Abstract
Digital pathology (DP) has disrupted the practice of traditional pathology, including applications in education, research, and clinical practice. Contemporary whole slide imaging (WSI) devices include technological advances that help address some of the challenges facing modern pathology, such as increasing workloads with fewer subspecialized pathologists, expanding integrated delivery networks with global reach, and greater customization when working up cases for precision medicine. This review focuses on integral hardware components of 43 market available and soon-to-be released digital WSI devices utilized throughout the world. Components such as objective lens type and magnification, scanning camera, illumination, and slide capacity were evaluated with respect to scan time, throughput, accuracy of scanning, and image quality. This analysis of assorted modern WSI devices offers essential, valuable information for successfully selecting and implementing a digital WSI solution for any given pathology practice.
Keywords: Digital pathology, hardware, microscopy, slide scanning, whole slide imaging, whole slide scanner
INTRODUCTION
The coronavirus disease-2019 (COVID-19) pandemic brought forth renewed insights into the widespread applicability of digital pathology (DP) solutions, namely that of whole slide imaging (WSI) systems. Global disruption of routine anatomic pathology (AP) procedures in clinical and academic settings ignited deeper exploration into the role of DP as a “safeguard to clinical services and pathology-based research,”[1] as well as the expanded employment of WSI as an educational tool.[2] Although DP solutions have been used with particular fervor throughout the pandemic, these tools have been progressively garnering merit in the practice of pathology since their inception in the 1970s.[3] From the rise of telepathology (1980s) to the advent of WSI (2000s) and its pairing with artificial intelligence and machine-learning algorithms (late 2010s), DP has quickly become a central focus to AP laboratories.[4,5]
The WSI device hardware itself has advanced through multiple generations since the introduction of the first virtual microscopes more than 20 years ago.[6,7,8,9,10] Starting with the commercial availability of the Aperio (Leica) T1 in 2001, each successive generation of WSI devices has been demarcated by graduated improvements in multiple functions, including scan speed, throughput, image quality, slide capacity, telepathology capabilities, and z-stacking. The WSI has been used for multiple applications, including education, remote teleconsultation, tumor boards/multidisciplinary conferences, biobanking, archiving, image analysis, and, more recently, primary diagnosis.[11] In 2017, the Philips Intellisite Pathology Solution (PIPS) was the first WSI system to be granted a United States Food and Drug Administration (FDA) De Novo pathway classification for the primary digital diagnosis of formalin-fixed paraffin-embedded tissue (FFPE) within surgical pathology.[6,12] Since then, WSI devices have become increasingly commoditized, with a growing number of models undergoing studies for near-term US FDA 510(k) clearance and European Union Conformité Européenne (EU CE) mark approval.[6]
There has been a paucity of recent publications that review the features of WSI devices and their components.[13,14,15] The objectives of this work are: (1) to offer an up-to-date technical comparison of currently available WSI hardware; (2) to provide a perspective on the function of individual WSI system hardware components for addressing demands in pathology; (3) to bridge the gap between vendor technical jargon and practical applications in pathology settings; (4) to aid in the pathology department’s/practice’s WSI device selection; and (5) to speculate on the further evolution of these tools as related to future pathology practice.
Overall, WSI hardware, and in particular the WSI device itself, is the primary focus of this review. The majority of the devices mentioned henceforth are referred to by the manufacturing vendor (current to the date of this publication). Please note the authors do not endorse any one vendor over another, nor should the inclusion of a specific WSI device be taken as a recommendation over any current or future WSI devices not listed. The WSI device specifications such as slide capacity, illumination, objective magnification, scanning methods and scan time/throughput are evaluated with respect to clinical and nonclinical use cases.
THE WHOLE SLIDE IMAGING PROCESS
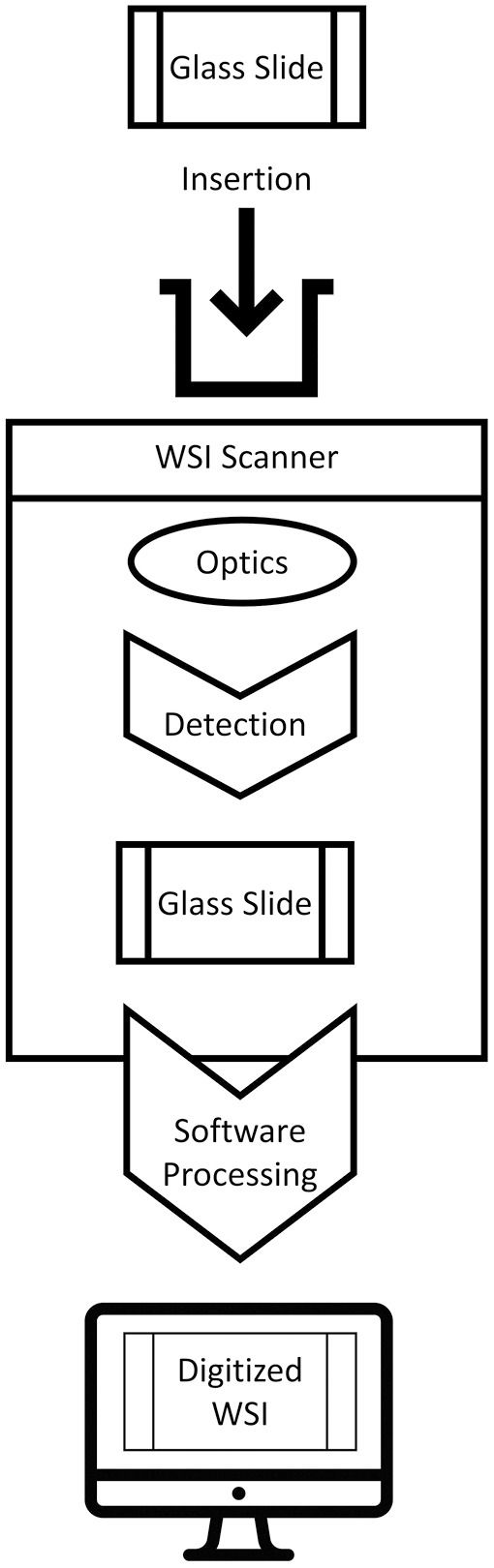
The WSI devices scan glass slides of histology and cytology origin and digitally reproduce them as virtual image objects, which are then evaluated using a WSI viewer on a capable workstation and connected display.[6] Historically, WSI devices were divided into two main categories: motorized microscopes and slide scanners; however, over time, these distinctions have been largely replaced by a device’s primary DP use case, such as high-throughput scanning, live-view microscopy for telepathology, or whole-mount scanning.[13,16]
From a process perspective, a WSI systems follow a “pixel pathway” that traces the flow of image data from the glass slide to a human reader.[17] The primary elements of the WSI pixel pathway can be separated into two primary subsystems, the image acquisition and workstation environments, that work in tandem to create and display WSI. In short, the image acquisition subsystem is composed of an illumination source (e.g., brightfield), imaging optics, robotics to load and manipulate the glass slide during the scanning process, and one or more digital cameras to read barcodes, focus, and capture specific fields of view (FOVs) that are then stitched together into a composite WSI. The stored image data file can be subsequently accessed by image review software (either via a server-based image management system or on a local machine with a WSI viewer) and presented on an appropriate display within the workstation environment subsystem.[17]
From a technical perspective, there are several major hardware elements integral to the creation of a WSI that one should be familiar with when evaluating WSI devices. These include components of slide feeding/handling, focusing, digital imaging (camera) sensors, and scanning method [Figure 1].[18]
Figure 1.
WSI creation (original illustration)
These elements will be further explored later within the context of current WSI device offerings.
WSI FEEDER AND SLIDE HANDLING
WSI acquisition begins with the loading of one or more physical glass slides into a slide feeder.[19] Advancements in slide-loading mechanisms have evolved in tandem with the demands of departments tasked with high-case volumes.[2,20] Such departments, in seeking to mitigate cost[21] and optimize efficiency, command high-throughput devices with rapid automatic loading and batch scanning capabilities to drive workflow and enhance resource management.[2,20,22,23] Many WSI instruments are capable of batch scanning and continuous or random-access processing, allowing for the concurrent uploading of slides during image capture of others.[14]
These features allow for the maximization of productivity via “walk-away” functionality, enabling operators to freely delegate themselves to other tasks while image acquisition is executed in the periphery.[2] Consideration of departmental volume, WSI device slide capacity, slide type (e.g., wet vs. dry), and dimension are of additional importance in consideration of the WSI device most capable of addressing certain departmental objectives.[2]
The total slide capacity of WSI devices currently available on the market ranges from 1, for example, Grundium Ocus 40[24] to 1000 slides, for example, 3D Histech Pannoramic 1000.[25] Positive correlation has been noted between increased slide-loading capacity and corresponding market price.[11] Total slide capacity is among the most critical of physical WSI device characteristics to evaluate in view of institutional workload, particularly departments bearing substantial caseloads.[20] The loading capacity of multidimensional slide capable devices decreases when using slides of larger dimensions. Most modern WSI devices are equipped to handle slides of standard dimension (1′′ × 3′′ or approximately 25 mm x 75 mm). Others are able to accommodate “mix-and-match” handling of both single (standard) and “double-wide” (2′′ × 3′′ or approximately 50 mm × 75 mm) slides in a single tray (accommodating both slide dimensions in a single scanning session), for example, Glissando POL (Objective Imaging), Hamamatsu NanoZoomer S60, Huron Tissuescope IQ. The advantages of WSI devices equipped to handle a diversity of slide dimensions have been historically met with difficulty in batch processing larger numbers of slides, particularly when involving greater variations in media.[11,20] Advances in technology, however, have mitigated shortfalls in batch processing capabilities.[14] Some WSI devices are capable of handling a litany of non-standard “large” or “mega” sized whole mount slides ranging up to 6′′ × 8′′ (150 mm × 200 mm), for example, Huron Tissuescope CF.
LOADING AND HANDLING
Auto-loading functionality in WSI devices designed to handle multiple slide dimensions may be restricted to a singular (typically standard) slide dimension. The Leica AT Turbo, though discontinued from this vendor’s current vendor portfolio, is still utilized in some academic centers.[26] Built for medium-to-high volume departments, it features an autoloading capacity of 400 standard slides and direct manual insertion capability for double-wide slides. Continuous autoloading functionality present in several medium-to-high volume WSI devices enables the user to upload slides while others are in the process of being scanned without interruption, thereby vastly improving output, workload, and workflow in high-volume laboratories.[27]
Some vendors may offer alternate configurations for their WSI models. These subsystem arrangements are designed for further homing of efficiency toward achieving laboratory objectives with sample volume, throughput, and workflow requirements in mind. Leica offers two subsystem configurations for its Aperio VERSA model (VERSA 8 and VERSA 200) that, respectively, offer an 8- and 200-slide batch capacity, allowing for the unattended scanning of 8 or 200 slides. Other vendors may offer additional, independently purchasable components that are integrable with their WSI devices. Such components amplify the capabilities of the WSI device that they are linked to. Huron’s TissueSnap, designed to optimize workflow, offloads imaging setup tasks (e.g., preview scanning, verification, batch processing) in order to maximize throughput of its linked WSI device. Multiple TissueSnap preview stations in different locations may be linked to one WSI device for maximal optimization of workflow.[28]
Slide thickness (approximately 1 mm) remains relatively consistent among standard and nonstandard slides. Many WSI devices have coverslip requirements that slightly bolster slide thickness and are often the cause for rescanning due to residual debris present on the coverslip or slide, interfering with appropriate finding of tissue.[29] Inappropriate coverslip thickness may also interfere with objective lens and magnification faculty, particularly when involving objectives with a numerical aperture (NA) of 0.4 or higher, and magnifications of X2 or greater.[30] Digitization capabilities have not yet surpassed the periodic addition of artifact along the pixel pathway to the final WSI destination. Coverslip debris may lead to artifacts causing global out-of-focus areas (“OOF”) that can impact diagnostics and accuracy of image analysis. Some WSI devices, for example, Leica Aperio GT 450, have developed mechanisms for enhanced tissue finding and image composition (the Aperio GT450 presents a 99.5% tissue finding accuracy rate regardless of any coverslip or slide pen marks, dust, and residue that may be present).[29]
In order to mitigate coverslip interference, particularly when handling wet slides (e.g., frozen sections), loading into a horizontal tray rather than a vertical rack is recommended.[2] Some WSI devices, for example, 3D Histech Pannoramic Midi II offer both tray and rack options that are suitable for situations requiring such discernment.
In choosing an appropriate WSI device for departmental use, dimensional considerations are not only relegated to that of the physical slide but also extend to those of the WSI device itself.
Dimensional constraints imposed by the WSI device slide handling capability and WSI device instrument size are of pertinence when evaluating system utility within a department.[2] Spatial constraints, particularly those prevalent in frozen section areas and others offering limited counter space, may be mitigated by smaller capacity portable or desktop WSI devices, for example, Grundium Ocus 40, Motic SL5. Higher-capacity and throughput WSI devices often command greater real estate within the laboratory [Table 1].
Table 1.
WSI device size (dimensions and weights listed in descending order)a
| Large-sized WSI devices | Dimensions (W × D × H) | Weight (kg) |
|---|---|---|
| 3D Histech Pannoramic 1000 | 154 x 100 x 91 cm | 270 |
| Roche Ventana iScan HT | 90 x 70 x 65 cm | 170 |
| Huron TissueScope CF | 60 x 55 x 70 cm | 150 |
| Philips Ultra Fast Scanner (UFS) | 93 x 66 x 59 cm (W x L x H) | 140 |
| Huron NanoZoomer S60 | 69 x 68 x 70 cm (W x L x H) | 79 |
| Huron NanoZoomer S360 | 75 x 69 x 63 cm (W x L x H) | 78 |
| Huron NanoZoomer S210 | 78 x 64 x 58 cm (W x L x H) | 69 |
| Huron TissueScope LE120 | 61 x 89 x 74 cm (W x L x H) | 68 |
| Huron NanoZoomer XR | 100 x 73 x 74 cm (W x L x H) | 67 |
| Leica Aperio GT 450 | 53 x 58 x 50 cm (W x D x H) | 64 |
| Medium-sized WSI devices | Dimensions (W x D x H) | Weight (kg) |
| Leica Aperio AT2 | 41 x 65 x 60 cm | 59 |
| Leica Aperio AT Turbo | 41 x 65 x 60 cm | 59 |
| Leica Aperio VERSA, 200 slide capacity | 68 x 68 x 59 cm | 58 |
| Roche Ventana DP200 | 50 x 68 x 46 cm (W x D x H) | 48 |
| 3D Histech Pannoramic 250 Flash III | 68 x 69 x 55 cm (W x D x H) | 46 |
| Huron TissueScope iQ | 60 x 54 x 43 cm (W x L x H) | 44 |
| Huron Tissuescope LE | 61 x 56 x 40 cm (W x L x H) | 39 |
| Roche Ventana iScan Coreo | 46 x 47 x 51 cm (W x D x H) | 39 |
| Leica Aperio LV1 | 44 x 43 x 54 cm (W x L x H) | 35 |
| Sakura VisionTek/ VisionTek M6 | 41 x 52 x 46 cm (W x D x H) | 35 |
| Motic EasyScan Infinity 100 | 70 x 40 x 42 cm (W x L x H) | 33 |
| Small-sized WSI devices (Model) | Dimensions (W x D x H) | Weight (kg) |
| 3D Histech Pannoramic Scan II | 74 x 53 x 45 cm | 26 |
| Leica Aperio VERSA, 8 slide capacity | 68 x 34 x 59 cm | 26 |
| Leica Aperio CS2 | 32 x 50 x 47 cm | 25 |
| 3D Histech Pannoramic Midi II | 70 x 50 x 50 cm | 23 |
| Huron NanoZoomer SQ | 36 x 45 x 38 cm | 20 |
| MikroScan SL5 | 30 x 36 x 24 cm | 16 |
| Motic EasyScan Pro 6 | 65 x 40 x 42 cm | 16 |
| Huron TissueScope PE | 40 x 46 x 47 cm | 15 |
| Motic EasyScan One | 21 x 40 x 42 cm | 13 |
| 3D Histech Pannoramic Desk II | 38 x 31 x 25 cm | 12 |
| Grundium Ocus/ Ocus 20/ Ocus 40 | 18 x 18 x 19 cm | 3.5 |
| PrimeHisto XE Histology Slide Scanner | 28 x 17 x 08 cm | 2 |
aAll efforts were made to provide accurate data for tables included in this article throughout its composition. Values are approximated and are subject to change. We suggest contacting vendor representation for the most recent, up-to-date confirmation, correction, and/or amendment of any of the following values featured here. Areas left blank are those that we were unable to allocate information for during the time of compilation. All the following information was sourced from vendor-affiliated commercial and informational resources, for example, WSI device user manuals.
As these devices are ideal in departments with higher volumes of consultations and corresponding workload, their high-throughput functionality is often not sacrificed for medium-to-lower throughput options with more compact dimensions. In these instances, spatial floor requirements may be mitigated by high-capacity WSI devices that do not have external PC requirements, for example, Phillips UFS. The heavy weight of some WSI devices (e.g., Pannoramic 1000) may require reinforcing of the floor or table on which they rest. It is of importance that such tables must be free of vibrations that may affect the scanner.
Slides are loaded into holders[20] also known as “racks,”[31]“cartridges,”[6]“cassettes,”[32] or “trays.”[33] Several racks of vertically stacked slides may be present in rotating “carousel” form in high-capacity WSI devices, for example, Leica Aperio GT450 [Table 2].
Table 2.
WSI device slide capacity, dimensional compatibility, loading mechanism, and PC inclusion
| Vendor | WSI device | Slide capacity and compatibility | Loading mechanism | PC includedc |
|---|---|---|---|---|
| 3D Histech | Pannoramic Desk II | 1 single (standard)a or 1 double-wideb | Manual | No |
| Pannoramic Midi II | 12 standard | Automatic | No | |
| Pannoramic Scan II | 150 standard | Automatic/continuous | No | |
| Pannoramic Confocal | 11 standard (0.5–1.5 mm thickness)d | Automatic/continuous | No | |
| Pannoramic 250 Flash III | 300 standard | Automatic/continuous | No | |
| Pannoramic 1000 | 1000 standard or, 200 double-wide | Automatic/ continuous | No | |
| Grundium | Ocus | 1 standard | Manual | Embedded Nvidia visual computer; Touch screen support |
| Ocus 20 | 1 standard | Manual | Embedded Nvidia visual computer; Touch screen support |
|
| Ocus 40 | 1 standard | Manual | Embedded Nvidia visual computer; Touch screen support |
|
| Huron | TissueScope LE120 | 120 standard or, 60 double-wide or, ≤ 10 whole-mount slides up to 150 mm x 200 mm (6′′ x 8′′) | Automatic/ continuous | PC included- integrated touch screen |
| TissueScope iQ | 400 standard or, 200 double-wide (can mix and match standard and double-wide slide cartridges) | Automatic/ continuous | No | |
| TissueScope CF | 12 standard or, 6 double-wide or, Four 3′′ x 4′′ or, Two 4′′ x 5′′ or, One 5′′ x 7′′ or, One 6′′ x 8′′ |
Automatic/ continuous | No | |
| TissueScope LE | 12 standard or, 3 double-wide or, one whole mount up to 6′′ x 8′′ |
Automatic | No | |
| TissueScope PE | 2 standard or, 1 double-wide |
Manual | No | |
| Hamamatsu | NanoZoomer SQ | 1 standard | Manual | No |
| NanoZoomer S60 | 60 standard or, 30 double-wide |
Automatic | No | |
| NanoZoomer S210 | 210 standard | Automatic | No | |
| NanoZoomer XR | 320 standard | Automatic/ continuous | No | |
| NanoZoomer S360 | 360 standard | Automatic/ continuous | No | |
| Mikroscan | MikroScan SL5 | 1 or 2 standard | Manual | No |
| MikroScan SL5 - 20 | 20 standard or, 10 double-widee |
Automatic/ continuous | 4K-resolution monitor included; PC requirement |
|
| Motic | EasyScan Pro 1 and EasyScan Pro 6 |
1 and 6 standard, respectively, Vet Mode (76 x 50 mm slide) optional for EasyScan Pro 6 |
Automatic (6 slide module) | Included: All-in-One 23.8’’ LED Monitor |
| EasyScan One | 1 standard slide | Manual | No | |
| EasyScan Infinity 60 and EasyScan Infinity 100 |
60 and 102 standard, respectively, Vet Mode (76 x 50mm slide) optional in both modules |
Automatic/ continuous | Included: All-in-One 23.8’’ LED Monitor |
|
| Objective Imaging |
Glissando 20SL | 20 standard or 10 “mega,” e.g., double-width | Automatic | Internal mini-PC with Ethernet connection and touchscreen monitor |
| Glissando POL | 2 standard or 1 double-width | Manual; may be retrofitted or included with optional 20 slide autoloader | Integrated Windows 10 mini-PC with Ethernet connection | |
| Glissando Desktop Scanner | 2 standard or 1 double-width | Manual; may be retrofitted or included with optional 20 slide autoloader | Integrated Windows 10 mini-PC with Ethernet connection | |
| Leica | Aperio VERSA | 8 standard, 200 standard with autoloader | From 8 slide stage to 200 slide autoloader | PC included |
| Aperio AT Turbo | 400 standard or 1 double-wide | Automatic (for standard) and manual (for double-wide) | ||
| Aperio AT2 | 400 standard or 1 double-wide slide | Manual: One standard or one double-wide when using the slide tray Automatic: Up to 400 standard when using the AutoLoader |
No | |
| Aperio AT2 DX | 400 standard or 1 double-wide slide or one double-wide when using the slide tray Automatic: Up to 400 standard when using the AutoLoader |
Manual: One standard | Viewing Station included: contains Aperio AT2 DX scanner console software and medical grade monitors with custom ICC color calibration; PC not included | |
| Aperio CS2 | 5 standard or 1 double-wide slide | Manual/Automatic single-slide capacity (five-slide tray standard) | No | |
| Aperio GT 450 | 450 standard slides | Automatic/ continuous | PC included with optional Vendor-Calibrated Viewing Monitors; touch screen | |
| Aperio LV1 | 4 standard | Manual | No | |
| Philips | UFS | 300 standard | Automatic/ continuous | Operated via an integrated LCD touch-screen |
| Roche | Ventana DP200 | 6 standard or, 3 double-wide |
Manual | No |
| Ventana iScan Coreo | 160 standard | Autoloading/continuous | Yes; integrated touchscreen and controller Yes; integrated touchscreen and controller |
|
| Sakura | VisionTek (Plan NeoFluar) | 4 standard | Manual | No |
| VisionTek M6 | 4 standard | Manual | No | |
| OptraScan | Ultra 320 | 320 single; able to accommodate double-wide | Automatic/ continuous | Yes; integrated |
aStandard width: 25.0 to 26.0 mm
b Double-wide width: 51.0 to 52.0 mm
c Noninclusion denotes external PC requirement
dTypical slide thickness (unless mentioned otherwise): 0.90 to 1.2 mm; typical slide length (unless mentioned otherwise): 75.0 to 76.0 mm
eAn upcoming release of the MikroScan SL5 slide holder will support 2′′ x 3′′ slides, allowing 10 2′′ x 3′′ slides or a combination of 1′′ x 3′′ and 2′′ x 3′′ slides to be loaded into the device; 20 standard-slide autoloading functionality; remotely located users have complete control of the autoloader.
slide holder is placed on a horizontal “XY” stage within the WSI device that is robotically driven by miniature motors. Slides are held in place via either mechanical attachment, lateral pressure, or vacuum systems.[13] During the process of image acquisition, the XY stages linearly guide the slides underneath a “Z” stage-bearing focusing optics (i.e., lens objectives) mounted vertically above the XY stage. Cameras rapidly acquire digital snapshots of the slide within their field of view (“FOV”) (typically 1 mm)[2,32,34] as the XY stages move along the planes of their axis to cover the entire slide area. The Z axis also serves to aid in focusing the camera by holding it within a stable position as rapid-fire digital images are acquired [Figure 2].[32]
Figure 2.
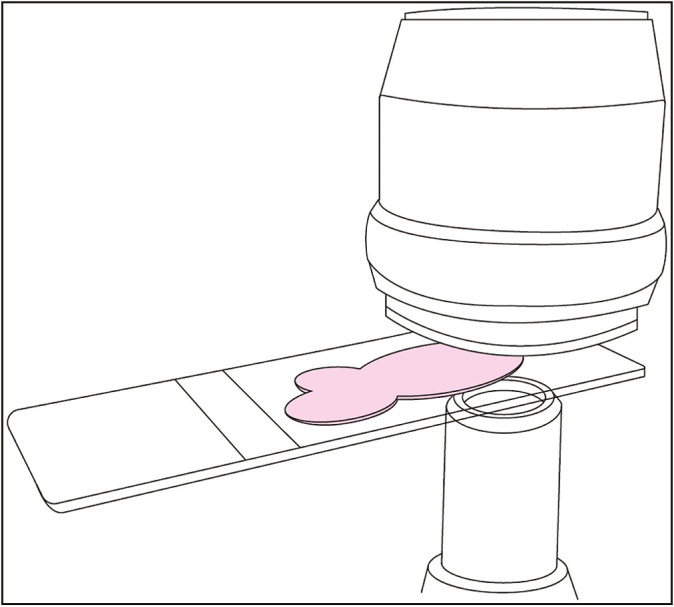
Stage movement and image capture (original illustration, based on 3D Histech Pannoramic 250 Flash III camera and stage). Glass slide with specimen (pink) driven through the WSI scanner via continuous movement of the motorized stage. A lens objective is shown above the glass slide. Continuous stage movement is accompanied by rapid, repetitive image capture via a camera depicted below the glass slide. The 3D Histech Pannoramic 250 Flash III has an image capture rate of 130 frames per second[35]
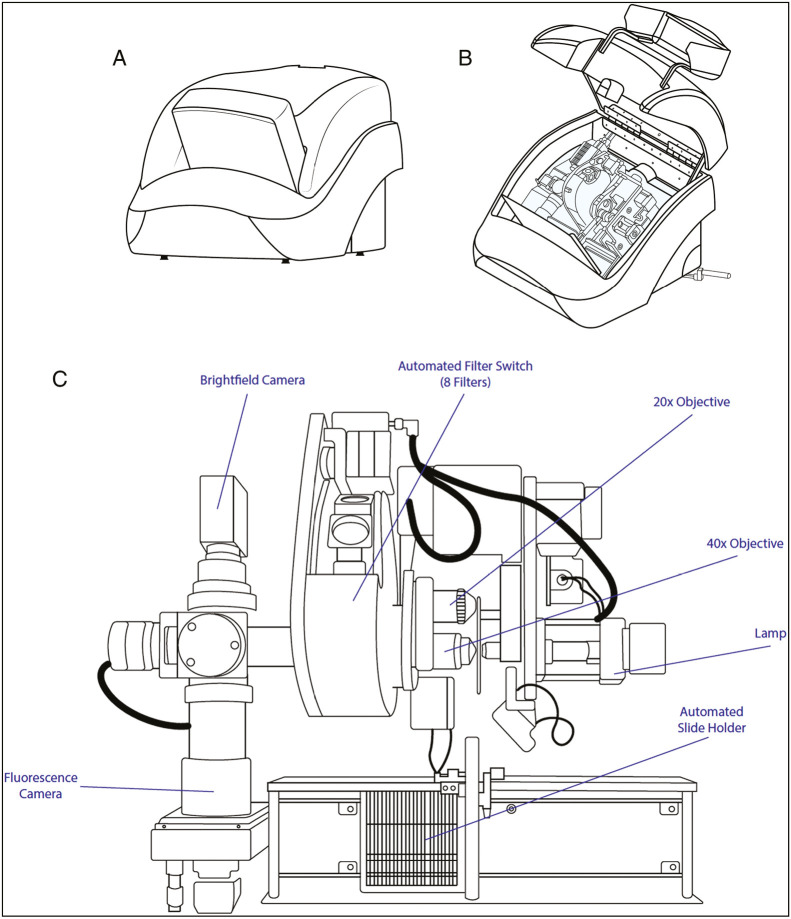
XYZ configurations vary per the unique internal spatial constraints of each WSI device. Camera shape and size, illumination (e.g., brightfield, fluorescent), lens objectives, and slide holders (varying in size due to factors such as total capacity and compatibility with mixed-slide dimensions) are some of several integral components of each WSI device that ultimately dictate the configuration of the custom stages that must maneuver adroitly among such components [Figure 3].
Figure 3.
WSI device exterior and interior (original illustration based on 3D Histech Pannoramic 250 Flash III Configuration). WSI Device Exterior (A) and interior (B, C) components consisting of multiple objectives along with fluorescence and brightfield capabilities (Modified from 3D Histech Pannoramic 250 Flash III).[35]
The WSI models offering configurations compatible with different slide dimensions or objective magnifications benefit from the implementation of custom stages that will allow for such diversity in functionality. An appropriately implemented custom XYZ stage may act to enhance imaging functionality, for example, implementation of a newly developed XYZ stage in the 3D Histech Pannoramic line of scanners conferred the devices with an extended focal range.[36] Stages of the modern era allow for the automatic, dynamic positioning of a wide range of tissue specimens, thereby acting to not only assist but also enhance the image capture of such specimens.
IMAGE QUALITY AND RESOLUTION
Overview of whole slide imaging system components influencing image resolution
The inherently subjective nature of digital image “quality” is often explicated by “resolution,” a metric not entirely separated from its innate arbitrariness[37] due to the many components[38,39] of the WSI system that ultimately influences its outcome.[40] In parsing the often nebulous, yet coveted ideal of image quality, it is important to note the complex interplay between objective lens magnification, sensor pixel size, and the viewing monitor that will ultimately be used to display the final image.[17,41,42]
“Microns per pixel” is a direct corollary of glass slide digitization quality as it bears a direct relation to optical magnification and pixel size (increasing with greater optical magnification and smaller pixel size), which is the standard metric of classifying resolution.[42]
In assessing the resolution of a WSI device, one must consider the entire WSI system. Magnification does not equate to resolution, nor is optical resolution interchangeable for digital resolution.[42] The NA of the objective lens along with light wavelength are the primary arbiters of optical resolution in purely analog settings.[42,43,44,45] Digital resolution, however, comprises the NA of the objective lens, objective magnification, digital camera sensor and pixel size, and pixel density of the viewing monitor (e.g., pixels per inch/ pixels per centimeter).[41,42,44]
Resolution determines the extent to which the smallest object can be resolved. Magnification indicates the size of the resolved object.[46] The minimum distance at which two distinct objects can be identified as separate events and the smallest level of detail discernible within that area encapsulates WSI resolution and forms the basis of its evaluation.[44,47,48,49]
“Gears” driving the WSI machine along the pixel pathway, including objective aperture(s), camera sensors, and viewing stations, for example, monitors are capable of being assessed for their resolute robustness within the parameters of this definition, are quintessential factors of digital image resolution and should be of consideration when choosing a WSI device. Objective lenses with NAs that are capable of high resolving power will be stifled by sensors that have lower resolution than their objective’s NA. A low-quality sensor will yield an improper acquisition of digital image data that will ultimately be unsalvageable by any other mechanism or component along the pixel pathway, for example, a high-resolution monitor is not capable of compensating for digital information lost, at the inception of its capture, by a low-resolution camera. Camera sensor resolution is hitherto a particularly vital determinant of final digital image resolution, though it does not dictate image quality without the joint influence of optical magnification and viewing station resolution.[44]
Cameras, sensors, and connectivity
The WSI device cameras demagnify captured images onto an image plane where charge-coupled devices (CCD), Complementary Metal Oxide Semiconductor (CMOS), or “scientific” CMOS (sCMOS) image sensors measure light energy to convert the measured information into a digital signal.[34,50,51]
Some vendors, for example, 3D Histech, have incorporated newer sCMOS sensors (first released to the public in 2009) into modern WSI device models. CCD, CMOS, and sCMOS sensors each present unique advantages and shortcomings.[52,53,54,55,56]
Scientific CMOS sensors have offered utility in DP through unique specifications delivering superior sensitivity, resolution, field-of-view, and frame rates.[20] However, sCMOS sensors have been demonstrated to induce extra readout, for example, the time required to digitize a single pixel (pixels/s), and pattern noise, that is, random, incongruent color and brightness variation when compared with older yet robust CCD sensors. These shortcomings in sCMOS capability have exhibited increases in artifact production, poorer imaging ability, and hindrance of fluorescent signaling. However, a litany of positive attributes and associated pitfalls are present within all sensor formats; therefore, modern WSI devices often utilize combinations of CCD, CMOS, and sCMOS cameras in unison to facilitate optimal digital slide creation (e.g., overview and tissue finding capabilities) and workflow organization (e.g., barcode scanning).[57,58,59]
For example, the Point-Grey “Grasshopper 3” camera line used in several WSI systems combines high-resolution CCD and CMOS sensors for optimal brightfield and fluorescent imaging.
Vendors may refine calibration or derive mechanisms, for example, enhanced illumination techniques, to take advantage of capabilities unique to sensors such as sCMOS while mitigating the drawbacks typically associated with such a modus,[60] for example, 3D Histech Pannoramic Confocal.[61,62]
The rapid advancement of technology has enabled sensor manufacturers to engineer solutions that mitigate many of the initial drawbacks present on the initial release of sCMOS technology while in tandem buttressing the capabilities of CCD and CMOS sensors. Innovative evolution in sensor capabilities has quelled debate concerning advantages and disadvantages of sensor modalities when selecting a WSI device. In evaluating WSI system functionality and output, it is of importance to understand the role of these components on the system in its entirety rather than to evaluate the system as a projection of these components.
Sensors drive an analog-to-digital conversion of photons that have been gathered through rapid optical (camera lens) capture of the physical slide specimen. The formed digital signal is then transmitted to a processing unit, that is, computer (after wavelength discrimination of photons via a filter), where a virtual slide image is generated.[63] Shorter wavelengths correlate with higher resolving power to elicit detail, for example, resolution, and vice versa.[42,64] Most sensors designed for brightfield illumination utilize blue, green, and red lights (respectively, ordered from shortest to longest wavelength) controlled and corrected for via an automated filter switch to enhance specimen resolving ability [Table 3].[45]
Table 3.
Assorted WSI cameras and sensors
| Vendor | WSI device model | Scanning camera resolution as depicted by megapixels (MP), sensor size (′′), and pixel size (height x width) and/or illumination |
|---|---|---|
| 3D Histech | Pannoramic Desk II | Brightfield: 5 MP 12-bit camera with RGB illumination |
| Pannoramic MIDI II | Brightfield: CMOS 5 MP 12 bit OR sCMOS 4.2 MP 16-bit camera with RGB illumination Fluorescent: 5 MP 12 bit OR 4.2 MP 16-bit camera with wideband/6 channel LED Sensor size: 1/2′′ 2056 x 1544 |
|
| Pannoramic Scan II | Brightfield: CMOS 5 MP 12 bit OR sCMOS 4.2 MP 16-bit camera with RGB illumination Fluorescent: 5 MP 12 bit OR 4.2 MP 16-bit camera with wideband/6 channel LED (2048 x 2048) |
|
| Pannoramic Confocal | Brightfield: sCMOS 5.5 MP 16-bit camera with RGB illumination Fluorescent: 5.5 MP 16-bit camera with 6 channel LED (2560 x 2160) |
|
| Pannoramic 250 Flash III |
Brightfield: CMOS 12 MP 12-bit camera with Xenon Flash illumination Fluorescent: 4.2 MP sCMOS 16-bit camera with 6 channel LED Lumencor SPECTRA solid state light engine; (2048 x 2048) |
|
| Pannoramic 1000 | Brightfield: sCMOS (12mp sensor) 12 MP 12-bit camera with Xenon Flash illumination (4096 x 3072) | |
| Grundium | Ocus | 6 MP (image sensor) |
| Ocus 20 | 12 MP (image sensor) | |
| Ocus 40 | 12 MP (image sensor) | |
| Mikroscan | MikroScan SL5 | 1920 x 1080 |
| Motic | EasyScan Pro 6 | 5 MP CCD (2/3-inch sensor) with 10W LED |
| EasyScan One | 5 MP CCD (2/3-inch sensor) with 10W LED | |
| EasyScan Infinity 100 | 5 MP (2/3-inch sensor) with 10W LED (CCD sensor) Three Camera System: 2/3-inch CCD sensor, 5MP; 10W LED; 2448 x 2048, 3.45 x 3.45μm/pixel, USB3 connectivity, 15fps |
|
| Objective | Glissando 20SL | 2048 x 2048 |
| Imaging | (medium capacity) | |
| Glissando POL | 2048 x 2048 | |
| Glissando Desktop Scanner |
2048 x 2048 | |
| Leica | Aperio VERSA | ANDOR Zyla 5.5 Monochrome camera for fluorescent scanning: USB3, CMOS, 5.5 MP; High sensitivity, high quantum efficiency: 6.5um pixels, (2560 x 2160), 40fps Point Grey Grasshopper 3 Color Bayer Camera for brightfield scanning: USB 3, CMOS, 4.1 MP, 5.5um pixels, (2048 x 2048), 90fps |
| Aperio GT 450 | 4k Trilinear camera; White LED illumination | |
| Aperio LV1 | 12 MP color digital camera Display update rate: 15 fps High-power LED illumination |
fps = frames per second/frame rate
Scanning cameras are connected via a camera “bus” that transfers image data from the sensor to the digital image processing unit.[65] Connection bus methods commonly utilized in past WSI devices, for example, IEEE 1394/1394b (FireWire/ FireWire 800) and USB 2.0, have been predominantly replaced by the widely adopted advanced transmission capabilities of USB 3.0 (first made public in 2010 demonstrating superior data transfer rates of up to 5 gigabits per second/640 megabytes per second, 10 times faster than that of its USB 2.0 predecessor). We may see incorporation of the most recent (though not currently market available) iteration of USB specifications into WSI devices of the near future (USB 4.0, released in 2019). USB 4.0, which touts transfer speeds of approximately 40,960 megabytes per second (≥40 gigabits per second), out clocks the 800 megabyte per second transfer rate of its FireWire predecessor, which, though once commonplace as a WSI system camera interface, is now quickly becoming obsolete.[13,66]
Currently, “Camera Link” is the sensor interface offering the highest rate of data transfer, for example, throughput, of all camera bus modalities used in WSI devices. Cameras utilizing “line” scanning techniques of digital image creation, a method requiring large bandwidth volume and particularly intensive synchronization requirements, may use a camera link bus in order to effectively transfer data. Cameras with higher “bit” depth, frame rate, and resolution (factors predisposing intricate, detailed image composition) also require consideration of appropriate camera bus interface, as these factors necessitate the transfer of greater levels of information, for example, pco.edge 4.2 sCMOS camera featured in current 3D Histech Vendor lineup (16 bit, high frame rate, increased exposure time) is interfaced via Camera Link.
Gigabyte ethernet (“GigE”) port connectivity offers similar bandwidth (approximately 100 MB/s) to that of IEEE 1394b and is included in some WSI device offerings from vendors such as Grundium, Huron, Roche, Sakura, and 3D Histech.[67] Several WSI devices are compatible with image viewing software, allowing the accessibility of stored data from a remote location via internet or intranet connection (e.g., NDP.view2 for Hamamatsu’s Nanozoomer series). Other devices (e.g., Leica Aperio LV1, MikroScan SL5, Grundium Ocus series) allow remote users full control of the instrument, including the ability to change magnification, switch between slides, and adjust fine focus. This remote, directly operable functionality, is executed through a strong Local Area Network (LAN) with internet access.[68]
The Grundium Ocus series connects to the network via 801.11ac Wi-Fi or 1GigE ethernet and is accessible anywhere through a secure internet connection. Aided by the assistance of a technician who operates the device locally (e.g., places slides into the scanner), a pathologist may then log into the Ocus from a remote location, control the device, view the slide, and produce a report that may be delivered to a surgeon. Multiple pathologists may access the same WSI device from different locations by using the same connectivity. In countries where devices such as the Ocus are approved for clinical diagnostics, the remote capability of these tools has been noted to relieve the number of intraoperative consultations in hospitals with no on-site pathologists as well as in prevention of two-stage surgeries and patient transfers.[69] Scanning camera resolution is indicated by the size of the digital image the camera produces, often interpreted by multiplying the vertical by horizontal (height x width) pixel area denoting the number (millions) of pixels in a single image, that is, megapixels (MP).
Larger camera sensor sizes confer larger FOV and greater camera resolution by utilizing less magnification to achieve the former and accomplish the latter. Most WSI camera sensors range from ½ to ¾ inches in size, as this range confers optimal balance of virtual image magnification, size, and resolution. The FOV latitude is a pertinent factor when considering image quality output. All sensors, regardless of size, will capture a smaller, magnified, cropped rectangular field of view centrally superimposed over the larger circular FOV provided from the vantage point of the lens objective (akin to the view seen from microscope eyepieces) due to the comparatively smaller dimensions of the sensor. Smaller sensors confer a smaller FOV and a greater degree of magnification than larger sensors, with the latter consequently demonstrating a more robust platform for appropriate image capture quality.
Discernable FOV, that is, the predominantly intact area in which resolution is unmarred by optical aberrations and factors predisposes to a reduction of image quality. Progressive obfuscation of image quality begins toward the periphery of the specified FOV area and increases beyond its boundaries. This area of obfuscation is still observable to the viewer, though with markedly less clarity as the area within the FOV boundaries. Although field size specifications are a relatively good indicator of image quality within specified FOV parameters, they are not an absolute indicator of image scope, one that extends beyond these parameters.
As camera sensors of all sizes inherently confer varying degrees of magnification and restricted (less than 100%) FOV when compared with the circular (maximal) objective lens view, particularly in the case of smaller sensors, a demagnification process must occur to enhance the sensor’s digital measuring capability if a larger FOV is desired.
Demagnification is executed via “C-mount” camera adapters equipped with reduction lenses that adjust the FOV displayed on the viewing monitor, thereby enhancing digital measuring capabilities. C-mount lens application to a camera sensor of appropriately corresponding dimensions will maximize FOV, thereby allowing the image captured by the sensor to closely match that seen from the objective lens (the magnification of which will also influence FOV). The 1 x C-mount adapters contain no reduction lens and produce a highly cropped (approximately 10% of maximal FOV) image as, in this instance, camera sensor magnification is left unmitigated [Figure 4].
Figure 4.
Field of view (original illustration). (A) Maximal field of view elicited by the lens objective. (B) Superimposed sensor FOV. (C) FOV demarking image area seen by viewer
The WSI devices that include C-mount adapters often include an assortment of ports suited to their unique hardware specifications. C-mount adapter compatibility is vendor-specific, that is, engineered to accommodate camera manufacturer specifications that often vary and are therefore not interchangeable with those of differing brands. C-mount replacements are readily available online and per associated WSI vendor and can be acquired with relative ease at a comparatively less cost than other WSI system components [Table 3].
Objective lenses and magnification
Objective lenses, among the primary pillars of resolution along the pixel pathway, present with many standard iterations of magnification that have unique forte in specific departmental applications. An objective magnification of x20 demonstrates strength in routine viewing of surgical pathology and immunohistochemistry slides, including those stained with hematoxylin and eosin (H&E).[44] An objective magnification of x40 demonstrates a comparatively higher diagnostic accuracy with cytology slide imaging and the digitization of in situ hybridization slides (requiring effective resolving capability between points less than 0.5 µm).[44]
Digital WSI vendors will often incorporate one or more analog objective lenses, each designed for a specific linear magnification power (e.g., ×20, ×40), within any of their WSI models. Further focusing and magnification via an objective lens may occur virtually using WSI software. These objectives may be classified according to their lens configurations, which fall under three distinct categories: Plan Achromat (Plan “Achro”), Plan Fluorite (Plan “Fl,” “Fluor,” “Fluar”), and Plan Apochromat (Plan “Apo”) [Table 4].
Table 4.
Resolution modified by C-mount
| Point Grey Grasshopper 3 (GS3-U3-51S5M-C) Global Shutter Type CMOS (Sony IMX250)a |
|---|
| Pixel size: 3.45μm × 3.45μm |
| Pixel resolution with 20x objective and 0.63x C-mount adapter: 0.27μm |
| Pixel resolution with 20x objective and 1x C-mount adapter: 0.17μm |
aScanning camera featured in Pannoramic Midi II (3D Histech). Camera interfaced (“bussed”) with USB 3.0 Base camera resolution (pixel size) with 20x objective corrected by 0.63x and 1x C-mount adapter
Objective lenses may differ in composition pertaining to the type of glass material used in their construction, the amount of material used, and the shape of the lens as well as any coating(s) applied to it. Differences in lens objective construction result in differences in light transmission efficiency and the degree of light ray correction, for example, how light rays are ultimately displayed on an image. Regardless of the type of objective used, its function is to magnify and resolve the image of the specimen under examination. In choosing an objective, consideration of sample preparation and properties to be examined as well as objective types, properties, and their varying corrections are essential.[46]
The NA of the objective and illuminating light wavelength directly correlate to the resolving power of the objective, that is, image resolution at this stage of the scanning process.[45]
A wider objective opening angle will allow more diffracted light from the image to be captured, thereby allowing for smaller details to be resolved. Objectives with higher NA are best suited for fluorescent applications, as fluorescent brightness increases with an increasing NA that serves to capture larger cones of light emission through greater illumination of the specimen. Higher NA is also correlated with a smaller depth of field (not to be confused with depth of focus, or image depth, which increases with decreasing depth of field and increasing objective magnification).[70]
As objective lenses are curved, their shape inherently predisposes to the creation of a curved image plane. As an ideal image plane is one that is flat, the curved nature of the objective lens is compensated for via a steeply curved lens surface on its posterior aspect, and via a concave meniscus on its anterior. Objectives with this type of correction, one that corrects for field flatness for optimal specimen viewing, are designated by the prefix “plan.”
Objectives also naturally present with color artifacts, that is, chromatic aberrations or color “fringes.” Objectives that have been color corrected via the combination of unique glasses, each holding different color refraction properties, are followed by the suffix “apochromat.” Historically, apochromatic lenses would correct for three to four spectral lines.[71] Some modern WSI models include apochromatic lenses correcting for up to 14 spectral lines, that is, fully color corrected, for example, Carl Zeiss apochromatic.
Plan-apochromatic objective lenses are often the costliest; however, they offer the greatest correction for chromatic (i.e., color) and spherical (i.e., lens shape) aberrations.
Fluorite (“fluar,” “fluotar,” “neofluar”) or semi-apochromatic objectives are typically chromatically corrected for red and blue and spherically corrected for blue and green. The fluorite elements in these lenses make them excellent candidates for applications requiring polarization.[72] The incorporation of these elements into this objective design also confers advantages in fluorescent and confocal applications.[3,43,73]
Plan-apochromatic objectives are most commonly seen in modern WSI devices, followed by fluorite objectives. Both have far higher degrees of correction than basic achromatic objectives, which are seldom used in WSI scanning.
Objectives designed with fluid immersion capabilities increase optical resolving power, thereby producing higher resolution images for mediums that benefit from such techniques. Immersion techniques prevent light deflection occurring in the air-filled area between the front lens of the objective and coverslip, causing light to bend or refract. As a result of this refraction, scattered rays of light are lost to the image as they are not directed through the objective lens. Immersion techniques replace the air gap with a fluid, for example, oil, allowing the light to travel through the new medium and into the objective instead of dissipating into air. As this once-diffracted light can now be collected and homed into the objective, the resolving power is increased, thus yielding higher resolution, for example, higher NA. The refractive index of the imaging medium between the objective lens and coverglass specimen is used to determine NA, with air delineating a refractive index of 1.0. Most dry objectives do not exceed an NA of 0.95; however, immersion objectives are able to overcome the air medium, often achieving refractive indexes of ≥1 (closely matching that of biological tissue), for example, C-Apochromat 1.2 NA oil immersion lens (3D Histech Pannoramic 1000).
High NA is also advantageous in confocal applications. The Pannoramic Confocal uses an “LD C”-Apochromat objective (long-distance, confocal) with an NA of 1.1 to achieve enhanced penetration through the use of infrared light.[74]
Specimens requiring oil immersion are often found in hematopathology (blood or bone marrow smears) and microbiology settings (e.g., gram stain).[2,75,76]
Water immersion has been demonstrated to markedly improve both optical sectioning and contrast in surgical samples obtained from Mohs surgery for basal cell skin carcinoma and from breast cancer lumpectomy through the enhancement of incident angle afforded by such techniques.[77]
Water immersion objectives also allow for safe scanning of glass slides without coverslip.
Though water immersion objectives are incompatible in most currently offered WSI devices, certain devices, for example, 3D Histech Pannoramic Confocal, are engineered for such options (this particular model is compatible with a C-Apochromatic water-immersion lens objective). The Pannoramic 1000 model from the same vendor offers a soon-to-be-released upgrade that will support this modality in the immediate future.
It is of importance to note that immersion mediums are not interchangeable for one another (e.g., water for air or vice-versa), nor are they substituted for mediums that may appear to have similar properties (e.g., low-viscosity immersion media such as anisol instead of appropriate immersion oil). Unsuitable immersion medium will cause a deviation in refraction and light dispersion, causing image aberration.[78] It is also important to note that attempting to use immersion oil with “dry” objectives, for example, those not designed for oil immersion microscopy, will result in damage to the lens.
“Infinity” optical design, that is, infinity conjugate (indicated by “ICS/UCS” marking on objective), first spearheaded by Reichert in the 1930s and then later adopted by microscopy specialist manufacturers such as Zeiss and Leica, is now widely used in mainstream microscopy and applied to digital WSI.[79] Infinite optical design takes advantage of light sourced from a point placed at infinity (e.g., camera sensor), rather than a fixed image distance (as seen in “finite” conjugate design used in basic microscopy), which is then focused to a specific position, for example, the area under inspection (slide specimen) by the lens objective.[80,81] Lens objectives produce parallel light paths that are then brought into focus at an intermediate plane via a tube lens (e.g., c-mount extension) between the objective and eyepiece. This technique allows for auxiliary components such as illumination (e.g., polarizers) and filters to be added to the parallel optic light pathways passing through the objective without significantly compromising focus and allowing for variations in magnification power.[82,83]
Some vendors have developed proprietary mechanisms to improve image clarity and contrast through the modification of lens objectives in WSI systems utilizing infinity conjugate design. Motic offers a Color Corrected Infinity Optics CCIS ® design that mitigates color fringing effects commonly manifested in traditional infinity optics systems through the employment of multiple layers of objective lens coatings. The resulting digital image is one that affords a similar expression of lucidity synonymous to that seen through the eyepieces of an analog microscope.
Despite outward similarities, minor lens specification discrepancies exist among brands, particularly those involving tube length. The same prudence exercised when handling medium-specific immersion objectives must be applied to that of infinity-corrected lenses from different manufacturers, all of which should be considered specific to their associated WSI device and are currently not interchangeable with lenses from another manufacturer.
Other vendors have approached achieving optimal image sharpness by employing digital, rather than physical, modifications toward enhancing color correction. Grundium employs a “color stacking” technique through which individual, full-resolution images of each color layer (red, green, blue, e.g., “RGB”) are automatically layered together to compose an image with three times more pixels, rendering greater levels of detail when compared with that of conventional techniques employing RGB imaging.
Image capture through digital microscopy via the use of a particular objective and its magnification is completed in a distinct fashion as compared with that of analog imaging. As soon as an image is scanned and uploaded through the use of a camera and objective lens, it can be magnified further using the power of software imaging techniques. Image capture resolution may be demonstrated via enhanced software magnification by using the initial image captured through an objective lens as a basis for which to further digitally magnify.
Imaging illumination
Brightfield and fluorescent scanning are the two primary techniques of illumination offered by WSI vendors. Some WSI models offer illumination mechanisms to either buttress the capabilities of these standard illumination functions, for example, confocal imaging capacity offering superior depth resolution (in comparison to wide-field fluorescence imaging)[84] featured in the 3D Histech Pannoramic Confocal, or adjunctive illumination for enhanced scanning capability, for example, polarized light application in the Objective Imaging Glissando POL.
Tungsten-halogen lamps have traditionally been used in brightfield applications to illuminate specimens of interest.[85,86] Tungsten-filament bulbs have been progressively replaced by the use of white-light emitting diodes (LEDs), ensuring a consistent color balance at any light level in modern WSI devices.[87]
Fluorescent applications that require specimens to be illuminated with wavelengths below 400 nanometers (within the ultraviolet spectrum extending beyond the range of tungsten–halogen illumination) that traditionally have made use of xenon arc lamps, mercury vapor lamps, and lasers. The WSI devices with fluorescent imaging capabilities are also increasingly employing comparatively advanced LED illumination mechanisms featuring wider bandwidth (allowing for the excitation of a multitude of fluorescent probes), quicker selection of specific wavelengths, reduced heat emission, and more compact design.[71,87,88]
Digital display
As the display station, for example, monitor, is the final destination of the WSI pixel pathway, its resolute caliber plays a substantial role in final image interpretation. Larger monitors and those with higher pixel resolution result in larger FOVs conducive to easier navigation and faster identification of salient regions of interest (ROI) within an image. Observer position (e.g., distance from monitor, posture) is an additional influential factor toward the effective and accurate interpretation of digital imagery.[42]
The American Telemedicine Association recommends viewing monitors to be color calibrated, suggesting use of the MacBeth color chart.[89,90]
Color-calibrated images are generally preferred by pathologists and have been suggested to improve diagnostic confidence and speed.[20,91]
Color variation and the mechanisms by which modern WSI vendors have attempted to achieve a basis for its standardization in image viewing are as tantamount to image quality as resolution. Specimen thickness, staining, WSI device, viewer, and display all affect color variation.[92]
The digital display, a hardware component (monitor) through which an observer interprets a WSI, is distinct from that of a WSI “viewer,” a web-based server component of WSI.[93] The FDA currently restricts FDA-cleared WSI systems to using only medical-grade (MG) displays.[91]
Currently offered displays, regardless of their classification (MG, consumer, and professional), are associated with a litany of descriptive metrics by which their parameters may be assessed. This assessment is often technically cumbersome and appears as esoteric to the average pathologist and consumer.[91]
It has been suggested that when seeking to interpret a multitude of display parameters, luminance, contrast, color accuracy, resolution, and “just noticeable difference”[94] are of particular importance in selecting an appropriate monitor for WSI viewing.[91]
SCANNING AND FOCUS METHODS
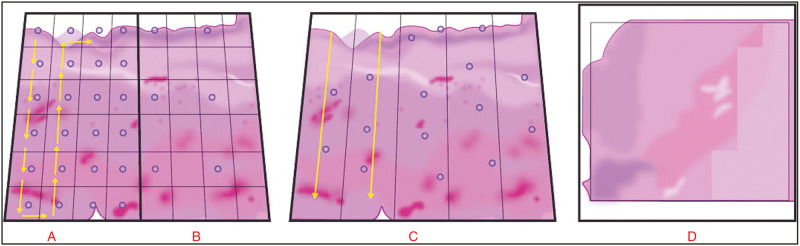
The WSI systems acquire images from physical slides via “tile” (e.g., area) and “line” scanning methods. Both approaches result in the creation of a single WSI from the combination of either square images, for example, “tiles” or image strips, for example, “lines” first captured by the camera sensor and then assembled (“stitched”) via software [Figure 5].
Figure 5.
Scanning methods: line vs. tile (original illustration). (A)Tile/area scanning utilizing focus points in every field. (B) Tile/ area scanning utilizing focus in every “nth” field”. (C) Line scanning utilizing “focus map” technique in which focus points dictate direction of scanning (indicated by yellow arrows). (D) Image “stitching” after digital data acquisition
Historically, proponents of the line-scanning approach have noted fewer optical aberrations due to comparatively less generated seams required for stitching.[11] Advances in technology have created a smaller to negligible disparity in the virtual image quality achieved from the two methods.[11] These advances include improvements in robotic stage technology, resulting in increasing stage accuracy and enhanced line, tile, and stitching techniques, some of which may be vendor proprietary, for example, the United States patent held by Grundium for novel enhanced image stitching.[95]
Certain WSI vendors employ enhanced tile and line scanning techniques, for example, TDI line scanning (Hamamatsu, Phillips, Roche, Leica) and area scanning (3D Histech) in their current WSI models.
TDI, that is, “time, delay, and integration,” line scanning employs sensors (e.g., CMOS) that utilize multiple line scan stages to allow for faster imaging speeds than standard line scanning techniques. TDI line scanning is less dependent on illumination (e.g., LED) effort while achieving high speeds and sensitivity in scanning capability.[80] Past WSI devices intended for fluorescence imaging have preferentially utilized tile scanning instead of line scanning techniques due to the comparatively poor image quality elicited by the latter.[96] The multiple stages of exposure captured by TDI line scan cameras have facilitated the generation of clear images for fluorescence applications, with modern WSI devices now capable of using either tile or line scanning techniques for such purposes.
Autofocusing is a feature in many modern WSI devices.[97] In tile scanning, focus points can be placed on every tile, although this process takes the most time. They may also be placed on every “nth” tile, reducing scan time. Focus maps, automatic refocusing, and automatic tissue recognition features are utilized by many modern WSI devices to create high-quality scans.
“Z-stack” or layered scanning along the Z-axis plane has become an increasingly desirable feature of WSI devices tasked to evaluate cytology smears. The WSI devices with Z-stacking capability optimize image capture through enhancement and broadening of focus. This is executed through multiplanar scanning of the physical slide at various focal planes along the vertical (Z)- axis and “stacking” these images to form a detailed, digitized composite [Figure 6].[23] Current WSI offerings are capable of Z-stack scanning of up to 30 layers, for example, 3D Histech Pannoramic 250 Flash III. The unique, three-dimensional aspect of cervicovaginal cytology smears and the thick nature of others pose challenges that are seldom evident when scanning surgical pathology slides. Unlike surgical pathology slides, which can be captured effectively over a narrow focal range, virtual cytopathology slide quality has demonstrated comparative inferiority when imaged within this range.[22]
Figure 6.
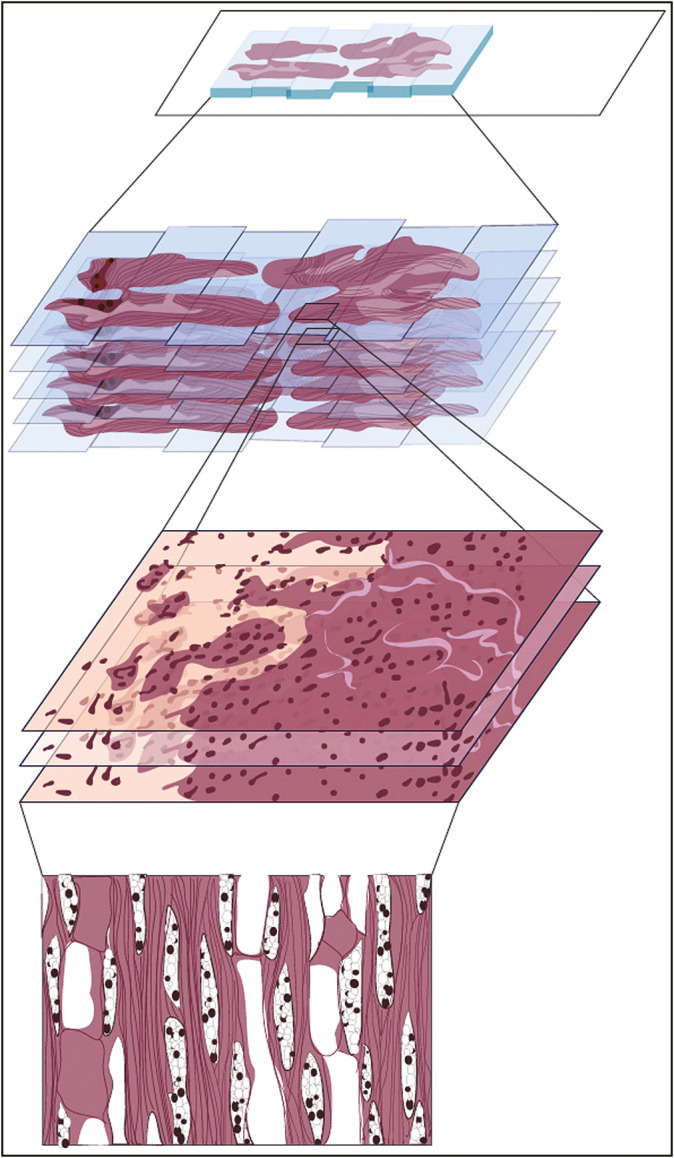
“Z-Stack/ extended depth of field” (original illustration). “Z-stacking,” a method of scanning by which a series of images are captured at various focal planes, i.e., “slices,” that are then combined to most effectively portray samples with 3D structures, such as clumps of cells or thick tissue. Some WSI scanning methods are built upon the Z-stack technique, e.g., “Extended Depth of Field (EDF),” or extended focus, which further enhances Z-stacking by combining the sharpest points of focus from each slice to maximize the depth of sharpness in the final image, demonstrated to be useful in cytology applications.
Some vendors may utilize proprietary methods of scanning or focusing, for example, Grundium, Hologic, Hamamatsu, Hologic. Leica has incorporated a proprietary “Real-Time Focusing (RTF)” method into their latest WSI models, for example, Aperio GT450, a method that combines an imaging line sensor and a focusing line sensor to enhance automatic tissue finding. Roche uses a 3rd-party patented “dynamic focus” system in their Ventana DP200 that utilizes line scanning methods to deliver enhanced z-stack functionality and high-resolution imaging for smaller depths of focus.[98]
Others may incorporate enhanced methods of Z-stacking into their WSI device offerings, for example, “Extended Focus,” in which the sharpest image from each focus, that is, “Z” level, of each image field is selected and combined into one single image [Figure 7].
Figure 7.
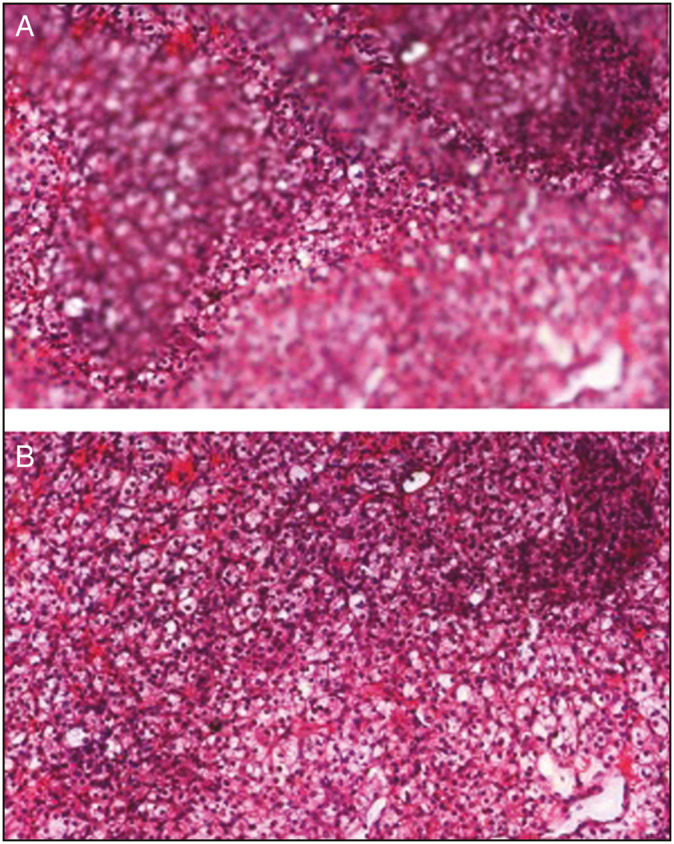
Extended focus (figure is not an original illustration and has been sourced from a 3D Histech company presentation). (A and B) Application of extended focus courtesy of 3D Histech
Such a technique acts to amend imperfections and provide clarity through dispersion of a maximum depth of sharpness evenly throughout an image, though at the cost of overall reduction in digitization speed. This is also true for Z-stack technique and any enhanced focus method.
Nearly every component of the scanning process coalesces to ultimately determine the speed at which images are scanned and the throughput of the WSI device. These factors are frequently altered by the user who may choose to make use of settings most optimal toward achieving a particular scanning objective. Objective magnification and lens type, area size to be scanned, scanning and focusing method, image capture, processing, and data collection and transfer are some of the multitudes of factors affecting the ultimate scan speed and throughput of a WSI device [Tables 5 and 6].
Table 5.
Objective lenses and resolution
| Vendor | WSI device model | Objective type/numerical aperture (NA) | Image capture magnification (per objective lens) | Image resolution (μm/ pixel) (per image capture magnification) |
|---|---|---|---|---|
| 3D Histech | Pannoramic Desk II | 20x (NA 0.8) or 40x (NA 0.95) | 58x | 0.172 |
| Pannoramic MIDI II | 20x (NA 0.8) or 40x (NA 0.95) | 52x or 110x/31x or 62x | 0.172 or 0.087/0.325 or 0.162 | |
| Pannoramic Scan II | 20x (NA 0.8) or 40x (NA 0.95) | 52x or 110x/31x or 62x | 0.172 or 0.087/0.325 or 0.162 | |
| Pannoramic Confocal |
20x (NA 0.8) or 40x (NA 1.2) | 31x/62x | 0.325/0.162 | |
| Pannoramic 250 Flash III |
20x (NA 0.8) or 40x (NA 0.95) | 41x/82x | 0.242/0.121 | |
| Pannoramic 1000 | 20x (NA 0.8) or 40x (NA 0.95) | 41x/82x | 0.25/0.12 | |
| Grundium | Ocus | 20x (NA 0.4) | 0.48 | |
| Ocus 20 | 20x (NA 0.4) | 20x | 0.5 | |
| Ocus 40 | 40x (NA 0.75)a | 40x | 0.25 | |
| Huron | TissueScope LE120 |
20x (NA 0.75) | 20x/40x | 0.4/0.2 |
| TissueScope iQ | 20x (NA 0.75) | 20x/40x | 0.4/0.2 | |
| TissueScope CF | 20x (NA 0.6) | 1x/2.5x/5x/10x/20x/40x | 10.0/5.0/2.0/1.0/.50/.25 | |
| Tissuescope LE | 20x (NA 0.75) | 20x/40x | 0.4/0.2 | |
| TissueScope PE | 20x (NA 0.75) | 1x/2.5x/5x /10x/20x/40x | 10.0/5.0/2.0/1.0/.50/.25 | |
| Hamamatsu | NanoZoomer SQ | 20x (NA 0.75) | 20x/40x | 0.46/0.23 |
| NanoZoomer S60 | 20x (NA 0.75) | 20x/40x | 0.46/0.23 | |
| NanoZoomer S210 | 20x (NA 0.75) | 20x/40x | 0.46/0.23 | |
| NanoZoomer XR | 20x (NA 0.75) | 20x/40x | 0.46/0.23 | |
| NanoZoomer S360 | 20x (NA 0.75) | 20x/40x | 0.46/0.23 | |
| Mikroscan | MikroScan SL5 | 2x (NA 0.06), 4x (NA 0.10), 10x (NA 0.25), 20x (NA 0.50), and 40x (NA 0.65) | 2x/4x/10x/20x/40x | 4.54/2.27/0.908/0.454/0.227 |
| Motic | EasyScan Pro 6 | 10x (NA 0.3), 20x (0.75), 40x (0.75) | 20x/40x/80x | 0.52/0.26/0.13 |
| EasyScan One | 10x (NA 0.3), 20x (NA 0.75), 40x (NA 0.75) | 20x/40x/80x | 0.52/0.26/0.13 | |
| EasyScan Infinity 100 | 10x (NA 0.3), 20X (0.75), 40x (0.75) | 20x/40x/80x | 0.52/0.26/0.13 | |
| Objective Imaging |
Glissando 20SL (medium capacity) 0.75) | Single lens 20x (NA 0.75), 40x (NA | 20x/40x | 0.55/0.275 |
| Glissando POL | Single-plan fluorite objective lens for polarized light applications; choose from 4x, 10x, 20x, or 40x magnification; for bright-field: 20x lens/ NA 0.75 Plan Apo | 4x/10x/20x/40x | 1.23/0.49/0.25/0.12 | |
| Glissando Desktop Scanner |
Single lens, 20x/0.75 NA Plan Apo, or 40x/0.75 NA Plan Fluor | 20x/40x | 0.55/0.275 | |
| Leica | Aperio VERSA | HCb Plan Fluotar: 1.25x, 5x, 10x, 20x, 40x, 63x (oil), 63x (dry) HC Plan Apo: 20x, 40x (dry), 40x (oil) |
||
| Aperio AT Turbo | 20x/0.75NA Plan Apo (40x scanning with 2x automatic optical mag changer) | 20x/40x | 0.5/0.25 | |
| Aperio AT2 | 20x/0.75 NA Plan Apo (40x scanning with 2x optical mag changer) | 20x/40x | 0.5c/0.25 | |
| Aperio CS2 | 20x/0.75 NA Plan Apo (40x scanning with 2x optical magnification changer) | 20x/40x | 0.5/0.25 | |
| Aperio GT 450 | 40x | Overview image/40x | 13.0/0.26 | |
| Aperio LV1 | 1.25x (NA 0.03), 5x (NA 0.16) and 20x (NA 0.4) ECd Plan-Neofluar | Overview image/2.5x/5x 10x/20x/40x/63x (digital magnification) | 10.0/2.16/1.08/0.54/0.27 0.138/0.086 | |
| Philips | UFS | 20x NA of 0.75 Plan Apo | 40x | 0.25 |
| Roche | Ventana DP200 | 20x and 40x | 40x | 0.23 |
| Ventana iScan | 4x (0.1NA), 10x (0.3NA), 20x | 4x/10x/20x/40x | 2.5/1.0/0.46/0.23 | |
| Coreo | (0.50NA), 40x (0.75NA) | |||
| Ventana iScan HT | 20x and 40x | 40x | 0.23 | |
| Sakura | VisionTek (Plan NeoFluar) | 2.5x(0.075 NA), 10x (0.3 NA), 20x (0.5 NA), 40x (magnification not objective) | Overview camera resolution at 0.45x/Live view and scanning camera resolution at 2.5x/10x/20x | 12.0/2.20/0.550/0.275 |
| VisionTek M6 | 2.5x, 5x (0.16 NA), 10x, 20x (0.5 NA), 40x (0.75 NA), 63x (magnification not objective) | Overview camera:e 0.45x = Live view and scanning camera:f 2.5x/5x/10x/20x/40x | Overview camera: 12.0 Live view and scanning camera: 2.2/1.10/0.55/0.275/0.138 |
|
| OptraScan | Ultra 320 | 40x (0.75 NA) | 20x/40x | 0.50/0.25 |
aAchieves 40x magnification via utilization of a 0.75 NA 20x wide field objective
bLeica Microsystems HC System (Harmonic Compound System)
c0.5 μm per pixel is equivalent to 50,000 pixels per inch by using a 20x objective for the Leica Aperio AT Turbo, Aperio AT2, Aperio CS2 WSI Devices
d“Enhanced Contrast” Plan-Neofluar objective from Zeiss
eOverview Camera (Sakura VisionTek): CMOS; 5MP (2560x1920); pixel size 2.2 μm x 2.2 μm
fLive view and scanning camera (Sakura VisionTek): CCD camera; pixel size 5.5 μm x 5.5 μm
Table 6.
Scan modes, speed, and throughput
| Vendor | |||
|---|---|---|---|
|
| |||
| 3D Histech | Illumination, scanning methods, and focus modes | Scan speeda | Throughputb |
| Pannoramic Desk II | Brightfield; Tile/area Scanning; Z-stack and Extended Focus |
6 min and 30 s | |
| Pannoramic MIDI II | Brightfield, Fluorescent; Tile/ area Scanning | 3 min and 23 s Fluorescent (10 x 10mm area): 6 min at 31x; 22 min at 62x |
15 slides per hour |
| Pannoramic Scan II | Brightfield, Fluorescent Tile/area scanning |
2.5 min at 40x; 5.5 min at 60x Fluorescent (10 x 10mm area): 6 min at 31x; 31 min at 62x |
20 slides per hour |
| Pannoramic Confocal | Brightfield, Fluorescent, and Confocal Tile/area Scanning |
8 min Fluorescent (10 x 10mm area): 18 min at 31x; 70 min at 62x |
7 slides per hour |
| Pannoramic 250 Flash III | Brightfield and Fluorescent; Tile (Area) Scanning; Z-Stack (up to 30+ layers), Extended Focus | 35 s at 20x; 1 min and 35 s at 40x Fluorescent (15 x 15mm area): 12 min at 30x; 40 min at 60x Fluorescent (10 x 10mm area): 5 min at 31x; 15 min at 62x |
54 slides per hour; 36 slides per hour at 40x 750 slides per day (using 20x objective/40x optical equivalent magnification, 10000 μm focus point distance, 15×15 mm average sample size, single-layer local scanning) |
| Pannoramic 1000 | Brightfield Area/Tile Scanning | <1 min at 20x or 40x | 100 slides per hour/ 2000 slides per day at >20x or 40x (using single-layer scanning) |
| Grundium | |||
| Ocus | Brightfield; manual focus (coarse), electronic focus (fine); patented novel stitching technique; Z-stack function | 2 min | |
| Ocus 20 | Brightfield; fully electronic focusing; z-stack, high (5μm) depth-of-field, patented novel stitching technique; Z-stack function | 1 min | |
| Ocus 40 | Brightfield; fully electronic focusing; patented novel stitching technique; Z stack Function | 3 min | |
| Huron | |||
| TissueScope LE120 Slide Scanner |
Brightfield; automatic tissue detection and focus; Z-stack capability | <1 min | |
| TissueScope iQ Intelligent Slide Scanner |
Brightfield; automatic tissue detection and focus; Z-stack capability | <1 min | |
| TissueScope CF | Brightfield and confocal fluorescence scanning | <1 min | |
| Tissuescope LE Slide Scanner | Brightfield; tile scanning; z-stack capability; Pre-focus map | <1 min | |
| TissueScope PE | Brightfield; automatic focus | 300 s at 20x or 40x | |
| Hamamatsu | |||
| NanoZoomer SQ | Pre-focus map; TDI line scanning; z-stack; brightfield | 150 s; 275 s at 40x | Daily capacity of 1 to 30 slides |
| NanoZoomer S60 | Pre-focus map; TDI line scanning; z stack; brightfield and fluorescent | 60 s; 150 s at 40x | Daily capacity of 30 to 200 slides |
| NanoZoomer S210 | Pre-focus map; TDI line scanning; z-stack; brightfield | 60 s; 150 s at 40x | Daily capacity of 50 to 300 slides |
| NanoZoomer XR | “Dynamic” pre-focusing, pre-focus map; TDI line scanning; z-stack: brightfield and fluorescent (option) | 25 s; 35 s at 40x | |
| NanoZoomer S360 | Brightfield; TDI line scanning; Pre-focus map; z-stack; |
30 s at 20x or 40x | 82 slides per hour at 20x or 40x (with 5 focus points) Daily capacity of 300 to 1000 slides |
| Mikroscan | |||
| MikroScan SL5 | Manual and automated focusing; auto-detection of tissue | 45 s | |
| Motic | |||
| EasyScan Pro 6 | Fast Realtime Autofocus; Automatic and Manual ROI mode; High-Precision Single-Field Focusing/EDF; Z-stack |
10x objective (20x magnification): 60 s 20x objective (40x magnification): 160 s 40x objective (80x magnification): 640 s |
|
| EasyScan One | Medium Fast Realtime Autofocus; Z-stack; EDF |
60 s; 160 s at 40x | |
| EasyScan Infinity 100 | Fast Realtime Autofocus; Z-stack; EDF |
10x objective (20x magnification): 60 s 20x objective (40x magnification): 160 s 40x objective (80x magnification): 640 s |
|
| Objective Imaging | |||
| Glissando 20SL (medium-capacity) | |||
| Glissando POL | Automatic region definition and focus setup | ||
| Glissando Desktop Scanner | Automatic tissue detection and focus setup; Z-stack | ||
| Leica | |||
| Aperio VERSA | Brightfield, 7-channel fluorescence and FISH; tile scanning; z stack capability | 206 s | |
| Aperio AT Turbo | Line scanning; brightfield | 90 s; 270 s at 40x | Up to 50 slides per hour (Sustained high-throughput rate of 33 slides per hour); 20 slides per hour at 40x |
| Aperio AT2 | TDI line scan; brightfield automatic focus; z stack capability of up to 25 layers | < 60 sc (time to view) | Sustained high-throughput rate of 50 slides per hour; 20 slides per hour at 40x Entire carousel may be scanned in less than 8 h |
| Aperio AT2 DX | TDI line scan; brightfield automatic focus; z-stack capability of up to 25 layers | < 60 s (time to view) | Sustained high-throughput rate of 50 slides per hour; 20 slides per hour at 40x; Entire carousel may be scanned in less than 8 h |
| Aperio CS2 | Brightfield; TDI line scanning; automatic focus; z stack capability | 90 s (time to view) | |
| Aperio GT 450 | Brightfield; TDI line scanning | 32 s at 40x | 81 slides per hour at 40x |
| Aperio LV1 | Brightfield; tile scanning; automatic focus; z-stack capability |
90 s; 180 s at 40x | |
| Philips | |||
| UFS | Brightfield; TDI line scanning; continuous autofocus | 35 s; 60 s at 40x equivalent | Up to 60 slides per hour (480 slides in an 8-h shift) |
| Roche | |||
| Ventana DP200 | Brightfield; Z-stack | 36 s; 73 s at 40x Time to view: <49 s at 20x; <85 s at 40x |
|
| Ventana iScan Coreo | Brightfield; Z-stack | 120 s; 450 s at 40x | |
| Ventana iScan HT | Brightfield; Z-stack up to 15 layers | 45 s; 72 s at 40x | 80 slides per hour |
| Sakura | |||
| VisionTek (Plan NeoFluar) | Brightfield; Z-stack | 3 min | |
| VisionTek M6 | Brightfield; Z-stack | 1.5 min | |
| Hologic | |||
| Genius Digital Diagnostics (CE-marked for diagnostic use in Europe and is not currently available for sale in the United States) | Unique volumetric scanning technology for simultaneous acquisition of multiple z-stack images | ||
| OptraScan | |||
| Ultra 320 | Brightfield; Real Time Autofocus 52 s at 40x (normal focusing technique), High Precision Autofocus, EDF, Z-stack |
||
aAll scan times/ throughput values are typically standardized for a 15 x 15mm area of interest (AOI) at 20x magnification utilizing brightfield technique for one standard-dimension slide unless indicated otherwise
bUnless stated otherwise, throughput is for the brightfield technique with an image capture magnification of 20x over an AOI of 15x15mm. Additional variables affecting throughput are disclosed when available, e.g., scanning layers, number of focus points, focus point distance etc. Throughput may vary greatly per user-configured modifications applied to any of the metrics/methods of digital WSI scanning included in this table along with many other variables that are not included, for example, slide media. Throughput is typically quoted as “slides per hour”; however, some vendors approach throughput through the viewpoint of a “typical” workday, for example, 8 h, documented as “daily capacity or slides-per-day,” for a WSI device
cTime-to-view: total amount of time allotted for the complete process of scanning, digitization, and viewing of a WSI (as per the “pixel pathway”), with the appearance of a WSI on a digital viewer, for example, “digital slide tray” denoting end-time
Image formats
When choosing a WSI device, it is important to take into account the format of the WSI file it generates, and whether these are supported by open-source libraries (e.g., Openslide and Bio-Formats), which are necessary for custom software programming involved in digital image analysis through traditional and deep learning methods. These libraries will enable software developers to access different regions of the image for various image processing and analysis steps. Although most whole slide image formats can be accessed through open-source libraries, a few are proprietary and are not readily supported; these may entail workarounds and significant additional programming effort in computer-aided diagnosis software development.[99]
DISCUSSION
The first historical record providing insight into the origin of the compound microscope dates to the year 1590. Almost 100 years elapsed from this time until any scientific work of “great and lasting value” was conducted using these tools. Anthony Van Leeuwenhoek, the Dutchman now hailed as the “father of microbiology,” autodidactically crafted his own lenses up to 300x magnification that vastly usurped the capabilities of all other devices available at that time (the best of which achieved 20–30x life-size magnifications).[100]
His curiosity propelled work in the biological sciences that did not receive recognition for its significance during his lifetime. More than 100 more years passed before microscopes became standard fixtures in laboratories. It has been postulated that had people known of the true significance of Leeuwenhoek’s observations, then the drive to push microscope technology would have been ignited much earlier.[100]
Slightly more than 20 years have passed since the development of the first automated WSI system by Wezel and Gilbertson in 1999.[15,44,101] During this short time, static images have been usurped by the advanced capabilities of robotic microscopes. Limitations in these technologies have rapidly given way to whole-slide scanners that are capable of producing images comparable to conventional microscopy.[102] The adoption of these instruments in an amalgam of forums has led to increased consumer interest, challenges to be addressed, and the developments that continue to solve them. Often, these challenges arise in the form of misconceptions. Perceptions regarding changes in traditional workflow following substantial capital expenditure requirements for the implementation of WSI systems are among the primary challenges to be overcome.[59,103] In addition, early studies have demonstrated decreased efficiency in signing out cases digitally due to the altered controls used for navigating through a slide.[26] However, there are indications to the contrary citing study design, level of experience looking at cases digitally, network speed, and workstation setup as factors contributing to improved diagnostic time.[104]
Most current vendor offerings of commercial digital WSI systems range from $30,000 to $250,000, a price point that has remained stagnant for approximately the past decade.[58] This expense is only further compounded by costs incurred through supplementary acquisition contracts (e.g., service agreements), staffing of additional personal (e.g., file clerks, system assistants), digital storage implementation, IT infrastructure, maintenance, training, hardware, and software integration expenses.[103] Return on investment is a primary concern in this regard. Many WSI devices included in our review have been featured in recent studies assessing benchmark metrics by which the overall efficiency, operational utility, and projected savings realized as a result of DP deployment within large anatomical departments are evaluated.[31,59] Assessment of these quantitative metrics yielded results demonstrating a decrease in the number of glass slide requests (an indication of estimated turnaround time, itself decreased), decrease in patient confirmatory testing, and decreases in costs incurred by operational and off-site assets (e.g., vendor services). A projected savings of ≥ $267,000 per year was calculated throughout a 5-y period of implementation in one such study, resulting in an overall projected cost savings of $1.3 million. Qualitative ancillary outcomes such as increased pathologist satisfaction as a result of digital implementations were also realized. The WSI devices used in these studies included the Leica Aperio AT2 (primarily for clinical operations), 3D Histech Pannoramic 250 and Confocal, Hamamatsu Nanozoomer S60, and VisionTek M6.
Although the cost of commercial modern WSI systems has remained static for the past decade rendering their acquisition difficult for many research laboratories, efforts have been expounded toward mitigating these expenses. Recent exploration into this objective resulted in the building of an effective WSI setup using low-cost components for $2500.[80,105] For low-volume scenarios, manually scanning a glass slide through a setup involving a conventional microscope attached to a smartphone is even possible, with an expected setup cost of only $100.[106] The PrimeHisto XE Histology slide scanner is a commercially available WSI device that is available at a highly cost-effective price point of $685.[107] Driven by an external PC (not included) requiring a simple software download (included with purchase), the PrimeHisto XE is a simple, yet effective user-friendly device that is capable of achieving image capture magnifications of up to 75x with negligible compromise to resolution.
Such efforts set the stage for a future in which open-access hardware can be utilized to create affordable, and thereby easier to obtain WSI devices accessible to a wider consumer base.[108,109]
The modern era of diagnostic pathology is one replete with events promoting the progressively visible and necessary significance of digital WSI applications. The public-health emergency after the COVID-19 crisis sparked closer investigations into the implementation and, most importantly, validation of novel digital workflows for remote use.[110] The stymied progression of digital WSI validation has posed an emblematic, yet tangible barrier preventing the widespread implementation of clinically approved digital diagnostic systems. Such efforts for validation, in the overarching sense, have been underpinned by a complex interplay of pathologists, information technologists, and laboratory management systems as well as policy makers, vendors of DP solutions, and stakeholders influencing innovation within the current landscape of DP.[111] Many of the WSI devices included in this review are approved for clinical use by equivalent regulatory bodies throughout the globe; however, they are still relegated to research-use-only status within the United States.[112]
Concordance with traditional light microscopy has been demonstrated by digital WSI.[113,114,115] Despite increasing interest in the validation of current DP systems for use in regulated clinical and nonclinical environments,[116] success remained mired within a sluggish sea of bureaucracy. Currently, the Leica Aperio AT2 DX (Aperio AT2 model with additional “diagnostic” Aperio ImageScope DX clinical viewing software clinically validated in more than 2000 cases) and Philips UFS are the only two digital WSI devices officially approved by the FDA for primary diagnosis in surgical pathology (though not for remote use). The CLIA regulations (last amended in 2003)[117] prevent pathologists from remotely using digital WSI systems from non-CLIA certified sites, creating a substantially limiting outcome. Minimization of human intervention has been suggested to streamline digital WSI system implementation.[112] The COVID-19 pandemic ushered in the implementation and validation of digital WSI with expedience. A memorandum issued by the Centers for Medicare and Medicaid Services opened laboratory accessibility, allowing pathologists to review WSIs remotely from non-CLIA sites.[17] Vital data resultantly accrued, supporting the application and necessity of digital WSI system capabilities during disasters of similar nature, or otherwise, in the future.
Although FDA approval does not dictate health-care practice, its pertinence as applied to WSI system marketing and intended purpose is highly relevant to the implementation of these systems within health-care settings. The WSI devices, prior to temporary legislative amendments implemented during COVID-19 (resulting primarily via efforts from the Digital Pathology Association), were predominantly designated as “Class III,” that is, “high risk” devices by the FDA, subject to the most rigid and inflexible designation pertaining to medical devices. Class III devices bear the unique distinction of subjection to FDA premarket approval (PMA) approval required to evaluate the safety and effectiveness of such devices, a process consisting of a 180-day approval period, markedly limiting their implementation. Failure to meet the standards of this evaluation results in the inability for WSI vendors to market their device as one purposed for clinical settings. A milestone achievement has been reached within the past year, with WSI devices purposed for primary diagnosis being approved for “de novo” submission through the FDA, giving such devices a less stringent “Class II” status and markedly expediting their track toward clinical approval.[17]
The significance of the observations achievable through modern WSI technology are now vastly understood, and the drive to push its capabilities ever further have been supplemented by Moore’s law and an ever-increasing conglomerate of pathologists and those pushing the technological forefront forward.[118] These two bodies, though recently distinct, are now intertwined.
Digital imaging has advanced substantially since 1999. Exciting prospects on the forefront will continue to fertilize its continued evolution. Limitations of our current time will soon cease to exist. Advances in autofocusing capabilities that lead to increased accuracy and speed of scanning have been recently explored.[119] Enhancements in WSI color performance and multispectral imaging are also near upon the horizon.[44,87,96]
Although recent reviews of WSI devices have been sparse, the increasing frequency by which new models and technology are released ultimately renders any publication of this nature rapidly outdated.[15] The knowledge required to interpret an image produced by a WSI scanning device is only further enhanced by knowledge of the device itself, its components, and its operation, to build a foundation on which the future of WSI may be erected.
CONCLUSIONS
Through the analysis of multiple metrics encompassing the components, design, purpose, and clinical context of 43 selected modern WSI devices, we conclude that such devices may be effectively implemented within a diverse array of departmental settings. In examining, detailing, and providing a relevant context for hardware specifications and the individual and combined capacity of these components in executing a litany of WSI applications, we have concluded that our selection of WSI devices is well equipped to handle the rigors of low-to-high-volume departments, endeavors in organized research and education, and independent efforts exerted by those seeking to explore the gamut of applications afforded by digital WSI. Digital WSI solutions have evolved in tandem with modern-day challenges in pathology that increasingly call upon the aid of such systems, for example, shorter turnaround times, increasing reporting complexity, and looming workforce shortage tasked with a globally aging demographic and higher incidences of disease.[120] Such solutions have primarily come to fruition within the past decade in conjunction with technological advancements, allowing for their construction as well as an increasing number of studies demonstrating the merits of their implementation.[6,14,23,121] We believe that the benefits of digital WSI are demonstrated through our documentation of integral WSI device components and their corresponding metrics. Such benefits include enhanced diagnostic ability through advancements in image capture and display equipment, reduction in valuable laboratory resources such as physical storage, and higher capacity, speed, and throughput offered by current WSI devices leading to increased productivity and efficiency. Though not a focus of our review, these benefits are further amplified by software solutions offering storage, viewing, and analysis capabilities. We also conclude that the abilities of digital WSI devices have markedly improved over the past 15 years. A 2006 review of digital WSI demonstrated an average scan time of one standard slide to be approximately 1 h at 40x magnification.[13] Our review has demonstrated markedly decreased scan times recorded using the same metric constraints (with multiple WSI devices capable of achieving <1 min, some as low as 30 s). We conclude that the future of WSI devices will entail almost-instant scan times when using the similar traditional constraints (e.g., single-layer scanning, 20x or 40x magnification, 15 × 15mm AOI), and markedly increased speed for applications currently burdening total scan time (e.g., z-stack, extended focus). Recent expedience of advancements in WSI may be viewed as a product of the modern departmental landscape, one edging toward complete adoption and implementation of DP. Integral components of laboratories utilizing DP tools, for example, information technology (IT) experts, laboratory managers, and pathologists, have shared an increasingly cohesive relationship with the WSI vendors and developers who possess vested interest in the burgeoning, global DP market primed to benefit from their innovations. Departments seeking to adopt DP may further direct the competitive focus of these parties toward addressing the specific needs of the global pathology market. Such efforts in open communication may precipitate the engineering of devices that are best suited toward addressing the needs of pathologists and their departments. High laboratory costs in conjunction with digital engineering complexities arising in certain arenas of DP, for example, cytopathology, have served as focal points on which WSI developers may then devise a solution. Two such examples included in our review include the Genius Digital Diagnostics Solution by Hologic (to be released), and the Ultra 320 WSI device by OptraScan. Both systems are purposed for cytopathology, with the Ultra 320 recently arriving to the market as the first cost-effective high-volume WSI device for cytology. The Genius Digital Diagnostics solution, also equipped for high-volume departments, aims at enhancing acumen in DP cytology diagnostics through incorporating artificial intelligence algorithms in its WSI device. These are two of the many modern WSI devices suggesting that (a) advancements in such devices, for example, decreased costs, enhanced functionality, will inevitability continue to evolve, and (b) this evolution may be further amplified throughout the course of time if the needs of pathologists are communicated to those in the technology sector who are capable, and motivated to address such needs.
The need for digital solutions in pathology, primarily that of WSI, was further demonstrated during the COVID-19 crisis, which resulted in regulatory approval by the FDA for select digital WSI solutions. The FDA approval for such devices, though limited, has been emblematic of the change in regulatory opinion long desired for their increased implementation, an effort that may be buttressed by this recent breakthrough in official validation. Further success demonstrated through implementation of digital WSI devices within clinical settings is necessary to acquire the attention of such regulatory bodies as well as pathologists who may question the capabilities of digital WSI in comparison to traditional WSI. With regard to the latter group, we believe that trust in such devices is granted through knowledge of their components as well as the many applications within pathology that are addressed through their design [Table 7].
Table 7.
Modern WSI device features and applications
| Vendor | |
|---|---|
|
| |
| 3D Histech | Features/uses |
| Pannoramic Desk II | Affordable, entry-level slide scanner for research laboratories transitioning to digital pathology, medical school and resident education, and clinical diagnostics. User-friendly, compact design for teleconsultation and frozen section scanning. Upgraded 5MP CMOS image capture camera from its predecessor (Pannoramic Desk) 4MP CIS camera, delivering 20% faster scanning speeds when using the same native optical resolution. |
| Pannoramic MIDI II | Low-capacity, low-to-moderate speed device for low-volume applications. Features automatic switching between brightfield and fluorescence scanning based on profile content. |
| Pannoramic Scan II | High-capacity scanner with “all-around” functionality, including automatic switching between brightfield and fluorescence scanning based on profile content. Designed for labs processing around 35,000 slides per year. |
| Pannoramic Confocal | Designed for research laboratories. Features automatic switching between brightfield and fluorescence scanning based on profile content and structure illumination and confocal imaging for molecular pathology applications at low running costs. |
| Pannoramic 250 Flash III |
High-throughput, high-speed, high-capacity scanner for high-volume applications. Recommended for labs processing around 110,000 slides per year. Features automatic switching between brightfield and fluorescence scanning based on profile content. |
| Pannoramic 1000 | High-volume, high-speed device featuring automated, “walk away” slide scanning for a whole day’s scanning needs. Recommended for labs processing around 200,000 slides per year. |
| Grundium | |
| Ocus | Entry-level scanner; scanned images and full user interface of the device are easily shareable online. Application areas: Pathology (frozen section, histology, etc.), Clinical research, Veterinary pathology, AI diagnosis, Marine sciences, Environmental research, Geology, Material sciences. Easy laptop, tablet, and smartphone connectivity (for all Ocus models) |
| Ocus 20 | High-speed, low-cost, compact design. Designed especially for intra-operational frozen section and ROSE applications. Additional applications include all areas listed for the Grundium Ocus. |
| Ocus 40 | Designed for versatile applications requiring very high image resolution; particularly suitable for integration as the imaging component in systems of multiple labs with centralized examination of samples. Applications include pathology (cytology, fecal, blood, etc.) and all areas listed for the Grundium Ocus and Ocus 20. |
| Huron | |
| TissueScope LE120 Slide Scanner | High-capacity, high-speed, mixed-slide scanner with continuous automatic setup and nonstop operation for high-volume applications requiring high throughput. |
| TissueScope iQ | High-capacity, high-speed, mixed-slide scanner for high-volume applications requiring high throughput. Key part of Huron’s |
| Intelligent Slide Scanner |
Scan, Index, Search platform for pathology, combining slide scanning with Huron’s AI-powered image search engine (available fall 2021) |
| TissueScope CF | High-speed, high-capacity device with confocal and brightfield imaging; mixed-slide functionality |
| TissueScope LE Slide Scanner |
High-speed, low-capacity, brightfield scanner designed to support low-volume applications |
| TissueScope PE | Cost-effective, low-capacity, low-speed, compact desktop scanner for low-volume applications |
| Hamamatsu | |
| NanoZoomer SQ | Compact, low-cost, single-slide automatic scanning; designed for limited installation spaces |
| NanoZoomer S60 | Medium-capacity WSI device with mixed-slide compatibility. Brightfield with optional fluorescence imaging module Users can superimpose brightfield and fluorescent images of the entire tissue image. |
| NanoZoomer S210 | Well-balanced, high-capacity, automatic scanning; low-cost |
| NanoZoomer XR | Dynamic Pre-Focusing (DPF) method (patent pending) tracks and keeps specimens in focus while scanning. Automatic slide quality check feature evaluates scanned digital slides and generates a quality focus score to determine the need for automatic rescanning, eliminating the need for manual intervention. |
| NanoZoomer S360 | High-throughput; high-speed, high-capacity scanner designed for mass processing in hospitals and clinical laboratories |
| MikroScan | |
| MikroScan SL5 | Compact, portable, cost-effective, high-speed WSI device with a complete set of 5 objectives for a wide range of applications. Dual modes include: a live robotic microscope for the examination of specimens on glass slides or a static digital pathology scanning mode for the creation of digitized tissue samples. |
| Motic | |
| EasyScan Pro 6 | High-speed, low-capacity scanner with “walk-away” functionality. An upcoming release of the slide holder will support 2′′ x 3′′ slides, allowing 10 2′′ x 3′′ slides or a combination of 1′′ x 3′′ and 2′′ x 3′′ slides to be loaded into the device; 20 standard-slide autoloading functionality; remotely located users will have complete control of the autoloader. |
| EasyScan One | Low-capacity, low-cost desktop scanner featuring a high-numerical aperture plan apochromatic objective (20x/0.75) to maximize color fidelity and resolution power; A large 2/3″ CMOS sensor confers large fields of view while delivering image detail equivalent to a high power 60X lens. |
| EasyScan Infinity 100 | 60 or 102 slide capacity options; both modules feature similar design to the EasyScan Pro 6 and include “walk-away” functionality. |
| Objective imaging | |
| Glissando 20SL (medium capacity) | Medium-capacity, high-speed, auto-loading “walk-away” scanning functionality with compact design. Multiple holder design offers avoiding any direct internal handling of glass for safe operation. Available in 20x or 40x scanning objective configurations. |
| Glissando POL | Features user-configurable illumination and camera settings. Simple manual switching between imaging modalities for dual-purpose brightfield and polarized light scanning. |
| Glissando Desktop Scanner | Compact, high-speed, low-cost scanner for low-volume operations. |
| Leica | |
| Aperio VERSA | Comprehensive, scalable (8-200 slide capacity) WSI device designed for IHC, ISH, and Fluorescent Tissue-Based Research, including Multiplex Whole Slide Scanning. Batch setup and automation for unsupervised “walk-away” scanning. Features advanced tissue detection for faint, lightly stained brightfield and fluorescent samples. Includes 2-D scanning technology, ideal for co-localization studies, and an automated oiler for 40x or 63x magnification oil scans. Offers precision scanning of brightfield and fluorescent samples, with accuracy and resolution required for FISH. Developed to support the diverse imaging needs of research facilities (from tissue-based and proteomic markers, to subcellular, molecular, and in situ hybridization probes). |
| Aperio AT Turbo | Intended for medium-to-high volume departments seeking improvements in turnaround time and reduction in departmental costs without compromise to laboratory space; high first-time scan success rate; digital slides are available to view in 90 s or less (at 20x magnification for a 15 x 15mm area) |
| Aperio AT2 | 98% first-time success rates yield fewer rescans; quiet device with minimal movement of slides in loader; features automatic adjustable tissue finder and skip blank stripes technology, resulting in smaller file sizes and faster scanning. Designed as a platform for research institutions, featuring a user-friendly “pathologist’s cockpit,” easily integrated into laboratory information systems (LIS) |
| Aperio AT2 DX | High-capacity slide scanner with Aperio ImageScope DX clinical viewing software, and a high-performance viewing workstation including ICC color-calibrated medical grade monitors. Designed for high-volume clinical labs. Can be used standalone, or in conjunction with Aperio Path DX case management clinical workflow software, for a streamlined, clinical workflow. Designed to aid diagnostics in high-volume clinical labs. |
| Aperio CS2 | Low-speed and low-capacity scanner designed for low-volume applications; automatic tissue finding functionality |
| Aperio GT 450 | Patented “Real-time automatic focusing” technique combines imaging and focusing line sensors aided by proprietary algorithms to continuously determine best focus value in relation to objective height (continuously adjusting in real-time for optimal image capture); automatic tissue finding function; priority rack scanning and automated image quality check (Aperio GT 450) |
| Aperio LV1 | Cost-effective; designed for research use. Compact desktop design lends to small footprint. Minimal IT requirements, fast implementation. |
| Philips | |
| UFS | High-speed Automated “walk away” scanning” Continuous autofocus; Automatic tissue detection |
| Roche | |
| Ventana DP200 | Moderate-speed, medium-capacity, high-speed scanner |
| Ventana iScan | Low-speed, high-capacity; continuous access to slide rack; autoloader with automated slide detection for walk-away scanning |
| Coreo | |
| Ventana iScan HT | High-throughput, high-speed scanner for high-volume applications; continuous random access to slide rack |
| Sakura | |
| VisionTek (Plan NeoFluar) | Low-capacity, low-speed; 3 objective design |
| VisionTek M6 | Low-capacity; moderate-speed; 3 objective design |
| Hologic | |
| Genius Digital Diagnostics WSI system (CE-marked for diagnostic use in |
Artificial intelligence-enhanced digital WSI system purposed for cytology; 400 standard slide capacity; high-speed, high-magnification; scans ThinPrep Gyn, Non-Gyn, and UroCyte slides; automated labeling, aliquoting, and uncapping/capping; integrated touch screen interface with slide inventory display. The WSI device is part of a scalable package/ complete solution, including an image management server and a review station for local or remote case review. |
| Europe and is not currently available for sale in the United States) | First CE-marked digital cytology platform combining a new artificial intelligence (AI) algorithm with advanced volumetric imaging technology to help cytotechnologists and pathologists identify pre-cancerous lesions and cancer cells |
| OptraScan | |
| Ultra 320 | Affordable, fully integrable, high-speed, high-volume scanner; part of a complete solution purposed for cytology and pathology with patented composite imaging for cytology samples; solution includes image analysis suite for breast, prostate, brain, renal, and lung (IHC and H&E) |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Browning L, Colling R, Rakha E, Rajpoot N, Rittscher J, James JA, et al. Digital pathology and artificial intelligence will be key to supporting clinical and academic cellular pathology through COVID-19 and future crises: The PathLAKE consortium perspective. J Clin Pathol. 2021;74:443–7. doi: 10.1136/jclinpath-2020-206854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans AJ, Depeiza N, Allen SG, Fraser K, Shirley S, Chetty R. Use of whole slide imaging (WSI) for distance teaching. J Clin Pathol. 2021;74:425–8. doi: 10.1136/jclinpath-2020-206763. [DOI] [PubMed] [Google Scholar]
- 3.Montironi R, Cimadamore A, Scarpelli M, Cheng L, Lopez-Beltran A, Mikuz G. Pathology without microscope: From a projection screen to a virtual slide. Pathol Res Pract. 2020;216:153196. doi: 10.1016/j.prp.2020.153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pallua JD, Brunner A, Zelger B, Schirmer M, Haybaeck J. The future of pathology is digital. Pathol Res Pract. 2020;216:153040. doi: 10.1016/j.prp.2020.153040. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein RS. Prospects for telepathology. Hum Pathol. 1986;17:433–4. doi: 10.1016/s0046-8177(86)80028-4. [DOI] [PubMed] [Google Scholar]
- 6.Pantanowitz L, Sharma A, Carter AB, Kurc T, Sussman A, Saltz J. Twenty years of digital pathology: An overview of the road travelled, what is on the horizon, and the emergence of vendor-neutral archives. J Pathol Inform. 2018;9:40. doi: 10.4103/jpi.jpi_69_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimes GJ, McClellan SA, Goldman J, Vaughn GL, Conner DA, Kujawski E, et al. Applications of virtual reality technology in pathology. Stud Health Technol Inform. 1997;39:319–27. [PubMed] [Google Scholar]
- 8.Ferreira R, Moon B, Humphries J, Sussman A, Saltz J, Miller R, et al. The virtual microscope. Proc AMIA Annu Fall Symp. 1997:449–53. [PMC free article] [PubMed] [Google Scholar]
- 9.Montalto MC, McKay RR, Filkins RJ. Autofocus methods of whole slide imaging systems and the introduction of a second-generation independent dual sensor scanning method. J Pathol Inform. 2011;2:44. doi: 10.4103/2153-3539.86282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balis UGJ. Selecting the Optimal Digital System for Your Department based on Case Volume and Projected Uses. Michigan Medicine. [Last accessed on 2021 Sep 10]. Available from: https://www.pathologyinformatics.org/docs/PI_December_Balis_2019_final_v_2.03.pdf .
- 11.Sucaet Y, Waelput W. Hardware and software. In: Sucaet Y, Waelput W, editors. Digital Pathology. Springer International Publishing: Cham; 2014. pp. 15–29. [Google Scholar]
- 12.U.S. Food and Drug Administration. FDA allows marketing of first whole slide imaging system for digital pathology. [Last accessed on 2021 Sep 10]. Available from: https://www.fda.gov/news-events/press-announcements/fda-allowsmarketing-first-whole-slide-imaging-system-digital-pathology .
- 13.Rojo MG, García GB, Mateos CP, García JG, Vicente MC. Critical comparison of 31 commercially available digital slide systems in pathology. Int J Surg Pathol. 2006;14:285–305. doi: 10.1177/1066896906292274. [DOI] [PubMed] [Google Scholar]
- 14.Farahani N, Parwani A, Pantanowitz L. Whole slide imaging in pathology: Advantages, limitations, and emerging perspectives. Pathol Lab Med Int. 2015:23–33. doi:10.2147/plmi.s59826. [Google Scholar]
- 15.Aeffner F, Adissu HA, Boyle MC, Cardiff RD, Hagendorn E, Hoenerhoff MJ, et al. Digital microscopy, image analysis, and virtual slide repository. Ilar J. 2018;59:66–79. doi: 10.1093/ilar/ily007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna MG, Parwani A, Sirintrapun SJ. Whole slide imaging: Technology and applications. Adv Anat Pathol. 2020;27:251–9. doi: 10.1097/PAP.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 17.Abels E, Pantanowitz L. Current state of the regulatory trajectory for whole slide imaging devices in the USA. J Pathol Inform. 2017;8:23. doi: 10.4103/jpi.jpi_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. Technical performance assessment of digital pathology whole slide imaging devices––guidance for industry and food and drug administration staff. [Last accessed on 2021 Sep 10]. Available from: https://www.fda.gov/media/90791/download .
- 19.Rockville: U. S. Food and Drug Administration; 2016. [Last accessed on 2021 Jul 9]. Center for Devices Radiological Health. Technical performance assessment of digital pathology whole slide imaging devices: Guidance for industry and Food and Drug Administration staff. Available from: https://www.fda.gov/media/90791/download . [Google Scholar]
- 20.Bertram CA, Klopfleisch R. The pathologist 2.0: An update on digital pathology in veterinary medicine. Vet Pathol. 2017;54:756–66. doi: 10.1177/0300985817709888. [DOI] [PubMed] [Google Scholar]
- 21.Galloway M, Nadin L. Benchmarking and the laboratory. J Clin Pathol. 2001;54:590–7. doi: 10.1136/jcp.54.8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saco A, Ramírez J, Rakislova N, Mira A, Ordi J. Validation of whole-slide imaging for histolopathogical diagnosis: Current state. Pathobiology. 2016;83:89–98. doi: 10.1159/000442823. [DOI] [PubMed] [Google Scholar]
- 23.Melo RCN, Raas MWD, Palazzi C, Neves VH, Malta KK, Silva TP. Whole slide imaging and its applications to histopathological studies of liver disorders. Front Med (Lausanne) 2019;6:310. doi: 10.3389/fmed.2019.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundium Ocus®40. Grundium. [Last accessed on 2021 Jul 9]. Available from: https://grundium.com/ocus40/
- 25.Pannoramic 1000. 3DHISTECH Ltd. [Last accessed on 2021 Jul 9]. Available from: https://www.3dhistech.com/research/pannoramic-digital-slide-scanners/pannoramic-1000/
- 26.Hanna MG, Reuter VE, Hameed MR, Tan LK, Chiang S, Sigel C, et al. Whole slide imaging equivalency and efficiency study: Experience at a large academic center. Mod Pathol. 2019;32:916–28. doi: 10.1038/s41379-019-0205-0. [DOI] [PubMed] [Google Scholar]
- 27.Chandra M. Digital pathology slides in medical education. Indian J Dermatopathol Diag Dermatol. 2014;1:17. [Google Scholar]
- 28.TissueSnap™ Preview Station. Huron Digital Pathology. [Last accessed on 2021 Sep 10]. Available from: https://www.hurondigitalpathology.com/tissuesnap/
- 29.Kohlberger T, Liu Y, Moran M, Chen PC, Brown T, Hipp JD, et al. Whole-slide image focus quality: Automatic assessment and impact on AI cancer detection. J Pathol Inform. 2019;10:39. doi: 10.4103/jpi.jpi_11_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cromey D. The importance of #1.5 thickness coverslips for microscopy. The University of Arizona. [Last accessed on 2021 Jul 9]. Available from: http://microscopy.arizona.edu/sites/default/files/sites/default/files/upload/coverslips_for_microscopy.pdf .
- 31.Hanna MG, Reuter VE, Ardon O, Kim D, Sirintrapun SJ, Schüffler PJ, et al. Validation of a digital pathology system including remote review during the COVID-19 pandemic. Mod Pathol. 2020;33:2115–27. doi: 10.1038/s41379-020-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Developing positioning systems for pathology scanners. Parker Daedal Engineered Solutions. [Last accessed on 2021 Jul 9]. Available from: https://olympuscontrols.com/wp-content/uploads/2016/08/Olympus-Controls_Motion-Control_Developing-Positioning-Systems-for-Pathology-Scanners.pdf .
- 33.Ghaznavi F, Evans A, Madabhushi A, Feldman M. Digital imaging in pathology: Whole-slide imaging and beyond. Annu Rev Pathol. 2013;8:331–59. doi: 10.1146/annurev-pathol-011811-120902. [DOI] [PubMed] [Google Scholar]
- 34.Zheng G, Ou X, Yang C. 0.5 gigapixel microscopy using a flatbed scanner. Biomed Opt Express. 2013;5:1–8. doi: 10.1364/BOE.5.000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pannoramic 250 Flash III. 3DHistech. [Last accessed on 2021 Jul 9]. Available from: https://www.3dhistech.com/research/pannoramic-digital-slide-scanners/pannoramic-250-flash-iii/
- 36.Pannoramic Desk II DW. 3DHistech Ltd. [Last accessed on 2021 Jul 9]. Available from: https://www.3dhistech.com/research/pannoramic-digital-slide-scanners/pannoramic-desk-ii-dw/
- 37.Gu K, Liu M, Zhai G, Yang X, Zhang W. Quality assessment considering viewing distance and image resolution? IEEE Trans Broadcast. 2015;61:520–31. doi:10.1109/tbc.2015.2459851. [Google Scholar]
- 38.Nieuwenhuizen RP, Lidke KA, Bates M, Puig DL, Grünwald D, Stallinga S, et al. Measuring image resolution in optical nanoscopy. Nat Methods. 2013;10:557–62. doi: 10.1038/nmeth.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fournier C, Denis L, Fournel T. On the single point resolution of on-axis digital holography. J Opt Soc Am A Opt Image Sci Vis. 2010;27:1856–62. doi: 10.1364/JOSAA.27.001856. [DOI] [PubMed] [Google Scholar]
- 40.Costa PM. Microphotography and image processing: Creating artwork.In: Costa PM, editor. The Handbook of Histopathological Practices in Aquatic Environments. 2018:119–33. [Google Scholar]
- 41.Reu PL, Sweatt W, Miller T, Fleming D. Camera system resolution and its influence on digital image correlation. Exp Mech. 2014;55:9–25. doi:10.1007/s11340-014-9886-y. [Google Scholar]
- 42.Sellaro TL, Filkins R, Hoffman C, Fine JL, Ho J, Parwani AV, et al. Relationship between magnification and resolution in digital pathology systems. J Pathol Inform. 2013;4:21. doi: 10.4103/2153-3539.116866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piston DW. Choosing objective lenses: The importance of numerical aperture and magnification in digital optical microscopy. Biol Bull. 1998;195:1–4. doi: 10.2307/1542768. [DOI] [PubMed] [Google Scholar]
- 44.Zarella MD, Bowman D, Aeffner F, Farahani N, Xthona A, Absar SF, et al. A practical guide to whole slide imaging: A white paper from the digital pathology association. Arch Pathol Lab Med. 2019;143:222–34. doi: 10.5858/arpa.2018-0343-RA. [DOI] [PubMed] [Google Scholar]
- 45.Yagi Y, Gilbertson JR. The importance of optical optimization in whole slide imaging (WSI) and digital pathology imaging. Diagn Pathol. 2008;3(Suppl 1):S1. doi: 10.1186/1746-1596-3-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeRose JA, Doppler M. Guidelines for understanding magnification in the modern digital microscope era. Micros Today. 2018;26:20–33. [Google Scholar]
- 47.Bruce A, Alexander J, Julian L, Martin R, Keith R, Peter W. Looking at the structure of cells in the microscope. In: Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, editors. Molecular Biology of the Cell. 4th edition. 2002. Garland Science. [Google Scholar]
- 48.Urone PP, Hinrichs R. College Physics. OpenStax; 2021. [Last accessed on 2021 Sep 10]. Limits of resolution: The Rayleigh criterion. Available from: https://opentextbc.ca/openstaxcollegephysics/chapter/limits-of-resolutionthe-rayleigh-criterion/ [Google Scholar]
- 49.Themelis G, Yoo JS, Soh KS, Schulz R, Ntziachristos V. Real-time intraoperative fluorescence imaging system using light-absorption correction. J Biomed Opt. 2009;14:064012. doi: 10.1117/1.3259362. [DOI] [PubMed] [Google Scholar]
- 50.Riley RS, Ben-Ezra JM, Massey D, Slyter RL, Romagnoli G. Digital photography: A primer for pathologists. J Clin Lab Anal. 2004;18:91–128. doi: 10.1002/jcla.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta S, Patel A, Mehta J. 2015 International Conference on Communications and Signal Processing (ICCSP) Melmaruvathur: IEEE; 2015. CCD or CMOS image sensor for photography; pp. 291–4. [Google Scholar]
- 52.A comparison of EMCCD vs sCMOS cameras. Oxford Instruments. [Last accessed on 2021 Jul 9]. Available from: https://andor.oxinst.com/learning/view/article/comparing-scmos .
- 53.Sikkel MB, Kumar S, Maioli V, Rowlands C, Gordon F, Harding SE, et al. High speed sCMOS-based oblique plane microscopy applied to the study of calcium dynamics in cardiac myocytes. J Biophotonics. 2016;9:311–23. doi: 10.1002/jbio.201500193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallace J. Photonics products: Scientific CMOS cameras: sCMOS cameras reach new levels of capability. Laser Focus World. [Last accessed on 2021 Jul 9]. Available from: https://www.laserfocusworld.com/detectors-imaging/article/16555375/photonics-products-scientific-cmos-cameras-scmoscameras-reach-new-levels-of-capability .
- 55.Janesick J, Putnam G. Developments and applications of high-performance CCD and CMOS imaging arrays? Ann Rev Nuc Part Sci. 2003;53:263–300. doi:10.1146/annurev.nucl.53.041002.110431. [Google Scholar]
- 56.Dave L. CCD vs.CMOS: Facts and fiction. Photon Spec. 2001;1:154–158. [Google Scholar]
- 57.Guo X, Lin H, Yu Z, McCloskey S. Computer Vision––ECCV 2014 Workshops. Cham: Springer; 2015. Barcode imaging using a light field camera; pp. 519–32. [Google Scholar]
- 58.Isse K, Lesniak A, Grama K, Roysam B, Minervini MI, Demetris AJ. Digital transplantation pathology: Combining whole slide imaging, multiplex staining and automated image analysis. Am J Transplant. 2012;12:27–37. doi: 10.1111/j.1600-6143.2011.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanna MG, Reuter VE, Samboy J, England C, Corsale L, Fine SW, et al. Implementation of digital pathology offers clinical and operational increase in efficiency and cost savings. Arch Pathol Lab Med. 2019;143:1545–55. doi: 10.5858/arpa.2018-0514-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.EMCCD vs sCMOS––Cameras for Confocal Microscopy. [Last accessed on 2021 Jul 10]. Available from: https://andor.oxinst.com/learning/view/article/emccd-vsscmos-cameras-for-spinning-disk-confocal-microscopy .
- 61.Zhang Z, Wang Y, Piestun R, Huang ZL. Characterizing and correcting camera noise in back-illuminated sCMOS cameras. Opt Express. 2021;29:6668–90. doi: 10.1364/OE.418684. [DOI] [PubMed] [Google Scholar]
- 62.Pannoramic Confocal. [Last accessed on 2021 Jul 10]. Available from: https://www.3dhistech.com/research/pannoramic-digital-slide-scanners/pannoramic-confocal/
- 63.Bigas M, Cabruja E, Forest J, Salvi J. Review of CMOS image sensors? Microelectron J. 2006;37:433–451. doi:10.1016/j.mejo.2005.07.002. [Google Scholar]
- 64.Abramowitz M, Davidson MW. Numerical Aperture and Resolution. Olympus Corporation. [Last accessed on 2021 Jul 9]. Available from: https://www.olympus-lifescience.com/en/microscope-resource/primer/anatomy/numaperture/
- 65.Choosing the Right Camera Bus. National Instruments Corp. [Last accessed on 2021 Jul 9]. Available from: https://www.ni.com/en-lb/innovations/whitepapers/12/choosing-the-right-camera-bus.html#section-681366672 .
- 66.USB 3.0 — The Camera Interface of the Future (?) [Last accessed on 2021 Jul 9]. Available from: https://www.baslerweb.com/en/vision-campus/interfaces-and-standards/usb3-interface-future/
- 67.Acquiring from GigE Vision Cameras with Vision Acquisition Software. National Instruments Corp. [Last accessed on 2021 Sep 10]. Available from: https://www.ni.com/en-lb/innovations/white-papers/06/acquiring-from-gigevision-cameras-with-vision-acquisition-softw.html .
- 68.McClintock DS. WSI: Effects on Workflow from the AP Labs Perspective. [Last accessed on 2021 Sep 10]. Available from: https://www.viethmms.com/~apin/docs/53McClintockThurs.pdf .
- 69.Sacco A, Esposito F, Marchetto G, Kolar G, Schwetye K. On edge computing for remote pathology consultations and computations. IEEE J Biomed Health Inform. 2020;24:2523–34. doi: 10.1109/JBHI.2020.3007661. [DOI] [PubMed] [Google Scholar]
- 70.Liao M, Lu D, Pedrini G, Osten W, Situ G, He W, et al. Extending the depth-of-field of imaging systems with a scattering diffuser. Sci Rep. 2019;9:7165. doi: 10.1038/s41598-019-43593-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNamara G, Difilippantonio M, Ried T, Bieber FR. Microscopy and image analysis. Curr Protoc Hum Genet. 2017;94:4.4.1. doi: 10.1002/cphg.42. 4.4.89. [DOI] [PubMed] [Google Scholar]
- 72.Objectives for Research Microscopy. ZEISS. [Last accessed on 2021 Jul 9]. Available from: https://www.zeiss.com/microscopy/us/service-support/glossary/objectivesfor-research-microscopy.html .
- 73.Fu X, Lennerz J, Onozato M, Iafrate A, Yagi Y. Evaluation of a confocal WSI scanner for FISH slide imaging and image analysis. Diagnos Pathol. 2017;3:1–23. [Google Scholar]
- 74.Objectives: High Performance ZEISS Objectives for Microscopy. ZEISS. [Last accessed on 2021 Jul 9]. Available from: https://www.zeiss.com/microscopy/us/products/microscope-components/objectives.html .
- 75.Chen ZW, Kohan J, Perkins SL, Hussong JW, Salama ME. Web-based oil immersion whole slide imaging increases efficiency and clinical team satisfaction in hematopathology tumor board. J Pathol Inform. 2014;5:41. doi: 10.4103/2153-3539.143336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caprette DR. Microscopy with Oil Immersion. Rice University. [Last accessed on 2021 Jul 9]. Available from: https://www.ruf.rice.edu/~bioslabs/methods/microscopy/oilimm.html .
- 77.Yoshitake T, Giacomelli MG, Quintana LM, Vardeh H, Cahill LC, Faulkner-Jones BE, et al. Rapid histopathological imaging of skin and breast cancer surgical specimens using immersion microscopy with ultraviolet surface excitation. Sci Rep. 2018;8:4476. doi: 10.1038/s41598-018-22264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruce-Chwatt LJ. Anisol: A convenient immersion medium for microscopy. Bull World Health Organ. 1959;20:151–3. [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Gross H. Systematic design of microscope objectives? Part I: System review and analysis. Adv Opt Technol. 2019;8:313–47. doi:10.1515/aot-2019-0002. [Google Scholar]
- 80.Shakeri SM, Hulsken B, van Vliet LJ, Stallinga S. Optical quality assessment of whole slide imaging systems for digital pathology. Opt Express. 2015;23:1319–36. doi: 10.1364/OE.23.001319. [DOI] [PubMed] [Google Scholar]
- 81.Lu Q, Liu G, Xiao C, Hu C, Zhang S, Xu RX, et al. A modular, open-source, slide-scanning microscope for diagnostic applications in resource-constrained settings. PLOS One. 2018;13:e0194063. doi: 10.1371/journal.pone.0194063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schade K-H, Euteneuer P, Müller-Rentz A. Optimization of the Interplay of Optical Components for Aberration Free Microscopy. Leica Microsystems. [Last accessed on 2021 Jul 9]. Available from: https://www.leicamicrosystems.com/science-lab/optimization-of-the-interplay-ofoptical-components-for-aberration-free-microscopy/
- 83.Rottenfusser R, Dragoo T, Davidson MW. Infinity Optical System Basics. ZEISS. [Last accessed on 2021 Jul 9]. Available from: https://www.zeiss.com/microscopy/us/solutions/reference/all-tutorials/basic-microscopy/infinityoptical-system-basics.html .
- 84.Wang YL, Grooms NWF, Civale SC, Chung SH. Confocal imaging capacity on a widefield microscope using a spatial light modulator. PLOS One. 2021;16:e0244034. doi: 10.1371/journal.pone.0244034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Der Graaff L, Boyaval F, Van Vliet LJ, Stallinga S. SPIE Photonics Europe. Strasbourg: Society of Photo-Optical Instrumentation Engineers; 2018. Fluorescence imaging for whole slide scanning using LED-based color sequential illumination. International Society for Optics and Photonics 106790D. International Society for Optics and Photonics 106790D. doi: 10.1117/12.2306776. [Google Scholar]
- 86.Tungsten-Halogen Lamps. [Last accessed on 2021 Jul 10]. Available from: http://zeiss-campusmagnet.fsu.edu/articles/lightsources/tungstenhalogen.html .
- 87.Cheng WC, Saleheen F, Badano A. Assessing color performance of whole-slide imaging scanners for digital pathology? Color Res Appl. 2019;44:322–34. doi:10.1002/col.22365. [Google Scholar]
- 88.Davidson MW. Education in Microscopy and Digital Imaging. ZEISS. [Last accessed on 2021 Jul 9]. Available from: http://zeiss-campus.magnet.fsu.edu/articles/lightsources/leds.html .
- 89.Pantanowitz L. Digital images and the future of digital pathology. J Pathol Inform. 2010;1:14. doi: 10.4103/2153-3539.68332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pantanowitz L, Dickinson K, Evans AJ, Hassell LA, Henricks WH, Lennerz JK, et al. American telemedicine association clinical guidelines for telepathology. J Pathol Inform. 2014;5:39. doi: 10.4103/2153-3539.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abel JT, Ouillette P, Williams CL, Blau J, Cheng J, Yao K, et al. Display characteristics and their impact on digital pathology: A current review of pathologists’ future “microscope”. J Pathol Inform. 2020;11:23. doi: 10.4103/jpi.jpi_38_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inoue T, Yagi Y. Color standardization and optimization in whole slide imaging. Clin Diag Pathol. 2020;4:14. doi: 10.15761/cdp.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng W-C, Lam S, Gong Q, Lemaillet P. Evaluating whole-slide imaging viewers used in digital pathology? Electron Imag. 2020;2020:372. doi:10.2352/issn.2470-1173.2020.9.Iqsp-372. [Google Scholar]
- 94.Livada B. Digital medical diagnostic displays. Int J Electr Eng Comput. 2020;4:33–42. doi:10.7251/IJEEC2001033L. [Google Scholar]
- 95.Grundium has been issued a US patent for image stitching. [Last accessed on 2021 Jul 10]. Available from: https://grundium.com/blogs/the-grundium-image-stitching-method-is-patented .
- 96.Indu M, Rathy R, Binu MP. “Slide less pathology”: Fairy tale or reality? J Oral Maxillofac Pathol. 2016;20:284–8. doi: 10.4103/0973-029X.185921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McKay RR, Baxi VA, Montalto MC. The accuracy of dynamic predictive autofocusing for whole slide imaging. J Pathol Inform. 2011;2:38. doi: 10.4103/2153-3539.84231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ltd F. CASE STUDY: How Roche is leading the digital pathology scanner market with FFEI’s line-scan camera and tracking expertise. [Last accessed on 2021 Sep 10]. Available from: https://www.ffei.co.uk/wp-content/uploads/2018/12/Ebook-Roche-VENTANA-DP-200-case-study-Final-Dec2018.pdf .
- 99.Supported Image Formats. Supported Image Formats. QuPath. [Last accessed on 2021 Sep 10]. Available from: https://qupath.readthedocsio/en/latest/docs/intro/formats.html .
- 100.Ball CS. The early history of the compound microscope. Bios. 1966;37:51–60. [Google Scholar]
- 101.Babaie M, Kalra S, Sriram A, Mitcheltree C, Zhu S, Khatami A, et al. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition Workshops. London: IEEE; 2017. Classification and retrieval of digital pathology scans: A new dataset; pp. 8–16. [Google Scholar]
- 102.Iyengar JN. Whole slide imaging: The futurescape of histopathology. Indian J Pathol Microbiol. 2021;64:8–13. doi: 10.4103/IJPM.IJPM_356_20. [DOI] [PubMed] [Google Scholar]
- 103.Liu Y, Pantanowitz L. Digital pathology: Review of current opportunities and challenges for oral pathologists. J Oral Pathol Med. 2019;48:263–9. doi: 10.1111/jop.12825. [DOI] [PubMed] [Google Scholar]
- 104.Vodovnik A. Diagnostic time in digital pathology: A comparative study on 400 cases. J Pathol Inform. 2016;7:4. doi: 10.4103/2153-3539.175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gullapalli R, Gullapalli A, Mubeen A, Parwani AV. Building a low-cost whole slide imaging (WSI) system in a basic research lab: Lessons and successes. J Pathol Inform. 2021;12:37. [Google Scholar]
- 106.Yu H, Gao F, Jiang L, Ma S. Development of a whole slide imaging system on smartphones and evaluation with frozen section samples. JMIR Mhealth Uhealth. 2017;5:e132. doi: 10.2196/mhealth.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.PrimeHisto XE Histology Slide Scanner. Carolina Biological Supply Company. [Last accessed on 2021 Sep 10]. Available from: https://www.carolina.com/microscope-slide-classroom-resources/primehisto-xe-histology-slide-scanner/489780.pr .
- 108.Guo C, Bian Z, Jiang S, Murphy M, Zhu J, Wang R, et al. OpenWSI: A low-cost, high- throughput whole slide imaging system via single-frame autofocusing and open-source hardware. Opt Lett. 2019:45. doi:10.1364/ol.45.000260. [Google Scholar]
- 109.Schubert M. Erasing Pathology’s Borders. The Pathologist. [Last accessed on 2021 Sep 10]. Available from: https://thepathologist.com/inside-the-lab/erasing-pathologys-borders .
- 110.Ardon O, Reuter VE, Hameed M, Corsale L, Manzo A, Sirintrapun SJ, et al. Digital pathology operations at an NYC Tertiary Cancer Center during the first 4 months of COVID-19 pandemic response? Acad Pathol. 2012;8 doi: 10.1177/23742895211010276. doi:10.1177/23742895211010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Betmouni S. Diagnostic digital pathology implementation: Learning from the digital health experience. Digit Health. 2021;7:20552076211020240. doi: 10.1177/20552076211020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fraggetta F, Garozzo S, Zannoni GF, Pantanowitz L, Rossi ED. Routine digital pathology workflow: The Catania experience. J Pathol Inform. 2017;8:51. doi: 10.4103/jpi.jpi_58_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alassiri A, Almutrafi A, Alsufiani F, Al Nehkilan A, Al Salim A, Musleh H, et al. Whole slide imaging compared with light microscopy for primary diagnosis in surgical neuropathology: A validation study. Ann Saudi Med. 2020;40:36–41. doi: 10.5144/0256-4947.2020.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mukhopadhyay S, Feldman MD, Abels E, Ashfaq R, Beltaifa S, Cacciabeve NG, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: A multicenter blinded randomized noninferiority study of 1992 cases (pivotal study) Am J Surg Pathol. 2018;42:39–52. doi: 10.1097/PAS.0000000000000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goacher E, Randell R, Williams B, Treanor D. The diagnostic concordance of whole slide imaging and light microscopy: A systematic review. Arch Pathol Lab Med. 2017;141:151–61. doi: 10.5858/arpa.2016-0025-RA. [DOI] [PubMed] [Google Scholar]
- 116.García-Rojo M, De Mena D, Muriel-Cueto P, Atienza-Cuevas L, Domínguez-Gómez M, Bueno G. New European Union regulations related to whole slide image scanners and image analysis software. J Pathol Inform. 2019;10:2. doi: 10.4103/jpi.jpi_33_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. CLIA Law & Regulations. [Last accessed on 2021 Sep 10]. Available from: https://www.cdc.gov/clia/law-regulations.html .
- 118.Evans M, Tanière P. From Moore’s law to molecular alterations The Pathologist. [Last accessed on 2021 Jul 9]. Available from: https://thepathologist.com/inside-the-lab/from-moores-law-to-molecular-alterations .
- 119.Li N, Lv T, Sun Y, Liu X, Zeng S, Lv X. High throughput slanted scanning whole slide imaging system for digital pathology. J Biophotonics. 2021;14:e202000499. doi: 10.1002/jbio.202000499. [DOI] [PubMed] [Google Scholar]
- 120.Lujan G, Quigley JC, Hartman D, Parwani A, Roehmholdt B, Meter BV, et al. Dissecting the business case for adoption and implementation of digital pathology: A white paper from the digital pathology association. J Pathol Inform. 2021;12:17. doi: 10.4103/jpi.jpi_67_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng CL, Tan PH. Digital pathology in the diagnostic setting: Beyond technology into best practice and service management. J Clin Pathol. 2017;70:454–7. doi: 10.1136/jclinpath-2016-204272. [DOI] [PubMed] [Google Scholar]