Abstract
Objectives:
Routine screening for HIV and hepatitis C virus (HCV) among specified age cohorts is recommended. New York State requires consent before screening for HIV but not HCV. We sought to estimate the effect of the consent requirement on screening rates for HIV.
Methods:
We performed a retrospective study of patients hospitalized in 2015-2016 at a tertiary care hospital in the Bronx, New York, during a period when prompts in the electronic health record facilitated screening for HIV and HCV among specified age cohorts. We compared proportions of patients eligible for screening for HIV and/or HCV who underwent screening and used generalized estimating equations and a meta-analytic weighted average to estimate an adjusted risk difference between undergoing HIV screening and undergoing HCV screening.
Results:
Among 11 938 hospitalized patients eligible for HIV and/or HCV screening, 38.5% underwent screening for HIV and 59.1% underwent screening for HCV. The difference in screening rates persisted after adjusting for patient and admission characteristics (adjusted risk difference = 22.0%; 95% CI, 20.6%-23.4%).
Conclusions:
Whereas the requirement for consent was the only difference in the processes of screening for HIV compared with screening for HCV, differences in how the 2 viruses are perceived may also have contributed to the difference in screening rates. Nevertheless, our findings suggest that requiring consent continues to impede progress toward the public health goal of routine HIV screening.
Keywords: HIV screening, HCV screening, routine screening, consent
Both HIV and hepatitis C virus (HCV) are viruses that disproportionately affect historically marginalized populations, including racial, ethnic, and sexual minority groups and injection drug users.1,2 To identify undiagnosed cases of these treatable infections, routine screening among specific age-defined populations is recommended for both viruses, regardless of perceived risk. Since 2006, the Centers for Disease Control and Prevention has recommended screening all people aged 13-64 at least once for HIV, 3 and beginning in 2012, it recommended screening all people born during 1945-1965 at least once for HCV. 4
To increase uptake of these recommendations, New York State mandates offering HIV and HCV screening at least once to eligible patients receiving health services, including during hospitalization.5,6 Despite a similar public health rationale underlying the mandates, a critical difference exists in the processes of testing for HIV and testing for HCV in New York State. Screening for HCV, similar to virtually all diagnostic tests performed during a medical evaluation, does not require explicit patient consent before performing a test. 5 Screening for HIV, however, at minimum requires that a patient be notified that an HIV test will be performed and be given an opportunity to decline testing. 6 Although regulations have been revised multiple times to streamline HIV screening, 7 implementation of the mandate remains suboptimal.8,9 Although previous studies have estimated the effect of written consent on rates of HIV screening, few studies have examined whether an effect persists in the context of verbal consent.
Considering the similarities between HIV and HCV in terms of modes of transmission, availability of effective treatment, and populations affected, and a similar mandate for routine screening distinguished only by the requirement for consent for an HIV test, we sought to estimate the effect of this differential policy on screening rates for HIV by comparing rates of screening for HIV with rates of screening for HCV.
Methods
Study Design
We conducted a retrospective cohort study comparing rates of HIV screening and HCV screening among adult patients hospitalized from April 1, 2015, through June 30, 2016. The institutional review board of Albert Einstein College of Medicine approved the study.
Setting and Population
We performed the study at an academic, tertiary care hospital of the Montefiore Health System, the largest provider of medical care in the Bronx, New York. The health system has had an electronic health record (EHR) system, which houses and shares clinical data, including laboratory values, across its inpatient, outpatient, and emergency departments (EDs), since 1997. Prevalence estimates for HIV in the Bronx and for chronic HCV in New York City in 2015 were 2.0% 10 and 1.4%, 11 respectively.
Inclusion and Exclusion Criteria
We included all hospitalized patients during the study period who were eligible for routine HIV or HCV screening. We excluded patients if an HIV or HCV test was performed during the ED portion of their hospitalization, because the ED workflow for screening is different from the inpatient workflow for screening, and we aimed to compare workflows as similar as possible to each other.
Although the New York State mandate to offer HIV and HCV screening applies to diverse health care settings, as an observational study attempting to isolate the independent effect of consent on rates of routine screening, our study focused on hospitalized patients in a single center to minimize heterogeneity in the process for offering and performing these tests.
HIV and HCV Screening During the Study Period
During the study period, automated EHR prompts and order sets facilitated screening for HIV among patients aged 21-64 and screening for HCV among patients born during 1945-1965. The prompts for HIV and HCV screening appeared only if the patient was in the appropriate age cohort and had no previous result for the respective test in the EHR. Prompts appeared to health care providers when they placed other orders for the patient. Each prompt displayed text informing the health care provider of the screening mandate for either HIV or HCV and noted that the patient had no previous test result on record. When the prompt was acknowledged, an order set appeared with options to either order an HIV or HCV test or document why screening was not indicated. The only differences between the HIV and HCV prompts and order sets were that (1) the HIV prompt indicated the need for verbal consent, whereas the HCV prompt did not, and (2) the HIV order set allowed health care providers to order the test themselves or request that an HIV counselor offer testing and obtain consent, whereas the HCV order set had no option for counselor involvement. The HIV and HCV prompts were introduced approximately 1 year and 1 month before the study period, respectively. We began the study period 1 month after introduction of the HCV prompt to decrease the likelihood that observed differences in testing would be explained by health care providers’ familiarity with the different prompts.
Outcome
The primary outcome was the proportion of patients tested before discharge among patients eligible for HIV and/or HCV screening.
Data Source and Variables
Data were extracted from the EHR. Patient and admission characteristics included sex, age, race/ethnicity, admitting service, length of stay, eligibility for the HIV and/or HCV prompt, and whether an HIV and/or HCV test was performed.
Eligibility for HIV and HCV Screening Prompts
Patients were classified as belonging to 1 of 3 age groups: HIV screening recommended (age 13-64 at time of admission), HCV screening recommended (year of birth 1945-1965), or HIV and HCV screening recommended (age 13-64 and year of birth 1945-1965). Patients were then classified, on the basis of their previous testing, according to the prompt or prompts for which they were eligible. Therefore, patients were ultimately classified as belonging to 1 of 5 groups: group A, appropriate age and year of birth for both HIV and HCV screening, without previous test for either (eligible for both HIV and HCV prompts); group B, appropriate age and year of birth for both HIV and HCV screening, with previous HCV test but without previous HIV test (eligible for HIV prompt); group C, appropriate age and year of birth for both HIV and HCV screening, with previous HIV test but without previous HCV test (eligible for HCV prompt); group D, appropriate age for HIV screening, without previous HIV test (eligible for HIV prompt); and group E, appropriate year of birth for HCV screening, without previous HCV test (eligible for HCV prompt). In sum, group A was used to estimate a within-group difference in screening rates of HIV and HCV, whereas the other groups were used to estimate between-group differences.
Statistical Analysis
The unit of analysis was a unique patient. Patients with multiple admissions during the study period were included at their earliest admission. To estimate the risk difference between HIV and HCV screening, we used generalized estimating equation models with the identity link function. Because eligibility for HIV and HCV screening is defined by different criteria for age and year of birth, by definition the patient groups would differ according to age. To account for this and other differences in baseline characteristics across the groups that may influence the likelihood of being screened for HIV and/or HCV, we adjusted the models for age, sex, race/ethnicity, length of stay (dichotomized at <3 days vs ≥3 days), and specialty of the hospital unit to which the patient was admitted (ie, admission service). First, we estimated the adjusted within-group risk difference in screening for HIV and HCV in group A, whose members were eligible for both prompts. We considered this within-group estimate the reference risk difference. Then, to assess the generalizability of this reference estimate beyond patients eligible for both prompts, we estimated the adjusted risk differences in screening across the groups only eligible for either the HIV or HCV prompt. Specifically, we estimated between-group differences using all combinations of the groups eligible only for either the HIV or HCV prompt: ie, group B vs group C, group B vs group E, group C vs group D, and group D vs group E. Although results from the between-group comparisons are not independent, to test whether the adjusted risk differences seen across the comparisons were homogenous, we applied the Cochran Q test and the I 2 test. 12 Based on these results, we estimated the overall risk difference and 95% CI between screening for HIV and screening for HCV by combining the results from the within- and between-group comparisons based on a fixed-effect meta-analytic weighted average. 13
Because the study was designed to compare rates of HIV and HCV screening in a setting where conditions for screening for the 2 infections were as similar as possible, we excluded patients who were screened for HIV in the ED because the ED had no similar HCV screening protocol. However, we included eligible patients who were offered but declined HIV screening while in the ED because the inpatient HIV prompts remained active for these patients. To assess whether exclusion of those patients substantially influenced our results, we performed a sensitivity analysis that repeated the aforementioned analyses using a study cohort that did not exclude otherwise eligible patients screened for HIV in the ED.
Results
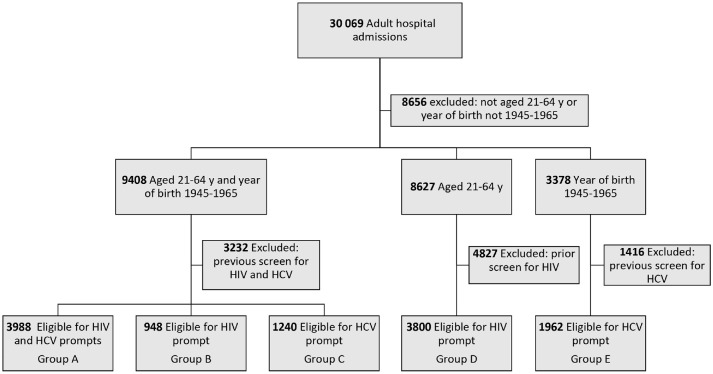
During the study period, 21 413 patients aged 21-64 and/or with year of birth 1945-1965 were hospitalized, and 11 938 (55.8%) were eligible for HIV screening, HCV screening, or both (Figure). Overall, 6053 (50.1%) patients were female, 3478 (29.1%) self-identified as non-Hispanic Black, and 3499 (29.3%) self-identified as Hispanic; 7762 (65.0%) were admitted to a medicine service (Table 1).
Figure.
Flow of patients in a study comparing routine HIV and HCV screening to estimate the effect of required consent on HIV screening rates among hospitalized patients, Bronx, New York, 2015-2016. All data were extracted from the electronic health record system of Montefiore Health System, Bronx, New York. Patients in group B were previously screened for HCV. Patients in group C were previously screened for HIV. Abbreviation: HCV, hepatitis C virus.
Table 1.
Patient and admission characteristics by screening eligibility in a study on routine HIV and hepatitis C virus (HCV) screening to estimate the effect of required consent on HIV screening rates among hospitalized patients, Bronx, New York, 2015-2016 a
| Characteristic | Group A (n = 3988) | Group B (n = 948) | Group C (n = 1240) | Group D (n = 3800) | Group E (n = 1962) |
|---|---|---|---|---|---|
| Age, median (IQR), y | 57.7 (53.9-61.5) | 58.7 (54.8-62.2) | 56.8 (53.2-60.6) | 38.9 (30.6-45.3) | 67.8 (66.4-69.3) |
| Sex | |||||
| Female | 1931 (48.4) | 526 (55.5) | 688 (55.5) | 1947 (51.2) | 961 (49.0) |
| Male | 2057 (51.6) | 422 (44.5) | 552 (44.5) | 1853 (48.8) | 1001 (51.0) |
| Race/ethnicity | |||||
| Non-Hispanic Black | 1189 (29.8) | 318 (33.5) | 431 (34.8) | 962 (25.3) | 578 (29.5) |
| Non-Hispanic White | 558 (14.0) | 97 (10.2) | 81 (6.5) | 353 (9.3) | 327 (16.7) |
| Hispanic | 970 (24.3) | 335 (35.3) | 518 (41.8) | 1153 (30.3) | 523 (26.7) |
| Other b | 869 (21.8) | 140 (14.8) | 147 (11.9) | 916 (24.1) | 366 (18.7) |
| Missing/unknown | 402 (10.1) | 58 (6.1) | 63 (5.1) | 416 (10.9) | 168 (8.6) |
| Admission service | |||||
| Medicine | 2697 (67.6) | 639 (67.4) | 953 (76.9) | 2006 (52.8) | 1467 (74.8) |
| Surgery | 1049 (26.3) | 260 (27.4) | 222 (17.9) | 1375 (36.2) | 398 (20.3) |
| Neurology, psychiatry, or gynecology | 242 (6.1) | 49 (5.2) | 65 (5.2) | 419 (11.0) | 97 (4.9) |
| Length of stay, median (IQR), d | 3 (1-6) | 3 (2-6) | 3 (1-5) | 2 (1-5) | 3 (2-7) |
Abbreviation: IQR, interquartile range.
All data were extracted from the electronic health record system of Montefiore Health System, Bronx, New York. Group A, appropriate age and year of birth for both HIV and HCV screening but had no previous test for either (eligible for both HIV and HCV prompts); group B, appropriate age and year of birth for both HIV and HCV screening, had previous HCV test but no previous HIV test (eligible for HIV prompt); group C, appropriate age and year of birth for both HIV and HCV screening, had previous HIV test but no previous HCV test (eligible for HCV prompt); group D, appropriate age for HIV screening but no previous HIV test (eligible for HIV prompt); and group E, appropriate year of birth for HCV screening but no previous HCV test (eligible for HCV prompt). Values are number (percentage) unless otherwise indicated. Percentages may not add to 100 because of rounding.
“Other” category consists of multiracial and Asian.
Among the 11 938 screening-eligible patients, 3988 (33.4%) were aged 21-64 with year of birth 1945-1965 and were eligible for both HIV and HCV screening (group A), 948 (7.9%) were aged 21-64 with year of birth 1945-1965 and were eligible for HIV screening only (group B), 1240 (10.4%) were aged 21-64 with year of birth 1945-1965 and were eligible for HCV screening only (group C), 3800 (31.8%) were aged 21-64 (with year of birth other than 1945-1965) and were eligible for HIV screening only (group D), and 1962 (16.4%) had year of birth 1945-1965 (with ages other than 21-64) and were eligible for HCV screening only (group E).
Overall, 3360 (38.5%) of 8736 patients eligible for HIV screening and 4252 (59.1%) of 7190 patients eligible for HCV screening had a test before discharge (Table 2). Among HIV screening–eligible groups, the proportion tested ranged from 37.0% to 40.1%, and among the HCV screening–eligible groups, the proportion tested ranged from 57.8% to 59.9%. The observed risk differences in the proportions of eligible patients tested for HIV and HCV across the multiple groups ranged from 17.6% to 22.6%. From the generalized estimating equation models adjusted for differences in demographic and admission characteristics, the adjusted risk difference ranged from 19.5% to 22.6% across the various comparisons. Tests for heterogeneity among the adjusted risk differences yielded a Cochran Q test of 3.20 (df = 4, P = .53) and I 2 of 0%, indicating minimal heterogeneity; an overall adjusted risk difference was estimated to be 21.8% (95% CI, 20.4%-23.3%). Results of the sensitivity analysis including patients tested for HIV during the ED portion of their hospitalization did not meaningfully affect the inferences of the primary analysis.
Table 2.
Proportions of patients eligible for screening who underwent the recommended testing, with observed and adjusted risk differences, in a study on routine HIV and HCV screening to estimate the effect of required consent on HIV screening rates among hospitalized patients, Bronx, New York, 2015-2016 a
| Group(s) compared | Received an HCV test before discharge, % | Received an HIV test before discharge, % | Observed risk difference, % | Adjusted risk difference, % (95% CI) b |
|---|---|---|---|---|
| Group A | 59.6 | 37.0 | 22.6 | 22.6 (20.9-24.3) |
| Group C vs Group B | 59.9 | 38.0 | 21.9 | 21.0 (16.8-25.2) |
| Group C vs Group D | 59.9 | 40.1 | 19.8 | 18.8 (14.2-23.3) |
| Group E vs Group B | 57.8 | 38.0 | 19.8 | 22.1 (15.1-29.1) |
| Group E vs Group D | 57.8 | 40.1 | 17.6 | 19.5 (13.5-25.5) |
| Overall | 59.1 | 38.5 | 20.6 | 21.8 (20.4-23.3) |
Abbreviation: HCV, hepatitis C virus.
All data were extracted from the electronic health record system of Montefiore Health System, Bronx, New York. Group A, appropriate age and year of birth for both HIV and HCV screening, had no previous test for either (eligible for both HIV and HCV prompts); group B, appropriate age and year of birth for both HIV and HCV screening, had previous HCV test but no previous HIV test (eligible for HIV prompt); group C, appropriate age and year of birth for both HIV and HCV screening, had previous HIV test but no previous HCV test (eligible for HCV prompt); group D, appropriate age for HIV screening but no previous HIV test (eligible for HIV prompt); and group E, appropriate year of birth for HCV screening but no previous HCV test (eligible for HCV prompt).
Models adjusted for age, sex, race/ethnicity, admission service (medicine/surgery/other), and length of stay.
Discussion
In the context of a strategy using automated EHR prompts to facilitate routine HIV and HCV screening for hospitalized patients, we found that a significantly greater proportion of people eligible for HCV screening was tested as compared with people eligible for HIV screening. Given the similarities between HIV and HCV as treatable viral infections with shared modes of transmission and that the processes for HIV and HCV screening were identical apart from the requirement for consent before HIV screening but not HCV screening, our findings suggest that the disparity in screening is likely attributable, at least in part, to that requirement. Because HIV screening is the first step to enable people who are infected to enter care and identify people who are not infected but may benefit from HIV prevention, our findings further suggest that the requirement for consent is hindering progress toward 2 key public health goals—increasing the proportion of people with HIV who are aware of their status and increasing the proportion of people at risk for HIV who access prevention services. 14
Other studies have examined whether consent imposes a barrier to the performance of HIV screening. Like ours, most of these studies were observational, but unlike ours, most addressed the effect of written consent, as opposed to the current regulations in New York State that allow for verbal consent.15 -17
For example, Ehrenkranz et al 15 in 2009 used survey data from the 2004 Behavioral Risk Factor Surveillance System to test for an association between residence in a state requiring written consent and self-reported HIV testing. They found that people living in states requiring written consent had 15% lower odds (odds ratio = 0.85; 95% CI, 0.80-0.90) of reporting HIV testing in the previous year as compared with people living in states not requiring written consent. 15 Similarly, a study in 2008 from San Francisco found that replacement of written consent with verbal consent was associated with a 44% increase in average monthly rates of HIV testing. 16 As a final example from an observational study, an analysis of changes implemented in 2005 to streamline the HIV written consent process in New York State found a 31.4% increase in the rate of testing associated with the change. 17 A randomized controlled trial in 2011-2013 assessed whether differences in how HIV testing is offered to ED patients were associated with the likelihood of patient acceptance. The study evaluated 3 offers, all predicated on verbal consent: opt-in (patients had to proactively request an HIV test), “active choice” (open-ended offer to patients to be tested), or opt-out (patients informed they would be tested unless they declined). The study found significant differences in acceptance according to offer type, with 38.0%, 51.3%, and 65.9% accepting testing in the opt-in, active choice, and opt-out arms, respectively. 18 It should be noted that the conditions being compared in each of these studies (ie, written consent vs no written consent,15,16 streamlined vs nonstreamlined written consent, 17 and opt-in vs active choice vs opt-out) 18 are different from each other and from the conditions in our study (verbal consent for HIV vs no consent for HCV), complicating direct comparisons of the findings.
Although these studies demonstrate that simplification of consent is associated with increased rates of HIV screening, we must consider factors other than consent that may have contributed to the difference in rates of HIV and HCV screening in our study. One consideration is that the New York State mandate to offer HIV screening preceded that of HCV screening, and the prompt for HIV screening was implemented approximately 1 year earlier than the prompt for HCV screening. Therefore, it is possible that health care provider fatigue with the HIV prompt relative to the newer HCV prompt contributed to the observed difference. Although we did not assess changes in rates of HIV or HCV testing over time during our study, the overall rate of HIV screening (38.5%) is similar to what we observed in a study conducted 1 year previously, in which 32.4% of eligible hospitalized patients were screened for HIV after introduction of that prompt. 19 The similarity of these findings suggests that health care provider fatigue with the HIV prompt did not have a substantial effect on our observed difference in screening.
We argue that the only difference in the workflow between routine screening for HIV and HCV was the requirement for consent before HIV testing and, therefore, differences in screening rates may be attributable to that requirement. However, our study did not account for differences in how HIV and HCV are perceived, which may also affect health care providers’ decisions to offer screening and patients’ decisions to accept screening. Specifically, the requirement for consent before HIV testing is historically tied to stigma associated with that condition, 20 and the consent process itself may invoke that stigma. Although the existing requirement may be viewed as a safeguard of patient autonomy, revising it would allow HIV screening to be more consistent with many other conditions for which routine screening is recommended without requiring explicit consent. Like HIV, some of these conditions, such as diabetes, also require lifelong management and have important implications on long-term health outcomes. Normalizing the process of HIV screening may counteract the legacy of stigma fostered in part by the exceptional requirements governing its implementation. 20 In addition, although both HIV and HCV are highly treatable conditions, a cure for HCV can be achieved after 2 or 3 months of treatment, whereas sustained viral suppression for HIV requires lifelong therapy. Therefore, we do not believe that our study can completely isolate the effect of perceived differences between the 2 infections on screening-related behavior. However, because HIV and HCV share certain similarities, including modes of transmission, availability of effective therapy, and an association with stigmatized behaviors, we believe that differences in perceptions affecting the decision to pursue screening for these conditions would be minimized.
Limitations
Our study had several limitations. First, because patients were classified as eligible for HIV screening and/or HCV screening based on previous testing in our health system, patients may have been misclassified if they were diagnosed with or screened for HIV and/or HCV outside our health system. We believe this limitation is attenuated for several reasons. One, ours is the largest health care system in the region, and the EHR system includes laboratory results beginning in 1997. Therefore, it is reasonable to believe that a substantial proportion of data on previous HIV/HCV testing would be captured in our system. Two, because patients frequently misunderstand whether they have been previously screened for HIV or HCV,21,22 reliance on self-report as a reason to exclude someone from screening is suboptimal, particularly in a region with a high prevalence of HIV and HCV.
A second limitation was that although our primary outcome was the proportion of screening-eligible patients who were tested, the available data did not allow us to distinguish between people not tested because a test was not offered and people not tested because they declined to receive the test when the test was offered. This distinction is less concerning for HCV than for HIV because, like most other laboratory tests for hospitalized patients not requiring consent, if a test was not performed it was most likely because it was not ordered. We do not believe that distinguishing whether an HIV test was never offered or was offered but the patient declined meaningfully affects our conclusions, because in either case, removal of consent would address each of these barriers.
A third limitation was that although we adjusted our analyses for several key factors known to be associated with undergoing HIV testing (including sex, age, and race/ethnicity), 23 as a retrospective observational study, unmeasured confounders may have affected our findings. Finally, our study reflects rates of routine screening among hospitalized patients in a region with a high prevalence of HIV and HCV. As such, our findings may not be generalizable to screening strategies in other clinical contexts, particularly outpatient settings, or regions with different HIV and HCV epidemiology.
Conclusion
We found that the rate of routine HCV screening far exceeded that of HIV screening for hospitalized patients in a setting where the only distinction in the screening strategies was the requirement for consent before HIV screening but not before HCV screening. Although New York State has made important policy changes to support routinization of HIV screening during the past decade, our findings suggest that the requirement for consent before HIV testing continues to negatively affect rates of HIV screening, impeding achievement of the goals to increase the proportion of people infected with HIV who are aware of their status and link people who are uninfected to appropriate HIV prevention strategies. Lawmakers must consider these findings as they continue refining policies to align with public health goals.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by National Institutes of Health grant K23MH106386 (U.R.F.), National Institute on Drug Abuse grant R01 034086 (A.H.L.), and the Gilead FOCUS Program for Scaling up HIV and HCV Testing (U.R.F. and A.H.L.).
ORCID iD: Uriel R. Felsen, MD, MPH, MS  https://orcid.org/0000-0002-4957-5767
https://orcid.org/0000-0002-4957-5767
References
- 1. Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2018. HIV Surveill Rep. 2020;31:1-119. Accessed December 15, 2020. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-31/index.html [Google Scholar]
- 2. Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2018. Accessed December 15, 2020. https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm
- 3. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17. [PubMed] [Google Scholar]
- 4. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32. [PubMed] [Google Scholar]
- 5. New York State Department of Health. NYS hepatitis C testing law frequently asked questions. 2015. Accessed June 17, 2019. https://www.health.ny.gov/diseases/communicable/hepatitis/hepatitis_c/rapid_antibody_testing/docs/testing_law_faqs.pdf
- 6. New York State Senate. Section 2781 of the New York State Public Health Laws. Accessed December 8, 2020. https://www.nysenate.gov/legislation/laws/PBH/2781
- 7. O’Connell DA, Martin EG, Cutler B, Birkhead GS. The evolution of HIV testing requirements in New York State, 1989-2013. J Acquir Immune Defic Syndr. 2015;68(suppl 1):S5-S9. doi: 10.1097/QAI.0000000000000422 [DOI] [PubMed] [Google Scholar]
- 8. Lazariu V, Parker MM, Leung SY, et al. New York State 2010 HIV testing law: an evaluation of testing rates using laboratory data. J Acquir Immune Defic Syndr. 2015;68(suppl 1):S10-S14. doi: 10.1097/QAI.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 9. Newton-Dame R, Wang JJ, Kim MS, Edelstein ZR, Cutler B, Tsoi BW. Evaluating the 2010 New York State HIV testing law in NYC ambulatory practices using electronic health records. J Acquir Immune Defic Syndr. 2015;68(suppl 1):S15-S20. doi: 10.1097/QAI.0000000000000407 [DOI] [PubMed] [Google Scholar]
- 10. New York City Department of Health and Mental Hygiene. HIV/AIDS annual surveillance statistics. Accessed December 15, 2019. http://www1.nyc.gov/site/doh/data/data-sets/hiv-aids-annual-surveillance-statistics.page
- 11. Bocour A, Greene SK, Laraque F, Winters A. Estimating the prevalence of chronic hepatitis C virus infection in New York City, 2015. Epidemiol Infect. 2018;146(12):1537-1542. doi: 10.1017/S095026881800170X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Academic Press; 2014. [Google Scholar]
- 14. Azar AM. Ending the HIV epidemic: a plan for America. 2019. Accessed April 2, 2019. https://www.hhs.gov/blog/2019/02/05/ending-the-hiv-epidemic-a-plan-for-america.html
- 15. Ehrenkranz PD, Pagán JA, Begier EM, Linas BP, Madison K, Armstrong K. Written informed-consent statutes and HIV testing. Am J Prev Med. 2009;37(1):57-63. doi: 10.1016/j.amepre.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zetola NM, Grijalva CG, Gertler S, et al. Simplifying consent for HIV testing is associated with an increase in HIV testing and case detection in highest risk groups, San Francisco, January 2003–June 2007. PLoS One. 2008;3(7):e2591. doi: 10.1371/journal.pone.0002591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wing C. Effects of written informed consent requirements on HIV testing rates: evidence from a natural experiment. Am J Public Health. 2009;99(6):1087-1092. doi: 10.2105/AJPH.2008.141069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montoy JC, Dow WH, Kaplan BC. Patient choice in opt-in, active choice, and opt-out HIV screening: randomized clinical trial. BMJ. 2016;532:h6895. doi: 10.1136/bmj.h6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Felsen UR, Cunningham CO, Heo M, Futterman DC, Weiss JM, Zingman BS. An expanded HIV testing strategy leveraging the electronic medical record uncovers undiagnosed infection among hospitalized patients. J Acquir Immune Defic Syndr. 2017;75(1):27-34. doi: 10.1097/QAI.0000000000001299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bayer R, Philbin M, Remien RH. The end of written informed consent for HIV testing: not with a bang but a whimper. Am J Public Health. 2017;107(8):1259-1265. doi: 10.2105/AJPH.2017.303819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Privette AR, Ferguson PL, Olsen J, Gay S, Richey LE. “That can’t be!”: perceptions of HIV and hepatitis C screening during admission to an acute care surgery service. J Emerg Trauma Shock. 2019;12(3):185-191. doi: 10.4103/JETS.JETS_103_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albrecht E, Frascarolo P, Meystre-Agustoni G, et al. An analysis of patients’ understanding of “routine” preoperative blood tests and HIV screening. Is no news really good news? HIV Med. 2012;13(7):439-443. doi: 10.1111/j.1468-1293.2012.00993.x [DOI] [PubMed] [Google Scholar]
- 23. Olatosi B, Siddiqi KA, Conserve DF. Towards ending the human immunodeficiency virus epidemic in the US: state of human immunodeficiency virus screening during physician and emergency department visits, 2009 to 2014. Medicine. 2020;99(2):e18525. doi: 10.1097/MD.0000000000018525 [DOI] [PMC free article] [PubMed] [Google Scholar]