Abstract
This network meta-analysis aimed at assessing the influence of tramadol on the intravaginal ejaculatory latency time (IELT) and sexual satisfaction score (SSS) in treating patients with premature ejaculation (PE). The PubMed, Embase, Cochrane Library databases (until July 2021), and original references of the included articles was systematically retrieved. The PRISMA checklist was followed. Finally, 14 articles including 1971 patients were included in this analysis. The results indicated that patients who were treated with tramadol (50 mg, 62 mg, 89 mg, and 100 mg) were superior to those treated with placebo in terms of IELT (p = .003, p < .00001, p < .00001, and p < .00001, respectively), but 25 mg tramadol did not show a significant advantage (p = .06). Patients who were treated with tramadol (50 mg and 100 mg) had a better efficacy than who were treated with 25 mg tramadol in the IELT (p < .00001 and p < .00001), but the effect of 50 mg tramadol and 100 mg tramadol were not significantly different (p = .17). The tramadol group had the better effect than the placebo group in the SSS (p < .0001). And 50 mg tramadol showed a significant improvement compared with 20 mg paroxetine, as assessed by the IELT (p = .03) and SSS (p = .03). Safety assessments including adverse events suggested that tramadol was well tolerated. Tramadol showed a better improvement of IELT and SSS than placebo or paroxetine, and 50 mg tramadol may be a more reasonable therapeutic dose for patients with PE.
Keywords: Tramadol, meta-analysis, premature ejaculation, intravaginal ejaculatory latency time, sexual satisfaction score
Premature ejaculation (PE) is a common male sexual dysfunction with a prevalence of about 20% to 30% (Porst et al., 2007). PE was reported to be associated with poor satisfaction with sexual intercourse, ejaculation-related pain, and interpersonal distress (Giuliano et al., 2008; McMahon et al., 2011). Based on the generally accepted classification standard, PE can be divided into lifelong PE and acquired PE. A variety of biological and psychological factors was known to be related to the etiology of PE (Coskuner & Ozkan, 2021).
There were many treatments for PE, including topical medications, cream, spray, and systemic therapy. Among them, tramadol has shown good potential in the treatment of PE (Gur et al., 2016). Tramadol is an effective synthetic opioid analgesic with central effect (McMahon, 2015). Within 1.6–1.9 hr of oral administration, it is almost completely absorbed and reaches the peak concentration (Safarinejad & Hosseini, 2006). Stimulation of u-opioid receptor and inhibition of norepinephrine and serotonin reuptake might be the two main reasons for tramadol’s delayed ejaculation (Marcou et al., 2005). Clinically, the effect of tramadol in treating PE has been already evaluated in several clinical trials. There was still a lack of network meta-analysis to confirm the effect and safety of tramadol in treating PE. We conducted the network meta-analysis to explore the potential value of its therapeutic effects.
Materials and Methods
Study Protocol
The preferred reporting items of the system review and meta-analysis (PRISMA) was applied as guideline for this study (Moher et al., 2010).
Trial Selection
Embase (until July 2021), PubMed (until July 2021), and Cochrane Library Database (until July 2021) were searched to collect clinical trials involving tramadol in treating men with PE. The search terms were as follows: (“tramadol”[MeSH Terms] OR “tramadol”[All Fields] OR “tramadol s”[All Fields] OR “tramadole”[All Fields]) AND (“premature ejaculation”[MeSH Terms] OR (“premature”[All Fields] AND “ejaculation”[All Fields]) OR “premature ejaculation”[All Fields]). There were no restrictions on the language for the included articles. References to relevant articles were also searched. Four reviewers independently selected articles (LY, ZZ, CY, and ZX). If the title and abstract were insufficient to determine whether study met the inclusion criteria, the full text needed to be read. Two reviewers (ZZ and WY) performed data extraction, and three reviewers (LY, CY and ZX) performed data review.
Inclusion and Exclusion Criteria
Trials met the following inclusion criteria: (a) tramadol in treating PE was evaluated; (b) the effective data were provided, mainly including the total number of each group and clinical outcomes; (c) the design type was clinical trial. The study was excluded when the following criteria were met: not a clinical trial, such as review, comment, letter and animal experiment; no effective comparison; no valid data.
Quality Assessment
The Cochrane Handbook for Systematic Reviews of Interventions 2nd Edition (Cumpston et al., 2019) was used to access the quality of included studies. The quality of each study was classified as one of three degrees: +: the study was considered to have a low risk of bias, if it met all the quality criteria; ?: the study was considered to have a moderate risk of bias, if any item of the criteria was met partly or remained unclear; or -: the study was considered to have a high risk of bias, if any item of the criteria was not met. All authors assessed the quality of articles and agreed with the final results.
Data Extraction
The following data were collected: (a) trial design and country; (b) name of first author and publishing year; (c) sample size; (d) method of therapy, dosage, scheme, duration of therapy, inclusion criteria, and whether to calculate sample size; (e) intravaginal ejaculatory latency time (IELT, starting from the time of intromission until ejaculation) and sexual satisfaction score (SSS) (range was 0–5; “0” = completely dissatisfied, “5” = very satisfied), and adverse events (AEs).
Statistical Analysis
The meta-analysis was carried out by using Rev Man v5.4.0. The mean difference (MD) was applied to estimate continuous outcomes with 95% confidence intervals (CI). The odds ratio (OR) with 95% CI was applied to estimate dichotomous outcomes. A fixed-effect model was adopted if the result showed p > .05. Otherwise, a random-effect model was adopted. We used Cochrane’s Q test and I2 statistics to analyze the heterogeneity. p ≤ .05 or I2 ≥ 50% reflected a significant heterogeneity, and a random-effect model would be used. Moreover, p < .05 indicated that the difference between the experimental group and the control group was statistically significant. Due to insufficient data, subgroup analysis was not performed in this study.
Results
Characteristics of Trials and Risk of Bias
According to the inclusion and exclusion criteria, 14 articles (Alghobary et al., 2010; Bar-Or et al., 2012; Eassa & El-Shazly, 2013; Eid, 2011; Gameel et al., 2013; Hamidi-Madani et al., 2018; Kaynar et al., 2012; Khan & Rasaily, 2013; Kurkar et al., 2015; Saadat et al., 2015; Safarinejad & Hosseini, 2006; Salem et al., 2008; Ur Rehman et al., 2020; Xiong et al., 2011) including 1971 patients were used to access the effect of tramadol in treating men with PE (Figure 1). Details of the 14 articles are presented in Table 1. The risk of bias summary and graph are presented in Figures 2 and 3. The network plot of the intervention comparisons for PE is shown in Figure 4.
Figure 1.
The flow diagram of selection process.
Table 1.
Details of Included Studies.
| Study | Study Design | Country | Treatment | Dosage | Sample Size | Scheme | Treatment Cycle | Inclusion Criteria | Sample Size Calculations |
|---|---|---|---|---|---|---|---|---|---|
| Mohammad RS (2006) | RCT | Iran | Tramadol; Placebo | 50 mg | 32; 32 | 2 hr before sexual activity | 8 weeks | Potent married men aged 20–52 years who had an IELT of less than 2 min that occurred in more than 90% of coitus. | No |
| Emad AS (2008) | Single blind crossover study | USA | Tramadol; Placebo | 25 mg | 30; 30 | 1–2 hr before sexual activity | 8 weeks | Patients were IELT <2 min in 80% of intercourse attempts, with at least one intercourse episode per week. | No |
| Moheiddin A (2010) | RCT | Egypt | Tramadol; Paroxetine | 50 mg; 20 mg | 17; 18 | 2–3 hr before sexual activity | 6 weeks | Only cases with lifelong PE were included. PE was defined according to the definition of DSM-IV-RT. | No |
| David BO (2011) | RCT | USA | Tramadol; Tramadol; Placebo | 62 mg; 89 mg | 206; 198; 200 | 2–8 hr before sexual activity | 12 weeks | The population baseline median IELT was <60 s in each treatment arm. | No |
| XIONG GG (2011) | RCT | China | Tramadol; Placebo | 50 mg | 36; 36 | 2 hr before intercourse | 8 weeks | Potent married men who had an IELT of <2 min over a 3-month period had been in a stable relationship with uncontrolled ejaculation. | No |
| Eid MA (2011) | Single blind base study | Egypt | Tramadol; Paroxetine; Placebo | 50 mg; 20 mg | 50; 50; 50 | 3–4 hr before sexual activity | 3 weeks | Patients with IELT of less than 2 min in 80% of intercourses for 3 weeks for at least 10 successive intercourses. | No |
| Mehmet K (2012) | Single blind crossover study | Turkey | Tramadol; Placebo | 25 mg | 30; 30 | 2 hr before intercourse | 8 weeks | All participants had primary PE and stable relationship with a partner for 6 months and possible sexual attempts of once a week or more. | No |
| Amil HK (2013) | RCT | India | Tramadol; Placebo | 100 mg | 30; 30 | Daily for 4 weeks and then 2 or 8 hr before intercourse for 4 weeks | 8 weeks | Patients with IELT of less than 1 min with minimal sexual stimulation before or after ejaculation. | No |
| Bayoumy IE (2013) | RCT | Egypt | Tramadol; Tramadol; Tramadol; | 25 mg; 50 mg; 100 mg | 100; 100; 100 | 2–3 hr before intercourse | 24weeks | Men aged 25–50 years with a stable monogamous heterosexual relationship with regular sexual intercourse at least twice per week with a cooperative female partner. | No |
| Tarek AG (2013) | RCT | Egypt | Tramadol; Paroxetine; Placebo | 50 mg; 20 mg | 30; 30; 30 | 2 hr before intercourse; 4 hr before intercourse | 4 weeks | Patients included were those with PE for >1 year and who had an IELT of <2 min in >75% of episodes of vaginal sexual intercourse over a 2-week period. | Yes |
| Adel K (2015) | RCT | Egypt | Tramadol; Tramadol; Placebo | 50 mg; 100 mg | 60; 60; 60 | 2–3 hr before intercourse | 8 weeks | Participants were potent with no history of erectile dysfunction, and had a stable, regular (≥1 sexual attempts per week), single‑partner heterosexual relationship for ≥1 year. | Yes |
| Seyyed HS (2015) | RCT | Iran | Tramadol; Placebo | 100 mg | 20; 20 | 2 hr before intercourse | 3 weeks | Men who had an IELT of <1 min had a stable relationship with uncontrolled ejaculation. | Yes |
| Ali HM (2018) | RCT | Iran | Tramadol; Paroxetine; Placebo | 50 mg; 20 mg | 50; 50; 50 | 2–3 hr before intercourse | 12 weeks | Men were between 18 to 55 years old, married, and had a stable relationship for at least 6 months with uncontrolled ejaculation that occurred before or within 1 min of vaginal intercourse. | No |
| Muhammad FUR (2020) | RCT | PAK | Tramadol; Paroxetine | 50 mg; 20 mg | 53; 53 | 2 hr before intercourse; 4 hr before intercourse | 8 weeks | Potent married men aged 30 to 40 years who had been in a relationship for at least 6 months with uncontrolled ejaculation within 1 min of vaginal intromission. | Yes |
Note. RCT = randomized controlled trial; PE = premature ejaculation; IELT = intravaginal ejaculatory latency time; DSM-IV-TR = Diagnostic and Statistical Manual, Fourth Edition, Text Revision.
Figure 2.

The summary of risk of bias.
Figure 3.
The graph of risk of bias.
Figure 4.

Network plot of the intervention comparisons for premature ejaculation.
Efficacy
IELT
25 mg Tramadol Versus Placebo
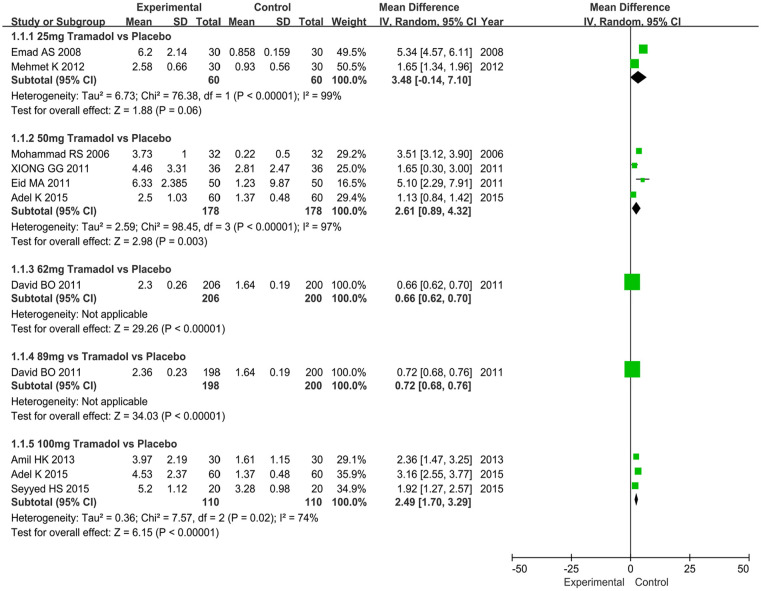
Two articles including a cohort of 120 men (tramadol group: 60 men; placebo group: 60 men) contained data on the IELT. The analysis showed an MD of 3.48 and 95% CI of −0.14 to 7.10 (p = .06), which implied that the tramadol group had no significant difference in improving IELT compared with the placebo group (Figure 5).
Figure 5.
Forest plots showing the comparison tramadol (25 mg, 50 mg, 62 mg, 89 mg, and 100 mg) with placebo in terms of intravaginal ejaculatory latency time for patients with premature ejaculation.
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
50 mg Tramadol Versus Placebo
Four articles including a cohort of 356 men (tramadol group: 178 men; placebo group: 178 men) contained data on the IELT. The analysis showed an MD of 2.61 and 95% CI of 0.89 to 4.32 (p = .003), which implied that the tramadol group had a significant improvement in the IELT compared with the placebo group (Figure 5).
62 mg Tramadol Versus Placebo
The change of IELT was supplied by one article including a cohort of 406 men (tramadol group: 206 men; placebo group: 200 men). The analysis drew an MD of 0.66 and 95% CI of 0.62 to 0.70 (p < .00001), which implied that the tramadol group had a significant improvement in the IELT compared with the placebo group (Figure 5).
89 mg Tramadol Versus Placebo
The change of IELT was supplied by one article including a cohort of 398 men (tramadol group: 198 men; placebo group: 200 men). The analysis drew an MD of 0.72 and 95% CI of 0.68 to 0.76 (p < .00001), which implied that the tramadol group had a significant improvement in the IELT compared with the placebo group (Figure 5).
100 mg Tramadol Versus Placebo
The change of IELT was supplied by three articles including a cohort of 220 men (tramadol group: 110 men; placebo group: 110 men). The analysis drew an MD of 2.49 and 95% CI of 1.70 to 3.29 (p < .00001), which implied that the tramadol group had a significant improvement in the IELT compared with the placebo group (Figure 5).
25 mg Tramadol Versus 50 mg Tramadol
The change of IELT was supplied by one article including a cohort of 200 men. The analysis drew an MD of −10.26 and 95% CI of −10.76 to −9.76 (p < .00001), which implied that 50 mg tramadol had a significant improvement in the IELT compared with 25 mg tramadol (Figure 6).
Figure 6.
Forest plots showing the effect of tramadol (25 mg vs. 50 mg, 25 mg vs. 100 mg, 50 mg vs. 100 mg, 62 mg vs. 89 mg, and 50 mg vs. 20 mg paroxetine) in terms of intravaginal ejaculatory latency time for patients with premature ejaculation.
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
25 mg Tramadol Versus 100 mg Tramadol
The change of IELT was supplied by one article including a cohort of 200 men. The analysis drew an MD of −23.32 and 95% CI of −24.05 to −22.59 (p < .00001), which implied that 100 mg tramadol had a significant improvement in the IELT compared with 25 mg tramadol (Figure 6).
50 mg Tramadol Versus 100 mg Tramadol
Two articles including a cohort of 320 men contained data on the IELT. The analysis showed an MD of −7.54 and 95% CI of −18.35 to −3.27 (p = .17), which implied that 50 mg tramadol had no significant differences in improving IELT compared with 100 mg tramadol (Figure 6).
62 mg Tramadol Versus 89 mg Tramadol
The change of IELT was supplied by one article including a cohort of 395 men. The analysis drew an MD of −0.06 and 95% CI of −0.11 to −0.01 (p = .01), which implied that 89 mg tramadol had a significant improvement in the IELT compared with 62 mg tramadol (Figure 6).
50 mg Tramadol Versus 20 mg Paroxetine
The change of IELT was supplied by five articles including a cohort of 401 men. The analysis drew an MD of 1.44 and 95% CI of 0.15 to 2.73 (p = .03), which implied that 50 mg tramadol had a significant improvement in the IELT compared with 20 mg paroxetine (Figure 6).
SSS
Tramadol Versus Placebo
The change of SSS was supplied by seven articles including a cohort of 802 men (tramadol group: 404 men; placebo group: 398 men). The forest plot demonstrated that tramadol had a greater effect in improving the SSS (MD 1.91, 95% CI [0.96, 2.86], p < .0001) compared with placebo (Figure 7).
Figure 7.
Forest plots showing the result of sexual satisfaction score (tramadol vs. placebo and tramadol vs. paroxetine).
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
Tramadol Versus Paroxetine
The change of SSS was supplied by three articles including a cohort of 266 men (tramadol group: 133 men; paroxetine group: 133 men). The analysis implied that the tramadol group had a greater effect in improving the SSS (MD 2.24, 95% CI [0.27, 4.20], p = .03) compared with the paroxetine group (Figure 7).
AEs
The analysis of AEs was supplied by eight articles including a cohort of 942 men (tramadol group: 474 men; placebo group: 468 men). No serious adverse reactions were found in both groups. The analysis showed an OR of 6.15 and 95% CI of 3.52 to 10.76 (p < .00001), which implied that tramadol had a significant difference in the number of AEs compared with placebo (Figure 8).
Figure 8.
Forest plots showing the adverse events of tramadol versus placebo.
Note. M-H = Mantel-Haenszel; CI = confidence interval; df = degrees of freedom.
Discussion
PE didn’t only affect the male partner, but it had also a major impact on quality of life of their sexual partners, including anxiety, anger, and loss of confidence (McMahon, 2016; Sharma et al., 2021). It was necessary to explore an effective treatment for PE. Drugs used to treat PE were more challenging in terms of the concept of treatment and the speculation of cause. This meta-analysis was performed to explore the influence of tramadol on the IELT and SSS in treating patients with PE from the perspective of evidence-based medicine.
Finally, 14 articles including 1971 patients were involved in this analysis. The results indicated that patients who were treated with tramadol (50 mg, 62 mg, 89 mg, and 100 mg) were superior to those treated with placebo in terms of IELT (p = .003, p < .00001, p < .00001, and p < .00001, respectively), but 25 mg tramadol did not show a significant advantage (p = .06). Patients who were treated with tramadol (50 mg and 100 mg) had a better efficacy than those who were treated with 25 mg tramadol in respect to IELT (p < .00001 and p < .00001), but the effects of 50 mg tramadol and 100 mg tramadol were not significantly different (p = .17). The tramadol group had the better effect than the placebo group in the SSS (p < .0001). Besides, 50 mg tramadol showed a significant improvement compared with 20 mg paroxetine, as assessed by IELT (p = .03) and SSS (p = .03). Safety assessments including AEs suggested that tramadol was well tolerated.
Tramadol was an opioid analgesic for the treatment of moderate and severe pain. Four regimens (50, 62, 89, and 100 mg) significantly prolonged the IELT compared with placebo. But high dosage (100 mg) did not show a significant advantage than low dosage (50 mg). Furthermore, low dosage regimen was well accepted and tolerated regarding its AEs. Therefore, this analysis identified that 50 mg tramadol might be a suitable regimen for PE, which should be carefully weighed against the risk of drug dependence (Epstein et al., 2006).
Tramadol has been studied as a potential drug for PE, with several clinical trials reporting the greater improvement of IELT with different doses of daily or on-demand tramadol (Althof et al., 2014; Hisasue, 2016). Though the mechanism of action of tramadol was not completely clear, its efficacy may come from anti-nociceptive and anesthetic effects, as well as regulating the central nervous system by inhibiting the reuptake of serotonin and norepinephrine (Szkutnik-Fiedler et al., 2012). James et al. (2015) identified that tramadol had a significant improvement in some cases for patients with PE such as compared with placebo, paroxetine daily and on demand, phosphodiesterase 5 inhibitors, local anesthetics, and behavioral therapy. Tan et al. (2021) performed a meta-analysis and reported that on-demand tramadol revealed a better effect than on-demand paroxetine for patients with PE, and patients in both groups showed good tolerance. However, doctors should pay attention to whether patients were at risk of addiction. Takeshita and Litzinger (2009) reported that tramadol wasn’t recommended to be used in combination with selective serotonin reuptake inhibitors because it had the risk of causing patients to develop serotonin syndrome.
In the included clinical trials, tramadol significantly increased IELT of patients with different degrees of PE in a dose-dependent manner, improved the satisfaction of patients with sexual activities, and the drug was well tolerated. One randomized controlled trial (RCT) reported that tramadol revealed a significant dose-related effect and AEs profile compared with placebo in treating PE (Kurkar et al., 2015). One RCT reported that 300 patients with lifelong PE were given either placebo or tramadol at different dosages for 24 weeks and found that IELT significantly increased in each group compared with baseline, suggesting that tramadol at various doses was effective and tolerable, with AEs related to constipation, nausea, headache, dizziness, dry mouth, and vomiting (Eassa & El-Shazly, 2013). Tramadol could become a choice in patients with mild-severe PE because of its anti-nociceptive and anesthetic effects (Kaynar et al., 2012). Despite the risk of abuse and dependence, these events were rare, especially in the case of short-term intermittent use of small doses (Kaynar et al., 2012). However, the possibility of drug addiction and other effects on sexual function should be fully considered before prescribing this drug.
The limitations of this meta‑analysis should be acknowledged. This study could not infer the long-term effect and safety of tramadol in treating men with PE. Selection bias, subjective factors, publication bias, study design, population characteristic, sample size, ethnic difference, and non-fixed duration may also affect final results. Our findings should be confirmed with RCTs with long-term follow-up, sufficient sample size, and fixed duration/dose.
Conclusions
Compared with placebo and paroxetine, tramadol showed a greater improvement of IELT and SSS. Besides, 50 mg tramadol may be a more reasonable therapeutic dose for patients with PE.
Supplemental Material
Supplemental material, sj-pdf-1-jmh-10.1177_15579883211057713 for The Influence of Tramadol on Intravaginal Ejaculatory Latency Time and Sexual Satisfaction Score in Treating Patients With Premature Ejaculation: A Network Meta-Analysis by Youyi Lu, Zhongbao Zhou, Xiaoyi Zhang, Yuanshan Cui, Yong Zhang and Yongqiang Wang in American Journal of Men’s Health
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Yantai science and technology project, Code: 2019YD025; National Nature Science Foundation of China, Code: 81801429.
Ethics Approval and Consent to Participate: The authors have no ethical conflicts to disclose.
ORCID iD: Zhongbao Zhou  https://orcid.org/0000-0002-9810-8145
https://orcid.org/0000-0002-9810-8145
Supplemental Material: Supplemental material for this article is available online.
References
- Alghobary M., El-Bayoumy Y., Mostafa Y., Mahmoud el H. M., Amr M. (2010). Evaluation of tramadol on demand vs. Daily paroxetine as a long-term treatment of lifelong premature ejaculation. Journal of Sexual Medicine, 7(8), 2860–2867. doi: 10.1111/j.1743-6109.2010.01789.x [DOI] [PubMed] [Google Scholar]
- Althof S. E., McMahon C. G., Waldinger M. D., Serefoglu E. C., Shindel A. W., Adaikan P. G., Torres L. O. (2014). An update of the international society of sexual medicine’s guidelines for the diagnosis and treatment of premature ejaculation (pe). Sexual Medicine, 2(2), 60–90. doi: 10.1002/sm2.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or D., Salottolo K. M., Orlando A., Winkler J. V. (2012). A randomized double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. European Urology, 61(4), 736–743. doi: 10.1016/j.eururo.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Coskuner E. R., Ozkan B. (2021). Premature ejaculation and endocrine disorders: A literature review. World Journal of Mens Health. doi: 10.5534/wjmh.200184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumpston M., Li T., Page M. J., Chandler J., Welch V. A., Higgins J. P., Thomas J. (2019). Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database of Systematic Reviews, 10, Ed000142. doi: 10.1002/14651858.Ed000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eassa B. I., El-Shazly M. A. (2013). Safety and efficacy of tramadol hydrochloride on treatment of premature ejaculation. Asian Journal of Andrology, 15(1), 138–142. doi: 10.1038/aja.2012.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid M. A. A., Hossam H, Ismail, Nashaat N, Shehada, Sameh Y. (2011). Comparative study between tramadol (50 mg) on demand and paroxitine hcl (20 mg) on demand in the treatment of premature ejaculation. Human Andrology, 1(2), 69–73. doi: 10.1097/01.XHA.0000399371.38752.73 [DOI] [Google Scholar]
- Epstein D. H., Preston K. L., Jasinski D. R. (2006). Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biological Psychology, 73(1), 90–99. doi: 10.1016/j.biopsycho.2006.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameel T. A., Tawfik A. M., Abou-Farha M. O., Bastawisy M. G., El-Bendary M. A., El-Gamasy Ael N. (2013). On-demand use of tramadol, sildenafil, paroxetine and local anaesthetics for the management of premature ejaculation: A randomised placebo-controlled clinical trial. Arab Journal of Urology, 11(4), 392–397. doi: 10.1016/j.aju.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano F., Patrick D. L., Porst H., La Pera G., Kokoszka A., Merchant S., Polverejan E. (2008). Premature ejaculation: Results from a five-country european observational study. European Urology, 53(5), 1048–1057. doi: 10.1016/j.eururo.2007.10.015 [DOI] [PubMed] [Google Scholar]
- Gur S., Kadowitz P. J., Sikka S. C. (2016). Current therapies for premature ejaculation. DRUG Discovery Today, 21(7), 1147–1154. doi: 10.1016/j.drudis.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Hamidi-Madani A., Motiee R., Mokhtari G., Nasseh H., Esmaeili S., Kazemnezhad E. (2018). The efficacy and safety of on-demand tramadol and paroxetine use in treatment of life long premature ejaculation: A randomized double-blind placebo-controlled clinical trial. Journal of Reproduction & Infertility, 19(1), 10–15. [PMC free article] [PubMed] [Google Scholar]
- Hisasue S. (2016). The drug treatment of premature ejaculation. Translational Andrology and Urology, 5(4), 482–486. doi: 10.21037/tau.2016.06.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaynar M., Kilic O., Yurdakul T. (2012). On-demand tramadol hydrochloride use in premature ejaculation treatment. Urology, 79(1), 145–149. doi: 10.1016/j.urology.2011.09.031 [DOI] [PubMed] [Google Scholar]
- Khan A. H., Rasaily D. (2013). Tramadol use in premature ejaculation: Daily versus sporadic treatment. Indian Journal of Psychological Medicine, 35(3), 256–259. doi: 10.4103/0253-7176.119477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkar A., Elderwy A. A., Abulsorour S., Awad S. M., Safwat A. S., Altaher A. (2015). A randomized, double-blind, placebo-controlled, crossover trial of “on-demand” tramadol for treatment of premature ejaculation. Urology Annals, 7(2), 205–210. doi: 10.4103/0974-7796.150481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcou T. A., Marque S., Mazoit J. X., Benhamou D. (2005). The median effective dose of tramadol and morphine for postoperative patients: A study of interactions. Anesthesia And Analgesia, 100(2), 469–474. doi: 10.1213/01.Ane.0000142121.24052.25 [DOI] [PubMed] [Google Scholar]
- Martyn-St James M., Cooper K., Kaltenthaler E., Dickinson K., Cantrell A., Wylie K., Hood C. (2015). Tramadol for premature ejaculation: A systematic review and meta-analysis. BMC Urology, 15, 6. doi: 10.1186/1471-2490-15-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C. G. (2015). Current and emerging treatments for premature ejaculation. Sexual Medicine Reviews, 3(3), 183–202. doi: 10.1002/smrj.49 [DOI] [PubMed] [Google Scholar]
- McMahon C. G. (2016). Emerging and investigational drugs for premature ejaculation. Translational Andrology and Urology, 5(4), 487–501. doi: 10.21037/tau.2016.04.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C. G., Althof S. E., Kaufman J. M., Buvat J., Levine S. B., Aquilina J. W., Porst H. (2011). Efficacy and safety of dapoxetine for the treatment of premature ejaculation: Integrated analysis of results from five phase 3 trials. Journal of Sexual Medicine, 8(2), 524–539. doi: 10.1111/j.1743-6109.2010.02097.x [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. International Journal of Surgery, 8(5), 336–341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Porst H., Montorsi F., Rosen R. C., Gaynor L., Grupe S., Alexander J. (2007). The premature ejaculation prevalence and attitudes (pepa) survey: Prevalence, comorbidities, and professional help-seeking. European Urology, 51(3), 816–823; discussion 824. doi: 10.1016/j.eururo.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Saadat S. H., Ahmadi K., Panahi Y. (2015). The effect of on-demand caffeine consumption on treating patients with premature ejaculation: A double-blind randomized clinical trial. Current Pharmaceutical Biotechnology, 16(3), 281–287. doi: 10.2174/1389201016666150118133045 [DOI] [PubMed] [Google Scholar]
- Safarinejad M. R., Hosseini S. Y. (2006). Safety and efficacy of tramadol in the treatment of premature ejaculation: A double-blind, placebo-controlled, fixed-dose, randomized study. Journal of Clinical Psychopharmacology, 26(1), 27–31. doi: 10.1097/01.jcp.0000195110.79027.3f [DOI] [PubMed] [Google Scholar]
- Salem E. A., Wilson S. K., Bissada N. K., Delk J. R., Hellstrom W. J., Cleves M. A. (2008). Tramadol hcl has promise in on-demand use to treat premature ejaculation. Journal of Sexual Medicine, 5(1), 188–193. doi: 10.1111/j.1743-6109.2006.00424.x [DOI] [PubMed] [Google Scholar]
- Sharma A. P., Sharma G., Tyagi S., Devana S. K., Mavuduru R. S., Bora G. S., Singh S. K. (2021). Safety and efficacy of "on-demand" tramadol in patients with premature ejaculation: An updated meta-analysis. International Braz J Urol, 47(5), 921–934. doi: 10.1590/s1677-5538.Ibju.2020.0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkutnik-Fiedler D., Kus K., Balcerkiewicz M., Grześkowiak E., Nowakowska E., Burda K., Sadowski C. (2012). Concomitant use of tramadol and venlafaxine - evaluation of antidepressant-like activity and other behavioral effects in rats. Pharmacological Reports, 64(6), 1350–1358. doi: 10.1016/s1734-1140(12)70932-5 [DOI] [PubMed] [Google Scholar]
- Takeshita J., Litzinger M. H. (2009). Serotonin syndrome associated with tramadol. Primary Care Companion to The Journal of Clinical Psychiatry, 11(5), 273. doi: 10.4088/PCC.08l00690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H., Zhou Z., Cui Y., Feng F., Zhang Y. (2021). A systematic review and meta-analysis of randomized controlled trials of “on-demand” use of tramadol vs “on-demand” use of paroxetine in the management of patients with premature ejaculation. International Journal of Clinical Practice, 75(11), e14825. doi: 10.1111/ijcp.14825 [DOI] [PubMed] [Google Scholar]
- Ur Rehman M. F., Imran Zaidi A., Ul Haq T., Rafique S., Ali F. (2020). Comparison of the efficacy of tramadol and paroxetine in the management of premature ejaculation. Cureus, 12(9), e10725. doi: 10.7759/cureus.10725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G. G., Wu F. H., Chen S. H., Yao W. L. (2011). [safety and efficacy of tramadol hydrochloride with behavioral modification in the treatment of premature ejaculation]. Zhonghua Nan Ke Xue, 17(6), 538–541. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jmh-10.1177_15579883211057713 for The Influence of Tramadol on Intravaginal Ejaculatory Latency Time and Sexual Satisfaction Score in Treating Patients With Premature Ejaculation: A Network Meta-Analysis by Youyi Lu, Zhongbao Zhou, Xiaoyi Zhang, Yuanshan Cui, Yong Zhang and Yongqiang Wang in American Journal of Men’s Health