Abstract
The bilin-containing photoreceptor TePixJ, a member of the cyanobacteriochrome (CBCR) family of phytochromes, switches between blue-light-absorbing and green-light-absorbing states in order to drive phototaxis in T. elongatus. Its photoswitching process involves the formation of a thioether linkage between the C10 carbon of phycoviolobilin and the sidechain of Cys494 during the change in state from green-absorbing to blue-absorbing forms. Complex changes in the binding pocket propagate the signal to other domains for downstream signaling. Here, we report time-resolved circular dichroism experiments in addition to pump-probe absorption measurements for interpretation of the biophysical mechanism of the green-to-blue photoconversion process of this receptor.
Keywords: Photoreceptor, pump-probe spectroscopy, circular dichroism, phytochrome
Introduction:
Phytochromes from plants and cyanobacteria and the related cyanobacteriochromes (CBCRs), which are only found in cyanobacteria, form two state photochromic systems that drive numerous cellular signaling processes in their biological systems.1–10 Each contains a conserved GAF domain that houses the tetrapyrrole bilin chromophore.11–15 However, canonical phytochromes contain a PAS-GAF-PHY domain architecture in order to maintain light sensitivity, while CBCRs only require the GAF domain for light sensitivity.16,17 A third class, PAS-less phytochromes, contain GAF and PHY domains but not a PAS domain.18,19 CBCRs display a large range of spectral tuning, with members of the class having light absorption states throughout the visible range.16,20–23 CBCRs and phytochromes switch between signaling states via bilin isomerization. Canonically, the chromophores are covalently bound to the protein via a conserved Cys residue thioether linkage which is necessary for chromophore binding. Upon light absorption, the bilin chromophore isomerizes between the 15Z and 15E states.24–26 This isomerization drives structural rearrangements and signaling activation in the photoreceptor’s output domain.4,14,27–35
CBCR receptors are spectrally tuned via binding pocket chemistry changes which can take a variety of different forms.21,23,32,36–42 As an example, AnPixJ, a homologue of TePixJ, also from T. elongatus, binds phycocyanobilin instead of phycoviolobilin and has very similar chromophore orientations to TePixJ in the Pg state, but instead of converting to a blue light absorbing state when excited as TePixJ does, it becomes red-absorbing with similar chromophore structure to the Cph1 photoreceptor’s red-absorbing state.29,43 This spectral tuning and the relatively small size of these photoreceptors makes them attractive targets for optogenetic tool development.44–49
DXCF CBCRs are a subset of the CBCR family named for their Asp-Xaa-Cys-Phe motif. The cysteine residue in this motif is used to covalently bind to the chromophore and shorten the conjugation relative to the red/far-red absorbing states of canonical phytochromes. Spectral tuning in these DXCF CBCRs is accomplished via a combination of bilin modification and binding pocket interactions which modulate the absorption wavelength.21,29,37,43,50 DXCF CBCRs have varied output functions including shade avoidance,51 regulating phycoerythrin levels,52 control of cell aggregation,53 and phototaxis.36,54
Blue/green CBCRs are a subset of the DXCF family and have absorption maxima in the blue and green wavelength regime. This is accomplished via a second thioether bond from the Cys in the DXCF motif to the chromophore which shortens the conjugated π system of the chromophore, allowing the chromophore to absorb blue light. This second thioether is lost upon photoconversion in the blue to green direction, and regained in the reverse reaction.20,55–57 This photoconversion process has been shown to be very slow in the blue to green direction, which requires loss of the second thioether linkage, while being more representative of typical photoconversion processes in the green to blue direction, which forms the second thioether bond.58,59 Such a disparity in photoconversion rates indicates divergent paths and different rate limiting steps.
In order to understand the structural motions of the photoconversion process and determine the binding pocket behavior, we have performed pump-probe absorption and circular dichroism (CD) studies of the blue/green CBCR TePixJin the green to blue direction.15,55,60 TePixJ is thought to be involved in phototaxis signaling in T. elongatus, due to its output domain being homologous with chemotaxis scaffold domains.60 It has been well characterized structurally but not spectroscopically. Blue (Pb) and green (Pg) end state structures of the GAF domain have been solved by both NMR and X-ray crystallography, while the only blue/green CBCR that has been studied via pump-probe spectroscopy is Tlr0924.29,55,58,59,61,62 For an overview of the structural and chromophore bonding changes involved in photoconversion, see Supplemental Figure S1.
The binding pocket motions that take place between the two structurally characterized end states can be tracked via time-resolved circular dichroism (TRCD), as the GAF domain exhibits differing helicity in the two end states, as well as a change at the phenylalanine peak at 256nm (Figure 1B). Additionally, the chromophore environment changes can also be tracked via TRCD in the visible regime as previously shown for oat phytochrome.63,64 The circular dichroism measurements allow us to link chromophore environment (in the visible regime) and protein structure (in the ultra-violet regime) changes to the light absorption state, which allow new insights into the signaling mechanism of these blue/green CBCRs.
Figure 1.
Steady state spectra of PixJ in Pb (blue) and Pg (green). A) Absorbance data of PixJ. Pb Amax is 422 nm. Pg Amax is 530 nm. The broadness of the Pb peak may indicate multiple chromophore species. B) UV-CD of Pb and Pg. Differences in helicity (< 240 nm) and phenylalanine CD signal (256 nm and inset) are distinguishable. C) Vis-CD of Pb and Pg. The CD spectral characteristics reflect the chromophore environmental differences between the two states.
Methodology:
Protein Expression and Purification.
PixJ GAF domain was expressed with a C-terminal His6-tag in BL21 E. coli cells as described previously.55 Using the same construct as Burgie et al., a 10 mL LB culture with 100 mg/mL Ampicillin and 50 mg/mL Kanamycin antibiotic concentrations was inoculated from −80°C glycerol stock and incubated overnight at 37°C. The following evening at midnight (typical start time when using shared lab space with windows, this method ensures the protein will only be handled after dark) the 10 mL starter culture was split between eight flasks of 500 mL TB spiked with 2 mL glycerol and 2 mM magnesium chloride. These new inoculates were grown at 37°C for four hours (until 4:00 am) to an optical density at 600 nm of 0.8. At this time, the temperature was decreased to 16°C and 10 mg of 5-aminolevulinic acid (5-ALV) was added to each flask, and growth was continued in the dark. This step allows the E. coli to produce heme, which will then be converted into the chromophore. An hour after addition of 5-ALV, IPTG was added to 1 mM final concentration in each flask. This step induces two genes under the control of the lactose repressor and T7 promoter that will convert the heme into the chromophore. The final induction step occurs one hour after IPTG was added with the addition of 2 g of L-arabinose to each flask. This step activates the expression of the TePixJ protein itself, which is under the control of pBAD. Sixteen hours later the cells were pelleted via centrifugation and placed in the −80°C freezer to aid with sonication/lysis.
Cells were lysed via sonication in 500 mM sodium chloride, 20 mM HEPES, pH 7.0, buffer and purified by Ni:NTA affinity column via imidazole step gradient. TePixJ protein elutes at 70 mM imidazole concentration. Ni:NTA chromatography was followed by size exclusion chromatography to generate pure samples. TePixJ monomers elute in the 85 mL range on the GE Superdex column in buffers above 250 mM sodium chloride. Below 100 mM sodium chloride, the protein has a tendency to stick on the column (see supplemental for typical protein purification gels and chromatogram). Expression yield is 2 mg of purified protein per liter expression culture. All expression and purification steps were completed in a dark room under red light in order to preserve the dark-adapted Pb state of TePixJ.
It is known that producing TePixJ via E. coli expression can cause some contamination of PCB in the sample, as documented in Ishizuka et al. 2011.56 From difference peak height analysis of our sample with samples of known PVB to PCB ratios from the same manuscript, we estimate our PCB contamination to be between five and ten percent.
Time-resolved Circular Dichroism and Absorption Spectroscopy.
The green (Pg- or E-state) to blue (Pb- or Z-state) TePixJ photoreaction was triggered by a 140 μJ pulse (3 ns pulse width, full width at half maximum) of 532 nm light generated by a Nd:YAG laser (Pro-250–10 Spectra Physics, Santa Clara, CA). Upon photoinitiation, the spectral changes accompanying the Pg to Pb reaction were monitored by either an absorption (TROD) or a circular dichroism (TRCD) probe as a function of time. The TROD apparatus is the basic skeleton (flash lamp, lenses, spectrograph, and CCD) of the TRCD system, absent the two polarizers and the strain plate (as shown in Supplemental Figure S2).
The TRCD system has been described in detail previously and will be discussed only briefly here.65 A xenon flash lamp probe beam passes through two orthogonally oriented MgF2 polarizers that are positioned before and after a strain plate, two lenses, and the sample in a flow cell or cuvette. Left and right elliptically polarized light are produced by the strain plate when it is oriented at +45°C or −45° relative to the polarization axis of the first polarizer, respectively. A focusing and a re-collimating lens, positioned around the sample, focuses the probe beam to a 1 mm diameter at the sample, where it overlaps with the 2 mm laser beam that is focused down from its initial 9 mm diameter. After passing through the sample, the probe beam continues through the second MgF2 polarizer, is collected by a spectrograph and then detected by a CCD (Andor Technology, Belfast, Ireland). The spectrograph (Monospec 27, Jarrell Ash, Grand Junction, CO) was equipped with a 1 mm slit for experiments in the visible (300–750 nm) and far-UV (200–300 nm) regions and a 1.5 mm slit for measurements in the near-UV wavelength range (240–340 nm). For near- and far-UV measurements, a grating with 600 grooves/mm (300 nm blaze) was used with the spectrograph; in contrast, for experiments in the visible region, a 150 grooves/mm holographic grating (200–800 nm) was used.
PixJ samples for time-resolved experiments were prepared using appropriate aliquots of a stock protein solution of 20 mg/mL in 20 mM HEPES, 50 mM sodium chloride, and 5 mM TCEP (pH 8) diluted with the corresponding buffer solution. For far-UV experiments the protein samples had an optical density at 220 nm of about 0.3 in a 10 μm quartz demountable cell. TePixJ samples for the near-UV experiments were prepared for measurements in a 200 μm quartz demountable cell or a 2 mm flow cell equipped with fused-silica windows (OD270 = 0.8). In the visible region, protein samples with OD530 of 0.6–0.7 were prepared for data collection in the same 2 mm flow cell. To confirm the protein’s ability to cycle between the two chromophore states, the TePixJ sample was converted between Pg and Pb before each experiment with high intensity continuous wave (CW) light (Model 9741–50 Low-Noise Illuminator, Cole Parmer, Chicago, IL). Either a 420 nm or a 530 nm interference filter was used with the CW illuminator to obtain Pg or Pb, respectively. For these photoconversions, equilibrium absorption spectra of the protein solutions were measured to determine the purity of Pg and Pb. Samples were checked to ensure that they maintained proper photoconversion throughout the experiment. Absorption spectra were also measured before and after each time-resolved experiment to confirm the integrity of the initial state (green versus blue) of the protein. All equilibrium absorption spectra were measured on a JASCO V-750 UV/VIS spectrophotometer (JASCO, Inc., Easton, MD), and all experiments were performed at room temperature of 20°C.
For time-resolved experiments that used a 2 mm flow cell, a peristaltic pump was used to recycle the sample between the cell and the sample reservoir. The TRCD and TROD data obtained with a flow cell were collected with a 10 s delay between each laser flash. During this delay, the sample was simultaneously flowed out of the laser beam path and illuminated with 420 nm light from the CW illuminator. These actions served to convert any Pb, formed by the laser or by the thermal back reaction from the initial Pg state, back to Pg and to assure that each incident laser pulse converted pure Pg. Similar precautions were used when the 10 and 200 μm demountable cells were employed. The demountable cells were mounted on an x-y stage so that between laser flashes repositioning and irradiating (with 420 nm CW light) the cell would ensure that the laser beam photoconverted pure Pg. TROD data were collected at 21 time delays from 250 ns to 840 ms after laser photoexcitation. Six time points were measured for chromophore TRCD data – 1, 2, 5, 10, 80 and 840 ms after the initial photo-event. For the TROD and TRCD measurements in this wavelength region, light was cut off below 350 nm (O-51) and 300 nm (O-53) (Corning Glass Works, Corning, NY), respectively, to avoid second order effects. Near-UV TRCD data were accumulated at 5, 10, 25, 80, 840 ms, and 4 s after laser excitation. TRCD data at eight time delays (1, 2, 5, 10, 50, 80, and 840 ms and 4 s) were measured in the far-UV region after photoexcitation. An additional far-UV data point, obtained after CW photoconversion from Pg to Pb on the TRCD apparatus and arbitrarily assigned to 30 s was added for data analysis.
Equilibrium CD spectroscopy.
Far- and near-UV, and visible equilibrium CD spectra were measured with a CD spectrophotometer (J1500, JASCO Inc., Easton, MD) using 10 μm, 200 μm, and 2 mm path length quartz cuvettes in the spectral regions from 180–300, 240–340, and 300–700 nm, respectively. Samples for these measurements were prepared in a similar way as for time-resolved experiments, with final absorbance values of ~0.3, 0.3, and 0.7 at 220, 270, and 530 nm, respectively. Data were collected using a digital integration time of 1 s, data pitch of 0.1 nm, bandwidth of 4 nm, and a scan speed of 200 nm/min. Three to 7 sets of data were obtained for measurements in each of the spectral regions. UV-vis spectra of the protein samples were measured simultaneously on the CD instrument, as well as on the dedicated UV/VIS spectrophotometer.
Data Analysis.
Difference TROD data were calculated by subtracting the pre-trigger absorption spectrum from the spectra of the photoexcited Pg species measured at each time delay. Subsequently, the data were analyzed using singular value decomposition (SVD) and global analysis algorithms written in the mathematical software package MATLAB (The MathWorks, Inc., Natick, MA). The SVD and global kinetic analyses methods have been described in detail previously.64,65 Thus, these methods will only be mentioned briefly here. SVD is a matrix approach that distinguishes between experimental noise and true kinetic intermediates that can be extracted from the spectral variations in the data. This method breaks down A, the data matrix, into mathematically independent spectral, U, and temporal, V, components that are weighted by a singular value, S, based on its significance to the data. How the spectral intermediates behave as a function of time in the data are determined and fit to a product of T, time, and B, spectral, functions, with A = BT, by using global kinetic analysis. B, the spectral components, is sometimes referred to as “b-spectra”, but more often called decay-associated spectra and will be referred to as such here.
The TRCD data decreases in signal to noise from the visible to the far- and near-UV wavelength regions for two major reasons. First, the far-UV region is particularly light-intensity-challenged because of light absorption by the buffer components (HEPES and TCEP) and the protein sample, which has a significantly higher extinction coefficient in this region than in the near-UV and visible wavelengths. Second, the signal change is about 13% in the far-UV and nearly 20% for both the near-UV and the visible regions. Combined, these factors contribute to much smaller and noisier circular dichroism spectral changes compared to the TROD data. For this reason, kinetic analysis was approached by exponential fitting using SigmaPlot (Version 13.0, Systat Software, Inc., San Jose, CA), despite the fact that SVD is particularly useful for separating noise from data. “Single wavelength” data were calculated from the multi-wavelength data (SI Figures S6–S8) by averaging about 6 nm around the wavelength of interest. The “single wavelengths” used in the exponential analyses are 220 nm (217–223 nm), 256 nm (253–259 nm), and 356 nm (353–359 nm). These wavelengths were chosen because they are spectral extrema, as shown in Figure 1. See SI Figure S6 for a justification for using this analysis method and SI Figure S9 for analysis of the 530 nm band.
Results and Discussion:
Figure 2 shows TROD data measured from 250 ns to 840 ms after initiation of the Pg to Pb photoreaction with 532nm laser light. (Steady state absorption spectra of the E and Z species shown in Figure 1). These data are best fit to three exponential components with time constants (and amplitudes) of 600 ± 130 ns (0.054 ± 0.03), 955 ± 150 μs (0.095 ± 0.01), and 10 ± 2.3 ms (0.48 ± 0.02). A recent study of the photoconversion of TePixJ determined much faster kinetics for the photoreceptor.66 The nature of why their rates are faster is currently unknown. Most likely it is due to a higher reaction temperature, as photoreceptors are known to be highly temperature sensitive.55,67–69At the first measured time point, 250 ns, a red-shifted intermediate is observed (Figure 2). This should be the Lumi-G state, that is, the state where the chromophore’s D-ring has isomerized. This initial state has also been reported for Tlr0924, the TePixJ homologue.59,62 Lumi-G transitions to 15Z-MetaG1 with a time constant of 600 ns. In this state, it is understood that the chromophore has isomerized at the D-ring after absorbing the photon and has begun to flex or move in the binding pocket, thus changing the absorption wavelength.
Figure 2.
Time-resolved absorption spectra measured at 21 time-delays after initiation of the Pg to Pb photoreaction (A). Grey arrows show the direction of the spectral changes from 250 ns (grey dots on green line) to 840 ms (grey dots on blue line) after photoexcitation. The horizontal arrow indicates a broadening of the absorbance around 560 nm ~1 ms before the band decreases in intensity. TROD data were best fit to three exponential processes using SVD analysis with time constants of 600 ± 130 ns, 955 ± 150 μs, and 10 ± 2 ms. See SI Figure 5 for comparison of residuals. The decay-associated spectra for these three exponential lifetimes are shown in (B). The spectrum measured at long times, t∞, can be overlaid with the difference spectrum (data not shown) calculated from the equilibrium Pg and Pb spectra shown in Figure 1A.
A second, more strongly absorbing, red-shifted peak (~570 nm) is observed with a time constant of 955 μs. This peak appears much later than a similar intermediate observed in Tlr0924, which appears in 5.3 μs.59 Our interpretation of this peak is that the chromophore has stabilized in the binding pocket in a slightly different and more planar orientation, hence lowering the energy landscape of the chromophore. Other phytochromes and cyanobacteriochrome receptors have been suggested to also transition through this intermediate.58,59 In our mechanism, we refer to this state as 15Z-MetaG2. It is unclear at present why the TePixJ intermediate occurs so much later than its relative Tlr0924, but we believe it to be one of two possibilities. First, it could be a product of different construct sizes used in the two experiments. The Tlr0924 experiments were performed on the full-length protein, while the TePixJ result is for GAF domain only.58,59 There could be some effect from other domains, since the structure is still unknown.
It seems more likely, however, that binding pocket differences are more likely the cause, since the end state in the visible absorption (the formation of the blue-absorbing species) is faster in TePixJ than Tlr0924, where downstream domains are expected to have more influence on kinetics.59
The final state observed in the TROD experiments is the appearance of the final blue absorbing state, which is observed at 10 ms. This is slower than non-Tlr0924 phytochromes and CBCRs, but is quicker than the published results for Tlr0924.59,63,64 The difference in the timing of the photoconversion also could be the result of only using the GAF domain of TePixJ, whereas the Tlr0924 measurements used the full-length protein. The visible TROD results, as a whole, are in line with previous studies on phytochromes, both in the spectral intermediates observed and the order in which they appear.
In our pump-probe absorbance experiments in the other direction (Pb to Pg), we observe very different rates of photoconversion and intermediates, indicating divergent mechanisms for photoconversion (Supplemental Figure S3). The Pb to Pg photoconversion event takes four seconds to complete, much longer than the Pg to Pb reaction. This rate disparity indicates that the Pb to Pg photoconversion process is distinct from the Pg to Pb conversion and not limited by protein motion, as we would expect the time regimes for the inverse protein motion to be on the same timescale, instead of two orders of magnitude slower. Therefore, we believe that in the Pb to Pg case, the rupture of the thioether bond is inhibited and rate-limiting.
Nanosecond Time-resolved Circular Dichroism Spectroscopy of the Chromophore Region (356 and 530 nm).
In order to draw conclusions about the local structure around the chromophore, TRCD experiments were performed in the 300–750 nm spectral region. (For steady state spectra of both end states, see Figure 1C). CD in this region observes the chromophore and its relationship with the binding pocket during the photoconversion, as binding pocket protein residues affect the energy landscape of the chromophore.
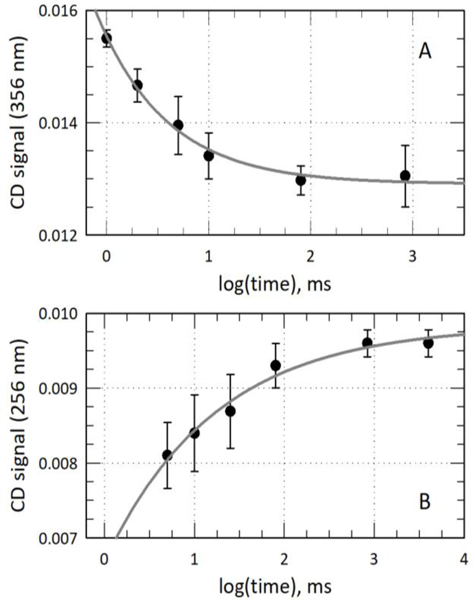
Figure 3A shows the chromophore transitions from the starting energy state to final energy state in this wavelength range with a time constant of 6 ± 2 ms, which is slightly shorter than the appearance of the blue absorbing state from the time-resolved absorbance data. This result suggests that the chromophore makes its way across the binding pocket from the earliest detectable motion in 6 ms, and the chromophore comes to rest just before the Cys494 side chain forms the covalent bond and changes the absorption spectrum.
Figure 3.
TRCD kinetics of the Pg to Pb photoreaction followed at 356 nm (A) and 256 nm (B), respectively. The data were best fit to single exponential functions with time constants of 6 ± 2 and 13 ± 5 ms, respectively.
Analysis of the visible chromophore band (530 nm) was not as clear-cut as for the 356 nm band because of interference by a band of unknown origin at 480 nm. A rough estimate of the kinetics of the 530 nm yields a single exponential lifetime of 9 ms. See SI Figure S8 for more information.
Nanosecond Time-resolved Near-UV (256 nm) Circular Dichroism Spectroscopy.
Near-UV (240–340 nm) TRCD spectroscopy was used to follow the motion of the DXCF motif phenylalanine (Phe495).70 Near-UV CD has been used to track structural changes in aromatic sidechain groups, especially during changes in solvation.71,72 Mutagenesis of Phe495 to tyrosine and alanine have been shown to lower chromoprotein stability in TePixJ.55 F495A breaks the WT photocycle. Phe495 is directly adjacent to Cys494, which makes the covalent bond that forms between the B and C rings during the photoconversion from Pg to Pb. This Phe goes from solvent exposed in the Pg state to buried under the chromophore in the Pb state, which requires a 180° rotation of the loop containing Cys494 and Phe495 (Supplementary Figure S4). This shift leads to an increase in CD signal as the Phe sidechain becomes constrained underneath the C-ring of the chromophore. As shown in Figure 3B, the data, while noisy because of the small signal change upon photoconversion of Pg, has a 13 ± 5 ms time constant, similar to that of the formation of the blue-absorbing species in our TROD data (Figure 2, τ = 10 ± 2 ms). This indicates that as the Cys attaches, the Phe is buried under the chromophore in the binding pocket. This is consistent with the formation of the covalent bond taking place concurrently with Cys494 and Phe495 coming to their final resting place in the binding pocket. Due to the challenges involved in collection and interpretation of TRCD data in this spectral region, chromophore contribution to this spectral feature cannot be ruled out at this time. Future experiments will be required to confirm the Phe495 kinetics via other methods, such as time-resolved crystallography or time-resolved cryo-electron microscopy.
Nanosecond Time-resolved Far-UV Circular Dichroism Spectroscopy
To follow global secondary structural changes, TRCD data were collected in the far-UV regime (200–300 nm). The kinetics shown in Figure 4 indicate that the protein backbone motion trails the absorbance change as the protein searches for new energy minima in a relatively flat landscape. This is consistent with the hypothesis that the Pg to Pb photoconversion is rate-limited by the protein motion required for rearrangement of the binding pocket.
Figure 4.
Far-UV TRCD kinetics of the Pg to Pb photoreaction probed at 220 nm. These data were best fit to a single exponential process with a time constant of 47 ± 15 ms.
Mechanism of green to blue photoconversion accounting for structural rearrangements of the protein.
Our mechanism, shown in Figure 5, is based on our spectroscopy and published crystal and NMR structures and fills in the microsecond to millisecond time gap observed via other techniques, such as femtosecond flash photolysis, time-resolved IR, and cryo-trapping. More rapidly than we can observe, the chromophore is excited and enters the 15E-G* state and then isomerizes into the 15Z-LumiG state on the order of picoseconds. Thus, the TROD experiments follow the tail of the decay of this Lumi state and its passage into 15Z-MetaG1, a more strongly absorbing red-shifted state than LumiG, as the chromophore stabilizes into a slightly lower energy state. At this point, the chromophore is sliding along the binding pocket. This slide is known to be required from the crystal and NMR structures of the Pb and Pg states, where the chromophore slides and rotates significantly towards the front of the binding pocket during formation of Pb.29,55,61 The chromophore reaches its final resting place after 6 ms, in a state we refer to as 15Z-MetaG3. At the beginning of the photoconversion process, Cys494 is pointed away from the binding pocket, as is the adjacent Phe, Phe495, whose motion is tracked with near-UV CD at 256nm. We use this Phe as a proxy for the loop motion and the adjacent cysteine residue. That helix must swing around and upward in order to form the thioether linkage and create the photocycle. This motion happens on the same time scale as the chromophore absorbance change, but physically must take place first based on the structure. Therefore, we assign the state to be 15Z-MetaG4. After the Cys is in place, the thioether linkage forms rapidly, within the uncertainty of the near-UV measurement. However, although the chromophore has reached its final resting place in the binding pocket, the protein has not finished moving at this point, so we refer to this intermediate as 15Z-Bmolten. Finally, the protein finishes its own transition to the blue-absorbing state and comes to rest after 47ms. Only then do we believe that the 15Z-B state has finally been achieved. We believe these motions to be conserved for not just other cyanobacteriochrome receptors, but phytochrome systems in general, as their bilin chromophores slide in the binding pocket canonically to photoconvert and activate their signaling domain.
Figure 5.
Mechanism of Pg to Pb photoconversion accounting for time-resolved absorption and CD kinetics. Light at 532 nm is absorbed at the chromophore, exciting it. Based on the work of Hardman et al. (33), the chromophore isomerizes faster than we can observe in the experiments reported here and becomes the Lumi state, around 5 ps. We then observe 600 ns and 955 μs intermediates in the TROD data that involve small absorbance peak shifts. Visible TRCD data report a process with a rate of 6 ms, indicating that the environmental asymmetry of the chromophore changes before its electrostatic environment. Near UV-CD data show a rate of 13 ms, a lifetime that is similar to the 10 ms reported by the TROD measurements. This indicates that structural reorganization, Phe495 is buried, occurs concurrently with electrostatic changes, Cys494 attachment. Finally, slow protein rearrangements continue with a rate constant of 47 ms. These data are consistent with the theory that protein motion limits the rate of photoconversion.
Conclusions.
Based on our experimental results and previous structural work, we show a mechanism of photoconversion where the protein re-arrangements are rate limiting and play a major role in the medium to late time-scale kinetics of TePixJ. Because our TRCD measurements focus on protein structural changes, previously unobserved intermediates explain the photoconversion process between the microsecond regime and the final formation of Pb. Others, specifically Hardman et al.,59 had observed the fast kinetics of chromophore light absorption and isomerization for this class in their work on Tlr0924, the closest homologue to TePixJ, as well as the slow, microsecond appearance of the red-shifted intermediate. However, their work does not use experimental methods that can provide data on what happens between this microsecond intermediate and the end state. Based on structural work completed by the Vierstra lab and others,29,55,61,73 structural motions that must take place during photoconversion can be inferred, but not when or in what order such changes occur. With the incorporation of our new data, we can comprehensively model the green to blue photoconversion of TePixJ on these timescales. Similar motions must be occurring in the homologue Tlr0924 during its photoconversion as well, and phytochromes in general must incorporate some of these motions, as the GAF domain slides the bilin chromophore across itself and must account for similar isomerization processes.27,34,74,75
Once the new intermediates are accounted for, the photoconversion mechanism clearly becomes an intricate dance between the protein and the chromophore, as the protein leads the newly isomerized chromophore into a different energy minimum and rearranges itself to accommodate the new location in the binding pocket, with motions large enough to be detectable and attributed to shifts in helicity. This protein rearrangement appears to be rate limiting in this case, as the blue absorption species appears rapidly after the formation of relevant intermediates in the circular dichroism spectroscopy. We hypothesize that many photoconversion processes that happen on these millisecond timescales are protein structural motion-limited by nature, as evidenced by SAXS/WAXS studies on other photoreceptors.27,30,75–78 The transmission of light absorbing events to protein signaling via recognition of altered conformational states is thus coming into focus.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank E.S. Burgie and R. D. Vierstra for providing the TePixJ constructs and instructions for expression and purification of said constructs, as well as insightful discussions pertaining to the biological implications of the photoconversion mechanism of the blue/green CBCRs.
Funding Sources
This work was supported by a grant from the NSF to the Science and Technology Center, BioXFEL, Contract 1231306 (to G.N.P.). This work was partially supported by a training fellowship for Jonathan Clinger from the Keck Center of the Gulf Coast Consortia, on the Houston Area Molecular Biophysics Program, National Institute of General Medical Sciences (NIGMS) T32GM008280.
ABBREVIATIONS
- CBCR
cyanobacteriochrome receptor
- GAF
cGMP phosphodiesterase/Adenylate cyclase/FhlA
- PAS
Per/ARNT/Sim
- PHY
phytochrome specific
- CD
circular dichroism
- TRCD
time-resolved circular dichroism
- 5-ALV
5-aminolevulinic acid
- IPTG
Description Isopropyl β-d-1-thiogalactopyranoside
- TROD
time-resolved optical density
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- TCEP
Tris(2 carboxyethyl) phosphine
- SAXS
small angle X-ray scattering
- WAXS
wide angle X-ray scattering
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
The authors declare no competing financial interest.
REFERENCES
- (1).Burgie ES; Vierstra RD Phytochromes: An Atomic Perspective on Photoactivation and Signaling. Plant Cell 2014, 26 (12), 4568–4583. 10.1105/tpc.114.131623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Leivar P; Monte E. PIFs: Systems Integrators in Plant Development. Plant Cell 2014, 26 (1), 56–78. 10.1105/tpc.113.120857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Chen M; Chory J. Phytochrome Signaling Mechanisms and the Control of Plant Development. Trends Cell Biol. 2011, 21 (11), 664–671. 10.1016/j.tcb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rockwell NC; Su Y-S; Lagarias JC PHYTOCHROME STRUCTURE AND SIGNALING MECHANISMS. Annu. Rev. Plant Biol 2006, 57 (1), 837–858. 10.1146/annurev.arplant.56.032604.144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Karniol B; Wagner JR; Walker JM; Vierstra RD Phylogenetic Analysis of the Phytochrome Superfamily Reveals Distinct Microbial Subfamilies of Photoreceptors. Biochem. J 2005, 392 (1), 103–116. 10.1042/BJ20050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yeh K; Wu SH; Murphy JT; Lagarias JC A Cyanobacterial Phytochrome Two-Component Light Sensory System. Science (80-. ). 1997, 277 (5331), 1505–1508. 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]
- (7).Rockwell NC; Lagarias JC A Brief History of Phytochromes. ChemPhysChem. Wiley-VCH Verlag April 26, 2010, pp 1172–1180. 10.1002/cphc.200900894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Rockwell NC; Lagarias JC Phytochrome Evolution in 3D: Deletion, Duplication, and Diversification. New Phytol. 2020, 225 (6), 2283–2300. 10.1111/nph.16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wiltbank LB; Kehoe DM Diverse Light Responses of Cyanobacteria Mediated by Phytochrome Superfamily Photoreceptors. Nature Reviews Microbiology. Nature Publishing Group January 1, 2019, pp 37–50. 10.1038/s41579-018-0110-4. [DOI] [PubMed] [Google Scholar]
- (10).Li FW; Melkonian M; Rothfels CJ; Villarreal JC; Stevenson DW; Graham SW; Wong GKS; Pryer KM; Mathews S. Phytochrome Diversity in Green Plants and the Origin of Canonical Plant Phytochromes. Nat. Commun 2015, 6 (1), 1–12. 10.1038/ncomms8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Montgomery BL; Lagarias JC Phytochrome Ancestry: Sensors of Bilins and Light. Trends in Plant Science. Elsevier Current Trends August 1, 2002, pp 357–366. 10.1016/S1360-1385(02)02304-X. [DOI] [PubMed] [Google Scholar]
- (12).Lagarias JC; Rapoport H. Chromopeptides from Phytochrome. The Structure and Linkage of the PR Form of the Phytochrome Chromophore. J. Am. Chem. Soc 1980, 102 (14), 4821–4828. 10.1021/ja00534a042. [DOI] [Google Scholar]
- (13).Hanzawa H; Shinomura T; Inomata K; Kakiuchi T; Kinoshita H; Wada K; Furuya M. Structural Requirement of Bilin Chromophore for the Photosensory Specificity of Phytochromes A and B. Proc. Natl. Acad. Sci 2002, 99 (7), 4725–4729. 10.1073/pnas.062713399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).van Thor JJ; Mackeen M; Kuprov I; Dwek RA; Wormald MR Chromophore Structure in the Photocycle of the Cyanobacterial Phytochrome Cph1. Biophys. J 2006, 91 (5), 1811–1822. 10.1529/biophysj.106.084335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ishizuka T; Narikawa R; Kohchi T; Katayama M; Ikeuchi M. Cyanobacteriochrome TePixJ of Thermosynechococcus Elongatus Harbors Phycoviolobilin as a Chromophore. Plant Cell Physiol. 2007, 48 (9), 1385–1390. 10.1093/pcp/pcm106. [DOI] [PubMed] [Google Scholar]
- (16).Yoshihara S; Katayama M; Geng X; Ikeuchi M. Cyanobacterial Phytochrome-like PixJ1 Holoprotein Shows Novel Reversible Photoconversion between Blue- and Green-Absorbing Forms. Plant Cell Physiol. 2004, 45 (12), 1729–1737. 10.1093/pcp/pch214. [DOI] [PubMed] [Google Scholar]
- (17).Hirose Y; Shimada T; Narikawa R; Katayama M; Ikeuchi M. Cyanobacteriochrome CcaS Is the Green Light Receptor That Induces the Expression of Phycobilisome Linker Protein. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (28), 9528–9533. 10.1073/pnas.0801826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Wu SH; Lagarias JC Defining the Bilin Lyase Domain: Lessons from the Extended Phytochrome Superfamily. Biochemistry 2000, 39 (44), 13487–13495. 10.1021/bi001123z. [DOI] [PubMed] [Google Scholar]
- (19).Anders K; Daminelli-Widany G; Mroginski MA; Von Stetten D; Essen LO Structure of the Cyanobacterial Phytochrome 2 Photosensor Implies a Tryptophan Switch for Phytochrome Signaling. J. Biol. Chem 2013, 288 (50), 35714–35725. 10.1074/jbc.M113.510461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rockwell NC; Martin SS; Feoktistova K; Lagarias JC Diverse Two-Cysteine Photocycles in Phytochromes and Cyanobacteriochromes. Proc. Natl. Acad. Sci 2011, 108 (29), 11854–11859. 10.1073/pnas.1107844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Rockwell NC; Martin SS; Gulevich AG; Lagarias JC Phycoviolobilin Formation and Spectral Tuning in the DXCF Cyanobacteriochrome Subfamily. Biochemistry 2012, 51 (7), 1449–1463. 10.1021/bi201783j. [DOI] [PubMed] [Google Scholar]
- (22).Enomoto G; Nomura R; Shimada T; Ni-Ni-Win; Narikawa R; Ikeuchi M. Cyanobacteriochrome SesA Is a Diguanylate Cyclase That Induces Cell Aggregation in Thermosynechococcus. J. Biol. Chem 2014, 289 (36), 24801–24809. 10.1074/jbc.M114.583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Song J-Y; Lee HY; Yang HW; Song J-J; Lagarias JC; Park Y-I Spectral and Photochemical Diversity of Tandem Cysteine Cyanobacterial Phytochromes. J. Biol. Chem 2020, 295 (19), 6754–6766. 10.1074/jbc.RA120.012950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Claesson E; Wahlgren WY; Takala H; Pandey S; Castillon L; Kuznetsova V; Henry L; Panman M; Carrillo M; Kübel J; et al. The Primary Structural Photoresponse of Phytochrome Proteins Captured by a Femtosecond X-Ray Laser. Elife 2020, 9. 10.7554/eLife.53514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yang X; Ren Z; Kuk J; Moffat K. Temperature-Scan Cryocrystallography Reveals Reaction Intermediates in Bacteriophytochrome. Nature 2011, 479 (7373), 428–432. 10.1038/nature10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Song C; Psakis G; Lang C; Mailliet J; Gärtner W; Hughes J; Matysik J. Two Ground State Isoforms and a Chromophore D-Ring Photoflip Triggering Extensive Intramolecular Changes in a Canonical Phytochrome. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (10), 3842–3847. 10.1073/pnas.1013377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Heyes DJ; Hardman SJO; Pedersen MN; Woodhouse J; De La Mora E; Wulff M; Weik M; Cammarata M; Scrutton NS; Schirò G. Light-Induced Structural Changes in a Full-Length Cyanobacterial Phytochrome Probed by Time-Resolved X-Ray Scattering. Commun. Biol 2019, 2 (1), 1. 10.1038/s42003-018-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Velazquez Escobar F; Utesch T; Narikawa R; Ikeuchi M; Mroginski MA; Gärtner W; Hildebrandt P. Photoconversion Mechanism of the Second GAF Domain of Cyanobacteriochrome AnPixJ and the Cofactor Structure of Its Green-Absorbing State. Biochemistry 2013, 52 (29), 4871–4880. 10.1021/bi400506a. [DOI] [PubMed] [Google Scholar]
- (29).Narikawa R; Ishizuka T; Muraki N; Shiba T; Kurisu G; Ikeuchi M. Structures of Cyanobacteriochromes from Phototaxis Regulators AnPixJ and TePixJ Reveal General and Specific Photoconversion Mechanism. Proc. Natl. Acad. Sci 2013, 110 (3), 918–923. 10.1073/pnas.1212098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Björling A; Berntsson O; Lehtivuori H; Takala H; Hughes AJ; Panman M; Hoernke M; Niebling S; Henry L; Henning R; et al. Structural Photoactivation of a Full-Length Bacterial Phytochrome. Sci. Adv 2016, 2 (8), e1600920. 10.1126/sciadv.1600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Schmidt A; Sauthof L; Szczepek M; Lopez MF; Escobar FV; Qureshi BM; Michael N; Buhrke D; Stevens T; Kwiatkowski D; et al. Structural Snapshot of a Bacterial Phytochrome in Its Functional Intermediate State. Nat. Commun 2018, 9 (1), 4912. 10.1038/s41467-018-07392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Rockwell NC; Martin SS; Lagarias JC There and Back Again: Loss and Reacquisition of Two-Cys Photocycles in Cyanobacteriochromes. Photochem. Photobiol 2017, 93 (3), 741–754. 10.1111/php.12708. [DOI] [PubMed] [Google Scholar]
- (33).Yang X; Stojković EA; Kuk J; Moffat K. Crystal Structure of the Chromophore Binding Domain of an Unusual Bacteriophytochrome, RpBphP3, Reveals Residues That Modulate Photoconversion. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (30), 12571–12576. 10.1073/pnas.0701737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Yang X; Kuk J; Moffat K. Conformational Differences between the Pfr and Pr States in Pseudomonas Aeruginosa Bacteriophytochrome. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (37), 15639–15644. 10.1073/pnas.0902178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Takala H; Björling A; Berntsson O; Lehtivuori H; Niebling S; Hoernke M; Kosheleva I; Henning R; Menzel A; Ihalainen JA; et al. Signal Amplification and Transduction in Phytochrome Photosensors. Nature 2014, 509 (7499), 245–248. 10.1038/nature13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Song J-Y; Cho HS; Cho J-I; Jeon J-S; Lagarias JC; Park Y-I Near-UV Cyanobacteriochrome Signaling System Elicits Negative Phototaxis in the Cyanobacterium Synechocystis Sp. PCC 6803. Proc. Natl. Acad. Sci 2011, 108 (26), 10780–10785. 10.1073/pnas.1104242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Enomoto G; Hirose Y; Narikawa R; Ikeuchi M. Thiol-Based Photocycle of the Blue and Teal Light-Sensing Cyanobacteriochrome Tlr1999. Biochemistry 2012, 51 (14), 3050–3058. 10.1021/bi300020u. [DOI] [PubMed] [Google Scholar]
- (38).Bussell AN; Kehoe DM Control of a Four-Color Sensing Photoreceptor by a Two-Color Sensing Photoreceptor Reveals Complex Light Regulation in Cyanobacteria. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (31), 12834–12839. 10.1073/pnas.1303371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lim S; Rockwell NC; Martin SS; Dallas JL; Lagarias JC; Ames JB Photoconversion Changes Bilin Chromophore Conjugation and Protein Secondary Structure in the Violet/Orange Cyanobacteriochrome NpF2163g3. Photochem. Photobiol. Sci 2014, 13 (6), 951–962. 10.1039/c3pp50442e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ma Q; Hua H-H; Chen Y; Liu B-B; Krämer AL; Scheer H; Zhao K-H; Zhou M. A Rising Tide of Blue-Absorbing Biliprotein Photoreceptors - Characterization of Seven Such Bilin-Binding GAF Domains in Nostoc Sp. PCC7120. FEBS J. 2012, 279 (21), 4095–4108. 10.1111/febs.12003. [DOI] [PubMed] [Google Scholar]
- (41).Lim S; Yu Q; Gottlieb SM; Chang CW; Rockwell NC; Martin SS; Madsen D; Lagarias JC; Larsen DS; Ames JB Correlating Structural and Photochemical Heterogeneity in Cyanobacteriochrome NpR6012g4. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (17), 4387–4392. 10.1073/pnas.1720682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Narikawa R; Kohchi T; Ikeuchi M. Characterization of the Photoactive GAF Domain of the CikA Homolog (SyCikA, Slr1969) of the Cyanobacterium Synechocystis Sp. PCC 6803. In Photochemical and Photobiological Sciences; Royal Society of Chemistry, 2008; Vol. 7, pp 1253–1259. 10.1039/b811214b. [DOI] [PubMed] [Google Scholar]
- (43).Song C; Narikawa R; Ikeuchi M; Gärtner W; Matysik J. Color Tuning in Red/Green Cyanobacteriochrome AnPixJ: Photoisomerization at C15 Causes an Excited-State Destabilization. J. Phys. Chem. B 2015, 119 (30), 9688–9695. 10.1021/acs.jpcb.5b04655. [DOI] [PubMed] [Google Scholar]
- (44).Möglich A; Moffat K. Engineered Photoreceptors as Novel Optogenetic Tools. Photochemical and Photobiological Sciences. Royal Society of Chemistry September 28, 2010, pp 1286–1300. 10.1039/c0pp00167h. [DOI] [PubMed] [Google Scholar]
- (45).Ramakrishnan P; Tabor JJ Repurposing Synechocystis PCC6803 UirS–UirR as a UV-Violet/Green Photoreversible Transcriptional Regulatory Tool in E. Coli. ACS Synth. Biol 2016, 5 (7), 733–740. 10.1021/acssynbio.6b00068. [DOI] [PubMed] [Google Scholar]
- (46).Blain-Hartung M; Rockwell NC; Moreno MV; Martin SS; Gan F; Bryant DA; Lagarias JC Cyanobacteriochrome-Based Photoswitchable Adenylyl Cyclases (CPACs) for Broad Spectrum Light Regulation of CAMP Levels in Cells. J. Biol. Chem 2018, 293 (22), 8473–8483. 10.1074/jbc.RA118.002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Olson EJ; Tzouanas CN; Tabor JJ A Photoconversion Model for Full Spectral Programming and Multiplexing of Optogenetic Systems. Mol. Syst. Biol 2017, 13 (4), 926. 10.15252/msb.20167456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Kuwasaki Y; Miyake K; Fushimi K; Takeda Y; Ueda Y; Nakajima T; Ikeuchi M; Sato M; Narikawa R. Protein Engineering of Dual-Cys Cyanobacteriochrome AM1_1186g2 for Biliverdin Incorporation and Far-Red/Blue Reversible Photoconversion. Int. J. Mol. Sci 2019, 20 (12), 2935. 10.3390/ijms20122935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Blain-Hartung M; Rockwell NC; Lagarias JC Light-Regulated Synthesis of Cyclic-Di-GMP by a Bidomain Construct of the Cyanobacteriochrome Tlr0924 (SesA) without Stable Dimerization. Biochemistry 2017, 56 (46), 6145–6154. 10.1021/acs.biochem.7b00734. [DOI] [PubMed] [Google Scholar]
- (50).Hasegawa M; Fushimi K; Miyake K; Nakajima T; Oikawa Y; Enomoto G; Sato M; Ikeuchi M; Narikawa R. Molecular Characterization of DXCF Cyanobacteriochromes from the Cyanobacterium Acaryochloris Marina Identifies a Blue-Light Power Sensor. J. Biol. Chem 2018, 293 (5), 1713–1727. 10.1074/jbc.M117.816553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Enomoto G; Ikeuchi M. Blue-/Green-Light-Responsive Cyanobacteriochromes Are Cell Shade Sensors in Red-Light Replete Niches. iScience 2020, 23 (3), 100936. 10.1016/j.isci.2020.100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Wiltbank LB; Kehoe DM Two Cyanobacterial Photoreceptors Regulate Photosynthetic Light Harvesting by Sensing Teal, Green, Yellow, and Red Light. MBio 2016, 7 (1). 10.1128/mBio.02130-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Enomoto G; Ni-Ni-Win; Narikawa R; Ikeuchi M. Three Cyanobacteriochromes Work Together to Form a Light Color-Sensitive Input System for c-Di-GMP Signaling of Cell Aggregation. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (26), 8082–8087. 10.1073/pnas.1504228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Yang Y; Lam V; Adomako M; Simkovsky R; Jakob A; Rockwell NC; Cohen SE; Taton A; Wang J; Clark Lagarias J; et al. Phototaxis in a Wild Isolate of the Cyanobacterium Synechococcus Elongatus. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (52), E12378–E12387. 10.1073/pnas.1812871115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Burgie ES; Walker JM; Phillips GN; Vierstra RD A Photo-Labile Thioether Linkage to Phycoviolobilin Provides the Foundation for the Blue/Green Photocycles in DXCF-Cyanobacteriochromes. Structure 2013, 21 (1), 88–97. 10.1016/j.str.2012.11.001. [DOI] [PubMed] [Google Scholar]
- (56).Ishizuka T; Kamiya A; Suzuki H; Narikawa R; Noguchi T; Kohchi T; Inomata K; Ikeuchi M. The Cyanobacteriochrome, TePixJ, Isomerizes Its Own Chromophore by Converting Phycocyanobilin to Phycoviolobilin. Biochemistry 2011, 50 (6), 953–961. 10.1021/bi101626t. [DOI] [PubMed] [Google Scholar]
- (57).Ulijasz AT; Cornilescu G; von Stetten D; Cornilescu C; Velazquez Escobar F; Zhang J; Stankey RJ; Rivera M; Hildebrandt P; Vierstra RD Cyanochromes Are Blue/Green Light Photoreversible Photoreceptors Defined by a Stable Double Cysteine Linkage to a Phycoviolobilin-Type Chromophore. J. Biol. Chem 2009, 284 (43), 29757–29772. 10.1074/jbc.M109.038513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Hauck AFE; Hardman SJO; Kutta RJ; Greetham GM; Heyes DJ; Scrutton NS The Photoinitiated Reaction Pathway of Full-Length Cyanobacteriochrome Tlr0924 Monitored Over 12 Orders of Magnitude. J. Biol. Chem 2014, 289 (25), 17747–17757. 10.1074/jbc.M114.566133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Hardman SJO; Hauck AFE; Clark IP; Heyes DJ; Scrutton NS Comprehensive Analysis of the Green-to-Blue Photoconversion of Full-Length Cyanobacteriochrome Tlr0924. Biophys. J 2014, 107 (9), 2195–2203. 10.1016/j.bpj.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ishizuka T; Shimada T; Okajima K; Yoshihara S; Ochiai Y; Katayama M; Ikeuchi M. Characterization of Cyanobacteriochrome TePixJ from a Thermophilic Cyanobacterium Thermosynechococcus Elongatus Strain BP-1. Plant Cell Physiol. 2006, 47 (9), 1251–1261. 10.1093/pcp/pcj095. [DOI] [PubMed] [Google Scholar]
- (61).Cornilescu CC; Cornilescu G; Burgie ES; Markley JL; Ulijasz AT; Vierstra RD Dynamic Structural Changes Underpin Photoconversion of a Blue/Green Cyanobacteriochrome between Its Dark and Photoactivated States. J. Biol. Chem 2014, 289 (5), 3055–3065. 10.1074/jbc.M113.531053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Freer LH; Kim PW; Corley SC; Rockwell NC; Zhao L; Thibert AJ; Lagarias JC; Larsen DS Chemical Inhomogeneity in the Ultrafast Dynamics of the DXCF Cyanobacteriochrome Tlr0924. J. Phys. Chem. B 2012, 116 (35), 10571–10581. 10.1021/jp302637u. [DOI] [PubMed] [Google Scholar]
- (63).Bjorling SC; Zhang CF; Farrens DL; Song PS; Kliger DS Time-Resolved Circular Dichroism of Native Oat Phytochrome Photointermediates. J. Am. Chem. Soc 1992, 114 (12), 4581–4588. 10.1021/ja00038a020. [DOI] [Google Scholar]
- (64).Chen E; Lapko VN; Song P-S; Kliger DS Dynamics of the N-Terminal α-Helix Unfolding in the Photoreversion Reaction of Phytochrome A †. Biochemistry 1997, 36 (16), 4903–4908. 10.1021/bi9627065. [DOI] [PubMed] [Google Scholar]
- (65).Chen E; Goldbeck RA; Kliger DS Nanosecond Time-Resolved Polarization Spectroscopies: Tools for Probing Protein Reaction Mechanisms. Methods 2010, 52 (1), 3–11. 10.1016/j.ymeth.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Hardman SJO; Heyes DJ; Sazanovich IV; Scrutton NS Photocycle of Cyanobacteriochrome TePixJ. Biochemistry 2020, 59 (32), 2909–2915. 10.1021/acs.biochem.0c00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Fujii Y; Tanaka H; Konno N; Ogasawara Y; Hamashima N; Tamura S; Hasegawa S; Hayasaki Y; Okajima K; Kodama Y. Phototropin Perceives Temperature Based on the Lifetime of Its Photoactivated State. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (34), 9206–9211. 10.1073/pnas.1704462114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Legris M; Klose C; Burgie ES; Rojas CC; Neme M; Hiltbrunner A; Wigge PA; Schäfer E; Vierstra RD; Casal JJ Phytochrome B Integrates Light and Temperature Signals in Arabidopsis. Science (80-. ). 2016, 354 (6314), 897–900. 10.1126/science.aaf5656. [DOI] [PubMed] [Google Scholar]
- (69).Jung JH; Domijan M; Klose C; Biswas S; Ezer D; Gao M; Khattak AK; Box MS; Charoensawan V; Cortijo S; et al. Phytochromes Function as Thermosensors in Arabidopsis. Science (80-. ). 2016, 354 (6314), 886–889. 10.1126/science.aaf6005. [DOI] [PubMed] [Google Scholar]
- (70).Woody RW; Dunker AK Aromatic and Cystine Side-Chain Circular Dichroism in Proteins. In Circular Dichroism and the Conformational Analysis of Biomolecules; Springer US: Boston, MA, 1996; pp 109–157. 10.1007/978-1-4757-2508-7_4. [DOI] [Google Scholar]
- (71).Chen E; Kliger DS Time-Resolved near UV Circular Dichroism and Absorption Studies of Carbonmonoxymyoglobin Photolysis Intermediates. Inorganica Chim. Acta 1996. 10.1016/0020-1693(95)04860-x. [DOI] [Google Scholar]
- (72).Ianeselli A; Orioli S; Spagnolli G; Faccioli P; Cupellini L; Jurinovich S; Mennucci B. Atomic Detail of Protein Folding Revealed by an Ab Initio Reappraisal of Circular Dichroism. J. Am. Chem. Soc 2018, 140 (10), 3674–3682. 10.1021/jacs.7b12399. [DOI] [PubMed] [Google Scholar]
- (73).Sethe Burgie E; Clinger JA; Miller MD; Brewster AS; Aller P; Butryn A; Fuller FD; Gul S; Young ID; Pham CC; et al. Photoreversible Interconversion of a Phytochrome Photosensory Module in the Crystalline State. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (1), 300–307. 10.1073/pnas.1912041116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Yang X; Ren Z; Kuk J; Moffat K. Temperature-Scan Cryocrystallography Reveals Reaction Intermediates in Bacteriophytochrome. Nature 2011, 479 (7373), 428–431. 10.1038/nature10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Takala H; Björling A; Berntsson O; Lehtivuori H; Niebling S; Hoernke M; Kosheleva I; Henning R; Menzel A; Ihalainen JA; et al. Signal Amplification and Transduction in Phytochrome Photosensors. Nature 2014, 509 (7499), 245–248. 10.1038/nature13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Cho HS; Schotte F; Dashdorj N; Kyndt J; Henning R; Anfinrud PA Picosecond Photobiology: Watching a Signaling Protein Function in Real Time via Time-Resolved Small- and Wide-Angle X-Ray Scattering. J. Am. Chem. Soc 2016, 138 (28), 8815–8823. 10.1021/jacs.6b03565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Choudry U; Heyes DJ; Hardman SJO; Sakuma M; Sazanovich IV; Woodhouse J; De La Mora E; Pedersen MN; Wulff M; Weik M; et al. Photochemical Mechanism of an Atypical Algal Phytochrome. ChemBioChem 2018, 19 (10), 1036–1043. 10.1002/cbic.201800016. [DOI] [PubMed] [Google Scholar]
- (78).Takala H; Niebling S; Berntsson O; Björling A; Lehtivuori H; Häkkänen H; Panman M; Gustavsson E; Hoernke M; Newby G; et al. Light-Induced Structural Changes in a Monomeric Bacteriophytochrome. Struct. Dyn 2016, 3 (5), 054701. 10.1063/1.4961911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.