Abstract
The importance of defining sex differences across various biological and physiological mechanisms is more pervasive now than it has been over the past 15–20 years. As the muscle biology field pushes to identify small molecules and interventions to prevent, attenuate, or even reverse muscle wasting, we must consider the effect of sex as a biological variable. It should not be assumed that a therapeutic will affect males and females with equal efficacy or equivalent target affinities under conditions where muscle wasting is observed. With that said, it is not surprising to find that we have an unclear or even a poor understanding of the effects of sex or sex hormones on muscle wasting conditions. Although recent investigations are beginning to establish experimental approaches that will allow investigators to assess the impact of sex-specific hormones on muscle wasting, the field still needs rigorous scientific tools that will allow the community to address critical hypotheses centered around sex hormones. The focus of this review is on female sex hormones, specifically estrogens, and the roles that these hormones and their receptors play in skeletal muscle wasting conditions. With the overall review goal of assembling the current knowledge in the area of sexual dimorphism driven by estrogens with an effort to provide insights to interested physiologists on necessary considerations when trying to assess models for potential sex differences in cellular and molecular mechanisms of muscle wasting.
Keywords: cachexia, disuse, estradiol, sarcopenia, skeletal muscle
INTRODUCTION
Skeletal muscle wasting, also referred to as muscle atrophy, occurs in response to a variety of conditions. The onset, duration, and magnitude of muscle wasting vary depending on the condition (i.e., muscle disuse, cachexia, or aging) and may by influenced by sex. One cause of sexual dimorphism is sex hormones: estrogens, progestins, and androgens. The focus of this review is on female sex hormones, specifically estrogens, and the roles that these hormones and their receptors play in skeletal muscle wasting conditions. The overall goal of this review is to advance readership knowledge in the area of sexual dimorphism driven by estrogens to enable physiologists to better elucidate cellular and molecular mechanisms of muscle wasting. First, we provide an overview of estrogen biology focusing on mechanisms of action in skeletal muscle (Fig. 1). Second, we present data from conditions that induce skeletal muscle wasting including disuse, sarcopenia, cachexia, and injury demonstrating how estrogens may or may not be involved. Finally, we end with a summary of estrogenic therapeutic interventions that may become beneficial in muscle wasting conditions as the field continues to unravel sex hormone mechanisms contributing to the maintenance of skeletal muscle mass.
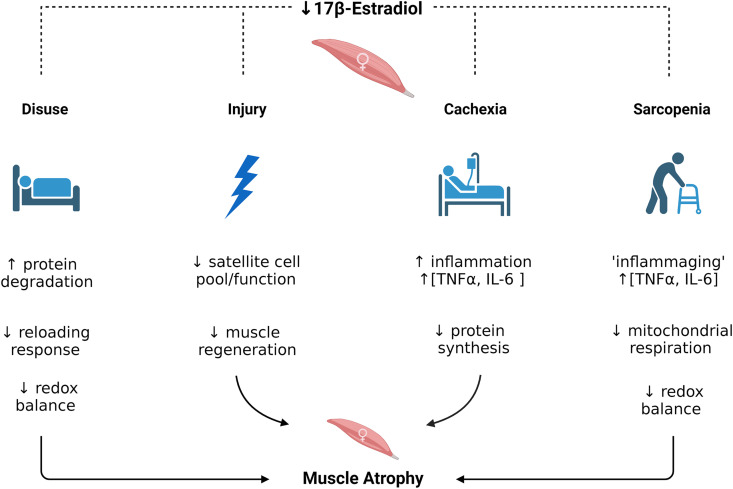
Figure 1.
Effects of 17β-estradiol deficiency on conditions of skeletal muscle wasting caused by disuse, injury, cachexia, and sarcopenia. [Images were generated at Biorender.com and published with permission.]
OVERVIEW OF ESTROGEN BIOLOGY
Estrogens are primarily produced in and secreted from the granulose cells of the ovaries in females and the Sertoli cells of the testes in males in response to follicle-stimulating hormone released from the anterior pituitary (1–4). In both sexes, adipose, fibroblast, mesenchymal stromal, and epithelial cells also produce estrogens locally (5). The production of estrogens in all cells is dependent on the expression and abundance of the enzyme aromatase (5–7). Aromatase regulates the conversion of androgens into estradiol [17-β estradiol (E2)] and estrone (E1), which are converted to estriol (E3) during pregnancy (1, 8). Aromatase expression and activity have been detected in human (9) and rat (10) skeletal muscle homogenates, suggesting that muscle may have the ability to convert testosterone to estrogen locally; in contrast, aromatase mRNA was not detected in mouse skeletal muscle (11). Immunofluorescence staining for aromatase indicated that aromatase was localized to either the sarcolemma or subsarcolemmal zone of skeletal muscle fibers from pre- and postmenopausal women (12). Important to note is that aromatase activity in nonfiber cell types within a skeletal muscle could contribute to skeletal muscle androgen-to-E2 action (13). For example, aromatase expression has been detected in isolated vascular smooth muscle cells (14, 15), endothelial cells (16), and macrophages (17), cell types that are known to be within skeletal muscle tissue. Thus, whether or not muscle cells (fibers) per se contain aromatase, particularly in rodent muscle, is not clear but likely as a tissue, skeletal muscle, has the ability to convert androgens to estrogens because of nonfiber cells in the environment containing aromatase.
In reproductive aged females, circulating concentrations of estrogens fluctuate during the menstrual [human (18, 19)] or estrous cycle [rodent (20–22)]. The menstrual cycle consists of the follicular and luteal phases (19). The estrous cycle is divided into four stages: proestrus and estrus, which make up the follicular phase, and the metestrus and diestrus, which consist of the luteal phase (22). E2 peaks at the end of the follicular phase (28–475 pg/mL) in women (19) and during proestrus in rodents (rats: ∼35 pg/mL, mice: ∼8 pg/mL) in rodents (21). Accurate measurement of female hormones is notoriously difficult due to a high degree of variability across the women’s lifespan [e.g., E2 can range from <20 pg/mL in postmenopausal women to 20,000 pg/mL during pregnancy (23)] (Table 1). In rodent models, challenges include the compressed 5-day cycle of fluctuating E2, small volumes of blood (serum) sample available to assay, and commercial assays that are developed primarily for human samples having potential less specificity for rodent blood. To address these issues, commonly employed methods such as ELISA, enzyme immunoassay, or radioimmunoassay that have limited accuracy, specificity, reproducibility, and standardization (29) are now being replaced by chromatographic methods combined with mass spectrometry in both human and rodent research (30).
Table 1.
Reported serum estradiol, estrone, and progesterone levels measured by GC- or LC-MS/MS from human and rodent blood
| Estradiol, pg/mL | Estrone, pg/mL | Progesterone, pg/mL | Method | References | |
|---|---|---|---|---|---|
| Women | |||||
| Premenopausal | 15–350 | 17–200 | 1,000–45,000 | LC-MS/MS | (24, 25) |
| Postmenopausal | 1–9 | 5–33 | LC-MS/MS | (26) | |
| <5 yr | 5 | 40 | LC-MS/MS | (27) | |
| >5 yr | 1.3 | 35 | (27) | ||
| Men | 10–40 | 15–60 | LC-MS/MS | (24, 28) | |
| Rats | |||||
| Female (3–4 mo) | <1–45 | 1–15 | 5–20 | GC-MS/MS | (21) |
| Mice | |||||
| Female (3–4 mo) | 1–12 | 1–2 | 10–60 | GC-MS/MS | (21) |
<5 yr and >5 yr indicate data from women who were postmenopausal for a time less than or greater than 5 yr since their last menstrual cycle. GC, gas chromatography; LC, liquid chromatography, MS/MS, tandem mass spectrometry.
The impact of female sex hormones, primarily E2, on skeletal muscle has been studied to a limited extent. There is some evidence in muscle physiology, as in many other systems (31, 32), that cycling levels of estrogens do not have a significant influence on biological variability. For example, in women, the fluctuation of sex hormones during the menstrual cycle did not elicit variation in muscle contractile properties including strength, power, and fatigability (33, 34). Similarly, female mice did not demonstrate alterations in running power, running economy (at >50% max oxygen consumption), or grip strength across the various phases of the estrous cycle (35). The phase of the estrous cycle did not impact cardiac function (e.g., heart rate, cardiac output, or stroke volume) or system mean arterial blood pressure in female rats (36). However, when challenged by hemorrhage shock, cardiac functions were depressed in all phases except during the proestrus phase, the phase of the cycle when E2 levels are the highest (36). This latter observation suggests that fluctuation of E2 during the normal menstrual or estrous cycle under homeostatic conditions may have little to no impact on physiological muscle function, but rather changes in E2 concentrations may play a more critical role in the ability of tissue to respond to an acute stress, perhaps such as those that elicit skeletal muscle wasting. A complexity to consider is that some acute stressors associated with muscle atrophy can induce alterations in the estrous cycle. For example, hindlimb unloading in rats causes altered duration and frequency of the estrous cycle (37, 38). In contrast, animals enduring the stress of space flight had very little change in the estrous cycle (39) suggesting that the investigator may need to determine how the acute stress model affects the estrous cycle to accurately assess the role of E2 in female muscle during conditions of muscle wasting.
Estrogen Transport, Receptors, and Mechanism of Action
In humans, E2 is transported in the bloodstream to target tissues primarily by being reversibly bound to sex-hormone-binding globulin (SHBG; ∼55%) or carrier proteins such as albumin (∼42%) with only ∼3% being free and nonprotein bound (40, 41). The “free hormone hypothesis” proposes that the biological activity of hormones is determined by the free concentration of E2 in the bloodstream (42, 43). However, whether E2 bound to SHBG or carrier proteins alters the biological activity of E2 in humans is currently unknown (42). Although rats and mice lack SHGB, experiments using transgenic mice expressing SHBG (SHBG-tg) provide support to the free hormone hypothesis (43). E2 treatment of ovariectomized (OVX) SHGB-tg mice increased circulating E2 concentrations; yet the elevated E2-SHBG levels failed to prevent the loss of uterine mass like E2 treatment to nontransgenic OVX mice did (43). These results suggest that the concentration of free, nonprotein-bound E2 levels is important for biological activity.
Estrogens are cholesterol derivatives that when free unbound can diffuse across membranes of target cells and bind intracellular or membrane-bound receptors to mediate physiological effects (40). There are three known estrogen receptors (ER): ERα, ERβ, and G protein-coupled receptor (GPER, formally designated as GPR30). Each of the estrogen receptors have been identified in numerous tissues and cell types throughout the body including skeletal muscle (44–48).
ERα and ERβ are both members of the nuclear receptor protein family (49) yet differ in the COOH-terminal ligand binding domain and in the NH2-terminal transactivation domain, binding affinity for E2, and tissue distribution and expression levels (40, 47). The ESR1 gene encodes for ERα protein and produces at least two variants (66 and 46 kDa; for review, see Refs. 50–53). The ERα (66 kDa) protein contains 595 amino acids and is highly expressed in the uterus and ovaries but is also found in the prostate stroma, testes, epididymis, breast, skeletal muscle, hypothalamus, pituitary, liver, pancreas, adipose, heart, bone, lungs, and blood vessels. The smaller ERα (46 kDa) variant has a truncated NH2-terminal resulting in a missing activation function (AF)-1 domain. This 46-kDa variant has not been described in skeletal muscle but has been detected in a membrane fraction of cardiac muscle (54), with other studies in nonmuscle tissue, suggesting it can be found in the plasma membrane (55, 56). The ESR2 gene encodes for ERβ, with the full-length protein containing 530 amino acids (60 kDa; for review, see Ref. 57). Multiple variants of ERβ have been identified with various masses because of alternative splicing, which results in the lack of the ligand-dependent AF-2 domain (53, 58). ERβ is highly expressed in ovaries and has also been identified in prostate epithelium, testes, epididymis, bone marrow, hypothalamus, adipose, lungs, bone, and blood vessels (5, 45, 47).
GPER is a 375-amino-acid (42 kDa) protein encoded by the GPER1 gene (59, 60). Although single nucleotide polymorphism variations of GPER have been detected and are associated with cancer predisposition and progression (61), little is known if the variants play a role in normal physiological functions. GPER is a seven transmembrane-domain G protein-coupled receptor with a subcellular localization on the plasma membrane; however, it has also been suggested to be localized on the endoplasmic reticulum (60, 62). GPER is expressed in the central nervous system, reproductive tissues, pancreatic islets, adipose, liver, skeletal muscle, heart, intestine, and inflammatory cells (48).
ER and GPER knockout mice have shown that these receptors have differing roles in estrogen physiology and distinct intracellular signaling mechanisms (63, 64). The “classic” or genomic mechanism of estrogen-mediated biological effects involves E2 diffusing directly across the cell membrane into the cytosol or nucleus and binding to ERα or ERβ (50, 53). The E2-ER then forms a dimer and binds to estrogen response elements (ERE) in the promoter and enhancer regions of genes, thereby regulating the transcription of estrogen responsive genes (40, 65, 66). In addition to the classic genomic mechanisms, E2 induces rapid activation of numerous protein-kinase cascades that can lead to indirect changes in gene expression because of protein phosphorylation. These nongenomic actions involve extracellular signal-regulated pathways such as phospholipase C (PLC)/protein kinase C (PKC) and p38/mitogen-activated protein kinase (MAPK) (60, 67). These pathways are thought to be primarily linked with membrane-associated ERs as well as the more recently identified GPER (60, 67). For more details on estrogen genomic and nongenomic mechanisms, see thorough reviews by Arnal et al. (50), Fuentes and Silveyra (67), and Olde and Leeb-Lundberg (48).
Estrogen Receptors in Skeletal Muscle
Identifying estrogen receptors in skeletal muscle tissue and especially cells (fibers) has been challenging because of 1) unreliable antibodies for ERα and ERβ producing false-positive results (54), 2) ∼60% of total protein in skeletal muscle being actin and myosin, making detection of low abundance proteins such as ERs difficult (68, 69), and 3) whole skeletal muscle tissue homogenates being “contaminated” by multiple cell types in the tissue that are known to express estrogen receptors [e.g., neurons (70), endothelial cells (71), and hematopoietic cells (72, 73)]. This latter challenge makes interpretation of the detection and quantification of ER proteins or mRNA distinctly in myofibers difficult.
To attribute ERs to specific cell types in skeletal muscle, strategies of fractionation, immunohistochemistry, or in situ hybridization can be used (with a caveat of understanding the challenges of working with ER antibodies or that mRNA localization should not be used as a substitute for protein localization). For example, the use of cellular protein fractionation has identified ERα and ERβ in both the crude nuclear and cytosolic/membrane fractions in skeletal muscle homogenate from postmenopausal women (74). Yet, immunostaining of the vastus lateralis muscle of children, adult men and women, and postmenopausal women has identified the primary localization of both ERα and ERβ to be in the myonuclei of muscle fibers (75). Both ERα and ERβ have been identified via immunofluorescence in mouse (76) and rat (77) skeletal muscles with localization in the nuclei. We and others have delivered fluorescently labeled ERα as means to determine ERα localization and circumvent ER antibodies challenges (54, 78). Following the electroporation of cDNA that encodes for GFP-tagged ERα (46 kDa) or ERα (66 kDa) into mouse skeletal muscle fibers, we largely found that both variants localize to the nucleus almost exclusively (78) in agreement with data from others (54). Although the degree to which ERα and ERβ are expressed on the sarcolemma has yet to be determined, membrane-bound GPER expression has been identified in human and rodent skeletal muscle and myotubes (12, 44, 79–81). Additionally, ERα and ERβ have been identified in satellite cells and play a key role in satellite cell dynamics during muscle regeneration (82, 83).
In addition to expression in the nucleus, competitive binding assays using the C2C12 skeletal muscle cell line suggest that there are nonclassic estrogen binding sites in mitochondria and microsomes (84). Immunolocalization of ERα and ERβ in C2C12 myoblast suggest that ERα is localized in the nucleus, microsomes, cytosol, and mitochondria whereas ERβ is primarily expressed in the mitochondria and to a lesser extent in the cytosol, nucleus, and microsomes (85). These nonclassic receptor localizations of ERα and ERβ have been proposed to contribute to E2 antiapoptotic effects in skeletal muscle (86), and thus, could play a role in maintaining skeletal muscle mass during muscle wasting conditions.
Recent evidence suggests that E2 can affect skeletal muscle independent of the ERs by localizing directly in the membranes affecting the fluidity of the membrane structure (87). In this case, E2 appears to have the ability to integrate with the mitochondrial membrane and influence mitochondrial energetics through effects on membrane fluidity (87). It is unclear at this point, if E2 can integrate with any other membranes such as the sarcolemma. However, since the structure of E2 is similar to cholesterol and the sarcolemma is enriched in cholesterol, it is conceivable that E2 may locate there as well and affect mechanisms that regulate skeletal muscle properties such as muscle mass.
THE INFLUENCE OF ESTROGEN ON MUSCLE WASTING
The hormonal profile is cyclic in women for the majority of their lifespan with the cyclic nature becoming less pronounced as ovarian estrogen and progesterone production decrease with age. This can result in women spending approximately one-third of their lives in a postmenopausal, E2-deficient state (88), and studies suggest that there is an accelerated loss of muscle mass associated with the onset of menopause (89–91). E2 deficiency is relevant for younger women as well as it can be a consequence of eating disorders, energy imbalances due to high-load exercise training, chemotherapy, or hysterectomy, for example. Thus, the mechanism by which E2 deficiency in females of any age couples with events that induces muscle wasting such as disuse or injury is critical to understand. Studies examining sex differences may hint at possible hormonal influences on muscle mass; however, additional research is needed to determine the cellular and molecular mechanisms underlying E2-mediated effects on muscle wasting as there are numerous other differences between the sexes than simply sex hormones. Below we present four conditions that cause skeletal muscle wasting and experimental results indicating sex differences and/or an influence by E2, as well as some mechanisms by which they occur.
Muscle Disuse Atrophy
Muscle mass is largely determined by the coordinated balance between muscle protein synthesis and muscle protein degradation. During periods of prolonged disuse (e.g., bedrest, limb immobilization, spaceflight), rates of muscle protein synthesis decrease significantly with concurrent elevations in degradation resulting in muscle atrophy. Because of the historical preference of using males in nonclinical investigations of disuse pathology, it was previously accepted that males and females exhibit similar cell signaling responses to inductions of atrophic pathways (92). However, recent works have highlighted sex-based disparities in the mechanisms that regulate disuse atrophy, suggesting an increased susceptibility to muscle wasting in females (93). Yoshihara et al. (94) found greater reductions in soleus muscle mass and cross-sectional area (CSA) in female rats than in males following 7 days of hindlimb unloading (HLU). Similarly, HLU led to significantly greater reductions in Type IIb myofiber CSA in the tibialis anterior muscles of female mice compared with males (95). Future research should investigate potential sexual dimorphisms in muscle wasting during long periods of physical inactivity considering the shorter duration of unloading (1–7 days) within these studies.
Mechanisms that may underlie greater loss of muscle mass in female rodents include lower rates of fractional protein synthesis and mRNA profile changes indicative of muscle catabolism during the first 24 h of disuse (95). Specifically, unloaded muscles of female rodents exhibited increased levels of ubiquitinated proteins because of alterations in the atrophic Foxhead box O3 (FoxO3a)/ubiquitin-proteasome pathway (94, 96). It appears that the enhanced severity of muscle wasting in females following disuse is attributed partly to elevated protein ubiquitination and degradation, especially during the initial phase of inactivity. Additional future research is necessary to determine whether these mechanisms are mediated by E2 as ubiquitination of the ERs has been demonstrated to disrupt receptor stability and function, ultimately impairing E2-ER signaling (97).
The seemingly greater predisposition of females than males to disuse-induced muscle wasting is further evident in conditions of E2 deficiency, such as that which occurs following the onset of menopause. Menopausal-induced E2 deficiency is modeled most often in preclinical rodent studies via bilateral surgical removal of the ovaries [i.e., ovariectomy (OVX)], resulting in substantial reductions of circulating ovarian hormones (98). Studies have shown that reloading of hindlimb skeletal muscles of OVX rats following HLU resulted in failure of muscle to recover atrophied muscle mass compared with ovary-intact rats (99, 100). Ovarian E2 deficiency was associated with impaired myofiber growth, myofiber regeneration, and extracellular matrix remodeling during muscle recovery from HLU (101). Importantly, impairments in the recovery of skeletal muscle mass from mechanical unloading can be rescued by E2 treatment pinpointing E2 as the major ovarian hormone impacting muscle mass (100, 101). Furthermore, E2 treatment facilitated the muscle mass recovery response of OVX rats during a rehabilitation exercise program (102), an effect also seen in postmenopausal women who were subjected to dual interventions of E2-based hormone therapy (HRT) and exercise [E2-related therapies; Sipilä et al. (103)]. A summary of the current knowledge surrounding the effects of HRT on muscle mass is discussed below.
In contrast to rodents, men but not women exhibit a loss in fiber size under conditions of reduced physical activity; however, women demonstrate lower muscle power output after physical inactivity compared with men (104). The authors suggested that in response to this type of muscle disuse (i.e., physical inactivity), actomyosin cross-bridge kinetics are slowed leading to reductions in myofiber force production and shortening velocity in fibers from women (104). These effects were further attributed partly to greater oxidative stress on contractile proteins in females than in males. Interestingly, estrogens appear to have tissue-specific effects on reactive oxygen species (ROS) emission potential, with E2 decreasing ROS emission in skeletal muscle (105). Thus, it can be speculated that any modulation in E2 levels or E2-ER signaling could lead to alterations in redox balance resulting in decreased muscle mass and/or strength. Overall, such studies indicate that across nonclinical models and in humans, the regulation of skeletal muscle mass in females following periods of disuse appears to be affected by ovarian hormone status and that ovarian function is important to recover from disuse that induces muscle atrophy.
Sarcopenia
Sarcopenia is defined as the age-associated reduction in skeletal muscle mass and strength that can lead to a decline in functional independence and an increased risk of disability, hospitalization, and mortality rates in aged adults (106). Factors contributing to sarcopenia include increased inflammation and oxidative stress, impaired mitochondrial function, satellite cell senescence, elevated apoptosis and proteasome activity, and suppressed protein synthesis and myocyte regeneration (107). Sarcopenic mechanisms appear to be sex dependent as elderly men exhibit more severe muscle wasting than elderly women because of male’s relatively larger muscle mass (108). However, the extent to which alterations in sex hormones contribute to the progression of sarcopenia remains to be fully elucidated. Despite the wide degree of variation among techniques and populations involved in longitudinal studies of sarcopenia, median rates of muscle mass loss in men and women aged ≥75 yr have been reported as 0.80–0.98% and 0.64–0.70% per year, respectively (109). It is important to note these data do not account for lifestyle factors (e.g., diet, degree of physical activity, socioeconomic status) among included population, which can impact severity of sarcopenia observed between sexes.
A chronic state of low-grade inflammation is a hallmark of aging (often termed “inflammaging”) and is marked by increased levels of proinflammatory cytokines such as tumor necrosis factor α (TNFα) and interleukin 6 (IL-6) (110). TNFα and IL-6 are well-characterized inflammatory biomarkers of aged skeletal muscle that stimulate muscle catabolism directly via activation of the UPS or indirectly by inducing muscle insulin resistance and inhibiting protein synthesis (111, 112). Although TNFα has been demonstrated to activate the UPS through NF-κB signaling in skeletal muscle (110), its contribution to muscle wasting during aging does not appear to occur through this pathway (113). Importantly, elevations in proinflammatory cytokines coupled with extended periods of physical inactivity (113) are strong predictors of morbidity and mortality in the aged population (114, 115).
Currently, there is no means to prevent sarcopenia. Thus, many groups are pursuing potential pharmacological agents and therapeutic interventions to minimize age-related muscle wasting. It is widely accepted that androgens exhibit anabolic effects on skeletal muscle in sarcopenic men (116), but unfortunately the ability of estrogens to prevent muscle wasting in sarcopenic women is not readily known. Previous research has highlighted potential mechanisms that may occur with the loss of ovarian-derived E2 and senescent female skeletal muscle. For example, E2 deficiency is associated with apoptosis-linked microRNAs (117) and increased levels of aforementioned cytokines (109, 111). Accordingly, E2 treatment in OVX rats decreased levels of proinflammatory cytokines and reduced muscle damage as evidenced by lower serum creatine kinase expression compared with that in untreated OVX rats (118). These data may position early anti-inflammatory treatment as an especially useful therapeutic to minimize the development of sarcopenia in the aged female population because of the apparent exacerbation of sarcopenic muscle wasting by ovarian loss of E2.
In addition to inflammation, alterations in mitochondrial energetic regulation influence the onset of sarcopenia (119–121). A significant body of literature across a variety of tissues has demonstrated myriad effects of estrogens and E2-ER signaling on mitochondrial function (e.g., ATP production, ROS emission, membrane potential regulation, calcium handling) as well as mitochondrial biogenesis (122). Thus, it is logical to consider that alterations in skeletal muscle mitochondria during aging may be mediated by decreases in E2 and ultimately contribute to the onset of sarcopenia in women. A number of groups have found that skeletal muscle from OVX mice demonstrate reduced ADP-stimulated mitochondrial respiration and an oxidative shift in redox homeostasis compared with control mice (105, 123). Loss of ovarian-derived E2 results in reduced complex I activity in skeletal muscle alongside an oxidative shift in the glutathione ratio and an increase in ROS emission, all of which were reversed with E2 treatment in OVX mice (87). As OVX mice are not commonly aged, it may be difficult to distinguish between the independent effects of decreased E2 and aging on mitochondrial function. However, elevated ROS emission potential is often found in various models of muscle wasting and in sarcopenia, suggesting that increased ROS emission due to E2 deficiency may contribute to or exacerbate sarcopenic muscle wasting (reviewed in Ref. 124).
Determining the manner in which E2 influences mitochondria in skeletal muscle has led to conflicting results concerning the role of ERα. The conditional deletion of ERα in cultured myotubes resulted in lower respiratory values (125), whereas in an inducible skeletal muscle-specific ERα knockout mouse model, no differences were found across multiple different mitochondrial respiratory measures (78). Similar results were confirmed by Torres et al. (87) who found that E2 associated with the mitochondrial membrane affected the fluidity of the membrane, thus influencing activity of the respiratory complexes. Furthermore, the association of E2 with the mitochondrial membrane occurred independent of ERα (87). It remains to be empirically determined if loss of E2 is a major leader to the onset of sarcopenia due to excessive ROS emission from muscle mitochondria; however, circumstantial evidence suggests it is possible and future experiments are needed.
Muscle Cachexia
Cachexia is a complex syndrome that is characterized by the unintentional loss of body weight of >5% or >2% for people with a body mass index <20 kg/m2, or >2% with individuals already presenting with established sarcopenia (appendicular muscle index: men <7.26 kg/m2; women <5.45 kg/m2) (126, 127). Muscle cachexia is associated with several diseases including autoimmune diseases (e.g., rheumatoid arthritis, multiple sclerosis), cancer (type and stage), chronic obstructive pulmonary disease, congestive heart failure, diabetes, renal failure, and sepsis (128, 129). Cachexia is considered to be caused by the dysregulation of metabolic, immunological, and neurological factors and involves multiple pathways (for review, see Refs. 129–134).
Elucidating the potential of E2 to alter cachexia by comparing men and women is difficult to determine because of diagnostic age (e.g., pre- vs. postmenopausal women), type of primary disease, body composition sex differences, level of physical activity, and pharmaceutical interventions that may be sex specific. Yet, given these limitations, the susceptibility and progression of cachexia seem to differ between the sexes (107, 135). For example, men with rheumatoid arthritis have a greater deficit in muscle mass than women (136); yet women with multiple sclerosis have less loss in muscle fiber cross-sectional area compared with men (137). In a study comparing muscle wasting in men and women patients with lung cancer, results indicated that although both sexes had a similar ∼6% total percent body weight loss over 6 mo, significantly more men (61%) than women (38%) were classified as sarcopenic (138). Similarly, in patients with progressive cachexia defined as >10% weight loss, men had a greater reduction in muscle mass loss compared with women (139). Taken together, these studies hint at the possibility that sex-specific hormones may be relevant to the prevention and/or treatment of muscle cachexia (130, 140).
The immune system is known to play a central role in the development of cachexia (128, 132, 133), with proinflammatory cytokines such as IL-1, IL-6, and TNF-α, all being associated with the progression of cachexia (141) from multiple origins [autoimmune (142), cancer (132), sepsis (131, 132, 140–143)]. E2 is a known regulator of the immune system (144, 145), and treatment with E2 has been demonstrated to alter expression of both IL-6 and TNF-α in humans (146) and mice (147) during conditions associated with cachexia. Thus, it has been purported that E2 could be protective against the cachexia (135); yet studies to directly examine muscle mass in response to estrogen treatment in diseases associated with cachexia are lacking. Future nonclinical and clinical studies are needed to determine if an estrogenic treatment can mitigate inflammation-induced progression of cachexia and further to substantiate molecular mechanisms involved.
Some mechanistic insight on how estrogens influence cachexia can be gleaned from studies using rodent models. Similar to studies in humans, a study examining cachexia in a nonclinical tumor-bearing mouse model (ApcMin/+) indicates that male and female mice differ with male ApcMin/+ mice having greater reductions in body mass than females (148). Additionally, it was hypothesized that the sex difference in muscle loss was IL-6 dependent as male ApcMin/+ mice expressed higher (∼58%) circulating IL-6 levels than female ApcMin/+ mice (148). Hetzler et al. (149) further demonstrated that tumor-bearing acyclic mice had elevated muscle cytokine gene expression (TNFα and IL-6) compared with female mice with normal estrous cycles. Ovariectomized colorectal cancer (azoxymethane/dextran sulfate sodium-treated) mice that were treated with E2 had decreased expression of both TNFα and IL-6 expression (147), additionally suggesting that estrogen alters cytokine expression and thus could be beneficial to reducing the progression of cachexia.
Direct impact of E2 on muscle wasting in a cachexic model is shown in a study by Counts and coworkers (150). They examined muscle cachexia in a nonclinical tumor-bearing mouse model (ApcMin/+) and showed that E2 treatment of OVX cachexic mice leads to greater muscle mass compared with those mice not treated with E2 (151). Additionally, the loss of body and muscle masses was less in female cachexic mice with normal estrous cycles compared with cachexic mice with abnormal cycles and presumably low E2 (149, 151). Collectively, these data demonstrate that estrogen is beneficial for maintaining muscle mass during ApcMin/+-induced cachexia. Counts et al. (151) proposed that the beneficial estrogenic effects on muscle mass may occur through the mechanistic target of rapamycin (mTOR) pathway. Skeletal muscle of E2-treated ApcMin/+ mice had an increased activation of mTORC targets including eukaryotic initiation factor 4E binding protein-1 (4E-BP1:T37/44) and ribosomal S6 kinase 1 (rpS6:S240/244), suggesting an increase in protein synthesis rates (151). Interestingly, sepsis, which induces elevated cytokine expression and results in cachexia, blunts muscle protein synthesis by impairing mTOR phosphorylation of 4E-BP1 (150, 152). In contrast, when cytokine production is inhibited 4E-BP1 phosphorylation and protein synthesis are increased (152, 153). Taken together, such results indicate that E2 alterations of mTOR signaling may be a secondary effect of E2 modulating the immune system. More research is needed to further determine the extent that E2 directly regulates mTOR signaling in muscle as well as the extent to which the beneficial effects of E2 treatment on muscle cachexia are involved in other cachexia-inducing diseases (autoimmune, renal failure, etc.).
Skeletal Muscle Injury
Skeletal muscle incurs injuries throughout life and requires repeated regeneration to regain mass and contractile function. With aging, the capacity to efficiently regenerate and recover from injury is impaired and contributes to a decrease in muscle mass (154–156). A type of stem cell specific to skeletal muscle (MuSC), also known as the satellite cell, mediates skeletal muscle regeneration. MuSCs are quiescent until activated in response to a growth or injury signal and then transition from quiescence into the myogenic program to proliferate and repair the muscle (for review, see Ref. 157). Decreases in MuSC number, proliferation, or activation hamper the muscle regeneration process leading to a loss in muscle mass (156, 158).
In humans and mice, there is recent evidence that a deficiency of ovarian hormones is related to a decline in the number of MuSCs (82). Both ERα and ERβ have been detected in MuSC (82, 83). The loss of ERα in MuSC results in upregulation of apoptotic genes (e.g., p53, p38, and DIABLO) and a reduction in MuSC population of up to 56% (82). The deletion of ERβ in MuSC does not alter the number of MuSC per muscle fiber but rather results in a suppression of MuSC proliferation and impaired expression of cyclinA2 (Ccna2), a cell-cycle regulator (83). Taken together, estrogens can impact the muscle regeneration process via receptor-mediated mechanisms within MuSCs.
In female mice lacking estrogens, the loss of MuSC is detrimental to muscle mass adaptation following repeat injuries (158), suggesting that effect of E2 on MuSC is beneficial for muscle mass adaptation. In addition to estrogen cell-autonomous mechanisms, estrogen molecules are a part of and have been implicated in modulating the MuSC microenvironment known as the niche (82, 159–161). The importance of estrogens in the MuSC niche is demonstrated via in vivo transplantation studies whereby injured muscle is regenerated from donor MuSCs. Specifically, MuSC harvested from control female mice transplanted into an E2-depleted environment (injured muscle in an OVX mouse) had cell engraftment reduced 75% compared with those transplanted into an E2-repleted environment indicating incomplete regeneration (82). Moreover, the transfer of MuSC harvested from an E2-depleted environment (OVX mouse) into an E2-present environment rescued MuSC engraftment (82). The MuSC niche consists of extracellular matrix, vascular and neural networks, immune cells, and circulating hormones (155, 161). In response to injury, E2 has been implicated in altering extracellular matrix (162), vascular (163) and neural (164) networks, and immune cells (165). However, how E2 modulates the MuSC niche and how alterations in the niche contribute to the maintenance or adaptation of muscle mass need further investigation. Furthermore, to what extent estrogenic mechanisms in MuSC are the same or differ from that of muscle fibers is not clear, though both likely impact the mass of muscle.
E2-RELATED THERAPEUTIC INTERVENTIONS, CONSIDERATIONS, AND ALTERNATIVES
Evidence is beginning to accumulate that E2 may be an important factor to consider in the treatment of muscle wasting. Although interrogation of mechanisms regulating muscle mass by E2 continues, there are several interventions to consider including elevating E2 signaling using hormone replacement therapy (HRT) and selective estrogen receptor modulators (SERMs) or through the reduction in E2 levels with aromatase inhibition. Here we will give an overview of these estrogenic interventions.
Estrogen-Based Hormone Replacement Therapy/Treatments
Estradiol is the most common form of HRT for the treatment of menopause and is prescribed via several different modes of administration (e.g., tablets, injections, and topical creams) and dosages (88, 166). The use of HRT has been reported to increase the levels of circulating estrogens. However, the physiological implications for the mode of administration and dosage for clinical benefits still need clarification (88, 167, 168). Healthy pre- and postmenopausal women treated with a transdermal E2 patch (0.1 mg E2 per day) for 14 days had an ∼1.3-fold increase in the ratio of E2-to-estrone in their serum yet had no effect on protein fractional synthesis rate or expression of genes involved in the regulation of muscle mass (e.g., MYOD1, FST, MSTN, and FOXO3) (169). The use of a conjugated equine estrogen of 0.625 mg daily for 1 yr has been shown to elevate E2 serum levels ∼2.7-fold in healthy postmenopausal women compared with women who received placebo (170). A recent meta-analysis investigating the effect of HRT on muscle mass demonstrated that a HRT of 0.625 mg of E2 in women >50 yr was associated with ∼0.06–0.2 kg greater muscle mass retention compared with the postmenopausal control group not receiving HRT (171). Although the analysis did not prove statistically significant, it does point to the potential benefits of E2-based HRT on maintaining muscle mass in healthy postmenopausal women. In another study, healthy pre- and postmenopausal women treated with a transdermal E2 patch (0.1 mg E2 per day) for 14 days had an ∼1.3-fold increase in the ratio of E2-to-estrone in their serum; yet there was no effect on protein fractional synthesis rate or expression of genes involved in the regulation of muscle mass (e.g., MYOD1, FST, MSTN, and FOXO3) (169). Thus, more clinical studies are needed to determine if HRT effects on muscle mass would be beneficial during conditions of muscle wasting including but not restricted to menopause.
Few studies have examined the effect of E2 treatment on skeletal muscle mass in ovarian hormone deficient rodents. Experimental variables such as age of rodents and E2 method of administration, dosage, and duration of treatment differ greatly among studies and complicate interpretation of muscle mass data. For example, reported E2 dosages in mice range from 0.02 µg/day to 20 mg/day (172–174). Dosing is critical because resulting serum levels can be nonphysiological and not comparable with ovary-intact, control animals. As an example, an E2 dose of ∼120 µg/day raised serum E2 levels ∼88-fold in OVX mice over ovary-intact mice (174). In another study, a dose of ∼4.2 µg/day raised serum E2 levels ∼4-fold in OVX mice and resulted in an increase in uterine mass beyond physiological levels as well as adverse effects on kidney physiology (172). Taken together, these studies suggest that an excessive dose of E2 can limit the risk-benefit ratio and complicates the interpretation of E2 effects in preclinical models.
Following ovariectomy in adult female mice, there is an increase skeletal muscle mass due to an increase in collagen and/or nonprotein content (99, 175–177). This increase in muscle weight gain by nonprotein content is attenuated with E2 treatment of ∼3 µg/day that yielded E2 serum levels ∼2- to 4-fold greater in OVX than ovary-intact mice (175, 178). The duration of ovarian hormone deficiency and that of E2 treatment in these mouse studies are relatively short, on the order of 2–12 wk, and may demonstrate transient effects on muscle mass. Little is known on how long-term OVX with or without E2 treatment affects muscle mass in preclinical animal models. In summary, although estrogen-based HRT is clinically relevant for women, the effects and mechanisms of any effects on skeletal muscle mass are not clear and further research is warranted.
Selective Estrogen Receptor Modulators
Selective estrogen receptor modulators (SERMs) have emerged as alternative therapeutic agents that show promise in treating muscle wasting associated with estrogen deficiency because of their more favorable side effect profile than estrogens (179). SERMs are a class of ER ligands that act as tissue- and cell-specific estrogen agonists/antagonists via conformational changes of ERs that are induced on ligand binding (180). Mechanisms of action of SERMs are reliant on different cell signaling properties and ER cofactors, which result ultimately in several physiological effects that are tissue dependent. Examples of SERMs commonly used in the clinic include tamoxifen (TAM; first-generation SERM), raloxifene (RAL; second-generation SERM), and bazedoxifene (BZA; third-generation SERM). Specifically, TAM and RAL have been shown to improve numerous pathogenic factors involved in mouse models of myopathy (181–183), which may hold implications in ameliorating declines in skeletal muscle mass. However, evidence surrounding the direct effects of SERMs on skeletal muscle in estrogen-deficient rodents and humans remains scarce. Thus, further research is necessary to determine the efficacy of this drug class in the treatment of muscle wasting.
Aromatase Inhibition
Aromatase inhibitor therapy is a common adjuvant therapy for patients with breast cancer and postmenopausal breast cancer survivors to reduced E2 levels (184, 185). Breast cancer patients who underwent aromatase inhibition demonstrated maintenance of total body fat and an increase in lean body mass and free testosterone levels compared with no-aromatase inhibition controls (185). Similarly, an increase in body mass and an increase in testosterone are observed in rats treated with an aromatase inhibitor (186) and in aromatase knockout mice (187, 188). Collectively, muscle mass may be maintained while taking aromatase inhibitors because of the rise in free testosterone (185) and therefore should be a consideration if using aromatase inhibitor in the study of estrogenic effects.
SUMMARY
The purpose of the review is to summarize the current understanding of E2’s impact on skeletal muscle wasting and highlight aspects of sex dimorphism on the topic. Our review of the literature has elucidated that the field needs additional studies to directly assess effects of E2 on the susceptibility of skeletal muscle atrophy or wasting. Several of our interpretations of the literature require some form of extrapolation or assumption regarding the muscle wasting conditions that we report on particularly when considering the estrogenic mechanisms that may be involved across the several models used to study wasting. That is, although sex differences may suggest that estrogen is involved, there are clearly many other aspects that differ between females and males than just sex hormones. Ultimately, determining if sex-based differences exist and the mechanisms that drive them will be necessary as the field pushes toward developing approaches to prevent, attenuate, and ideally reverse muscle wasting.
GRANTS
This study was funded by National Institutes of Health Grants R61AR078100 and RO1AR066660 (to E.E.S.), RO1AG031743 and RO1AG062899 (to D.A.L.), and T32AR007612 (to S.L.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L.M. and E.C.M. prepared figures; S.L.M., E.C.M., D.A.L., and E.E.S. drafted manuscript; S.L.M., E.C.M., D.A.L., and E.E.S. edited and revised manuscript; S.L.M., E.C.M., D.A.L., and E.E.S. approved final version of manuscript.
REFERENCES
- 1.Chan HJ, Petrossian K, Chen S. Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and—resistant breast cancer cells. J Steroid Biochem Mol Biol 161: 73–83, 2016. doi: 10.1016/j.jsbmb.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, Moriya T, Hirakawa H, Evans DB, Hayashi S, Ohuchi N, Sasano H. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res 67: 3945–3954, 2007. doi: 10.1158/0008-5472.CAN-06-3105. [DOI] [PubMed] [Google Scholar]
- 3.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev 97: 995–1043, 2017. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlowski M, Sarao MS. Physiology, follicle stimulating hormone. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2021. http://www.ncbi.nlm.nih.gov/books/NBK535442/. [PubMed] [Google Scholar]
- 5.Barakat R, Oakley O, Kim H, Jin J, Ko CJ. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep 49: 488–496, 2016. doi: 10.5483/bmbrep.2016.49.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15: 342–355, 1994. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 7.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase—a brief overview. Annu Rev Physiol 64: 93–127, 2002. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med 19: 197–209, 2013. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larionov AA, Vasyliev DA, Mason JI, Howie AF, Berstein LM, Miller WR. Aromatase in skeletal muscle. J Steroid Biochem Mol Biol 84: 485–492, 2003. doi: 10.1016/S0960-0760(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 10.Aizawa K, Iemitsu M, Maeda S, Mesaki N, Ushida T, Akimoto T. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med Sci Sports Exerc 43: 2072–2080, 2011. doi: 10.1249/MSS.0b013e31821e9d74. [DOI] [PubMed] [Google Scholar]
- 11.Kim NR, David K, Corbeels K, Khalil R, Antonio L, Schollaert D, Deboel L, Ohlsson C, Gustafsson JÅ, Vangoitsenhoven R, Van der Schueren B, Decallonne B, Claessens F, Vanderschueren D, Dubois V. Testosterone reduces body fat in male mice by stimulation of physical activity via extrahypothalamic ERα signaling. Endocrinology 162: bqab045, 2021. doi: 10.1210/endocr/bqab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pöllänen E, Sipilä S, Alen M, Ronkainen PHA, Ankarberg-Lindgren C, Puolakka J, Suominen H, Hämäläinen E, Turpeinen U, Konttinen YT, Kovanen V. Differential influence of peripheral and systemic sex steroids on skeletal muscle quality in pre- and postmenopausal women. Aging Cell 10: 650–660, 2011. doi: 10.1111/j.1474-9726.2011.00701.x. [DOI] [PubMed] [Google Scholar]
- 13.Liao ZH, Huang T, Xiao JW, Gu RC, Ouyang J, Wu G, Liao H. Estrogen signaling effects on muscle-specific immune responses through controlling the recruitment and function of macrophages and T cells. Skelet Muscle 9: 20, 2019. doi: 10.1186/s13395-019-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res 84: 1285–1291, 1999. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 15.Snyder GD, Krishna UM, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol 283: H1936–H1942, 2002. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- 16.Diano S, Horvath TL, Mor G, Register T, Adams M, Harada N, Naftolin F. Aromatase and estrogen receptor immunoreactivity in the coronary arteries of monkeys and human subjects. Menopause 25: 1201–1207, 2018. doi: 10.1097/GME.0000000000001219. [DOI] [PubMed] [Google Scholar]
- 17.Villaggio B, Soldano S, Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin Exp Rheumatol 30: 934–938, 2012. [PubMed] [Google Scholar]
- 18.Romani W, Patrie J, Curl LA, Flaws JA. The correlations between estradiol, estrone, estriol, progesterone, and sex hormone-binding globulin and anterior cruciate ligament stiffness in healthy, active females. J Womens Health (Larchmt) 12: 287–298, 2003. doi: 10.1089/154099903321667627. [DOI] [PubMed] [Google Scholar]
- 19.Verdonk SJE, Vesper HW, Martens F, Sluss PM, Hillebrand JJ, Heijboer AC. Estradiol reference intervals in women during the menstrual cycle, postmenopausal women and men using an LC-MS/MS method. Clin Chim Acta 495: 198–204, 2019. doi: 10.1016/j.cca.2019.04.062. [DOI] [PubMed] [Google Scholar]
- 20.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology 130: 805–810, 1992. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson ME, Vandenput L, Tivesten Å, Norlén A-K, Lagerquist MK, Windahl SH, Börjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology 156: 2492–2502, 2015. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 22.Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 6: 5, 2020. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanczyk FZ, Clarke NJ. Measurement of estradiol—challenges ahead. J Clin Endocrinol Metab 99: 56–58, 2014. doi: 10.1210/jc.2013-2905. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem 50: 373–384, 2004. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 25.Denver N, Khan S, Homer NZM, MacLean MR, Andrew R. Current strategies for quantification of estrogens in clinical research. J Steroid Biochem Mol Biol 192: 105373, 2019. doi: 10.1016/j.jsbmb.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertelsen B-E, Kellmann R, Viste K, Bjørnevik AT, Eikesdal HP, Lønning PE, Sagen JV, Almås B. An ultrasensitive routine LC-MS/MS method for estradiol and estrone in the clinically relevant sub-picomolar range. J Endocr Soc 44: bvaa047, 2020. doi: 10.1210/jendso/bvaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VH, Ridgway EC, Wierman ME. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography tandem mass spectrometry. Steroids 76: 177–182, 2011. doi: 10.1016/j.steroids.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsing AW, Stanczyk FZ, Belanger A, Schroeder P, Chang L, Falk RT, Fears TR. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev 16: 1004–1008, 2007. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- 29.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab 98: 1376–1387, 2013. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel-Khalik J, Björklund E, Hansen M. Simultaneous determination of endogenous steroid hormones in human and animal plasma and serum by liquid or gas chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 928: 58–77, 2013. doi: 10.1016/j.jchromb.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35: 565–572, 2011. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40: 1–5, 2014. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Dasa MS, Kristoffersen M, Ersvær E, Bovim LP, Bjørkhaug L, Moe-Nilssen R, Sagen JV, Haukenes I. The female menstrual cycles effect on strength and power parameters in high-level female team athletes. Front Physiol 12: 600668, 2021. doi: 10.3389/fphys.2021.600668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janse de Jonge XA, Boot CRL, Thom JM, Ruell PA, Thompson MW. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol 530: 161–166, 2001. doi: 10.1111/j.1469-7793.2001.0161m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguiar AS Jr, Speck AE, Amaral IM, Canas PM, Cunha RA. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci Rep 8: 10742, 2018. doi: 10.1038/s41598-018-29050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, Choudhry MA, Hsieh Y-C, Hu S, Rue LW 3rd, Bland KI, Chaudry IH. Estrus cycle: influence on cardiac function following trauma-hemorrhage. Am J Physiol Heart Circ Physiol 291: H2807–H2815, 2006. doi: 10.1152/ajpheart.00195.2006. [DOI] [PubMed] [Google Scholar]
- 37.Rosa‐Caldwell ME, Mortreux M, Kaiser UB, Sung D‐M, Bouxsein ML, Dunlap KR, Greene NP, Rutkove SB. The estrous cycle and skeletal muscle atrophy: investigations in rodent models of muscle loss. Exp Physiol, 2021. doi: 10.1113/EP089962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tou JCL, Grindeland RE, Wade CE. Effects of diet and exposure to hindlimb suspension on estrous cycling in Sprague-Dawley rats. Am J Physiol Endocrinol Physiol 286: E425–E433, 2004. doi: 10.1152/ajpendo.00287.2003. [DOI] [PubMed] [Google Scholar]
- 39.Hong X, Ratri A, Choi SY, Tash JS, Ronca AE, Alwood JS, Christenson LK. Effects of spaceflight aboard the International Space Station on mouse estrous cycle and ovarian gene expression. NPJ Microgravity 7: 11, 2021. doi: 10.1038/s41526-021-00139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med 346: 340–352, 2002. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 41.Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol 230: R13–R25, 2016. doi: 10.1530/JOE-16-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bikle DD. The free hormone hypothesis: when, why, and how to measure the free hormone levels to assess vitamin D, thyroid, sex hormone, and cortisol status. JBMR Plus 5: e10418, 2021. doi: 10.1002/jbm4.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurent MR, Hammond GL, Blokland M, Jardí F, Antonio L, Dubois V, Khalil R, Sterk SS, Gielen E, Decallonne B, Carmeliet G, Kaufman JM, Fiers T, Huhtaniemi IT, Vanderschueren D, Claessens F. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep 6: 35539, 2016. doi: 10.1038/srep35539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One 5: e10164, 2010. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couse JF, Lindzey J, Grandien K, Gustafsson J-Å, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 138: 4613–4621, 1997. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda K, Horie-Inoue K, Inoue S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J Steroid Biochem Mol Biol 191: 105375, 2019. doi: 10.1016/j.jsbmb.2019.105375. [DOI] [PubMed] [Google Scholar]
- 47.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138: 863–870, 1997. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 48.Olde B, Leeb-Lundberg LMF. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab 20: 409–416, 2009. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Kininis M, Kraus WL. A global view of transcriptional regulation by nuclear receptors: gene expression, factor localization, and DNA sequence analysis. Nucl Recept Signal 6: e005, 2008. doi: 10.1621/nrs.06005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnal J-F, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P, Katzenellenbogen B, Katzenellenbogen J. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev 97: 1045–1087, 2017. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 51.Kos M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ERα gene promoter region. Mol Endocrinol 15: 2057–2063, 2001. doi: 10.1210/me.15.12.2057. [DOI] [PubMed] [Google Scholar]
- 52.Lung DK, Reese RM, Alarid ET. Intrinsic and extrinsic factors governing the transcriptional regulation of ESR1. Horm Cancer 11: 129–147, 2020. doi: 10.1007/s12672-020-00388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yaşar P, Ayaz G, User SD, Güpür G, Muyan M. Molecular mechanism of estrogen–estrogen receptor signaling. Reprod Med Biol 16: 4–20, 2016. doi: 10.1002/rmb2.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pugach EK, Blenck CL, Dragavon JM, Langer SJ, Leinwand LA. Estrogen receptor profiling and activity in cardiac myocytes. Mol Cell Endocrinol 431: 62–70, 2016. doi: 10.1016/j.mce.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor α 46-kDa isoform in human endothelial cells. Circulation 107: 120–126, 2003. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- 56.Yang JZ, O’Flatharta C, Harvey BJ, Thomas W. Membrane ERα-dependent activation of PKCα in endometrial cancer cells by estradiol. Steroids 73: 1110–1122, 2008. doi: 10.1016/j.steroids.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol 63: 165–192, 2001. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 58.Hirata S, Shoda T, Kato J, Hoshi K. Isoform/variant mRNAs for sex steroid hormone receptors in humans. Trends Endocrinol Metab 14: 124–129, 2003. doi: 10.1016/S1043-2760(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 59.DeLeon C, Wang DQ-H, Arnatt CK. G Protein-coupled estrogen receptor, GPER1, offers a novel target for the treatment of digestive diseases. Front Endocrinol (Lausanne) 11: 578536, 2020. doi: 10.3389/fendo.2020.578536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo J, Liu D. Does GPER really function as a G protein-coupled estrogen receptor in vivo? Front Endocrinol (Lausanne) 11: 148, 2020. doi: 10.3389/fendo.2020.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulhaq ZS, Soraya GV, Milliana A, Tse WKF. Association between GPER gene polymorphisms and GPER expression levels with cancer predisposition and progression. Heliyon 7: e06428, 2021. doi: 10.1016/j.heliyon.2021.e06428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barton M. Position paper: the membrane estrogen receptor GPER—clues and questions. Steroids 77: 935–942, 2012. doi: 10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Prossnitz ER, Hathaway HJ. What have we learned about GPER function in physiology and disease from knockout mice? J Steroid Biochem Mol Biol 153: 114–126, 2015. doi: 10.1016/j.jsbmb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rouhimoghadam M, Lu AS, Salem AK, Filardo EJ. Therapeutic perspectives on the modulation of G-protein coupled estrogen receptor, GPER, function. Front Endocrinol (Lausanne) 11: 591217, 2020. doi: 10.3389/fendo.2020.591217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett 238: 1–14, 2006. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Hamilton KJ, Hewitt SC, Arao Y, Korach KS. Estrogen hormone biology. Curr Top Dev Biol 125: 109–146, 2017. doi: 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 116: 135–170, 2019. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cutler AA, Dammer EB, Doung DM, Seyfried NT, Corbett AH, Pavlath GK. Biochemical isolation of myonuclei employed to define changes to the myonuclear proteome that occur with aging. Aging Cell 16: 738–749, 2017. doi: 10.1111/acel.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deshmukh AS, Murgia M, Nagaraj N, Treebak JT, Cox J, Mann M. Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol Cell Proteomics 14: 841–853, 2015. doi: 10.1074/mcp.M114.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu C-L, Herndon C. New roles for neuronal estrogen receptors. Neurogastroenterol Motil 29: e13121, 2017. doi: 10.1111/nmo.13121. [DOI] [PubMed] [Google Scholar]
- 71.Stefano GB, Prevot V, Beauvillain JC, Cadet P, Fimiani C, Welters I, Fricchione GL, Breton C, Lassalle P, Salzet M, Bilfinger TV. Cell-surface estrogen receptors mediate calcium-dependent nitric oxide release in human endothelia. Circulation 101: 1594–1597, 2000. doi: 10.1161/01.cir.101.13.1594. [DOI] [PubMed] [Google Scholar]
- 72.Murphy AJ, Guyre PM, Wira CR, Pioli PA. Estradiol regulates expression of estrogen receptor ERα46 in human macrophages. PLoS One 4: e5539, 2009. doi: 10.1371/journal.pone.0005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, Hevener AL. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA 108: 16457–16462, 2011. [Erratum in Proc Natl Acad Sci USA 109: 645, 2012] doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park Y-M, Pereira R, Erickson C, Swibas T, Kang C, Van Pelt RE. . Time since menopause and skeletal muscle estrogen receptors, PGC-1α, and AMPK. Menopause 24: 815–823, 2017. doi: 10.1097/GME.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiik A, Ekman M, Johansson O, Jansson E, Esbjörnsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol 131: 181–189, 2009. doi: 10.1007/s00418-008-0512-x. [DOI] [PubMed] [Google Scholar]
- 76.Barros RPA, Machado UF, Warner M, Gustafsson J-A. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc Natl Acad Sci USA 103: 1605–1608, 2006. [Erratum in Proc Natl Acad Sci USA 103: 8298, 2006] doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bunratsami S, Udomuksorn W, Kumarnsit E, Vongvatcharanon S, Vongvatcharanon U. Estrogen replacement improves skeletal muscle performance by increasing parvalbumin levels in ovariectomized rats. Acta Histochemica 117: 163–175, 2015. doi: 10.1016/j.acthis.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Iñigo MR, Amorese AJ, Tarpey MD, Balestrieri NP, Jones KG, Patteson DJ, Jackson KC, Torres MJ, Lin CT, Smith CD, Heden TD, McMillin SL, Weyrauch LA, Stanley EC, Schmidt CA, Kilburg-Basnyat BB, Reece SW, Psaltis CE, Leinwand LA, Funai K, McClung JM, Gowdy KM, Witczak CA, Lowe DA, Neufer PD, Spangenburg EE. Estrogen receptor-α in female skeletal muscle is not required for regulation of muscle insulin sensitivity and mitochondrial regulation. Mol Metab 34: 1–15, 2020. doi: 10.1016/j.molmet.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pöllänen E, Kangas R, Horttanainen M, Niskala P, Kaprio J, Butler-Browne G, Mouly V, Sipilä S, Kovanen V. Intramuscular sex steroid hormones are associated with skeletal muscle strength and power in women with different hormonal status. Aging Cell 14: 236–248, 2015. doi: 10.1111/acel.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Alencar A, Lin M, Sun X, Sudo RT, Zapata-Sudo G, Lowe DA, Groban L. Activation of GPR30 improves exercise capacity and skeletal muscle strength in senescent female Fischer344 × Brown Norway rats. Biochem Biophys Res Commun 475: 81–86, 2016. doi: 10.1016/j.bbrc.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moreno-Ulloa A, Miranda-Cervantes A, Licea-Navarro A, Mansour C, Beltrán-Partida E, Donis-Maturano L, Delgado De la Herrán HC, Villarreal F, Álvarez-Delgado C. (−)-epicatechin stimulates mitochondrial biogenesis and cell growth in C2C12 myotubes via the G-protein coupled estrogen receptor. Eur J Pharmacol 822: 95–107, 2018. doi: 10.1016/j.ejphar.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collins BC, Arpke RW, Larson AA, Baumann CW, Xie N, Cabelka CA, Nash NL, Juppi HK, Laakkonen EK, Sipilä S, Kovanen V, Spangenburg EE, Kyba M, Lowe DA. Estrogen regulates the satellite cell compartment in females. Cell Rep 28: 368–381.e6, 2019. doi: 10.1016/j.celrep.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seko D, Fujita R, Kitajima Y, Nakamura K, Imai Y, Ono Y. Estrogen receptor β controls muscle growth and regeneration in young female mice. Stem Cell Reports 15: 577–586, 2020. doi: 10.1016/j.stemcr.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milanesi L, Russo de Boland A, Boland R. Expression and localization of estrogen receptor alpha in the C2C12 murine skeletal muscle cell line. J Cell Biochem 104: 1254–1273, 2008. doi: 10.1002/jcb.21706. [DOI] [PubMed] [Google Scholar]
- 85.Milanese L, Vasconcelos A, Russo de Boland A, Boland R. Expression and subcellular distribution of native estrogen receptor beta in murine C2C12 cells and skeletal muscle tissue. Steroids 74: 489–497, 2009. doi: 10.1016/j.steroids.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Boland R, Vasconsuelo A, Milanesi L, Ronda A, Boland A. 17β-estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids 73: 859–863, 2008. doi: 10.1016/j.steroids.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 87.Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin C-T, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR, Neufer PD. 17β-estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab 27: 167–179.e7, 2018. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paciuc J. Hormone therapy in menopause. Adv Exp Med Biol 1242: 89–120, 2020. doi: 10.1007/978-3-030-38474-6_6. [DOI] [PubMed] [Google Scholar]
- 89.Kitamura I, Koda M, Otsuka R, Ando F, Shimokata H. Six-year longitudinal changes in body composition of middle-aged and elderly Japanese: age and sex differences in appendicular skeletal muscle mass. Geriatr Gerontol Int 14: 354–361, 2014. doi: 10.1111/ggi.12109. [DOI] [PubMed] [Google Scholar]
- 90.Rolland YM, Perry HM, Patrick P, Banks WA, Morley JE. Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J Gerontol A Biol Sci Med Sci 62: 330–335, 2007. doi: 10.1093/gerona/62.3.330. [DOI] [PubMed] [Google Scholar]
- 91.Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP. Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc 37: 1195–1202, 2005. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- 92.Gao Y, Arfat Y, Wang H, Goswami N. Muscle atrophy induced by mechanical unloading: mechanisms and potential countermeasures. Front Physiol 9: 235, 2018. doi: 10.3389/fphys.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosa-Caldwell ME, Greene NP. Muscle metabolism and atrophy: let’s talk about sex. Biol Sex Differ 10: 43, 2019. doi: 10.1186/s13293-019-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshihara T, Natsume T, Tsuzuki T, Chang S-W, Kakigi R, Sugiura T, Naito H. Sex differences in forkhead box O3a signaling response to hindlimb unloading in rat soleus muscle. J Physiol Sci 69: 235–244, 2019. doi: 10.1007/s12576-018-0640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosa‐Caldwell ME, Lim S, Haynie WA, Brown JL, Deaver JW, Morena Da Silva F, Jansen LT, Lee DE, Wiggs MP, Washington TA, Greene NP. Female mice may have exacerbated catabolic signalling response compared to male mice during development and progression of disuse atrophy. J Cachexia, Sarcopenia Muscle 12: 717–730, 2021. doi: 10.1002/jcsm.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kondakova IV, Shashova EE, Sidenko EA, Astakhova TM, Zakharova LA, Sharova NP. Estrogen receptors and ubiquitin proteasome system: mutual regulation. Biomolecules 10: 500, 2020. doi: 10.3390/biom10040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koebele SV, Bimonte-Nelson HA. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas 87: 5–17, 2016. doi: 10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol (1985) 100: 286–293, 2006. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- 100. Brown M, Foley A, Ferreria JA. Ovariectomy, hindlimb unweighting, and recovery effects on skeletal muscle in adult rats. Aviat Space Environ Med 76: 1012–1018, 2005. [PubMed] [Google Scholar]
- 101.McClung JM, Davis JM, Carson JA. Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp Physiol 92: 219–232, 2007. doi: 10.1113/expphysiol.2006.035071. [DOI] [PubMed] [Google Scholar]
- 102.Brown M, Ferreira JA, Foley AM, Hemmann KM. A rehabilitation exercise program to remediate skeletal muscle atrophy in an estrogen-deficient organism may be ineffective. Eur J Appl Physiol 112: 91–104, 2012. doi: 10.1007/s00421-011-1925-0. [DOI] [PubMed] [Google Scholar]
- 103.Sipilä S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 101: 147–157, 2001. doi: 10.1042/CS20000271. [DOI] [PubMed] [Google Scholar]
- 104.Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, Maugan DW, Ades PA, Beynnon BD, Toth MJ. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol 592: 4555–4573, 2014. doi: 10.1113/jphysiol.2014.279034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Torres MJ, Ryan TE, Lin C-T, Zeczycki TN, Neufer PD. Impact of 17β-estradiol on complex I kinetics and H2O2 production in liver and skeletal muscle mitochondria. J Biol Chem 293: 16889–16898, 2018. doi: 10.1074/jbc.RA118.005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson LJ, Liu H, Garcia JM. Sex differences in muscle wasting. Adv Exp Med Biol 1043: 153–197, 2017. doi: 10.1007/978-3-319-70178-3_9. [DOI] [PubMed] [Google Scholar]
- 108.Mizuno T, Matsui Y, Tomida M, Suzuki Y, Nishita Y, Tange C, Shimokata H, Imagama S, Otsuka R, Arai H. Differences in the mass and quality of the quadriceps with age and sex and their relationships with knee extension strength. J Cachexia, Sarcopenia Muscle 12: 900–912, 2021. [doi: 10.1002/jcsm.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3: 260, 2012. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mourkioti F, Rosenthal N. NF-κB signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med (Berl) 86: 747–759, 2008. doi: 10.1007/s00109-008-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M, Aubertin-Leheudre M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas 68: 331–336, 2011. doi: 10.1016/j.maturitas.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 112.Geraci A, Calvani R, Ferri E, Marzetti E, Arosio B, Cesari M. Sarcopenia and menopause: the role of estradiol. Front Endocrinol (Lausanne) 12: 682012, 2021. doi: 10.3389/fendo.2021.682012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomes MJ, Martinez PF, Pagan LU, Damatto RL, Cezar MDM, Lima ARR, Okoshi K, Okoshi MP. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget 8: 20428–20440, 2017. doi: 10.18632/oncotarget.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol 8: 1045, 2017. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bian A-L, Hu H-Y, Rong Y-D, Wang J, Wang J-X, Zhou X-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res 22: 25, 2017. doi: 10.1186/s40001-017-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Spiegeleer AD, Beckwée D, Bautmans I, Petrovic M; Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Drugs Aging 35: 719–734, 2018. doi: 10.1007/s40266-018-0566-y. [DOI] [PubMed] [Google Scholar]
- 117.Karvinen S, Juppi H-K, Le G, Cabelka CA, Mader TL, Lowe DA, Laakkonen EK. Estradiol deficiency and skeletal muscle apoptosis: possible contribution of microRNAs. Exp Gerontol 147: 111267, 2021. doi: 10.1016/j.exger.2021.111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. FASEB J 26: 1909–1920, 2012. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- 119.Hyatt HW, Powers SK. Mitochondrial dysfunction is a common denominator linking skeletal muscle wasting due to disease, aging, and prolonged inactivity. Antioxidants (Basel) 10: 588, 2021. doi: 10.3390/antiox10040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7: 2–12, 2008. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 121.Coen PM, Musci RV, Hinkley JM, Miller BF. Mitochondria as a target for mitigating sarcopenia. Front Physiol 9: 1883, 2018. doi: 10.3389/fphys.2018.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ventura-Clapier R, Piquereau J, Veksler V, Garnier A. Estrogens, estrogen receptors effects on cardiac and skeletal muscle mitochondria. Front Endocrinol (Lausanne) 10: 557, 2019. doi: 10.3389/fendo.2019.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jackson KC, Wohlers LM, Lovering RM, Schuh RA, Maher AC, Bonen A, Koves TR, Ilkayeva O, Thomson DM, Muoio DM, Spangenburg EE. Ectopic lipid deposition and the metabolic profile of skeletal muscle in ovariectomized mice. Am J Physiol Regul Integr Comp Physiol 304: R206–R217, 2013. doi: 10.1152/ajpregu.00428.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, Grune T. Sarcopenia—molecular mechanisms and open questions. Ageing Res Rev 65: 101200, 2021. doi: 10.1016/j.arr.2020.101200. [DOI] [PubMed] [Google Scholar]
- 125.Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS, Hevener AL. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 8: 334ra54, 2016. doi: 10.1126/scitranslmed.aad3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495, 2011. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 127.Ni J, Zhang L. Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag Res 12: 5597–5605, 2020. doi: 10.2147/CMAR.S261585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Londhe P, Guttridge DC. Inflammation induced loss of skeletal muscle. Bone 80: 131–142, 2015. doi: 10.1016/j.bone.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hasselgren P-O, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg 233: 9–17, 2001. doi: 10.1097/00000658-200101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Montalvo RN, Counts BR, Carson JA. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr Opin Support Palliat Care 12: 394–403, 2018. doi: 10.1097/SPC.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rausch V, Sala V, Penna F, Porporato PE, Ghigo A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis 10: 1, 2021. doi: 10.1038/s41389-020-00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang W, Huang J, Wu H, Wang Y, Du Z, Ling Y, Wang W, Wu Q, Gao W. Molecular mechanisms of cancer cachexia-induced muscle atrophy (review). Mol Med Rep 22: 4967–4980, 2020. doi: 10.3892/mmr.2020.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dalle S, Koppo K. Is inflammatory signaling involved in disease-related muscle wasting? Evidence from osteoarthritis, chronic obstructive pulmonary disease and type II diabetes. Exp Gerontol 137: 110964, 2020. doi: 10.1016/j.exger.2020.110964. [DOI] [PubMed] [Google Scholar]
- 134.Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer cachexia: its mechanism and clinical significance. IJMS 22: 8491, 2021. doi: 10.3390/ijms22168491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhong X, Zimmers TA. Sex differences in cancer cachexia. Curr Osteoporos Rep 18: 646–654, 2020. doi: 10.1007/s11914-020-00628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baker JF, Long J, Ibrahim S, Leonard MB, Katz P. Men are at greater risk of lean mass deficits in rheumatoid arthritis. Arthritis Care Res (Hoboken) 67: 112–119, 2015. doi: 10.1002/acr.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wens I, Dalgas U, Vandenabeele F, Krekels M, Grevendonk L, Eijnde BO. Multiple sclerosis affects skeletal muscle characteristics. PLoS One 9: e108158, 2014. doi: 10.1371/journal.pone.0108158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non−small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 91: 1133S–1137S, 2010. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 139.Stephens NA, Gray C, MacDonald AJ, Tan BH, Gallagher IJ, Skipworth RJE, Ross JA, Fearon KC, Greig CA. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clin Nutr 31: 499–505, 2012. doi: 10.1016/j.clnu.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 140.Kim YJ, Tamadon A, Park HT, Kim H, Ku S-Y. The role of sex steroid hormones in the pathophysiology and treatment of sarcopenia. Osteoporos Sarcopenia 2: 140–155, 2016. doi: 10.1016/j.afos.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yeh S-S, Schuster MW. Geriatric cachexia: the role of cytokines. Am J Clin Nutr 70: 183–197, 1999. doi: 10.1093/ajcn.70.2.183. [DOI] [PubMed] [Google Scholar]
- 142.Santo RCE, Fernandes KZ, Lora PS, Filippin LI, Xavier RM. Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 9: 816–825, 2018.doi: 10.1002/jcsm.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis. Virulence 5: 12–19, 2014. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294: 63–69, 2015. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maglione A, Rolla S, Mercanti SFD, Cutrupi S, Clerico M. The adaptive immune system in multiple sclerosis: an estrogen-mediated point of view. Cells 8: 1280, 2019. doi: 10.3390/cells8101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D'Elia H, Mattsson L-Å, Ohlsson C, Nordborg E, Carlsten H. Hormone replacement therapy in rheumatoid arthritis is associated with lower serum levels of soluble IL-6 receptor and higher insulin-like growth factor 1. Arthritis Res Ther 5: R202–R209, 2003. doi: 10.1186/ar761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Song C-H, Kim N, Lee SM, Nam RH, Choi SI, Kang SR, Shin E, Lee DH, Lee H-N, Surh Y-J. Effects of 17β-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem Pharmacol 164: 139–151, 2019. [Erratum in Biochem Pharmacol 168: 339–340, 2019] doi: 10.1016/j.bcp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 148.Hetzler KL, Hardee JP, Puppa MJ, Narsale AA, Sato S, Davis JM, Carson JA. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim Biophys Acta 1852: 816–825, 2015. doi: 10.1016/j.bbadis.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hetzler KL, Hardee JP, LaVoie HA, Murphy EA, Carson JA. Ovarian function’s role during cancer cachexia progression in the female mouse. Am J Physiol Endocrinol Physiol 312: E447–E459, 2017. doi: 10.1152/ajpendo.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism 56: 49–57, 2007. doi: 10.1016/j.metabol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 151.Counts BR, Fix DK, Hetzler KL, Carson JA. The effect of estradiol administration on muscle mass loss and cachexia progression in female ApcMin/+ mice. Front Endocrinol (Lausanne) 10: 720, 2019. doi: 10.3389/fendo.2019.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Physiol 293: E453–E459, 2007. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 153.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care 8: 255–263, 2005. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 154.Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. GER 63: 91–100, 2017. doi: 10.1159/000450922. [DOI] [PubMed] [Google Scholar]
- 155.Mashinchian O, Pisconti A, Le Moal E, Bentzinger CF. The muscle stem cell niche in health and disease. Curr Top Dev Biol 126: 23–65, 2018. doi: 10.1016/bs.ctdb.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 156.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature 490: 355–360, 2012. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Forcina L, Cosentino M, Musarò A. Mechanisms regulating muscle regeneration: insights into the interrelated and time-dependent phases of tissue healing. Cells 9: 1297, 2020. doi: 10.3390/cells9051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Larson AA, Baumann CW, Kyba M, Lowe DA. Oestradiol affects skeletal muscle mass, strength and satellite cells following repeated injuries. Exp Physiol 105: 1700–1707, 2020. doi: 10.1113/EP088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heo H-R, Chen L, An B, Kim K-S, Ji J, Hong S-H. Hormonal regulation of hematopoietic stem cells and their niche: a focus on estrogen. Int J Stem Cells 8: 18–23, 2015. doi: 10.15283/ijsc.2015.8.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505: 555–558, 2014. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salimena MC, Lagrota-Candido J, Quírico-Santos T. Gender dimorphism influences extracellular matrix expression and regeneration of muscular tissue in mdx dystrophic mice. Histochem Cell Biol 122: 435–444, 2004. doi: 10.1007/s00418-004-0707-8. [DOI] [PubMed] [Google Scholar]
- 163.Karas RH, Schulten H, Pare G, Aronovitz MJ, Ohlsson C, Gustafsson JA, Mendelsohn ME. Effects of estrogen on the vascular injury response in estrogen receptor α,β (double) knockout mice. Circ Res 89: 534–539, 2001. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- 164.Islamov RR, Hendricks WA, Katwa LC, McMurray RJ, Pak ES, Spanier NS, Murashov AK. Effect of 17β-estradiol on gene expression in lumbar spinal cord following sciatic nerve crush injury in ovariectomized mice. Brain Res 966: 65–75, 2003. doi: 10.1016/s0006-8993(02)04191-4. [DOI] [PubMed] [Google Scholar]
- 165.Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol 252: 57–67, 2008. doi: 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Delgado BJ, Lopez-Ojeda W. Estrogen. Treasure Island (FL): StatPearls Publishing, 2021. http://www.ncbi.nlm.nih.gov/books/NBK538260/. [PubMed] [Google Scholar]
- 167.Giordano Imbroll M, Gruppetta M. A current perspective into young female sex hormone replacement: a review. Expert Rev Endocrinol Metab 15: 405–414, 2020. doi: 10.1080/17446651.2020.1816820. [DOI] [PubMed] [Google Scholar]
- 168.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol 13: 220–231, 2017. doi: 10.1038/nrendo.2016.164. [DOI] [PubMed] [Google Scholar]
- 169.Smith GI, Yoshino J, Reeds DN, Bradley D, Burrows RE, Heisey HD, Moseley AC, Mittendorfer B. Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. J Clin Endocrinol Metab 99: 256–265, 2014. doi: 10.1210/jc.2013-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Edlefsen KL, Jackson RD, Prentice RL, Janssen I, Rajkovic A, O'Sullivan MJ, Anderson G. The effects of postmenopausal hormone therapy on serum estrogen, progesterone and sex hormone binding globulin levels in healthy post-menopausal women. Menopause 17: 622–629, 2010. doi: 10.1097/gme.0b013e3181cb49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Javed AA, Mayhew AJ, Shea AK, Raina P. Association between hormone therapy and muscle mass in postmenopausal women: a systematic review and meta-analysis. JAMA Netw Open 2: e1910154, 2019. doi: 10.1001/jamanetworkopen.2019.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Meng X, Dai X, Liao T-D, D'Ambrosio M, Wang F, Yang JJ, Yang X-P. Dose-dependent toxic effects of high-dose estrogen on renal and cardiac injury in surgically postmenopausal mice. Life Sci 88: 178–186, 2011. doi: 10.1016/j.lfs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Modder UIL, Riggs BL, Spelsberg TC, Fraser DG, Atkinson EJ, Arnold R, Khosla S. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur J Endocrinol 151: 503–510, 2004. doi: 10.1530/eje.0.1510503. [DOI] [PubMed] [Google Scholar]
- 174.Haghmorad D, Salehipour Z, Nosratabadi R, Rastin M, Kokhaei P, Mahmoudi MB, Amini AA, Mahmoudi M. Medium-dose estrogen ameliorates experimental autoimmune encephalomyelitis in ovariectomized mice. J Immunotoxicol 13: 885–896, 2016. doi: 10.1080/1547691X.2016.1223768. [DOI] [PubMed] [Google Scholar]
- 175.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol 102: 1387–1393, 2007. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 176.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol (1985) 100: 548–559, 2006. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 177.Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol’s beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol (1985) 110: 109–115, 2011. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol (1985) 100: 2012–2023, 2006. doi: 10.1152/japplphysiol.01583.2005. [DOI] [PubMed] [Google Scholar]
- 179.Nelson ER, Wardell SE, McDonnell DP. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis. Bone 53: 42–50, 2013. doi: 10.1016/j.bone.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.An K-C. Selective estrogen receptor modulators. Asian Spine J 10: 787–791, 2016. doi: 10.4184/asj.2016.10.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dorchies OM, Reutenauer-Patte J, Dahmane E, Ismail HM, Petermann O, Patthey-Vuadens O, Comyn SA, Gayi E, Piacenza T, Handa RJ, Décosterd LA, Ruegg UT. The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy. Am J Pathol 182: 485–504, 2013. doi: 10.1016/j.ajpath.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 182.Maani N, Sabha N, Rezai K, Ramani A, Groom L, Eltayeb N, Mavandadnejad F, Pang A, Russo G, Brudno M, Haucke V, Dirksen RT, Dowling JJ. Tamoxifen therapy in a murine model of myotubular myopathy. Nat Commun 9: 4849, 2018. doi: 10.1038/s41467-018-07057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu B, Shah SN, Lu P, Bollinger LE, Blaeser A, Sparks S, Harper AD, Lu QL. Long-term treatment of tamoxifen and raloxifene alleviates dystrophic phenotype and enhances muscle functions of FKRP dystroglycanopathy. Am J Pathol 188: 1069–1080, 2018. doi: 10.1016/j.ajpath.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 184.Tenti S, Correale P, Cheleschi S, Fioravanti A, Pirtoli L. Aromatase inhibitors-induced musculoskeletal disorders: current knowledge on clinical and molecular aspects. Int J Mol Sci 21: 5625, 2020. doi: 10.3390/ijms21165625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van Londen GJ, Perera S, Vujevich K, Rastogi P, Lembersky B, Brufsky A, Vogel V, Greenspan SL. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat 125: 441–446, 2011. doi: 10.1007/s10549-010-1223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vanderschueren D, van Herck E, Nijs J, Ederveen AG, De Coster R, Bouillon R. Aromatase inhibition impairs skeletal modeling and decreases bone mineral density in growing male rats. Endocrinology 138: 2301–2307, 1997. doi: 10.1210/endo.138.6.5216. [DOI] [PubMed] [Google Scholar]
- 187.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97: 12735–12740, 2000. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jones MEE, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17: 55–64, 2006. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]