ABSTRACT
Background
Early evidence suggests good response to pallidal deep brain stimulation (DBS) in DYT‐KMT2B.
Objectives
We aimed to conduct a systematic review and meta‐analysis to assess outcomes and identify predictors of good outcome following GPi‐DBS in DYT‐KMT2B.
Methods
We searched MEDLINE, Cochrane and MDS‐abstracts databases using the MeSH terms “KMT2B and DYT28”. We included studies that reported objective outcomes following GPi‐DBS in DYT‐KMT2B. The BFMDRS‐M (Burke‐Fahn‐Marsden Dystonia Rating Scale‐ Movement) total scores pre‐ and post‐surgery were used to quantify outcomes. We calculated pooled effects using a random effects meta‐analysis and used meta‐regression to identify potential effect modifiers. Multiple linear regression using individual patient data was used to identify predictors of good outcome (>50% improvement from baseline on BFMDRS‐M).
Results
Initial searches screened 132 abstracts of which 34 full‐text articles were identified to be of potential interest. Ten studies reporting 42 individual patients, met the inclusion/exclusion criteria and were included in the final review. The mean age at onset was 6.4 ± 5.7 years and 40% were male. The median follow‐up was 12 months (range: 1–264 months). GPi‐DBS resulted in median BFMDRS‐M improvement of 42.7% (range: −103.5% to 95.9%) postoperatively. Pooled proportion of patients experiencing clinical improvement >50% on BFMDRS‐M was 41% (95% CI: 27%–57%). Male gender [β: 22.6, 95% CI: 8.0–37.3, P = 0.004), and higher pre‐operative BFMDRS‐M score [β: 0.62, 95% CI: 0.36–0.87, P < 0.001) were independently associated with better outcome.
Conclusion
KMT2B‐associated dystonia responds effectively to pallidal stimulation. The outcome is better in males and those with more severe dystonia at baseline.
Keywords: dystonia, KMT2B, deep brain stimulation, BFMDRS, meta‐analysis
Deep Brain Stimulation (DBS) is the standard of care for patients with severe generalized and segmental dystonia refractory to medical therapy. 1 The globus pallidus interna (GPi) is the most commonly used target and conventionally, patients are selected for GPi‐DBS based on dystonia phenomenology and other associated clinical features. 2 With the advances in next generation sequencing and genomic‐array techniques, genetic information has been used successfully to guide selection of patients for DBS. 3 Even prior to the next generation sequencing era, it was known that patients with DYT‐ TOR1A do exceedingly well after GPi‐DBS with 60%–90% improvement in dystonia scores compared to baseline. 4 , 5 , 6 The outcomes in other monogenic dystonia syndromes including DYT‐THAP1 and DYT‐SGCE are less robust while some genetic dystonias such as DYT‐ATP1A3 are unlikely to respond to DBS at all. 7 , 8 , 9 DYT‐KMT2B is a recent addition to the list of monogenic dystonias with a predictable response to DBS. 10 The KMT2B (Histone‐lysine N‐methyltransferase 2B) gene is associated with early‐onset generalized dystonia with a complex phenotype including facial dysmorphism, intellectual disability and prominent gait and laryngeal dystonia with anarthria in many affected individuals. 11 , 12 , 13 This gene is being increasingly identified in dystonia cohorts worldwide and is now understood to be one of the commonest monogenic dystonia syndromes. 14 The dystonia is often medically refractory and early evidence from multiple groups suggests good response to pallidal stimulation in KMT2B dystonia, though only few patients have been systematically reported worldwide. The single largest reported series of patients with DYT‐KMT2B who underwent DBS included 18 patients and reported >30% improvement in dystonia severity in over 50% individuals. 15 Individual case reports and smaller series have reported generally good, yet heterogenous outcomes. 16 , 17 , 18 We aimed to systematically review the literature on the efficacy of GPi‐DBS in DYT‐KMT2B and perform a meta‐analysis of individual patient data to define the outcomes to GPi‐DBS and identify possible predictors of a good outcome after surgery in this population.
Methods
Search Strategy
This study followed the Preferred Reporting Items for Systematic Review and Meta‐analyses (PRISMA) guidelines. We conducted an initial literature search in January 2021, which was later updated in May 2021. The MEDLINE and Cochrane databases were queried with the MeSH terms “KMT2B” AND “DYT28.” Further studies were identified from reference lists of included papers and the database of meeting abstracts from the International MDS Congress (www.mdsabstracts.org). Manuscripts meeting all the following criteria were included: (1) the paper reported objective outcomes following GPi‐DBS in patients with genetically proven KMT2B dystonia; (2) individual patient BFMDRS‐M (Burke‐Fahn‐Marsden Dystonia Rating Scale‐ Movement) or total scores pre‐ and post‐surgery were available to quantify outcomes; (3) report was available in English language or English language translation was available. 19 All studies were included from the inception of the research database without any restriction regarding the type of study (original articles, care reports, case series, letters to editor). We excluded manuscripts that reviewed previously published data without reports of original data, did not report outcomes of GPi‐DBS, reported an on‐going study or were duplicate publications. The authors of published manuscripts were contacted to provide individual patient data in case it was not available.
Data Extraction
Two independent reviewers (RR, AS) screened the titles and abstracts of all manuscripts returned by the initial search. Full texts of relevant articles were reviewed and those meeting the inclusion and exclusion criteria were included in the review. Both the reviewers extracted the data independently using a pre‐specified tabular format. Any disagreement raised between the reviewers was resolved through discussion and referral to a third author (KG) where necessary, till mutual consensus amongst all authors was achieved.
Outcomes and Analysis
Only studies for which individual patient outcome data were available were used for quantitative synthesis. We extracted information on demographic details, clinical features, KMT2B genetic variants, disease duration, details of intervention, follow‐up duration, severity of dystonia and adverse events (AE). The primary outcome was the relative change from baseline in the BFMDRS‐M score at last available follow‐up. Data regarding clinical phenotype and genetic variant were summarized descriptively. We pooled the data for age, gender, duration of symptoms, disease duration at time of surgery, follow‐up duration and pre‐ and post‐operative BFMDRS‐M scores. Continuous variables were summarized as mean (SD) or median (range) and categorical variables as proportions. Relative change from baseline after surgery was computed as [(pre‐op BFMDRS—post‐op BFMDRS)/pre‐op BFMDRS] and expressed as percentage. Good outcome was defined as more than 50% relative change in the BFMDRS‐M score as compared to baseline. Statistical analysis was performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) employing the “meta” and “metafor” package. 20 The Der Simonian‐Laird model was used to calculate the pooled effect. Study heterogeneity was assessed using I2 value and Q statistic, with I2 < 25% considered low heterogeneity, 25%–50% moderate heterogeneity and >50% high degree of heterogeneity. The random‐effects model was used for the meta‐analysis if I2 was more than 50%. A P value less than 0.05 was considered statistically significant. Forest plots were charted for pooled results. Publication bias was assessed using funnel plot and the Egger's test. Meta‐regression was done to assess the effect of clinical variables on the primary outcome using the “metafor” package in R. Additionally, we built a multiple linear regression model using individual patient data to explore additional predictors of good outcome (pre‐op BFMDRS—post‐op BFMDRS). The following independent variables were included in the regression model: age at onset, gender, age at DBS, disease duration at DBS, follow‐up duration and pre‐operative BFMDRS‐M score.
Results
Initial searches identified 132 abstracts of which 34 were of potential interest. After full text review, 10 studies met the inclusion/exclusion criteria and were included in the final analysis (Fig. 1). 15 , 16 , 17 , 18 , 21 , 22 , 23 , 24 , 25 , 26 We found a total of 42 subjects with DYT‐KMT2B who underwent GPi‐DBS and had pre‐ and post‐operative outcome data available. Number of patients reported in individual studies ranged from 1 to 17. The mean age at onset of dystonia was 6.4 ± 5.7 years and 40% of the patients were male. Two patients had adult‐onset dystonia. Distribution of dystonia was generalized in 41 out of 42 and segmental in one patient with adult‐onset dystonia. Additional neurological and/or systemic involvement was noted in 38/42 patients (90.5%; Table SS1). All patients underwent bilateral GPi‐DBS. The mean age at DBS was 14.7 ± 10.0 years. Follow‐up duration ranged from 1 month to 264 months (median: 12 months) in individual patients. There was a significant difference in the pooled pre‐ and post‐operative BFMDRS‐M scores (Pre: 62.4 ± 27.6 and Post: 35.8 ± 25.3, P < 0.001) with median improvement of 42.7% (range: −103.5% to 95.9%) in dystonia at last available follow‐up.
FIG. 1.
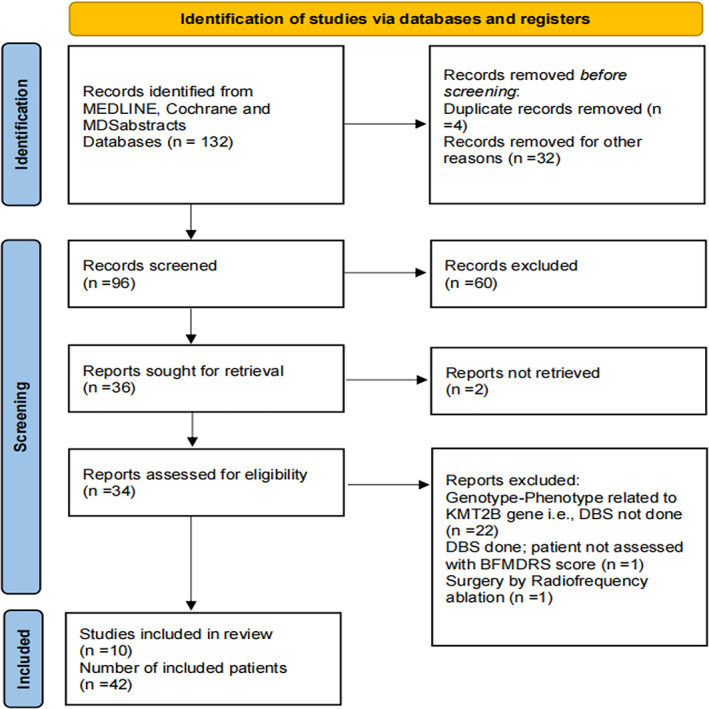
PRISMA flow diagram.
The pooled proportion of patients experiencing >50% clinical improvement on BFMDRS‐M score was 41% (95% CI: 27%–57%) (Fig. 2). Studies were homogenous with I2 value of 8%. Visual inspection of the funnel plot suggested relatively symmetrical distribution of studies (Fig. 3). Egger's regression test showed that the X‐axis intercept occurred at 1.079 with a two‐sided P‐value of 0.215, suggestive of no statistically significant publication bias. Meta‐regression suggested that male gender (P = 0.019) and follow‐up duration (P = 0.051) may be potential effect modifiers (Fig. 4).
FIG. 2.

Forest plot showing pooled proportion of clinical improvement following GPi‐DBS in patients with DYT‐KMT2B.
FIG. 3.

Funnel plot showing relatively symmetrical distribution, suggestive of no publication bias.
FIG. 4.

Bubble plot showing results of meta‐regression suggesting that male gender (A) and follow‐up duration (B) are potential effect modifiers.
We explored this further using individual patient data, and a multiple linear regression model was constructed to identify predictors of improvement in dystonia post‐operatively with age at onset, gender, age at DBS, disease duration at DBS, follow‐up duration and pre‐operative BFMDRS‐M score as independent variables (Table 1). The best‐fit model could explain 38% (adjusted R‐squared) of the variance in outcome. Male gender [β: 22.6, 95% CI: 8.0–37.3, P = 0.004), and higher pre‐operative BFMDRS‐M score [β: 0.62, 95% CI: 0.36–0.87, P < 0.001) were independently associated with greater change in BFMDRS‐M scores following surgery.
TABLE 1.
Predictors of good outcome after Gpi‐DBS in DTY‐KMT2B. Multiple linear regression model including the above independent variables could explain 38% of the observed variance in outcome (adjusted R‐squared = 0.38, Akaike Information Criterion (AIC) = 360.7). Values represent beta‐coefficients (95% CI)
| Variable | Coefficient (univariate) | Coefficient (multivariate) |
|---|---|---|
| Male gender | 11.50 (−4.50 to 27.50) P = 0.154 | 22.60 (7.95 to 37.26) P = 0.004 |
| Age at onset | −0.12 (−1.49 to 1.26) P = 0.863 | −1.05 (−20.03 to 17.93) P = 0.911) |
| Age at DBS | −0.08 (−0.86 to 0.71) P = 0.843 | 1.65 (−17.25 to 20.55) P = 0.860 |
| Duration at DBS | −0.06 (−1.00 to 0.87) P = 0.891 | −1.83 (−20.73 to 17.08) P = 0.845 |
| Follow up duration | 0.01 (−0.09 to 0.12) P = 0.794 | 0.00 (−0.09 to 0.10) P = 0.931 |
| Pre‐operative BFMDRS‐M score | 0.46 (0.22 to 0.71) P < 0.001 | 0.62 (0.36 to 0.87) P < 0.001 |
BFMDRS‐M, Burke Fahn Marsden Dystonia Rating Scale‐ Motor; DBS, Deep Brain Stimulation.
Adverse events were reported in 11 (26.2%) patients. Five patients had freezing of gait post‐operatively, including one patient with freezing of gait pre‐operatively. Electrode revision was required in four patients (two due to migrated electrode and two due to worsening dystonia with high impedance). One patient was reported to have electrode fracture and intraoperative lenticular hemorrhage, resulting in contralateral hemiparesis. One patient required surgical scar revision with extension cable replacement. Worsening dysphagia and dysphonia were reported in one patient. One patient developed status dystonicus 6 years after the initial surgery necessitating additional leads to be placed in the subthalamic nucleus.
Discussion
This systematic review and meta‐analysis suggest good outcomes following GPi‐DBS in patients with DYT‐KMT2B. Pooled data from patients with genetically confirmed DYT‐KMT2B show median improvement of 42.7% after GPi‐DBS. Significant improvement in motor symptoms (defined as more than 50%, relative change in the BFMDRS‐M score as compared to baseline) was seen in 41% of patients. Male gender and more severe disease pre‐operatively were associated with greater reduction in the BFMDRS‐M score following surgery.
Although not directly compared, the motor benefit from GPi‐DBS in DYT‐KMT2B is less than that reported for DYT‐TOR1A while substantially higher than that reported for other monogenic dystonias such as DYT‐THAP1 and DYT‐SCGE. 10 Coupled with the increasing frequency of recognition of DYT‐KMT2B in dystonia cohorts, these results reinforce the need to identify typical phenotypic features and offer genetic testing for KMT2B at low thresholds. It is unknown whether the differential outcomes of GPi‐DBS in monogenic dystonia are driven by downstream effects of the genetic variant and specific neuronal pathophysiological changes, or due to relatively distinct phenotypes associated with genes. 12 For instance, the predominant limb involvement in DYT‐TOR1A may be expected to respond better to GPi‐DBS, compared to cervical or laryngeal involvement in DYT‐THAP1. 27 DYT‐KMT2B is unique in that despite significant cranio‐bulbar involvement, which is a traditional clinical red flag for poor response to neurostimulation, patients show significant improvement in overall motor symptoms post GPi‐DBS. Gender‐specific effects are known after subthalamic nucleus (STN)‐DBS for Parkinson's disease (PD). For instance, studies have shown that the quality of life improves more in women than men after STN‐DBS for PD. 28 , 29 Such gender effects have not been generally reported with GPi‐DBS for dystonia, except for a trend towards better improvement in women with DYT‐THAP1. 10 Gender‐specific differences in phenotype or disease severity are not reported in DYT‐KMT2B, hence, this association merits further study. Earlier DBS and lesser baseline dystonia severity are known to be associated with better outcomes in isolated dystonia. 6 , 30 Contrary to this, higher dystonia severity was associated with better improvement in DYT‐KMT2B, a finding that vouches for the consideration of GPi‐DBS even in patients with advanced dystonia and long disease duration. There was a trend towards worsening dystonia severity as follow‐up duration increased, though this finding was not statistically significant. This may be due to the slowly progressive nature of disease in DYT‐KMT2B. As the median follow‐up duration in this study was 12 months, further long‐term observations are required to explore the stability of effects. Adverse events, though inconsistently reported, were similar to those previously reported with GPi‐DBS in isolated dystonia, including new‐onset freezing of gait and hardware related complications. 31 , 32
There are several limitations to this study. Being a relatively rare and recently identified disease, the number of patients with DYT‐KMT2B reported to have undergone GPi‐DBS is low, translating to a small sample size. All the studies were case reports or case series with no control group reported and the outcomes were not assessed in a blinded manner. While the BFMDRS‐M is a robust measure of dystonia severity, very few studies reported the BFMDRS‐D score or other measures to assess disability and quality of life after GPi‐DBS, which are important patient‐centric outcomes. Individual sub‐component scores of the BFMDRS‐M were not available to quantify the effect of GPi‐DBS on distinct anatomical locations, for instance limb dystonia or laryngeal dystonia. Confounding effects of co‐interventions like medications and botulinum toxin and effects of lead position or programming parameters could not be assessed as they were not consistently reported.
Conclusion
This systematic review summarizes the current body of evidence supporting the use of GPi‐DBS in DYT‐KMT2B that may guide patient selection and counseling. It also identifies critical gaps in knowledge regarding the effectiveness of GPi‐DBS in DYT‐KMT2B. Future randomized trials or prospective studies with blinded outcome assessments are warranted to capture broader outcomes, including measures of quality of life. Nevertheless, GPi‐DBS is an effective treatment for patients with generalized dystonia associated with DYT‐KMT2B and should be considered in all patients, particularly those with advanced disease and severe dystonia.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
R.R.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
K.G.: 1B, 2A, 1C, 2B, 2C, 3B
A.S.: 1A, 1B, 1C, 2B, 2C, 3A, 3B
D.M.R.: 1B, 2C, 3B
M.C.: 1C, 2C, 3B
B.B.K.: 1C, 2C, 3B
M.S.: 1B, 1C, 2B, 2C, 3B
A.K.S.: 1B, 1C, 2B, 2C, 3B
Disclosures
Ethical Compliance Statement
The authors confirm that approval of an institutional review board was not required for this work as it was a review of published data. Written informed consent was not required for this work as it was a review of published data. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The authors declare that there are no conflicts of interest relevant to this work. This research was funded by Department of Biotechnology, India (BT/PR26428/MED/12/783/2017) under the project entitled “The Indian Movement Disorder Registry and Biobank: Clinical and Genetic evaluation of movement disorders in Indian patients.”
Financial Disclosures for the Previous 12 Months
Roopa Rajan has received research grant support from DBT India, DST‐SERB India, Michael J Fox Foundation and AIIMS, New Delhi. Kanwaljeet Garg reports no relevant disclosures. Arti Saini reports no relevant disclosures. Divya M Radhakrishnan reports no relevant disclosures. Miryam Carecchio reports no relevant disclosures. Binukumar BK reports no relevant disclosures. Manmohan Singh reports no relevant disclosures. Achal K Srivastava reports no relevant disclosures.
Supporting information
Table S1. Clinical features and genetic variants in 42 patients with DYT‐KMT2B who underwent GPi‐DBS and had objective outcome data available.
References
- 1. Moro E, LeReun C, Krauss JK, et al. Efficacy of pallidal stimulation in isolated dystonia: a systematic review and meta‐analysis. Eur J Neurol 2017;24(4):552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh M, Agrawal M. Deep brain stimulation for tremor and dystonia. Neurol India 2020;68(8):187. [DOI] [PubMed] [Google Scholar]
- 3. Jinnah HA, Alterman R, Klein C, Krauss JK, Moro E, Vidailhet M, Raike R. Deep brain stimulation for dystonia: a novel perspective on the value of genetic testing. J Neural Transm (Vienna) 2017;124(4):417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brüggemann N, Kühn A, Schneider SA, et al. Short‐ and long‐term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology 2015;84(9):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coubes P, Roubertie A, Vayssiere N, Hemm S, Echenne B. Treatment of DYT1‐generalised dystonia by stimulation of the internal globus pallidus. Lancet 2000;355(9222):2220–2221. [DOI] [PubMed] [Google Scholar]
- 6. Andrews C, Aviles‐Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry 2010;81(12):1383–1389. [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Yu X. Deep brain stimulation for myoclonus dystonia syndrome: a meta‐analysis with individual patient data. Neurosurg Rev 2021;44(1):451–462. [DOI] [PubMed] [Google Scholar]
- 8. Tisch S, Kumar KR. Pallidal deep brain stimulation for monogenic dystonia: the effect of Gene on outcome. Front Neurol 2021;11:630391 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7820073/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danielsson A, Carecchio M, Cif L, et al. Pallidal deep brain stimulation in DYT6 dystonia: clinical outcome and predictive factors for motor improvement. J Clin Med 2019;8(12):2163 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6947218/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Artusi CA, Dwivedi A, Romagnolo A, et al. Differential response to pallidal deep brain stimulation among monogenic dystonias: systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry 2020;91(4):426–433. [DOI] [PubMed] [Google Scholar]
- 11. Zech M, Lam DD, Winkelmann J. Update on KMT2B‐related dystonia. Curr Neurol Neurosci Rep 2019;19(11):92. [DOI] [PubMed] [Google Scholar]
- 12. Meyer E, Carss KJ, Rankin J, et al. Mutations in the histone methyltransferase gene KMT2B cause complex early‐onset dystonia. Nat Genet 2017;49(2):223–237. [DOI] [PubMed] [Google Scholar]
- 13. Zech M, Jech R, Havránková P, et al. KMT2B rare missense variants in generalized dystonia. Mov Disord 2017;32(7):1087–1091. [DOI] [PubMed] [Google Scholar]
- 14. Lange LM, Junker J, Loens S, et al. Genotype–phenotype relations for isolated dystonia genes: MDSGene systematic review. Mov Disord 2021;36(5):1086–1103. [DOI] [PubMed] [Google Scholar]
- 15. Cif L, Demailly D, Lin J‐P, et al. KMT2B‐related disorders: expansion of the phenotypic spectrum and long‐term efficacy of deep brain stimulation. Brain 2020;143(11):3242–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao Z, Yao H, Bao X, Wen Y, Liu B, Wang S, Yang H. DYT28 responsive to Pallidal deep brain stimulation. Mov Disord Clin Pract 2020Jan;7(1):97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carecchio M, Invernizzi F, Gonzàlez‐Latapi P, et al. Frequency and phenotypic spectrum of KMT2B dystonia in childhood: a single‐center cohort study. Mov Disord 2019;34(10):1516–1527. [DOI] [PubMed] [Google Scholar]
- 18. Dafsari HS, Sprute R, Wunderlich G, et al. Novel mutations in KMT2B offer pathophysiological insights into childhood‐onset progressive dystonia. J Hum Genet 2019;64(8):803–813. [DOI] [PubMed] [Google Scholar]
- 19. Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Dystonia study group. Rating scales for dystonia: a multicenter assessment. Mov Disord 2003;18(3):303–312. [DOI] [PubMed] [Google Scholar]
- 20. R: The R Project for Statistical Computing [Internet]. [cited 2021 Jun 8]. Available from: https://www.r-project.org/.
- 21. Winslow N, Maldonado A, Zayas‐Rodriguez L, Lamichhane D. Adult‐onset KMT2B‐related dystonia responsive to deep brain stimulation. Mov Disord Clin Pract 2020;7(8):992–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X‐Y, Dai L‐F, Wan X‐H, et al. Clinical phenotypes, genotypes and treatment in Chinese dystonia patients with KMT2B variants. Parkinsonism Relat Disord 2020;77:76–82. [DOI] [PubMed] [Google Scholar]
- 23. Morsi O, Valero G, Jimenez J, et al. Globus pallidus deep brain stimulation (GPi‐DBS) in one patient with complex early‐onset dystonia and KMT2B mutation: a case report. Mov Disord 2018;33(suppl 2):S236. Available from: https://www.mdsabstracts.org/abstract/globus‐pallidus‐deep‐brain‐stimulation‐gpi‐dbs‐in‐one‐patient‐with‐complex‐early‐onset‐dystonia‐and‐kmt2b‐mutation‐a‐case‐report/. [Google Scholar]
- 24. Rajan R, Garg K, Saini A, et al. Pallidal deep brain stimulation for KMT2B related dystonia in an Indian patient. Ann Indian Acad Neurol 2021;24:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawarai T, Miyamoto R, Nakagawa E, et al. Phenotype variability and allelic heterogeneity in KMT2B‐associated disease. Parkinsonism Relat Disord 2018;52:55–61. [DOI] [PubMed] [Google Scholar]
- 26. Mun JK, Kim AR, Ahn JH, et al. Successful Pallidal stimulation in a patient with KMT2B‐related dystonia. J Mov Disord 2020;13(2):154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reese R, Volkmann J. Deep brain stimulation for the Dystonias: evidence, knowledge gaps, and practical considerations. Mov Disord Clin Pract 2017;4(4):486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hariz G‐M, Limousin P, Zrinzo L, et al. Gender differences in quality of life following subthalamic stimulation for Parkinson's disease. Acta Neurol Scand 2013;128(4):281–285. [DOI] [PubMed] [Google Scholar]
- 29. Dietrich AD, Koeppen JA, Buhmann C, et al. Sex disparities in the self‐evaluation of subthalamic deep brain stimulation effects on mood and personality in Parkinson's disease patients. Front Neurol 2020;11:776. 10.3389/fneur.2020.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y‐S, Ni L‐H, Fan R‐M, Yao M‐Y. Meta‐regression analysis of the long‐term effects of Pallidal and subthalamic deep brain stimulation for the treatment of isolated dystonia. World Neurosurg 2019;129:e409–e416. [DOI] [PubMed] [Google Scholar]
- 31. Krause P, Völzmann S, Ewert S, Kupsch A, Schneider GH, Kühn AA. Long‐term effects of bilateral pallidal deep brain stimulation in dystonia: a follow‐up between 8 and 16 years. J Neurol 2020;267(6):1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schrader C, Capelle H‐H, Kinfe TM, et al. GPi‐DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology 2011;77(5):483–488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical features and genetic variants in 42 patients with DYT‐KMT2B who underwent GPi‐DBS and had objective outcome data available.


