Abstract
The timing of cytokinesis relative to other mitotic events in the fission yeast Schizosaccharomyces pombe is controlled by the septation initiation network (SIN). During a mitotic checkpoint, the SIN is inhibited by the E3 ubiquitin ligase Dma1 to prevent chromosome mis-segregation. Dma1 dynamically localizes to spindle pole bodies (SPBs) and the contractile ring (CR) during mitosis though its role at the CR is unknown. Here, we examined whether Dma1 phosphorylation affects its localization or function. We found that preventing Dma1 phosphorylation by substituting the six phosphosites with alanines diminished Dma1 CR localization but did not affect its mitotic checkpoint function. These studies reinforce the conclusion that Dma1 localization to the SPB is key to its role in the mitotic checkpoint.
Keywords: cytokinesis, ubiquitin ligase, septation initiation network, spindle pole body, signaling, protein kinase, fission yeast
Introduction
The accurate division of a cell into two daughter cells with identical complements of genomic material is essential, as failure to do so can result in aneuploidy or cell death. In the fission yeast, Schizosaccharomyces pombe, the proper coordination of mitotic exit with cytokinesis is determined by the septation initiation network (SIN), a protein kinase cascade that normally initiates cytokinesis only after chromosome segregation is complete (for reviews see [1–3]). When the mitotic spindle cannot form, the SIN is inhibited to prevent cytokinesis from occurring before chromosomes have safely segregated [4, 5]. SIN inhibition during a mitotic error relies on the dimeric E3 ubiquitin ligase Dma1 [5–8], a member of the FHA and RING finger family [9].
Dma1 has a very complex localization pattern during the cell cycle. It dynamically localizes to spindle pole bodies (SPB) during mitosis [5, 10] and ubiquitinates the SIN scaffold protein, Sid4, that is detected exclusively at SPBs [6, 11]. Dma1 binding and ubiquitination of Sid4 requires CK1-mediated Sid4 phosphorylation, which provides a docking site for Dma1’s FHA domain [12]. Sid4 ubiquitination then antagonizes the SPB localization of the Polo-like kinase Plo1 [5, 6], the major SIN activator [13–15] so that SIN signaling is inhibited and cytokinesis is delayed. Dma1 also localizes to the site of cell division through an unknown mechanism and for an unknown purpose, first in precursor nodes that eventually form the cytokinetic ring (CR) [16], then to the CR; Dma1 leaves the site of division transiently during late anaphase, only to return prior to cell division [10]. Dma1 overproduction or tethering Dma1 to SPBs blocks SIN activity and results in cell death [5, 10, 17], indicating its levels, localization, and activity must be properly regulated.
We previously determined that Dma1 auto-ubiquitination influences its SPB localization and function in the mitotic checkpoint. Specifically, Dma1 auto-ubiquitination prevents its binding to Sid4 and therefore Dma1 auto-ubiquitination is implicated in checkpoint silencing [10]. Here, we investigated potential regulation of Dma1 by phosphorylation. We found that Dma1 is phosphorylated in vivo throughout the cell cycle and we identified six phosphorylated residues scattered throughout the protein. Four of the six sites can be targeted by a combination of three master kinases in vitro – Cdk1, Plo1, and CK2, but we obtained no evidence that they control Dma1 phosphorylation in vivo. Strikingly, neither phospho-ablating nor phospho-mimicking mutations at any or all of the six sites abolished Dma1 catalytic activity towards itself in vitro or its checkpoint substrate Sid4, as demonstrated by Sid4’s continued ubiquitination in phosphomutant strains. While we found that loss of phosphorylation altered Dma1’s localization to the CR in early mitosis, we did not detect an impact of phosphorylation on Dma1 localization to SPBs or its function in delaying cytokinesis during a mitotic arrest. Our results reinforce the conclusion that Dma1’s function in the mitotic checkpoint depends solely on its SPB localization.
Materials and Methods
Yeast methods
Yeast strains were grown in yeast extract with appropriate supplements [18]. Genes were tagged endogenously at the 3’ end of their open reading frame (ORF) with FLAG3:kanR, GFP:kanR, HA3-TAP:kanR, mNeonGreen:kanR, mCherry:natR, or HBH:kanR using pFA6 cassettes as previously described [19, 20]. For dma1 gene replacements, dma1::ura4+ was transformed with the appropriate pIRT2–dma1 mutant. Stable integrants were selected by resistance to 1.5 mg/ml 5-FOA. Mutants were identified by colony PCR with primers inside and outside of the 3’-flanking regions, and validated through DNA sequencing of the entire ORF.
For blocking nda3-KM311 strains in prometaphase, cultures were grown at the permissive temperature (32°C) and then shifted to the restrictive temperature (18°C) for 5.5 or 6 hrs. For quantitative imaging, cells were fixed for 10 minutes with 70% ethanol and washed with PBS. For blocking cdc10-V50, cdc25–22, and mts3–1 strains, cultures were grown at the permissive temperature (25°C) and then shifted to the restrictive temperature (36°C) for 3.5 hrs. For blocking strains with hydroxyurea (HU) in Figure 1, cultures were grown at 25°C, treated with a final concentration of 12 mM HU for 3 hrs and then dosed again with a final concentration of 6 mM HU for an additional 2 hrs.
Figure 1. Dma1 is phosphorylated through the cell cycle.
(A-B) Immunoblot analysis of Dma1-FLAG immunoprecipitates, treated (+) or not (−) with lambda phosphatase, from asynchronously growing cells (A) or cells arrested at a variety of cell cycle stages (G1 phase, cdc10-V50; S phase, HU; G2 phase, cdc25–22; metaphase, mts3–1; and prometaphase, nda3-KM311) (B). (C) Autoradiography and immunoblot analysis of Dma1-FLAG immunoprecipitates from the indicated strains, that were labeled in vivo with 32P-orthophosphate. (D) Phosphoamino acid analysis of wildtype Dma1 from C. The positions of phosphoserine, phosphothreonine and phosphotyrosine standards are indicated with dotted circles. (E) Schematic of Dma1 with phosphorylation sites identified by LC-MS/MS indicated. FHA = Forkhead-associated domain, RF = Ring Finger domain. (F) Immunoblot analysis of Dma1-FLAG immunoprecipitates from the indicated strains resolved on a PhosTag gel. (G) Immunoblot analysis of Dma1-FLAG immunoprecipitates from the indicated strains resolved on a PhosTag gel. “Treatment” denotes shifting cells from 25°C to 36°C for 1.5 hours followed by adding 10 uM 1-NMPP1 and 30 uM 3-BrBPP1 for 15 minutes.
Molecular biology methods
Plasmids were generated by standard molecular biology techniques. dma1 mutations were made either in the context of a gene fragment in the pIRT2 vector that included 500 bp upstream and downstream of the open reading frame or in the context of the open reading frame in pMAL2-c (New England Biolabs) or pET-His6-MBP preScission LIC cloning vector (HMPKS; Addgene plasmid # 29721) using a QuikChange site-directed mutagenesis kit (Agilent Technologies). In dma1–6A, the mutations are: S4A, T18A, S20A, S166A, S251A, and S266A; in dma1–6D/E, the mutations are: S4D, T18E, S20D, S166D, S251D, and S266D.
S. pombe protein methods
Cell pellets were washed once in NP-40 buffer (10 mM sodium phosphate pH 7.0, 1% Nonidet P40, 150 mM NaCl, 2 mM EDTA) with inhibitors (1.3 mM benzamidine, 1 mM PMSF, and 1 Complete Protease Inhibitor Cocktail Tablet, EDTA-free per 50 ml) and lysed by bead disruption. For denaturing lysis, 500 μl SDS lysis buffer (10 mM sodium phosphate pH 7.0, 1 % SDS, 1 mM DTT, 1 mM EDTA, 50 mM NaF, 100 μM sodium orthovanadate, 1 mM PMSF, and 4 μg/ml leupeptin) was added and samples were incubated at 95°C for 2 minutes, lysate was extracted with 800 μl NP-40 buffer and transferred to a fresh eppendorf tube. For native lysis, the lysate was extracted with 500 μl NP-40 buffer and again with 800 μl, then transferred to a fresh eppendorf tube. Extractions were followed by a 10 minute clearing spin.
Proteins were immunoprecipitated from protein lysates using an excess of antibody (listed below) and rotating at 4°C for 1 hour, followed by addition of Protein A or G Sepharose beads (GE Healthcare), as appropriate, and mixing at 4°C for 30 minutes. Samples were washed four times with NP-40 buffer. Antibodies: 4 μl of 1 μg/μl anti-GFP (IC9H4, a mouse monoclonal antibody produced in the Vanderbilt antibody and protein resource core), 2 μl of 1 μg/μl anti-FLAG (Sigma-Aldrich), or 3 μl rabbit anti-Sid4 antiserum (VU364) [12]. For phosphatase treatment, immunoprecipitated protein was washed twice with 25 mM HEPES-NaOH (pH 7.4) and 150 mM NaCl, then treated with lambda phosphatase (New England Biolabs) in 1× NEBuffer for PMP and 1 mM MnCl2 and incubated at 30°C for 30 to 60 minutes with agitation.
Proteins were resolved by PAGE (see below), transferred by electroblotting to a polyvinylidene difluoride (PVDF) membrane (Immobilon FL; Millipore, Bedford, MA), blocked with Odyssey Blocking Buffer (LI-COR Biosciences), and incubated with primary antibodies at the following concentrations/dilutions: 1:10,000 anti-Cdc2 (anti-PSTAIR, Sigma-Aldrich), 0.4 μg/ml anti-GFP (Roche), 1 μg/ml anti-FLAG (Sigma-Aldrich), 1:2000 anti-Sid4 serum, or 1:5000 anti-Dma1 serum (VU377) [10] overnight at 4°C. Primary antibodies were detected with secondary antibodies coupled to IRDye680 or IRDye800 (LI-COR Biosciences, Lincoln, NE) and visualized using an Odyssey Infrared Imaging System (LI-COR Biosciences). Resolving gels: 3–8% Tris-acetate PAGE used for Dma1-FLAG blotting except for a 10% SDS-PAGE containing 25 μM PhosTag in Figure 1F and 1G; 4–12% NuPAGE used for Sid4 and Dma1 ubiquitination assay blotting, 12% Tris-glycine PAGE used for Cdc2 blotting, 10% Tris-glycine PAGE used for MBP-Dma1 blotting.
Protein expression and purification
For phosphorylation of Dma1 variants shown in Figure S2A, the variant cDNAs were cloned into pMAL-2c and then transformed into Escherichia coli Rosetta2(DE3)pLysS cells. Maltose-binding protein (MBP)-Dma1 fusions were induced by addition of 0.8 mM IPTG and overnight incubation at 18°C. Bacterial cells were lysed by incubating with 300 μg/ml lysozyme for 20 minutes followed by sonication. Proteins were affinity purified on amylose resin (NEB E8021) in MBP column buffer (100 mM NaCl, 20 mM Tris-HCl, 1 mM EDTA, 1 mM DTT, and 1% Nonidet P40). Resin was washed with MBP column buffer and protein was eluted with MBP column buffer containing 10 mM maltose.
For all other experiments, dma1 variants were cloned into pET-His6-MBP preScission LIC cloning vector. Proteins were induced in Escherichia coli Rosetta2(DE3)pLysS cells by addition of 0.4 mM IPTG and overnight incubation at 18°C. Cells were lysed using 300 μg/mL lysozyme for 20 minutes followed by sonication. Proteins were affinity purified on pre-washed amylose resin (New England Biolabs) in MBP column buffer (100 mM NaCl, 20 mM Tris-HCl pH 7.4, mM EDTA, 1 mM DTT, and 1% Nonidet P40). Dma1 was cleaved from MBP by adding 1 μl (2 units) of PreScission protease to ~500 μg MBP-Dma1 on amylose beads (in 200 μl MBP column buffer) and incubating at 4°C overnight. The supernatant containing cleaved Dma1 was retrieved and the concentration of Dma1 was determined by SDS-PAGE using BSA as standard.
In vitro ubiquitination assay
Ubiquitination reactions included 0.2 μg recombinant Dma1 cleaved from MBP, 175 nM E1 (recombinant human His6-UBE1, R&D Systems), 0.19 μM E2 (recombinant human UbcH5α/UBE2D1, R&D Systems), 100 μg/mL methylated ubiquitin, 5 mM ATP, and 1× ubiquitination buffer (50 mM Tris-HCl pH 7.5, 2.5 mM MgCl2, 0.5 mM DTT). These 20 μL reactions were incubated with agitation at 30°C for 120 minutes before adding SDS sample buffer. Ubiquitination products and Dma1 were detected by immunoblotting with a 1:15,000 dilution of anti-Dma1 serum [10].
For ubiquitination assays of immunoprecipitated Dma1-GFP, the mock or λ-phosphatase treated immunoprecipitates were washed twice with ubiquitylation buffer before being combined in 20 μL ubiquitylation reactions as above. Reactions were incubated with agitation at 30°C for 90 minutes before adding SDS sample buffer to quench the reaction. To assess the extent of Dma1’s ubiquitin modification, proteins were separated by SDS-PAGE and detected by immunobloting with anti-GFP antibody and/or anti-Dma1 serum.
To determine abundances of Dma1 in immunoblotting experiments, signal intensities within identical squares or rectangles drawn around single or multiple bands were quantified using an Odyssey instrument. Experiments were performed twice.
Protein purification and mass spectrometry
Endogenously tagged versions of Dma1 (Dma1-TAP and Dma1-HBH) were purified as previously described [21–23] and analyzed by 2D-LC-MS/MS as previously described [24, 25]. RAW files were processed using two pipelines: 1) using Myrimatch (v 2.1.132) [26] and IDPicker (v 2.6.271.0) [27] as previously described [28] and 2) using TurboSEQUEST, Scaffold (v 4.4.7) and Scaffold PTM (v 3.0.0) as previously described [29].
In vivo radio-labeling
10 ml of S. pombe cells were grown in reduced (20 mM NaH2PO4) phosphate minimal media supplemented with the appropriate amino acids to mid-log phase. Cells were labeled with 5 mCi 32P-orthophosphate for 4 hours at 36°C. Denatured cell lysates were prepared and Dma1-FLAG was immunoprecipitated with anti-FLAG. Immunoprecipitates were resolved on a 6–20% gradient SDS polyacrylamide gel, transferred to a PVDF membrane and phosphorylated proteins were detected by autoradiography for 4 d at −80°C with intensifying screen. The membrane was then immunoblotted for anti-FLAG. 32-P-labeled Dma1 was subjected to partial acid hydrolysis while bound to the PVDF membrane [30], and the phosphoamino acids were separated in two dimensions by thin-layer electrophoresis at pH 1.9 and 3.5 [31].
In vitro kinase assays
Kinase reactions were performed in protein kinase buffer (10 mM Tris, pH 7.4, 10 mM MgCl2, and 1 mM DTT or NEB supplied buffer) with 5 μM cold ATP, 3 μCi of γ−32P-ATP, and recombinant CK2 (New England Biolabs), insect cell–produced Cdc2-Cdc13 or insect cell–produced Plo1 at 30°C for 30 minutes. Reactions were quenched by the addition of SDS sample buffer. Proteins were separated by SDS–PAGE and detected by Coomassie Blue (Sigma) staining or transferred to polyvinylidene fluoride (PVDF) membrane for detection by autoradiography.
Microscopy Methods
Images of S. pombe cells were acquired using a Personal DeltaVision microscope system (Cytiva Life Sciences, Marlborough, MA), which includes an Olympus IX71 microscope, 60×NA 1.42 Plan Apochromat and 100×NA 1.40 U Plan S Apochromat objectives, live-cell and standard filter wheel sets, a pco.edge 4.2 sCMOS camera, and softWoRx imaging software. Images were acquired at 25°C. All images were deconvolved with 10 iterations except for the images used for fluorescence quantification. Time-lapse imaging was performed using a CellASIC ONIX microfluidics perfusion system (Millipore Sigma, Burlington, MA). Cells were loaded into Y04C plates for 5 s at 8 psi, and YE liquid medium flowed into the chamber at 5 psi throughout imaging. For all images, Z-series optical sections were taken at 0.5 μm spacing and images were acquired every 2 minutes. All images for quantification were sum projected. Images in figures were deconvolved and max projected.
For Fig. S3, the events were defined as follows: “Arrival at division site” was the first frame in which Dma1-mNG was detected at division site. “Ring formation” was the first frame where a coherent ring was visible. “SPB blinks” was the first frame where Dma1-mNG leaves or dims at one or both SPBs. “Anaphase B begins” was the first frame where the SPBs move towards the cell poles as the spindle elongates. “Disappearance from division site” was the frame where Dma1-mNG no longer detected at the division site. “Spindle full length” was the frame where the SPBs are furthest apart during mitosis. “Re-appearance at division site” was the first frame in which Dma1-mNG returns to the division site.
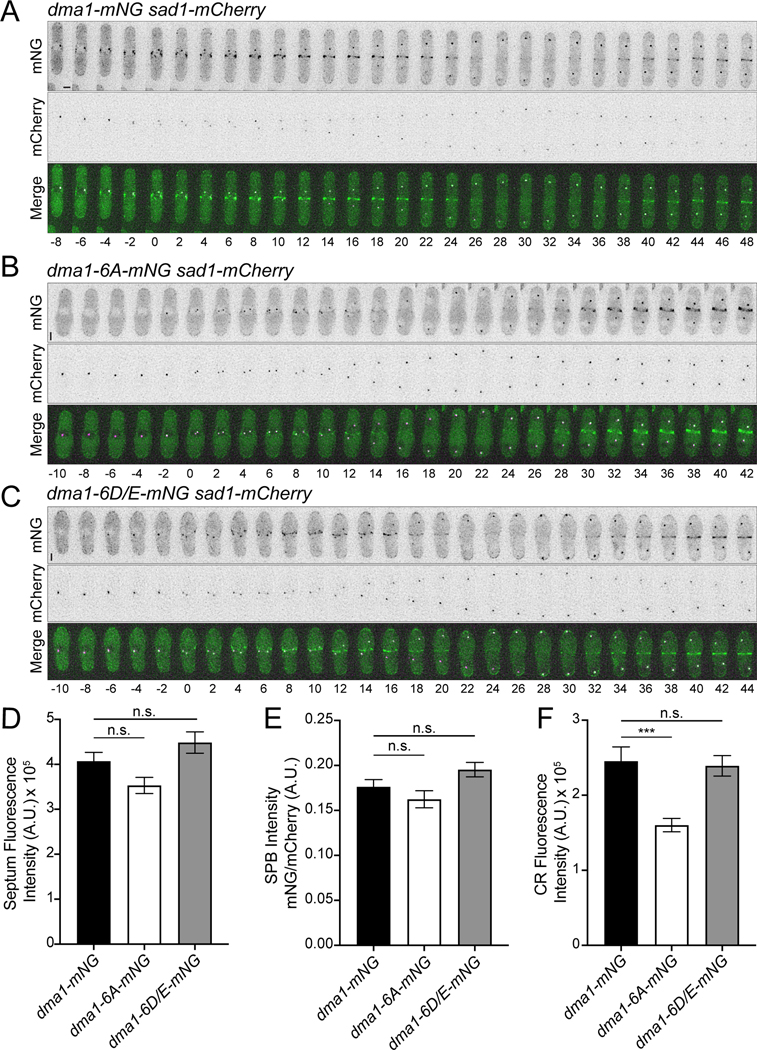
Fluorescence intensity measurements were made with ImageJ software (National Institutes of Health, Bethesda, MD). All intensity measurements were corrected for background. In each image used, background measurements were taken from an area without any cells. The intensity of the background was divided by the area to give the average intensity per pixel of the background. This was then multiplied by the area of the region of interest (ROI) used to measure fluorescence intensity and subtracted from that ROI’s raw intensity measurement to get the final intensity measurement corrected for background. For whole cell fluorescence measurements, the corrected intensity measurements were divided by the area of the ROI. For SPB intensity measurements both Dma1-mNG and Sad1-mCherry SPBs were corrected for background. Additionally, Dma1-mNG SPB intensity was divided by the Sad1-mCherry measurement of the same SPB. In Fig. 3D, constricted rlc1-mCherry was used to indicate cells with a septum for quantification. In Fig. 3E, sad1-mCherry and rlc1-mCherry were used to indicate cells at the end of anaphase B for quantification. For Fig. 3F, unconstricted rlc1-mCherry was used to indicate early mitotic CRs for quantification.
Figure 3. Dma1 phosphomutant localization during cell division.
(A-C) Representative montages of live-cell time-lapse imaging of the indicated strains. Images were acquired every 2 minutes. Time 0 = SPB separation and numbers indicate minutes from SPB separation. Scale bars, 2 μm. (D-F) Live cell imaging of dma1-mNG, dma1–6A-mNG, or dma1–6D/E-mNG with rlc1-mCherry and sad1-mCherry and quantification of (D) fluorescence intensity along the septum (when the CR marked by Rlc1-mCherry is fully constricted and a septum is present), (E) SPB intensity at the end of mitosis when two SPBs marked by Sad1-mCherry are well separated and next to the cell tips, or (F) CR fluorescence intensity when a fully formed and unconstricted CR is present. n ≥ 26 cells. Error bars represent SEM. One-way ANOVA. *** ≤ p = 0.001. (septum dma1-mNG vs. dma1–6A-mNG p = 0.15, dma1-mNG vs. dma1–6D/E-mNG p =0.33) (SPB dma1-mNG vs. dma1–6A-mNG p = 0.48, dma1-mNG vs. dma1–6D/E-mNG p = 0.25) (CR dma1-mNG vs. dma1–6D/E-mNG p = 0.95).
Checkpoint assay
Log phase cells growing in YE at 32°C were synchronized in S-phase by the addition of 12 mM hydroxyurea (Sigma) for 3 hours. The arrested cells were then filtered, washed with YE media to remove hydroxyurea while on the filter, and immediately incubated in pre-cooled YE media at 19°C. Septation indices were measured periodically (every 30 minutes or 1 hour) for up to 12 hours by counting live cells under a phase contrast microscope.
Quantification and Statistical Analysis
Calculations of mean, standard error of the mean (SEM), and statistical significances were performed with Prism 8.0 (GraphPad Software). Significance was defined by a p value equal to or less than 0.05. One-way ANOVA was used with Tukey’s post hoc test for multiple comparisons.
Results
Dma1 is phosphorylated throughout the cell cycle
Dma1 SPB localization and Dma1-mediated ubiquitination of Sid4 are cell cycle dependent [6, 10], leading us to explore the molecular mechanisms that regulate Dma1 activity. Because phosphorylation provides a rapid and reversible means of regulating protein function often coupled to ubiquitination [32–34], we examined whether Dma1 is phosphorylated in vivo. Using immunoblot analysis of Dma1 immunoprecipitates, treated with or without λ phosphatase, we detected Dma1 phosphorylation in asynchronous cells that could be observed by slower migration in SDS-PAGE (Figure 1A). Further examination of Dma1 in various cell cycle arrests showed that Dma1 phosphorylation occurs throughout the cell cycle (Figure 1B). In vivo labeling with 32P-orthophosphate followed by immunoprecipitation and phosphoamino acid analysis showed that the majority of Dma1 phosphorylation occurred on serine residues (Figure 1C and D).
Identification of Dma1 phosphorylation sites
To determine the sites of Dma1 phosphorylation, we performed LC-MS/MS analysis on Dma1 purified from S. pombe cells using a variety of epitope tags, lysis procedures, and cell cycle blocks. In all, six sites were identified: 1 threonine and 5 serines (Figure 1E and S1). Four of these sites (S4, T18, S251, S266) were also identified in global phosphoproteomics screens [35–37]. To investigate if this cohort of six was the full complement of Dma1 phosphorylation sites, we replaced dma1+ with a mutant version in which all six sites were replaced with alanines. While multiple species of Dma1 were detected on a PhosTag-containing gel, Dma1–6A co-migrated with the fastest migrating band (Figure 1F), indicating the absence of phosphorylation.
Dma1 is phosphorylated in vitro by several master kinases
Examination of the linear sequences surrounding the six identified phosphosites showed that four fit known kinase consensus sequences; S166 fits the Cdk1 consensus [38, 39], S251 fits the Plo1 consensus [40], and T18 and S20 fit the CK2 consensus [41, 42]. To determine if Cdk1, Plo1, and CK2 were indeed capable of phosphorylating Dma1, in vitro kinase assays were performed with recombinant Dma1 and commercially available or insect cell-produced kinases (Figure S2). Cdk1 phosphorylation of MBP-Dma1-S166A was diminished relative to MBP-Dma1 (Figure S2A). Similarly, Plo1 could not phosphorylate MBP-Dma1-S251A (Figure S2B). It is notable that S251 but not S166 was found to be specifically present during mitosis in two phosphoproteomic screens [35, 36]. Lastly, CK2 phosphorylation of MBP-Dma1 was diminished with the MBP-Dma1-T18A S20A mutant and lost with the MBP-Dma1-T18A S20A S266A mutant (Figure S2C), indicating that CK2 phosphorylation can occur not only at the predicted T18 and S20 sites but also at the previously unassigned S266 site. The S4 phosphorylation site does not fit a known kinase consensus sequence and it is not phosphorylated in vitro by Cdk1, Plo1 or CK2.
To determine whether these three kinases were involved in Dma1 phosphorylation in vivo, we combined analog-sensitive (cdc2-as, orb5-as) and temperature sensitive (plo1–1) alleles. Despite these kinases targeting Dma1 in vitro, we found no evidence that inhibiting any of them singly (not shown) or together (Figure 1G) changed Dma1 phosphostatus as monitored by SDS-PAGE mobility suggesting that these kinases are not responsible for regulating Dma1 phosphostatus in cells.
Dma1 phosphostate does not significantly influence its ubiquitination activity
We next investigated whether phosphorylation modulates Dma1 ubiquitination activity in vivo and in vitro. We constructed phospho-ablating and potentially phospho-mimetic dma1 mutations by replacing the six phosphorylated serine and threonine residues with alanines (dma1–6A), or aspartates for serines and glutamate for threonine (dma1–6D/E), respectively. These mutant constructs (dma1–6A and dma1–6D/E) were each integrated at the endogenous dma1 locus, and we then examined ubiquitination of Dma1’s known target, Sid4, in either asynchronously growing or pro-metaphase arrested cells. To distinguish Sid4 ubiquitination from Sid4 phosphorylation, Sid4 immunoprecipitates were treated with λ-phosphatase prior to immunoblotting [12, 43]. Although this assay is not quantitative, we found that Sid4 was ubiquitinated to similar levels as in wildtype in both dma1–6A and dma1–6D/E but was not ubiquitinated in dma1Δ (Figure 2A). This result indicates that the Dma1 phosphomutants are catalytically active towards substrate.
Figure 2. Role of phosphorylation in Dma1 ubiquitination activities.
(A) Immunoblot analysis of Sid4 immunoprecipitates treated with phosphatase from asynchronous (left) or nda3-arrested (right) cells of the indicated dma1 genotype. One representative of two separate experiments is shown. (B) Immunoblot analysis of the indicated recombinantly purified and MBP tag-cleaved Dma1 proteins that were incubated with E1 activating enzyme, E2 conjugating enzyme, ATP, and methylated ubiquitin. The Dma1 input prior to the ubiquitination assay is shown (right panel). The percentage of Dma1 auto-ubiquitination was determined by subtracting the amount of unmodified Dma1 (left panels) from the total Dma1 that was added to the reactions (right panels) as determined by quantification on an Odyssey instrument and then dividing ubiquitinated by the total. (C) Immunoblot analysis of the indicated Dma1-GFP immunoprecipitates from prometaphase arrested cells (nda3-KM311 cells shifted to 19°C for 6 hours) that were treated with λ-phosphatase or mock-treated followed by incubation with E1 activating enzyme, ATP, methylated ubiquitin, and with (+) or without (−) E2 conjugating enzyme. The ubiquitinated Dma1 species were quantified on an Odyssey instrument and divided by the total amount of Dma1 to determine the percentage of modified Dma1.
We next examined if phosphorylation affected the ability of Dma1 to auto-ubiquitinate in vitro. Full-length Dma1, which lacks phosphorylation, Dma1–6D/E, and two Dma1 loss-of-function mutants were purified from bacteria as MBP fusions and the MBP moiety was removed prior to auto-ubiquitination assays, which used methylated Ub to prevent chain elongation. The Dma1-I194A mutant that is predicted based on structural modeling to abrogate interaction with cognate E2 enzyme(s) [44] has much reduced E3 activity relative to wildtype in vivo and in vitro [6, 10] (Figure 2B). Dma1-F233 is a second amino acid within the predicted hydrophobic groove also likely to interact with E2 enzyme(s). As expected, the double mutant Dma1-I194A F233A had even lower levels of auto-ubiquitination activity compared to wildtype (Figure 2B). Dma1–6D/E auto-ubiquitination was modestly but reproducibly reduced relative to wildtype (Figure 2B). Specifically, while 82% of wildtype Dma1 became ubiquitinated on at least one site, 71% of Dma1–6D/E did. We also examined the effect of phosphorylation on Dma1 ubiquitination activity by immunoprecipitating Dma1-GFP from prometaphase-arrested cells and then treating the immunoprecipitates with lambda phosphatase or only vehicle prior to an in vitro auto-ubiquitination assay. We found that phosphatase-treated Dma1-GFP auto-ubiquitinated similarly to phosphorylated Dma1-GFP (Figure 2C). By measuring the amount of ubiquitinated species relative to unmodified Dma1 in each case, we found that there was no significant difference. Taken together, our data suggest that phosphorylation does not play an important role in regulating Dma1 E3 ubiquitin ligase activity.
A Dma1 phosphomutant has altered intracellular localization
We next tested if Dma1 phosphostatus affected its intracellular localization. Dma1, Dma1–6A and Dma1–6D/E were tagged with mNeonGreen (mNG). The overall levels of the phosphomutants were indistinguishable from wild type as determined by measuring total fluorescent intensities (Figure S3A). By live cell imaging, the kinetics of mitotic progression were found to be similar in all three strains (Figure S3B) and all Dma1 proteins were found to have similar kinetics of cortex, SPB, and CR localization (Figure 3A–C and Figure S3B). Also, all Dma1 variants localized to the site of division and SPBs with similar intensity after spindle breakdown, marked by SPBs starting to move toward each other (Figure 3D–E). However, we observed that Dma1–6A was significantly more difficult to detect at the first instance of CR localization early in mitosis than either Dma1 or Dma1–6D/E (Figure 3A–C). We therefore quantified the CR intensities of Dma1 proteins relative to a CR marker, Rlc1-mCherry. We found that indeed, Dma1–6A had significantly reduced CR localization in early mitosis relative to Dma1 and Dma1–6D/E (Figure 3F).
Dma1 phosphorylation does not affect its mitotic checkpoint function
Although immunoblotting showed that Sid4 was ubiquitinated in mitotically arrested cells producing any of the Dma1 phosphomutants (Figure 2A) and Dma1 phosphomutants appeared to localize normally to SPBs (Figure 3E), we examined whether the Dma1-dependent checkpoint was fully functional in the dma1–6A and dma1–6D/E mutants. dma1+, dma1–6A, dma1–6D/E, and dma1Δ cells that also contained the cold-sensitive nda3-km311 ß-tubulin mutant and wild-type cells were synchronized in S phase by treatment with hydroxyurea, shifted to 19°C to induce microtubule depolymerization, and septation kinetics were monitored. We observed that nda3-km311, dma1–6A nda3-km311 and dma1–6D/E nda3-km311 cells delayed septation relative to wildtype cells and that dma1Δ cells did not (Figure 4A). These data indicate that Dma1 phosphostate does not significantly influence its function in delaying septation during a mitotic arrest. Consistent with this, we found during a mitotic checkpoint arrest, the intensities of Dma1 and Dma1 phosphomutants at the SPB relative to the SPB marker, Sad1-mCherry, were very similar (Figure 4B).
Figure 4. Checkpoint assay.
(A) The indicated strains were synchronized in S phase with hydroxyurea and shifted to 19°C. The septation indices were measured every 1 hour (for early time points) or 30 minutes (for later time points) for 12 hours. One of the two experiments with similar results was shown. (B) Quantification of SPB intensity of arrested and ethanol fixed dma1-mNG nda3-KM311, dma1–6A-mNG nda3-KM311, or dma1–6D/E-mNG nda3-KM311 with rlc1-mCherry and sad1-mCherry. n ≥ 67 cells. Error bars represent SEM. One-way ANOVA. * ≤ p 0.05 (SPB dma1-mNG vs. dma1–6D/E-mNG p = 0.99).
Discussion
Dma1 signaling must be properly regulated to ensure that its checkpoint activity is quickly activated in the event of a mitotic spindle error and is also reversed when the error is resolved. Here we tested whether Dma1 phosphostate influences it signaling potential in the mitotic checkpoint that delays cytokinesis until chromosome segregation is complete. We found no evidence that phosphorylation influences Dma1 localization to SPBs, its ability to ubiquitinate itself or its substrate Sid4, or its mitotic checkpoint function.
Surprisingly, we found that preventing phosphorylation impacted the ability of Dma1 to localize normally to the CR. Though the binding partner(s) of Dma1 at the CR and its potential function there are unknown, our data suggest Dma1 phosphorylation modulates its interaction with the CR during early mitosis. The dma1–6A allele may serve as a useful separation of function allele for any future studies addressing this question. It is interesting that Dma1 returns to the division site upon mitotic exit at increased levels compared to early mitosis, and this latter division site localization does not depend on phosphorylation. This suggests that there are two different mechanisms localizing Dma1 to the division site differentially regulated by phosphorylation.
We determined that Dma1 is phosphorylated in vivo throughout the cell cycle and that this phosphorylation occurs on six sites. Some of these phosphosites were also identified in global phosphoproteomics screens designed to identify mitotic-specific phosphorylation and two mitotic kinases, Cdk1 and Plo1, phosphorylate Dma1 in vitro. It was therefore surprising that we did not detect a significant increase in Dma1 phosphorylation during mitosis nor an effect of eliminating Cdk1 or Plo1 kinase activity on Dma1 phosphostate. While other kinases may act redundantly, considering our data indicates the role of Dma1 phosphorylation is to target it to the CR, we speculate that the kinases responsible for phosphorylating Dma1 in vivo may be one of many kinases localized at the cell cortex rather than the SPB.
Phosphorylation of other E3 ubiquitin ligases has been reported to inhibit their E3 ligase activity or switch their substrate preference. As examples, IκB kinase phosphorylates the HECT E3 ligase ITCH to inhibit its E3 ligase activity [45] by impairing E2-E3 complex formation [46]. Protein kinase A-dependent phosphorylation of the E3 ligase Smad Ubiquitin Regulatory Factor 1 (Smurf1) changed its affinity for substrates, leading to reduced degradation of one substrate and increased degradation of another substrate [47]. Although our study suggests that dephosphorylation has little effect on Dma1 E3 ubiquitin ligase activity towards its currently known substrates in mitosis, Sid4 and itself, it does not rule out the possibility that Dma1 phosphorylation is important for ubiquitination of its meiotic substrates [48, 49] or influence its choice of E2 enzyme partner. Finally, a caveat to our study is that mutations designed to mimic phosphorylation in dma1–6D/E may not have done so [50], and thus the effect of constitutive Dma1 phosphorylation may not have been revealed.
Supplementary Material
Acknowledgements
The authors thank Drs. Sierra Cullati and Alaina Willet for comments on the manuscript and Dr. Willet for creating the graphical abstract. C.M.J. and A.E.J. were supported by the Cellular, Biochemical and Molecular Sciences Training Program (NIH T32GM08554). This work was supported by National Institutes of Health grant GM R35131799 to K.L.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 5-FOA
5-Fluoroorotic Acid
- BSA
Bovine serum albumin
- CK1
casein kinase 1
- CR
contractile ring or cytokinetic ring
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- FHA
forkhead-associated
- GFP
green fluorescent protein
- HBH
hexahistidine biotin hexahistidine
- HU
hydroxyurea
- IPTG
Isopropyl β- d-1-thiogalactopyranoside
- LC-MS
liquid chromatography mass spectrometry
- MBP
maltose-binding protein
- mNG
mNeonGreen
- NP-40
Nonidet P40
- ORF
open reading frame
- PBS
phosphate buffered saline
- PMP
protein metallophosphatase
- PMSF
phenylmethylsulfonyl fluoride
- PVDF
polyvinylidene difluoride
- RF
ring finger
- RING
really interesting new gene
- ROI
region of interest
- S. pombe
Schizosaccharomyces pombe
- SEM
standard error of the mean
- SIN
septation initiation network
- SPB
spindle pole body
- TAP
tandem-affinity purification
- YE
yeast extract
Footnotes
Data availability:
The data that support the findings of this study are either in the figures or supplementary material of this article or will be made available upon reasonable request of the corresponding author (kathy.gould@vanderbilt.edu).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Krapp A. & Simanis V. (2008) An overview of the fission yeast septation initiation network (SIN), Biochemical Society transactions. 36, 411–5. [DOI] [PubMed] [Google Scholar]
- 2.Johnson AE, McCollum D. & Gould KL (2012) Polar opposites: Fine-tuning cytokinesis through SIN asymmetry, Cytoskeleton (Hoboken). 69, 686–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simanis V. (2015) Pombe’s thirteen - control of fission yeast cell division by the septation initiation network, Journal of cell science. 128, 1465–74. [DOI] [PubMed] [Google Scholar]
- 4.Murone M. & Simanis V. (1996) The fission yeast dma1 gene is a component of the spindle assembly checkpoint, required to prevent septum formation and premature exit from mitosis if spindle function is compromised, Embo J. 15, 6605–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Guertin DA, Venkatram S, Gould KL & McCollum D. (2002) Dma1 prevents mitotic exit and cytokinesis by inhibiting the septation initiation network (SIN), Dev Cell. 3, 779–90. [DOI] [PubMed] [Google Scholar]
- 6.Johnson AE & Gould KL (2011) Dma1 ubiquitinates the SIN scaffold, Sid4, to impede the mitotic localization of Plo1 kinase, The EMBO journal. 30, 341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson AE, Collier SE, Ohi MD & Gould KL (2012) Fission yeast Dma1 requires RING domain dimerization for its ubiquitin ligase activity and mitotic checkpoint function, The Journal of biological chemistry. 287, 25741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullati SN & Gould KL (2019) Spatiotemporal regulation of the Dma1-mediated mitotic checkpoint coordinates mitosis with cytokinesis, Curr Genet. 65, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks L 3rd, Heimsath EG Jr., Loring GL & Brenner C. (2008) FHA-RING ubiquitin ligases in cell division cycle control, Cell Mol Life Sci. 65, 3458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CM, Chen JS, Johnson AE, Elmore ZC, Cullati SN, Beckley JR & Gould KL (2018) Relief of the Dma1-mediated checkpoint requires Dma1 autoubiquitination and dynamic localization, Mol Biol Cell. 29, 2176–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang L. & Gould KL (2000) Sid4p is required to localize components of the septation initiation pathway to the spindle pole body in fission yeast, Proc Natl Acad Sci U S A. 97, 5249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson AE, Chen JS & Gould KL (2013) CK1 is required for a mitotic checkpoint that delays cytokinesis, Current biology : CB. 23, 1920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkura H, Hagan IM & Glover DM (1995) The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells, Genes Dev. 9, 1059–73. [DOI] [PubMed] [Google Scholar]
- 14.Mulvihill DP, Petersen J, Ohkura H, Glover DM & Hagan IM (1999) Plo1 kinase recruitment to the spindle pole body and its role in cell division in Schizosaccharomyces pombe, Mol Biol Cell. 10, 2771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka K, Petersen J, MacIver F, Mulvihill DP, Glover DM & Hagan IM (2001) The role of Plo1 kinase in mitotic commitment and septation in Schizosaccharomyces pombe, Embo J. 20, 1259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JQ, Sirotkin V, Kovar DR, Lord M, Beltzner CC, Kuhn JR & Pollard TD (2006) Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast, J Cell Biol. 174, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YH, Wang GY, Hao HC, Chao CJ, Wang Y. & Jin QW (2017) Facile manipulation of protein localization in fission yeast through binding of GFP-binding protein to GFP, J Cell Sci. 130, 1003–1015. [DOI] [PubMed] [Google Scholar]
- 18.Moreno S, Klar A. & Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe, Methods Enzymol. 194, 795–823. [DOI] [PubMed] [Google Scholar]
- 19.Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A 3rd, Steever AB, Wach A, Philippsen P. & Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe, Yeast. 14, 943–51. [DOI] [PubMed] [Google Scholar]
- 20.Wach A, Brachat A, Poehlmann R. & Philippsen P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae, Yeast. 10, 1793–1808. [DOI] [PubMed] [Google Scholar]
- 21.Elmore ZC, Beckley JR, Chen JS & Gould KL (2014) Histone H2B ubiquitination promotes the function of the anaphase-promoting complex/cyclosome in Schizosaccharomyces pombe, G3 (Bethesda). 4, 1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould KL, Ren L, Feoktistova AS, Jennings JL & Link AJ (2004) Tandem affinity purification and identification of protein complex components, Methods. 33, 239–44. [DOI] [PubMed] [Google Scholar]
- 23.Tagwerker C, Flick K, Cui M, Guerrero C, Dou Y, Auer B, Baldi P, Huang L. & Kaiser P. (2006) A tandem affinity tag for two-step purification under fully denaturing conditions: application in ubiquitin profiling and protein complex identification combined with in vivocross-linking, Mol Cell Proteomics. 5, 737–48. [DOI] [PubMed] [Google Scholar]
- 24.McDonald WH, Ohi R, Miyamoto DT, Mitchison TJ & Yates JR III (2002) Comparison of three directly coupled HPLC MS/MS strategies for identification of proteins from complex mixtures: single-dimension LC-MS/MS, 2-phase MudPIT, and 3-phase MudPIT., International Journal of Mass Spectrometry. 219, 245–51. [Google Scholar]
- 25.Roberts-Galbraith RH, Chen JS, Wang J. & Gould KL (2009) The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring, J Cell Biol. 184, 113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabb DL, Fernando CG & Chambers MC (2007) MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis, J Proteome Res. 6, 654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma ZQ, Dasari S, Chambers MC, Litton MD, Sobecki SM, Zimmerman LJ, Halvey PJ, Schilling B, Drake PM, Gibson BW & Tabb DL (2009) IDPicker 2.0: Improved protein assembly with high discrimination peptide identification filtering, J Proteome Res. 8, 3872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLean JR, Kouranti I. & Gould KL (2011) Survey of the phosphorylation status of the Schizosaccharomyces pombe deubiquitinating enzyme (DUB) family, J Proteome Res. 10, 1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JS, Broadus MR, McLean JR, Feoktistova A, Ren L. & Gould KL (2013) Comprehensive proteomics analysis reveals new substrates and regulators of the fission yeast clp1/cdc14 phosphatase, Molecular & cellular proteomics : MCP. 12, 1074–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamps MP & Sefton BM (1989) Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins, Anal Biochem. 176, 22–7. [DOI] [PubMed] [Google Scholar]
- 31.Boyle WJ, van der Geer P. & Hunter T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two- dimensional separation on thin-layer cellulose plates, Methods Enzymol. 201, 110–49. [DOI] [PubMed] [Google Scholar]
- 32.Bohnert KA & Gould KL (2011) On the cutting edge: post-translational modifications in cytokinesis, Trends Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter T. (2007) The age of crosstalk: phosphorylation, ubiquitination, and beyond, Mol Cell. 28, 730–8. [DOI] [PubMed] [Google Scholar]
- 34.Holt LJ (2012) Regulatory modules: Coupling protein stability to phopshoregulation during cell division, FEBS Lett. 586, 2773–7. [DOI] [PubMed] [Google Scholar]
- 35.Koch A, Krug K, Pengelley S, Macek B. & Hauf S. (2011) Mitotic substrates of the kinase aurora with roles in chromatin regulation identified through quantitative phosphoproteomics of fission yeast, Sci Signal. 4, rs6. [DOI] [PubMed] [Google Scholar]
- 36.Swaffer MP, Jones AW, Flynn HR, Snijders AP & Nurse P. (2018) Quantitative Phosphoproteomics Reveals the Signaling Dynamics of Cell-Cycle Kinases in the Fission Yeast Schizosaccharomyces pombe, Cell Rep. 24, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tay YD, Leda M, Spanos C, Rappsilber J, Goryachev AB & Sawin KE (2019) Fission Yeast NDR/LATS Kinase Orb6 Regulates Exocytosis via Phosphorylation of the Exocyst Complex, Cell Rep. 26, 1654–1667 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno S. & Nurse P. (1990) Substrates for p34cdc2: in vivo veritas?, Cell. 61, 549–51. [DOI] [PubMed] [Google Scholar]
- 39.Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H. & Cantley LC (1994) Use of an oriented peptide library to determine the optimal substrates of protein kinases, Curr Biol. 4, 973–82. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima H, Toyoshima-Morimoto F, Taniguchi E. & Nishida E. (2003) Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate, J Biol Chem. 278, 25277–80. [DOI] [PubMed] [Google Scholar]
- 41.Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, Blenis J, Hunter T. & Cantley LC (1996) A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1, Mol Cell Biol. 16, 6486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meggio F, Marin O. & Pinna LA (1994) Substrate specificity of protein kinase CK2, Cell Mol Biol Res. 40, 401–9. [PubMed] [Google Scholar]
- 43.Chan KY, Alonso-Nunez M, Grallert A, Tanaka K, Connolly Y, Smith DL & Hagan IM (2017) Dialogue between centrosomal entrance and exit scaffold pathways regulates mitotic commitment, J Cell Biol. 216, 2795–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katoh S, Hong C, Tsunoda Y, Murata K, Takai R, Minami E, Yamazaki T. & Katoh E. (2003) High precision NMR structure and function of the RING-H2 finger domain of EL5, a rice protein whose expression is increased upon exposure to pathogen-derived oligosaccharides, J Biol Chem. 278, 15341–8. [DOI] [PubMed] [Google Scholar]
- 45.Perez JM, Chirieleison SM & Abbott DW (2015) An IkappaB Kinase-Regulated Feedforward Circuit Prolongs Inflammation, Cell Rep. 12, 537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez JM, Chen Y, Xiao TS & Abbott DW (2018) Phosphorylation of the E3 ubiquitin protein ligase ITCH diminishes binding to its cognate E2 ubiquitin ligase, J Biol Chem. 293, 1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng PL, Lu H, Shelly M, Gao H. & Poo MM (2011) Phosphorylation of E3 ligase Smurf1 switches its substrate preference in support of axon development, Neuron. 69, 231–43. [DOI] [PubMed] [Google Scholar]
- 48.Krapp A, Del Rosario EC & Simanis V. (2010) The role of Schizosaccharomyces pombe dma1 in spore formation during meiosis, J Cell Sci. 123, 3284–93. [DOI] [PubMed] [Google Scholar]
- 49.Krapp A. & Simanis V. (2014) Dma1-dependent degradation of SIN proteins during meiosis in Schizosaccharomyces pombe, J Cell Sci. 127, 3149–61. [DOI] [PubMed] [Google Scholar]
- 50.Dephoure N, Gould KL, Gygi SP & Kellogg DR (2013) Mapping and analysis of phosphorylation sites: a quick guide for cell biologists, Molecular biology of the cell. 24, 535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.