ABSTRACT
Background
In Parkinson's disease (PD) long‐term motor outcomes of subthalamic nucleus deep brain stimulation (STN‐DBS) are well documented, while comprehensive reports on non‐motor outcomes are fewer and less consistent.
Objective
To report motor and non‐motor symptoms after 5‐years of STN‐DBS.
Methods
We performed an open 5‐year extension study of a randomized trial that compared intraoperative verification versus mapping of STN using microelectrode recordings. Changes from preoperative to 5‐years of STN‐DBS were evaluated for motor and non‐motor symptoms (MDS‐UPDRS I‐IV), sleep disturbances (PDSS), autonomic symptoms (Scopa‐Aut), quality of life (PDQ‐39) and cognition through a neuropsychological test battery. We evaluated whether any differences between the two randomization groups were still present, and assessed preoperative predictors of physical dependence after 5 years of treatment using logistic regression.
Results
We found lasting improvement of off‐medication motor symptoms (total MDS‐UPDRS III, bradykinetic‐rigid symptoms and tremor), on‐medication tremor, motor fluctuations, and sleep disturbances, but reduced performance across all cognitive domains, except verbal memory. Reduction of verbal fluency and executive function was most pronounced the first year and may thus be more directly related to the surgery than worsening in other domains. The group mapped with multiple microelectrode recordings had more improvement of bradykinetic‐rigid symptoms and of PDQ‐39 bodily discomfort sub‐score, but also more reduction in word fluency. Older age was the most important factor associated with physical dependence after 5 years.
Conclusion
STN‐DBS offers good long‐term effects, including improved sleep, despite disease progression. STN‐DBS surgery may negatively impact verbal fluency and executive function.
Keywords: Parkinson's disease, STN‐DBS, long‐term, non‐motor symptoms, cognition
Subthalamic nucleus deep brain stimulation (STN‐DBS) is an established treatment for Parkinson's disease (PD) with motor fluctuations or tremor not responsive to levodopa. While short‐term effects have been well documented both on motor symptoms, 1 , 2 , 3 , 4 , 5 , 6 , 7 and non‐motor symptoms (NMS), 8 , 9 , 10 , 11 , 12 studies on long‐term effects of STN‐DBS (≥5 years) have mainly reported effects on motor symptoms. A sustained effect has been shown on bradykinesia, rigidity and tremor, but less so on axial symptoms like gait freezing, postural instability and dysarthria that worsen gradually in parallel with advancing disease. 13 , 14 , 15 , 16 , 17 , 18 Studies reporting long‐term effects on sleep and dysautonomia are lacking, and reports on the long‐term impact on cognitive functions are few and show conflicting results. 19
Many long‐term studies are small and some have relatively high drop‐out rates (in the range 37–82%), causing methodological challenges. 20 , 21 , 22 Drop‐outs from such studies occur more frequently among the poor‐performing patients (eg, nursing home residents) or deceased patients. This could lead to conclusions of a too favorable outcome and affect the evaluation of preoperative predictive factors.
Long‐term benefit varies among patients. Some studies on predictive factors of long‐term outcome have emerged, showing that younger age and better preoperative motor function and cognition are beneficial. 23 Preoperative levodopa responsiveness has been shown to predict short‐term motor outcome, 24 but not the outcome beyond 3 years. 25 Evidence that pin‐points the most important preoperative predictive factors are still lacking. The long‐term effects on non‐motor symptoms have been less studied, and few groups have reported on both motor and a wide range of non‐motor symptoms in the same cohort.
We have previously reported results from a randomized controlled trial with one‐year follow‐up of 60 PD patients undergoing STN‐DBS surgery, with targeting guidance either from single microelectrode (one central trajectory, or as few as needed to confirm STN signals; sMER) for target verification, or multiple simultaneously inserted MER for target mapping (mMER). 26 , 27 The mMER group had a significantly greater improvement after 1 year both in MDS‐UPDRS III medication‐off score and in two PDQ‐39 domains (activities of daily living and bodily discomfort).
Here we report motor and non‐motor outcomes after 5 years of STN‐DBS, both in the total study population and the two randomized groups. To our knowledge there are no studies that prospectively have evaluated the MDS‐UPDRS I‐IV scores after 5 years of STN‐DBS treatment, or have combined these scores with validated scales assessing sleep disturbances, autonomic dysfunction, cognition, and disease specific‐quality of life.
Methods
From April 2009 to December 2013, 76 patients had STN‐DBS surgery at Oslo University Hospital. Sixty patients were eligible and included in a prospective, randomized, double‐blind study comparing the use of single versus multiple simultaneous microelectrode‐recordings to guide the placement of the permanent electrode. The detailed description of the study design (including surgical procedure and causes for exclusions) and the main results on motor and quality of life‐outcomes at the one‐year follow‐up have been published previously. 26
Neurologic and Neuropsychiatric Evaluations
The Movement Disorder Society revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) was used preoperatively, and after 3 months, 1 year and 5 years of STN‐DBS to assess non‐motor experiences of daily living (I), motor experiences of daily living (II), motor examination (III) and the severity and impact of motor fluctuations (IV). 28 MDS‐UPDRS III was scored both after an overnight withdrawal of dopaminergic drugs (medication‐off), and after a levodopa dose approximately 1.5 times the patient's usual morning dose (medication‐on). Postoperative evaluations were performed in the stimulation‐on state. The MDS‐UPDRS III score was also divided into bradykinetic‐rigid symptoms [Items (I) 2–8 and 14], axial symptoms with known less response to levodopa (I 1 and 9–13) and tremor (I 15–18). 29 , 30 The Hoehn & Yahr scale (HY, 0–5) was scored in both the medication‐off and the medication‐on state. 31 Based on the HY‐scores at 5‐year follow‐up, patients were divided into two groups: Physically dependent (HY 4–5) versus physically independent (HY 1–3). Health‐related quality of life was assessed with the Parkinson's Disease Questionnaire‐39 (PDQ‐39), 32 , 33 with eight domain scores [mobility, activities of daily living (ADL), emotional well‐being, stigma, bodily discomfort, social support, cognition, and communication] and the mean across the domain scores [Summary index (SI)].
Sleep disturbances were assessed by the self‐rated Parkinson's Disease Sleep Scale (PDSS), 34 with 15 items scored from 0 (symptom severe and always present) to 10 (symptom‐free), maximum score 150. Autonomic symptoms were evaluated by the self‐rated questionnaire Scopa‐Aut, with 23 items assessing gastrointestinal symptoms (7 items), urinary symptoms (6), cardiovascular symptoms (3), thermoregulation (4), pupillomotor function (1) and sexual function (2 separate items for each gender). 35 Higher total scores express more severe symptoms (range 0–69). Levodopa equivalent daily doses (LEDD) were calculated at each follow‐up. 36 A comprehensive neuropsychological assessment was administered preoperatively and at the 1 and 5‐year follow‐up. The test battery covered the following six cognitive domains: attention/working memory [Digit Span and Number‐Letter Sequencing from the Wechsler Adult Intelligence Scale III (WAIS‐III)], 37 executive functions (Color‐Word Interference Test (CWIT) inhibition and inhibition/shifting conditions from the Delis‐Kaplan Executive Function System (D‐KEFS)), 38 processing speed (The Symbol Digit Modalities Test 39 and D‐KEFS CWIT; Color Naming and Word Reading), verbal learning and memory [Hopkins Verbal Learning Test‐Revised (HVLT‐R)], 40 visual learning and memory [Brief Visuospatial Memory Test‐Revisited (BVMT‐R)], and verbal fluency (phonemic and category fluency from D‐KEFS). The memory tests included total acquisition score for the three learning attempts, delayed recall and recognition. Parallel versions of the HVLT‐R and BVMT‐R were employed across time points to minimize re‐test effects. A composite global cognitive score was calculated expressing the mean of the individual domain scores. Raw scores were transformed into standardized T‐scores (mean = 50, SD = 10) using the test publisher's normative data.
Statistical Analysis
The scores were assessed with tests for normality. Paired sample t‐test was performed to determine the within subject differences between preoperative, 1‐year and 5‐year follow‐up, or Wilcoxon Signed Rank Test for non‐normally distributed variables. Differences between the randomization groups of the mother study from preoperative to 5‐years were compared using independent t‐tests, or Mann–Whitney U‐test for non‐normally distributed variables. Bonferroni adjusted α‐value were used when appropriate due to repeated testing.
Exploratory correlation analyses were performed, studying both preoperative levodopa response, motor scores, patient‐related characteristics, and other less‐studied parameters like LEDD and non‐motor symptoms like sleep and dysautonomia, as suggested by previous publications. 41 Exploratory correlation analyses were performed with Pearson correlation or Spearman's rank order correlation, for normally and non‐normally distributed variables respectively, and independent sample t‐tests. The variables that showed significant correlations from these exploratory correlation analyses (≤0.05, 2‐tailed), and no multicollinearity, were subsequently included in a logistic regression analysis. All statistical analyses were performed using IBM SPSS.26.
Results
In the original study 60 patients were included (30 in each randomization group), with primary endpoints evaluated blindly 1 year postoperatively. 26 At the 5‐year follow‐up 54 patients were included in the per protocol analysis: Three patients had surgical site infections with hardware explantation and discontinued neurostimulation within the first postoperative year, while at 5 years one patient was lost due to dementia and long travel distance and two patients had clinical follow‐up, but not by protocol (Fig. 1). Some patients did not finish the complete protocol, which is reflected in reduced “n” for the different variables. No preoperative differences were found between the patients who completed all questionnaires (n = 42) and those who failed to complete one or more questionnaires. For full baseline data of the total population and the two randomization groups, see Table 1.
FIG. 1.
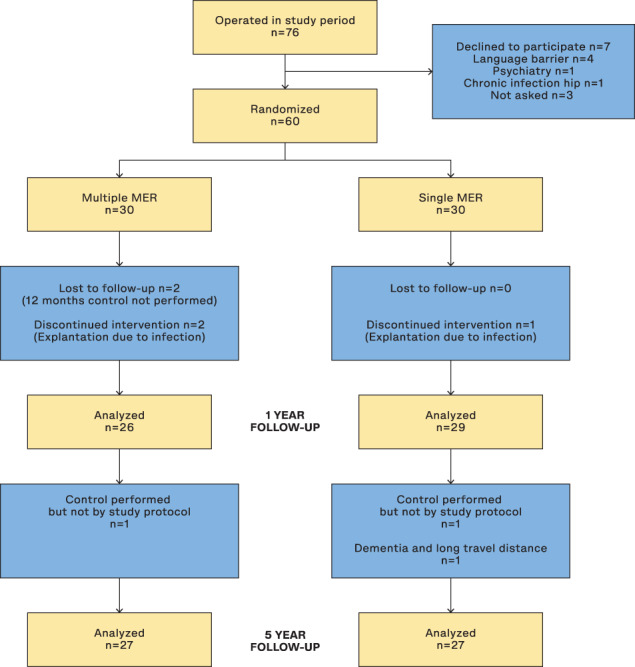
Participant flow‐chart. Patients designated as “Lost to follow‐up” (n = 2, multiple MER group) at 1 year are not the same individuals as those that dropped out at the 5‐year follow‐up.
TABLE 1.
Baseline characteristics of the 54 patients examined at 5 years postoperatively
| Total | sMER | mMER | |
|---|---|---|---|
| n = 54 | n = 27 | n = 27 | |
| Gender [n (%)] | |||
| Male | 39 (72) | 17 (63) | 22 (82) |
| Female | 15 (28) | 10 (37) | 5 (19) |
| Age at surgery | 63 (44–71) | 62 (44–71) | 63 (49–70) |
| Disease duration (yr) | 12 (4–23) | 11 (4–23) | 11 (4–17) |
| LEDD | 1248 (428–2490) | 1338 (874–2259) | 1248 (428–2490) |
| MDS‐UPDRS I | 10 (2–24) | 10 (1–25) | 10 (3–24) |
| MDS‐UPDRS II | 16 (1–31) | 16 (0–31) | 17 (9–32) |
| MDS‐UPDRS III | |||
| Off | 49.0 (28–75) | 44 (28–66) | 52 (28–75) |
| On | 13.0 (2–45) | 13 (3–37) | 13 (2–45) |
| MDS‐UPDRS IV | 10 (0–16) | 10 (1–15) | 9 (0–16) |
| PDQ‐39 | n = 53 | n = 27 | n = 26 |
| 23.4 (5.7–59.4) | 23.1 (5.7–59.4) | 25.3 (7.8–49.4) | |
|
Mattis dementia Rating scale |
n = 45 142 (131–144) |
n = 20 142 (131–144) |
n = 25 142 (134–144) |
| Neuropsychological testing: | |||
| Attention/working memory | n = 50 | n = 26 | n = 24 |
| 46.7 (31.5–65.0) | 46.7 (35.0–65.0) | 47.5 (31.5–65.0) | |
| Executive function | n = 50 | n = 26 | n = 24 |
| 45.8 (30.9–67.5) | 45.8 (30.9–57.5) | 47.9 (31.7–67.5) | |
| Processing | n = 49 | n = 25 | n = 24 |
| 47.3 (25.3–57.1) | 47.7 (35.8–55.4) | 46.1 (25.3–57.1) | |
| Verbal memory | n = 50 | n = 26 | n = 24 |
| 43.0 (20.0–61.3) | 42.3 (20.0–59.3) | 46.0 (28.7–61.3) | |
| Visual memory | n = 50 | n = 26 | n = 24 |
| 45.5 (24.5–67.5) | 45.5 (29.0) | 45.5 (24.5–67.5) | |
| Verbal fluency | n = 50 | n = 26 | n = 24 |
| 53.3 (38.4–80) | 50.0 (38.4–80) | 57.5 (41.7–78.3) | |
| Global | n = 50 | n = 26 | n = 24 |
| 46.1 (36.6–62.1) | 44.2 (38.6–62.1) | 48.0 (36.6–60.5) | |
Values are medians (min‐max). n = 54 except for cognitive domains.
Abbreviations: LEDD, Levodopa equivalent daily doses; MDS‐UPDRS, The Movement Disorder Society revision of the Unified Parkinson's Disease Rating Scale.
At the 5‐year follow‐up, significant improvements compared to baseline were found for MDS‐UPDRS part III medication‐off total score and the bradykinetic‐rigid and tremor sub‐scores (all P < 0.001), whereas the axial symptom sub‐score was not improved (Table 2, Fig. 2). The MDS‐UPDRS part IV was also significantly improved at 5 years (P < 0.001). MDS‐UPDRS part IV and the tremor sub‐score off‐and on‐medication were all relatively unchanged from the 1‐year to the 5‐year follow‐up. The on‐medication MDS‐UPDRS III total score and bradykinetic‐rigid and axial sub‐scores all worsened compared to preoperative scores (all P < 0.001).
TABLE 2.
One‐ and five‐year outcomes for 54 patients treated with STN‐DBS
| Preop. | 1 yr | P‐value (preop.‐1 yr) | 5 yr | P‐value (1 yr to 5 yr) | P‐value (preop.‐5 yr) | ||
|---|---|---|---|---|---|---|---|
| Motor symptoms and LEDD | |||||||
| MDS‐UPDRS III | OFF | n = 54 | n = 52 | <0.001 a | n = 53 | <0.001 a | <0.001 a |
| 49.1 (12.2) | 19.2 (9.1) | 34.8 (13.7) | |||||
| ON | n = 54 | n = 52 | 0.006 a | n = 51 | <0.001ab | <0.001ab | |
| 14.5 (8.9) | 11.4 (6.9) | 25.8 (12.1) | |||||
| Bradyk.‐Rigid | OFF | 33.4 (7.6) | 13.8 (6.6) | <0.001 a | 24.8 (9.2) | <0.001 a | <0.001 a |
| ON | 11.1 (6.3) | 8.8 (5.3) | 0.002 a | 18.5 (8.6) | <0.001 a | <0.001 a , b | |
| Tremor | OFF | 7.7 (5.9) | 1.8 (2.9) | <0.001 a , b | 1.7 (3.7) | 0.881 | <0.001 a |
| ON | 1.6 (2.9) | 0.4 (1.0) | 0.001 a , b | 0.7 (1.5) | 0.339 b | 0.013 a , b | |
| Axial | OFF | 8.0 (5.2) | 3.5 (3.5) | <0.001 a , b | 8.2 (5.6) | <0.001 a , b | 0.860 |
| ON | 1.9 (2.2) | 2.1 (2.1) | 0.825 | 6.8 (4.9) | <0.001 a , b | <0.001 a , b | |
| MDS‐UPDRS IV | n = 54 | n = 52 | n = 53 | ||||
| 9.7 (3.5) | 2.4 (3.5) | <0.001 a | 2.9 (3.2) | 0.223 | <0.001 a , b | ||
| MDS‐UPDRS II | n = 54 | n = 52 | n = 48 | ||||
| 16.9 (7.2) | 11.3 (6.8) | <0.001 a | 19.5 (10.4) | <0.001 a | 0.056 | ||
| LEDD | n = 54 | n = 52 | n = 54 | ||||
| 1289 (425) | 633 (331) | <0.001 a | 659 (397) | 0.509 | <0.001 a | ||
| Non‐motor symptoms | |||||||
| MDS‐UPDRS I | n = 54 | n = 52 | n = 47 | ||||
| 11.0 (6.0) | 8.8 (5.3) | 0.001 a | 10.8 (7.0) | 0.008 a | 0.785 | ||
| PDSS | n = 52 | n = 49 | n = 46 | ||||
| 94.3 (21.2) | 107.5 (21.5) | <0.001 a | 110.6 (23.3) | 0.108 | <0.001 a | ||
| Scopa‐Aut | n = 52 | n = 50 | n = 45 | ||||
| 16.0 (7.5) | 14.5 (7.7) | 0.110 | 16.6 (8.7) | 0.021 | 0.172 | ||
| Cognitive scores | |||||||
| Attention/working memory | n = 50 | n = 48 | n = 45 | ||||
| 47.1 (7.4) | 46.4 (8.2) | 0.140 | 42.7 (8.2) | <0.001 a | <0.001 a | ||
| Executive function | n = 50 | n = 47 | n = 31 | ||||
| 46.9 (7.7) | 42.5 (9.3) | <0.001 a | 40.7 (9.4) | <0.001 a | 0.001 a | ||
| Processing | n = 49 | n = 47 | n = 33 | ||||
| 45.7 (7.4) | 42.5 (7.4) | 0.001 a | 35.7 (9.2) | <0.001 a | <0.001 a | ||
| Verbal memory | n = 50 | n = 48 | n = 46 | ||||
| 43.2 (10.1) | 41.5 (10.6) | 0.206 | 40.3 (9.9) | 0.593 | 0.050 | ||
| Visual memory | n = 50 | n = 48 | n = 44 | ||||
| 46.6 (10.2) | 46.9 (11.0) | 0.891 | 41.1 (13.2) | <0.001 a | 0.001 a | ||
| Word fluency | n = 50 | n = 48 | n = 41 | ||||
| 55.0 (10.7) | 48.6 (10.4) | <0.001 a | 42.6 (11.9) | <0.001 a | <0.001 a | ||
| Global | n = 50 | n = 48 | n = 46 | ||||
| 47.4 (6.0) | 44.7 (7.4) | <0.001 a | 39.9 (8.4) | <0.001 a | <0.001 a | ||
| Quality of life | |||||||
| PDQ‐39 SI | n = 53 | n = 50 | n = 46 | ||||
| 26.0 (11.9) | 19.7 (13.8) | <0.001 a | 27.3 (15.2) | <0.001 a | 0.652 | ||
| Mobility | 35.7 (21.1) | 27.3 (25.2) | 0.015 a | 43.3 (29.3) | 0.002 a | 0.147 | |
| ADL | 38.1 (21.2) | 23.4 (19.3) | <0.001 a | 37.1 (28.6) | 0.003 a | 0.872 | |
| Emotional | 18.0 (15.6) | 19.1 (19.0) | 0.635 | 24.0 (20.1) | 0.382 | 0.097 | |
| Stigma | 24.8 (21.3) | 14.9 (19.1) | 0.001 | 13.2 (18.1) | 0.538 | 0.008 a , b | |
| Social support | 11.6 (16.9) | 12.4 (16.4) | 0.679 | 10.5 (14.0) | 0.498 | 0.740 | |
| Cognition | 24.5 (17.0) | 19.8 (17.1) | 0.014 a | 26.1 (21.1) | 0.084 | 0.729 | |
| Communication | 18.2 (16.3) | 24.8 (23.2) | 0.040 | 36.0 (22.4) | 0.034 | <0.001 a | |
| Bodily discomfort | 45.8 (22.4) | 26.0 (23.1) | <0.001 a | 28.6 (24.1) | 0.665 | <0.001 a | |
Significant also after Bonferroni adjusted α‐value of 0.017 for repeated testing.
Wilcoxon signed rank test.
Abbreviations: MDS‐UPDRS, The Movement Disorder Society revision of the Unified Parkinson's Disease Rating Scale; LEDD, Levodopa equivalent daily doses; PDSS, Parkinson's Disease Sleep Scale; Scopa‐Aut, Scales for Outcomes in Parkinson's disease—Autonomic Dysfunction; PDQ‐39, Parkinson's Disease Questionnaire‐39.
FIG. 2.
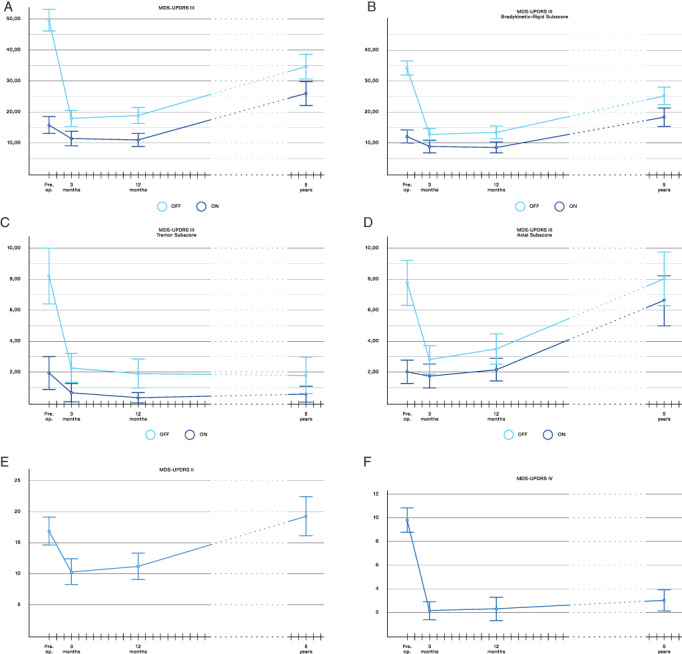
Score changes of motor symptoms through the study period. MDS‐UPDRS, The Movement Disorder Society revision of the Unified Parkinson's Disease Rating Scale. (A) MDS‐UPDRS part III off‐ and on‐medication, further shown as (B) bradykinetic‐rigid symptoms, (C) tremor symptoms and (D) axial symptoms. (E) Motor experiences of daily living (MDS‐UPDRS part II) and (F) motor fluctuations (MDS‐UPDRS IV).
Mean (SD) voltage (V) was at 1 year for left/right hemisphere 2.9(0.7)/2.7(0.8), and at 5 years 3.2(0.8)/3.1(0.7), (p < 0.007, paired t‐test 1 versus 5 years, both hemispheres). Frequency/pulse width were at 1 year mean 135 Hz/63 microseconds and at 5 years 130 Hz/61 microseconds. One of the two middle contacts was used in 91% of electrodes at 1 year and 94% at 5 years. No significant differences were found between randomization groups. LEDD was at 5 years reduced by mean (SD) 49 (27) % (P < 0.001), thus at a similar level as 12 months postoperatively [reduction 50 (23) %].
Figure 3 displays the changes over time for MDS‐UPDRS I, PDSS, and Scopa‐Aut total scores. After 5 years of STN‐DBS, sustained significant sleep improvement was observed, both for the total PDSS score (P < 0.001) and the sub‐scores “overall quality of nights sleep,” “sleep onset and maintenance insomnia,” “nocturnal restlessness,” “nocturnal motor symptoms,” and “daytime dozing” (also significant after Bonferroni adjustment). Both mean MDS‐UPDRS I and Scopa‐Aut total score had at 5 years returned to about the same level as preoperatively (Table 2). The Scopa‐Aut sub‐score of thermoregulatory function was significantly improved at 1 year as published previously. 27 At 5 years it was still better than preoperatively, but no longer statistically significant (P = 0.043, Bonferroni adjusted α‐value <0.017). Pupillomotor score was worse than preoperatively (P = 0.006).
FIG. 3.
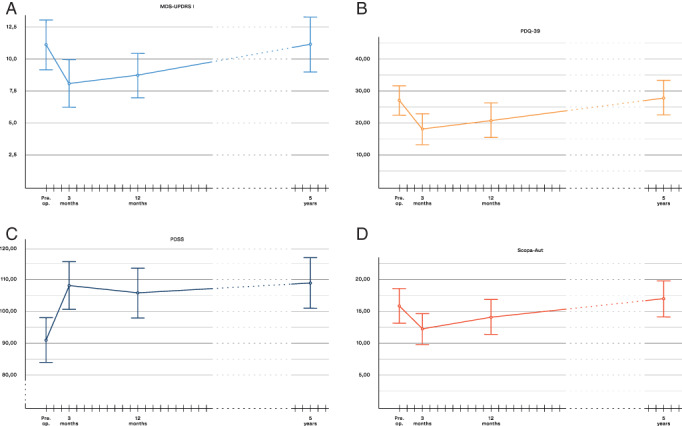
Change of non‐motor symptoms and quality of life through the study period. MDS‐UPDRS, The Movement Disorder Society revision of the Unified Parkinson's Disease Rating Scale. PDQ‐39, Parkinson's Disease Questionnaire‐39. PDSS, Parkinson's Disease Sleep Scale. Scopa‐Aut, Scales for Outcomes in Parkinson's disease—Autonomic Dysfunction. (A) Non‐motor experiences of daily living (MDS‐UPDRS part I). (B) health‐related quality of life (PDQ‐39 SI). (C) Sleep disturbances (PDSS). (D) Autonomic symptoms (Scopa‐Aut).
During the 5 years of follow‐up, test scores across cognitive domains were significantly reduced, except verbal memory (Table 2). In Figure 4, box plots illustrating changes between preoperative to 1 year (A), and 1 year to 5 years (B), are presented. The score reduction was significantly smaller during period A (timeline of 1 year) than period B (timeline 4 years) for attention/working memory (P = 0.024), processing speed (0.001), visual memory (0.001) and global score (0.011), while there were no differences between period A and period B for executive function (0.174) or verbal fluency (0.223).
FIG. 4.
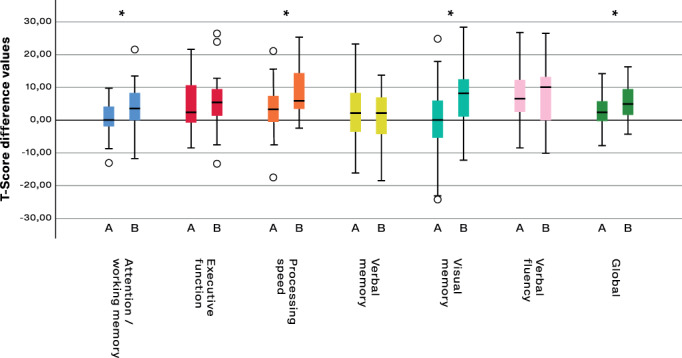
Comparison of score changes in six neuropsychological domains from preoperative to 1 year postoperative (A), and from 1 year to 5 years of STN‐DBS (B). *significant difference between period A and period B. Scores are presented as changes in T‐scores which are raw scores transformed into standardized scores using the test publisher's normative data. Boxes represent the first to the third quartile, the vertical line through the box is the median. Whiskers represent the minimal and maximal non‐outliers, while the circles are outliers.
PDQ‐39 Summary Index score had at 5 years returned to about the same level as preoperatively (Fig. 3 and Table 2). The PDQ‐39 sub‐scores stigma and bodily discomfort were, however, still improved compared to preoperatively (P = 0.008 and P < 0.001 respectively), whereas the communication sub‐score had worsened (P < 0.001).
The variables that changed significantly from preoperative to the 5‐year follow‐up were evaluated for any differences between the randomization groups. At 5 years, the mMER group had a greater improvement in the MDS‐UPDRS III bradykinetic‐rigid sub‐score medication‐off [mean (SD) 11.4 (10.4) vs. 5.7 (9.0), P = 0.043] and a greater improvement of the PDQ‐39 sub‐score bodily discomfort [27.4 (30.7) vs. 9.8 (19.8), P = 0.025], compared to the sMER group. The reduction of verbal fluency was significantly larger in the mMER group compared to the sMER group in period A, but not period B. No other significantly different cognitive changes were found between randomization groups.
At 5 years, 43 patients were physically independent (HY 1–3) and 11 patients physically dependent (HY 4–5, or information from the electronic medical journal that they were physically dependent). Exploratory analyses comparing preoperative characteristics that might predict this outcome showed that patients in the physically independent group had been significantly younger (60 vs. 65 years, P = 0.043), had better PDSS score (98.0 vs. 78.7, P = 0.008], lower LEDD [1227 vs. 1531, P = 0.033], higher medication‐on tremor score [2.0 vs 0.1, P < 0.001], and better MDS‐UPDRS I score [10.1 vs 14.4 (P = 0.032)] (independent samples t‐test). A logistic regression analysis was performed entering these variables. The model as a whole explained between 37% (Cox and Snell R Squared) and 59% (Nagelkerke R Squared) of the variance in physical independence versus dependence after 5 years of STN‐DBS (goodness of fit of the model P < 0.001, and correctly classified 87% of cases). Age at surgery (P = 0.046, odds ratio 1.251) was the only preoperative characteristic to make a unique statistically significant contribution to the model. Odds Ratio (P‐value) were for the other preoperative variables: PDSS 0.933 (P = 0.60), LEDD 1.002 (P = 0.078), MDS‐UPDRS III tremor on‐medication 0.379 (P = 0.211), and MDS‐UPDRS I 0.926 (P = 0.438).
Discussion
In this prospective study 54 PD patients treated with STN‐DBS were followed for 5 years. Our main findings were significant and lasting improvement in off‐medication motor symptom scores, on‐medication tremor, motor fluctuations, reduced dopaminergic medication and improved sleep. The findings from neuropsychological testing indicate negative impact on the cognitive domains verbal fluency and executive function, but whether this reflect subjective experiences or affect daily functioning is uncertain.
Our findings on motor symptoms confirm previous studies, showing sustained effect on tremor and motor fluctuations. Concomitantly, there was a gradual worsening of axial symptoms such as speech, gait and balance, and to some degree of bradykinetic‐rigid symptoms. 18 , 42 Particularly the axial symptoms are known to be clinical markers of disease progression and to become less responsive to levodopa. The rate of motor symptom progression in our study are in line with published data documenting an average increase of MDS‐UPDRS III off‐medication scores of around 2.4 points/yr in a de novo PD cohort. 43 Good effect on tremor and stable low dopaminergic medication at 5 years were also recently reported in a study of very early DBS treatment (mean 2.1 ± 1.3 years of dopaminergic treatment before surgery). 44 Thus, this seems to be a consistent finding across different disease stages.
Interestingly, sleep also remains significantly improved after 5 years of STN‐DBS, and do not worsen even though bradykinetic‐rigid symptoms worsen and other signs of disease progression are quite evident. This may support the notion that the favorable effect of STN‐DBS on sleep disturbances is not merely due to improved motor symptoms. Few other studies report long‐term effect on sleep after STN‐DBS. A retrospective study of 10 patients showed no significant change of sleep symptoms compared to baseline after 5 years. 45 However, also a recent study of 61 patients, of whom 46 completed 3‐year follow‐up, showed improvement in total PDSS, overall quality of nights sleep, sleep onset and maintenance insomnia, and nocturnal motor symptoms. 46
Little is known of long‐term impact on autonomic symptoms after STN‐DBS. In a general PD population Scopa‐Aut scores have been shown to increase by age, disease duration and severity. 47 , 48 , 49 In our study, however, the total score did not change from preoperative to the 5‐year follow‐up. Only excessive sweating was significantly improved at 1‐year follow‐up, 27 though not sustained at 5 years.
Non‐motor activities of daily living (MDS‐UPDRS I) score did not change significantly from preoperative to the 5‐year follow‐up, but showed less progression than in the de novo cohort which progressed by 0.92 points/yr. 43
Considering our results on the non‐motor symptoms (significantly improved sleep, less progression in MDS‐UPDRS I and Scopa‐Aut total score than described in general PD populations), STN‐DBS seems to have some beneficial effect on these symptoms even in the long‐term.
Impaired cognition is part of the multitude of symptoms associated with PD. One study found cognitive impairment even in 24% of newly diagnosed PD patients (versus 4% of healthy controls). 50 Dementia increases with disease duration and has been reported to develop in a similar rate in DBS operated and non‐operated patients. 51 , 52 On the other hand, a study that compared STN‐DBS treated patients with non‐operated patients found worsening of cognitive function, but improved quality of life. 53 Many studies have reported cognitive impairments in STN‐DBS treated PD cohorts, although there is conflicting evidence both regarding the domains affected and the magnitude of change. A review by Combs et al. found small declines in psychomotor speed, memory, attention, executive functions, and overall cognition, while moderate declines were found for verbal fluency. 54 Mehanna et al. conclude that worsening of one or more cognitive functions is rare after DBS, but evidence from available studies are conflicting. 19 Reduction in verbal fluency is the most consistent finding across studies. 19 , 42
In our study, we observed significant worsening across all cognitive domains from preoperative assessment to the five‐year follow‐up, except for verbal memory. Decline in verbal fluency and executive function from preoperative to the one‐year follow‐up were of a similar magnitude as the decline over the next 4 years in total. This could indicate a more direct effect of the STN‐DBS surgery. Processing speed, attention/working memory, visual memory and global score were significantly more reduced during the last 4 years of follow‐up compared to the first year, thus more likely reflect disease progression. Differences in neuropsychological evaluation methods, surgical procedures, final lead placement and postoperative stimulation settings may all contribute to the variable results on cognition across studies. 55 , 56
Disease‐specific Quality of life measured by PDQ‐39 Summary Index has consistently been found to be improved at 1‐year follow‐up, but no longer at the 5‐year follow‐up. 42 The subdomains of PDQ‐39 are less frequently reported. We found that the domains stigma and bodily discomfort improved, whereas communication worsened after 5 years of STN‐DBS. PDQ‐39 scores collected prospectively over longer time periods should, however, be interpreted with caution, as quality of life measures may be influenced by a range of life events unrelated to the treatment effect. We agree with other authors that PDQ‐39 is probably not a good measure of the best timing of STN‐DBS surgery, or its efficacy.
In the previously published randomized controlled 1‐year phase of our study, we showed that the group mapped intraoperatively with multiple microelectrode recordings (mMER) had a significantly greater improvement both of motor symptoms (MDS‐UPDRS III off‐medication) and of the PDQ‐39 sub‐scores ADL and bodily discomfort, compared to the group evaluated with single MER for target verification only (sMER). 26 After 5 years, the mMER group still had more improvement of bradykinetic‐rigid symptoms and the PDQ‐39 bodily discomfort sub‐score than the sMER group. Clinically meaningful change in the PDQ‐39 sub‐score bodily discomfort has been estimated to be ≥2.1 points. 57 Thus, the differences between the randomization groups seem to be clinically meaningful after 5 years. To our knowledge long‐term results comparing these two methods of peroperative target guidance have not been previously reported.
Witt et al. showed that when electrode trajectories intersected the caudate nucleus there was increased risk of decline in global cognition and working memory, 58 whereas Smith et al. did not find correlation between cognitive decline and the number of microelectrode trajectories, location of the electrode tip or the stimulation parameters. 59 In our study, the mMER group had on average more reduction in verbal fluency than the sMER group, which may indicate a negative effect of multiple trajectories on verbal fluency and lend some support to the findings of Witt et al. However, to which extent the cognitive changes detected by specific neuropsychological tests affect the patient's daily functioning is uncertain. In a non‐randomized study comparing single and multiple microelectrode recordings, larger reductions in verbal fluency and memory were found in the multiple microelectrode group. 60 However, in open interviews with patients and partners these symptoms were mainly not reported as clinically relevant. Thus, uncertainties still exist, regarding to which degree cognitive changes are related to the surgery itself, stimulation, reduction of dopaminergic drugs or the expected progression of PD, and the impact these changes may have on the patients daily functioning and quality of life.
An important issue that does not seem to be fully resolved yet, concerns the correct selection of PD candidates for STN‐DBS surgery and which preoperative factors that may predict a worse long‐term outcome. In 20 patients followed for 8 years, the patients who developed postural instability had preoperatively both more postural instability and higher dopaminergic medication. 17 Executive dysfunction also correlated negatively with postural instability. A recent study with mean follow‐up of 8.4 ± 6.3 years, showed that preoperative higher frontal cognitive score and off‐medication motor score predicted good motor outcome. 22 A review of studies with >5 years follow‐up concludes that more preoperative axial features and higher off‐medication gait score predicts negative long‐term motor outcomes, whereas younger age at disease onset is a positive predictive factor. 61 High age as a negative predictive factor seems intuitive because elderly patients have less cognitive reserve, a higher incidence of levodopa‐resistant symptoms and shorter life expectancy. In the present study, younger age at surgery was the most important predictor of physical independence at 5 years, while the group that became physically dependent, preoperatively used higher doses of dopaminergic treatment, had less tremor symptoms on‐medication, more severe sleep symptoms, and more non‐motor ADL symptoms. Disease duration was not a predictor. The reason for this is not certain, but probably reflects that some patients progress faster than others, and because STN‐DBS is not disease modifying, those patients will have less beneficial long‐term results.
A weakness in many long‐term studies is high drop‐out rates, possibly leading to bias because the patients with worse mobility and cognitive outcome do not come for follow‐up. In one study 17 of 50 patients died during follow‐up, and they were on average older at the time of surgery. 62 Also in other long‐term studies, results are reported on a significantly lower proportion of patients than those operated in the time period. 20 , 21 , 22 A strength of our study is the prospective design and the fact that we can account for all our patients. In the prediction analysis for physical independence versus dependence, all 54 patients were included for the predictive variables (except for PDSS with n = 52). However, it is challenging to assess the most affected patients with certain neuropsychological tests, specifically in the domains of executive function and processing speed. This may represent a potential bias. Therefore, these scores should be regarded as a “best outcome,” rather than a full representation of outcome. For the first year there were, however, relatively few drop‐outs.
In conclusion, our study confirms good long‐term effect of STN‐DBS on motor symptoms and fluctuations and also on sleep disturbances. Progression of the underlying degenerative disease process is, however, evident also in this cohort. Our findings also indicate that STN‐DBS surgery might have a negative impact on verbal fluency and executive function. Regarding preoperative risk–benefit evaluation, we confirm that age is a key factor, but level of dopaminergic treatment and magnitude of non‐motor symptoms also deserve to be considered. These factors seem to be more important than disease duration or the exact magnitude of the preoperative levodopa response. This response is important for short term results, but does not seem to predict long‐term outcomes. We propose to carefully consider which are the key factors in the preoperative risk–benefit evaluation, especially in patients older than 65 years. Further prospective studies designed specifically to evaluate predictive factors are needed, which include patient characteristics, operation method, potential future biomarkers and non‐motor symptom evaluations.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution. (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique. (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
S.B.: 1B, 1C, 2A, 2B, 2C, 3A, 3B
M.T.: 1A, 1B, 1C, 2C, 3B
R.B.: 1C, 3B
T.W.R.: 1C, 3B
A.K.: 1C, 3B
E.D.: 1A, 1B, 2C, 3B
S.A.: 1A, 2B, 2C, 3B
I.M.S.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The study was approved by the Regional Committee for Medical and Health Research Ethics (REC South East, project no. 6.2009.46), and registered at ClinicalTrials.gov (Identifier NCT00855621, first received March 3, 2009). All participants gave written informed consent prior to inclusion.
Funding Sources and Conflicts of Interest
M.T. was supported by a grant from South‐Eastern Regional Health Authority Norway. I.M.S has received funding from two private donations to the Research section of Department of Neurology during this work, and honoraria from lectures in scientific meetings/educational courses arranged by Medtronic, Boston Scientific and Movement Disorders Society. S.B., R.B., T.W.R., A.K., E.D., S.A.: The authors declares that there are no conflicts of interest relevant to this work and that there are no additional disclosures to report.
Financial Disclosures for the Previous 12 months
E.D. has received honoraria for lectures from AbbVie. I.M.S. has received funding from two private donations to the Research section of Department of Neurology during this work, and honoraria from lectures in scientific meetings/educational courses arranged by Allergan and Boston Scientific. S.B., M.T., R.B., T.W.R., A.K., and S.A. declare that there are no additional disclosures to report.
Acknowledgments
The authors thank all study participants. Statistician Are Hugo Pripp has advised and guided the authors on the statistical analysis.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Deuschl G, Schade‐Brittinger C, Krack P, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2. Kleiner‐Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta‐analysis of outcomes. Mov Disord 2006;21(Suppl:14):S290–S304. 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 3. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301:63–73. 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open‐label trial. Lancet Neurol 2010;9:581–591. 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant‐current device in Parkinson's disease: an open‐label randomised controlled trial. Lancet Neurol 2012;11:140–149. 10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- 6. Schupbach WM, Maltête D, Houeto JL, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology 2007;68:267–271. 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 7. Timmermann L, Jain R, Chen L, et al. Multiple‐source current steering in subthalamic nucleus deep brain stimulation for Parkinson's disease (the VANTAGE study): a non‐randomised, prospective, multicentre, open‐label study. Lancet Neurol 2015;14:693–701. 10.1016/S1474-4422(15)00087-3. [DOI] [PubMed] [Google Scholar]
- 8. Fasano A, Daniele A, Albanese A. Treatment of motor and non‐motor features of Parkinson's disease with deep brain stimulation. Lancet Neurol 2012;11:429–442. 10.1016/S1474-4422(12)70049-2. [DOI] [PubMed] [Google Scholar]
- 9. Klingelhoefer L, Samuel M, Chaudhuri KR, Ashkan K. An update of the impact of deep brain stimulation on non motor symptoms in Parkinson's disease. J Parkinsons Dis 2014;4:289–300. 10.3233/JPD-130273. [DOI] [PubMed] [Google Scholar]
- 10. Kim HJ, Jeon BS, Paek SH. Nonmotor symptoms and subthalamic deep brain stimulation in Parkinson's disease. J Mov Disord 2015;8:83–91. 10.14802/jmd.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurtis MM, Rajah T, Delgado LF, Dafsari HS. The effect of deep brain stimulation on the non‐motor symptoms of Parkinson's disease: a critical review of the current evidence. NPJ Parkinsons Dis 2017;3:16024. 10.1038/npjparkd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dafsari HS, Silverdale M, Strack M, et al. Nonmotor symptoms evolution during 24 months of bilateral subthalamic stimulation in Parkinson's disease. Mov Disord 2018;33:421–430. 10.1002/mds.27283. [DOI] [PubMed] [Google Scholar]
- 13. Krack P, Batir A, van Blercom N, et al. Five‐year follow‐up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 2003;349:1925–1934. 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 14. Constantinescu R, Eriksson B, Jansson Y, et al. Key clinical milestones 15 years and onwards after DBS‐STN surgery‐a retrospective analysis of patients that underwent surgery between 1993 and 2001. Clin Neurol Neurosurg 2017;154:43–48. 10.1016/j.clineuro.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 15. Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten‐year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol 2011;68:1550–1556. 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- 16. Zibetti M, Merola A, Rizzi L, et al. Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson's disease. Mov Disord 2011;26:2327–2334. 10.1002/mds.23903. [DOI] [PubMed] [Google Scholar]
- 17. Fasano A, Romito LM, Daniele A, et al. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain 2010;133:2664–2676. 10.1093/brain/awq221. [DOI] [PubMed] [Google Scholar]
- 18. Lau B, Meier N, Serra G, et al. Axial symptoms predict mortality in patients with Parkinson disease and subthalamic stimulation. Neurology 2019;92:e2559–e2570. 10.1212/WNL.0000000000007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehanna R, Bajwa JA, Fernandez H, Wagle Shukla AA. Cognitive impact of deep brain stimulation on Parkinson's disease patients. Parkinsons Dis 2017;2017:3085140. 10.1155/2017/3085140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizzone MG, Fasano A, Daniele A, et al. Long‐term outcome of subthalamic nucleus DBS in Parkinson's disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord 2014;20:376–381. 10.1016/j.parkreldis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 21. Jiang JL, Chen SY, Hsieh TC, Lee CW, Lin SH, Tsai ST. Different effectiveness of subthalamic deep brain stimulation in Parkinson's disease: a comparative cohort study at 1 year and 5 years. J Formos Med Assoc 2015;114:835–841. 10.1016/j.jfma.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 22. Cavallieri F, Fraix V, Bove F, et al. Predictors of long‐term outcome of subthalamic stimulation in Parkinson disease. Ann Neurol 2020;89:587–597. 10.1002/ana.25994. [DOI] [PubMed] [Google Scholar]
- 23. Fukaya C, Watanabe M, Kobayashi K, et al. Predictive factors for long‐term outcome of subthalamic nucleus deep brain stimulation for Parkinson's disease. Neurol Med Chir (Tokyo) 2017;57:166–171. 10.2176/nmc.oa.2016-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lyons KE, Davis JT, Pahwa R. Subthalamic nucleus stimulation in Parkinson's disease patients intolerant to levodopa. Stereotact Funct Neurosurg 2007;85:169–174. 10.1159/000099076. [DOI] [PubMed] [Google Scholar]
- 25. Piboolnurak P, Lang AE, Lozano AM, et al. Levodopa response in long‐term bilateral subthalamic stimulation for Parkinson's disease. Mov Disord 2007;22:990–997. 10.1002/mds.21482. [DOI] [PubMed] [Google Scholar]
- 26. Bjerknes S, Toft M, Konglund AE, et al. Multiple microelectrode recordings in STN‐DBS surgery for Parkinson's disease: a randomized study. Mov Disord Clin Pract 2018;5:296–305. 10.1002/mdc3.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bjerknes S, Skogseid IM, Hauge TJ, Dietrichs E, Toft M. Subthalamic deep brain stimulation improves sleep and excessive sweating in Parkinson's disease. NPJ Parkinsons Dis 2020;6:29. 10.1038/s41531-020-00131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 29. Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord 2013;28:668–670. 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 30. Ricciardi L, de Angelis A, Marsili L, et al. Hypomimia in Parkinson's disease: an axial sign responsive to levodopa. Eur J Neurol 2020;27:2422–2429. 10.1111/ene.14452. [DOI] [PubMed] [Google Scholar]
- 31. Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19:1020–1028. 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 32. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ‐39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 33. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 1995;4:241–248. [DOI] [PubMed] [Google Scholar]
- 34. Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA‐AUT. Mov Disord 2004;19:1306–1312. 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 36. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 37. Wechsler D. WAIS‐III Administration and Scoring Manual: Wechsler Adult Intelligence Scale‐Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 38. Ellis DC. The Delis‐Kaplan Executive Function System: D‐KEFS Examiner's Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 39. Smith A. Symbol Digits Modalities Test. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 40. Brandt J, Benedict RH. Hopkins Verbal Learning Test‐Revised. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 41. Lang AE, Houeto JL, Krack P. Deep brain stimulation: preoperative issues. Mov Disord 2006;21(Suppl 14):S171–S196. 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]
- 42. Limousin P, Foltynie T. Long‐term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol 2019;15:234–242. 10.1038/s41582-019-0145-9. [DOI] [PubMed] [Google Scholar]
- 43. Holden SK, Finseth T, Sillau SH, Berman BD. Progression of MDS‐UPDRS scores over five years in de novo Parkinson disease from the Parkinson's progression markers initiative cohort. Mov Disord Clin Pract 2018;5:47–53. 10.1002/mdc3.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hacker ML, Turchan M, Heusinkveld LE, et al. Deep brain stimulation in early‐stage Parkinson disease: five‐year outcomes. Neurology 2020;95:e393–e401. 10.1212/WNL.0000000000009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang LL, Liu JL, Fu XL, et al. Long‐term efficacy of subthalamic nucleus deep brain stimulation in Parkinson's disease: a 5‐year follow‐up study in China. Chin Med J (Engl) 2015;128:2433–2438. 10.4103/0366-6999.164925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choi JH, Kim HJ, Lee JY, et al. Long‐term effects of bilateral subthalamicnucleus stimulation on sleep in patients with Parkinson's disease. PLoS One 2019;14:e0221219. 10.1371/journal.pone.0221219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient‐reported autonomic symptoms in Parkinson disease. Neurology 2007;69:333–341. 10.1212/01.wnl.0000266593.50534.e8. [DOI] [PubMed] [Google Scholar]
- 48. Merola A, Romagnolo A, Rosso M, et al. Autonomic dysfunction in Parkinson's disease: A prospective cohort study. Mov Disord 2018;33:391–397. 10.1002/mds.27268. [DOI] [PubMed] [Google Scholar]
- 49. Arnao V, Cinturino A, Valentino F, et al. In patient's with Parkinson disease, autonomic symptoms are frequent and associated with other non‐motor symptoms. Clin Auton Res 2015;25:301–307. 10.1007/s10286-015-0306-x. [DOI] [PubMed] [Google Scholar]
- 50. Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005;65:1239–1245. 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 51. Kim HJ, Jeon BS, Paek SH, et al. Long‐term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson's disease. J Neurol 2014;261:1090–1096. 10.1007/s00415-014-7321-z. [DOI] [PubMed] [Google Scholar]
- 52. Lilleeng B, Gjerstad M, Baardsen R, Dalen I, Larsen JP. The long‐term development of non‐motor problems after STN‐DBS. Acta Neurol Scand 2015;132:251–258. 10.1111/ane.12391. [DOI] [PubMed] [Google Scholar]
- 53. Smeding HM, Speelman JD, Huizenga HM, Schuurman PR, Schmand B. Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson's disease. J Neurol Neurosurg Psychiatry 2011;82:754–760. 10.1136/jnnp.2007.140012. [DOI] [PubMed] [Google Scholar]
- 54. Combs HL, Folley BS, Berry DTR, et al. Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson's disease: a meta‐analysis. Neuropsychol Rev 2015;25:439–454. 10.1007/s11065-015-9302-0. [DOI] [PubMed] [Google Scholar]
- 55. Kumar R, Lang AE, Rodriguez‐Oroz MC, et al. Deep brain stimulation of the globus pallidus pars interna in advanced Parkinson's disease. Neurology 2000;55:S34–S39. [PubMed] [Google Scholar]
- 56. Tsai ST, Lin SH, Lin SZ, Chen JY, Lee CW, Chen SY. Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson's disease. Neurosurgery 2007;61:E1024–E1029; discussion E1029‐1030. 10.1227/01.neu.0000303198.95296.6f. [DOI] [PubMed] [Google Scholar]
- 57. Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ‐39 Parkinson's disease questionnaire. Age Ageing 2001;30:299–302. 10.1093/ageing/30.4.299. [DOI] [PubMed] [Google Scholar]
- 58. Witt K, Granert O, Daniels C, Volkmann J, Falk D, van Eimeren T, Deuschl G. Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson's disease: results from a randomized trial. Brain 2013;136:2109–2119. 10.1093/brain/awt151. [DOI] [PubMed] [Google Scholar]
- 59. Smith KM, O'Connor M, Papavassiliou E, Tarsy D, Shih LC. Phonemic verbal fluency decline after subthalamic nucleus deep brain stimulation does not depend on number of microelectrode recordings or lead tip placement. Parkinsonism Relat Disord 2014;20:400–404. 10.1016/j.parkreldis.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 60. Temel Y, Wilbrink P, Duits A, et al. Single electrode and multiple electrode guided electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. Neurosurgery 2007;61:346–355; discussion 355–347. 10.1227/01.neu.0000303993.82149.98. [DOI] [PubMed] [Google Scholar]
- 61. Rodriguez‐Oroz MC, Moro E, Krack P. Long‐term outcomes of surgical therapies for Parkinson's disease. Mov Disord 2012;27:1718–1728. 10.1002/mds.25214. [DOI] [PubMed] [Google Scholar]
- 62. Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long‐term outcome of 50 consecutive Parkinson's disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord 2008;14:114–119. 10.1016/j.parkreldis.2007.06.012. [DOI] [PubMed] [Google Scholar]


