Abstract
Early brain injury (EBI) is considered an important cause of morbidity and mortality after aneurysmal subarachnoid hemorrhage (aSAH). As a factor in EBI, microcirculatory dysfunction has become a focus of interest, but whether microcirculatory dysfunction is more important than angiographic vasospasm (aVS) remains unclear. Using data from 128 cases, we measured the time to peak (TTP) in several regions of interest on digital subtraction angiography. The intracerebral circulation time (iCCT) was obtained between the TTP in the ultra-early phase (the baseline iCCT) and in the subacute phase and/or at delayed cerebral ischemia (DCI) onset (the follow-up iCCT). In addition, the difference in the iCCT was calculated by subtracting the baseline iCCT from the follow-up iCCT. Univariate analysis showed that DCI was significantly increased in those patients with a prolonged baseline iCCT, prolonged follow-up iCCT, increased differences in the iCCT, and with severe aVS. Poor outcome was significantly increased in patients with prolonged follow-up iCCT and increased differences in the iCCT. Multivariate analysis revealed that increased differences in the iCCT were a significant risk factor that increased DCI and poor outcome. The results suggest that the increasing microcirculatory dysfunction over time, not aVS, causes DCI and poor outcome after aneurysmal aSAH.
Keywords: Aneurysmal subarachnoid hemorrhage, delayed cerebral infarction, early brain injury, intracranial circulation time, microcirculatory dysfunction
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) comprises 3–4% of all strokes and it remains associated with severe mortality higher than 25%.1–3 Previously, it had been hypothesized that the main cause of delayed cerebral ischemia (DCI) and poor outcome was angiographic vasospasm (aVS) of the major cerebral arteries. However, the results of the CONSCIOUS-1 study, which was a randomized, blinded clinical trial using an endothelin antagonist, clazosentan, led us to doubt that aVS is the only factor causing DCI and poor outcome. 4 As a result, early brain injury (EBI), which consists of neuronal apoptosis, impaired autoregulation, brain edema, cortical spreading depression (CSD) and microcirculatory dysfunction, has become a focus of interest.5–12
Before the CONSCIOUS-1 study, there had already been several experimental and clinical studies showing that microcirculatory dysfunction due to microthrombosis and/or microvasospasm could contribute to DCI after SAH.13–15 After the CONSCIOUS-1 trial, investigations of microcirculatory dysfunction after SAH increased, and its role in DCI and outcome appears to be conclusive.10,13–16 However, whether microcirculatory dysfunction or aVS plays the more important role in DCI and outcome has not been concluded clinically, because it is difficult to establish a clear clinical index for evaluating microcirculatory dysfunction. Some studies have indicated that cerebral circulation time (CCT) can reflect the status of microcirculatory impairment.17–19 Several studies using computed tomography perfusion have examined the relationship between DCI and indicators related to the CCT, such as the mean transit time (MTT) and the time to peak (TTP), but they did not reach a definite conclusion about their relationship to DCI, and a consensus among studies could therefore not be reached.20–32 Digital subtraction angiography (DSA) is recognized as the most reliable method for the evaluation of CCT and aVS. While two studies investigated the correlation between CCT measured by DSA exclusively during the ultra-early phase within 24 hours after the ictus, and no consistent result emerged of the relationship between CCT and DCI.20,22,32–35 Importantly, they did not examine CCT during the subacute phase.22,32
In order to clarify the correlation between microcirculatory dysfunction and DCI, it is necessary to evaluate the CCT together with aVS by means of DSA during both an ultra-early and a subacute phase. The purpose of the present study was to clarify the role of microcirculatory dysfunction in DCI after aSAH by measuring CCT and aVS in patients enrolled in two prospective, double-blinded, randomized placebo-controlled trials (RCT). These were performed in four of our neurosurgical institutions and they tested the efficacies of cilostazol and statin.36,37 We defined the “ultra-early phase” as within 24 hours after the SAH onset, and all patients in these two RCTs underwent DSA twice, in the preoperative, ultra-early phase and in the subacute phase between 7 to 11 days.32,38,39
Methods
Study population
Participants who used cilostazol or pitavastatin in two multicenter RCTs from 2012 to 2016 were enrolled.36,37 The RCTs included 256 patients with ruptured cerebral aneurysms, and 128 respective control cases served for the analysis in the present study. Both RCTs were conducted according to the Declaration of Helsinki and the ethical guidelines on clinical studies in four neurosurgical institutions in Aomori prefecture, Japan. The protocol for these studies was assessed and approved by the Committee of Medical Ethics of the Hirosaki University Graduate School of Medicine. Both studies were registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN, No. 000014402 and No. 000015977). All participants or their legal representatives obtained written, informed consent before treatment.
Eligibility for the study was based on fulfillment of the inclusion criteria of adult patients aged between 20 and 80 years who were admitted within 24 hours after the onset of aneurysmal SAH and treated by aneurysmal neck clipping or coil embolization within 48 hours after the ictus. The other inclusion criteria were a diffuse (long axis > 20 mm) or localized (long axis <20 mm), thick (short axis > 4 mm) SAH clot on a CT scan done within 24 hours after of the ictus, and Hunt and Hess (H & H) grades I–IV as evaluated before clipping or coil embolization. The exclusion criteria were the current use of drugs that could affect intracerebral circulation time such as antiplatelet, anticoagulant agents, endothelin antagonist, clazosentan, and any kind of statin. The other exclusion criteria were preexisting cerebral damage from past stroke or traumatic brain injury confirmed on CT scan, postoperative neurological deficits due to a clipping or coiling procedure, major neurological deficits and severe H&H grading due to concomitant or acute hydrocephalus and/or intracerebral hematoma induced by aneurysmal rupture, pre-existing severe hepatic, renal, pulmonary or cardiac disease, and pregnancy.
Standard of care
After aneurysmal clipping or coil embolization, the cases were randomly assigned to the placebo or drug treatment group. In the control group, the administration of placebo orally twice per day was started within 72 hours after the ictus and continued for 14 days. The circulating blood volume was kept within normovolemia, and all participants were treated three times per day with intravenous administration of 30 mg of fasudil hydrochloride, which has a vasodilatory effect due to Rho-kinase inhibition and is recommended under the Japanese stroke guidelines for the management of aneurysmal SAH. 40 On the other hand, none of the participants received oral or intravenous administration of nimodipine for prevention of DCI.
Rescue therapy was initiated when DCI was diagnosed as described below. The standardized procedures included induced hypertension and endovascular infusion of fasudil, followed by balloon angioplasty if adequate vasodilation was not achieved by vasodilator infusion. Acute hydrocephalus during the subacute phase was treated with external ventricular drainage or a sustained lumbar drain.
Clinical assessment
A baseline DSA was determined on admission within 24 hours after the ictus and a follow-up DSA was performed again 9 ± 2 days after the SAH ictus and/or within four hours after the onset of DCI in order to assess aVS and iCCT. DCI was defined as clinical deterioration of more than two points on the Glasgow Coma Scale (GCS), the development of new, focal neurological signs, or both, when the cause was felt to be ischemia attributable to vasospasm after other possible causes of worsening had been excluded. 41 All cerebral angiography was performed by flat panel DSA (AXIOM-Artiszee®, Siemens Health Care). Using standard angiographic methods (transfemoral route) for all patients, image acquisition was performed via a 4 Fr catheter with the tip positioned at the C3–4 level. The 2D DSA series were acquired at a rate of 16 frames per second, as is routine in our department. For image acquisition, 6 ml of contrast material (Iohexol 300, GE Healthcare, Japan) were injected in all series by mechanical injection at a flow rate of 8 ml/s for two seconds. The diameters of the proximal cerebral arteries, that is the bilateral C1 segments of the internal carotid artery, M1 segments of the middle cerebral artery, and A1 segments of the anterior cerebral artery, were measured, and the percent reduction in the arterial diameter was calculated by comparing the baseline DSA to the follow-up DSA. The severity of cerebral vasospasm was categorized as none or mild: 0–25% decrease; moderate: 25–50% decrease; and severe: over 50% decrease in arterial diameter on follow-up images compared to the baseline DSA. 42
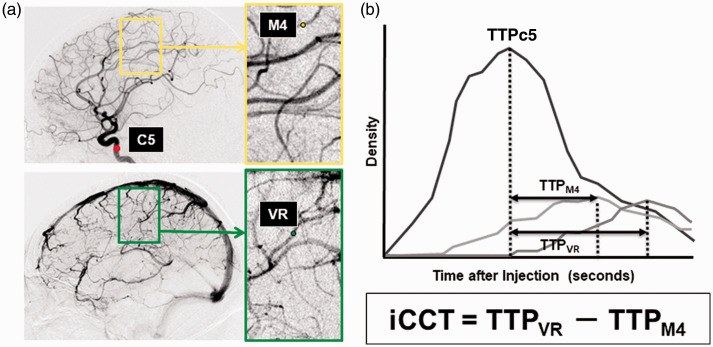
DSA images were saved to a separate DVD in the Digital Imaging and Communications in Medicine (DICOM) format. Representative, consecutive DICOM images from DSA were loaded to a personal computer and we analyzed the time-density curve to measure time to peak (TTP) and intracerebral circulation time (iCCT) with post-processing image analysis software (U11437 ver. 1313. Hamamatsu Photonics Co., Ltd.) The regions of interest (ROIs) consisting of four pixels were placed at the vertical intracavernous portion of the internal carotid artery (ROIC5), at the cortical segment of the rolandic artery (ROIM4), and at the rolandic vein (ROIVR). The time-density curve was analyzed at the selected ROIs with regard to the image intensity divided by the run-time of the DSA in order to measure the TTP (Figure 1). TTP at ROIM4 (TTPM4) and TTP at ROIVR (TTPVR) were defined as the time from TTP at the carotid siphon (TTPC5) to the maximum level of the contrast density at each ROI. We then defined the iCCT as the difference in time between TTPM4 and TTPVR. We defined the baseline iCCT as that measured in the ultra-early phase after the ictus, and the follow-up iCCT was measured in the subacute phase on 7–11 days after the ictus. In addition, the difference in the iCCT was obtained by subtracting the baseline iCCT value from the follow-up iCCT value for each individual patient. 39
Figure 1.
Measurement of iCCT.
A, ROIs were set in the vertical intracavernous portion of the internal carotid artery (C5), the cortical segment of the rolandic artery (M4), and the rolandic vein (VR) on the images of lateral projection. B, Time-density curve of the contrast media in each ROI representing the various times to peak (TTP), and intracerebral circulation time (iCCT).
Neurological examination and CT scan were performed on admission within 24 hours of SAH onset and 6–12 hours after the aneurysm treatment procedure. A follow-up examination was performed 7 ± 2 days, 14 ± 2 days, 1 and 3 months after the ictus, and whenever neurological worsening occurred. Clinical outcome was assessed by the Glasgow Outcome Scale (GOS). Poor outcome was predefined as severe disability (SD), vegetative state (VS) and death (D), and favorable outcome was predefined as good recovery (GR) and moderate disability (MD). This was assessed by two independent, blinded site investigators.
Based on these parameters, we compared aVS, the baseline iCCT, the follow-up iCCT, and the differences in the iCCT with the clinical characteristics of DCI and poor outcome. The factors affecting aVS and iCCT were also investigated.
Statistical analysis
Intergroup differences were analyzed using the Student's t-test, the Mann–Whitney U test, Pearson chi-square test, or the Fisher exact probability test. A two-sided probability value <0.05 was considered significant. To identify independent factors affecting DCI and outcome, multivariate analyses were performed by including possible confounding factors using a logistic regression model, and a receiver operating characteristics (ROC) curve analysis of iCCT was performed to assess a threshold value that allows identification of patients at risk for DCI. Sensitivity and specificity were calculated for this threshold value, as was the area under the curve (AUC) for quantifying diagnostic accuracy. The confidence interval was set at 95%. Multivariate analyses were performed by including possible confounding factors with a p < 0.2.36,43 All analyses used the statistics program JMP Pro ver. 13.
Results
A total of 128 patients were enrolled in this study. The baseline characteristics of age, sex, hypertension, smoking, IVH, ICH, aneurysmal clipping, H&H grade, severity of the SAH clot, and aneurysm location were comparable between patients with and without DCI and patients with and without unfavorable outcome (Table 1). All thirty of the participants with DCI underwent induced hypertension and received endovascular infusion of fasudil, and eight were followed by balloon angioplasty. Patients with unfavorable outcome had a significantly more severe H&H grade as compared to patients with favorable outcome (p < 0.01). The patients with DCI had significantly more severe aVS as compared to patients without DCI (p = 0.04); however, the rate of severe aVS did not differ between patients with favorable and with unfavorable outcome.
Table 1.
Demographics including intracerebral circulation time and incidence of endpoints for patients.
| DCI |
p Value | Outcome |
p Value | |||
|---|---|---|---|---|---|---|
| - | + | Unfavorable | Favorable | |||
| Number of subjects | 98 | 30 | 23 | 105 | ||
| Age, y, mean ± SD | 59 ± 11 | 62 ± 9 | 0.74 | 61 ± 11 | 60 ± 10 | 0.66 |
| Female | 64 (65%) | 16 (53%) | 0.17 | 11 (48%) | 69 (66%) | 0.09 |
| Hypertension | 45 (46%) | 14 (24%) | 0.55 | 11 (48%) | 48 (46%) | 0.06 |
| Smoker | 43 (44%) | 17 (57%) | 0.15 | 8 (35%) | 52 (50%) | 0.15 |
| IVH | 8 (8%) | 4 (13%) | 0.30 | 4 (17%) | 8 (8%) | 0.14 |
| ICH | 11 (11%) | 4 (13%) | 0.49 | 4 (17%) | 11 (10%) | 0.27 |
| Clipping | 89 (91%) | 26 (87%) | 0.36 | 21 (91%) | 94 (90%) | 0.58 |
| Hunt & Hess grade | 0.53 | <0.01** | ||||
| Grade I or II | 60 (61%) | 18 (60%) | 5 (22%) | 73 (70%) | ||
| Grade III | 28 (29%) | 9 (30%) | 12 (52%) | 25 (24%) | ||
| Grade IV | 10 (10%) | 3 (10%) | 6 (26%) | 7 (6%) | ||
| SAH on baseline CT | 0.19 | 0.09 | ||||
| Thick local | 8 (8%) | 3 (10%) | 1 (4%) | 10 (10%) | ||
| Thin diffuse | 1 (1%) | 1 (3%) | 0 (0%) | 2 (2%) | ||
| Thick diffuse | 89 (91%) | 26 (87%) | 22 (96%) | 93 (88%) | ||
| Aneurysm location | 0.70 | 0.22 | ||||
| ACA | 38 (39%) | 11 (37%) | 6 (26%) | 43 (41%) | ||
| ICA | 35 (36%) | 9 (30%) | 6 (26%) | 38 (36%) | ||
| MCA | 21 (20%) | 7 (23%) | 9 (39%) | 19 (18%) | ||
| VA / BA | 4 (4%) | 3 (10%) | 2 (9%) | 5 (5%) | ||
| Angiographic VS | 0.04* | 0.12 | ||||
| Non-mild-moderate | 68 (69%) | 15 (50%) | 12 (52%) | 71 (68%) | ||
| Severe | 30 (31%) | 15 (50%) | 11 (48%) | 34 (32%) | ||
| iCCT | ||||||
| Baseline iCCT, second ± SD | 3.6 ± 1.2 | 4.1 ± 1.2 | 0.04* | 4.2 ± 1.1 | 3.8 ± 1.2 | 0.74 |
| Follow up iCCT, second ± SD | 3.4 ± 0.6 | 4.0 ± 0.5 | <0.01** | 4.2 ± 0.9 | 3.3 ± 1.0 | <0.01** |
| iCCT differences, second ± SD | 0.45 ± 0.89 | 0.23 ± 1.9 | <0.01** | −0.03 ± 1.7 | 0.45 ± 1.1 | <0.01** |
ACA: anterior cerebral artery; DCI: delayed cerebral ischemia; ICA: internal cerebral artery; iCCT: intracerebral circulation time; ICH: intracerebral hemorrhage; IVH: intraventricular hemorrhage; MCA: middle cerebral artery; VA/BA: vertebral artery/basilar artery; VS: vasospasm *p < 0.05, **p < 0.01.
The baseline and follow up DSA were performed 9.7 ± 12.5 hours and 8.5 ± 2.3 days after the SAH onset, respectively. The mean value of the baseline iCCT showed significant prolongation in the patients with DCI as compared to patients without DCI (p = 0.04), but showed no significant difference between patients with favorable and with unfavorable outcome. The follow-up iCCT showed significant prolongation in the patients with DCI (p < 0.01) and in the patients with unfavorable outcome (p < 0.01) as compared to patients without DCI and patients with favorable outcome. There was a significant increase in the differences in the iCCT in the patients with DCI (p < 0.01) and in the patients with unfavorable outcome (p < 0.01).
Assessment of the correlation of iCCT and aVS with DCI and outcome
Based on the above results, the factors that affect DCI and outcome were examined by multivariate analysis.
DCI
Multivariate logistic regression analysis revealed that the follow-up iCCT (OR 5.93, 95% CI 1.42–10.8; p = 0.024) and the differences in the iCCT (OR 8.84, 95% CI 1.69 –37.2; p = 0.015) were significant factors affecting the development of DCI, but factors such as the baseline iCCT, age, IVH, H & H grade, SAH hematoma on CT, clipping, and severe aVS did not attain statistical significance (Table 2).
Table 2.
Multivariate analysis of factors affecting endpoints.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Factor | p Value | OR | 95% CI | p Value |
| DCI | ||||
| Baseline iCCT | 0.042* | 1.89 | 0.18–3.89 | 0.139 |
| Follow-up iCCT | <0.01** | 5.93 | 1.42–10.8 | 0.024* |
| iCCT differences | <0.01** | 8.84 | 1.69–37.2 | 0.015* |
| Age > 65 | 0.742 | 1.64 | 0.32–8.41 | 0.548 |
| IVH | 0.297 | 1.15 | 0.12–10.61 | 0.901 |
| Clipping | 0.356 | 0.69 | 0.09–5.07 | 0.722 |
| H&H G3-4 | 0.531 | 2.76 | 0.64–11.8 | 0.174 |
| SAH thick diffuse | 0.187 | 1.69 | 0.14–20.6 | 0.678 |
| Severe aVS | 0.039* | 2.88 | 0.15–12.1 | 0.148 |
| Unfavorable outcome | ||||
| Follow-up iCCT | <0.01** | 5.62 | 0.74–42.3 | 0.093 |
| iCCT differences | <0.01** | 11.5 | 1.01–130 | 0.048* |
| DCI | <0.01** | 8.98 | 1.17–68.8 | 0.035* |
| Age > 65 | 0.658 | 0.98 | 0.12–8.17 | 0.987 |
| IVH | 0.135 | 15.3 | 0.41–577 | 0.140 |
| Clipping | 0.576 | 0.13 | 0.03–1.08 | 0.056 |
| H&H G3-4 | <0.01** | 9.87 | 1.01–96.8 | 0.049* |
| SAH thick diffuse | 0.088 | 1.02 | 0.01–155 | 0.994 |
| Severe aVS | 0.122 | 6.01 | 0.77–33.8 | 0.092 |
DCI: delayed cerebral ischemia; H&H G: Hunt & Hess grading; iCCT: intracerebral circulation time; IVH: intraventricular hemorrhage; aVS: angiographic vasospasm; 95% CI: 95% confidence intervals; OR: odds ratio.
*p < 0.05, **p < 0.01.
Outcome
Multivariate logistic regression analysis revealed that differences in the iCCT (OR 11.5, 95% CI 1.01–130; p = 0.048), DCI (OR 8.98, 95% CI 1.17–68.8; p = 0.035) and H & H grade (OR 9.87, 95% CI 1.01–96.8; p = 0.049) were significant risk factors for unfavorable outcome (Table 2).
Analysis of the factors affecting an increase of iCCT differences
In order to investigate the factors affecting differences in iCCT, the diagnostic threshold value for the follow-up iCCT and differences in iCCT for the development of DCI were evaluated. Sensitivity and specificity were then calculated. According to the threshold value, we divided the patients into two groups, and multivariate analyses were performed.
Threshold values for the iCCT differences
The ROC curve analysis revealed 4.02 seconds for the follow-up iCCT (73% sensitivity, 76% specificity, AUC 0.73), and 0.38 seconds for differences in the iCCT (89% sensitivity, 88% specificity, AUC 0.90) as possible threshold values that caused unfavorable outcome. The baseline characteristics for the patients as divided into two groups are shown in Table 3. Significant differences between patients with and without an increase in differences in the iCCT were shown in hypertension (p = 0.037), H&H grade (p = 0.041) and severe aVS (p = 0.017).
Table 3.
Demographics and vasospasm-related data for iCCT differences.
| Follow-up iCCT prolongation |
p Value | Increase in iCCT differences |
p Value | |||
|---|---|---|---|---|---|---|
| - | + | - | + | |||
| Number of subjects | 95 | 33 | 103 | 25 | ||
| Age, y, mean ± SD | 59 ± 12 | 60 ± 10 | 0.68 | 61 ± 11 | 58 ± 12 | 0.76 |
| Female | 62 (65%) | 18 (55%) | 0.19 | 65 (63%) | 15 (60%) | 0.47 |
| Hypertension | 41 (43%) | 18 (55%) | 0.18 | 43 (42%) | 16 (64%) | 0.04* |
| Smoker | 46 (48%) | 14 (42%) | 0.35 | 47 (46%) | 13 (52%) | 0.36 |
| IVH | 9 (9%) | 3 (9%) | 0.63 | 8 (8%) | 4 (16%) | 0.18 |
| ICH | 11(12%) | 4 (12%) | 0.58 | 13 (13%) | 2 (8%) | 0.40 |
| Clipping | 85 (89%) | 30 (91%) | 0.56 | 93 (90%) | 22 (88%) | 0.49 |
| Hunt & Hess grade | 0.01* | 0.04* | ||||
| Grade I or II | 64 (67%) | 14 (42%) | 67 (65%) | 11 (44%) | ||
| Grade III | 26 (27%) | 11 (33%) | 29 (28%) | 8 (32%) | ||
| Grade IV | 5 (5%) | 8 (24%) | 7 (7%) | 6 (24%) | ||
| SAH on baseline CT | 0.30 | 0.23 | ||||
| Thick local | 9 (9%) | 2 (6%) | 10 (10%) | 1 (4%) | ||
| Thin diffuse | 2 (2%) | 0 (%) | 2 (2%) | 0 (63%) | ||
| Thick diffuse | 84 (89%) | 31 (94%) | 91 (88%) | 24 (96%) | ||
| Aneurysm location | 0.13 | 0.25 | ||||
| ACA | 39 (41%) | 10 (30%) | 40 (39%) | 9 (36%) | ||
| ICA | 33 (35%) | 11 (33%) | 35 (34%) | 9 (36%) | ||
| MCA | 18 (19%) | 10 (30%) | 22 (21%) | 6 (24%) | ||
| VA / BA | 5 (5%) | 2 (7%) | 6 (6%) | 1 (4%) | ||
| Angiographic VS | 0.02* | 0.02* | ||||
| Non-mild-moderate | 67 (71%) | 16 (48%) | 72 (70%) | 11 (44%) | ||
| Severe | 28 (29%) | 17 (52%) | 31 (30%) | 14 (56%) | ||
ACA: anterior cerebral artery; DCI: delayed cerebral ischemia; ICA: internal cerebral artery; iCCT: intracerebral circulation time; ICH: intracerebral hemorrhage; IVH: intraventricular hemorrhage; MCA: middle cerebral artery; VA/BA: vertebral artery/basilar artery; VS: vasospasm *p < 0.05, **p < 0.01.
Multivariate analysis
Severe aVS was a significant risk factor in the patients with a follow-up iCCT prolongation (OR 3.48, 95% CI 1.05–11.5; p = 0.04), but other confounding factors such as sex, hypertension, H&H grade, and aneurysm location did not attain a significant statistical difference (Table 4). None of the cofounding factors such as hypertension, H&H grade, IVH, and severe aVS factor demonstrated a significant difference in the patients with an increase in differences in the iCCT (Table 4).
Table 4.
Multivariate analysis of affecting factors of increase in the iCCT differences.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Factor | p Value | OR | 95% CI | p Value |
| Follow-up iCCT | ||||
| Female | 0.19 | 0.64 | 0.19–2.13 | 0.47 |
| Hypertension | 0.18 | 1.17 | 0.34–3.99 | 0.79 |
| H&H > G3 | 0.014* | 2.96 | 0.89–9.82 | 0.08 |
| Aneurysm location | 0.13 | 1.19 | 0.26–5.41 | 0.82 |
| Severe aVS | 0.017* | 3.48 | 1.05–11.5 | 0.04* |
| Increase in iCCT differences | ||||
| Hypertension | 0.037* | 1.36 | 0.40–4.93 | 0.62 |
| H&H > G3 | 0.041* | 2.32 | 0.68–8.32 | 0.17 |
| IVH | 0.18 | 1.61 | 0.22–33.5 | 0.67 |
| Severe aVS | 0.015* | 2.47 | 0.14–8.64 | 0.14 |
H&H G: Hunt & Hess grading; iCCT: intracerebral circulation time; IVH: intraventricular hemorrhage; aVS: angiographic vasospasm; 95% CI: 95% confidence intervals; OR: odds ratio *p < 0.05, **p < 0.01.
Discussion
After the CONSCOUS-1 trial, a meta-analysis using 14 double-blinded RCTs that investigated the effects of various agents on aVS, DCI and clinical outcome in SAH patients also demonstrated that pharmaceutical treatments significantly decrease the occurrence of aVS, but not that of poor outcome. Another meta-analysis of six RCTs studying the effects of various statins showed the same result as the above analysis.44,45
The results of the present study also suggest that aVS is not important enough to contribute to a reduction in the incidence of DCI and poor outcome. Thus, many recent studies have shown that EBI, which consists of neuronal apoptosis, impaired autoregulation, brain edema, cortical spreading depression (CSD), and microcirculatory dysfunction primarily affects DCI instead of aVS.6,9–11,15,17,46 The present study was performed to clarify the role of microcirculatory dysfunction on DCI and outcome by analyzing CCT, which is considered an indicator of microcirculatory dysfunction.17,47–50
Two studies have examined the relationship between CCT and DCI by using DSA that was performed only in the ultra-early phase after the ictus.22,32 However, the results differed between the two studies with regard to any correlation between increased CCT and DCI. In one study, the prolongation of peripheral CCT was significantly correlated with the occurrence of DCI. 32 The other study measured whole brain CCT and showed no significant difference between the CCT and the development of DCI. 22 Neither of these studies tested the correlation between aVS and CCT. Therefore, a correlation between microcirculatory dysfunction in the ultra-early phase and DCI is suggested but is not definitive. This determination corresponds to the results of the baseline iCCT of the present study, in which a significant correlation of the baseline iCCT with DCI was obtained only when univariate analysis was applied. This result suggests that although there is a possibility of microcirculatory disturbance in the ultra-early phase, severe H & H grade may be more meaningful for outcome in actual practice than iCCT prolongation, as shown in Table 2.
On the other hand, there are no studies on CCT during the subacute phase using DSA. In the present study, the follow-up iCCT measured in the subacute phase was correlated with DCI more than was the baseline iCCT measured during the ultra-early phase. A correlation of the follow-up iCCT with poor outcome, however, was not obtained. These uncertain correlations between microcirculatory dysfunction and DCI and poor outcome not only in the ultra-early phase but also in the subacute phase may be due to the fact that the physiological variation in the CCT of healthy individuals was reported to range between 4.67 s ± 1.08 s and 5.11 s ± 1.00 s.51,52 This range could therefore influence a one-time evaluation of CCT by masking abnormal changes.
Therefore, chronological differences in microcirculatory impairment from the ultra-early phase to the subacute phase were also investigated in the present study. The results showed that an increase in differences in the iCCT could be a significant risk factor for DCI and for poor outcome, which was demonstrated not only by univariate analysis but also by multivariate analysis. These results suggest that the differences in impaired microcirculation themselves, as they are aggravated from the ultra-early phase onward to the subacute phase, are important in DCI onset and poor outcome.
DCI and poor outcome may be affected not only by microcirculatory dysfunction during the ultra-early phase, but also by persistent microcirculatory dysfunction occurring as a part of EBI and continuing until the subacute phase with gradual aggravation. Therefore, differences in the iCCT during the clinical course can serve as an index for predicting DCI onset and poor outcome.
In terms of the mechanisms for microcirculatory impairments, previous clinical and experimental studies revealed that microthrombosis and microvasospasm occurred during both the ultra-early and the subacute phase.13,15,16,41,53–58 Microthrombosis can be induced by systemic hypercoagulability, increased platelet-activation, endothelial cell damage, and microcirculatory stasis.13–16,53–63 Microthrombosis can be induced by systemic hypercoagulability, increased platelet-activation, endothelial cell damage, and microcirculatory stasis.13–16,46,53–58,64 Microvasospasm can also be induced by vasoconstrictors derived from activated platelets or SAH clots, vasodilatory dysfunction due to endothelial impairment, and changed vascular reactivity to vasodilatory and/or vasocontractile stimuli.15,16,55,59–63 Regarding the relationship between microthrombosis and microvasospasm, the current study in vivo revealed that microvasospasm gradually progresses toward the subacute phase, and induces microthrombosis.16,65 An analysis of the factors affecting the prolongation of the iCCT in the present study revealed that aVS was the only factor influencing the follow-up iCCT. Endothelial damage to the major cerebral arteries induced by aVS might activate circulating platelets, thereby resulting in microcirculatory disturbance.
To treat microthrombosis, antiplatelet agents such as aspirin, ozagrel sodium, dipyridamole, and ticlopidine have been tried; however, a systematic review showed some improvement in DCI and outcome could be obtained by single agent administration, but without any unequivocally significant difference. 66 On the other hand, a recent systematic review in which the majority of cases were treated by coil embolization also showed a significant reduction in poor outcome.67,68 Although a recent retrospective study showed that double antiplatelet therapy might be effective, a large-scale RCT will be required to verify its significance.69,70 As another treatment method for microthrombosis, there have been reports showing the effectiveness of ADAMTS13, which has an antiplatelet effect by inhibiting VWF-dependent platelet aggregation. This, however, is still in the experimental stage and has not been clinically applied. 71 Additionally, as a treatment for microthrombosis with anticoagulants, low-dose intravenous heparin infusion has been reported and is associated with a decreased risk of DCI. 72 Based on the above, the efficacy of antiplatelet or anticoagulants agents seems promising, and further investigations on the proper types of agents and appropriate combination of agents as polypharmacy is necessary in order to obtain a more definite clinical effect.
At the same time, there is currently no clinically implemented treatment for microvasospasm. Although the effectiveness of NO inhalation therapy for microvessel spasm has been experimentally proven, its clinical application has not been verified.19,73,74 Several RCTs have shown the efficacy of cilostazol, one of the antiplatelet drugs.36,75 In addition to exerting antiplatelet action by inhibiting PDE3, cilostazol also results in vascular smooth muscle dilatation, and in vitro studies demonstrated that its vasodilatory action is particularly strong in microarteries. 73 Therefore, the efficacy of cilostazol for DCI and poor outcome may be partially attributed to its ameliorative action on microvasospasm. 76 In the future, it will be necessary to elucidate the mechanism of microvasospasm in more detail and to develop a therapeutic method.
As mentioned above, it is necessary to continue to explore treatment methods for microcirculatory disturbance, but methods related to the evaluation of microcirculatory disturbance need to be optimized. In this study, we performed post hoc analysis using DSA-based iCCT to elucidate the pathogenesis of microcirculatory disturbance after aSAH. However, frequent DSA is difficult to perform in clinical practice, and the analysis of the iCCT is not easy because it requires complicated image processing. Currently, new techniques are available as alternatives to DSA. These include arterial spin labelling (ASL) perfusion magnetic resonance imaging (MRI) sequences and/or time-resolved near-infrared spectroscopy, which offer longitudinal, non-invasive measurement of cerebral blood flow and microvascular, dynamic cerebral autoregulation.77–79 These methods can be used for frequent examinations or continuous monitoring, and are expected to be non-invasive methods for the analysis of microcirculatory disturbance in patients with aSAH.
Conclusion
An increase in differences in the iCCT showed a significant correlation with the occurrence of DCI and poor outcome, independent of aVS in aSAH patients. This suggests that greater microcirculatory dysfunction from the ultra-early phase to the subacute phase can be a causative factor for DCI development and poor outcome.
Acknowledgements
We thank Mark Inglin (University of Basel) for his editorial assistance.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: MN, NM, NS, and HO were involved in study design and data interpretation. MN, NM, and HO were involved in the data analysis. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
ORCID iD: Masato Naraoka https://orcid.org/0000-0001-6490-527X
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and Meta-analysis. Lancet Neurol 2010; 9: 167–176. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001; 124: 249–278. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998; 50: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008; 39: 3015–3021. [DOI] [PubMed] [Google Scholar]
- 5.Chou SHY, Feske SK, Simmons SL, et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res 2011; 2: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill J, Zhang JH. Subarachnoid hemorrhage: is it time for a new direction? Stroke 2009; 40: S86–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan P, Xu L, Zhang H, et al. A review of hematoma components clearance mechanism after subarachnoid hemorrhage. Front Neurosci 2020; 14: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rass V, Helbok R. Early brain injury after poor-grade subarachnoid hemorrhage. Curr Neurol Neurosci Rep 2019; 19: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pluta R, Hansen-Schwartz J, Dreier J, et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res 2009; 31: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep 2017; 19 [DOI] [PubMed] [Google Scholar]
- 11.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 12.Wang FANG, Hu QIN, Chen C-H, et al. The protective effect of cerebralcare granule® on brain edema, cerebral microcirculatory disturbance, and neuron injury in a focal cerebral ischemia rat model. Microcirculation 2012; 19: 260–272. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Kimura M, Souma M, et al. Cerebral microthrombosis in symptomatic cerebral vasospasm – a quantitative histological study in autopsy cases. Neurol Med Chir (Tokyo) ) 1990; 30: 309–316. [DOI] [PubMed] [Google Scholar]
- 14.Ohkuma H, Suzuki S, Kimura M, et al. Role of platelet function in symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1991; 22: 854–859. [DOI] [PubMed] [Google Scholar]
- 15.Ohkuma H, Itoh K, Shibata S, et al. Morphological changes of intraparenchymal arterioles after experimental subarachnoid hemorrhage in dogs. Neurosurgery 1997; 41: 230–236. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich B, Müller F, Feiler S, et al. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab 2012; 32: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohkuma H, Manabe H, Tanaka M, et al. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2000; 31: 1621–1627. [DOI] [PubMed] [Google Scholar]
- 18.Caspers J, Rubbert C, Turowski B, et al. Timing of mean transit time maximization is associated with neurological outcome after subarachnoid hemorrhage. Clin Neuroradiol 2017; 27: 15–22. [DOI] [PubMed] [Google Scholar]
- 19.Lenz IJ, Plesnila N, Terpolilli NA. Role of endothelial nitric oxide synthase for early brain injury after subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab 2021; 41: 1669–1681. [DOI] [PMC free article] [PubMed]
- 20.Iseda T, Nakano S, Yoneyama T, et al. Angiographic cerebral circulation time before and after endovascular therapy for symptomatic vasospasm. Clin Radiol 2000; 55: 679–683. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Schaaf I, Wermer MJ, Van Der Graaf Y, et al. CT after subarachnoid hemorrhage: relation of cerebral perfusion to delayed cerebral ischemia. Neurology 2006; 66: 1533–1538. [DOI] [PubMed] [Google Scholar]
- 22.Burkhardt J-K, Chen X, Winkler EA, et al. Early hemodynamic changes based on initial color-coding angiography as a predictor for developing subsequent symptomatic vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg 2018; 109: e363–e373. [DOI] [PubMed] [Google Scholar]
- 23.Starnoni D, Maduri R, Hajdu SD, et al. Early perfusion computed tomography scan for prediction of vasospasm and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg 2019; 130: e743–e752. [DOI] [PubMed] [Google Scholar]
- 24.Dong L, Zhou Y, Wang M, et al. Whole-brain CT perfusion on admission predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Eur J Radiol 2019; 116: 165–173. [DOI] [PubMed] [Google Scholar]
- 25.Sviri GE, Britz GW, Lewis DH, et al. Dynamic perfusion computed tomography in the diagnosis of cerebral vasospasm. Neurosurgery 2006; 59: 319–324. [DOI] [PubMed] [Google Scholar]
- 26.Rijsdijk M, Van Der Schaaf IC, Velthuis BK, et al. Global and focal cerebral perfusion after aneurysmal subarachnoid hemorrhage in relation with delayed cerebral ischemia. Neuroradiology 2008; 50: 813–820. [DOI] [PubMed] [Google Scholar]
- 27.Killeen RP, Mushlin AI, Johnson CE, et al. Comparison of CT perfusion and digital subtraction angiography in the evaluation of delayed cerebral ischemia. Acad Radiol 2011; 18: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chai W, Sun X, Lv F, et al. Clinical study of changes of cerebral microcirculation in cerebral vasospasm after SAH. Acta Neurochir Suppl 2011; 110: 225–228. [DOI] [PubMed]
- 29.Lagares A, Cicuendez M, Ramos A, et al. Acute perfusion changes after spontaneous SAH: a perfusion CT study. Acta Neurochir (Wien) 2012; 154: 405–411. [DOI] [PubMed] [Google Scholar]
- 30.Hickmann A-K, Langner S, Kirsch M, et al. The value of perfusion computed tomography in predicting clinically relevant vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurosurg Rev 2013; 36: 267–278. [DOI] [PubMed] [Google Scholar]
- 31.Sanelli PC, Anumula N, Johnson CE, et al. Evaluating CT perfusion using outcome measures of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2013; 34: 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gölitz P, Hoelter P, Rösch J, et al. Ultra-early detection of microcirculatory injury as predictor of developing delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Clin Neuroradiol 2018; 28: 501–507. [DOI] [PubMed] [Google Scholar]
- 33.Udoetuk JD, Stiefel MF, Hurst RW, et al. Admission angiographic cerebral circulation time may predict subsequent angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery 2007; 61: 1152–1161. [DOI] [PubMed] [Google Scholar]
- 34.Lin CJ, Chang FC, Tsai FY, et al. Stenotic transverse sinus predisposes to poststenting hyperperfusion syndrome as evidenced by quantitative analysis of peritherapeutic cerebral circulation time. AJNR Am J Neuroradiol 2014; 35: 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitt MR, Morton RP, Haynor DR, et al. Angiographic perfusion imaging: real-time assessment of endovascular treatment for cerebral vasospasm. J Neuroimaging 2014; 24: 387–392. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda N, Naraoka M, Ohkuma H, et al. Effect of cilostazol on cerebral vasospasm and outcome in patients with aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled trial. Cerebrovasc Dis 2016; 42: 97–105. [DOI] [PubMed] [Google Scholar]
- 37.Naraoka M, Matsuda N, Shimamura N, et al. Long-acting statin for aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled trial. J Cereb Blood Flow Metab 2018; 38: 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong GKC, Boet R, Ng SCP, et al. Ultra-early (within 24 hours) aneurysm treatment after subarachnoid hemorrhage. World Neurosurg 2012; 77: 311–315. [DOI] [PubMed] [Google Scholar]
- 39.Han Y, Ye F, Long X, et al. Ultra-early treatment for poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. World Neurosurg 2018; 115: e160–e171. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya M, Suzuki Y, Sugita K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg 1992; 76: 571–577. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Chen J, Toyota Y, et al. Ultra-Early cerebral thrombosis formation after experimental subarachnoid hemorrhage detected on T2∗ magnetic resonance imaging. Stroke 2021; 52: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dankbaar JW, Rijsdijk M, van der Schaaf IC, et al. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology 2009; 51: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inagawa T. Risk factors for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a review of the literature. World Neurosurg 2016; 85: 56–76. [DOI] [PubMed] [Google Scholar]
- 44.Etminan N, Vergouwen MD, Ilodigwe D, et al. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2011; 31: 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen J, Huang K-Y, Zhu Y, et al. Effect of statin treatment on vasospasm-related morbidity and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 2017; 127: 291–301. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki S, Suzuki M, Iwabuchi T, et al. Role of multiple cerebral microthrombosis in symptomatic cerebral vasospasm: with a case report. Neurosurgery 1983; 13: 199–203. [DOI] [PubMed] [Google Scholar]
- 47.Anzabi M, Angleys H, Aamand R, et al. Capillary flow disturbances after experimental subarachnoid hemorrhage: a contributor to delayed cerebral ischemia? Microcirculation 2019; 26: e12516. [DOI] [PubMed] [Google Scholar]
- 48.Østergaard L, Aamand R, Karabegovic S, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2013; 33: 1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herz DA, Baez S, Shulman K. Pial microcirculation in subarachnoid hemorrhage. Stroke 1975; 6: 417–424. [DOI] [PubMed] [Google Scholar]
- 50.Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 2012; 32: 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greitz T. A radiologic study of the brain circulation by rapid serial angiography of the carotid artery. Acta Radiol Suppl 1956; 46: 1–123. [PubMed] [Google Scholar]
- 52.Lin CJ, Hung SC, Guo WY, et al. Monitoring peri-therapeutic cerebral circulation time: a feasibility study using color-coded quantitative DSA in patients with steno-occlusive arterial disease. AJNR Am J Neuroradiol 2012; 33: 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedrich V, Flores R, Muller A, et al. Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage. Brain Res 2010; 1354: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pisapia JM, Xu X, Kelly J, et al. Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp Neurol 2012; 233: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabri M, Ai J, Lakovic K, et al. Mechanisms of microthrombi formation after experimental subarachnoid hemorrhage. Neuroscience 2012; 224: 26–37. [DOI] [PubMed] [Google Scholar]
- 56.Sabri M, Ai J, Knight B, et al. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 2011; 31: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vergouwen MDI, Knaup VL, Roelofs JJTH, et al. Effect of recombinant ADAMTS-13 on microthrombosis and brain injury after experimental subarachnoid hemorrhage. J Thromb Haemost 2014; 12: 943–947. [DOI] [PubMed] [Google Scholar]
- 58.Andereggen L, Neuschmelting V, von Gunten M, et al. The role of microclot formation in an acute subarachnoid hemorrhage model in the rabbit. Biomed Res Int 2014; 2014: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohkuma H, Suzuki S. Histological dissociation between intra- and extraparenchymal portion of perforating small arteries after experimental subarachnoid hemorrhage in dogs. Acta Neuropathol 1999; 98: 374–382. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Xie G, Zhou C, et al. Baincalein alleviates early brain injury after experimental subarachnoid hemorrhage in rats: possible involvement of TLR4/NF-κB-mediated inflammatory pathway. Brain Res 2015; 1594: 245–255. [DOI] [PubMed] [Google Scholar]
- 61.Wang K-C, Tang S-C, Lee J-E, et al. Impaired microcirculation after subarachnoid hemorrhage in an in vivo animal model. Sci Rep 2018; 8: 13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu H, Dienel A, Schöller K, et al. Microvasospasms after experimental subarachnoid hemorrhage do not depend on endothelin a receptors. Stroke 2018; 49: 693–699. [DOI] [PubMed] [Google Scholar]
- 63.Joerk A, Ritter MM, Langguth N, et al. Propentdyopents as heme degradation intermediates constrict mouse cerebral arterioles and are present in the cerebrospinal fluid of patients with subarachnoid hemorrhage. Circ Res 2019; 124: e101–e114. [DOI] [PubMed] [Google Scholar]
- 64.Stein SC, Browne KD, Chen X-H, et al. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery 2006; 59: 781–788. [DOI] [PubMed] [Google Scholar]
- 65.Uhl E, Lehmberg J, Steiger H-J, et al. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery 2003; 52: 1307–1315. [DOI] [PubMed] [Google Scholar]
- 66.D, Mees S, van den Bergh WM, Algra A, et al. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 4: CD006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cagnazzo F, Derraz I, Lefevre P-H, et al. Antiplatelet therapy in patients with aneurysmal SAH: impact on delayed cerebral ischemia and clinical outcome. A meta-analysis. AJNR Am J Neuroradiol 2019; 40: 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Enriquez-Marulanda A, Salem MM, Ravindran K, et al. Effect of premorbid antiplatelet medication use on delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a propensity score-matched study. Cureus 2019; 11: e5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagahama Y, Allan L, Nakagawa D, et al. Dual antiplatelet therapy in aneurysmal subarachnoid hemorrhage: association with reduced risk of clinical vasospasm and delayed cerebral ischemia. J Neurosurg 2018; 129: 702–710. [DOI] [PubMed] [Google Scholar]
- 70.Clarke JV, Suggs JM, Diwan D, et al. Microvascular platelet aggregation and thrombosis after subarachnoid hemorrhage: a review and synthesis. J Cereb Blood Flow Metab 2020; 40: 1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vergouwen MDI, Bakhtiari K, Van Geloven N, et al. Reduced ADAMTS13 activity in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2009; 29: 1734–1741. [DOI] [PubMed] [Google Scholar]
- 72.Kole MJ, Wessell AP, Ugiliweneza B, et al. Low-dose intravenous heparin infusion after aneurysmal subarachnoid hemorrhage is associated with decreased risk of delayed neurological deficit and cerebral infarction. Neurosurgery 2021; 88: 523–530. [DOI] [PubMed] [Google Scholar]
- 73.Terpolilli NA, Feiler S, Dienel A, et al. Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J Cereb Blood Flow Metab 2016; 36: 2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balbi M, Vega MJ, Lourbopoulos A, et al. Long-term impairment of neurovascular coupling following experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 2020; 40: 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Senbokuya N, Kinouchi H, Kanemaru K, et al. Effects of cilostazol on cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a multicenter prospective, randomized, open-label blinded end point trial. J Neurosurg 2013; 118: 121–130. [DOI] [PubMed] [Google Scholar]
- 76.Saber H, Desai A, Palla M, et al. Efficacy of cilostazol in prevention of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a meta-analysis. J Stroke Cerebrovasc Dis 2018; 27: 2979–2985. [DOI] [PubMed] [Google Scholar]
- 77.Milej D, He L, Abdalmalak A, et al. Quantification of cerebral blood flow in adults by contrast-enhanced near-infrared spectroscopy: validation against MRI. J Cereb Blood Flow Metab 2020; 40: 1672–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elting JWJ, Tas J, Aries MJH, et al. Dynamic cerebral autoregulation estimates derived from near infrared spectroscopy and transcranial doppler are similar after correction for transit time and blood flow and blood volume oscillations. J Cereb Blood Flow Metab 2020; 40: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baker WB, Balu R, He L, et al. Continuous non-invasive optical monitoring of cerebral blood flow and oxidative metabolism after acute brain injury. J Cereb Blood Flow Metab 2019; 39: 1469–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]