Abstract
Background
An ever-increasing number of studies have reported an increased incidence of spontaneous pulmonary barotrauma such as pneumothorax, pneumomediastinum, and subcutaneous emphysema in patients with COVID-19. We conducted this systematic review and meta-analysis to assess the value and significance of the available data.
Methods
A thorough systematic search was conducted to identify studies of barotrauma in hospitalized patients with COVID-19. Data analysis of case reports was done using a statistical package for the social sciences (SPSS) version 22, and meta-analysis was performed using CMA-3.
Results
We identified a total of 4488 studies after thorough database searching.118 case reports and series, and 15 observational studies were included in the qualitative analysis. Fifteen studies were included in the quantitative analysis. The observational studies reported barotrauma in 4.2% (2.4–7.3%) among hospitalized patients; 15.6% (11–21.8%) among critically ill patients; and 18.4% (13–25.3%) in patients receiving invasive mechanical ventilation, showing a linear relationship of barotrauma with the severity of the disease. In addition, barotrauma was associated with a longer length of hospital stay, more extended ICU stay, and higher in-hospital mortality. Also, a slightly higher odds of barotrauma was seen in COVID-19 ARDS compared with non-COVID-19 ARDS.
Conclusion
COVID-19 pneumonia is associated with a higher incidence of barotrauma. It presents unique challenges for invasive and non-invasive ventilation management. Further studies are required to unravel the underlying pathophysiology and develop safer management strategies.
Keywords: COVID-19, Coronavirus Disease 2019; IMV, Invasive Mechanical Ventilation; ARDS, Acute Respiratory Distress Syndrome; NIPPV, Non-invasive Positive Pressure Ventilation; ICU, Intensive Care Unit
Keywords: COVID-19, Barotrauma, Pneumothorax, Pneumomediastinum, Pneumopericardium, Subcutaneous emphysema, Severe acute respiratory syndrome coronavirus-2, SARS-Cov-2
Highlights
-
•
Barotrauma was reported among 4.2% (2.4–7.3) of patients hospitalized with COVID-19.
-
•
Among critically ill patients with COVID-19, barotrauma was reported in 15.6% (11–21.8).
-
•
A higher incidence of barotrauma was reported among patients who received mechanical ventilation [18.4%, (13–25.3)].
1. Introduction
Pulmonary barotrauma refers to the spontaneous rupture of alveoli and the subsequent release or dissection of air into the various extra alveolar spaces resulting in pneumothorax, pneumomediastinum, pulmonary interstitial emphysema, pneumatocele or air cyst formation, subcutaneous emphysema, pneumopericardium, and or pneumoperitoneum. An increasing number of barotrauma cases have been reported with COVID-19 pneumonia in hospitalized patients. Some studies have correlated COVID-19 related barotrauma with a longer length of hospitalization, longer ICU stay, and higher mortality [1]. Barotrauma has been reported among COVID-19 patients requiring invasive mechanical ventilation (IMV), non-invasive positive pressure ventilation (NIPPV), and other forms of respiratory support ranging from supplemental oxygenation by nasal cannula to heated high flow nasal cannula [[1], [2], [3]]. Risk factors, pathophysiology, and clinical implications of barotrauma in patients with COVID-19 are not well understood. Thus, to fully evaluate the available data, we sought to perform this systematic review and meta-analysis. We have included studies (case reports, case series, cohort, and case-control studies) from the onset of the COVID-19 pandemic from December 31, 2019, up until May 4, 2021.
2. Methods
We used Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for the systematic review of available literature [4]. The study protocol was registered in the International prospective register of systematic reviews (PROSPERO) with ID: CRD42021261038 [5]. The completed PRISMA 2020 checklist is uploaded for review purposes with manuscript submission. Additionally, the quality of the systematic review is self-assessed using AMSTAR 2 criteria and submitted [6].
2.1. Literature search
Electronic databases including PubMed, PubMed Central, Embase, and Scopus were used to search relevant articles published until May 4, 2021, using the following MeSH terms and appropriate Boolean operators as ((emphysema) AND (COVID-19)) OR ((barotrauma[MeSH Terms])) AND (SARS COV-2[MeSH Terms])). Electronic search details are available in supplementary file 1.
2.2. Selection of studies
2.2.1. Type of studies
We included case reports, case series, cohort studies, and case-control studies. Patients included in the studies fulfilled the following criteria; (1) more than 17 years of age, (2) confirmed diagnosis of COVID-19 based on a positive reverse transcriptase polymerase chain reaction (RT-PCR) nasopharyngeal swab (3) and spontaneous pneumothorax, pneumomediastinum, pneumopericardium, pneumoperitoneum, hydropneumothorax, or subcutaneous emphysema either at their initial presentation or during treatment of COVID-19 pneumonia. Editorials, comments, viewpoints, systematic reviews, meta-analyses, and articles lacking full text and adequate data were excluded.
2.2.2. Outcome measures
The primary outcome of interest was the incidence and type of barotrauma event. The secondary outcomes of interest were the impact of barotrauma on the clinical outcome in terms of hospitalization, length of ICU stay, and hospital mortality. Further, we compared the incidence of barotrauma between COVID-19 ARDS vs. Non-COVID-19 ARDS (i.e., ARDS from other etiologies).
2.2.3. Data extraction and management
All the stages of data extraction were done according to the PRISMA guideline. The first two authors individually screened published articles based on inclusion and exclusion criteria using Covidence [7]. Discrepancies were resolved by mutual consent obtained from the third author. Data were extracted onto a standardized form designed in Microsoft Excel to collect the pertinent information, including the first author, type of study, site of study, year of publication, age, gender, associated comorbidities, the incidence of barotrauma, type of barotrauma, cause of barotrauma, treatment modality for barotrauma, and clinical outcomes in terms of length of hospitalization, length of ICU stay, and in-hospital mortality. The sample size, mean age, percentage of male and female, associated comorbidities, type of barotrauma, and the clinical outcomes were included for the cohort studies. Further analysis of data from case reports was done in Statistical Package for the Social Sciences (SPSS) version 22. For meta-analysis of observational studies, comprehensive meta-analysis version-2 (CMA-3) was used.
2.2.4. Risk of bias (quality) assessment
The quality of individual articles was evaluated using the Joanna Briggs Institute (JBI) [8] critical appraisal for cohort studies, case-control studies, and case series (Supplementary file 1, Tables 1–3) [1,2,[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]].
2.2.5. Strategy for data synthesis
The handling of data and combining results of the studies was done using proportion and the Random effect model using CMA-3 software. Additionally, data from the case reports were summarized, and descriptive analysis was done using Statistical Package for the Social Sciences (SPSS) version 22.
3. Results
A total of 4488 studies were identified after thorough database searching, and 1304 duplicates were removed. Title and abstracts of 3184 studies were screened, and 2892 irrelevant studies were excluded. Full-text eligibility of 292 studies was assessed, and 159 studies were excluded for definite reasons. A total of 118 case reports and series and 15 observational studies were included in the qualitative analysis, and 15 studies were included in the quantitative analysis. The following information is depicted in the PRISMA flow diagram (Fig. 1).
Fig. 1.
Prisma flow diagram.
3.1. Narrative summary
There were ten cohort studies, three case-control studies, and two case series among included studies. In addition, three studies were done in the US and Italy, two studies in Turkey, while there was a single study from the UK, Kuwait, Germany, Iran, India, Spain, and Mexico. Three studies reported outcomes in critically ill patients. Six studies were performed on patients receiving invasive mechanical ventilation. Two studies included patients with varying clinical severity receiving different respiratory support modalities. The detailed narrative summary is presented in Table 1, Table 2.
Table 1.
Narrative summary of the included observational studies.
| Author | Type of study | Place of study | Study subjects Characteristics | Total cases | Barotrauma cases | Pneumothorax cases | Pneumomediastinum cases | Subcutaneous emphysema (SE) cases |
Pneumopericardium |
|---|---|---|---|---|---|---|---|---|---|
| Belletti A [16] et al., 2021 (all IMV) | Observational Study | Italy | Total: N:116; Barotrauma: 28 Age: Barotrauma (B): 62 (57–70) Other (O): 62 (54–69) Sex: B: Male (23/28) O: Male (75/88) Hypertension: B: 8/28 O: 44/88 DM: B: 5/28 O: 14/88 Active Smoker: B:1/28 O: 2/88 Past Smoker: B:2/28 O: 7/88 |
116 |
28/116 Time from intubation to barotrauma: 14.0 ± 11.0 |
22/28 (78.5%) Chest tube: 18/22(81.8%) Conservative: 4/22(18.2%) One chest tube patient additionally required minithoracotomy (4.5%) |
13/28 (46.42%) Conservative: 12/13 Surgical repair for TEF: 1/13 |
– | – |
| Elsaaran H [17] et al., 2021 (critical Admitted COVID) | Retrospective cohort | Kuwait | N: 343; B: 54; O: 289 Age: B: 55.3 (15.0); O: 56.0 (13.3) Sex: Male B: 42/54; O: 243/289 DM: B: 20/54; O: 123/289 HTN: B: 24/54; O:131/289 |
343 | 54/343 | 49/54 | 8/54 | – | 2/54 |
| Jones E [18] et al., 2020 (severe COVID) | retrospective cohort | UK | N: 83; B: 8 Age: B: 54.5(37.8–57.4); O: 57.8 (50.4–65.2) Sex: Male: B: 8/8; O: 53/75 Smoking: B: (2/8); O: 21/28 |
83 | 8/83 | 4/8 | 7/8 | 8/8 | |
| Kahn MR [19] et al., 2021 (ICU COVID) | retrospective cohort | USA | Total: 75; B: 16; O: 59 Age: B: 54(15); O: 60 (16) Sex, Female: B: 18/59; O: 2/16 DM: B:9/16; O:34/59 HTN: B: 6/16; O: 30/59 Tobacco Use: B: 2/16; O: 5/59 |
75 | 16/75 (Same patients might have presented with multiple types of barotrauma) | 9/16 | 10/16 | 6/16 | 4/16 |
| Lemmers DHL [20] et al., 2020 (IMV COVID) | cohort | Italy | Age: B: 64(60–70); O: 67 (59–71) Sex, Male: B:15/23; O: 118/146 DM: B:3/23; O:23/146 HTN: 11/23; O: 79/146 |
169 | 23/169 | – | – | – | – |
| Loffi M [21] et al., 2020 (all Admitted) | retrospective cohort | Italy | Age: B: 72(49–80); O: 64 (53–75) Sex, Male; B:5/6; O: 68/96 DM: B: 0/6; O: 11/96 HTN: B: 1/6; O: 37/96 Smoking: B: 2/6; O: 25/96 |
102 | 6/102 (pneumo-mediastinum only) | – | 6/6 | 3/6 | 1/6 |
| McGuinness G [1] et al., 2020 (IMV COVID) | retrospective cohort | USA | Total: 601; B: 89 Age: B: 58(54,61); O: 64 (62,65) Sex: Male: B: 65/89; O: 361/512 Smoking: Never Smoked: 51/89; Past Smoker: 22/89; Current Smoker: 4/89 |
601 | 89/601 | 54/89 | 59/89 | – | 14/89 |
| Miro O [2] et al., 2021 (All COVID) | case control | Spain | Total Barotrauma: 40 Age: B: 66(47–74); O: 61 (46–77) Sex, Male: B:29/40; O: 205/400 DM: B: 7/40; O: 74/400 HTN: B: 15/40; O: 168/400 Smoking: B: (4/40); O: 26/400 |
40 | 40/- | 37/40 | 6/40 | 6/40 | – |
| Ozdemir S [9] et al., 2020 (IMV COVID) | retrospective study | Turkey | N: 107; Barotrauma: 8 Age: B: 61 (53–63.5); O: 60 (51–70) Sex, Male: B:8/8; O: 62/99 |
107 | 8/107 | 8/8 | 1/8 | 2/8 | – |
| Ozsoy IE [10] et al., 2021 (Not clear) | retrospective study | Turkey | Total: 70; Barotrauma: 20 Age: B: 57.7 ± 14.1; O: 60.6 ± 13.6 Sex, Male B: 10/20; O: 22/40; DM: B: (7/20); O: 22/40 HTN: B: (5/20); O: 32/40 Smoking B: 4/40 O: 26/400 |
20 | 20/- (Same patients might have presented with multiple types of barotrauma) |
4/20 | 20/20 | 14/20 | – |
| Rodriguez-Arciniega TG [14] et al., 2020 | Case control | Mexico | Total: 271 Barotrauma (SPM):9 Age: B:57(CI, 42.8–71.11), O: 59.5 (CI, 58.02–61.77) Sex, Male B: 7/9, O: 165/262 HTN: B: 5/9, O: 154/262 DM: B: 3/9, O: 110/262 Smoking: B: 1/9, O: 36/262 |
271 | 9/271 (3.32%) All 9 patients SPM | 4/9 | 9/9 | – | – |
| Swain SK [11] et al., 2020 (IMV) | Retrospective cohort | India | N: 262; Barotrauma: 64 Age: 61 ± 14 Sex: Male: 26 DM: 18 HTN: 22(57.89%) |
262 | 64/262 | 42/64 | 32/64 | 38/64 | – |
| Tofigh AM [12] et al., 2020 (ICU) | Observational study | Iran | Barotrauma: 7 Age: 61.71 ± 14.15 Male: 6/7 DM: 4/7 HTN: 2/7 |
7 | 7 | 7 All cases of B/L pneumothorax All cases B/L chest tubes |
– | 7 | – |
| Udi J [15] et al., 2020 (All Post-IMV) | Case control | Germany | Total: 20; Barotrauma: 8 Age: B:62 (47–76), O: 61 (38–77) Male; B: 6/8, O: 7/12 |
20 | 8/20 | 5/8 All > chest tube insertion | 5/8 | 2/8 | 1/8 |
| Wong K [13] et al., 2020 (ICU) | Retrospective cohort | USA | Barotrauma: 75 Age: 62.8 (25–90) Male: 55 (73.3%) HTN: 39/75 DM: 20/75 |
75 | 75 | 75 | 27/75 | – | – |
Table 2.
Outcomes among COVID-19 with barotrauma.
| Author | Hospital LOS | ICU LOS | In-Hospital Mortality | Mortality | Discharged |
|---|---|---|---|---|---|
| Belletti A [16] et al., 2021 | B: 41.5 (28.0–69.5) O: 28.0 (15.0–44.0) |
B: 28.0 (14.5–51.0) O: 12.0 (7.5–21.0) |
NR | At F/U for median period of 34.0 (28.0–42.0) days B:17/28 O: 34/88 |
NR |
| Elsaaran H [17] et al., 2021 |
Mean (SD) B: 16.4 (8.5) O: 20.1 (14.0) |
Mean (SD) B: 14.9 (7.8) O: 13.9 (12.1) |
B: 38/54 O: 153/289 |
NR | NR |
| Jones E [18] et al., 2020 | NR | NR | NR | At F/U at three months period B: 5/8 O:34/75 |
B: 2/8 O:40/75 |
| Kahn MR [19] et al., 2021 | B:26(23–45) O: 14 (9–19) |
B: 17 (15–30.5) O:7 (3–13) |
B:9/16 O: 22/59 |
At 28-day F/U B:4/16 O: 18/59 |
B: 4/16 O: 26/59 |
| Lemmers DHL [20] et al., 2020 |
Median (IQR) B:18 (12–28) O: 14(8–23) |
Median (IQR) B:11(6–21) O: 9(5–18) |
B:13/23 O: 73/146 |
NR | NR |
| Loffi M [21] et al., 2020 | NR | NR | B: 1/6 O: 11/96 |
NR | B: 5/6 O: 85/96 |
| McGuinness G [1] et al., 2020 |
(95% CI) B: 25(22,28) O: 18 (17,19) |
NR | B:47/89 O: 298/512 |
NR | B:15/89 O: 116/512 |
| Miro O [2] et al., 2021 | NR | NR | B:13/40 O: 55/400 |
NR | NR |
| Ozdemir S [9] et al., 2020 | NR | NR | B:4/8 O: 68/99 |
NR | NR |
| Ozsoy IE [10] et al., 2021 |
Median (IQR) B:20.5 (12.3–27.8) O: 9 (8–14) |
NR | B: 12/20 O: 2/50 |
NR | NR |
| Rodriguez-Arciniega TG [14] et al., 2020 |
Mean (SB: 95%CI) B: 16.8 (13.9: 6.11–27.48) O: 12.06 (6.7: 11.24–12.87) |
NR | B: 3/9 O: 96/262 |
NR | NR |
| Swain SK [11] et al., 2020 | NR | NR | B: 25/38 | NR | NR |
| Tofigh AM [12] et al., 2020 | NR | NR | B: 7/7 | NR | NR |
| Udi J [15] et al., 2020 | NR | NR | B: 2/8 | NR | NR |
| Wong K [13] et al., 2020 | B: 48.7 | NR | B: 57/75 | NR | 18/75 |
3.2. Proportion of barotrauma
Among the included studies reporting barotrauma, pooling of the individual study data using random-effect model showed barotrauma in 4.2% (2.4–7.3%); 15.6% (11–21.8%); and 18.4% (13–25.3%) of hospitalized patients, severely ill or critically ill patients, and patients receiving invasive mechanical ventilation, respectively (Fig. 2).
Fig. 2.
The proportion of barotrauma among observational studies, random effect model.
3.3. Types of barotrauma reported
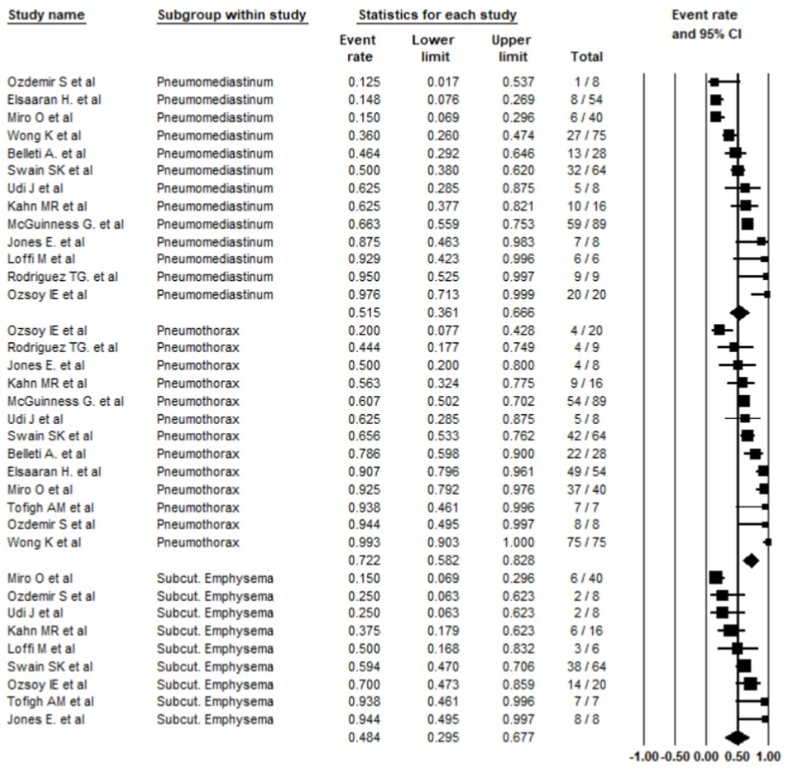
Pneumothorax was the most common form of barotrauma reported at 72.2% (58.2–82.8%); followed by pneumomediastinum at 51.5% (36.1–66.6%), and subcutaneous emphysema reported in 48.4% (29.5–67.7%) (Fig. 3). Among the included studies, three studies also reported 12 cases of pneumopericardium.
Fig. 3.
Common types of barotrauma reported, random effect model.
3.4. Length of hospitalization
Eight studies reported the length of hospitalization (LoH) [1,9,11,12,14,15,18,21]. LoH was longer in COVID-19 patients in all studies than non-barotrauma COVID-19 patients, except Elsaaran H [12]. Due to varying severities of patients enrolled in different studies carrying significant biological heterogeneities, the length of hospital stay outcome is not pooled.
3.5. Length of ICU stay
Belletti, Elsaaran, Kahn, and Lemmers reported the ICU length of stay [11,12,14,15]. All four of these studies reported a longer duration of ICU LOS in the barotrauma group.
3.6. Mortality
Thirteen studies reported in-hospital mortality [1,2,9,10,12,[14], [15], [16], [17], [18], [19], [20], [21]]. Total mortality was 58.7% in the barotrauma group compared to 40.6% in the control (non-barotrauma) group.
3.7. Barotrauma in COVID-19 ARDS vs. Non-COVID-19 ARDS
Only two studies reported the incidence of barotrauma among COVID-19 ARDS and non-COVID-19 ARDS patients. However, pooling the data using a random-effect model showed a slightly higher barotrauma odds ratio in COVID-19 ARDS than non-COVID-19 (OR, 3.31; CI, 0.66–16.65, I2: 83.95%) (Supplementary file 1, Fig. 1).
3.8. Descriptive summary of the case reports and case series
Data from 118 case reports and series including 197 COVID-19 patients were included in this descriptive analysis. Among 197 cases; 83.2% (n, 164) were male, 69% (n, 136) cases with pneumothorax (82; unilateral, 40; bilateral, and 14 with no mention of laterality). Right-sided pneumothoraces (n = 55) was more commonly reported than left-sided pneumothorax (n = 26) (Fig. 4). Among pneumothorax cases, tension pneumothorax was present in 19 cases. Similarly, pneumomediastinum were reported in 49.7% (n, 98), subcutaneous emphysema in 37.1% (n, 73), and pneumoperitoneum in 6.1% (n, 12) cases (Supplementary file 1, Fig. 2). 57.4% (n, 113) required a formal chest tube placement. 2.5% of patients (n, 5) required needle thoracostomy in the setting of tension pneumothorax. 35.5% (n, 70) were treated conservatively and did not require invasive treatment. It is worthwhile noting that more than half of the patients developed barotrauma in the setting of the invasive mechanical ventilation (IMV) support (52.8%, n = 104), while 42.6% (n, 84) patients developed without IMV use.
Fig. 4.
Laterality of pneumothorax in case studies.
4. Discussion
Herein we report a comprehensive meta-analysis on the incidence, type of barotrauma, and its impact on clinical outcomes among patients with COVID-19 pneumonia. We have included 118 case reports, case series, and 15 observational studies.
Pneumothorax and pneumomediastinum have been previously associated with coronavirus pneumonia during the SARS epidemic of 2002–2004 [22]. The incidence of barotrauma during the 2002–2004 epidemic varied from 5 to 34% [[23], [24], [25]]. Barotrauma has been increasingly recognized and reported from the onset of the COVID-19 pandemic [[26], [27], [28]]. One of the reasons for the higher incidence could be attributed to the broader use of chest computed tomography (CT) and its sensitivity to detect extra-alveolar gas collections [29]. We observed a linear association of increased barotrauma incidence with increasing disease severity observed as 4.2% (2.4–7.3%) among hospitalized patients, 15.6% (11–21.8%) among critically ill patients in ICU, and 18.4% (13–25.3%) among patients receiving invasive mechanical ventilation.
A higher incidence of barotrauma was observed in COVID-19 ARDS than non-COVID-19 ARDS in some studies, despite the use of lung-protective ventilation strategy in both groups [30]. The findings of our meta-analysis substantiated the conclusions of the individual studies regarding the increased occurrence of barotrauma in COVID-19 ARDS. Conventionally, barotrauma has been associated with high transpulmonary pressure, especially high tidal volume and high positive end-expiratory pressure in ARDS patients [31]. However, Kahn et al. observed no difference in mean airway pressure, positive inspiratory pressure, positive end-expiratory pressure (PEEP), tidal volume, or minute ventilation during 0 or 14 days in patients who sustained barotrauma with those who did not [14]. Thus, we speculate the higher occurrence of barotrauma could be attributed to alveolar viral and inflammatory injury.
COVID-19 induces an elevated level of tumor necrosis factor alfa (TNF-alfa) and Interleukin-6 (IL-6) [32]. In animal models, TNF-alfa has been shown to induce apoptosis-driven alveolar damage [33,34]. Further in the setting of IMV, there is a proportional association between cytokine production, PEEP, and tidal volume [35]. These findings suggest that higher PEEP and higher tidal volume can further enhance the cytokine response worsening alveolar injury predisposing to barotrauma. A linear association between the occurrence of barotrauma and the clinical severity of disease was observed in our meta-analysis. This can be a manifestation of the degree of inflammatory response. Due to the paucity of data among the included studies, we were unable to perform a subgroup analysis of group differences in the incidence of barotrauma among patients receiving NIPPV vs. IMV, or the incidence of barotrauma between different ventilator modes, and settings. In the comprehensive review of case reports and case series, 42.6% of cases in individual cases developed barotrauma without IMV use. Thus, although purely speculative, the higher incidence of barotrauma in COVID-19, even without the use of positive pressure ventilation, could also be a unique manifestation of inflammatory burden, alveolar injury, and its pathophysiologic sequelae. Further studies are warranted to understand the precise pathophysiology.
Spontaneous barotrauma was associated with a longer length of hospitalization, more prolonged ICU stays, and increased mortality. Also, cumulative analysis of case reports showed that more than half of pneumothoraces required formal chest tube placement. In addition, a small subgroup of patients developed tension pneumothorax requiring emergent needle thoracostomy. Thus, pneumothoraces with COVID-19 subjected patients undergoing invasive procedures, including emergent life-saving procedures like needle thoracostomy. Hence, clinicians should be well aware of the higher risk of barotrauma in patients with COVID-19, even without positive pressure ventilation use. In addition, spontaneous barotrauma should be considered high in the differential in COVID-19 patients with an acute decline in clinical status.
5. Limitations
Our meta-analysis has several limitations, including a small number of retrospective cohort and case-control studies. There is also significant heterogeneity in the study designs and demographics. The included observational studies have their inherent limitations. Baseline characteristics, mechanical ventilator settings, assessment of organ dysfunction, and clinical outcomes were not individually reported across studies. Reported clinical outcomes in the included studies could be affected by several confounding variables [36]. Further, a higher detection of barotrauma could result from increased use of chest CT during the pandemic. It would have been clinically valuable to compare the incidence of barotrauma between chest radiography and chest CT and different ventilator modes and settings. However, it could not be performed due to the lack of data in the included studies. The presence of comorbidities primarily and severity of organ dysfunction could have significantly influenced the clinical outcomes [36].
6. Conclusion
The incidence of barotrauma in COVID-19 ARDS was higher than ARDS from other etiologies. Barotrauma was seen in COVID-19 among patients receiving IMV, NIPPV, and supplemental oxygenation by other non-invasive modalities. The pathophysiology of viral pneumonia is complex and poorly understood. It is still unrecognized if there are inherently unique mechanisms to COVID-19, which predisposes patients to an exaggerated inflammatory response resulting in alveolar injury that affects respiratory mechanics, resulting in barotrauma at a higher rate than other viral pneumonia. Barotrauma in COVID-19 patients was associated with a longer length of hospitalization, more extended ICU stay, and higher mortality. Further in-vitro and in-vivo studies are required to understand the pathophysiology and develop safer ventilation and treatment strategies in COVID-19 pneumonia.
7. Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The data analyzed during the current study are available within manuscript and supplementary files.
Funding
This article did not receive any specific grant from funding agencies in the public, commercial, or other sectors.
Authors' contributions
DBS, and YRS contributed to the concept and design, analysis, and interpretation of data. DBS, PB, AA, NP, RD, and SK contributed to the literature search, data extraction, review, and initial manuscript drafting. YRS, WAYM, RA, MGK, guided and supervised in different stages and contributed in the interpretation of data, revising the manuscript for important intellectual content and approval of the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.103221.
Contributor Information
Dhan Bahadur Shrestha, Email: medhan75@gmail.com.
Yub Raj Sedhai, Email: YubRaj.Sedhai@vcuhealth.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.McGuinness G., Zhan C., Rosenberg N., Azour L., Wickstrom M., Mason D.M., Thomas K.M., Moore W.H. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297 doi: 10.1148/RADIOL.2020202352. E252–E262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miró Ò., Llorens P., Jiménez S., Piñera P., Burillo-Putze G., Martín A., Martín-Sánchez F.J., García-Lamberetchs E.J., Jacob J., Alquézar-Arbé A., Mòdol J.M., López-Díez M.P., Guardiola J.M., Cardozo C., Lucas Imbernón F.J., Aguirre Tejedo A., García Á García, Ruiz Grinspan M., Llopis Roca F., González del Castillo J., Salmerón P.P., García Lamberechts E.J., López Grima M.L., Juan Gómez M.Á., Millán J., Lázaro L.S., Peña B., Román F., Noceda J., Cano Cano M.J., Serra R.S., José Fortuny Bayarri M., José Salvador Suárez F., Tejera M.G., Herrera L.A., Caballero Mateos A.M., Meléndez N., Albero P.B., Jiménez B., del Río R., García C.P., Amador P.S., Brasó Aznar J.V., Ruiz López J.L., Ponce M.C., Fernández E.D., Martínez L.E., de Reynoso Rodríguez M., Gómez A.P., Bellver E.G., Rizzi M., Suarez C.C., Argilag L.B., Roset-Rigat A., Haro-Bosch A., Rovira M.A., Arbaizar P.F., Tost J., Lorenzo I.C., Masó S.M., Palau A., Tur R.G., Muñoz M.A., Ferrer E.S., García L.L., Alarcón Jiménez B.S., Quesada S.F., Huerta A., Fragiel M., Álvarez S.M., Martínez Virto A.M., Galán C. del A., Jiménez G.F., Rodríguez E.Á., Villa T.A., Venegas de L'Hotellerie M.J., Cabezas V.P., Mocanu C., Nieto P.G., Alonso M.Á., Marco C.L., Gaforio A.F., Galán B.H., Martín S.G., Somohano F.V., Laguna N.L., Panadero R.P., Fuentes de Frutos M., Castillo C.G., Castañeda A.B., Escudero S.G., López Díez M.P., Oliva Ramos J.R., Herrero D.S., Flórez R.C., Chaib F.B., Mohamedi Abdelkader I.S., Tornero A.P., Chia A.N., Pérez E.M., Martín V.R., García Soto A.B., Padial E.D., García J.P., Fernández de Simón Almela A., López R.C., José López Díaz J., Maza Vera M.T., Calveiro R.R., Lucas-Galán F.J., Moreno M.R., Martínez F.G., Olmeda D.M., Juárez R., Rodríguez M.E., Fernández Rodríguez J.F., Monzo J.P., González N.C., Velarde Herrera D.M., Martínez Bautista B.M., Niembro Valdés A.P., Álvarez L.A., Motto E.Q., García N.T., Sánchez Nicolás J.A., Aragües P.L., Ruiz de Lobera N., Ferreras Amez J.M. Frequency, risk factors, clinical characteristics, and outcomes of spontaneous pneumothorax in patients with coronavirus disease 2019: a case-control, emergency medicine-based multicenter study. Chest. 2021;159:1241–1255. doi: 10.1016/j.chest.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doğan B.İ., Mahleç Anar C., Sertoğullarından B., Turan M.O. Pneumomediastinum as a complication of COVID-19 disease: a case report. Tuberk. Toraks. 2021;69:94–97. doi: 10.5578/tt.20219911. [DOI] [PubMed] [Google Scholar]
- 4.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88:105906. doi: 10.1016/J.IJSU.2021.105906. [DOI] [PubMed] [Google Scholar]
- 5.D. Shrestha, P. Budathoki, A. Adhikari, N. Pokharel, R. Dhakal, S. Kafle, Barotrauma in COVID-19: a systematic review and meta-analysis, PROSPERO 2021 CRD42021261038. (n.d.). https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=261038 (accessed September 11, 2021). [DOI] [PMC free article] [PubMed]
- 6.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:4008. doi: 10.1136/BMJ.J4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. , (n.d.). www.covidence.org (accessed May 13, 2021).
- 8.Critical-appraisal-tools-Critical Appraisal Tools | Joanna Briggs Institute., ((n.d.)).
- 9.Rodriguez-Arciniega T.G., Sierra-Diaz E., Flores-Martinez J.A., Alvizo-Perez M.E., Lopez-Leal I.N., Corona-Nakamura A.L., Castellanos-Garcia H.E., Bravo-Cuellar A. Frequency and risk factors for spontaneous pneumomediastinum in COVID-19 patients. Front. Med. 2021;8:662358. doi: 10.3389/fmed.2021.662358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udi J., Lang C.N., Zotzmann V., Krueger K., Fluegler A., Bamberg F., Bode C., Duerschmied D., Wengenmayer T., Staudacher D.L. Incidence of barotrauma in patients with COVID-19 pneumonia during prolonged invasive mechanical ventilation - a case-control study. J. Intensive Care Med. 2021;36:477–483. doi: 10.1177/0885066620954364. [DOI] [PubMed] [Google Scholar]
- 11.Belletti A., Palumbo D., Zangrillo A., V Fominskiy E., Franchini S., Dell'Acqua A., Marinosci A., Monti G., Vitali G., Colombo S., Guazzarotti G., Lembo R., Maimeri N., Faustini C., Pennella R., Mushtaq J., Landoni G., Scandroglio A.M., Dagna L., De Cobelli F. Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J. Cardiothorac. Vasc. Anesth. 2021 doi: 10.1053/j.jvca.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsaaran H., AlQinai S., AlTarrah D., Abdulrasoul M., Al-Youha S., Almazeedi S., Al-Haddad M., Jamal M.H., Al-Sabah S. Prevalence and risk factors of barotrauma in Covid-19 patients admitted to an intensive care unit in Kuwait; a retrospective cohort study. Ann. Med. Surg. 2021;63:102141. doi: 10.1016/j.amsu.2021.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones E., Gould A., Pillay T.D., Khorasanee R., Sykes R., Bazo-Alvarez J.C., Cox C., Shurovi B., Isted A., Simpson T., Jennings M., Breeze R., Khaliq W. Subcutaneous emphysema, pneumomediastinum, and pneumothorax in critically ill patients with coronavirus disease 2019: a retrospective cohort study. Crit. Care Explor. 2020;2 doi: 10.1097/cce.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn M.R., Watson R.L., Thetford J.T., Wong J.I., Kamangar N. High incidence of barotrauma in patients with severe coronavirus disease 2019. J. Intensive Care Med. 2021 doi: 10.1177/0885066621989959. 885066621989959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemmers D.H.L., Abu Hilal M., Bnà C., Prezioso C., Cavallo E., Nencini N., Crisci S., Fusina F., Natalini G. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6 doi: 10.1183/23120541.00385-2020. 00385–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loffi M., Regazzoni V., Sergio P., Martinelli E., Stifani I., Quinzani F., Robba D., Cotugno A., Dede M., Danzi G.B. Spontaneous pneumomediastinum in COVID-19 pneumonia. Monaldi Arch. Chest Dis. 2020;90:604–607. doi: 10.4081/monaldi.2020.1399. [DOI] [PubMed] [Google Scholar]
- 17.Özdemir S., Bilgi D.Ö., Köse S., Oya G. Pneumothorax in patients with coronavirus disease 2019 pneumonia with invasive mechanical ventilation. Interact. Cardiovasc. Thorac. Surg. 2021;32:351–355. doi: 10.1093/icvts/ivaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozsoy I.E., Tezcan M.A., Guzeldag S., Ozdemir A.T. Is spontaneous pneumomediastinum a poor prognostic factor in covid-19? J. Coll. Physicians Surg. Pak. 2021;31:132–137. doi: 10.29271/jcpsp.2021.02.132. [DOI] [PubMed] [Google Scholar]
- 19.Swain S.K., Behera I.C., Das S.R. Incidence of subcutaneous emphysema in the head, neck and thoracic region of intubated covid-19 patients: our experiences. Int. J. Curr. Res. Rev. 2021;13:19–24. doi: 10.31782/IJCRR.2021.13420. [DOI] [Google Scholar]
- 20.Tofigh A.M., Shojaei S., Bagherpour J.Z., Mirkheshti A., Tahmasebi H. Pneumothorax as an ominous side effect in COVID-19 patients under mechanical ventilation: report of seven patients. J. Cell. Mol. Anesth. 2020;5:202–205. doi: 10.22037/jcma.v5i3.30402. [DOI] [Google Scholar]
- 21.Wong K., Kim D.H., Iakovou A., Khanijo S., Tsegaye A., Hahn S., Narasimhan M., Zaidi G. Pneumothorax in COVID-19 acute respiratory distress syndrome: case series. Cureus. 2020;12:3–9. doi: 10.7759/cureus.11749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ooi G.C., Daqing M. SARS: radiological features. Respirology. 2003;8:15–19. doi: 10.1046/j.1440-1843.2003.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P., Ephtimios I.E., Kitai I., Mederski B.D., Shadowitz S.B., Gold W.L., Hawryluck L.A., Rea E., Chenkin J.S., Cescon D.W., Poutanen S.M., Detsky A.S. Clinical features and short-term outcomes of 144 patients with SARS in the greater toronto area. J. Am. Med. Assoc. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 24.Fowler R.A., Lapinsky S.E., Hallett D., Detsky A.S., Sibbald W.J., Slutsky A.S., Stewart T.E. Critically ill patients with severe acute respiratory syndrome. J. Am. Med. Assoc. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 25.Kao H.K., Wang J.H., Sung C.S., Huang Y.C., Lien T.C. Pneumothorax and mortality in the mechanically ventilated SARS patients: a prospective clinical study. Crit. Care. 2005;9:440–445. doi: 10.1186/cc3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahluwalia A.S., Qarni T., Narula N., Sadiq W., Chalhoub M.N. Bilateral pneumothorax as possible atypical presentation of coronavirus disease 2019 (COVID-19) Respir. Med. Case Reports. 2020;31:101217. doi: 10.1016/j.rmcr.2020.101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira J.G., Rapparini C., Gomes B.M., Pinto L.A.C., Freire M.S. da S.E. Pneumothorax as a late complication of COVID-19. Rev. Inst. Med. Trop. Sao Paulo. 2020;62 doi: 10.1590/s1678-9946202062061. e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen M.L., van Manen M.J.G., Cretier S.E., Braunstahl G.-J. Pneumothorax in patients with prior or current COVID-19 pneumonia. Respir. Med. Case Reports. 2020;31:101187. doi: 10.1016/j.rmcr.2020.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kafle S., Shrestha E., Pokharel N., Budhathoki P., Shrestha D.B., Vittorio T. Pneumomediastinum and subcutaneous emphysema in an adult male from Nepal infected with COVID-19. Cureus. 2021;13 doi: 10.7759/CUREUS.16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajdev K., Spanel A.J., McMillan S., Lahan S., Boer B., Birge J., Thi M. Pulmonary barotrauma in COVID-19 patients with ARDS on invasive and non-invasive positive pressure ventilation. J. Intensive Care Med. 2021;36:1013–1017. doi: 10.1177/08850666211019719. [DOI] [PubMed] [Google Scholar]
- 31.Ioannidis G., Lazaridis G., Baka S., Mpoukovinas I., Karavasilis V., Lampaki S., Kioumis I., Pitsiou G., Papaiwannou A., Karavergou A., Katsikogiannis N., Sarika E., Tsakiridis K., Korantzis I., Zarogoulidis K., Zarogoulidis P. Barotrauma and pneumothorax. J. Thorac. Dis. 2015;7:S38–S43. doi: 10.3978/j.issn.2072-1439.2015.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure clinical and translational report complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slutsky A.S. Ventilator-Induced lung injury: from barotrauma to biotrauma. Respir. Care. 2005;50(5):646–659. [PubMed] [Google Scholar]
- 34.Chen L., Xia H.F., Shang Y., Yao S.L. Molecular mechanisms of ventilator-induced lung injury. Chin. Med. J. (Engl). 2018;131:1225–1231. doi: 10.4103/0366-6999.226840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan C., Chen L., Lu C., Zhang W., Xia J.A., Sklar M.C., Du B., Brochard L., Qiu H. Lung recruitability in COVID-19–associated acute respiratory distress syndrome: a single-center observational study. Am. J. Respir. Crit. Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ketcham S.W., Sedhai Y.R., Miller H.C., Bolig T.C., Ludwig A., Co I., Claar D., McSparron J.I., Prescott H.C., Sjoding M.W. Causes and characteristics of death in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome: a retrospective cohort study. Crit. Care. 2020;24 doi: 10.1186/s13054-020-03108-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study are available within manuscript and supplementary files.