Abstract
Objective:
Due to previously reported trastuzumab safety concerns and the scant data available in endometrial cancer patients, we sought to assess the safety, tolerability and toxicity profile of trastuzumab in patients with advanced/recurrent uterine serous carcinoma (USC) that overexpress HER2/neu in our multicenter randomized phase II trial.
Methods:
Patients were randomized 1:1 to receive carboplatin/paclitaxel (C/P) for 6 cycles ± trastuzumab (T) with the experimental arm continuing to receive single agent trastuzumab maintenance treatment until disease progression/toxicity. Progression-free-survival was the primary endpoint; overall-survival and toxicity were secondary endpoints. Adverse events (AEs) were compared between treatment arms.
Results:
There were 28 patients in the C/P arm and 32 patients in the experimental (C/P+T) arm. Fifty-eight patients (97%) experienced 977 treatment-related AEs of which 875 (89.6%) were low-grade (grade 1–2) and 102 (10.4%) were high-grade (grade 3–5). The mean±standard deviation of AEs per patient was 15.5±16.3 in the C/P arm and 17.0±16.0 in the C/P+T arm. Gastrointestinal AEs were the most common in both arms (n=155, 15.7%) of which 94.2% were low-grade (n=146). Importantly, no significant difference between treatment arms was detected in any system-organ class of AE including cardiac AE. Five (17%) of 29 patients who received prolonged trastuzumab maintenance therapy had no sign of cumulative toxicity after an average (range) of 5.1 (4.2 – 6.3) years.
Conclusions:
Trastuzumab appears to be safe and has a manageable toxicity profile both when used in combination with chemotherapy and when used for single agent maintenance in patients with HER2/neu positive USC. This safety profile is reassuring given the proven efficacy of trastuzumab in advanced/recurrent HER2/neu positive USC.
Keywords: Trastuzumab, uterine serous carcinomas, HER2/neu, tolerability, side effects
INTRODUCTION
In the United States, endometrial cancer is the most prevalent of all gynecologic malignancies, accounting for an estimated 65,620 cases and 12,590 deaths in 2020 alone [1]. Endometrial cancer remains one of the only malignancies in which both the incidence and deaths continue to rise [1–3]. Uterine serous carcinoma (USC) is an aggressive, high-grade histologic subtype of endometrial cancer that portends a poor clinical prognosis. In fact, approximately 70% of patients with USC present with stage III or IV disease [4,5]. Although USC accounts for only 10% of all endometrial carcinomas, it is responsible for a disproportionate 39% of all uterine cancer-related deaths and has an overall 5-year survival rate of 45%, compared to 91% for those with the more common histologic subtype, endometrioid adenocarcinoma [6–8].
HER2/neu, the molecular target of the monoclonal antibody trastuzumab, is a tyrosine kinase receptor that plays an integral role in the coordination of the complex ErbB signaling network that is responsible for the regulation of cell growth, survival and proliferation [9]. HER2 overexpression and amplification is present in approximately 30% of USC and appears to be a poor prognostic factor similar to that seen in breast cancer [10–18]. Traditionally, USC has been treated with hysterectomy and surgical staging followed by platinum/taxane combination chemotherapy [19–22]. However, initial response rates to the most commonly used chemotherapy regimen, carboplatin and paclitaxel, can be as low as 20–60% for previously untreated advanced stage disease and an even worse response rate of 10–15% for those with recurrent disease [23].
A recent multicenter randomized phase II trial demonstrated that trastuzumab in combination with carboplatin/paclitaxel resulted in a significant improvement in both progression-free survival and overall survival for patients with both advanced and recurrent HER2/neu-positive USC [24,25]. As a result, the National Comprehensive Cancer Network’s Uterine Neoplasm Guidelines endorses the addition of trastuzumab to standard cytotoxic chemotherapy as the preferred regimen for the treatment of HER2/Neu-positive, advanced or recurrent USC [26].
HER2/neu amplification or overexpression is present in approximately 15 percent of primary invasive breast cancers [27]. Women with both early-stage and metastatic HER2-positive breast cancer are treated with trastuzumab-containing regimens because of several studies demonstrating substantial clinical benefit for these patients [28–31]. Large randomized trials of adjuvant trastuzumab for HER2-positive breast cancer reported an increase in the risk of cardiac toxicities, including congestive heart failure (0.6% to 3.8%) and left ventricular ejection-fraction declines (4.1% to 30.1%) [28,33–36]. Additionally, 20%−40% of breast cancer patients have an infusion reaction during the first treatment with trastuzumab, although most are mild, and only 0.3% exhibiting features suggestive of anaphylaxis [37–41]. Other rarer side effects of trastuzumab (less than 1%), are acute respiratory distress syndrome (ARDS), subacute interstitial pneumonia, and organizing pneumonia [42–44]. Although pulmonary toxicity is infrequent, it may be life-threatening, and discontinuation of trastuzumab is advised if such pulmonary side effects are present [45–47].
Despite the vast amount of information present in the literature regarding the safety, tolerability, and toxicity profile of trastuzumab in breast cancer patients, there is very limited information available on those with endometrial cancer in general and USC in particular who are treated with trastuzumab. In fact, GOG 181B, which was a phase II trial evaluating trastuzumab in women with advanced or recurrent HER2-positive endometrial carcinoma, is the only study to date that reports on the side-effect profile of treatment with trastuzumab for HER2-positive endometrial cancer [48]. In this study, Fleming et al. found a total of 33 adverse effects in their HER2-positive cohort of 34 patients. There were two deaths that were thought to be attributed to trastuzumab treatment, and both were associated with cardiovascular events (one infarction and one cardiopulmonary arrest) [48]. Other grade-3 and −4 adverse events included anemia and other hematologic events, plus gastrointestinal, metabolic, pain, and pulmonary issues [48]. Nevertheless, patients in GOG 181B were on study only for a few months before trial closure, highlighting the fact that there is no information available on the long-term tolerability of trastuzumab in USC patients, who tend to be older in age than Type I endometrial cancer patients. An important secondary objective of the randomized phase II trial was “to assess the safety profile of trastuzumab in USC patients” [24,25]. In this report, we fulfill that objective by characterizing the tolerability and toxicity profile of trastuzumab treatment found in our randomized phase II trial of patients treated with trastuzumab for advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress HER2/neu.
METHODS
Study design and conduct
The patient eligibility criteria and study design for this investigator-initiated randomized phase II study (NCT01367002) have been previously described in detail [24]. In brief, at a total of 11 participating academic institutions within the United States, patients were randomized 1:1 by the lead study institution using minimization to balance the treatment arms for study site, disease status (advanced versus recurrent uterine serous carcinoma), and residual tumor after debulking within the advanced-disease group [26]. Patients were scheduled to receive intravenous carboplatin area under the curve (AUC) 5 and paclitaxel 175 mg/m2 over 3 hours every 21 days with or without trastuzumab at 8mg/kg for the first dose and 6 mg/kg in subsequent cycles until disease progression or prohibitive toxicity. The first subject was enrolled in August of 2011, after which (1) the accrual rate was slower than planned, and (2) observed progression-free survival exceeded original expectations. The study was closed to further accrual in March of 2017 with a total of 61 enrolled subjects. Efficacy analysis commenced in August of 2017. The current updated analysis was performed at the time of 43 progressions and 38 deaths (Figure 1).
Figure 1:
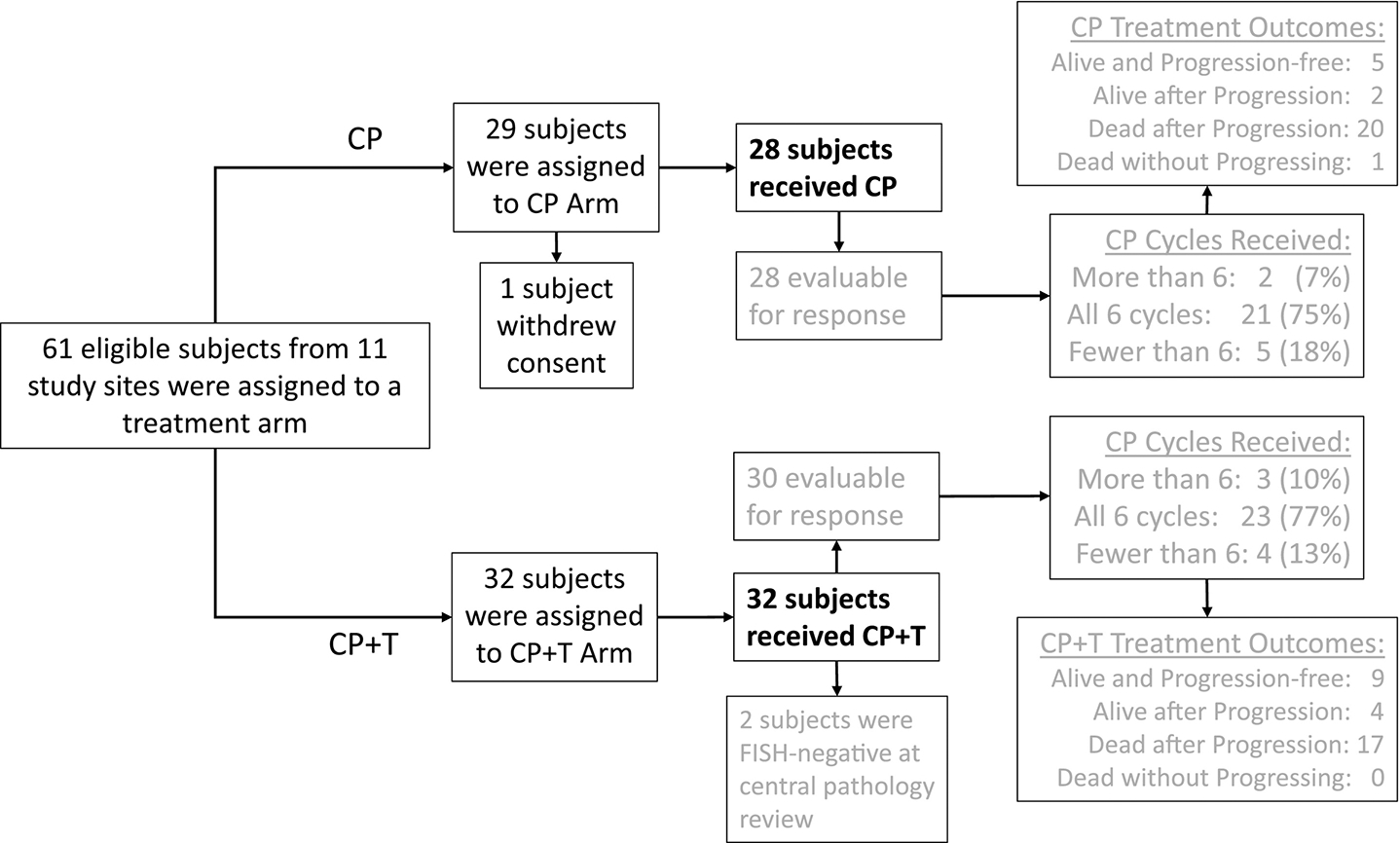
CONSORT diagram. Adapted from Fader AN, Roque DM, Siegel E, et al. Randomized phase II trial of carboplatin-paclitaxel compared to carboplatin-paclitaxel-trastuzumab in advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress HER2/Neu (NCT01367002): updated overall survival analysis.
Eligibility
All patients were 18 years or older and had FIGO 2009 stage III-IV recurrent (any previous stage) HER2/Neu-positive uterine serous carcinoma as defined by an immunohistochemistry score of 3+ or 2+ with gene amplification confirmed by fluorescence in situ hybridization (FISH). HER2/Neu-positive status was determined using paraffin-embedded tumor tissue from either primary surgery or from recurrent disease [49]. Scoring was performed according to guidelines set forth by the 2007 American Society of Clinical Oncology/College of American Pathologists for breast cancer [50]. Specimens were centrally reviewed for HER2/Neu+ and confirmed to contain ≥10% uterine serous carcinoma by two gynecologic pathologists. Patients may have been either optimally or suboptimally debulked after primary surgery. Patients were enrolled within 8 weeks after surgery or diagnosis of recurrent disease. Patients were required to exhibit an Eastern Cooperative Oncology Group performance status of 0 to 2, adequate bone marrow, renal function, and hepatic function. All patients diagnosed with recurrence were required to have measurable disease, defined as at least one target lesion per RECIST v1.1 [50,51]. A treatment-free interval of >6 months from last carboplatin/paclitaxel was required in those with recurrent disease. Patients with recurrent disease may not have received >3 prior chemotherapies for treatment of their uterine cancer. The schemata for treatment modification are provided in the full protocol.
Endpoints
All adverse events were classified using the Medical Dictionary for Regulatory Activities version 20.0 and onward. The severity of any toxic effect was graded in accordance with the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0 [51]. All adverse events and serious adverse events were collected and documented for each patient from the day informed consent was obtained until their end-of-treatment visit. New serious adverse events (including deaths) were collected for 30 days after the last dose of study treatment. To assess the safety profile of trastuzumab in USC patients enrolled on NCT01367002, we performed two analyses First, we analyzed the adverse events reported by patients in both treatment arms and evaluated the overall tolerability of the addition of trastuzumab to the standard chemotherapy regimen of carboplatin and paclitaxel for the treatment of advanced-stage and recurrent uterine serous carcinoma. Second, we analyzed the adverse events reported by patients in Arm 2 who received single-agent-trastuzumab maintenance after chemotherapy. All data were entered into our OnCore clinical-trials management system using case report forms specifically for NCT01367002 and collecting TRAEs (treatment related adverse events), grade, and drug attribution information.
Statistical analyses
Descriptive statistics were used to summarize and describe the basic features of the data collected. The number and proportion of patients who experienced at least one AE was reported in each treatment arm. For each patient, the number of AEs of any grade, of low grade (Grades 1 or 2), and of high grade (Grades 3, 4, or 5) was counted, and these three counts per patient were summarized by treatment arm as the sum, mean, standard deviation, and the “five-number summary” consisting of the mean, quartiles, and range.
RESULTS
As previously published by Fader et al., between August 2011 and January 2017, sixty-one subjects were enrolled, of which three participants were excluded due to withdrawal of consent (n=1) or failure to confirm HER2/Neu positivity by FISH following 2+ immunohistochemistry (n=2) at the time of central review. This left 58 subjects (28 in the carboplatin/paclitaxel (C/P) arm and 30 in the carboplatin/paclitaxel +trastuzumab (C/P+T) arm) evaluable for response to treatment [24] (Figure 1). Treatment arms did not differ significantly in regard to race, ethnicity, study site, or disease status (advanced versus recurrent disease), radiation or optimal debulking among advanced-disease subjects, or number of prior lines of chemotherapy among recurrent-disease subjects. However, the patients in the trastuzumab arm were seven years younger (median 66 years; interquartile range of 64–69 years) compared to the control arm (median 73 years; interquartile range of 68–78 years) [24]. Nonetheless, there was no statistically significant difference in the number of patients who experienced any adverse event due to treatment when stratified by age ≥70 years and age <70 years. Additionally, although most of the patients in both study arms were Caucasian, there were no significant differences in race or ethnicity between treatments arms, as previously published [24].
For our final analysis of adverse events due to treatment, we included the two aforementioned patients with immunohistochemical HER2/Neu positivity that were ultimately found to be FISH-negative upon central review. This was deemed acceptable for two reasons: the primary outcome of the current manuscript is toxicity and these two patients received trastuzumab. Thus, there were 60 patients evaluable for toxicity, 28 of whom received C/P and 32 of whom received C/P+T. Fifty-eight (97%) of them reported at least one treatment-related adverse event (AE) during the chemotherapy phase of treatment, including 27 (96%) on the C/P arm versus 31 (97%) on the C/P+T arm. These 58 patients experienced a total of 977 treatment-related AEs during chemotherapy (Tables 1A, 1B, and 1C), of which 875 (89.6%) were low-grade (grade 1–2) and 102 (10.4%) were high-grade (grade 3–5). The number of AEs per patient had a mean ±SD [standard deviation] of 15.5 ±16.3 on the C/P arm and 17.0 ±16.0 on the C/P+T arm (Table 1A). Of the 977 AEs, 433 (44.3%) were in the C/P arm and 544 (55.7%) were in the C/P+T arm (Table 1B). When grouped by system-organ class, the most common AE in both arms consisted of gastrointestinal disorders (n=155, 15.7%), the overwhelming majority of which (n=146) were low-grade (Table 1C). Gastrointestinal disorders also showed the most treatment-arm imbalance for any AEs per patient, with a mean ±SD of 3.16 ±3.20 on the C/P+T arm versus 1.93 ±2.67 on C/P alone, but this 64% increase in the presence of trastuzumab failed to attain statistical significance. Importantly, none of the other 18 system-organ classes showed a noteworthy treatment-arm imbalance in the number of AEs of any grade per patient (range in p-values: 0.17 – 0.84).
Table 1.
Demonstrates all adverse events for both Arm I and Arm II Please note in Table 1c the investigations category can best be described as abnormal lab findings, or other abnormal procedural findings (i.e. ejection fraction decreased is categorized here as well as urine output decreased).
| A. Distribution of AEs Per Patient (any grade) During Chemotherapy | ||||||||
| Treatment Arm | Sum | Mean | Standard Deviation | Minimum | First Quartile | Median | Third Quartile | Maximum |
| Carboplatin/Paclitaxel only (C/P) | 433 | 15.46 | 16.26 | 0 | 6.5 | 8.5 | 18 | 73 |
| Carboplatin/Paclitaxel +Trastuzumab (C/P+T) | 544 | 17.00 | 16.01 | 0 | 8 | 10.5 | 25.5 | 72 |
| BOTH ARMS | 977 | 16.28 | 16.01 | 0 | 7 | 10 | 20.5 | 73 |
| B: Adverse Events (AEs) by Grade and Treatment Arm | ||||||||
| Number of AEs by Grade | Number of AEs, All Grades | |||||||
| TREATMENT ARM | 1 | 2 | 3 | 4 | 5 | |||
| Carboplatin/Paclitaxel only (C/P) | 273 | 121 | 34 | 3 | 2 | 433 | ||
| Carboplatin/Paclitaxel +Trastuzumab (C/P+T) | 343 | 138 | 52 | 11 | . | 544 | ||
| BOTH ARMS | 616 | 259 | 86 | 14 | 2 | 977 | ||
| C: Adverse Events (AEs) by Grade and System Organ Class | ||||||||
| Number of AEs by Grade | Number of AEs, All Grades | |||||||
| SYSTEM ORGAN CLASS (CATEGORY) | 1 | 2 | 3 | 4 | 5 | |||
| Gastrointestinal disorders | 109 | 37 | 9 | . | . | 155 | ||
| Investigations | 74 | 44 | 25 | 4 | . | 147 | ||
| Blood and lymphatic system disorders | 38 | 27 | 17 | 7 | . | 89 | ||
| General disorders and administration site conditions | 65 | 23 | . | . | . | 89 | ||
| Skin and subcutaneous tissue disorders | 47 | 33 | 1 | . | . | 81 | ||
| Metabolism and nutrition disorders | 54 | 16 | 10 | . | . | 80 | ||
| Musculoskeletal and connective tissue disorders | 54 | 21 | 1 | . | . | 76 | ||
| Nervous system disorders | 59 | 8 | 1 | . | . | 68 | ||
| Respiratory, thoracic and mediastinal disorders | 33 | 10 | 3 | 1 | . | 47 | ||
| Vascular disorders | 10 | 8 | 9 | . | 2 | 29 | ||
| Infections and infestations | 12 | 11 | 4 | 2 | . | 29 | ||
| Renal and urinary disorders | 14 | 11 | 3 | . | . | 28 | ||
| Psychiatric disorders | 21 | 1 | 2 | . | . | 24 | ||
| Reproductive system and breast disorders | 7 | 4 | . | . | . | 11 | ||
| Eye disorders | 5 | 2 | . | . | . | 7 | ||
| Ear and labyrinth disorders | 5 | . | . | . | . | 5 | ||
| Injury, poisoning and procedural complications | 4 | . | . | . | . | 4 | ||
| Immune system disorders | 1 | 3 | . | . | . | 4 | ||
| Cardiac disorders | 4 | . | . | . | . | 4 | ||
| ALL SYSTEM-ORGAN CLASSES | 616 | 259 | 86 | 14 | 2 | 977 | ||
The low-grade AEs per patient had means ±SDs of 14.07 ±15.31 and 15.03 ±13.68, respectively, on the C/P and C/P+T arms Table 2). When low-grade AEs were subgrouped by system-organ class, gastrointestinal disorders revealed the most treatment-arm imbalance. The average number of low-grade gastrointestinal AEs per patient was 1.6-fold higher with C/P+T(mean ±SD = 2.97 ±3.07) than with C/P alone (mean ±SD = 1.82 ±2.58), but this increase was considered clinically inconsequential given that these AE were low grade and secondary to the prolonged duration of treatment in the trastuzumab arm. None of the 18 other system-organ classes revealed an appreciable treatment-arm imbalance in the number of low-grade AEs per patient.
Table 2:
Demonstrates grade 1–2 adverse events for both Arm I and Arm II
|
A. Distribution of Low-Grade (1–2) AEs Per Patient During Chemotherapy
| ||||||||
| Treatment Arm | Sum | Mean | Standard Deviation | Minimum | First Quartile | Median | Third Quartile | Maximum |
|
| ||||||||
| C/P | 394 | 14.07 | 15.31 | 0 | 5.5 | 8.5 | 17 | 68 |
|
| ||||||||
| C/P+T | 481 | 15.03 | 13.68 | 0 | 7 | 10 | 20.5 | 57 |
|
| ||||||||
| Both arms | 875 | 14.58 | 14.35 | 0 | 6 | 9 | 18 | 68 |
|
| ||||||||
|
| ||||||||
|
B. Number of Low-Grade AEs reported
| ||||||||
| System-Organ Class | Grade 1 | Grade 2 | Sum | |||||
|
| ||||||||
| Gastrointestinal disorders | 109 | 37 | 146 | |||||
| Investigations | 74 | 44 | 118 | |||||
| Blood and lymphatic system disorders | 38 | 27 | 65 | |||||
| General disorders and administration site conditions | 65 | 23 | 88 | |||||
| Skin and subcutaneous tissue disorders | 47 | 33 | 80 | |||||
| Metabolism and nutrition disorders | 54 | 16 | 70 | |||||
| Musculoskeletal and connective tissue disorders | 54 | 21 | 75 | |||||
| Nervous system disorders | 59 | 8 | 67 | |||||
| Respiratory, thoracic and mediastinal disorders | 33 | 10 | 43 | |||||
| Vascular disorders | 10 | 8 | 18 | |||||
| Infections and infestations | 12 | 11 | 23 | |||||
| Renal and urinary disorders | 14 | 11 | 25 | |||||
| Psychiatric disorders | 21 | 1 | 22 | |||||
| Reproductive system and breast disorders | 7 | 4 | 11 | |||||
| Eye disorders | 5 | 2 | 7 | |||||
| Ear and labyrinth disorders | 5 | 0 | 5 | |||||
| Injury, poisoning and procedural complications | 4 | 0 | 4 | |||||
| Immune system disorders | 1 | 3 | 4 | |||||
| Cardiac disorders | 4 | 0 | 4 | |||||
|
| ||||||||
| ALL System-Organ Hasses | 616 | 259 | 875 | |||||
For high grade AEs per patient, the means ±SDs on the C/P and C/P+T arms were 1.39 ±2.39 and 1.97 ±3.43, respectively (Table 3). When high-grade AEs were subgrouped by system-organ class, the blood and lymphatic-system disorders demonstrated the most treatment-arm imbalance. The average number of high-grade blood-and-lymph AEs per patient was 3.3-fold higher with C/P+T (mean ±SD = 0.59 ±1.01) than with C/P alone (mean ±SD = 0.18 ±0.55). This difference was however clinically inconsequential and likely related to chemotherapy and the prolonged duration of treatment in the trastuzumab arm. Importantly, none of the other system-organ-class comparisons for high-grade adverse events demonstrated a noteworthy significant imbalance between treatment arms.
Table 3:
Demonstrates grade 3–5 adverse events for both Arm I and Arm II
| A. Distribution of High-Grade (3–5) AEs Per Patient During Chemotherapy | ||||||||
| Treatment Arm | Sum | Mean | Standard Deviation | Minimum | First Quartile | Median | Third Quartile | Maximum |
| C/P | 39 | 1.39 | 2.39 | 0 | 0 | 0 | 1.5 | 10 |
| C/P+T | 63 | 1.97 | 3.43 | 0 | 0 | 0.5 | 3 | 15 |
| Both arms | 102 | 1.70 | 2.98 | 0 | 0 | 0 | 2 | 15 |
| B. Number of High-Grade AEs reported | ||||||||
| System-Organ Class | Grade 3 | Grade 4 | Grade 5 | Sum | ||||
| Gastrointestinal disorders | 9 | 0 | 0 | 9 | ||||
| Investigations | 25 | 4 | 0 | 29 | ||||
| Blood and lymphatic system disorders | 17 | 7 | 0 | 24 | ||||
| General disorders and administration site conditions |
1 | 0 | 0 | 1 | ||||
| Skin and subcutaneous tissue disorders | 1 | 0 | 0 | 1 | ||||
| Metabolism and nutrition disorders | 10 | 0 | 0 | 10 | ||||
| Musculoskeletal and connective tissue disorders |
1 | 0 | 0 | 1 | ||||
| Nervous system disorders | 1 | 0 | 0 | 1 | ||||
| Respiratory, thoracic and mediastinal disorders | 3 | 1 | 0 | 4 | ||||
| Vascular disorders | 9 | 0 | 2 | 11 | ||||
| Infections and infestations | 4 | 2 | 0 | 6 | ||||
| Renal and urinary disorders | 3 | 0 | 0 | 3 | ||||
| Psychiatric disorders | 2 | 0 | 0 | 2 | ||||
| Reproductive system and breast disorders | 0 | 0 | 0 | 0 | ||||
| Eye disorders | 0 | 0 | 0 | 0 | ||||
| Ear and labyrinth disorders | 0 | 0 | 0 | 0 | ||||
| Injury, poisoning and procedural complications | 0 | 0 | 0 | 0 | ||||
| Immune system disorders | 0 | 0 | 0 | 0 | ||||
| Cardiac disorders | 0 | 0 | 0 | 0 | ||||
| ALL System-Organ Classes | 86 | 14 | 2 | 102 | ||||
Regarding cardiovascular AEs, four patients experienced a total of four AEs, all of which were low-grade. There was only one in the C/P arm and only three in the C/P+T arm (Table 4). There were also no instances of congestive heart failure. There was one investigation for a grade-3 left ventricular dysfunction deemed to be unrelated to study drug by both the patient’s cardiologist and the study’s Principal Investigator.
Table 4:
Adverse cardiac events for both Arm I and Arm II
| Table (Supp) Cardiac AEs | |||
|---|---|---|---|
| Any Cardiac AE (During Chemotherapy) | |||
| Arm | Yes | No | |
| 1 | 1 | 27 | |
| 2 | 3 | 29 | |
| Cardiac AE Type* (N) (During Chemotherapy) | |||
| Arm | Palpitations | Sinus Tachycardia | Vent. Arrhythmia |
| 1 | 0 | 1 | 0 |
| 2 | 2 | 0 | 1 |
| Any Cardiac AE (Trastuzumab Maintenance) | |||
| Arm | Left Ventricular systolic dysfunction | ||
| Yes | No | ||
Of the thirty-two patients that were randomized to the C/P+T arm and treated with trastuzumab during their chemotherapy, twenty-nine of them (91%) received single-agent maintenance trastuzumab after finishing frontline chemotherapy. Of the three patients who did not, two were the HER2/neu 2+ but FISH-negative patients who were excluded from the original efficacy analysis. All except two of the 29 patients began single-agent trastuzumab on Cycle 7. The two exceptions both had recurrent measurable disease, received carboplatin and paclitaxel through Cycle 9, and began their single-agent trastuzumab on Cycle 10. The 29 patients have experienced 617 post-chemotherapy cycles up through June 24th, 2020 and have received trastuzumab during 615 of them to yield an average of 21.2 cycles (range: 1–94 cycles) of trastuzumab thus far (Table 4). At the time of this writing (mid-March of 2021), 5 (17%) of the 29 USC patients who began maintenance treatment with single-agent trastuzumab continue to receive it every 3 weeks per protocol. These 5 patients have been on trastuzumab maintenance for 4.2, 4.7, 4.9, 5.5, and 6.3 years. One subject skipped trastuzumab on Cycles 41 and 50 and received late paclitaxel instead. Another patient received late paclitaxel concurrently with trastuzumab from Cycle 25 through Cycle 29 of her trastuzumab-alone treatment. Those 5 cycles are counted as single-agent trastuzumab for purposes of this analysis. No other patient received a second agent during their trastuzumab alone post chemotherapy segment.
DISCUSSION
In a previous multicenter randomized phase II trial, the addition of trastuzumab to carboplatin and paclitaxel resulted in a significant improvement in PFS and OS in advanced/recurrent HER2/neu-positive USC [24,25]. Due to previous safety concerns regarding trastuzumab and its reported adverse cardiac events, we evaluated the safety of the addition of trastuzumab to carboplatin and paclitaxel followed by maintenance trastuzumab in elderly patients with advanced or recurrent USC, as a follow up analysis of the original phase II study [24,25, 28, 33–35]. Our data suggest both the safety and tolerability of trastuzumab, which has been shown to improve oncologic outcomes for HER2/Neu 3+ patients with advanced or recurrent USC. Thus far, five of 29 patients who began trastuzumab maintenance have remained on treatment for an average (range) of 5.1 (4.2 – 6.3) years.
Though most of the patients were Caucasian, the treatment arms were well-balanced in regard to ethnicity and race, as previously published [24]. Elderly patients (≥70 years old in this analysis) were more likely to experience a high-grade AE in both arms. Although the average number of high-grade AEs per patient was 3.3-fold higher in Arm II (mean = 0.59 high-grade AEs per patient) compared to Arm I (mean = 0.18 high-grade AEs per patient) this increase was clinically inconsequential since no patient discontinued therapy due to these AEs and such an increase in number was likely related to the prolonged duration of treatment in the trastuzumab arm. Our adverse-events investigation suggest that trastuzumab is very well-tolerated in elderly patients with USC. In that respect, it differs from the published results in breast cancer, a tumor where patients often receive cardiotoxic agents (i.e. anthracyclines) as standard of care, and where trastuzumab treatment has been shown to increase the incidence of adverse events. It is worth noting that the median age in a large Cochrane review was 49 years, which is much younger than the median age (67 years) of our experimental arm [30,24]. Thus, despite our much more elderly population, our data suggests that trastuzumab was not only efficacious but also highly tolerable in this group of patients not previously exposed to Adriamycin (doxorubicin).
In addition, even though the majority of patients in our study had at least one AE, either during treatment or during post-treatment maintenance with trastuzumab, the majority of these adverse events were low-grade AEs, most commonly being fatigue, nausea, and constipation. These are all extremely common AEs with most chemotherapeutic regimens, each with their own available treatments and prophylactic regimens to decrease their incidence. There was an investigation due to decreased EF in a patient with a history of complete heart block with a pacemaker and cardiologic workup including LVEF monitoring every 3 months per protocol revealed the cause as pacemaker-induced cardiomyopathy. Thus it was not deemed to be a direct toxicity of trastuzumab.
Most of the available literature in breast cancer has limited the window of usage of trastuzumab to 1 year in the adjuvant setting [28–30]. Though there is insufficient evidence based on a small number of trials, a shorter duration of therapy was associated with lower cardiotoxicity in the aforementioned Cochrane review [34]. Despite this, even though our cohort of patients received an average of 21.2 cycles and maximum of 94 (at the time of this writing, five patients continue to receive trastuzumab treatment every 21 days), there was no clinically significantly increase in toxicity, particularly cardiac toxicity. Based on the limited data currently available, one would anticipate a greater number of adverse effects due to the cumulative number of cycles in combination with the elderly median age of our population, which we unexpectedly did not find. As mentioned above, an important difference between our study and the existing literature in breast cancer is that most trials reporting significant cardiac toxicity with trastuzumab included anthracyclines, which are an important contributor to cardiac toxicity. Both a history of prior anthracycline receipt and, especially, concurrent anthracycline use are known to increase cardiac toxicity, so much that the NCCN recommends the latter be avoided [53]. Standard first line chemotherapy (both adjuvant and neoadjuvant) consists of carboplatin and paclitaxel in USC; anthracyclines were avoided altogether, and patients were excluded from this study if they had a prior malignancy, thus this would not confound our results [24]. In our patient cohort there was only one patient that had a grade 3 event while receiving maintenance trastuzumab and this event was not considered related to treatment. Thus, we found no evidence of increased cardiac toxicity of trastuzumab in this elderly cohort of patients with USC.
Our study has several important limitations. The sample size could impact the rate of adverse events, as they were not the primary endpoint and thus our analysis was not powered to detect them. This was due to slow patient accrual and unexpected efficacy in the parent trial [24]. Additionally, the patients in the control arm had a higher median age than the experimental arm; however, the high-grade AE rate was higher in the older population, thus indicating that this did not confound our results.
The safety demonstrated herein is particularly promising given the proven utility of trastuzumab in advanced and/or recurrent HER2/neu positive USC. Pertuzumab will be used in combination with trastuzumab in an upcoming NRG/CTEP trial in the same population and we are hopeful that results will not be limited by patient toxicity (NCT01367002). In conclusion, trastuzumab appears to be very well-tolerated treatment when used in combination with standard chemotherapy and as single-agent maintenance in patients with HER2/neu positive advanced/recurrent uterine serous carcinoma.
Highlights.
Our patients received an average of 21.2 and maximum of 94 cycles of Trastuzumab without significant toxicity.
The majority of adverse events were low-grade, most commonly fatigue, nausea, and constipation.
Patients who received trastuzumab maintenance had no sign of increased toxicity after an average of 5.1 years of continued treatment.
Elderly patients (≥70 years old) were more likely to experience a high-grade adverse event in both treatment arms.
Trastuzumab in combination with chemotherapy appears to be safe and has a manageable toxicity profile in patients with HER2/Neu-positive USC.
Financial support
This work was supported in part by by grants from NIH U01 CA176067-01A1, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation, the Guido Berlucchi Foundation and the Dominique Cicchetti Foundation to A.S. This investigation was also supported by NIH Research Grant CA-16359 from NCI and Stand-up-to-cancer (SU2C) convergence grant 2.0 to A.S. Santin.
Footnotes
Conflicts of Interest statement
All authors fulfill the conditions required for authorship
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020 CA Cancer J Clin. 2020. 2020; 70:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Wethington SL, Fader AN. ProMisE on the horizon: molecular classification of endometrial cancer in young women. Gynecol Oncol. 2019;153(3):465–466. doi: 10.1016/j.ygyno.2019.05.001 [DOI] [PubMed] [Google Scholar]
- [3].Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J Natl Cancer Inst. 2018;110(4):354–361. doi: 10.1093/jnci/djx214 [DOI] [PubMed] [Google Scholar]
- [4].Slomovitz BM, Burke TW, Eifel PJ, et al. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol 2003; 91:463. [DOI] [PubMed] [Google Scholar]
- [5].Podratz KC, Mariani A. Uterine papillary serous carcinomas: the exigency for clinical trials. Gynecol Oncol 2003; 91:461. [DOI] [PubMed] [Google Scholar]
- [6].Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006; 95 Suppl 1:S105. [DOI] [PubMed] [Google Scholar]
- [7].Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer 2006; 94:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kosary C Cancer of the Corpus Uterus. Uterus SEER Surviv Monogr Cancer Surviv Adults US SEER Program 1988–2001 1988–2001 NCI SEER Program Natl Cancer Inst; Bethesda MD. 2007. [Google Scholar]
- [9].Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501 [DOI] [PubMed] [Google Scholar]
- [10].Santin AD, Bellone S, Siegel ER, et al. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): a major prognostic indicator in uterine serous papillary cancer. Am J Obstet Gynecol 2005; 192(3): 813–818. doi: 10.1016/j.ajog.2004.10.605 [DOI] [PubMed] [Google Scholar]
- [11].Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod Pathol 2013. December; 26(12): 1605–12. doi: 10.1038/modpathol.2013.113. Epub 2013 Jun 14 [DOI] [PubMed] [Google Scholar]
- [12].Grushko TA, Filiaci VL, Mundt AJ, et al. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;108(1):3–9. doi: 10.1016/j.ygyno.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Santin AD, Bellone S, Gokden M, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2002;8(5):1271–1279. [PubMed] [Google Scholar]
- [14].Khalifa MA, Mannel RS, Haraway SD, Walker J, Min KW. Expression of EGFR, HER-2/neu, P53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol Oncol. 1994;53(1):84–92. doi: 10.1006/gyno.1994.1092 [DOI] [PubMed] [Google Scholar]
- [15].Odicino FE, Bignotti E, Rossi E, et al. HER-2/neu overexpression and amplification in uterine serous papillary carcinoma: comparative analysis of immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and fluorescence in situ hybridization. Int J Gynecol Cancer. 2008;18(1):14–21. doi: 10.1111/j.1525-1438.2007.00946. [DOI] [PubMed] [Google Scholar]
- [16].Díaz-Montes TP, Ji H, Smith Sehdev AE, et al. Clinical significance of Her-2/neu overexpression in uterine serous carcinoma. Gynecol Oncol. 2006;100(1):139–144. doi: 10.1016/j.ygyno.2005.08.017 [DOI] [PubMed] [Google Scholar]
- [17].Singh P, Smith CL, Cheetham G, Dodd TJ, Davy MLJ. Serous carcinoma of the uterus-determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: its significance and clinical correlation. Int J Gynecol Cancer 2008;18(6):1344–1351. doi: 10.1111/j.1525-1438.2007.01181. [DOI] [PubMed] [Google Scholar]
- [18].Buza N, Roque DM, Santin AD. HER2/neu in Endometrial Cancer: A Promising Therapeutic Target with Diagnostic Challenges. Arch Pathol Lab Med. 2014. March;138(3):343–50. doi: 10.5858/arpa.2012-0416-RA [DOI] [PubMed] [Google Scholar]
- [19].Boruta DM, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115(1):142–153. doi: 10.1016/j.ygyno.2009.06.011 [DOI] [PubMed] [Google Scholar]
- [20].Fader AN, Drake RD, O’Malley DM, et al. Platinum/taxane-based chemotherapy with or without radiation therapy favorably impacts survival outcomes in stage I uterine papillary serous carcinoma. Cancer. 2009;115(10):2119–2127. doi: 10.1002/cncr.24247 [DOI] [PubMed] [Google Scholar]
- [21].Ramondetta L, Burke TW, Levenback C, Bevers M, Bodurka-Bevers D, Gershenson DM. Treatment of uterine papillary serous carcinoma with paclitaxel. Gynecol Oncol. 2001;82(1):156–161. doi: 10.1006/gyno.2001.6211 [DOI] [PubMed] [Google Scholar]
- [22].Fader AN, Nagel C, Axtell AE, et al. Stage II uterine papillary serous carcinoma: Carboplatin/paclitaxel chemotherapy improves recurrence and survival outcomes. Gynecol Oncol. 2009;112(3):558–562. doi: 10.1016/j.ygyno.2008.11.016 [DOI] [PubMed] [Google Scholar]
- [23].Hoskins PJ, Swenerton KD, Pike JA, et al. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(20):4048–4053. doi: 10.1200/JCO.2001.19.20.4048 [DOI] [PubMed] [Google Scholar]
- [24].Fader AN, Roque DM, Siegel E, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(20):2044–2051. doi: 10.1200/JCO.2017.76.5966 [DOI] [PubMed] [Google Scholar]
- [25].Fader AN, Roque DM, Siegel E, et al. Randomized phase II trial of carboplatin-paclitaxel compared to carboplatin-paclitaxel-trastuzumab in advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress Her2/Neu (NCT01367002): updated overall survival analysis. J Clin Oncol. 2018: October;36(20):2044–2051/JCO.2017.76.596629584549 [Google Scholar]
- [26].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology-Uterine Neoplasms. Version 4.2019. 2019. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed October 6, 2019.
- [27].Noone AM, Cronin KA, Altekruse SF, et al. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiol Biomarkers Prev 2017; 26:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011; 365:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673. [DOI] [PubMed] [Google Scholar]
- [30].Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012; :CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014; 32:3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384:164. [DOI] [PubMed] [Google Scholar]
- [33].Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol 2009; 27:6129. [DOI] [PubMed] [Google Scholar]
- [34].Dang C, Guo H, Najita J, et al. Cardiac Outcomes of Patients Receiving Adjuvant Weekly Paclitaxel and Trastuzumab for Node-Negative, ERBB2-Positive Breast Cancer. JAMA Oncol 2016; 2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002; 20:1215. [DOI] [PubMed] [Google Scholar]
- [36].Nowsheen Somaira et al. “Incidence, Diagnosis, and Treatment of Cardiac Toxicity from Trastuzumab in Patients with Breast Cancer.” Current breast cancer reports vol. 9,3 (2017): 173–182. doi: 10.1007/s12609-017-0249-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, et al. Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol 2001; 12:1545. [DOI] [PubMed] [Google Scholar]
- [38].Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783. [DOI] [PubMed] [Google Scholar]
- [39].Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17:2639. [DOI] [PubMed] [Google Scholar]
- [40].Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20:719. [DOI] [PubMed] [Google Scholar]
- [41].Cook-Bruns N Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology 2001; 61 Suppl 2:58. [DOI] [PubMed] [Google Scholar]
- [42].Vahid B, Mehrotra A. Trastuzumab (Herceptin)-associated lung injury. Respirology 2006; 11:655. [DOI] [PubMed] [Google Scholar]
- [43].Radzikowska E, Szczepulska E, Chabowski M, Bestry I. Organising pneumonia caused by transtuzumab (Herceptin) therapy for breast cancer. Eur Respir J 2003; 21:552. [DOI] [PubMed] [Google Scholar]
- [44].Bettini AC, Tondini C, Poletti P, et al. A case of interstitial pneumonitis associated with Guillain-Barré syndrome during administration of adjuvant trastuzumab. Tumori 2008; 94:737. [DOI] [PubMed] [Google Scholar]
- [45].Bettini AC, Tondini C, Poletti P, et al. A case of interstitial pneumonitis associated with Guillain-Barré syndrome during administration of adjuvant trastuzumab. Tumori 2008; 94:737. [DOI] [PubMed] [Google Scholar]
- [46].Pepels MJ, Boomars KA, van Kimmenade R, Hupperets PS. Life-threatening interstitial lung disease associated with trastuzumab: case report. Breast Cancer Res Treat 2009; 113:609. [DOI] [PubMed] [Google Scholar]
- [47].Herceptin (trastuzumab for injection). FDA approved package insert. US National Library of Medicine. www.dailymed.nlm.nih.gov (Accessed on December 02, 2010).
- [48].Fleming FG, Sill MW, Darcy KM, McMeekin DS, Thigpen JT, Adler LM, Berek JS, Chapman JA, DiSilvestro PA, Horowitz IR, Fiorica JV. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010; 116(1): 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138(2):241–256. doi: 10.5858/arpa.2013-0953-SA [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- [51].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- [52].Common Terminology Criteria for Adverse Events (CTCAE) - CTCAE_4.03_2010–06-14_QuickReference_5×7.pdf. Accessed October 3, 2016. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf [Google Scholar]
- [53].Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Isakoff SJ, Krishnamurthy J, Lyons J, Marcom PK, Matro J, Mayer IA, Moran MS, Mortimer J, O’Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Young JS, Burns JL, Kumar R, Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology, J Natl Compr Canc Netw. 18 (2020) 452–478. 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]


