Abstract
Background:
An antibiogram is a summary of antibiotic susceptibility patterns for selected bacterial pathogens and antibiotics. The New Hampshire Department of Health and Human Services’ Division of Public Health Services (DPHS) sought to create an annual state antibiogram to monitor statewide antibiotic resistance trends, guide appropriate empiric antibiotic prescribing, and inform future statewide antibiotic stewardship.
Methods:
Through legislative authority, DPHS required hospital laboratories to report antibiogram data annually. DPHS convened an advisory group of infectious disease and pharmacy stakeholders and experts to develop a standardized reporting form for bacteria and antibiotic susceptibility, which was disseminated to all 26 hospitals in New Hampshire. We combined the reported data into a statewide antibiogram, and we created clinical messaging to highlight findings and promote rational antibiotic prescribing among health care providers.
Results:
All hospital laboratories in New Hampshire submitted annual antibiogram data for 2016 and 2017, including more than 30 000 and 20 000 bacterial isolates recovered from urine and nonurine cultures, respectively, each year. The advisory group created clinical messages for appropriate treatment of common infectious syndromes, including uncomplicated urinary tract infections, community-acquired pneumonia, skin and soft-tissue infections, intra-abdominal infections, and health care–associated gram-negative aerobic infections. The statewide antibiograms and clinical messaging were widely disseminated.
Conclusions:
The small size of New Hampshire, a centralized public health structure, and close working relationships with hospitals and clinical partners allowed for efficient creation and dissemination of an annual statewide antibiogram, which has fostered public health–clinical partnerships and built a foundation for future state-coordinated antibiotic stewardship. This process serves as a model for other jurisdictions that are considering antibiogram development.
Keywords: antimicrobial resistance, antibiogram, hospital data, antibiotic stewardship, state-level collaboration, coordinated stewardship
Antibiotic-resistant infections threaten public health and account for thousands of deaths annually in the United States. 1 The national threat posed by emerging antibiotic-resistant infections led to the creation of the National Strategy and Action Plan for Combating Antibiotic-Resistant Bacteria in September 2014. 2
An estimated 30% to 50% of all outpatient antibiotic prescriptions written are unnecessary,3,4 and many hospitalized patients receive potentially inappropriate broad-spectrum antibiotics in the United States,5,6 which drives antibiotic resistance. Historically, designing and implementing antibiotic stewardship initiatives has occurred at the level of local health care facilities. These local-level initiatives have been effective in reducing antibiotic use in individual facilities without increasing adverse patient outcomes.7 -9 An additional need, however, exists for regional, coordinated approaches to prevent the emergence and spread of antibiotic-resistant infections. 10
Nationally, efforts are under way to contain emerging multidrug-resistant organisms, 11 such as carbapenem-resistant Enterobacteriaceae,12,13 Candida auris,14,15 and bacteria carrying the mcr-1 resistance gene.16,17 State and local public health agencies are appropriately strengthening antibiotic-resistance surveillance, investigation, and response activities to identify trends in overall antibiotic resistance.
To address the need for a coordinated regional approach, the New Hampshire Department of Health and Human Services’ Division of Public Health Services (DPHS) convened a multidisciplinary workgroup to promote and coordinate antibiotic resistance and antibiotic stewardship throughout the state. One priority goal of the group was to better understand statewide antibiotic-resistance patterns through creation of an annual state antibiogram. An antibiogram is a summary of antibiotic susceptibility patterns for selected bacterial pathogens and antibiotics. The group collected local hospital antibiotic susceptibility data to create an antibiogram that would be used to (1) conduct regional and state-level surveillance on antibiotic-resistance trends to proactively address increasing resistance trends across geographic regions, (2) provide data and summary guidance to support appropriate empiric antibiotic prescribing, and (3) guide and gauge the efficacy of state and regional antibiotic stewardship initiatives.
Methods
In fall 2016, DPHS, the New England Quality Innovation Network–Quality Improvement Organizations, and the Foundation for Healthy Communities convened a multidisciplinary workgroup, referred to as the Antimicrobial Resistance Advisory Workgroup (ARAW). DPHS charged the ARAW to help coordinate and provide direction for antibiotic stewardship initiatives in New Hampshire. ARAW consists of more than 70 stakeholders from various professions, including experts in the fields of medicine and infectious disease, veterinary medicine, nursing, pharmacy, dentistry, infection prevention, microbiology, and public health and representatives of various quality improvement and regulatory agencies.
Historically, DPHS had requested that hospitals voluntarily report antibiogram data; however, primarily because of differences in how hospitals construct their own antibiograms, DPHS could not meaningfully combine these data. In November 2016, DPHS updated administrative rules that govern reportable conditions to require hospital laboratories to report their antibiogram data annually to DPHS. To maximize compliance among hospital laboratories, DPHS also engaged hospital microbiologists through the Laboratory Response Network to understand the laboratory systems and limitations for susceptibility reporting. The process for creating and disseminating the state antibiogram consisted of 8 steps (Figure 1).
Figure 1.
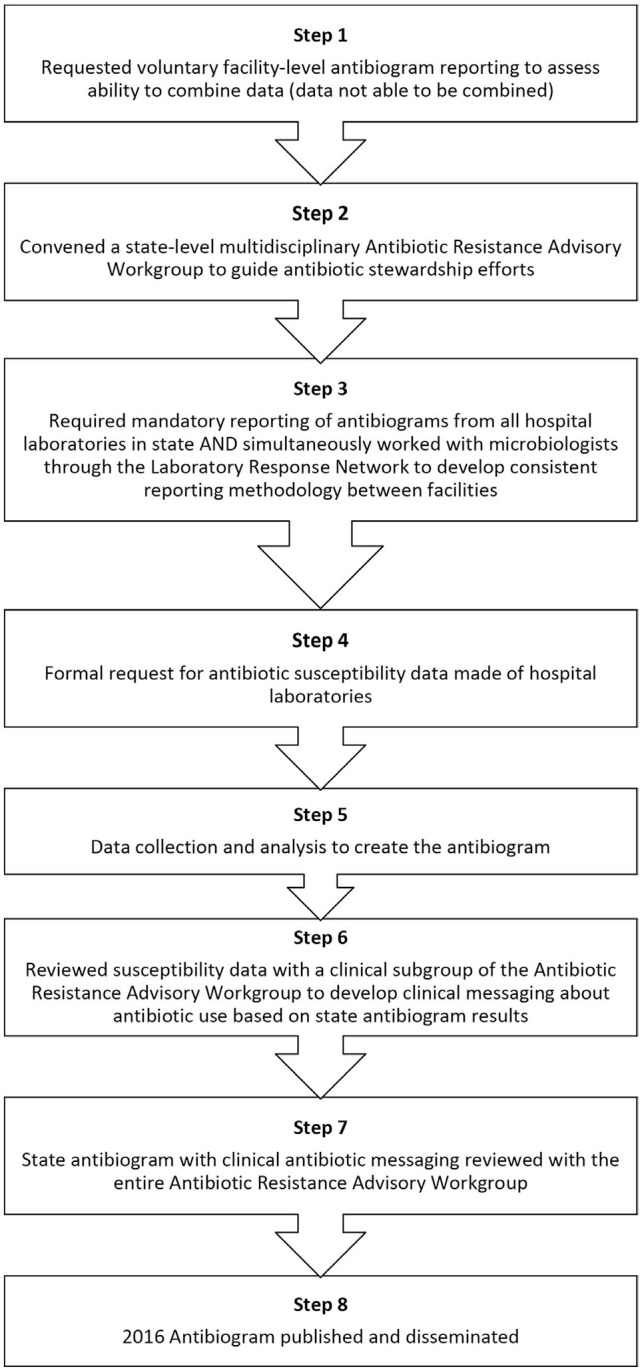
Eight-step process of creating a state antibiogram for New Hampshire.
Data Collection and Review
We used Excel (Microsoft Corporation) to create a fillable form, listing selected gram-negative and gram-positive bacteria in each row and selected antibiotics in each column. We separated antibiotic susceptibility data for urine and nonurine isolates to allow for use of these data to create guidance on treating urinary tract infections distinct from guidance on treating other common infections.
In May 2017, DPHS sent the fillable form for calendar year 2016 to all 26 hospital microbiology laboratories and 1 Veterans Affairs facility. DPHS requested that data be reported in accordance with Clinical and Laboratory Standards Institute (CLSI) guidance on the creation of cumulative antibiograms, 18 with the exception that the form asked each laboratory to include data on organisms with fewer than 30 isolates, because data from all facilities would be combined and analyzed. The form instructed that susceptibility data should be obtained directly from the laboratory’s automated antimicrobial susceptibility testing instrument to avoid excluding data potentially suppressed by the laboratory’s information system. DPHS contacted the manufacturers of the systems (Vitek and MicroScan) to ask them to provide written directions to guide microbiologists in the data collection process and provide in-person technical assistance, if requested. To facilitate the combining of data, DPHS asked hospital microbiologists to report the total number of isolates tested for each organism and number (instead of percentage) of isolates susceptible to each antibiotic–organism combination. Hospital microbiology laboratories combined data for inpatient and outpatient isolates. DPHS did not ask hospitals to separate data by location or unit because hospital microbiology laboratories indicated that fulfilling such a request would be difficult and labor intensive.
In 2018, we made a similar request of microbiology laboratories for calendar year 2017 data to create the 2017 state antibiogram. On the basis of our experience and feedback in collecting the 2016 data, we refined the data request process when we requested the 2017 data. Specifically, we made minor changes to better align with laboratory instrument output by arranging antibiotics alphabetically instead of by class, collected data on the percentage and number of isolates susceptible, and requested the data in January (instead of May) to better align with when hospital laboratories create their own facility antibiograms.
With each data set, the New Hampshire DPHS Hospital Associated Infections (HAI) program conducted an internal assessment to identify outliers or implausible data by comparing the percentages on susceptibility among all hospitals for each antibiotic–organism combination. The HAI program staff discussed any questionable data, such as <100% susceptibility of Staphylococcus aureus to vancomycin, with individual submitting microbiologists. Although vancomycin-intermediate and vancomycin-resistant S aureus have been reported, neither had been reported in New Hampshire in either 2016 or 2017. By validating data with submitting microbiologists, we identified potentially misreported bacterial resistance (rather than antibiogram reporting errors).
Antibiogram Development
After review and validation of each hospital’s antibiotic susceptibility data, we manually combined all laboratory data into an Excel file, separating isolates from urine and nonurine sources and gram-positive from gram-negative organisms. We followed CLSI standards for the creation of the state antibiogram documents. 18 We censored from reporting combinations of organisms with intrinsic resistance to certain antibiotics or nonclinically appropriate combinations. We created 4 antibiograms: 2 antibiograms showing the percentage of isolates susceptible for each antibiotic–organism combination (1 for urine isolates and 1 for nonurine isolates) and 2 antibiograms comparing the total number of isolates susceptible with the total number of isolates reported (1 for urine isolates and 1 for nonurine isolates). Per CLSI standards, we did not report any antibiotic–organism combination with fewer than 30 isolates from all hospitals combined. For the 2016 state antibiogram, if fewer than 3 hospitals reported a particular antibiotic–organism combination, we did not report the data, but we added a note indicating that the data may not be geographically representative. For the 2017 state antibiogram, because of concerns about the reliability and representativeness of the data, the subject matter expert workgroup elected to censor the data if fewer than 3 hospitals reported a particular antibiotic–organism combination.
Messaging and Communication
After finalizing the antibiograms, we convened an infectious disease physician and pharmacist subgroup of the ARAW to review the data and develop key clinical messages about antibiotic prescribing for common infectious syndromes. These clinical messages referenced national guidelines while accounting for local resistance. The clinical messaging in the 2016 antibiogram focused on antibiotic prescribing recommendations for (1) urinary tract infections, (2) community-acquired pneumonia, (3) skin and soft-tissue infections, (4) intra-abdominal infections, and (5) health care–associated gram-negative aerobic infections (Table 1). Although some messages were reiterated in the 2017 antibiogram, we added other clinical messaging: a list of antibiotics whose susceptibilities could be used to predict the activity of other antibiotics, a chart of recommended duration of antibiotic treatment, and recommendations for penicillin allergy testing (Table 1). The entire ARAW reviewed and approved the final antibiograms along with the clinical messaging for each year. 19
Table 1.
Summary comparison of 2016 and 2017 antibiogram clinical messaging, New Hampshire
| Infection | 2016 antibiogram messaging | 2017 antibiogram messaging |
|---|---|---|
| Urinary tract infection | Asymptomatic bacteriuria should not be treated in most cases. • Nitrofurantoin and cephalexin are most likely to be active against Escherichia coli. • Fosfomycin can be considered for E coli and Enterococcus species. |
• Nitrofurantoin and cephalexin are more likely to be active against E coli. • Most Enterococcus species are susceptible to amoxicillin/ampicillin. a • Fosfomycin can be considered for E coli and Enterococcus species. • Treatment for uncomplicated urinary tract infections can be as short as 3-5 days (depending on antibiotic). Treatment for complicated urinary tract infections or pyelonephritis can be as short as 7 days. a • Asymptomatic bacteriuria should not be treated in most cases. |
| Pneumonia | • Azithromycin should not be prescribed if there is concern for pneumococcal pneumonia. • Preferred antibiotics to treat pneumococcal pneumonia: ° Amoxicillin ° Amoxicillin-clavulanate ° Cefuroxime • Avoid fluoroquinolones due to toxicity. • Ceftriaxone PLUS doxycycline or azithromycin recommended for hospitalized patients with community-acquired pneumonia. |
• Azithromycin should not be prescribed if there is concern for pneumococcal pneumonia. • Preferred antibiotics to treat pneumococcal pneumonia: ° Amoxicillin ° Amoxicillin-clavulanate ° Cefpodoxime (changed from 2016 due to increasing resistance) a • >Avoid fluoroquinolones due to toxicity. • Ceftriaxone PLUS doxycycline or azithromycin recommended for hospitalized patients with community-acquired pneumonia. • Vancomycin is not necessary for all episodes of hospital-acquired pneumonia. a • Treatment for community-acquired pneumonia can be as short as 5 days. Treatment for hospital-acquired pneumonia is 7 days. a |
| Skin and soft-tissue infection | • Most skin and soft-tissue infections are due to Streptococcus species or methicillin-susceptible Staphylococcus aureus, so first-line therapy is with cephalexin/cefazolin. • Trimethoprim-sulfamethoxazole or doxycycline are first-line therapy for methicillin-resistant S aureus skin and soft-tissue infections or abscess (clindamycin should not be used). |
• Most skin and soft-tissue infections are due to Streptococcus species or methicillin-susceptible S aureus, so first-line therapy is with cephalexin/cefazolin. • Trimethoprim-sulfamethoxazole or doxycycline are first-line therapy for methicillin-resistant S aureus skin and soft-tissue infections or abscess (clindamycin should not be used). • Treatment can be as short as 5 days. a |
| Intra-abdominal infections | • Pseudomonas is not a common pathogen in intra-abdominal infections. • Ceftriaxone PLUS metronidazole recommended for empiric inpatient treatment. • Piperacillin-tazobactam or cefepime PLUS metronidazole recommended for serious life-threatening infections. |
Not applicable |
| Other | • Restrict use of carbapenems. • Mild-moderate infections caused by extended spectrum beta-lactamase–producing organisms do not always require treatment with a carbapenem. Alternatives include trimethoprim-sulfamethoxazole, nitrofurantoin, fosfomycin, and ciprofloxacin. b |
• Restrict the use of carbapenems. • Restrict fluoroquinolone use given toxicities. a • More than 90% of patients with a penicillin allergy listed in their medical record are not truly allergic; therefore, based on an assessment, providers should consider a ° De-labeling the penicillin allergy a ° Providing a supervised penicillin challenge a ° Penicillin skin testing a |
Message was added in 2017.
Message not used in 2017.
The HAI program disseminated the final publications of the antibiograms and clinical messaging through various strategies and to various types of health care providers (Table 2). We posted the state antibiogram and clinical messaging to the DPHS/US Department of Health and Human Services website20,21 and sent the antibiogram and clinical messaging electronically through the Health Alert Network system to health care providers. ARAW members also helped with distribution to hospital staff members. In addition, the HAI program maintains listservs for hospital infection control practitioners, urgent care centers, assisted living facilities, and ambulatory surgical centers, all of which received notification of the antibiogram publication. The listservs at the New Hampshire Hospital Association, the Foundation for Healthy Communities, the New Hampshire Quality Innovation Network–Quality Improvement Organizations, and the New Hampshire Healthcare Association also received notifications. Finally, the HAI program and ARAW members presented the data and accompanying clinical recommendations at various professional organization meetings (a New Hampshire infection preventionist organization, the New Hampshire Healthcare Quality Assurance Commission, a meeting of hospital chief medical officers, and a New Hampshire collaborative antibiotic stewardship symposium).
Table 2.
Dissemination of the 2016 and 2017 antibiogram and executive summary in New Hampshire
| Type of recipient | Recipient |
|---|---|
| Health care providers | • Division of Public Health Services website • Health Alert Network message • Disseminated through Antimicrobial Resistance Advisory Workgroup network • Discussion and presentation to hospital chief medical officers (CMOs) during a regularly scheduled CMO meeting of the hospital association |
| Health care facilities and partners | • Hospital-associated infections program listservs targeting hospital infection control practitioners, urgent care, assisted living and nursing home facilities, and ambulatory surgical centers • Microbiologists through the hospital Laboratory Response Network • Healthcare Quality Assurance Commission meeting • New Hampshire Infection Control and Epidemiology Professionals meeting • New Hampshire Hospital Pharmacy Director’s meeting |
| Professional societies | • New Hampshire Medical Society • New Hampshire Dental Society • New Hampshire Infection Control and Epidemiology Professionals • New Hampshire Healthcare Association |
| Public health partner organizations | • New Hampshire licensing boards of medicine, pharmacy, nursing, dental, and veterinary medicine • New Hampshire Hospital Association and Foundation for Health Communities • New Hampshire Quality Improvement Network/Quality Improvement Organizations • Infection Prevention and Control Coalition, Nashua, New Hampshire • City of Manchester Health Department • City of Nashua Health Department |
Ethical Considerations
We collected only aggregate data with no patient identifiers and used these data for public health surveillance and quality improvement as mandated by state law; the work was exempt from approval by an institutional review board.
Results
All 26 acute care hospital laboratories in New Hampshire reported 2016 data, but 3 were excluded from the 2016 state antibiogram report because they did not report data in the requested format. All 26 New Hampshire hospitals and 1 Veterans Affairs microbiology laboratory reported 2017 data in the requested format and were included in the 2017 state antibiogram.
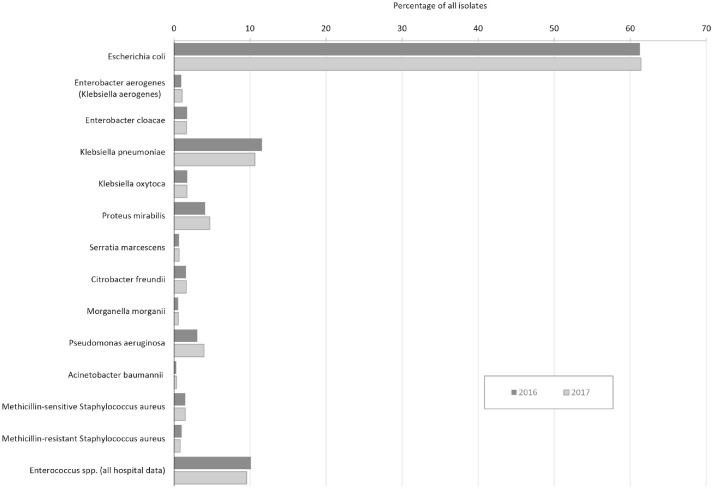
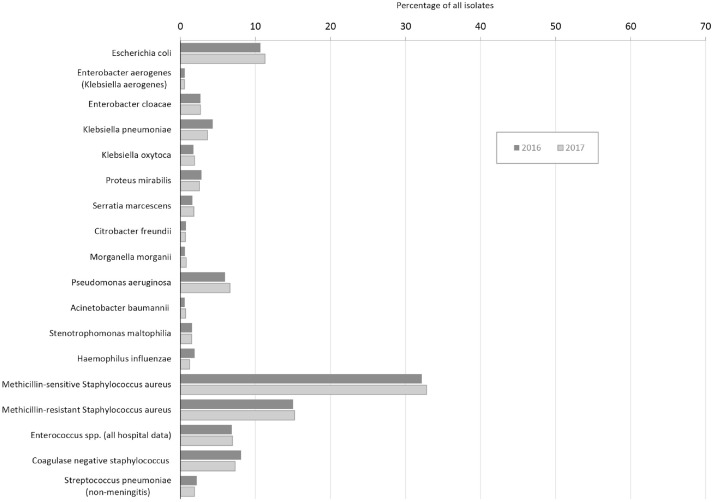
The 23 hospital laboratories reporting 2016 data submitted data on 39 707 bacterial isolates from urine sources and 20 280 bacterial isolates from nonurine sources. The 26 laboratories reporting 2017 data submitted data on 48 419 bacterial isolates from urine sources and 23 652 bacterial isolates from nonurine sources (Figures 2 and 3).
Figure 2.
Antibiogram isolates recovered from urine cultures in New Hampshire, 2016 and 2017. For each isolate, a percentage was calculated.
Figure 3.
Antibiogram isolates recovered from nonurine cultures in New Hampshire, 2016 and 2017.
Discussion
We presented data from a successful clinical–public health partnership that created a state antibiogram that can serve as a model for other states and public health jurisdictions. Hospitals create facility-level antibiograms to assist local health care providers with antibiotic prescribing and to inform local antibiotic stewardship programs. 7 Similarly, a regional or state antibiogram that combines data from multiple microbiology laboratories can be a resource for health care providers who may not have access to a local antibiogram. A regional antibiogram can also be an effective tool for regional surveillance of emerging antibiotic resistance in a wide range of organisms and can serve as a benchmark for antibiotic resistance across hospitals and regions, which can affect antibiotic prescribing.22 -27
The creation of the New Hampshire state antibiogram was key to our initiating of a regional coordinated antibiotic stewardship program. The establishment and collaboration with a multidisciplinary advisory workgroup and the Laboratory Response Network facilitated stewardship activities. At a time when regional and state collaborations are being promoted to combat antibiotic-resistant infections, the state generated interest and brought together clinical and public health partners in a shared goal of improving antibiotic prescribing and preventing antibiotic resistance. The small size of New Hampshire, its centralized public health structure, and its close working relationships with hospitals allowed for collaboration with microbiology laboratories to collect antibiogram data. Other public health jurisdictions have developed antibiograms or are exploring options and best practices for developing them.22 -30 To our knowledge, however, New Hampshire is the first state to use a combined antibiogram to proactively promote appropriate antibiotic prescribing through statewide clinical messaging that targets common infectious syndromes.
By relying on collaboration with clinical partners, the creation of the state antibiogram required minimal public health resources. Once the process for creating the antibiogram was established, it became more efficient and consistent in subsequent years. The process improved by seeking feedback from laboratory microbiologists who were submitting data and adjusting the data collection form.
Limitations
This study had several limitations. First, during the first year of data collection, the methods used by each clinical laboratory to collect and report data varied; some laboratories collected data directly from their antibiotic susceptibility testing instrument, but others collected data from their library information system, which potentially resulted in incomplete reporting of any suppressed results. Second, the first iteration of the state antibiogram was not a complete record of hospital laboratory antibiotic susceptibility results, because data from 3 hospital laboratories were excluded. The process was improved to allow for more consistent and complete reporting.
Third, we limited antibiotic susceptibility data to laboratory isolates from hospital inpatient and outpatient settings; we did not incorporate data from outpatient reference laboratory isolates. Therefore, the antibiograms in 2016 and 2017 may be skewed toward acute care or inpatient isolates and may not reflect overall antibiotic susceptibility across the entire continuum of care in New Hampshire, especially nursing homes. 31 However, they are the most complete picture to date of antibiotic resistance in New Hampshire. Future work will need to focus on collecting data from regional and national reference laboratories in a format that can be integrated into the state antibiogram.
Fourth, although the intention of New Hampshire’s DPHS is to analyze resistance patterns over time using antibiogram data, it has not yet performed this analysis because the reporting process and construction of the antibiogram changed from 2016 to 2017. Further analysis using subsequent years of data will be important.
Finally, DPHS has not formally evaluated the effect of the published state antibiogram. However, anecdotally, health care system partners have adopted and disseminated clinical messaging. Recently, a health care facility presented data on changes in facility-level antibiotic-prescribing patterns after publication of the first state antibiogram, but more research is needed for a formal evaluation.
Future Directions
The HAI program plans to continue to improve and enhance the data collection process, analysis, and utility of the state antibiogram. In future iterations, the program will include reference laboratory data, including more outpatient data, which will allow a more complete analysis of antibiotic resistance patterns. These data will improve representation of other types of health care facilities, including outpatient settings in which reference laboratories test isolates from patients outside the acute care setting.
The HAI program also plans to develop a streamlined and consistent data quality assurance process. As part of this process, the program will look for statistical changes in the data by coding the antibiogram into R, the statistical computing program, to decrease the amount of manual data entry and review, improve analysis capabilities, and implement year-to-year and regional geographic comparisons.
Lastly, DPHS is working to improve access to the antibiogram data and clinical messaging to make it more available at point of care (mobile application and/or pocket-sized booklets) and working with local stewardship partners to expand dissemination. A formal evaluation of the utility of the antibiogram is needed.
Practice Implications
The creation of a state antibiogram is an important tool for coordinating statewide antibiotic stewardship. The New Hampshire experience is a potential model for other states and jurisdictions, but national leadership and coordination is also needed to provide consistent guidance to states in the methods of creating and using regional antibiograms. In addition, once published data on regional resistance are available, future stewardship efforts must focus on furthering local antibiotic stewardship, public and health care provider messaging, and connecting antibiotic resistance patterns seen on the antibiograms with antibiotic use and prescribing.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hannah M. Leeman, MPH  https://orcid.org/0000-0003-2054-1739
https://orcid.org/0000-0003-2054-1739
References
- 1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 2. Centers for Disease Control and Prevention. U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria (National Action Plan). September 2014. Accessed November 10, 2020. https://www.cdc.gov/drugresistance/us-activities/national-action-plan.html
- 3. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi: 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Antibiotic Use in the United States 2017: Progress and Opportunities. US Department of Health and Human Services, Centers for Disease Control Prevention; 2017. [Google Scholar]
- 5. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. doi: 10.1001/jamainternmed.2016.5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200. [PMC free article] [PubMed] [Google Scholar]
- 7. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi: 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karanika S, Paudel S, Grigoras C, Kalbasi A, Mylonakis E. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother. 2016;60(8):4840-4852. doi: 10.1128/AAC.00825-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner B, Filice GA, Drekonja D, et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol. 2014;35(10):1209-1228. doi: 10.1086/599172 [DOI] [PubMed] [Google Scholar]
- 10. Slayton RB, Toth D, Lee BY, et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. MMWR Morb Mortal Wkly Rep. 2015;64(30):826-831. doi: 10.15585/mmwr.mm6430a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woodworth KR, Walters MS, Weiner LM, et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms—United States, 2006-2017. MMWR Morb Mortal Wkly Rep. 2018;67(13):396-401. doi: 10.15585/mmwr.mm6713e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151-1161. doi: 10.1128/AAC.45.4.1151-1161.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62(9):165-170. [PMC free article] [PubMed] [Google Scholar]
- 14. Tsay S, Welsh RM, Adams EH, et al. Notes from the field: ongoing transmission of Candida auris in health care facilities—United States. MMWR Morb Mortal Wkly Rep. 2011;22(1):95. doi: 10.1016/j.wem.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams E, Quinn M, Tsay S, et al. Candida auris in healthcare facilities, New York, USA, 2013-2017. Emerg Infect Dis. 2018;24(10):1816-1824. doi: 10.3201/eid2410.180649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vasquez AM, Montero N, Laughlin M, et al. Investigation of Escherichia coli harboring the mcr-1 resistance gene—Connecticut, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(36):979-980. doi: 10.15585/mmwr.mm6536e3 [DOI] [PubMed] [Google Scholar]
- 17. Kline KE, Shover J, Kallen AJ, Lonsway DR, Watkins S, Miller JR. Investigation of first identified mcr-1 gene in an isolate from a U.S. patient—Pennsylvania, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(36):977-978. doi: 10.15585/mmwr.mm6536e2 [DOI] [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline. Revision A4. Clinical and Laboratory Standards Institute; 2014. [Google Scholar]
- 19. New Hampshire Department of Health and Human Services, Division of Public Health Services. NH statewide antibiogram. Accessed November 11, 2020. www.dhhs.nh.gov/dphs/cdcs/hai/publications.htm
- 20. New Hampshire Department of Health and Human Services, Division of Public Health Services. State of New Hampshire 2016 state antibiogram. December 2017. Accessed October 28, 2020. https://www.dhhs.nh.gov/dphs/cdcs/hai/documents/antibiogram-sum-2016.pdf
- 21. New Hampshire Department of Health and Human Services, Division of Public Health Services. State of New Hampshire 2017 state antibiogram and implications for antibiotic prescribing. October 2018. Accessed October 28, 2020. https://www.dhhs.nh.gov/dphs/cdcs/hai/documents/antibiogram-sum-2017.pdf
- 22. Durante AJ, Maloney M, Leung VH, et al. Expanded susceptibility and resistance mechanism testing among carbapenem-resistant Enterobacteriaceae through a statewide antibiogram, a clinical and public health partnership. Infect Control Hosp Epidemiol. 2019;40(9):1071-1073. doi: 10.1017/ice.2019.179 [DOI] [PubMed] [Google Scholar]
- 23. Hostler CJ, Moehring RW, Ashley ESD, et al. Feasibility and value of developing a regional antibiogram for community hospitals. Infect Control Hosp Epidemiol. 2018;39(6):718-722. doi: 10.1017/ice.2018.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamma PD, Robinson GL, Gerber JS, et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol. 2013;34(12):1244-1251. doi: 10.1086/673974 [DOI] [PubMed] [Google Scholar]
- 25. Duffy J, Sievert D, Rebmann C, et al. Effective state-based surveillance for multidrug-resistant organisms related to health care–associated infections. Public Health Rep. 2011;126(2):176-185. doi: 10.1177/003335491112600208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guarascio AJ, Brickett LM, Porter TJ, Lee ND, Gorse EE, Covvey JR. Development of a statewide antibiogram to assess regional trends in antibiotic-resistant ESKAPE organisms. J Pharm Pract. 2019;32(1):19-27. doi: 10.1177/0897190017735425 [DOI] [PubMed] [Google Scholar]
- 27. Castrodale L, Hennessy T. Combined antibiogram for hospitals with 50+ beds—Alaska, 2002. Alaska Med. 2004;46(4):81-87. [PubMed] [Google Scholar]
- 28. Massachusetts Bureau of Infectious Disease and Laboratory Sciences. Massachusetts antibiograms: statewide antibiograms. 2019. Accessed October 28, 2020. https://www.mass.gov/service-details/massachusetts-antibiograms
- 29. State of Hawaii, Department of Health, Disease Outbreak Control Division. State antibiogram. Accessed October 28, 2020. https://health.hawaii.gov/docd/resources/reports/state-antibiogram
- 30. Washington State Department of Health. Antibiograms. Accessed October 28, 2020. https://www.doh.wa.gov/ForPublicHealthandHealthcareProviders/HealthcareProfessionsandFacilities/AntibioticResistance/Stewardship/Antibiograms
- 31. Hughes MA, Dosa DM, Caffrey AR, et al. Antibiograms cannot be used interchangeably between acute care medical centers and affiliated nursing homes. J Am Med Dir Assoc. 2020;21(1):72-77. doi: 10.1016/j.jamda.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]