to allow insertion of the recording electrode. Rats were immobilized on a stereotactic frame (David Kopf Instruments; Tujunga, CA); core body temperature was maintained at 35–37°C using a heating plate and monitored using a rectal probe (Physiotemp; Clifton, NJ).
Extracellular voltage potentials were recorded using a glass insulated tungsten electrode (FHC; Bowdoin, ME) that was lowered into the superficial (I–II) and deep laminae (III–IV) of the spinal cord via a micropositioner.4,5,12-14 Signals were digitally sampled at 25 kHz (Micro1401; CED; Cambridge, UK) and recorded using Spike2 software (CED; Cambridge, UK).33,68,69 Mechanosensitive neurons were identified by brushing the plantar surface of the forepaw;33,68–70 following identification, a series of non-noxious and noxious mechanical stimuli was applied to the forepaw ipsilateral to the injury consisting of a light brush, von Frey filaments (1.4, 4, 10, and 26 g) and a noxious pinch (60 g vascular clip).33,68–70 Before any stimulus was applied to the forepaw there was a 2-s baseline period, stimulation began with a light brushing (applied for 10 s), followed by application of a series of non-noxious and noxious von Frey filaments (1.4 g, 4 g, 10 g, and 26 g) each applied for five stimulations of 1-s followed by 1-s of recovery, and application of a noxious pinch (applied for 10-s), as previously described.33,68–70 In total, the non-noxious and noxious stimuli were applied for 30-s intervals.
Voltage recordings from each neuron were spike-sorted using Spike2 (CED; Cambridge, UK) to identify individual neurons. Both evoked and spontaneous activity were recorded since both types of neuronal activity are hallmarks of central sensitization.62,71,72 The number of evoked spikes was summed over the continuous 10-s stimulus period for each of the brush and pinch stimuli. For stimulation by the von Frey filaments, the number of evoked spikes was summed from the initial application of the filament until 1-s after the last application.33,68–70 For each evoked stimulus, the baseline firing in the 1-s period prior to stimulus application was quantified and subtracted from the total evoked spike count. Spontaneous activity was evaluated by totaling the number of spikes in the 2-s baseline period prior to the brush stimulation. 73 Neurons were classified based on their spontaneous activity during the baseline period before the application of the brushing: neurons exhibiting no baseline firing (0 spikes/sec), or active neurons exhibiting some level of spontaneous activity either at less than 1 spike/sec or at or more than 1 spike/sec. The distribution of spontaneously active neurons was compared between groups using a Pearson χ2 tests (4 × 3 contingency table) with Tukey post-hoc test.
Since a hallmark of central sensitization is an increase in the number of neurons responding to a wide range of inputs,68,70 each neuron for which recordings were made was phenotypically classified according to their evoked responses from von Frey stimulation. Wide dynamic range (WDR) neurons were taken as those that respond in a graded manner to stimuli of increasing strength; neurons were classified as low-threshold mechanoreceptive (LTM) neurons if they responded more to non-noxious filaments (1.4 g and 4 g) than noxious filaments (10 g and 26 g) and nociceptive specific (NS) neurons were those responding only to noxious filaments. 73 The evoked spike counts and spontaneous activity were also analyzed separately for the WDR and NS neuronal populations since both of these phenotypes respond differently to prolonged noxious stimulation having varied contributions to central sensitization.74,75 Evoked responses were compared between groups using a two-way nested ANOVA (group × stimulus), with neurons nested within rats and rats nested within groups, with Tukey post-hoc test.33,68–70 Pearson χ2 tests (4 × 3 contingency table) with Tukey post-hoc test evaluated differences in the distribution of neuron phenotypes in each group as well as the differences in distribution of spontaneously firing neurons in each group, separately for each neuron phenotype.
Spinal western blot and analyses
The C7 and C8 spinal cord tissue ipsilateral to procedures was collected on day 14 from the inhibitor-treated (NRC+sPLA 2 inhibitor n = 8) and PBS-treated (NRC+vehicle n = 8; sham+PBS n = 4) groups to assess glutamate transporter (GLT1, GLAST) and glutamate receptor (mGluR5, GluR1, NR1) expression. Spinal cord tissue from a normal unoperated rat (n = 1) was included in each blot to serve as a control. Tissue was homogenized in lysis buffer (50 mmol/L TrisHCl, 1% Triton X-100, 150 mmol/L NaCl, 1 mmol/L ethylenedia-minetetraacetic acid (EDTA, pH 8.0)) in the presence of protease and phosphatase inhibitors (Sigma-Aldrich Corp.; St Louis, MO).69,70 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed by loading spinal cord protein (37 µg per well) on a polyacrylamide gel (Invitrogen; Carlsbad, CA) and running for 75 min at 150 V. Protein was transferred from the poly-acrimide gel onto a polyvinylidene difluoride (PVDF) membrane using an iBlot (Invitrogen; Carlsbad, CA). Gels were divided based on their high-molecular weight (75–150 kDa; mGluR5, NR1, GluR1) or low-molecular weight (25–75 kDa; GLT1, GLAST) protein targets.
Membranes were then blocked for 1 h using an Intercept Blocking Buffer (Li-Cor Biosciences; Lincoln, NE) and incubated overnight at 4°C with primary antibodies for the secondary signaling molecule, the ionotropic glutamate receptors NMDA subunit NR1 (rabbit, 1:1000; Millipore; Billerica, MA) and GluR1 (rabbit, 1:1000; Millipore; Billerica, MA), the metabotropic glutamate receptor mGluR5 (rabbit, 1:1250; Millipore, Billerica, MA), the astrocytic glutamate transporters GLAST (rabbit, 1:2000; Abcam, Cambridge, MA) and GLT1 (rabbit, 1:500, Abcam, Cambridge, MA), or β-tubulin as a loading control (mouse, 1:2000; Covance, Princeton, NJ). The PVDF membrane was washed in tris-buffered saline (TBS) with 0.1% Tween, followed by a 2-h incubation at room temperature with goat anti-rabbit 800 and goat anti-mouse 680 fluorescent secondary antibodies (1:10,000; Li-Cor Biosciences, Lincoln, NE). Each membrane was imaged using an Odyssey Imaging System (Li-Cor Biosciences; Lincoln, NE). The fluorescence intensity of each target protein band was analyzed using the Odyssey 2.1 software and normalized to the corresponding β-tubulin fluorescence to control for the amount of protein loaded. Protein expression was calculated as fold-changes over corresponding protein levels in normal tissue and each protein of interest was compared between groups using separate one-way ANOVAs with a post hoc Tukey’s test.
Results
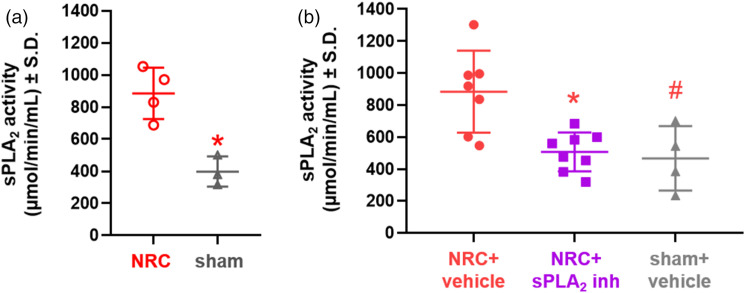
Spinal sPLA2 activity at day 7 after a painful nerve root compression (962.25 ± 175.30 µmol/min/mL) is significantly elevated (p = 0.005) over activity levels for a sham procedure (397.60 ± 193.96 µmol/min/mL) (Figure 1(a)).
Figure 1.
Spinal sPLA2 activity is elevated at days 7 and 14 after a painful nerve root compression and is attenuated at day 14 following spinal sPLA2 inhibition given at day 7. (a) Spinal sPLA2 activity at day 7 after a painful nerve root compression (NRC) is significantly elevated (p = 0.005) over sham control levels. (b) Inhibitor treatment at day 7 significantly reduces (*p = 0.026) spinal sPLA2 activity to sham levels on day 14. Spinal sPLA2 activity after NRC with vehicle treatment is also significantly higher (#p = 0.035) than for sham.
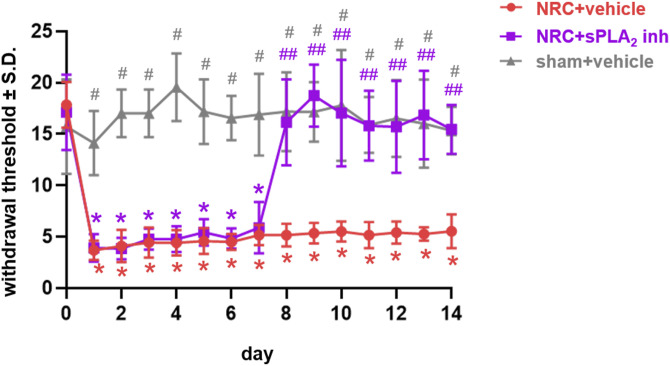
Inhibiting spinal sPLA2 7 days after a nerve root compression injury attenuates the behavioral sensitivity that is typically evident over 14 days after that injury (Figure 2). After nerve root compression, the withdrawal thresholds for the ipsilateral paw are significantly lower (p < 0.0001) than baseline (day 0) and corresponding sham thresholds (p < 0.0002) through day 7, and thresholds after a sham procedure are not different from baseline on any day tested (Figure 2). Following behavioral assessments on day 7, the withdrawal thresholds are significantly elevated (p < 0.0004) with inhibitor treatment over thresholds for the vehicle-treated group starting on day 8, returning to sham levels through day 14 (Figure 2). In contrast, thresholds with vehicle treatment on day 7 remain significantly lower than both their corresponding baseline (p < 0.0001) levels and the sham (p < 0.014) levels, as well as those for the inhibitor treatment (p < 0.0004) (Figure 2).
Figure 2.
Spinal sPLA2 inhibition at day 7 after painful nerve root compression abolishes existing sensitivity through day 14. Painful nerve root compression (NRC) significantly decreases withdrawal thresholds from baseline (*p < 0.0001) and relative to corresponding sham thresholds (#p < 0.0002) through day 7. Treatment with the PBS vehicle on day 7 does not alter withdrawal thresholds, which remain significantly lower than baseline (*p < 0.0001) and sham (#p < 0.014) through day 14. But, after intrathecal treatment with the sPLA2 inhibitor, thresholds are significantly elevated (##p < 0.0004) over thresholds for the vehicle treatment group and return to sham levels as early as day 8 lasting through day 14.
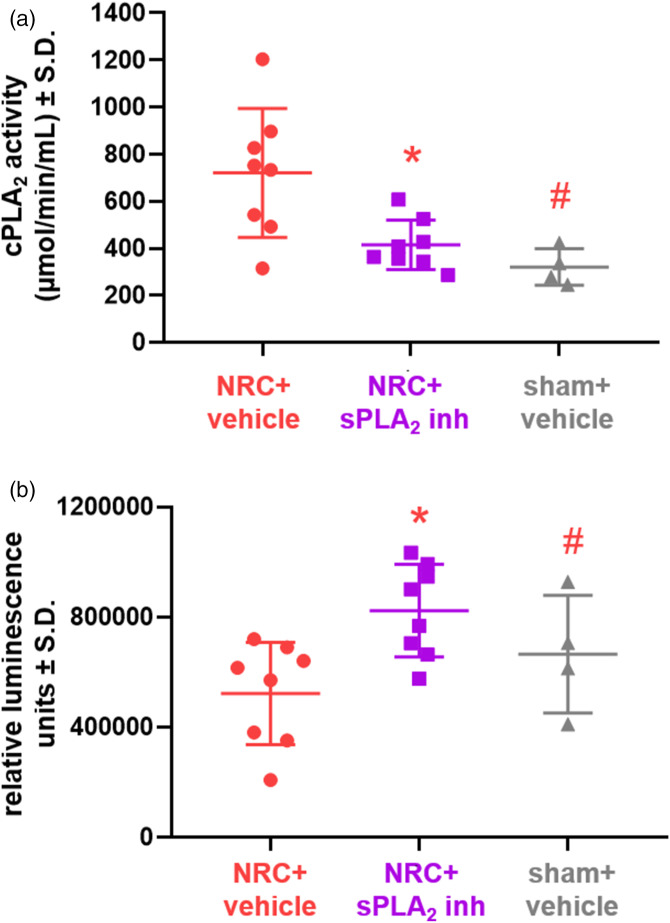
Both spinal sPLA2 and cPLA2 activity are modulated at day 14 after a painful root compression and a single intrathecal dose of TEA-PC at day 7 is sufficient to inhibit spinal sPLA2 and alter cPLA2 activity in the spinal cord (Figures 1 and 3). Spinal sPLA2 activity in the vehicle-treated NRC group is significantly higher (p = 0.035) than sham activity levels at day 14 (Figure 1(b)). However, spinal sPLA2 activity after inhibitor treatment at day 7 is reduced to sham levels and is significantly lower (p = 0.026) than corresponding levels in the vehicle-treated group (Figure 1(b)). Inhibitor treatment also brings spinal cPLA2 activity to sham levels (Figure 3(a)), which are significantly lower (p = 0.008) than the activity in the corresponding vehicle-treated injury group (Figure 3(a)). Spinal cPLA2 activity levels after NRC and vehicle treatment are also significantly elevated (p = 0.008) over those of the sham group. Spinal sPLA2 inhibition significantly increases (p = 0.006) spinal intracellular glutamate concentrations as compared to vehicle treatment (Figure 3(b)). Intracellular glutamate concentrations after vehicle treatment are also significantly lower (p = 0.022) than sham levels (Figure 3(b)). There are no differences in intracellular glutamate levels detected between the sham and inhibitor-treated NRC groups (Figure 3(b)).
Figure 3.
Intrathecal administration of the sPLA2 inhibitor reduces cPLA2 activity in the spinal cord and increases intracellular glutamate concentration after a painful nerve root compression (NRC). (a) Spinal cPLA2 activity in the vehicle treated group is significantly elevated over (#p = 0.008) sham activity levels at day 14; but, sPLA2 inhibition after NRC significantly reduces (*p = 0.018) spinal cPLA2 activity to sham levels. (b) Intracellular glutamate levels in spinal tissues after a painful NRC are significantly lower with vehicle treatment than for NRC with inhibitor treatment (*p = 0.006) and sham (#p = 0.022).
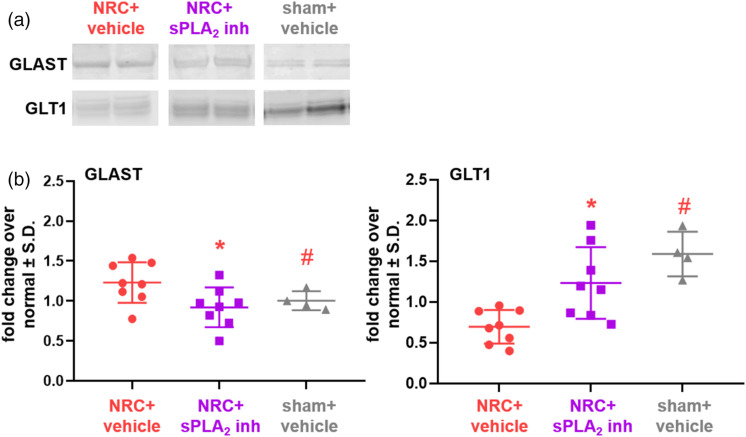
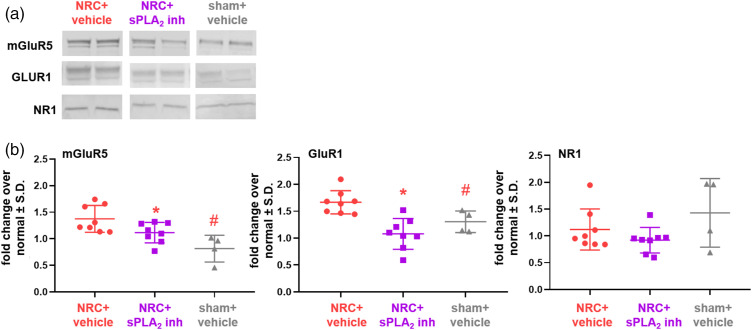
In addition to increasing intracellular glutamate concentration to sham levels (Figure 3(b)), spinal sPLA2 inhibition restores glutamate transporter expression and decreases expression of two of the glutamate receptors probed (Figures 4 and 5). Specifically, GLAST expression (60 kDa) is significantly lower (p = 0.034) in the inhibitor-treated group compared to the vehicle-treated group after NRC and is the same as sham levels, which are also significantly (p = 0.027) lower than the untreated group (Figure 4(b)). Those same groups track together for GLT1 (62 kDa) expression, with both the inhibitor-treated and sham control groups having significantly more (p < 0.0009) GLT1 than the group with NRC and vehicle treatment (Figure 4(b)). Of note, for treatment with vehicle, GLT1 expression in several samples is at normal levels at day 14 (Figure 4(b)). Expression of both the mGluR5 and GluR1 receptors is greater than normal levels with vehicle treatment (Figure 5) and is significantly higher (p = 0.01 mGluR5; p = 0.035 GluR1) than levels in the sham control group (Figure 5(b)). Spinal sPLA2 inhibition significantly reduces both mGluR5 (p = 0.002) and GluR1 (p = 0.0004) expression when compared to vehicle-treated levels (Figure 5(b)) and keeps them at sham levels. There were no differences in NR1 expression detected between groups.
Figure 4.
Spinal sPLA2 inhibition restores glial transporter expression at day 14 after nerve root injury. (a) Representative blots show spinal glutamate transporter expression for all groups. (b) Spinal GLAST expression with vehicle treatment after a painful root compression (NRC) is higher than levels of expression for sham (#p = 0.027), but inhibitor treatment after NRC significantly reduces (*p = 0.034) GLAST expression to sham levels. Conversely, vehicle treatment significantly downregulates GLT1 compared to sham control levels (#p < 0.0006), but GLT1 protein levels for NRC with inhibitor treatment are significantly (*p <0.009) higher than levels for untreated NRC and are not different from sham.
Figure 5.
At day 14, painful nerve root compression (NRC) increases mGluR5 and GluR1 expression that is reduced with spinal sPLA2 inhibition. (a) Representative blots show spinal mGluR5, GluR1, and NR1 expression. (b) Spinal sPLA2 inhibition significantly reduces both mGluR5 (*p < 0.01) and GluR1 (*p < 0.035) expression to sham levels, as compared to expression in vehicle-treated spinal cord. Whereas expression in the vehicle-treated NRC group for both receptors is significantly greater (#p = 0.002 mGluR5; #p = 0.0004 GluR1) than sham expression levels. NR1 expression is not different between any groups.
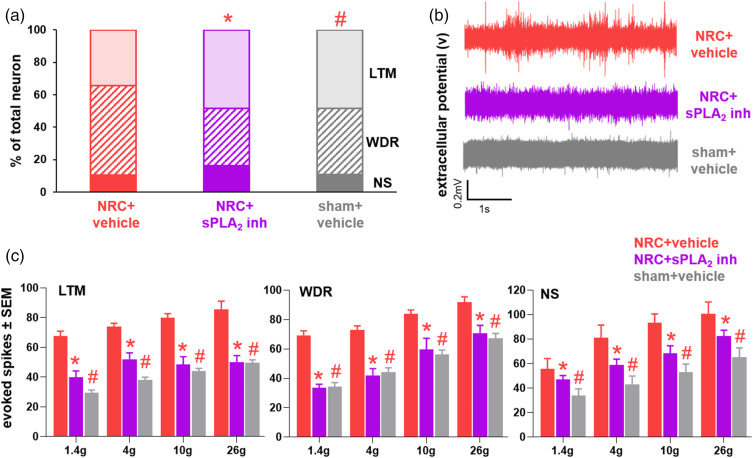
Recordings were made at day 14 from a total of 126 spinal neurons at an average depth of 442 ± 270 µm across all groups. Although a painful nerve root compression has 34.5% of the spinal neurons classified as LTM, 55.2% as WDR and 10.4% as NS at day 14, spinal sPLA2 inhibition restores the proportion of spinal neurons classified as WDR neurons to sham levels (Figure 6(a)). The distribution of neuronal phenotypes after inhibitor treatment is significantly different (p = 0.005) from the proportion of phenotypes in the vehicle-treated group (Figure 6(a)). The distribution of neuronal phenotypes in the vehicle-treated painful compression group is also significantly different (p = 0.027) from that of the sham group, with no differences observed in the distribution between the inhibitor-treated and sham groups (Figure 6(a)). In addition, spinal sPLA2 inhibition blocks the increase in evoked spikes for each neuronal phenotype that is usually evident and keeps activity at sham levels (Figure 6(b)). In particular, stimulation of the affected forepaw with both non-noxious (1.4, 4 g) and noxious (10 g, 26 g) filaments evokes significantly fewer spikes in the inhibitor-treated group and the nonpainful sham control compared to responses following a painful compression with vehicle treatment for all neuronal phenotypes, including LTM (p < 0.0005), WDR (p < 0.038), and NS (p < 0.029) (Figure 6(c)). In contrast, vehicle treatment with a painful root compression induces significantly more spikes (LTM p < 0.0007; WDR p < 0.039; NS p < 0.015) for each neuronal phenotype compared to the evoked responses in the sham group, for all filaments tested (Figure 6(c)). There are no differences in the evoked responses between the inhibitor-treated compression and sham groups for any filament.
Figure 6.
Spinal neuronal phenotype distribution and evoked activity at day 14 with a nerve root compression (NRC) are restored to sham levels with spinal sPLA2 inhibitor treatment at day 7. (a) The proportion of WDR neurons is greatest with for NRC with vehicle treatment and the distribution of LTM, WDR and NS neurons is significantly (#p = 0.027) different from sham and NRC with inhibitor treatment (*p = 0.005). Inhibitor treatment restores the phenotypic distribution to sham levels. (b) Representative voltage tracings from WDR neurons show inhibitor treatment reduces neuronal activity following 1.4 g stimulation as compared to vehicle. (c) sPLA2 inhibitor treatment significantly reduces evoked activity in all neuronal phenotypes (LTM *p < 0.0005; WDR *p < 0.038; NS *p < 0.029) compared to activity in the NRC with vehicle treatment, with no differences between the inhibitor treated and sham groups. Evoked activity in the vehicle-treated group is also significantly elevated (LTM #p < 0.0007; WDR #p < 0.039; NS #p < 0.015) for all filaments compared to sham for every neuronal phenotype.
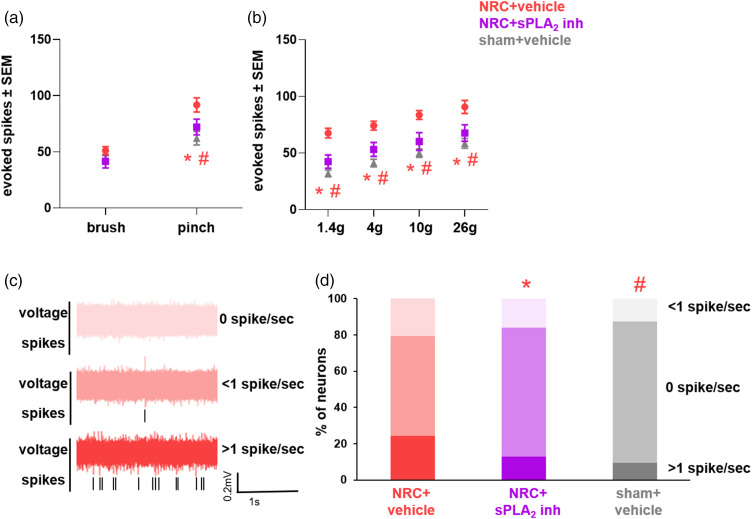
In addition to preventing the increases in activity and shift in the distribution of neuronal phenotypes (Figure 6), inhibiting spinal sPLA2 at day 7 also attenuates the overall dorsal horn neuronal hyperexcitability that is evident 14 days after a painful nerve root compression (Figure 7). Although there are no differences in neuronal firing evoked by the non-noxious light brush among groups (Figure 7(a)), neuronal activity elicited by a noxious pinch is significantly lower for the compression with inhibitor treatment at day 7 (p = 0.005) and sham (p = 0.0001) than the responses for the group undergoing a painful compression with vehicle treatment at day 7 (Figure 7(a)). Inhibitor treatment also significantly lowers (p < 0.026) evoked responses to sham levels in spinal dorsal horn neurons for stimulation with both the non-noxious and noxious filaments as compared to response of the vehicle-treated group (Figure 7(b)). The neuronal firing evoked in the group receiving compression with vehicle treatment at day 7 is significantly higher (p < 0.004) than that for the sham control group for all filaments tested (Figure 7(b)).
Figure 7.
Spinal sPLA2 inhibition at day 7 returns evoked and spontaneous dorsal horn activity to sham levels on day 14 after painful nerve root injury. (a) Differences in dorsal horn evoked activity are only observed after a noxious pinch, with both the inhibitor-treated (*p = 0.005) and sham (#p = 0.0001) groups exhibiting significantly fewer evoked spikes than the vehicle-treated nerve root compression (NRC) group. (b) Both non-noxious and noxious filament stimulation also elicit significantly more evokes spikes for NRC with vehicle treatment than sham (#p < 0.004); yet, inhibitor treatment significantly reduces (*p < 0.026) evoked activity to sham levels. (c) Example voltage traces from vehicle-treated neurons that display no baseline firing (0 spikes/sec) or active firing (either <1 or ≥1 spike/sec). (d) Spinal sPLA2 inhibition also restores the proportion of neurons that exhibit spontaneous activity to sham levels and is significantly different (*p = 0.038) than the proportion of neurons observed after NRC with vehicle treatment. At day 14, the proportion of neurons with spontaneous activity is also significantly different (#p = 0.006) in the vehicle NRC group than that in the sham control group.
In addition to regulating spinal neuron activity evoked by paw stimulation, neurons similarly exhibit different spontaneous activity responses across the groups (Figure 7(c) and (d)). Spinal sPLA2 inhibition reduces the increased spontaneous activity that is observed in the dorsal horn at day 14 with a painful nerve root compression (Figure 7(d)), with a greater proportion of neurons with no baseline firing or <1 spike/sec, as well as fewer active neurons with spontaneous activity (>1 spike/sec) (Figure 7(d)). The distribution of spontaneous firing rates among neurons is significantly different (p = 0.033) in the compression with inhibition treatment compared to the painful vehicle-treated group, but there are no differences in distribution compared to the sham control group (Figure 7(d)). The distribution of spontaneously active neurons in the sham control group is also significantly lower (p = 0.007) than for compression with vehicle treatment (Figure 7(d)). Spinal sPLA2 inhibition also selectively reduces spontaneous activity in the WDR neurons, with the proportion of inactive neurons (0 spikes/sec) increasing to 71.0% with treatment as compared to 55.2% in the vehicle-treated group. Similarly, the proportion of spontaneously active neurons (with any activity greater than 0) decreases to 29.0% after inhibitor treatment as opposed to 44.8% with vehicle treatment. The distribution of spontaneous activity responses following inhibitor treatment is significantly different (p = 0.038) than the distribution observed in the vehicle-treated groups and there are no differences (p > 0.05) in the distribution of spontaneous neurons between the inhibitor-treated and sham groups. The distribution of WDR neurons exhibiting spontaneous activity in the vehicle-treated injury group (70%) is also significantly higher (p = 0.006) than the distribution for the WDR neurons in the inhibitor-treated group (40%). There are no differences in the proportion of neurons exhibiting spontaneous activity among groups for either the LTM or NS neurons.
Discussion
Inhibiting spinal sPLA2 after painful neuropathic injury, at a time when both pain and abberant neuronal activity are established,9,64 attenuates both that behavioral sensitivity and neuronal hyperexcitability, in part by restoring the spinal glutamatergic signaling that is altered after a painful nerve root compression (Figures 2–7). The abolishment of behavioral sensitivity (Figure 2) occurs in conjunction with increased spinal intracellular glutamate concentration (Figure 3(b)) suggesting spinal sPLA2 may contribute to the excitotoxicity and glutamate spillover that promotes the neuronal hyperexcitability that maintains neuropathic pain.62,76 Since mounting evidence in neuronal cultures shows bioactive lipids modulate glutamatergic neurotransmission,55,77,78 inhibiting spinal sPLA2 may act by mechanisms that attenuate bioactive lipid generation. In fact, the decrease in cPLA2 activity in the spinal cord at day 14 with sPLA2 inhibition (Figure 1(b) and 3(b)) suggests that inhibiting sPLA2 alone may be sufficient to regulate hydrolysis of phospholipid membranes and liberation of bioactive lipids. This purported decrease in hydrolytic activity and likely generation of AA with sPLA2 inhibition may restore glial glutamate transporter expression (Figure 4), thereby restoring spinal glutamate uptake and increasing intracellular glutamate concentrations (Figure 3(b)) and reducing excitotoxicity. Spinal sPLA2 inhibition also reduces mGluR5 and GluR1 expression in conjunction with decreasing spinal neuronal hyperexcitability (Figures 5–7) which supports its neuromodulatory role in maintaining neuropathic pain.
Abolishing behavioral sensitivity is consistent with other reports that inhibiting spinal phospholipase activity reduces established neuropathic pain.25,32 However, inhibiting downstream proteins in the phospholipase A2 cascade, like cyclooxygenase (COX), is not sufficient to reduce such established pain. 25 Although spinal sPLA2 is elevated at day 7 after injury,13,23,32 spinal sPLA2 hydrolysis activity is also upregulated at both days 7 and 14 after a painful nerve root compression (Figure 1), suggesting that sPLA2-mediated lipid generation continues even after the initial injury and inflammatory response. Similarly, spinal cPLA2 activity is elevated at day 14 but that activity returns to sham levels with administration of the sPLA2 inhibitor (Figure 3(a)). Although the sPLA2 and cPLA2 isoforms primarily hydrolyze extracellular and intracellular phospholipids, respectively,42,79 there is substantial cross-talk between both isoforms and in vitro studies suggest the actions of cPLA2 depend on coordination with sPLA2.41,79,80 Studies in granulocytes 81 and mast cells 82 point to sPLA2-mediated release of lysophospholipids as amplifying cPLA2 activation, leading to increased synthesis of lipid mediators in inflammation. Inhibiting spinal sPLA2 is sufficient to also reduce spinal cPLA2 activity (Figure 3(b)) and attenuate pain (Figure 2). Interestingly, intrathecal cPLA2 inhibition by AACOCF3 after chronic constriction injury only attenuates pain with repeated twice daily dosing. 25 The difference in effective dosing may be attributed to the complex actions of sPLA2 outside the cell, including the release of not only AA but also saturated, monounsaturated, and polyunsaturated fatty acids (PUFAs) like docosahexaenoic acid (DHA) and platelet activating factor (PAF), which are all precursors to anti-inflammatory lipid mediators involved in the pathogenesis of neuropathic pain.42,82 Together with the regulation of intracellular cPLA2 activity, production of lipid mediators that contribute to neuropathic pain, and the relative ease of accessing extracellular sPLA2 versus intracellular cPLA2, inhibiting spinal sPLA2 is a more attractive treatment target.
Neuropathic pain is characterized by progressive downregulation of spinal glutamate transporter expression,25,33,83 contributing to the accumulation of extracellular glutamate that potentiates neuronal hyperexcitability. 84 Reduced spinal glutamate uptake is further mediated by endogenous lipids, including AA85,86 and DHA, 87 through direct competitive inhibition of the glutamate binding site in GLT1 and GLAST. Although AA and DHA concentrations were not assayed here, the decreased spinal sPLA2 and cPLA2 activity with spinal sPLA2 inhibition suggests decreased lipid mediator generation and increased intracellular glutamate uptake. The concurrent restoration of spinal GLAST and GLT1 transporter expression (Figure 4) implies a likely restoration of spinal glutamate uptake as well. Since GLT1 is responsible for the majority of glutamate uptake in the spinal cord, 85 the increase in GLAST after painful root compression may be a compensatory mechanism.33,88 GLT1 expression at day 14 after injury (Figure 4(b)) is not downregulated to the same extent as observed at day 7 in this same injury model by immunohistochemistry. 33 Despite differences in techniques, spinal GLT1 expression may depend on activation of the transcription factor, kappa B motif-binding phosphoprotein (KBBP) in astrocytes, which is regulated by pre-synaptic signaling of mGluR and GluR receptors. 89 Increased spinal mGluR5 and GluR1 at day 14 (Figure 5(b)) may increase pre-synaptic neurotransmitter and glutamate release 35 activating KBBP and initiating the translation of more GLT1 protein. For conditions with GLT1 at normal levels (Figure 5(b)) KBBP activation may be involved; probing the contribution of pre-synaptic inputs to the production of new astrocytic GLT1 after injury would test this hypothesis.
Increasing intracellular glutamate may also reduce activation of spinal glutamate receptors (Figure 3). These findings support the hypothesis that spinal sPLA2 indirectly contributes to glutamate receptor activation and neurotransmission by exacerbating excitotoxicity. Glutamate receptor activation is sensitive to extracellular glutamate levels, with mGluR5 and NR1 displaying tonic activation of receptors between 1–20 µM, 90 below the estimated excitotoxic glutamate concentration (20–100 µM) causing neural injury. Conversely, GluRs mediate most of the fast-excitatory neurotransmission and require the highest concentrations of extracellular glutamate (100–1000 µM). 90 Although intracellular glutamate levels serve as a proxy measurement for extracellular glutamate here, decreases in both spinal mGluR5 and the less-sensitive GluR1 receptor further supports sPLA2‘s role in increasing excitotoxic glutamate concentration and modulating glutamatergic signaling with painful injury. NR1 expression at day 14 is unaltered with painful root compression (Figure 5(b)) which differs from a report with a painful chronic sciatic nerve constriction. 91 However, since altered NR1 expression was reported in synaptosome-extracted tissues from a model of inflammatory pain rather than using whole tissue lysates, 92 the lack of changes in NR1 observed here may be due to tissue preparation techniques. In addition to increased NR1 expression, phosphorylation and activation of other NMDAR subunits are also implicated in the pathogenesis of central sensitization, 30 neither of which were evaluated here. Measuring the effects of inhibiting spinal sPLA2 on synaptic-level changes in NMDAR subunits and phosphorylation states of glutamate receptors would test whether spinal sPLA2 inhibition mediates the synaptic plasticity that maintains central sensitization.
Spinal sPLA2 inhibition decreases the proportion of spinal WDR neurons after injury (Figure 6(a)). The increase in WDR neurons after a painful nerve root compression33,68 (Figure 6) is a hallmark of central sensitization, 62 indicating a reorganization of high-threshold Aδ and C fibers synapsing with dorsal horn neurons.93,94 The increased response to graded stimulation (Figure 6(a)) also suggests expansion of WDR neurons’ receptive fields, which could account for the shift in neuronal phenotypic distribution after injury. 95 Restoring spinal excitatory signaling after painful root compression with the GLT1-agonist, ceftriaxone, similarly restores the proportion of WDR neurons after injury, 33 suggesting that increasing spinal glutamate uptake (Figures 3 and 4) may also reset the spinal reorganization that occurs with central sensitization. 62 The effects of the downstream effectors of phospholipase A2, including COX and prostaglandin E2 (PGE2), sensitize spinal dorsal horn neurons.96–98 PGE2 inhibition reduces evoked responses from WDR neurons by reducing EP2 receptor activation, 96 which may contribute to the reduced evoked activity after spinal sPLA2 inhibition (Figure 7). WDR neurons increase spontaneous activity after painful neuropathic injury,71,72 likely driving central sensitization after neuropathy. 99 Interestingly, spinal application of PGE2 alone is not sufficient to elicit spontaneous action potentials; 96 yet, inhibiting spinal glutamate uptake increases spontaneous action potentials in response to non-noxious and noxious stimulation. 100 These reports suggest that the reduced spontaneous activity observed with spinal sPLA2 inhibition (Figure 7) is likely due to its modulation of glutamate transporters, independent of the actions of its downstream inflammatory mediators.
Overall, inhibiting spinal sPLA2 provides a robust treatment approach for attenuating neuropathic pain and spinal neuronal hyperexcitability after painful nerve root injury. It is important to note that the effects of spinal sPLA2 inhibition on neuropathic pain were evaluated using only reflexive measurements and only in male Holtzman rats. Given growing evidence of the affective components of pain 101 and differences in pain processing between males and females, 102 further assessment of sensory and affective painful behaviors is needed in both genders in order to better define and translate sPLA2 inhibitors to the clinic. Similarly, despite the reduction observed in sPLA2 activity (Figure 3(a)), both the sPLA2 expression following administration of TEA-PC as well as the pharmacokinetic profile, including half-life, of the inhibitor were not tested in this study. Moreover, any conclusion is based on the concentration and formulation used here. Although this study used same dose as was found to prevent pain and/or spinal hypersensitivity, neither that work 21 nor this study explicitly tested specificity of the TEA-PC for sPLA2. Therefore, it is unknown exactly how the current effects occurred or how long they will last after a single intrathecal injection and will need to be further characterized in separate studies. It is possible that lipid conjugation of the prodrug (sPLA2 inhibitor, TEA) to the lipid phosphorylcholine (PC) may extend the half-life of the drug as observed in.103-105 In particular, the lipid prodrug of indomethacin, DP-155, was shown to improve half-life in brain tissue to 94 h compared to 24 h with indomethacin alone. 105 However, lipid products also were not directly measured here. Future studies are needed to determine the exact half-life extension of lipid conjugation to TEA since it will be needed to inform future dosing paradigms.
While a neuromodulatory role of sPLA2 after painful injury has been speculated based on in vitro findings and early intervention in vivo,21,32,55,77 current findings suggest spinal sPLA2 contributes to glutamatergic neurotransmission as well as exacerbates spinal excitotoxicity. Although the roles of lipid mediators like PAF, which stimulates glutamate release from pre-synaptic neurons and astrocytes,106,107 were not investigated, decreased activity of both PLA2 isoforms suggests that sPLA2 inhibition likely reduces generation of lipid mediators. As an emerging area of interest in understanding and treating pain, defining the neuromodulatory contributions of bioactive lipids may provide insights into novel mechanisms and therapeutic targets for pain. Nevertheless, these findings suggest spinal sPLA2 may be a novel and robust neuromodulatory target to treat persistent neuropathic pain.
Footnotes
Author Contributions: The study design and conceptualization was performed by SK and BAW. SK and PG performed our experimental techniques and data analysis. SK, PG, and BAW all contributed to the interpretation of findings and manuscript drafting. Final manuscript was put together by BAW.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Catherine D. Sharpe Foundation and the National Institute of Neurological Disorders and Stroke (R01NS199892).
ORCID iD
Beth A Winkelstein https://orcid.org/0000-0003-0414-0484
References
- 1.DiBonaventura M, Sadosky A, Concialdi K, et al. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res 2017; 10: 2525–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nuckley DJ, Konodi MA, Raynak GC, et al. Neural space integrity of the lower cervical spine: effect of normal range of motion. Spine 2002; 27: 587–595. [DOI] [PubMed] [Google Scholar]
- 3.Panjabi MM, Maak TG, Ivancic PC, et al. Dynamic intervertebral foramen narrowing during simulated rear impact. Spine 2006; 31: E128–E134. [DOI] [PubMed] [Google Scholar]
- 4.Abbed KM, Coumans JV. Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery 2007; 60: S28–S34. [DOI] [PubMed] [Google Scholar]
- 5.Caridi JM, Pumberger M, Hughes AP. Cervical radiculopathy: a review. HSS J 2011; 7: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashizume H, DeLeo JA, Colburn RW, et al. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine 2000; 25: 1206–1217. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S, Baba H, Uchida K, et al. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine 2005; 30: 1699–1705. [DOI] [PubMed] [Google Scholar]
- 8.Nagashima H, Morio Y, Yamane K, et al. Tumor necrosis factor-α, interleukin-1β, and interleukin-6 in the cerebrospinal fluid of patients with cervical myelopathy and lumbar radiculopathy. Eur Spine J 2009; 18: 1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson KJ, Guarino BB, Winkelstein BA. Transient nerve root compression load and duration differentially mediate behavioral sensitivity and associated spinal astrocyte activation and mGLuR5 expression. Neuroscience 2012; 209: 187–195. [DOI] [PubMed] [Google Scholar]
- 10.Rothman SM, Winkelstein BA. Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann Biomed Eng 2010; 38: 2563–2576. [DOI] [PubMed] [Google Scholar]
- 11.Rothman SM, Huang Z, Lee KE, et al. Cytokine mRNA expression in painful radiculopathy. J Pain 2009; 10: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekiguchi M, Kobayashi H, Sekiguchi Y, et al. Sympathectomy reduces mechanical allodynia, tumor necrosis factor-alpha expression, and dorsal root ganglion apoptosis following nerve root crush injury. Spine 2008; 33: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 13.Kartha S, Weisshaar CL, Philips BH, et al. Pre-treatment with Meloxicam prevents the spinal inflammation and oxidative stress in DRG neurons that accompany painful cervical radiculopathy. Neuroscience 2018; 388: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JR, Galie PA, Slochower DR, et al. Salmon-derived thrombin inhibits development of chronic pain through an endothelial barrier protective mechanism dependent on APC. Biomaterials 2016; 80: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman SM, Nicholson KJ, Winkelstein BA. Time-dependent mechanics and measures of glial activation and behavioral sensitivity in a rodent model of radiculopathy. J Neurotrauma 2010; 27: 803–814. [DOI] [PubMed] [Google Scholar]
- 16.Rothman SM, Guarino BB, Winkelstein BA. Spinal microglial proliferation is evident in a rat model of painful disc herniation both in the presence of behavioral hypersensitivity and following minocycline treatment sufficient to attenuate allodynia. J Neurosci Res 2009; 87: 2709–2717. [DOI] [PubMed] [Google Scholar]
- 17.König C, Morch E, Eitner A, et al. Involvement of spinal IL-6 trans-signaling in the induction of hyperexcitability of deep dorsal horn neurons by spinal tumor necrosis factor-alpha. J Neurosci 2016; 36: 9782–9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.König C, Zharsky M, Möller C, et al. Involvement of peripheral and spinal tumor necrosis factor α in spinal cord hyperexcitability during knee joint inflammation in rats. Arthritis Rheumatol 2014; 66: 599–609. [DOI] [PubMed] [Google Scholar]
- 19.Reeve AJ, Patel S, Fox A, et al. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000; 4: 247–257. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez E, Bär K-J, Ebersberger A, et al. Spinal prostaglandins are involved in the development but not the maintenance of inflammation-induced spinal hyperexcitability. J Neurosci 2001; 21: 9001–9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quindlen-Hotek JC, Kartha S, Winkelstein BA. Immediate inhibition of spinal secretory phospholipase A2 prevents the pain and elevated spinal neuronal hyperexcitability and neuroimmune regulatory genes that develop with nerve root compression. Neuroreport 2020; 31: 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson CI, Lucas KK, Hua X-Y, et al. Spinal phospholipase A2 in inflammatory hyperalgesia: role of the small, secretory phospholipase A2. Neuroscience 2005; 133: 543–553. [DOI] [PubMed] [Google Scholar]
- 23.Titsworth WL, Cheng X, Ke Y, et al. Differential expression of sPLA2 following spinal cord injury and a functional role for sPLA2-IIA in mediating oligodendrocyte death. Glia 2009; 57: 1521–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morioka N, Takeda K, Kumagai K, et al. Interleukin-1beta-induced substance P release from rat cultured primary afferent neurons driven by two phospholipase A2 enzymes: secretory type IIA and cytosolic type IV. J Neurochem 2002; 80: 989–997. [DOI] [PubMed] [Google Scholar]
- 25.Sung B, Wang S, Zhou B, et al. Altered spinal arachidonic acid turnover after peripheral nerve injury regulates regional glutamate concentration and neuropathic pain behaviors in rats. Pain 2007; 131: 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha JS, Dho SH, Youm TH, et al. Astrocytic phospholipase A2 contributes to neuronal glutamate toxicity. Brain Res 2014; 1590: 97–106. [DOI] [PubMed] [Google Scholar]
- 27.Yawn BP, Wollan PC, Weingarten TN, et al. The prevalence of neuropathic pain: clinical evaluation compared with screening tools in a community population. Pain Med 2009; 10: 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlton SM, Neugebauer V. Peripheral metabotropic glutamate receptors as drug targets for pain relief. Expert Opin Ther Targets 2002; 6: 349–361. [DOI] [PubMed] [Google Scholar]
- 29.Muir K. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol 2006; 6: 53–60. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H-Y, Chen S-R, Pan H-L. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol 2011; 4: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chacur M, Milligan ED, Sloan EM, et al. Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain 2004; 108: 180–191. [DOI] [PubMed] [Google Scholar]
- 32.Kartha S, Yan L, Ita ME, et al. Phospholipase A2 inhibitor-loaded phospholipid micelles abolish neuropathic pain. ACS Nano 2020; 14: 8103–8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson KJ, Gilliland TM, Winkelstein BA. Upregulation of GLT-1 by treatment with ceftriaxone alleviates radicular pain by reducing spinal astrocyte activation and neuronal hyperexcitability. J Neurosci Res 2014; 92: 116–129. [DOI] [PubMed] [Google Scholar]
- 34.Smith JR, Lee J, Winkelstein BA. Nerve root compression increases spinal astrocytic vimentin in parallel with sustained pain and endothelial vimentin in association with spinal vascular reestablishment. Spine 2017; 42: 1434–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inquimbert P, Bartels K, Babaniyi OB, et al. Peripheral nerve injury produces a sustained shift in the balance between glutamate release and uptake in the dorsal horn of the spinal cord. Pain 2012; 153: 2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Prim 2017; 3: 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol 2013; 74: 630–636. [DOI] [PubMed] [Google Scholar]
- 38.Osikowicz M, Mika J, Przewlocka B. The glutamatergic system as a target for neuropathic pain relief. Exp Physiol 2013; 98: 372–384. [DOI] [PubMed] [Google Scholar]
- 39.Iwagaki N, Miles GB. Activation of group I metabotropic glutamate receptors modulates locomotor-related motoneuron output in mice. J Neurophysiol 2011; 105: 2108–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banno T, Omura T, Masaki N, et al. Arachidonic acid containing phosphatidylcholine increases due to microglial activation in ipsilateral spinal dorsal horn following spared sciatic nerve injury. PLoS One 2017; 12: e0177595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellom M, Basselin M, Keleshian VL, et al. Dose-dependent changes in neuroinflammatory and arachidonic acid cascade markers with synaptic marker loss in rat lipopolysaccharide infusion model of neuroinflammation. BMC Neurosci 2012; 13: 50. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Murakami M, Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: an update. Biochimie 2013; 95: 43–50. [DOI] [PubMed] [Google Scholar]
- 43.Han WK, Sapirstein A, Hung CC, et al. Cross-talk between cytosolic phospholipase A2α (cPLA2α) and secretory phospholipase A2 (sPLA2) in hydrogen peroxide-induced arachidonic acid release in murine mesangial cells: sPLA2 regulates cPLA2α activity that is responsible for arachidonic acid releas. J Biol Chem 2003; 278: 24153. [DOI] [PubMed] [Google Scholar]
- 44.Gabayl E, Wolfl G, Shavitl Y, et al. Chronic blockade of interleukin-1 (IL-1) prevents and attenuates neuropathic pain behavior and spontaneous ectopic neuronal activity following nerve injury. Eur J Pain 2011; 15: 242–248. [DOI] [PubMed] [Google Scholar]
- 45.Liu N-K, Zhang YP, Titsworth WL, et al. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol 2006; 59: 606–619. [DOI] [PubMed] [Google Scholar]
- 46.Millán C, Torres M, Sánchez-Prieto J. Co-activation of PKA and PKC in cerebrocortical nerve terminals synergistically facilitates glutamate release. J Neurochem 2003; 87: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z, He Y, Wang ZJ. The beta-lactam antibiotic, ceftriaxone, inhibits the development of opioid-induced hyperalgesia in mice. Neurosci Lett 2012; 509: 69–71. [DOI] [PubMed] [Google Scholar]
- 48.Lerea LS, McNamara JO. Ionotropic glutamate receptor subtypes activate c-fos transcription by distinct calcium-requiring intracellular signaling pathways. Neuron 1993; 10: 31–41. [DOI] [PubMed] [Google Scholar]
- 49.O'Regan MH, Smith-Barbour M, Perkins LM, et al. A possible role for phospholipases in the release of neurotransmitter amino acids from ischemic rat cerebral cortex. Neurosci Lett 1995; 185: 191–194. [DOI] [PubMed] [Google Scholar]
- 50.Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J Physiol 1998; 513: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zerangue N, Arriza JL, Amara SG, et al. Differential modulation of human glutamate transporter subtypes by arachidonic acid. J Biol Chem 1995; 270: 6433–6435. [DOI] [PubMed] [Google Scholar]
- 52.Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev 2013; 93: 1621–1657. [DOI] [PubMed] [Google Scholar]
- 53.Miller B, Sarantis M, Traynelis SF, et al. Potentiation of NMDA receptor currents by arachidonic acid. Nature 1992; 355: 722–725. [DOI] [PubMed] [Google Scholar]
- 54.Grigoriev VV, Serkov IV, Beznosko BK, et al. Influence of derivatives of arachidonic and docosohexaenic acids on AMPA receptors in Purkinje neurons and cognitive functions in mice. Izv Akad Nauk Ser Biol 2010; 37: 312–315. [PubMed] [Google Scholar]
- 55.Decoster MA, Lambeau G, Lazdunski M, et al. Secreted phospholipase A2 potentiates glutamate-induced calcium increase and cell death in primary neuronal cultures. J Neurosci Res 2002; 67: 634–645. [DOI] [PubMed] [Google Scholar]
- 56.Yaksh TL, Kokotos G, Svensson CI, et al. Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release 2006. J Pharmacol Exp Ther 2006; 316: 466–475. [DOI] [PubMed] [Google Scholar]
- 57.Nicholson KJ, Quindlen JC, Winkelstein BA. Development of a duration threshold for modulating evoked neuronal responses after nerve root compression injury. Stapp Car Crash J 2011; 55: 1–24. [DOI] [PubMed] [Google Scholar]
- 58.Chang Y-W, Winkelstein BA. Schwann cell proliferation and macrophage infiltration are evident at day 14 after painful cervical nerve root compression in the rat. J Neurotrauma 2011; 28: 2429–2438. [DOI] [PubMed] [Google Scholar]
- 59.Chantranupong L, Saulnier JL, Wang W, et al. Rapid purification and metabolomic profiling of synaptic vesicles from mammalian brain. Elife 2020; 9: 59699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fowler SW, Ramsey AK, Walker JM, et al. Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiol Learn Mem 2011; 95: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leem JW, Kim HK, Hulsebosch CE, et al. Ionotropic glutamate receptors contribute to maintained neuronal hyperexcitability following spinal cord injury in rats. Exp Neurol 2010; 224: 321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 64.Smith JR, Syre PP, Oake SA, et al. Salmon and human thrombin differentially regulate radicular pain, glial-induced inflammation and spinal neuronal excitability through protease-activated receptor-1. PLoS One 2013; 8: e80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 66.Zeeman ME, Kartha S, Winkelstein BA. Whole-body vibration induces pain and lumbar spinal inflammation responses in the rat that vary with the vibration profile. J Orthop Res 2016; 34: 1439–1446. [DOI] [PubMed] [Google Scholar]
- 67.Ma W, Chabot J-G, Vercauteren F, et al. Injured nerve-derived COX2/PGE2 contributes to the maintenance of neuropathic pain in aged rats. Neurobiol Aging 2010; 31: 1227–1237. [DOI] [PubMed] [Google Scholar]
- 68.Nicholson KJ, Zhang S, Gilliland TM, et al. Riluzole effects on behavioral sensitivity and the development of axonal damage and spinal modifications that occur after painful nerve root compression. J Neurosurg Spine 2014; 20: 751–762. [DOI] [PubMed] [Google Scholar]
- 69.Crosby ND, Gilliland TM, Winkelstein BA. Early afferent activity from the facet joint after painful trauma to its capsule potentiates neuronal excitability and glutamate signaling in the spinal cord. Pain 2014; 155: 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kras JV, Kartha S, Winkelstein BA. Intra-articular nerve growth factor regulates development, but not maintenance, of injury-induced facet joint pain & spinal neuronal hypersensitivity. Osteoarthritis Cartilage 2015; 23: 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Kawamata M, Namiki A. Changes in properties of spinal dorsal horn neurons and their sensitivity to morphine after spinal cord injury in the rat. Anesthesiology 2005; 102: 152–164. [DOI] [PubMed] [Google Scholar]
- 72.Chu KL, Faltynek CR, Jarvis MF, et al. Increased WDR spontaneous activity and receptive field size in rats following a neuropathic or inflammatory injury: implications for mechanical sensitivity. Neurosci Lett 2004; 372: 123–126. [DOI] [PubMed] [Google Scholar]
- 73.Crosby ND, Weisshaar CL, Winkelstein BA. Spinal neuronal plasticity is evident within 1 day after a painful cervical facet joint injury. Neurosci Lett 2013; 542: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coghill RC, Mayer DJ, Price DD. Wide dynamic range but not nociceptive-specific neurons encode multidimensional features of prolonged repetitive heat pain. J Neurophysiol 1993; 69: 703–716. [DOI] [PubMed] [Google Scholar]
- 75.Martin WJ, Cao Y, Basbaum AI. Characterization of wide dynamic range neurons in the deep dorsal horn of the spinal cord in preprotachykinin-A null mice in vivo. J Neurophysiol 2004; 91: 1945–1954. [DOI] [PubMed] [Google Scholar]
- 76.Ji R-R, Nackley A, Huh Y, et al. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018; 129: 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolko M, Decoster MA, de Turco EBR, et al. Synergy by secretory phospholipase A2 and glutamate on inducing cell death and sustained arachidonic acid metabolic changes in primary cortical neuronal cultures. J Biol Chem 1996; 271: 32722–32728. [DOI] [PubMed] [Google Scholar]
- 78.Bazan NG, Allan G. Platelet-activating factor in the modulation of excitatory amino acid neurotransmitter release and of gene expression. J Lipid Mediat Cell Signal 1996; 14: 321–330. [DOI] [PubMed] [Google Scholar]
- 79.Sun GY, Shelat PB, Jensen MB, et al. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med 2010; 12: 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lai Y, Oslund RC, Bollinger JG, et al. Eosinophil cysteinyl leukotriene synthesis mediated by exogenous secreted phospholipase A2 group X. J Biol Chem 2010; 285: 41491–41500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim YJ, Kim KP, Han SK, et al. Group V phospholipase A2 induces leukotriene biosynthesis in human neutrophils through the activation of group IVA phospholipase A2. J Biol Chem 2002; 277: 36479–36488. [DOI] [PubMed] [Google Scholar]
- 82.Matsuzawa A, Murakami M, Atsumi G-i, et al. Release of secretory phospholipase a 2 from rat neuronal cells and its possible function in the regulation of catecholamine secretion. Biochem J 1996; 318: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci 2003; 23: 2899–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danbolt NC. Glutamate uptake. Prog Neurobiol 2001; 65: 1–105. [DOI] [PubMed] [Google Scholar]
- 85.Dumuis A, Sebben M, Fagni L, et al. Stimulation by glutamate receptors of arachidonic acid release depends on the Na+/Ca2+ exchanger in neuronal cells. Mol Pharmacol 1993; 43: 976–981. [PubMed] [Google Scholar]
- 86.Bezzi P, Domercq M, Brambilla L, et al. CXCR4-activated astrocyte glutamate release via TNFa: Amplification by microglia triggers neurotoxicity. Nat Neurosci 2001; 4: 702–710. [DOI] [PubMed] [Google Scholar]
- 87.Grintal B, Champeil-Potokar G, Lavialle M, et al. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: evidence for a signalling role of docosahexaenoic acid. Neurochem Int 2009; 54: 535–543. [DOI] [PubMed] [Google Scholar]
- 88.Lepore AC, O’donnell J, Kim AS, et al. Reduction in expression of the astrocyte glutamate transporter, GLT1, worsens functional and histological outcomes following traumatic spinal cord injury. Glia 2011; 59: 1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Gozen O, Watkins A, et al. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron 2009; 61: 880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Featherstone DE. Intercellular glutamate signaling in the nervous system and beyond. ACS Chem Neurosci 2010; 1: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang S, Lim G, Zeng Q, et al. Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. J Neurosci 2005; 25: 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang X, Yang H-B, Xie Q-J, et al. Peripheral inflammation increased the synaptic expression of NMDA receptors in spinal dorsal horn. Pain 2009; 144: 162–169. [DOI] [PubMed] [Google Scholar]
- 93.Baba H, Ji R-R, Kohno T, et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell. Neurosci 2003; 24: 818–830. [DOI] [PubMed] [Google Scholar]
- 94.Keller AF, Beggs S, Salter MW, et al. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain 2007; 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kondo E, Iwata K, Ogawa A, et al. Involvement of glutamate receptors on hyperexcitability of wide dynamic range neurons in the gracile nucleus of the rats with experimental mononeuropathy. Pain 2002; 95: 153–163. [DOI] [PubMed] [Google Scholar]
- 96.Baba H, Kohno T, Moore KA, et al. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J Neurosci 2001; 21: 1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hefferan MP, O’Rielly DD, Loomis CW. Inhibition of spinal prostaglandin synthesis early after L5/L6 nerve ligation prevents the development of prostaglandin-dependent and prostaglandin-independent allodynia in the rat. Anesthesiology 2003; 99: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 98.Staniaszek L, Norris L, Kendall D, et al. Effects of COX-2 inhibition on spinal nociception: the role of endocannabinoids. Br J Pharmacol 2010; 160: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Djouhri L, Koutsikou S, Fang X, et al. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 2006; 26: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weng H-R, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience 2006; 138: 1351–1360. [DOI] [PubMed] [Google Scholar]
- 101.Talbot K, Madden VJ, Jones SL, et al. The sensory and affective components of pain: are they differentially modifiable dimensions or inseparable aspects of a unitary experience? A systematic review. Br J Anaesth 2019; 123(2): e263–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020; 21: 353–365. [DOI] [PubMed] [Google Scholar]
- 103.Markovic M, Ben-Shabat S, Aponick A, et al. Lipids and lipid-processing pathways in drug delivery and therapeutics. Int J Mol Sci 2020; 21(9): 3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bech EM, Pedersen SL, Jensen KJ. Chemical Strategies for half-life extension of biopharmaceuticals: lipidation and its alternatives. ACS Med Chem Lett 2018; 9(7): 577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dvir E, Elman A, Simmons D, et al. DP-155, a lecithin derivative of indomethacin, is a novel nonsteroidal antiinflammatory drug for analgesia and Alzheimer’s disease therapy. CNS Drug Rev 2007; 13: 260–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling 1. J Lipid Res 2009; 50: S237–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tatulian SA. Toward understanding interfacial activation of secretory phospholipase A2 (PLA2): membrane surface properties and membrane-induced structural changes in the enzyme contribute synergistically to PLA2 activation. Biophys J 2001; 80: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]