Abstract
Introduction:
Several treatment options are available for the management of older adults with newly diagnosed patients with Multiple Myeloma (MM) who are ineligible for hematopoietic cell transplantation (tiMM). We aimed to identify treatment options that provide the best balance in terms of efficacy and safety.
Methods:
We searched bibliographic databases and meeting libraries for search terms reflecting newly diagnosed and older and/or transplant-ineligible patients from inception to October 21, 2018. Phase II/III randomized trials comparing at least two first line treatment regimens for newly diagnosed tiMM were included. We extracted data on efficacy (progression free survival, PFS, overall survival and overall response rate) and safety (grade ¾ toxicities) and conducted network meta-analysis using Bayesian methods and random effects models. Relative ranking of treatment regimens was assessed using Surface under the cumulative ranking (SUCRA) probabilities.
Results:
We identified 27 trials involving 12,194 patients. For PFS, the four most effective regimens were: Daratumumab, Bortezomib, Melphalan and Prednisone (SUCRA 0.960) followed by Daratumumab, lenalidomide and dexamethasone (Dara_RD, SUCRA 0.847), Bortezomib, melphalan, prednisone, thalidomide maintenance with bortezomib-thalidomide (SUCRA 0.834) and Bortezomib, Lenalidomide and Dexamethasone (SUCRA 0.739). Among these four most efficacious regimens, toxicity profile was most favorable for Dara_RD (median additional AEs per patient vs dexamethasone=0.74; 95% CrI 0.51-1.17; SUCRA 0.430).
Conclusion:
Among first line tiMM regimens, increasing efficacy is associated with increased toxicity. We provide relative ranking of these regimens for both efficacy and safety. Future studies should incorporate geriatric assessments and frailty biomarkers to refine treatment decision-making for each individual patient.
Keywords: First-line therapy, Multiple Myeloma, Transplant Ineligible, meta-analysis, systematic revie
Introduction:
Multiple Myeloma (MM) is the second most common hematological malignancy in the United States with 32,000 cases per year and accounting for 13,000 deaths in 2019(1). With a median age at diagnosis of 69 years, majority (63%) of are 65 years or older at diagnosis(1).
Initial treatment of patients with newly diagnosed MM depends on their ability to safely undergo high dose chemotherapy and autologous hematopoietic cell transplantation (HCT)(2). Randomized controlled trials have evaluated the benefit of HCT mostly among patients less than 65 years although recent evidence argues that chronological age by itself is not a contraindication for HCT(2). Conversely, those with advanced age or with significant comorbidities are considered ineligible for HCT (tiMM) (3).
Several first-line treatment options exist for tiMM(3). There is a lack of consensus as to what constitutes the best frontline regimen. Since these patients are often older and frail, an ideal regimen should balance maximal efficacy with minimal toxicity. Hence, in order to guide clinical decision-making, we performed this Bayesian network meta-analysis (NMA) of efficacy and toxicity data from all existing randomized controlled trials (RCT) among newly diagnosed tiMM.
Methods:
We performed this systematic review in accordance to a pre-published protocol (PROSPERO CRD42018115364). Our findings have been reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension statement for network meta-analysis(4).
Search Strategy and Selection Criteria:
A medical librarian (AG) designed search strategy using the following databases: Ovid Medline, Ovid EMBASE, PubMed, Web of Science Core Collection, and the Cochrane Library from inception of database to October 21 2018 (Supplementary Appendix). We used a combination of controlled vocabulary and keywords with various synonyms that reflected concepts of MM combined with newly diagnosed and older and/or transplant ineligible patients. Our search result was limited to English language and RCT as the publication type. Additionally, we performed a gray literature search through, 1) manual hand search of bibliographies of identified RCTs 2) trial registries (Clinicaltrials.gov, and World Health Organization, WHO International Clinical Trials Registry Platform) and (3) conference proceedings of American Society of Hematology, American Society of Clinical Oncology, European Hematology Association and European Society of Medical Oncology from 2016-2018.
Citations from all databases were imported into Endnote X8™ database. After removing, remaining set of articles was ingested into Covidence™, a screening and data extraction tool(5). Two independent screeners performed title and abstract review (SG & MRA) with a third screener (BD) to resolve ties.
Selection Criteria:
After preliminary screening, two authors (SG & MRA) independently reviewed full texts of potentially eligible studies to confirm final eligibility. We used the following selection criteria; a) All phase II and phase III randomized clinical trials comparing treatment regimens for the management of newly diagnosed patients with tiMM b) RCT including crossover, cluster and patient-randomized clinical trials c) excluded studies involving radiation therapy or surgery as primary therapy d) excluded studies employing bone-modifying agents as primary therapy.
Data Extraction:
Two authors in duplicate (SG and MRA) extracted data including study characteristics (first author, year of publication, sample size, treatment regimens, duration of follow up), baseline characteristics of the participants (age, sex, stage) and outcome data (efficacy and toxicity as defined below) in piloted forms. Quality assessment was done using Cochran’s risk of bias assessment tool(6).
Definition of Outcomes:
The primary efficacy outcome was progression free survival (PFS), defined as time from randomization to the date of first confirmed progression or date of death, whichever earlier. We quantified effect measure in terms of hazard ratio (HR) along with 95% credible interval (CrI). If HR was not reported in the primary study, we estimated HR using published Kaplan Meier survival curves using the method outlined by Guyot et al using R software(7). If multiple publications were available from the same study, one with the longest available follow-up results was used. Secondary efficacy outcomes included overall response rate (ORR) as defined by the International Myeloma Working Group (8), and overall survival (OS) defined as the time from randomization to the date of death.
The primary safety outcome was the rate of all cause and common grade 3 or 4 adverse events (AE) as reported in the primary studies.
Statistical analysis:
Using study-level data, a Bayesian random-effect hierarchical model was fit with non-informative priors of uniform distribution (0, 5) and adjusting for correlation between effects in multi-arm trials. We generated posterior samples using Markov Chain Monte-Carlo (MCMC) simulation technique running the analysis in four parallel chains. We used a series of 5,000 burn-in simulations to allow convergence and then a further 2,000 simulations (succeeding 5,000 simulations saved at an interval of 10 in each chain) to produce the outputs. Convergence was assessed using Gelman and Rubin’s MCMC Convergence Diagnostic with Potential Scale Reduction Factor (PSRF) value close to 1 indicating approximate convergence(9). The Bayesian model introduces a random effect representing any changes in the observed treatment effect that may be due to the comparison being made. The variability in this random effect was interpreted as incoherence. Assessment of inconsistency was performed by node-splitting approach, in which disagreements between direct and indirect estimates were assessed(10). The model goodness-of-fit was evaluated by the deviance information criterion (DIC).
For the analysis of efficacy, we used a complementary log-log link function for analysis of OS and PFS whereas for ORR we used a logit linked function. The model preserved randomized treatment comparisons within trials. For the analysis of toxicities, a multivariate Bayesian probit-normal regression model was fitted to the observed number of AEs reported by at least 50% of the studies, which were subjected to further analysis in an arm-based meta-analytic approach, based on the Bayesian hierarchical model for a univariate binary outcome proposed by Zhang et al(11) and its extension to multivariate outcomes by Hong et al(12). The oldest treatment (i.e Dexamethasone) was selected as a reference treatment against which relative efficacy and safety of all other regimens was estimated. The notation and modeling of toxicity analysis is described in Supplementary Appendix.
To ensure interpretability of the NMA results, the geometry of the network, results with probabilistic statements, and estimates of interventions effects along with their corresponding 95 % Credible Intervals (CrIs), as well as forest plots were presented. We ranked the intervention and reported each interventions’ probability of ranking first (being the best treatment) as well as the surface under the cumulative ranking curve (SUCRA) values(13). High SUCRA values are expected for the best treatments, and low SUCRA values are expected for the worst treatments. All statistical analyses were conducted using netmeta package in R version 3.5.1, JAGS 4.30 and WinBUGS 1.4.3.
Results:
After screening 5587 titles/abstracts and 128 full text articles, we identified 27 clinical trials for further analyses (Figure 1). This included 3 phase II studies (14–16) and 24 phase III studies encompassing a total of 12,194 patients evaluating 25 different treatment regimens. All but two studies defined tiMM as age ≥ 65 years (17, 18), significant comorbidity, physician’s discretion or patient decision. SWOG S0777 (19) allowed younger patients without intent for immediate stem cell transplant. The median age ranged from 63-79 years and 47% were females. Overall, 1679 of 6646 patients (25.3%) had an Eastern Cooperative Oncology Group performance status≥2; this proportion ranged from 9.9% to 50% across 10 evaluable studies. Similarly, 738 of 3370 across eight trials (21.9%) had high-risk cytogenetics. The baseline summary characteristics are shown in Table 1.
Figure 1:
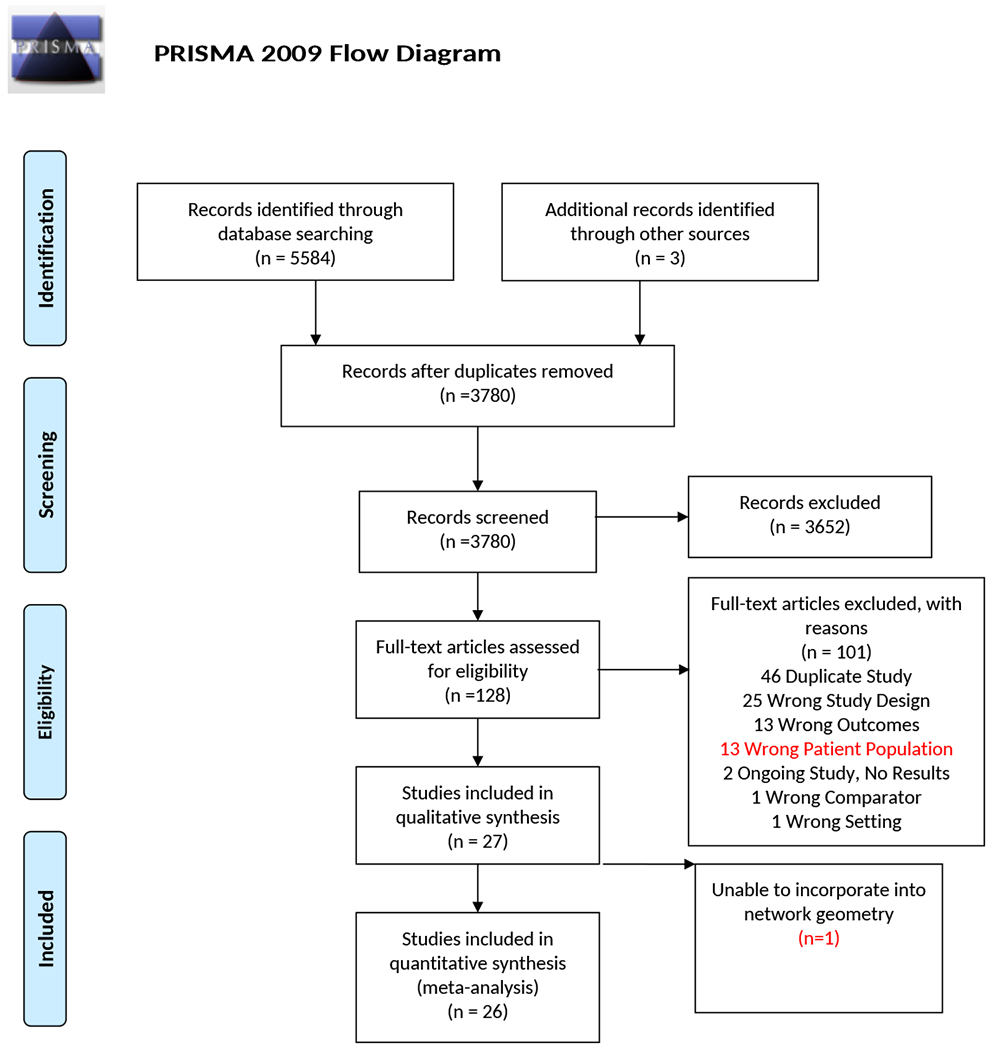
PRISMA flow diagram illustrating the process of study selection. After initial librarian guided search and removal of duplicates, two authors independently screened the title and abstract, followed by full text review of inclusion and exclusion criteria. A total of 27 studies were included in the final quantitative synthesis.
Table 1:
Summary Characteristics of clinical trials
| Trial Name or Author/Year | Experimental | Control | Number of patients (Exp/Ctrl) | Median age; years(range) | Female % | ISS III % | Median Follow up (months) | Primary end point |
|---|---|---|---|---|---|---|---|---|
| IFM 95-01/2006 | MP* | Dex vs Mel_Dex vs Dex_IFN | 122/127/118/121 | 70 (NA) | 49% | NA | 82.8 | OS |
| IFM 01-01/2006 | MPT* | MP | 113/116 | 78.5 (75-89) | 54% | 32.2% | 47.5 | OS |
| GIMEMA/2006 | MPT-T* | MP | 167/164 | 72 (NR) | 46% | NA | 38.3 vs 37.7 | RR/EFS |
| IFM 99-06/2007 | MPT* | MP vs Mel100 | 125/196/126 | NR | 46% | 30.9% | 51.5 | OS |
| MM003/2008 | TD* | Dex | 235/235 | 65 (38-83) | 49.4% | NA | 18 | ORR |
| VISTA/2008 | VMP* | MP | 304/295 | 71 (48-91) | 50% | 34.4% | 60.1 | TTP |
| HOVON49/2009 | MPT-T* | MP | 165/168 | 72 (65-87) | 44% | 27.1% | 39 | EFS |
| Ludwig / 2009 | TD | MP | 145/143 | 72 (54-86) | 50% | 67% | 28.1 | PFS |
| NMSG / 2010 | MPT-T* | MP | 182/175 | 74 (NA) | 44% | 42.2% | 42 | OS |
| PETHEMA / 2010 | VMP | VTP | 130/130 | 73 (NA) | NA | 33.4% | 32 | ORR |
| S0232 / 2010 | RD* | Dex | 97/95 | 54 (NA) | 43.8% | 26.0% | 47.2 | PFS |
| GIMEMA0305/2010 | VMPT-VT* | VMP | 254/257 | 71 (NA) | 50.7% | 25.5% | 23.2 | PFS |
| TMSG / 2011 | MPT* | MP | 58/57 | 70 (NA) | 46% | 41.7% | 23 | ORR/Toxicity |
| Sacchi / 2011 | MPT* | MP | 64/54 | 77 (66-89) | 53% | 27% | 30 | Unclear |
| MRC-IX / 2011 | CTD* | MP | 426/423 | 73 (57-89) | 44.3% | 44.1% | 44 | ORR/PFS/OS |
| MM-015 / 2012 | MPR_R* | MPR vs MP | 152/153/154 | 71 (65-92) | 50.3% | 49.9% | 30 | PFS |
| FIRST / 2014 | RD* | RD18 vs MPT | 535/541/547 | 73 (40-92) | 47% | 41.6% | 67 | PFS |
| San-Miguel / 2014 | VMP_Siltuximab | VMP | 52/54 | 70 (48-90) | NA | 53.7% | 22 | ORR |
| UPFRONT / 2015 | VMP | VD vs VTD | 167/168/167 | 73 (NR) | 50% | 33.6% | 42.7 | PFS |
| E1A06 / 2015 | MPR_R | MPT_T | 152/154 | 76 (54-92) | 45.4% | 31.2% | 40.7 | PFS |
| HOVON87 /2016 | MPR_R | MPT_T | 319/318 | 72 (60-91) | 45.7% | 26.3% | 36 | PFS |
| GEMOH/ 2016 | MPT | CTD vs TD | 32/32/18 | 71 (NA) | 56.1% | 40.5% | 37.5 | ORR |
| EMN01/ 2016 | MPR_R | CPR vs RD | 218/222/222 | 73 (NA) | 52.8% | 27.1% | 39 | PFS |
| Mateos/ 2016 | VMP_RD_seq | VMP_RD_alt | 118/115 | 74 (NA) | NA | 31,8% | 30.3 | PFS/Toxicity |
| S0777 / 2017 | VRD* | RD | 242/229 | 63 (NA) | 41.6% | 33.3% | 55 | PFS |
| ALCYONE/ 2018 | VMP_Dara* | VMP | 350/356 | 71 (40-93) | NA | 38.4% | 27.8 | PFS |
| MAIA/ 2019 | Dara_RD* | RD | 368/369 | 73 (45-90) | 47.9% | 29.4% | 28 | PFS |
Exp/Ctrl, Experiment/Control arm; ISS, International staging system; OS, overall survival; PFS, progression free survival; EFS, event free survival; ORR, overall response rate; TTP, time to progression.
Identifies superior regimen within each study based on the primary endpoint. Lack of asterisk indicates that there was no clear superior regimen among the various arms of the trial based on the primary endpoint.
The different study regimens were as follows: 1) Dexamethasone (Dex) 2) Dexamethasone-Interferon alpha (Dex_IFN) 3) Melphalan 100 (MEL100), 4) Melphalan Dexamethasone (Mel_DexD) 5) Melphalan Prednisone (MP) 6) Thalidomide Dexamethasone (TD) 7) Continuous Lenalidomide Dexamethasone (RD) 8) Lenalidomide Dexamethasone for 18 cycles (RD18) 9) Bortezomib Dexamethasone (VD) 10) Melphalan Prednisone Thalidomide (MPT) 11) Melphalan Prednisone Thalidomide followed by Thalidomide maintenance (MPT_T) 12) Melphalan Prednisone Lenalidomide (MPR) 13) Melphalan Prednisone Lenalidomide followed by Lenalidomide maintenance (MPR_R) 14) Cyclophosphamide Prednisone Lenalidomide (CPR) 15) Cyclophosphamide Thalidomide Dexamethasone (CTD) 16) Bortezomib Melphalan Prednisone (VMP) 17) Daratumumab Lenalidomide Dexamethasone (Dara_RD) 18) Bortezomib Thalidomide Prednisone (VTP) 19) Bortezomib Thalidomide Dexamethasone (VTD) 20) Bortezomib Lenalidomide Dexamethasone (VRD) 21) Bortezomib Melphalan Prednisone Siltuximab (VMP_Siltuximab) 22) Bortezomib Melphalan Prednisone Thalidomide followed by Bortezomib Thalidomide maintenance (VMPT_VT) 23) Bortezomib Melphalan Prednisone followed by Lenalidomide Dexamethasone in a sequential (VMP_RD_seq) or 24) alternating regimen (VMP_RD_alt) and 25) VMP plus Daratumumab (VMP_Dara).
Risk of Bias:
Of the 27 studies, 19 had low risk for bias in random sequence generation (selection bias, 70%) and 20 in allocation concealment (selection bias, 74%). All but four studies were open label studies and blinding of outcome assessment was done by nine studies (detection bias, 33%). All studies had low risk for bias of incomplete outcome data (attrition bias) or selective reporting (reporting bias) (Figure S1).
Network Geometry:
Twenty-six clinical trials comparing 23 treatment regimens were incorporated into one network as shown in Figure 2. One trial compared VMP and RD in sequential versus alternative regimens that could not be connected to the network geometry and excluded from further analysis (16). To decrease the nodes, we made following assumptions: a) we separated MPT regimens into fixed versus indefinite thalidomide regimens. Four of seven trials used thalidomide maintenance (14, 17, 18, 20–23). Only a minority of patients (8%) in Sacchi et al received thalidomide maintenance(14). The remaining three studies were reclassified as MPT_T arm similar to ECOG E1A06 (24) and HOVON 87(25) study. b) CTD arm in Hungria et al(26) and that studied by Morgan et al(27) were considered identical regimens, since the cumulative dosing of thalidomide, cyclophosphamide and dexamethasone were similar and c) In Magarotto et al(28), all patients received indefinite lenalidomide maintenance; hence the RD arm was considered equivalent to RD continuous in FIRST trial, SWOG S0777 and Zonder et al(19, 29, 30), and MPR arm was considered equivalent to MPR_R in ECOG E1A06(24) and HOVON 87(25) study.
Figure 2:
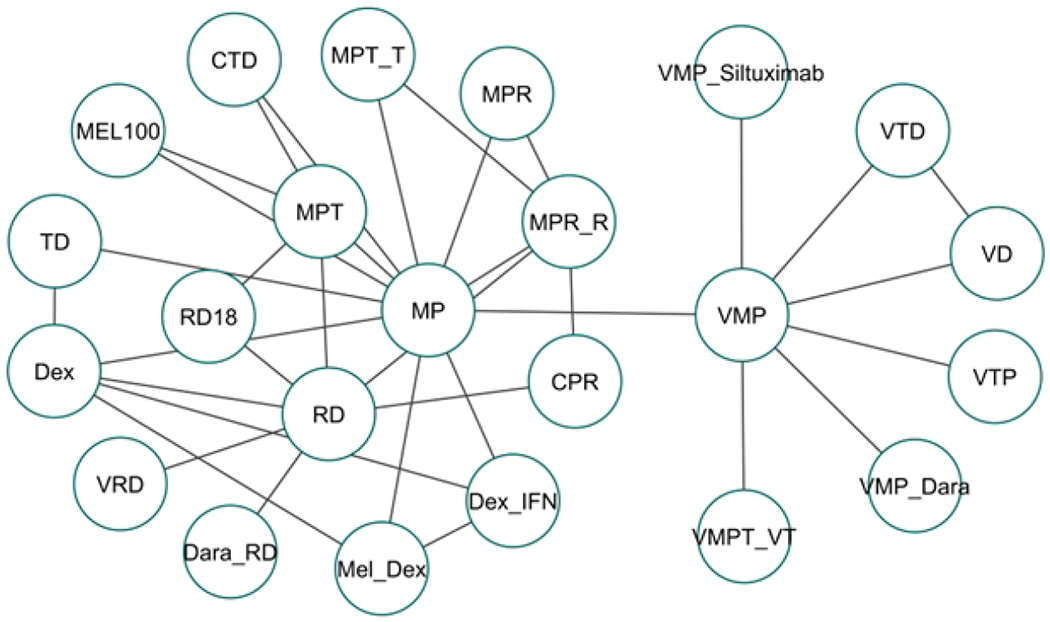
Network Geometry for analysis of progression free survival. The network geometry shows various pairwise comparisons of various treatment regimens across multiple clinical trials.
Validity of our Network Meta-analysis:
We assessed for the assumption of transitivity by conducting qualitative review of included studies. The eligibility criteria including definition of transplant ineligibility and other summary characteristics of the included trials are quite comparable as shown in Table 1. Next we tested for consistency, statistical agreement between the direct and indirect comparisons, using a node splitting approach, and found that for most comparisons, the direct and indirect comparisons did not show a significant difference. We found a good convergence of the MCMC simulation using Gelman-Rubin diagnostics with a PSRF of 1.01 (Table S4).
Network meta-analysis of the Efficacy outcome:
Table 2 and Figure 3 summarizes the efficacy outcomes of the various trials. We analyzed efficacy results using progression free survival (PFS) as our primary outcome as well as performed analysis for overall survival (OS) and overall response rates (ORR). Our network meta-analysis suggested that triplets and quadruplet combination regimens were highly effective compared to single agents or doublet therapies. The four most effective regimens in terms of PFS included VMP_Dara (SUCRA 0.960) followed by Dara_RD, SUCRA 0.847), VMPT_VT (SUCRA 0.834) and VRD (SUCRA 0.734). For overall survival, after updating recently published mature OS data from ALCYONE trial(31), VMP_Dara ranked the highest (SUCRA 0.908) followed by VRD (SUCRA 0.854) and Dara_RD (SUCRA 0.783), with the caveat that mature overall survival data was not yet available for Dara-RD regimen from the MAIA trial. Lastly, in terms of ORR, the top three regimens included VMP_Dara (SUCRA 0.955), Dara_RD (0.939) and VRD (SUCRA 0.819) (Table 2). Figure 3 summarizes these findings in terms of relative hazard ratios comparing each regimen against dexamethasone as the reference treatment.
Table 2:
Treatments ranked by the surface under the cumulative ranking curve (SUCRA). Treatments with higher values of SUCRA are more beneficial.
| Treatment Arm | SUCRA for PFS | SUCRA for ORR | SUCRA for OS | SUCRA for AE |
|---|---|---|---|---|
| VMP_Dara | 0.960 | 0.951 | 0.908 | 0.279 |
| Dara_RD | 0.848 | 0.940 | 0.783 | 0.430 |
| VMPT_VT | 0.835 | 0.812 | 0.644 | 0.224 |
| VRD | 0.739 | 0.818 | 0.837 | 0.331 |
| VTP | 0.728 | 0.583 | 0.719 | 0.713 |
| VTD | 0.693 | 0.790 | 0.517 | 0.751 |
| VMP | 0.639 | 0.575 | 0.619 | 0.410 |
| VMP_Siltuximab | 0.613 | 0.783 | 0.562 | 0.095 |
| MPR_R | 0.586 | 0.414 | 0.329 | 0.153 |
| VD | 0.569 | 0.632 | 0.491 | 0.856 |
| MPT_T | 0.550 | 0.304 | 0.391 | 0.373 |
| MPT | 0.536 | 0.358 | 0.600 | 0.300 |
| CPR | 0.534 | 0.405 | 0.580 | 0.498 |
| CTD | 0.475 | 0.538 | 0.616 | 0.596 |
| Mel_Dex | 0.372 | 0.444 | 0.471 | 0.815 |
| RD | 0.534 | 0.629 | 0.625 | 0.548 |
| RD18 | 0.368 | 0.613 | 0.656 | 0.678 |
| Mel100 | 0.257 | 0.326 | 0.318 | 0.000 |
| TD | 0.238 | 0.211 | 0.060 | 0.802 |
| MP | 0.222 | 0.038 | 0.250 | 0.705 |
| MPR | 0.217 | 0.214 | 0.176 | 0.074 |
| Dex_IFN | 0.088 | 0.059 | 0.243 | 0.907 |
| Dex | 0.038 | 0.056 | 0.229 | 0.971 |
PFS, progression free survival; ORR, overall response rate; OS, overall survival; AE, adverse events.
Figure 3:
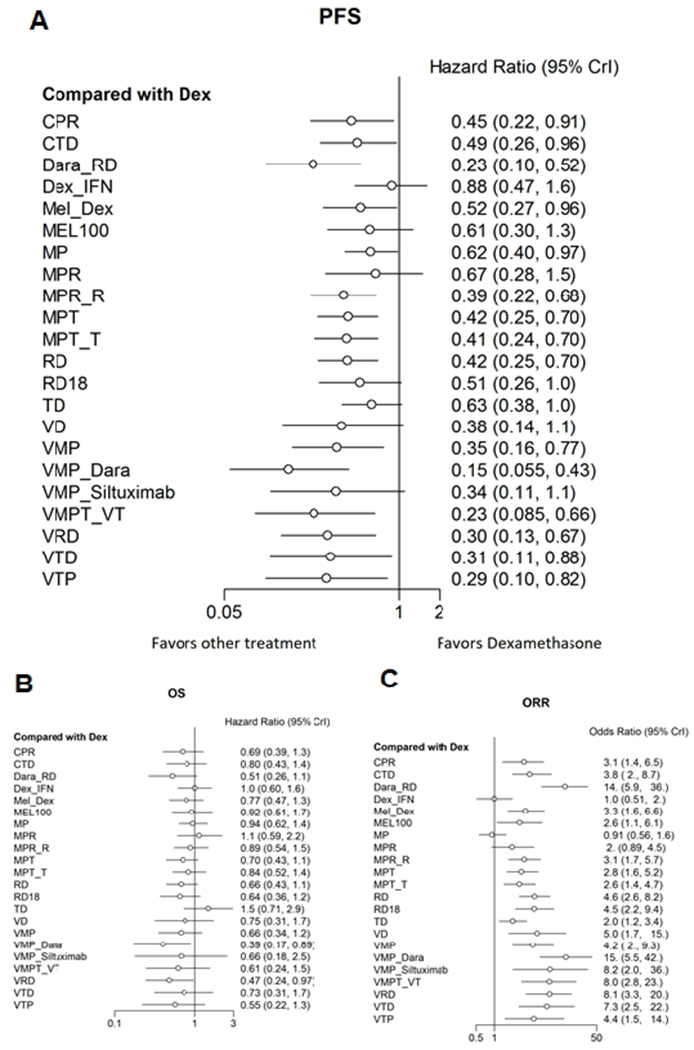
Forrest plot showing comparative efficacy outcomes among various frontline regimens for newly diagnosed transplant ineligible patients with Multiple Myeloma. As compared to dexamethasone, the four most effective regimens in terms of PFS (Panel A) included VMP_Dara (HR 0.15, 95% CI 0.06-0.43) followed by Dara_RD (HR 0.23, 95% CI 0.10-0.52), VMPT_VT (HR 0.23, 95% CI 0.09-0.66) and VRD (HR 0.30, 95% CI 0.13-0.67). Similar results were seen for Overall Survival (Panel B) and Overall Response Rate (Panel C).
Network meta-analysis of the Safety outcome:
We selected seven AEs reported by over 50% of the studies which were included in our toxicity analysis (Figure S2, Figure S3). Not surprisingly, combination regimens involving three or more drugs had increased toxicity rates as compared to single or two drug regimens. Our analysis showed that as compared to dexamethasone, the expected additional number (with 95% credible interval) of AEs for each study patient was the highest in the following arms: reduced intensity transplantation (MEL-100, 3.449, 95% CrI 3.31-3.59, SUCRA 0), followed by Melphalan, prednisone and lenalidomide, MPR (1.38, 95% CrI 1.09-1.81; SUCRA 0.074) and VMP plus Siltuximab (1.34, 95% CrI 0.92-1.97; SUCRA 0.095) (Table 2, Figure S2).
Association of efficacy with toxicity:
The association between efficacy and toxicity among the various study regimens is shown in Figure 4. Regimens with increased efficacy were also associated with increased rates of toxicity. Of the four most efficacious regimens, toxicity profile was most favorable for Dara_RD (median additional AEs per patient vs dexamethasone=0.74; 95% CrI 0.51-1.17; SUCRA 0.430) followed by VRD (median additional AE 0. 91; 95% CI 0.41-1.55; SUCRA 0.331), VMP_Dara (median additional AE 0.91; 95% CrI 0.65-1.34; SUCRA 0.279) and VMPT_VT (median additional AE 0.98; 95% CrI 0.79-1.24; SUCRA 0.224).
Figure 4:
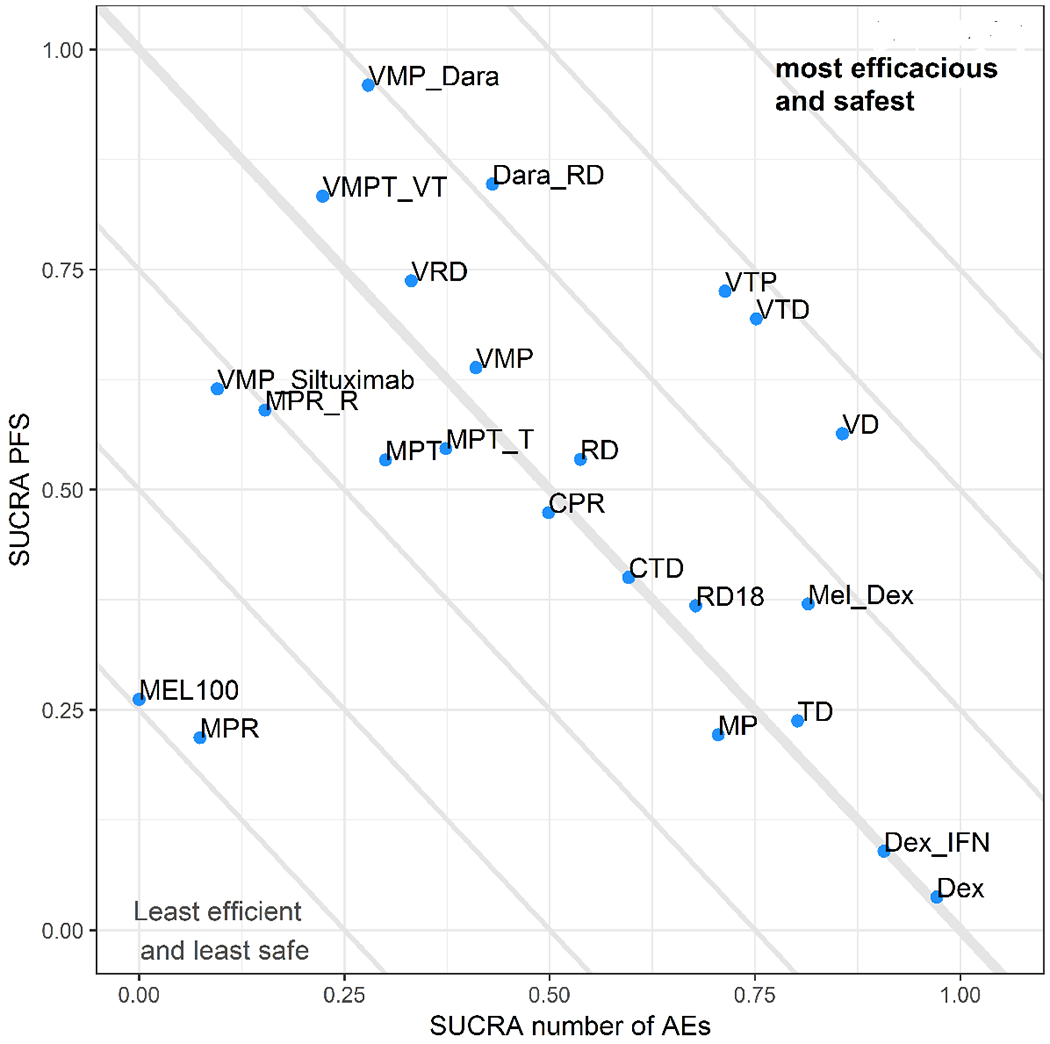
Scatter Plot of Surface under Cumulative Ranking Curve (SUCRA) for efficacy and safety. In this scatterplot, progression free survival is plotted on Y-axis and the number of grade ¾ adverse events is plotted on X-axis. Treatments close to upper-right corner tends to be beneficial in terms of both efficacy and safety. Among the four most efficacious regimens, Daratumumab, Lenalidomide, and Dexamethasone (Dara_RD) provides the best balance between efficacy and safety.
Sensitivity Analysis:
We tested the validity of our results against key assumptions by performing the following sensitivity analysis a) re-grouping the study by Waage et al(22), Palumbo et al(21) and Wijermans et al(23) as MPT instead of MPT_T b) separating CTD regimen studied by Hungria et al(26) and Morgan et al(27), characterizing the latter as an attenuated CTD regimen (CTDa) and c) removing SWOG S077 study(19) from our analyses. The key efficacy results remained largely unchanged in our sensitivity analyses. (Figures S4, S5, S6)
Discussion:
TiMM patients often involve older and frail patients where treatment efficacy must be balanced with toxicity. Our findings demonstrate that increasing efficacy of first-line regimens is often associated with increased toxicity as well. VMP_Dara, Dara-RD, VMPT_VT and VRD were likely to be the four most efficacious agents for tiMM. Among these four regimens, Dara-RD followed by VRD offered the best balance in terms of maximizing efficacy and minimizing toxicity. To our knowledge this is the first network meta-analysis that indirectly evaluated efficacy as well as toxicity results both of which are equally important for patients and providers for decision making.
Prior to our study, Weisel et al performed a systematic literature review and network meta-analysis of treatments for patients with tiMM (32). The authors identified and analyzed 17 clinical trials published before June 2015 and concluded that lenalidomide and dexamethasone (RD) was associated with a significant PFS and OS advantage versus other first line treatments. More updated results were recently reported by Blommenstein et al, additionally incorporating two major studies: SWOG S0777(19) and ALCYONE(33) study and analyzing a total of 24 studies published till date. The authors reported that the Dara-VMP and VMPT-VT were among the most effective first line treatments for tiMM (34). However, neither of these studies analyzed toxicity, an equally important outcome for patients and providers, leaving an important gap in the literature. In addition to providing results on both efficacy and safety outcomes, our study also incorporates the recently published practice changing MAIA study(35), hence generating a more updated summative evidence on this topic.
In our study, we found that a greater efficacy from newer treatment regimens was also associated with an increased risk of toxicity. Hence, there is a need to personalize treatment strategies based on their anticipated tolerance of treatment. Chronological age does not adequately capture this vulnerability due to marked heterogeneity in the aging process(36). In this regard, Comprehensive Geriatric Assessments (CGA) may be a useful tool to capture frailty and may help guide treatment selection among these patients. Recently, the International Myeloma Working Group (IMWG)(37) and others(38) have proposed frailty assessment tools that enable identification of frail individuals with MM at an increased risk of treatment related toxicities(36). Incorporation of frailty assessment tools in future clinical trials testing tiMM may provide additional guidance on clinical decision making in the future.
The validity of any NMA relies on certain key assumptions. First, we tested for transitivity, i.e the study population was sufficiently homogenous across trials. Our study population comprised of previously untreated patients with symptomatic Multiple Myeloma and definition of transplant ineligibility was fairly consistent across trials. We also found that the baseline characteristics of study participants were quite comparable across trials. We found good consistency, i.e statistical agreement between direct and indirect evidence as well as convergence i.e an indication that the data distribution fits the Bayesian model well. Lastly, our network geometry was simplified with certain assumptions, and we conducted appropriate sensitivity analyses to ensure that our results were not reliant on these assumptions.
Despite having several potential strengths, our study has few important limitations. While we used an exhaustive search of the literature, we could have missed unpublished literature resulting in publication bias. We did not report on cost of therapy, symptom burden and patient reported quality of life: all of which are equally important for this study population. Given the lack of individual patient data, we were unable to study the impact of patient characteristics and other covariates on study outcomes. Lastly, frailty was not uniformly measured across various studies and we were unable to study its impact on regimen related toxicity.
In conclusion, our study provides a comparative analysis of efficacy and safety endpoints of various frontline regimens for the management of patients tiMM. We found that triplet and quadruplet novel-agent based regimens had the greatest efficacy, but also led to increasing toxicity. While Dara-VMP was most likely to be the most efficacious regimen in terms of PFS, Dara-RD provided the best balance between efficacy and safety. Future studies should include incorporate geriatric assessment and frailty biomarkers to further refine treatment selection in this vulnerable population.
Supplementary Material
Acknowledgments
None of the authors have relevant conflicts of interest to disclose. Han Yu was supported by National Cancer Institute (NCI) grant P30CA016056
No relevant funding to declare
References:
- 1.National Cancer Institute Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Multiple Myeloma 2019. [Available from: https://seer.cancer.gov/statfacts/html/mulmy.html.
- 2.Mikhael J, Ismaila N, Cheung MC, Costello C, Dhodapkar MV, Kumar S, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol. 2019;37(14):1228–63. [DOI] [PubMed] [Google Scholar]
- 3.Mikhael J, Ismaila N, Cheung MC, Costello C, Dhodapkar MV, Kumar S, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. Journal of Clinical Oncology. 2019;37(14):1228–63. [DOI] [PubMed] [Google Scholar]
- 4.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 5.Cleo G, Scott AM, Islam F, Julien B, Beller E. Usability and acceptability of four systematic review automation software packages: a mixed method design. Systematic reviews. 2019;8(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Medical Research Methodology. 2012;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. [DOI] [PubMed] [Google Scholar]
- 9.Gelman A, Rubin DB. Inference from Iterative Simulation Using Multiple Sequences. Statistical Science. 1992;7(4):457–72. [Google Scholar]
- 10.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932–44. [DOI] [PubMed] [Google Scholar]
- 11.Lin L, Zhang J, Hodges JS, Chu H. Performing Arm-Based Network Meta-Analysis in R with the pcnetmeta Package. Journal of statistical software. 2017;80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong H, Chu H, Zhang J, Carlin BP. A Bayesian missing data framework for generalized multiple outcome mixed treatment comparisons. Research synthesis methods. 2016;7(1):6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. [DOI] [PubMed] [Google Scholar]
- 14.Sacchi S, Marcheselli R, Lazzaro A, Morabito F, Fragasso A, Di Renzo N, et al. A randomized trial with melphalan and prednisone versus melphalan and prednisone plus thalidomide in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplant. Leuk Lymphoma. 2011;52(10):1942–8. [DOI] [PubMed] [Google Scholar]
- 15.San-Miguel J, Blade J, Shpilberg O, Grosicki S, Maloisel F, Min CK, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. 2014;123(26):4136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateos MV, Martinez-Lopez J, Hernandez MT, Ocio EM, Rosinol L, Martinez R, et al. Sequential vs alternating administration of VMP and Rd in elderly patients with newly diagnosed MM. Blood. 2016;127(4):420–5. [DOI] [PubMed] [Google Scholar]
- 17.Beksac M, Haznedar R, Firatli-Tuglular T, Ozdogu H, Aydogdu I, Konuk N, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86(1):16–22. [DOI] [PubMed] [Google Scholar]
- 18.Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–70. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–18. [DOI] [PubMed] [Google Scholar]
- 21.Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513):825–31. [DOI] [PubMed] [Google Scholar]
- 22.Waage A, Gimsing P, Fayers P, Abildgaard N, Ahlberg L, Bjorkstrand B, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9):1405–12. [DOI] [PubMed] [Google Scholar]
- 23.Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160–6. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AK, Jacobus S, Fonseca R, Weiss M, Callander NS, Chanan-Khan AA, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood. 2015;126(11):1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zweegman S, van der Holt B, Mellqvist UH, Salomo M, Bos GM, Levin MD, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127(9):1109–16. [DOI] [PubMed] [Google Scholar]
- 26.Hungria VT, Crusoe EQ, Maiolino A, Bittencourt R, Fantl D, Maciel JF, et al. Phase 3 trial of three thalidomide-containing regimens in patients with newly diagnosed multiple myeloma not transplant-eligible. Ann Hematol. 2016;95(2):271–8. [DOI] [PubMed] [Google Scholar]
- 27.Morgan GJ, Davies FE, Gregory WM, Russell NH, Bell SE, Szubert AJ, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118(5):1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magarotto V, Bringhen S, Offidani M, Benevolo G, Patriarca F, Mina R, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127(9):1102–8. [DOI] [PubMed] [Google Scholar]
- 29.Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–17. [DOI] [PubMed] [Google Scholar]
- 30.Zonder JA, Crowley J, Hussein MA, Bolejack V, Moore DF Sr., Whittenberger BF, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood. 2010;116(26):5838–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395(10218):132–41. [DOI] [PubMed] [Google Scholar]
- 32.Weisel K, Doyen C, Dimopoulos M, Yee A, Lahuerta JJ, Martin A, et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leuk Lymphoma. 2017;58(1):153–61. [DOI] [PubMed] [Google Scholar]
- 33.Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378(6):518–28. [DOI] [PubMed] [Google Scholar]
- 34.Blommestein HM, van Beurden-Tan CHY, Franken MG, Uyl-de Groot CA, Sonneveld P, Zweegman S. Efficacy of first-line treatments for multiple myeloma patients not eligible for stem cell transplantation: a network meta-analysis. Haematologica. 2019;104(5):1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med. 2019;380(22):2104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zweegman S, Larocca A. Frailty in multiple myeloma: the need for harmony to prevent doing harm. Lancet Haematol. 2019;6(3):e117–e8. [DOI] [PubMed] [Google Scholar]
- 37.Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelhardt M, Domm AS, Dold SM, Ihorst G, Reinhardt H, Zober A, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102(5):910–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


