Abstract
Background: Although intravenous (IV) infiltration is relatively common, data regarding complications and outcomes of this problem remain limited. In addition, there is wide variation in institutional protocols for the management of IV infiltrations. Through retrospective review, we aim to delineate complications and outcomes, and propose an algorithm for the management of these injuries. Methods: We performed a retrospective review of all patients who had an IV infiltration at a tertiary care center’s inpatient and outpatient facilities between January 1, 2016, and December 31, 2018. Results: In all, 479 patients with 495 infiltrations were included, with a mean age of 36.7 years. The upper extremity was involved in 89.6% of events. Of all the events, 8.6% led to a superficial soft tissue infection, 3.2% led to necrosis or eschar formation, and 1.9% led to ulceration or full-thickness wound formation. There were zero cases of compartment syndrome. Only 5.1% resulted in any long-term defects; none resulted in a functional defect of the extremity. Patients with vascular disease did not experience worse outcomes compared with healthy individuals. Plastic or orthopedic surgery was consulted in 25.3% of events. No emergent surgical intervention was required, 7 (1.4%) required bedside procedures, and 7 (1.4%) patients underwent nonacute operations. Conclusions: A specialist was consulted in about one-quarter of IV infiltrations, yet none were surgical emergencies. Instead, most complications could be monitored and managed by a primary team. Therefore, we propose algorithms involving nursing staff, wound care teams, and primary physicians with limited specialist consultation to manage these injuries.
Keywords: IV infiltration, IV extravasation, compartment syndrome, treatment protocols, iatrogenic, eschar, necrosis, specialist consultation
Introduction
Intravenous (IV) infiltration, defined as the efflux of solutions from a vessel into the surrounding tissue during an infusion, 1 is a well-recognized complication of peripheral IV therapy. Extravasation of a vesicant has the potential to cause blisters, severe tissue injury, or necrosis. 2 In this article, the term infiltration will be used to include infiltration of vesicant or nonvesicant medications. Common vesicants include chemotherapeutic agents, vasoactive medications, contrast agents, antibiotics, and solutions containing calcium salts and/or 10% dextrose.1,3-6 Additional risk factors for infiltration include catheter placement in areas with little underlying soft tissue, the use of large-gauge needles, and frequent cannulation. 1 Signs and symptoms of injury include pain and swelling of the affected area with progression to blistering, blanching, and paresthesia. Infiltration may progress to eschar formation and skin ulceration or necrosis, with possible damage to deeper structures that provide function to the extremity, including tendons and nerves. 7 Although rare, increased intracompartmental pressure secondary to swelling or eschar formation has even been reported as a cause of compartment syndrome. As peripheral catheters are typically superficial to fascial compartments, compartment syndrome caused by fluid infiltration is highly unlikely. Nevertheless, it often remains a concern for consulting services. 8
Prior studies have demonstrated a wide range of incidence of infiltration injuries in hospitals, ranging from 0.1% to 6% of all patients who require IV access.9,10 Although the complications of infiltration injury have long been recognized, rates of these complications have not been well defined. A 2007 study of 69,657 computed tomographic (CT) contrast injections found that infiltration occurred in 0.7% of cases, with moderate-to-severe complications occurring in only 10 (0.01%) patients, despite the rapid infusion rate and large infusion volume of CT contrast. This low complication rate occurred despite surgical consultation occurring in only 0.07% of patients, which was at least in part due to an institutional management protocol that involved evaluations by radiologists prior to the consultation of surgeons and strict criteria for consultation. 11 However, this policy is not standard, and there is a wide variation in both institutional protocols and those suggested in the literature for the management of IV infiltration. These protocols often involve extremity surgeons (plastic or orthopedic) for evaluation of the patient, yet are not based on objective knowledge of the complications, morbidity, and associated need for procedural interventions.
Through a retrospective review, we aim to delineate complications, outcomes, and management of patients with IV infiltration in both outpatient and inpatient settings. We hypothesize that interventions are rarely necessary and that long-term sequelae are rare. Finally, we propose an institutional protocol for the acute and subacute management of IV infiltration injuries in an effort to reduce unnecessary consultations, which come at an unnecessary cost for the patient and the health care system.
Materials and Methods
We performed a retrospective cohort study of all patients who had an IV infiltration injury or were treated for an IV infiltration injury at a single major tertiary care center’s inpatient and outpatient facilities between January 1, 2016, and December 31, 2018. Patients were identified through the use of the Research Derivative (RD), a secure web-based application designed to support data capture for research studies. We used a combination of keywords and International Classification of Diseases codes to identify all patients with IV injury (Supplemental Table 1). Medical records were obtained and reviewed for these patients. If a patient presented with multiple instances of infiltration during the study period, each episode was individually recorded. Patients were excluded from the study if there was insufficient or unclear documentation, defined as 3 or more study variables being undocumented in the electronic medical record (EMR) for a single infiltration event.
Data on patient demographics and medical history, infiltration characteristics, interventions, and outcomes were recorded. For analysis of patient demographics, each patient was only included once in the analysis, regardless of the number of infiltration events that occurred. For analysis of outcomes, patients were not included if they died within a month of infiltration (3 patients excluded). When analyzing long-term complications, patients were excluded if there was no appropriate follow-up documented in the EMR, which was defined as either: (1) no definitive documentation of resolutions of symptoms; or (2) no definitive documentation of complications or long-term (30-day) sequelae. All data were reported using raw counts, measures of central tendency (mean and median), and measures of dispersion (95% confidence intervals, standard errors, interquartile ranges) where appropriate.
Short-term complications and long-term sequelae between patients with a history of diabetes mellitus, chronic kidney disease, and/or peripheral vascular disease were compared. In addition, outcomes resulting from vesicant versus nonvesicant IV infiltrations were compared, as well as between chemotherapeutic and nonvesicant solutions. Statistical significance was determined using 2-tailed Fisher exact tests (GraphPad). Institutional review board approval was granted for this study on January 4, 2018 (#180006).
Results
Patient Demographics
Six hundred eighty patients were identified; of these, 479 had sufficient documentation to be included for analysis. The median age of these 479 patients was 36.7 (± 28.2) years. Two hundred fifty-eight (53.9%) were women. Of the 475 patients included for analysis of outcomes, 105 (22.1%) had diabetes mellitus, 52 (10.9%) had chronic kidney disease, 16 (3.4%) had peripheral vascular disease, and 132 (27.8%) had 1 or more of the above.
Infiltrate Characteristics
There were a total of 495 recorded IV infiltration events among the cohort. The anatomical location of infiltration was recorded in 442 events (Supplemental Table 2). Of the infiltrations with a recorded anatomical location, 309 (70.0%) occurred in the upper arm, antecubital fossa, and forearm; 87 (19.7%) occurred in the hand. Thirty-six (8.1%) occurred in the lower extremity, 7 (1.6%) occurred in the scalp, 2 (0.5%) occurred in the neck, and 1 (0.2%) occurred in the chest wall. In 451 of the 495 infiltration events, laterality was recorded; 206 (45.7%) were on the left, 238 (52.8%) were on the right, and 7 (1.6%) occurred in the scalp without laterality; all scalp IV infiltrations were placed in pediatric patients.
Three hundred eighty-one (77.0%) infiltrations occurred in the inpatient setting, 37 (7.5%) occurred in the emergency department, 28 (5.7%) occurred in the outpatient setting, 27 (5.5%) occurred in the operating room, and 4 (0.8%) occurred in the post-anesthesia care unit. Finally, 19 (3.8%) occurred at an outside hospital, and the patient subsequently presented to our institution for care.
Outcomes
After accounting for patients who died within 30 days of an infiltration, 475 infiltration events were included for analysis of outcomes (Table 1). Of the immediate complications, the most common was a superficial soft tissue infection (including cellulitis, superficial thrombophlebitis, and 1 drainable soft tissue abscess), with 41 (8.6%) infected infiltration sites. Fifteen (3.2%) infiltrate sites developed necrosis or an eschar (Supplemental Figure 1a), whereas 9 (1.9%) progressed to ulceration or visible full-thickness wound formation (Supplemental Figure 1b). Two (0.4%) preceded a chronic disease exacerbation (1 infiltration prior to a gout attack, 1 prior to a flare up of Raynaud disease; both of these events were adequately treated and did not lead to long-term sequelae). Finally, 1 (0.2%) infiltration was implicated in the formation of a deep vein thrombosis, and 0 (0.0%) led to compartment syndrome.
Table 1.
Outcomes of Patients Experiencing an Infiltration Who Did Not Die Within a Month of Injury (n = 475).
| Outcome | No. (%) |
|---|---|
| Immediate complications | |
| Superficial soft tissue infection a | 41 (8.6) |
| Necrosis/Eschar | 15 (3.2) |
| Ulceration/Full-thickness wound formation | 9 (1.9) |
| Chronic disease exacerbation | 2 (0.4) |
| Deep vein thrombosis | 1 (0.2) |
| Compartment syndrome | 0 (0.0) |
| Long-term complications (n = 469) b | |
| Cosmetic defect c | 16 (3.4) |
| Contracture | 2 (0.4) |
| Chronic pain | 2 (0.4) |
| Chronic wound | 2 (0.4) |
| Persistent numbness | 1 (0.2) |
| Skin discoloration d | 1 (0.2) |
Includes cellulitis, superficial thrombophlebitis, and soft tissue abscesses.
Six additional patients were excluded from analysis of long-term complications due to insufficient follow-up recorded in the electronic medical record.
Includes long-term scars and visible wounds.
Secondary to iron infiltration.
An additional 6 infiltration events were excluded from the analysis of long-term complications due to a lack of appropriate follow-up. Of the 469 events in the analysis, 16 (3.4%) led to a cosmetic defect of a scar or visible wound. Two (0.4%) infiltrates led to an excessive skin contracture causing functional impairment, chronic pain, or chronic wound, whereas 1 (0.2%) infiltrate each led to persistent numbness or skin discoloration secondary to iron infiltration.
Infiltrations occurring in patients with a history of diabetes mellitus, chronic kidney disease, and/or peripheral vascular disease did not lead to worse outcomes when compared with patients without these conditions (Table 2). In addition, when comparing vesicant with nonvesicant medications (vesicant status of medications as reviewed in 2014 by Le and Patel 12 ), there was no difference in outcomes between the 2 cohorts (Table 3). However, infiltrations of chemotherapeutic agents led to a significantly increased rate of short-term complications and long-term sequelae when compared with infiltrations of nonvesicant medications (Table 4).
Table 2.
Comparison of Outcomes by Medical History (N = 475).
| Medical History | Short-term complications | Long-term sequelae |
|---|---|---|
| History of DM, CKD, or PVD (n = 132) | 19 (14.4%) | 4 (3.0%) |
| No history of DM, CKD, or PVD (n = 343) | 44 (12.8%) | 21 (6.1%) |
| P value (2-tailed Fisher exact test) | .65 (NS) | .25 (NS) |
Note. DM = diabetes mellitus; CKD = chronic kidney disease; PVD = peripheral vascular disease; NS = not significant.
Table 3.
Comparison of Outcomes Between Vesicant and Nonvesicant Infiltrations (N = 398).
| Medication type | Short-term complications | Long-term sequelae |
|---|---|---|
| Nonvesicant medication (n = 123) | 9 (7.3%) | 5 (4.1%) |
| Vesicant medication (n = 275) | 36 (13.1%) | 12 (4.4%) |
| P value (2-tailed Fisher exact test) | .17 (NS) | 1.00 (NS) |
Note. Seventy-seven infiltrations were of an unknown medication. NS = not significant.
Table 4.
Comparison of Outcomes Between Nonvesicant and Chemotherapeutic Infiltrations.
| Short-term complications | Long-term sequelae | |
|---|---|---|
| Nonvesicant medication (n = 123) | 9 (7.3%) | 5 (4.1%) |
| Chemotherapeutic medication (n = 17) | 9 (52.9%) | 6 (35.3%) |
| P value (2-tailed Fisher exact test) | .0001 | .0004 |
Interventions
One hundred twenty-five (25.3%) infiltrations at our institution led to a plastic or orthopedic surgical evaluation (Table 5). One hundred ten (22.2%) infiltrations required a pharmacologic intervention: there were 60 (12.1%) instances of hyaluronidase injections, 26 (5.3%) instances of systemic antibiotic administration due to suspected infection, 16 (3.2%) instances of phentolamine administration, 4 (0.8%) instances of chemotherapy-specific antidote administration, and 4 (0.8%) other pharmacologic interventions. A bedside debridement or incision and drainage (I&D) was required in 7 (1.4%) cases, with 488 (98.6%) cases not needing a bedside procedure. Seven (1.4%) patients underwent an operative procedure: 3 (0.6%) underwent debridement (with 1 patient requiring a repeat debridement), 3 (0.6%) underwent debridement and grafting, and 1 (0.2%) required an I&D of a resultant abscess. Zero (0.0%) infiltrations required a fasciotomy. There were 0 acute surgical interventions required in our cohort.
Table 5.
Management and Interventions of All Infiltration Injuries (N = 495).
| Intervention | No. (%) |
|---|---|
| Plastic or orthopedic surgery consulted | |
| Yes | 125 (25.3) |
| No | 370 (74.7) |
| Pharmacologic intervention | |
| Hyaluronidase injection | 60 (12.1) |
| Systemic antibiotics | 26 (5.3) |
| Phentolamine | 16 (3.2) |
| Chemotherapy-specific antidote | 4 (0.8) |
| Other | 4 (0.8) |
| Total | 110 (22.2) |
| Bedside debridement or incision and drainage | |
| Yes | 7 (1.4) |
| No | 488 (98.6) |
| Surgical intervention | |
| Debridement | 3 (0.6) |
| Debridement and grafting | 3 (0.6) |
| Incision and drainage of resultant abscess | 1 (0.2) |
| Fasciotomy or other acute intervention | 0 (0.0) |
| Total | 7 (1.4) |
Discussion
During the 3-year period, 680 patients at our inpatient and outpatient facilities were diagnosed with a peripheral IV infiltration. Four hundred seventy-nine patients with 495 infiltration events were included in our analysis. There was wide variation in patient age and comorbidities, and most infiltrations occurred in the inpatient setting. Notably, 89.6% of all documented infiltrations occurred in the upper extremity. Plastic or orthopedic surgery evaluated the patient at a rate of 25.3%. Despite this high frequency of specialized physician evaluation, a bedside procedure was only required in 1.4% of cases. In addition, there were no immediate complications requiring operative intervention, including no instances of compartment syndrome. The most common immediate complication was superficial soft tissue infection, which could be effectively recognized and managed by the admitting team, and 5.1% of infiltrations led to long-term sequelae. Six infiltrations required an eventual debridement with or without grafting. None of the noted complications required urgent or emergent surgical intervention. Of note, a patient’s medical history (diabetes mellitus, chronic kidney disease, and/or peripheral vascular disease) did not alter outcomes of peripheral IV infiltration injuries. In general, infiltrations of vesicant medications did not lead to worse outcomes compared with nonvesicant medications, although chemotherapeutic agents were the exception to this finding.
These findings are important when considering health care costs. In 2014, we demonstrated that the average charge associated with a new plastic surgery consultation was $155.68 if the provider billed for all services. 13 This represents a real cost burden to the patient or a revenue loss to the hospital if the consultation is not staffed or billed appropriately. This cost burden, in combination with the lack of immediate complications or acute interventions requiring the services of plastic or orthopedic surgeons, suggests that an institutional protocol that decreases consultations to specialists for IV infiltrations could decrease unnecessary use without changing outcomes.
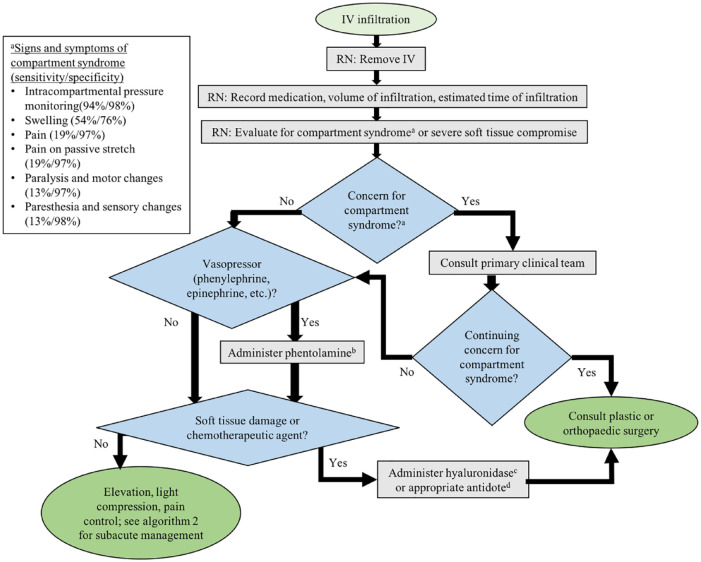
Our suggested protocol for the acute management of peripheral IV infiltration (Figure 1) involves a trained registered nurse evaluating the patient and removing the IV, with the primary team evaluating for compartment syndrome if necessary. If the primary physician is concerned about an acute pathology (eg, compartment syndrome), plastic or orthopedic surgery should be consulted without delay. In addition, phentolamine should be administered for vasopressor infiltration, and hyaluronidase should be administered for soft tissue damage per institutional protocol. Soft tissue damage should be evaluated by a plastic or orthopedic surgeon. The appropriate antidote according to the literature should be administered for chemotherapy infiltrations; plastic or orthopedic surgery should then be consulted for chemotherapy infiltrations due to the increased risk of both short-term and long-term complications. Once these concerns have been addressed, the patient should have elevation of the infiltrated area, application of light compression, appropriate analgesia, and referral to the protocol for subacute management (Figure 2).
Figure 1.
Our suggested protocol for the acute management of peripheral IV infiltration injuries. IV = intravenous; RN = registered nurse.
aSee figure for signs and symptoms of compartment syndrome. 14
bPhentolamine dose per institutional policy. At our institution, 5 mg of phentolamine is injected subcutaneously over 5 separate injections along the infiltration site.
cHyaluronidase dose per institutional policy. At our institution, a 15 unit/mL dilution of hyaluronidase is injected subcutaneously over 5 separate injections along the infiltration site.
dChemotherapy-specific antidotes are well studied and published in the literature 15 and should be used in the case of infiltration of chemotherapeutic agents.
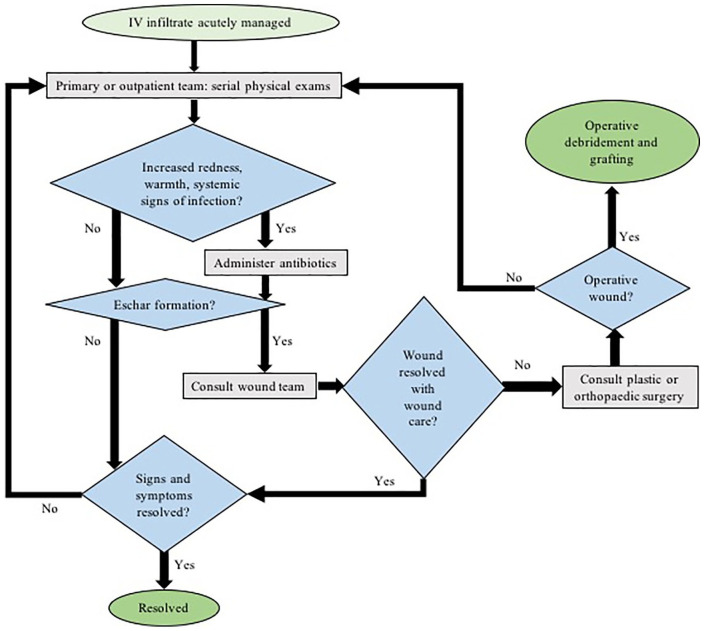
Figure 2.
Our suggested protocol for the subacute management of peripheral IV infiltration injuries. IV = intravenous.
Subacute management can be performed in the inpatient or outpatient setting, depending on the patient’s status. Subacute management involves monitoring for infection and administering appropriate antibiotic treatment, as well as monitoring for necrosis or eschar formation with the use of a bedside nurse or wound care specialist. If a wound care specialist is unavailable or the wound persists despite adequate wound care, then the plastic or orthopedic surgery team should be consulted. These 2 protocols allow for continuous monitoring and prevention of complications while decreasing unneeded surgical consultations.
Limitations
There are several limitations to this study. Not all patients with IV infiltration are captured in the RD due to deficient documentation; this is most clearly reflected in the outpatient setting. However, it is unlikely that compartment syndrome or other acute problems requiring intervention were missed by our method, as significant documentation is generated under those circumstances. In addition, certain medications known to be vesicants, such as CT contrast and vasopressors, may be overrepresented in our cohort, as those medications will be associated with more careful documentation when compared with nonvesicants. Our findings may not be generalizable to every patient care setting. Implementation of our suggested protocol is dependent on the availability of surgical teams, as well as a dedicated wound team for inpatients and outpatients, which some hospitals may not have. Finally, our study does not include data from intraosseous catheters, which are becoming increasingly used in trauma patients.
Conclusion
This study demonstrates that acute complications from peripheral IV infiltrations are rare; we suggest protocols for the acute and subacute management of these injuries. Our findings indicate that specialists, such as plastic and orthopedic surgeons, generally do not need to be involved in the care of peripheral IV infiltrations. Instead, their involvement can be deferred to a subacute or outpatient basis as needed. The data and protocols presented here may help to decrease health care costs without sacrificing patient outcomes stemming from peripheral IV injuries.
Supplemental Material
Supplemental material, HANDsupplementalfigure1 for Outcomes and Management of Peripheral Intravenous Infiltration Injuries by Joseph T. Gibian, Danny Zakria, Cooper March, Basil Schaheen and Brian C. Drolet in HAND
Supplemental material, Supplementary_material for Outcomes and Management of Peripheral Intravenous Infiltration Injuries by Joseph T. Gibian, Danny Zakria, Cooper March, Basil Schaheen and Brian C. Drolet in HAND
Acknowledgments
The authors of this study would like to thank Vanderbilt University Medical Center’s Department of Plastic Surgery. They would also like to thank Amanda Bailey, NP, for her contribution of pictures to this manuscript. In addition, thanks to Vanderbilt University Medical Center for their support.
Footnotes
Supplemental material is available in the online version of the article.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: No consent was required for this article, as the study was performed in a retrospective manner with no intervention.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Department of Plastic Surgery at Vanderbilt University Medical Center.
ORCID iD: Joseph T. Gibian  https://orcid.org/0000-0002-7059-1833
https://orcid.org/0000-0002-7059-1833
References
- 1. Al-Benna S, O’Boyle C, Holley J. Extravasation injuries in adults. ISRN Dermatol. 2013;2013:856541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doellman D, Hadaway L, Bowe-Geddes LA, et al. Infiltration and extravasation: update on prevention and management. J Infus Nurs. 2009;32(4):203-211. [DOI] [PubMed] [Google Scholar]
- 3. Brown AS, Hoelzer DJ, Piercy SA. Skin necrosis from extravasation of intravenous fluids in children. Plast Reconstr Surg. 1979;64:145-150. [DOI] [PubMed] [Google Scholar]
- 4. Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Der-matol. 1999;40:367-398; quiz 399-400. [DOI] [PubMed] [Google Scholar]
- 5. Subhani M, Sridhar S, DeCristofaro JD. Phentolamine use in a neonate for the prevention of dermal necrosis caused by dopamine: a case report. J Perinatol. 2001;21(5):324-326. [DOI] [PubMed] [Google Scholar]
- 6. Yosowitz P, Ekland DA, Shaw RC, et al. Peripheral intravenous infiltration necrosis. Ann Surg. 1975;182:553-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kreidieh FY, Moukadem HA, El Saghir NS. Overview, prevention and management of chemotherapy extravasation. World J Clin Oncol. 2016;7:87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pare JR, Moore CL. Intravenous infiltration resulting in compartment syndrome: a systematic review. J Patient Saf. 2018;14(2):e6-e8. [DOI] [PubMed] [Google Scholar]
- 9. MacCara ME. Extravasation: a hazard of intravenous therapy. Drug Intell Clin Pharm. 1983;17(10):713-717. [DOI] [PubMed] [Google Scholar]
- 10. Abolfotouh MA, Salam M, Bani-Mustafa A, et al. Prospective study of incidence and predictors of peripheral intravenous catheter-induced complications. Ther Clin Risk Manag. 2014;10:993-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang CL, Cohan RH, Ellis JH, et al. Frequency, management, and outcome of extravasation of nonionic iodinated contrast medium in 69,657 intravenous injections. Radiology. 2007;243(1):80-87. [DOI] [PubMed] [Google Scholar]
- 12. Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48(7):870-886. [DOI] [PubMed] [Google Scholar]
- 13. Drolet BC, Tandon VJ, Sargent R, et al. Revenue generation and plastic surgery training programs: 1-year evaluation of a plastic surgery consultation service. Plast Reconstr Surg. 2016;138(3):539e-542e. [DOI] [PubMed] [Google Scholar]
- 14. Duckworth AD, McQueen MM. The diagnosis of acute compartment syndrome: a critical analysis review. JBJS Rev. 2017;5(12):e1. [DOI] [PubMed] [Google Scholar]
- 15. Perez Fidalgo JA, Garcia Fabregat L, Cervantes A, et al. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl. 7):vii167-173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, HANDsupplementalfigure1 for Outcomes and Management of Peripheral Intravenous Infiltration Injuries by Joseph T. Gibian, Danny Zakria, Cooper March, Basil Schaheen and Brian C. Drolet in HAND
Supplemental material, Supplementary_material for Outcomes and Management of Peripheral Intravenous Infiltration Injuries by Joseph T. Gibian, Danny Zakria, Cooper March, Basil Schaheen and Brian C. Drolet in HAND