Abstract
Objective
This study was performed to compare the effectiveness and safety of vesselplasty versus vertebroplasty in the treatment of osteoporotic compression fractures with posterior wall rupture.
Methods
Patients who underwent treatment of a single osteoporotic vertebral compression fracture with posterior wall rupture from January 2016 to February 2020 were retrospectively reviewed. They were divided into a vesselplasty group (n = 17) and a vertebroplasty group (n = 43). Pain relief, radiographic outcomes, and bone cement leakage were compared between the two groups.
Results
There were no significant differences in the operation time, postoperative pain relief, vertebral compression recovery, or local Cobb angle improvement between the two groups. However, the overall bone cement leakage rate (29.4% vs. 67.4%) and spinal canal leakage rate (0.0% vs. 30.2%) were significantly lower in the vesselplasty group than vertebroplasty group.
Conclusions
Vesselplasty offers similar pain relief and vertebral compression recovery but lower spinal canal leakage compared with vertebroplasty. Vesselplasty is thus a better option than vertebroplasty for patients with osteoporotic compression fractures with posterior wall rupture.
Keywords: Osteoporotic compression fracture, vesselplasty, vertebroplasty, bone cement leakage, spinal canal leakage, safety, effectiveness
Introduction
Osteoporotic vertebral compression fractures (OVCFs) are common fractures in patients of advanced age.1,2 Affected patients experience significant spinal pain when changing their position. Conservative treatment can lead to pneumonia, bedsores, deep vein thrombosis, and other complications related to long-term bed rest. Vertebroplasty is a common surgical method for the treatment of OVCFs. 3 Bone cement leakage, especially leakage into the spinal canal, is a common complication of vertebroplasty. 4 Bone cement can compress the spinal cord or nerves with serious consequences.
Rupture of the posterior wall of the vertebral body is common in pathological fractures of the spine.5–7 It can also be seen in OVCFs. 8 Rupture of the posterior wall is a risk factor for bone cement leakage into the spinal canal. 9 To avoid this complication, great care should be taken when vertebroplasty is performed to treat OVCFs with posterior wall rupture. Theoretically, because of the restriction of the container, vesselplasty can reduce the chance of bone cement leakage when the posterior wall ruptures. However, few related studies have been performed to date. Moreover, although vertebroplasty has been shown to have a high rate of bone cement leakage, all existing articles compare vertebroplasty with kyphoplasty rather than vesselplasty.10,11 The present study was performed to compare the effectiveness and safety of vesselplasty and vertebroplasty in the treatment of OVCFs with posterior wall rupture.
Patients and methods
This study was approved by the institutional review board of Beijing Tsinghua Changgung Hospital (approval no. 21180-0-01). Considering the retrospective nature of the study, the need for informed consent was waived. The reporting of this study conforms to the STROBE guidelines. 12 We reviewed patients with a single OVCF with posterior wall rupture who were admitted to our hospital from January 2016 to February 2020. Patients with multiple spinal fractures and pathological fractures were excluded. All patients had preoperative and postoperative radiographs and preoperative computed tomography (CT) or magnetic resonance imaging (MRI) data. The fracture line had low density on CT and low signal intensity in the T1, T2, and fat-suppressed T2 sequences on MRI. Bleeding and edema signals were seen around the fracture line on MRI, showing low signal intensity on T1 images, mixed signal intensity on T2 images, and high signal intensity on fat-suppressed T2 images. If the fracture line extended to the posterior wall of the vertebral body on CT or MRI or the posterior wall of the vertebral body showed morphological changes, the posterior wall was considered to be ruptured (Figure 1). The patients were divided into two groups based on their treatment method, vesselplasty or vertebroplasty, which was chosen according to the surgeon’s preference. Low-viscosity bone cement was used for both vesselplasty and vertebroplasty. All patients were followed up by telephone.
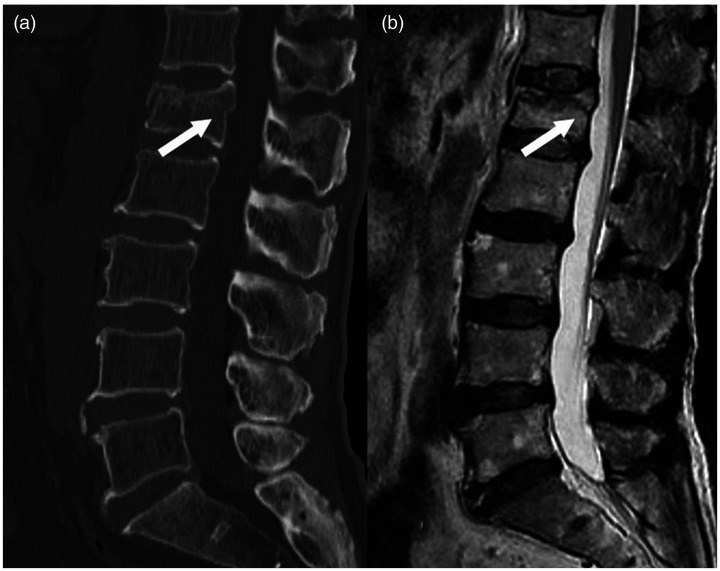
Figure 1.
Example of an osteoporotic vertebral compression fracture with posterior wall rupture. These images show the fracture line (arrow) extending to the posterior wall of the vertebral body on (a) computed tomography and (b) magnetic resonance imaging.
The vesselplasty procedure was performed as follows. The patient was placed in the prone position under local anesthesia. The working channel was inserted into the vertebral body through the unilateral pedicle with the help of a puncture needle. A precision drill was introduced, ensuring that the tip did not extend beyond the anterior edge of the vertebral body. The cement container was then inserted, and the cement was injected under fluoroscopic guidance. A small quantity of bone cement sometimes permeated through the container to better cross-link the surrounding bone and thus enhance stability (Figure 2). The injection system was a Vessel-X (Central Medical Technologies, Taipei, Taiwan). The vertebroplasty procedure was performed as follows. As for the vesselplasty procedure, the patient was placed in the prone position under local anesthesia. The puncture needle was inserted along the pedicle to the middle of the fractured vertebral body, and cement was injected under C-arm fluoroscopy (Figure 3). The injection system was a Mendec Spine Kit (Tecres, Verona, Italy).
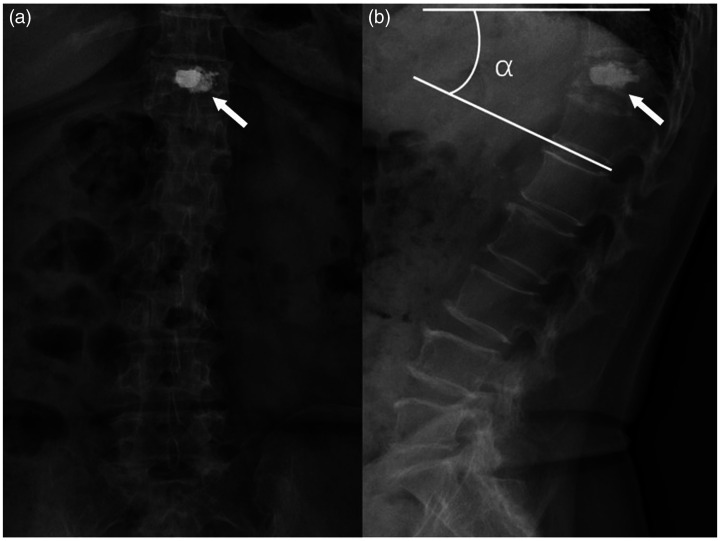
Figure 2.
(a) Anterior X-ray and (b) lateral X-ray after vesselplasty and illustration of the local Cobb angle (α). Some bone cement could permeate through the cement container to enhance stability (arrow).
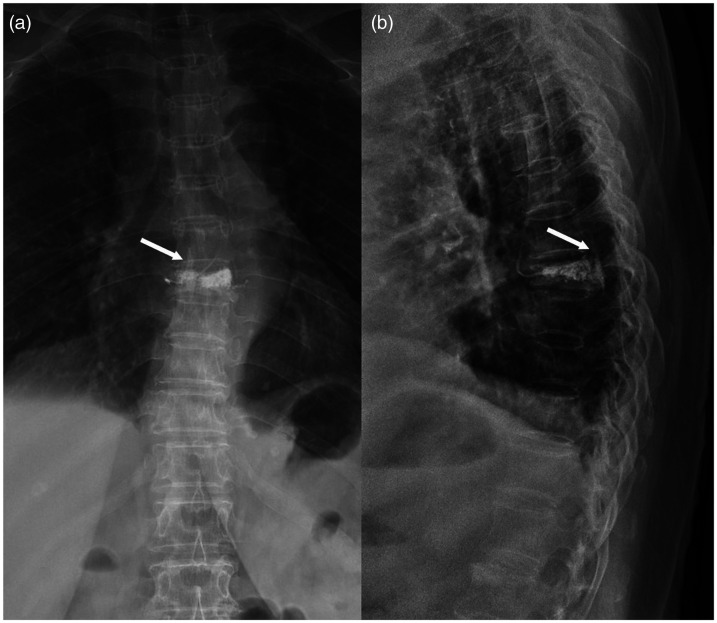
Figure 3.
(a) Anterior X-ray and (b) lateral X-ray after vertebroplasty and example of bone cement spinal canal leakage (arrow), which was in the spinal canal on the lateral X-ray and between the two pedicles on the anterior X-ray.
The following demographic and clinical characteristics of the patients in the two groups were compared: sex, age, body mass index, smoking status, Hounsfield unit value (T12 or L1), albumin concentration, fracture location (T11–L1 or elsewhere), intravertebral clefts, time to surgery, operation time, and follow-up time. The pain-related parameters included the visual analog scale (VAS) score before surgery, 24 hours after surgery, 6 months after surgery, and at the final follow-up. The postoperative pain relief rate was calculated as (preoperative VAS score − postoperative VAS score)/preoperative VAS score × 100%. This calculation method was used at 24 hours after surgery, 6 months after surgery, and at the final follow-up.
The imaging-related parameters included anterior vertebral compression, defined as the vertebral anterior margin height divided by the posterior margin height on the lateral X-ray; middle vertebral compression, defined as the vertebral middle height divided by the posterior margin height on the lateral X-ray; and the local Cobb angle (Figure 2), defined as the angle between the proximal vertebral superior endplate and the distal vertebral inferior endplate in the sagittal plane. These parameters were compared both before and after the operation. The differences in these parameters between the two groups were also compared.
The complication-related parameters included overall bone cement leakage, spinal canal leakage, intervertebral disc leakage, leakage in other areas, reoperation due to leakage, bone cement volume, nerve injury, and new vertebral fracture. Bone cement leakage was assessed on the postoperative X-ray. If abnormal bone cement was located in the spinal canal on the lateral X-ray and the corresponding bone cement on the anterior X-ray was located between the two pedicles, spinal canal leakage was diagnosed (Figure 3).
IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. A chi-square test was performed for categorical data. If the conditions of the chi-square test were not met, Fisher’s exact test was used. Student’s t test was performed for numerical data. A paired t test was used to analyze the changes in the VAS score, vertebral compression, and local Cobb angle in the same group before and after the procedure. A P value of <0.050 was considered statistically significant.
Results
In the vesselplasty group, 18 patients were initially screened and 1 patient was lost to follow-up; therefore, 17 patients were finally enrolled. In the vertebroplasty group, 47 patients were initially screened and 4 patients were lost to follow-up; therefore, 43 patients were finally enrolled. Table 1 shows the demographic characteristics of the two groups. There were no significant differences in sex, age, body mass index, smoking status, Hounsfield unit value, albumin concentration, proportion of thoracolumbar fractures, time to surgery, or intravertebral clefts between the two groups. The operation time was longer in the vesselplasty group than vertebroplasty group (46.76 ± 15.54 vs. 39.31 ± 14.60 minutes, respectively), but the difference was not significant. The mean follow-up time was 21.88 ± 10.87 months in the vesselplasty group and 20.81 ± 8.18 months in the vertebroplasty group, also without a significant difference.
Table 1.
Patients’ demographic characteristics.
| Characteristics | Vesselplasty | Vertebroplasty | P |
|---|---|---|---|
| Age, years | 74.41 ± 7.97 | 75.79 ± 8.09 | 0.553 |
| Female sex | 14 (82.4) | 34 (79.1) | 1.000 |
| Body mass index, kg/m2 | 24.19 ± 4.74 | 24.19 ± 3.42 | 1.000 |
| Current smoker | 2 (11.8) | 1 (2.3) | 0.191 |
| Hounsfield unit value, T12 or L1 | 80.66 ± 32.77 | 83.68 ± 24.30 | 0.734 |
| Albumin, g/L | 39.81 ± 4.11 | 40.32 ± 3.95 | 0.657 |
| T11–L1 | 12 (70.6) | 29 (67.4) | 0.813 |
| Intravertebral cleft | 7 (41.2) | 12 (27.9) | 0.319 |
| Time to surgery, days | 12.18 ± 10.21 | 13.05 ± 13.93 | 0.816 |
| Operation time, minutes | 46.76 ± 15.54 | 39.30 ± 14.43 | 0.083 |
| Follow-up duration, months | 21.82 ± 10.53 | 20.81 ± 8.18 | 0.693 |
Data are presented as mean ± standard deviation or n (%).
The VAS scores in the vesselplasty group and vertebroplasty group decreased from 7.94 ± 1.64 to 1.88 ± 2.37 and from 7.58 ± 2.16 to 1.88 ± 2.10 at 24 hours after the procedure, respectively. Postoperative pain was significantly relieved in both groups (P < 0.05). There were no significant differences in the VAS scores or pain relief rates between the two groups at different time points (Table 2).
Table 2.
Pain-related parameters.
| Vesselplasy | Vertebroplasty | P | |
|---|---|---|---|
| Visual analog scale score | |||
| Before the procedure | 7.94 ± 1.64 | 7.65 ± 1.95 | 0.590 |
| 24 hours after the procedure | 1.88 ± 2.37 | 1.88 ± 2.10 | 0.998 |
| P (before – 24 hours after) | 0.000 | 0.000 | |
| 6 months after the procedure | 0.88 ± 1.36 | 1.14 ± 1.85 | 0.605 |
| Follow-up | 0.82 ± 1.13 | 1.14 ± 1.89 | 0.521 |
| Pain relief rate | |||
| 24 hours after the procedure (%) | 76.58 ± 28.51 | 74.51 ± 35.09 | 0.829 |
| 6 months after the procedure (%) | 87.67 ± 18.51 | 83.25 ± 34.81 | 0.623 |
| Follow-up (%) | 88.37 ± 16.49 | 82.06 ± 37.13 | 0.504 |
Data are presented as mean ± standard deviation.
Both the anterior and middle vertebral compression in the two groups were improved after the procedure (P < 0.05). The difference before and after the procedure did not differ between the two groups. The postoperative local Cobb angles in the two groups were also significantly reduced (P < 0.05). There was no significant difference in the postoperative reduction between the two groups (Table 3).
Table 3.
Imaging-related parameters.
| Before operation | After operation | P | Difference | |
|---|---|---|---|---|
| Anterior compression | ||||
| Vesselplasty (%) | 58.17 ± 16.31 | 66.17 ± 9.36 | 0.017 | 8.01 ± 12.44 |
| Vertebroplasty (%) | 69.45 ± 15.18 | 76.38 ± 14.60 | 0.002 | 6.93 ± 13.64 |
| P | 0.779 | |||
| Middle compression | ||||
| Vesselplasty (%) | 60.48 ± 9.17 | 70.09 ± 7.78 | <0.001 | 9.61 ± 7.58 |
| Vertebroplasty (%) | 70.04 ± 11.61 | 75.81 ± 9.75 | 0.004 | 5.77 ± 12.35 |
| P | 0.238 | |||
| Local Cobb angle | ||||
| Vesselplasty | 19.72 ± 9.71 | 15.99 ± 8.79 | 0.017 | 3.73 ± 5.79 |
| Vertebroplasty | 15.22 ± 13.22 | 11.69 ± 12.97 | <0.001 | 3.53 ± 4.17 |
| P | 0.885 | |||
Data are presented as mean ± standard deviation.
The overall bone cement leakage rate in the vesselplasty group was 29.4%, which was significantly lower than that in the vertebroplasty group (67.4%) (P < 0.05). No bone cement leakage into the spinal canal occurred in the vesselplasty group. Thirteen patients in the vertebroplasty group had spinal canal leakage, with an incidence rate of 30.2%. The difference between the two groups was statistically significant (P < 0.05). There was no significant difference in intervertebral disc leakage. The rate of leakage in other areas was 23.5% in the vesselplasty group and 65.1% in the vertebroplasty group with a significant difference (P < 0.05). None of the patients in either group underwent reoperation because of bone cement leakage. The bone cement volume was significantly lower in the vesselplasty group than vertebroplasty group (3.79 ± 0.44 vs. 4.26 ± 0.85 mL, respectively; P < 0.05). The incidence of nerve injury and new vertebral fracture during follow-up was not different between the two groups (Table 4).
Table 4.
Complication-related parameters.
| Vesselplasty | Vertebroplasty | P | |
|---|---|---|---|
| Bone cement volume, mL | 3.79 ± 0.44 | 4.26 ± 0.85 | 0.008 |
| Bone cement leakage | |||
| Overall | 5 (29.4) | 29 (67.4) | 0.007 |
| Spinal canal | 0 (0.0) | 13 (30.2) | 0.012 |
| Intervertebral disc | 2 (11.8) | 3 (7) | 0.616 |
| Other area | 4 (23.5) | 28 (65.1) | 0.004 |
| Reoperation due to leakage | 0 (0.0) | 0 (0.0) | – |
| Nerve injury | 0 (0.0) | 1 (2.3) | 1.000 |
| New vertebral fracture | 1 (5.9) | 1 (2.3) | 0.490 |
Data are presented as mean ± standard deviation or n (%).
Discussion
Bone cement leakage is a common complication of vertebroplasty. Nieuwenhuijse et al. 13 found that the bone cement leakage rate in vertebroplasty reached 75.1%. Ryu et al. 14 reported that bone cement leaked into the epidural space in 26.5% of treated vertebrae in 40.3% of patients undergoing percutaneous vertebroplasty. Our study showed that in the vertebroplasty group, the overall bone cement leakage rate was 67.4% and the spinal canal leakage rate was 30.2%, which are similar to the above-reported data. According to Yeom et al., 4 bone cement leakage can be divided into three types: leakage via the basivertebral vein (type B), leakage via the segmental vein (type S), and leakage through a cortical defect (type C). Bone cement can leak into any place through a cortical defect. If cement is present in the foraminal area on the lateral X-ray, the positive prediction rate for spinal canal leakage is 86%. Bone cement leakage in the spinal canal can cause nerve injury with serious consequences. Intervertebral disc leakage can increase the probability of adjacent vertebral fracture. 15 Considering these facts, bone cement leakage in the present study was divided into spinal canal leakage, intervertebral disc leakage, and other area leakage. Spinal canal leakage usually occurs in the extradural area, but intradural leakage has also been reported. 16 Intravertebral clefts, cortical disruption, low cement viscosity, and high bone cement volume are major risk factors for cement leakage in vertebroplasty.13,14,17,18 In this study, the higher bone cement volume in the vertebroplasty group than in the vesselplasty group may have contributed to the higher bone cement leakage rate.
Vertebroplasty is associated with a high rate of bone cement leakage into the spinal canal in the treatment of OVCFs with posterior wall rupture. Ozsoy et al. 8 performed vertebroplasty to treat osteoporotic thoracolumbar fractures involving the posterior wall, and spinal canal leakage occurred in 1 of 12 patients. Vertebroplasty for the treatment of vertebral tumors with posterior wall rupture has been widely reported. Sun et al. 9 found that the risk of bone cement leakage was high when vertebroplasty was performed for metastatic spinal tumors with posterior wall deficiency. The risk increased with increasing severity of posterior wall defects. Some measures can be taken to reduce the probability of bone cement leakage. Cui et al. 19 stated that the bone cement volume should be reduced in patients with vertebral metastases with posterior wall rupture who undergo vertebroplasty. Basile et al. 6 reported that delayed bone cement injection was safe and effective in the treatment of osteolytic destruction or fracture of the posterior vertebral wall in patients with multiple myeloma. Dorsal leakage occurred in 2 of 34 vertebral bodies, but neurological symptoms were not aggravated.
The literature on vesselplasty is scarce. Vesselplasty has the advantage of a low bone cement leakage rate. 20 In 2007, Zheng et al. 21 conducted cadaver studies and found that treatment by the Vessel-X and kyphoplasty were similar in terms of restoring the mechanical strength and height of the fractured vertebra and that the Vessel-X was associated with less bone cement leakage. Flors et al. 22 applied the Vessel-X Bone Filling Container System to treat OVCFs and found that this treatment could significantly relieve pain, improve activity, and reduce the need for analgesia with no evidence of clinical complications. However, their study did not include patients with posterior wall rupture, nor did it establish a control group. In the present study, we compared vesselplasty and vertebroplasty for treatment of OVCFs with posterior wall rupture. We found that vesselplasty had a lower bone cement leakage rate, possibly because of the restraint effect of the cement container during injection. Notably, the vesselplasty group had a lower bone cement volume, which also decreased the possibility of bone cement leakage.
In this study, vesselplasty was performed exclusively with a unilateral approach. If vesselplasty is performed with a bilateral approach, two containers are used. This increases the cost and prolongs the operation time. In the unilateral approach, we attempted to place the container in the central part of the vertebral body to avoid deviating to the puncture side, thus obtaining a more balanced distribution of bone cement and ensuring a good treatment effect. Generally, vesselplasty takes less time to perform. However, the operation time was not statistically significant in our study. The reason for this may be the small sample size. In addition, vertebroplasty had been performed in our hospital for a longer time than vesselplasty. Our surgeons are more familiar with vertebroplasty procedures, thus reducing the operation time. This may also explain the lack of difference in the operation time in our study.
Vesselplasty is also suitable for the treatment of pathological vertebral fractures. Klingler et al. 23 reported that vesselplasty was beneficial to spinal stability, pain control, and improvement of body function in patients with pathological vertebral fractures, even in the case of posterior wall deficiency. In addition, no symptomatic bone cement leakage was found in their study. However, vesselplasty is also associated with a risk of bone cement leakage in the spinal canal. Yeh et al. 24 reported a case of an acute T6 compression fracture treated with vesselplasty. The balloon ruptured during the operation, and the bone cement leaked into the spinal canal. Despite emergency laminectomy and removal of the bone cement, the patient still developed paraplegia. Balloon rupture may be related to the heat generated from polymethyl methacrylate during polymerization.
Vertebral augmentation in the treatment of chronic OVCFs achieves short-term pain improvement and kyphotic angle recovery similar to those achieved for recent OVCFs. In the long term, however, a more severe kyphotic angle may rebound in patients with chronic OVCFs. 25 Cement leakage is generally similar in chronic OVCFs and recent OVCFs. Epidural vein leakage is dominant in recent OVCFs, whereas intervertebral disc leakage is dominant in chronic OVCFs.26,27 Two meta-analyses of Kummell’s disease were published this year. Zhang et al. 28 found that kyphoplasty and vertebroplasty had similar clinical effects in patients with Kummell’s disease but that kyphoplasty had less bone cement leakage, greater radiographic improvement, and more resource consumption. Cabrera et al. 29 reported that compared with vertebral augmentation alone, vertebral augmentation plus short-segment fixation might have better clinical and radiological outcomes for Kummell’s disease but is associated with more blood loss, a longer length of stay, and a longer operative time. A cleft in the vertebra is a typical imaging manifestation of chronic OVCFs. To avoid the influence of chronic OVCFs on our study results, we compared the proportion of clefts between the two groups and found no significant differences (Table 1).
The study has several limitations. First, OVCFs with posterior wall rupture are not common. Vesselplasty was introduced to our hospital only a short time before this study. The sample size in this study was small. However, there was a significant difference in the bone cement leakage rate between the two groups. The overall leakage rate was 29.4% in the vesselplasty group and 67.4% in the vertebroplasty group (P < 0.01). No spinal canal leakage occurred in the vesselplasty group, whereas the spinal canal leakage rate in the vertebroplasty group was 30.2%. In the future, randomized studies with large samples are needed to confirm this finding. Second, CT is certainly more accurate in assessing cement leakage than X-ray. However, to reduce the patient’s exposure to radiation and considering the low reoperation rate for bone cement leakage, we routinely perform X-ray examination, not CT, to assess radiological outcomes after vertebral augmentation. Thus, the true leakage rate may have been underestimated in this study. 30 Third, considering the short follow-up time in this study, long-term follow-up is needed to further compare the advantages and disadvantages of the two treatment methods.
Conclusions
Vesselplasty is a better treatment option than vertebroplasty for OVCFs with posterior wall rupture. Its overall bone cement leakage rate and spinal canal leakage rate are significantly lower. The pain relief and vertebral height recovery are similar between the two procedures.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Song-Hua Xiao https://orcid.org/0000-0001-6298-9633
References
- 1.Liu RQ, Chao AJ, Wang K, et al. Incidence and risk factors of medical complications and direct medical costs after osteoporotic fracture among patients in China. Arch Osteoporos 2018; 13: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Old JL andCalvert M.. Vertebral compression fractures in the elderly. Am Fam Physician 2004; 69: 111–116. [PubMed] [Google Scholar]
- 3.Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. Am J Neuroradiol 1997; 18: 1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 4.Yeom JS, Kim WJ, Choy WS, et al. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br Vol 2003; 85B: 83–89. [DOI] [PubMed] [Google Scholar]
- 5.Amoretti N, Diego P, Amelie P, et al. Percutaneous vertebroplasty in tumoral spinal fractures with posterior vertebral wall involvement: feasibility and safety. Eur J Radiol 2018; 104: 38–42. [DOI] [PubMed] [Google Scholar]
- 6.Basile A, Cavalli M, Fiumara P, et al. Vertebroplasty in multiple myeloma with osteolysis or fracture of the posterior vertebral wall. Usefulness of a delayed cement injection. Skeletal Radiol 2011; 40: 913–919. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Linden E Kroft LJM andDijkstra PDS.. Treatment of vertebral tumor with posterior wall defect using image-guided radiofrequency ablation combined with vertebroplasty: preliminary results in 12 patients. J Vasc Interv Radiol 2007; 18: 741–748. [DOI] [PubMed] [Google Scholar]
- 8.Ozsoy KM, Oktay K, Gezercan Y, et al . Percutaneous vertebroplasty for the treatment of osteoporotic thoracolumbar fractures with posterior body involved in elderly patients. Turk Neurosurg 2019; 29: 90–94. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, Yang Z, Xu Y, et al. Safety of percutaneous vertebroplasty for the treatment of metastatic spinal tumors in patients with posterior wall defects. Eur Spine J 2015; 24: 1768–1777. [DOI] [PubMed] [Google Scholar]
- 10.Liang L, Chen XL, Jiang WM, et al. Balloon kyphoplasty or percutaneous vertebroplasty for osteoporotic vertebral compression fracture? An updated systematic review and meta-analysis. Ann Saudi Med 2016; 36: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao G Liu X andLi F.. Balloon kyphoplasty versus percutaneous vertebroplasty for treatment of osteoporotic vertebral compression fractures (OVCFs). Osteoporosis Int 2016; 27: 2823–2834. [DOI] [PubMed] [Google Scholar]
- 12.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwenhuijse MJ Van Erkel AR andDijkstra S.. Cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: identification of risk factors. Spine J 2011; 11: 839–848. [DOI] [PubMed] [Google Scholar]
- 14.Ryu KS, Park CK, Kim MC, et al. Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg 2002; 96: 56–61. [DOI] [PubMed] [Google Scholar]
- 15.Lin EP, Ekholm S, Hiwatashi A, et al . Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. Am J Neuroradiol 2004; 25: 175–180. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YJ, Tan TS, Chen WH, et al. Intradural cement leakage - a devastatingly rare complication of vertebroplasty. Spine 2006; 31: E379–E382. [DOI] [PubMed] [Google Scholar]
- 17.Sun HB, Jing XS, Liu YZ, et al. The optimal volume fraction in percutaneous vertebroplasty evaluated by pain relief, cement dispersion, and cement leakage: a prospective cohort study of 130 patients with painful osteoporotic vertebral compression fracture in the thoracolumbar vertebra. World Neurosurg 2018; 114: E677–E688. [DOI] [PubMed] [Google Scholar]
- 18.Zhan Y, Jiang JZ, Liao HF, et al. Risk factors for cement leakage after vertebroplasty or kyphoplasty: a meta-analysis of published evidence. World Neurosurg 2017; 101: 633–642. [DOI] [PubMed] [Google Scholar]
- 19.Cui YP, Pan YX, Lei MX, et al. The first algorithm calculating cement injection volumes in patients with spine metastases treated with percutaneous vertebroplasty. Therap Clin Risk Manag 2020; 16: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Fiki M. Vertebroplasty, kyphoplasty, lordoplasty, expandable devices, and current treatment of painful osteoporotic vertebral fractures. World Neurosurg 2016; 91: 628–632. [DOI] [PubMed] [Google Scholar]
- 21.Zheng ZM, Luk KDK, Kuang GM, et al . Vertebral augmentation with a novel Vessel-X bone void filling container system and bioactive bone cement. Spine 2007; 32: 2076–2082. [DOI] [PubMed] [Google Scholar]
- 22.Flors L, Lonjedo E, Leiva-Salinas C, et al. Vesselplasty: a new technical approach to treat symptomatic vertebral compression fractures. Am J Roentgenol 2009; 193: 218–226. [DOI] [PubMed] [Google Scholar]
- 23.Klingler JH, Sircar R, Deininger MH, et al. Vesselplasty: a new minimally invasive approach to treat pathological vertebral fractures in selected tumor patients - preliminary results. Rofo-Fortschr Gebiet Rontgenstrahlen Bildgeb Verfahr 2013; 185: 340–350. [DOI] [PubMed] [Google Scholar]
- 24.Yeh KL, Wu SH, Wu SS, et al. Rare episode of cement leakage during vesselplasty in a case of vertebral compression fracture. World Neurosurg 2020; 137: 416–420. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Liu T, Yin P, et al. The therapeutic effects of percutaneous kyphoplasty on osteoporotic vertebral compression fractures with or without intravertebral cleft. Int Orthop 2019; 43: 359–365. [DOI] [PubMed] [Google Scholar]
- 26.Tanigawa N, Kariya S, Komemushi A, et al. Cement leakage in percutaneous vertebroplasty for osteoporotic compression fractures with or without intravertebral clefts. Am J Roentgenol 2009; 193: W442–W445. [DOI] [PubMed] [Google Scholar]
- 27.Jung JY Lee MH andAhn JM.. Leakage of polymethylmethacrylate in percutaneous vertebroplasty: comparison of osteoporotic vertebral compression fractures with and without an intravertebral vacuum cleft. J Comput Assist Tomogr 2006; 30: 501–506. [DOI] [PubMed] [Google Scholar]
- 28.Zhang BL, Chen GH, Yang XX, et al . Percutaneous kyphoplasty versus percutaneous vertebroplasty for neurologically intact osteoporotic Kummell’s disease: a systematic review and meta-analysis. Global Spine J 2021; 2192568220984129. DOI: 10.1177/2192568220984129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabrera JP, Camino-Willhuber G, Guiroy A, et al . Vertebral augmentation plus short-segment fixation versus vertebral augmentation alone in Kummell’s disease: a systematic review and meta-analysis. Neurosurg Rev 2021. DOI: 10.1007/s10143-021-01661-8. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt R, Cakir B, Mattes T, et al. Cement leakage during vertebroplasty: an underestimated problem? Eur Spine J 2005; 14: 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]