Wilson's disease (WD) is an autosomal recessive disorder with abnormal copper deposition occurring in various tissues subsequently leading to their dysfunction. It results from the defective functioning of a copper transporting P‐type ATPase, associated with mutations in the gene ATP7B. As the name suggests, Wilson's hepato‐lenticular degeneration primarily manifests with neurological, hepatic and psychiatric signs and symptoms. 1 The usual therapy is penicillamine, trientine and zinc but they are often not tolerated well. Ammonium tetrathiomolybdate (ATTM) is a chelating agent largely used in veterinary medicine for copper intoxications. 2 It has also proven to be effective over the years in WD. 3 , 4 , 5 It blocks the absorption of dietary copper in the gut lumen by forming complexes with copper and proteins, thus causing increased copper elimination in feces. We present a patient of WD, with prominent neurological features treated with ATTM and successful clinical and radiological improvement.
Case Report
A 25‐year‐old Caucasian woman, born out of a non‐consanguineous marriage, presented to the outpatient department with a one‐year history of a rapidly progressive tremor, which markedly affected her functional status. She had a brother with WD and a healthy daughter. Physical and neurological examination revealed bilateral Kayser‐Fleischer rings and rest tremor in the upper limbs, both proximal and distal, of moderate amplitude. A more significant tremor in the upper limbs appeared within a few seconds after adopting any antigravity posture or a simple action (“wing beating” tremor). After its onset, the tremor would be of increasing amplitude, and spread to the trunk, head and lower extremities. Tremor only disappeared if the upper limbs returned to the resting position. Occasionally, dystonic posturing of the right hand was observed (Video 1, segment 1). Rest of the neurological examination was within normal limits, and systemic examination did not reveal any abnormality. Global assessment scale for Wilson's disease (GAS) 6 showed Tier 1: L3, C0, M4, O0; Tier 2: 7 points (0/0/0/0/1/3/0/0/0/0/0/0/3/0).
VIDEO 1.
This video shows the severe “wing beating” and generalized tremor before treatment (segment 1) and its complete disappearance after treatment (segment 2).
Laboratory investigations revealed total serum copper level‐ 69 μg/dL (normal 70–140), “free” serum copper level (total serum copper—[serum ceruloplasmin x 3])‐ 45 μg/dL (normal 5–10), 24‐hour urinary copper excretion‐ 482 μg (normal <100) and serum ceruloplasmin level‐ 8 mg/dL (normal 18–53). She had subclinical liver disease (cirrhosis without portal hypertension, Child A) with normal liver function tests. Pre‐treatment brain MRI (Fig. 1) showed bilateral and symmetrical hypointensities in the globus pallidus and substantia nigra due to deposits of paramagnetic material and hyperintensities in the lateral thalamus, central midbrain, central pons and substantia nigra. Sanger sequencing of the whole ATP7B gene revealed the p.L1305P and p.H1069Q mutations in compound heterozygosity.
FIG 1.
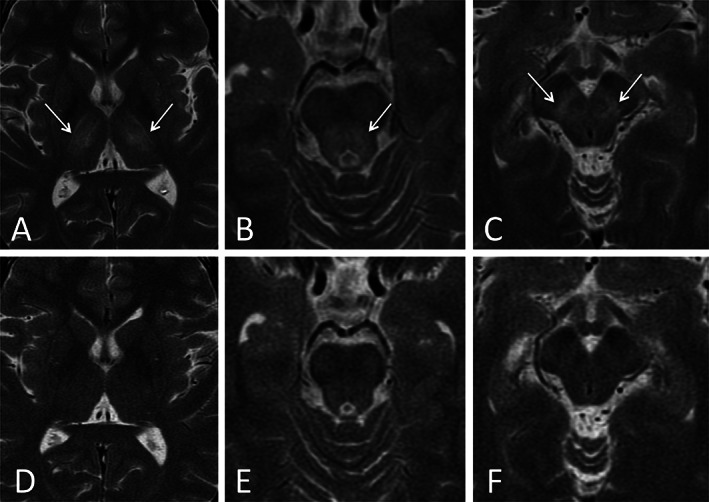
Axial T2‐weighted brain MR images showing pre‐treatment abnormal bilateral and symmetrical hyperintensities in the lateral thalamus (A), central midbrain (B) and substantia nigra (C) (white arrows) with post‐treatment complete resolution of abnormalities (D, E, F). The hypointensities discussed in the text are not visible in these images.
Initially, treatment was started with penicillamine 250 mg four times a day, pyridoxine 150 mg once a day and zinc 50 mg thrice a day. Penicillamine was eventually withdrawn because of neurological worsening and digestive intolerance, and replaced by ATTM 2 mg/kg of body weight per day in 6 divided doses, 3 times along with meals to prevent dietary copper absorption and thrice in‐between meals to chelate tissue copper. ATTM was administered as compassionate use because, at that time, trientine was not available in Spain. During therapy, as recommended, 1 , 7 we ensured an exhaustive control of the different copper pools. We considered: serum “free” copper level of 10–25 μg/dL as minimal risk of toxicity; 5–10 μg/dL as “decoppered” stage; and <5 μg/dL as copper deficiency.
The patient showed a marked improvement within 15 days of starting ATTM. She continued to remain asymptomatic when followed up 3 months later (Video 1, segment 2). GAS showed: Tier 1: L3, C0, M0, O0; Tier 2: 0 points. She reached the “decoppered” stage 4 months later. ATTM was then removed, leaving only maintenance zinc to ensure constant serum “free” copper levels between 5 and 10 μg/dL. Mild anemia and leukopenia (hemoglobin 6.1 g/dL, neutrophils 250/μL) appeared as adverse event at the end of ATTM treatment. It resolved 4 days after removing ATTM but required a blood transfusion. A brain MRI 5 months later showed only mild improvement of the initial abnormalities but 4 years later normal imaging was obtained (Fig. 1). The patient has remained asymptomatic during the 11 years of follow‐up.
Discussion
The availability of ATTM has always been a concern because it is not commercially obtainable in most of the countries. In this case report, we want to emphasize the rapid, complete and sustained neurological response over time with ATTM and zinc. Their combined use as a starting therapy, and zinc alone as maintenance therapy when the “decoppering” phase is over, was a good alternative to penicillamine. Also worth noting is the normalization of the brain imaging, suggesting removal of the abnormal copper deposition. A rigorous control of the different copper pools and adverse events was however mandatory.
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
IJP: 1A, 1B, 1C, 3A, 3B
AR: 1C, 3B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. Informed patient consent was obtained. The authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
No specific funding was received for this work and the authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
References
- 1. Mulligan C, Bronstein JM. Wilson disease. An overview and approach to management. Neurol Clin 2020;38(2):417–432. 10.1016/j.ncl.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 2. Humphries WR, Mills CF, Greig A, Roberts L, Inglis D, Halliday GJ. Use of ammonium tetrathiomolybdate in the treatment of copper poisoning in sheep. Vet Rec 1986;119(24):596–598. [PubMed] [Google Scholar]
- 3. Aggarwal A, Bhatt M. Advances in treatment of Wilson disease. Tremor Other Hyperkinet Mov 2018;8:525. 10.7916/D841881D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brewer GJ, Hedera P, Kluin KJ, et al. Treatment of Wilson disease with ammonium tetrathiomolybdate. III. Initial therapy in a total of 55 neurologically affected patients and follow‐up with zinc therapy. Arch Neurol 2003;60(3):379–385. 10.1001/archneur.60.3.379. [DOI] [PubMed] [Google Scholar]
- 5. De Fabregues O, Viñas J, Palasí A, et al. Ammonium tetrathiomolybdate in the decoppering phase treatment of Wilson's disease with neurological symptoms: A case series. Brain Behav 2020;10(5):e01596. 10.1002/brb3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aggarwal A, Aggarwal N, Nagral A, Jankharia G, Bhatt M. A novel global assessment scale for Wilson's disease (GAS for WD). Mov Disord 2009;24(4):509–518. 10.1002/mds.22231. [DOI] [PubMed] [Google Scholar]
- 7. Weiss KH, Stremmel W. Clinical considerations for an effective medical therapy in Wilson's disease. Ann N Y Acad Sci 2014;1315:81–85. 10.1111/nyas.12437. [DOI] [PubMed] [Google Scholar]


