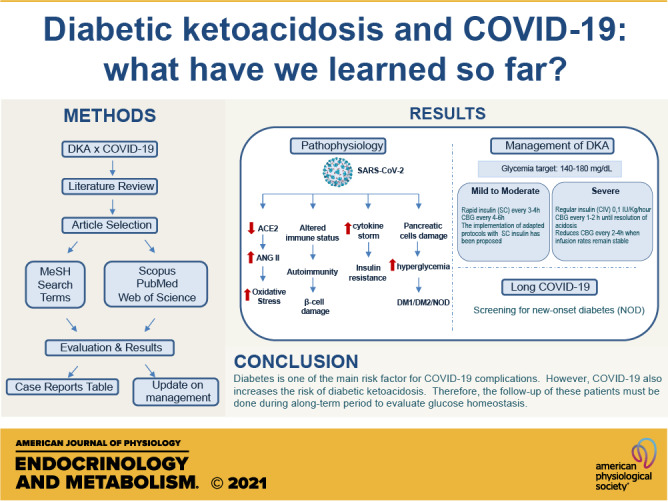
Keywords: COVID-19, diabetic ketoacidosis, diabetes mellitus, SARS-CoV-2
Abstract
In December 2019, a pandemic emerged due to a new coronavirus that imposed various uncertainties and discoveries. It has been reported that diabetes is a risk factor for worst outcomes of COVID-19 and also that SARS-CoV-2 infection was correlated with the occurrence of diabetic ketoacidosis (DKA) in patients. The aim of this work is to discuss this correlation emphasizing the main case reports from 2020 while exploring the management of DKA during the course of COVID-19. Web of Science, PubMed, and Scopus databases were searched using two sets of Medical Subject Heading (MeSH) search terms or Title/Abstract words: Coronavirus Infections (Coronavirus Infections, Middle East Respiratory Syndrome, COVID-19) and Diabetic Ketoacidosis (Diabetic Ketoacidosis, Diabetic Acidosis, Diabetic Ketosis). There is a clear correlation between COVID-19 and DKA. The SARS-Cov-2 infection may precipitate both a hyperglycemic state and ketoacidosis occurrence in patients with diabetes and nondiabetic patients, which may lead to fatal outcomes. DKA in patients with COVID-19 may increase risk and worse outcomes. Hence, the SARS-Cov-2 infection presents a new perspective toward the management of glycemia and acidosis in patients with diabetes and nondiabetic patients, highlighting the need for rapid interventions to minimize the complications from COVID-19 while reducing its spreading.
INTRODUCTION
In December 2019, cases of pneumonia emerged due to a respiratory infection caused by a new coronavirus (SARS-Cov-2) in the province of Wubei in China (1). The infection may occur mildly; however, some infected people may present complications, causing an acute respiratory syndrome, which has been demanding great hospital support due to the high rate of transmissibility of the virus. Therefore, this infection has generated great concern internationally (2).
Since the first studies, there have been some risk factors that may disturb the immune response against the coronavirus-related infection. Cardiovascular and cerebrovascular diseases, chronic obstructive pulmonary disease, and diabetes are some of the comorbidities of increased risk correlated to the worsening of patients with coronavirus (3). In patients with diabetes, especially with decompensated disease, there are reports of more severe cases and worse responses (4, 5).
Diabetes is a metabolic syndrome that contributes to a proinflammatory condition with an exacerbated immune response, such as the one reported in COVID-19 literature. On a hyperglycemic state, there is an increase in the virulence of pathogens, a decrease in the production of interleukins in response to infection, and a reduction in the phagocytic activity and polymorphonuclear leukocytes leading to an impaired prognosis of these patients in face of COVID-19 (6).
Diabetic ketoacidosis (DKA) is considered a fatal metabolic alteration attributed to hyperglycemia, with accumulation of ketone bodies and consequent acidosis. This condition is more common in type 1 diabetes (DM 1), although it can occur in patients with type 2 diabetes (DM 2) during the course of viral infections. Studies indicate that COVID-19 can accelerate the lipolysis and induce ketosis, favoring its development, especially in patients with diabetes who do not perform effective glycemic monitoring (7).
Therefore, monitoring patients with diabetes is essential to control the disease and to avoid possible complications of COVID-19, such as DKA. Telemedicine is beneficial for people with diabetes in this context, as it ensures the monitoring, while maintaining social distance and minimizing the risk of coronavirus transmission.
Given this scenario, with the emergence of an unprecedented pandemic in recent decades, the knowledge about COVID-19 is poorly consolidated. In addition, the literature about the effects of SARS-Cov-2 infection associated with DKA occurrence in the context of diabetes mellitus is not fully established. Hence, it is essential to review the pathophysiology of this new infection while discussing the medical outcomes of the main COVID-19 cases during 2020, especially with regards to the management of patients with diabetic ketoacidosis.
METHODOLOGY
The searches for the articles were performed in the Web of Science, PubMed, and Scopus databases using two sets of intersecting bibliographic search terms or Title/Abstract words: Coronavirus Infections (Coronavirus Infections, Middle East Respiratory Syndrome, COVID-19) and Diabetic Ketoacidosis (Diabetic Ketoacidosis, Diabetic Acidosis, Diabetic Ketosis). In addition, an original table was designed to collect, organize, and summarize the main case reports of 2020 associating diabetic ketoacidosis and COVID-19 to illustrate the main exam findings and patient’s management.
RESULTS AND DISCUSSION
Diabetes and COVID-19
Diabetes behaves as one of the main causes of morbidity and mortality in the world, and this condition is associated with several micro- and macrovascular complications that impact patients’ survival. Several studies indicate that patients with diabetes and, especially, those infected with the SARS-CoV-2 virus have higher rates of hospital admission, of developing severe pneumonia, and also higher mortality rates when compared with patients without comorbidities (6, 8).
Diabetes is a chronic inflammatory condition that directly affects the response to pathogens, mostly in those patients with uncontrolled glycemia because the innate and humoral responses are compromised. Hyperglycemia can affect immune function; conversely, a dysregulated immunological status is linked to macrovascular complications of diabetes mellitus. Thus, T2DM is associated with immunological dysregulation, which is potentially equivalent to accelerated aging and could, therefore, potentially explain the poor prognosis in patients with diabetes mellitus and COVID-19 (9).
The entry of the SARS-CoV-2 virus into the cell promotes a “cytokine storm” due to the triggering of inflammatory responses with recruitment of T helper cells and production of γ interferon. The increase in this cytokine pattern possibly turns patients with diabetes and COVID-19 more susceptible to organ injuries, which may lead to multiple organ failure, reported in the severe progression of the disease (10).
Diabetic Ketoacidosis
Diabetic ketoacidosis (DKA) is a potentially fatal metabolic complication of uncontrolled diabetes (11). DKA occurs in patients with a total or a partial insulin deficiency in the presence of a precipitating factor. In this scenario, some metabolic changes occur such as hyperglycemia, increased lipolysis, production of ketone bodies, glycosuria with progressive dehydration, alteration of electrolytes, and vomiting. Among the main precipitating factors of DKA, the inadequate use of insulin and infectious diseases are the most common (11, 12).
Recently, Dhatariya and Umpierrez suggested a revision of DKA criteria, proposing to change the blood glucose criterion from >250 mg/dL to >200 mg/dL because some patients with DKA had euglycemia (13–15). This condition occurs mainly in pregnant women with diabetes, in patients with altered gluconeogenesis due to alcohol abuse, and also in patients who use glucose-sodium cotransporter 2 (SGLT2) inhibitors (16).
Diabetic Ketoacidosis and COVID-19
Currently, the correlation between the SARS-CoV-2 infection and the precipitation of diabetic ketoacidosis (DKA) has been suggested in some patients (17). Although the mechanism is not clear, there are few possible explanations to the association between COVID-19 and this serious condition related to hyperglycemia. One of the main hypotheses includes the interaction between SARS-CoV-2 virus and the angiotensin-converting enzyme 2 (ACE2) (9).
ACE2 is the functional receptor of SARS-CoV-2 in human cells, and it is present in many tissues from the human body, including pancreatic islets (18). ACE2 main substrate is angiotensin 2 that suppresses insulin secretion and reduces the blood flow in the pancreatic islets. As a consequence, it causes decreased proliferation of these structures and induces local inflammation, apoptosis, and hyperglycemia. In contrast to angiotensin 2, angiotensin 1–7, the product of ACE2, has an opposite effect, causing increased insulin secretion and vasodilation (9). Therefore, the degradation of angiotensin 2 by ACE2 protects cells from the effect of renin angiotensin aldosterone system (RAAS) hyperactivation and reduces insulin resistance due to the decrease of the oxidative cellular stress (18).
Considering the protective role of ACE2, it is common that the expression of this enzyme is increased, mainly in people with huge carbohydrates and glucose intake, to compensate for insulin resistance (18). The connection between SARS-CoV-2 and ACE2 results in a decreased expression of this enzyme and an increased contact between the angiotensin 2 and the pancreatic tissue, resulting in long-term deleterious effects and justifying the hyperglycemia in patients with diabetes and even in nondiabetic patients (9). Furthermore, it has been reported that most of diabetic patients with SARS-CoV-2 infection that developed DKA had a good glycemic control before the hospitalization, reinforcing the correlation between the virus and the hyperglycemia (17).
Also, the direct interaction between SARS-CoV-2 virus and pancreatic islets is another reason that contributes to hyperglycemia manifestations in patients with COVID-19 (17). This can occur not only due to the damage to pancreatic B cells in response to the abundance of proinflammatory cytokines but also because of the autoimmunity caused by SARS-CoV-2 infection in genetically predisposed patients (18). As a consequence of pancreatic cells modification, hyperglycemia and acute diabetes have been reported in hospitalized patients with the SARS-CoV-2 infection (19). A study followed 39 patients with SARS-CoV, another virus of the same family of the COVID-19 agent, and showed that 20 of these people developed diabetes during the admission (19). After 3 yr of follow-up, two of the observed patients remained diabetic, suggesting that SARS-CoV infection caused transient pancreatic damage, culminating in diabetes mellitus (DM) development.
Considering the hyperglycemia caused by the period of SARS-CoV-2 infection, DKA must be addressed as a COVID-19 complication even in patients without DM diagnosis. To illustrate, one study including 658 hospitalized patients with COVID-19 diagnosis showed that 42 (6.4%) of them developed ketosis upon admission. Only 15 (35.7%) of these patients had a previous DM diagnosis, whereas the other 27 (64.3%) were nondiabetic patients. Among all the people followed in this research, only five developed DKA and two of them were not previously diagnosed with DM, suggesting that COVID-19 can accelerate lipolysis and induce both ketosis and DKA without a previous DM diagnosis (7).
On the other hand, DKA impact in patients with COVID-19 seems to be conflicting between studies. Although one research established a mortality of 50% in patients with DKA and COVID-19 (17), another one showed that patients with DKA were more likely to survive when compared with patients who have not developed DKA (20). It is known that patients with concomitant DKA and hyperosmolar hyperglycemic syndrome have higher mortality, but there are not enough data supporting the same correlation with COVID-19 (21). Even though more studies are needed, the evidence on SARS-CoV-2 suggests a huge possibility of interaction between COVID-19 and glucose metabolism leading to DKA precipitation in diabetic and even in nondiabetic patients. Therefore, we highlight in next session a collection of the main report cases associating COVID-19 with diabetic ketoacidosis.
Case Reports
A great range of report cases have been documented, during the last year, associating diabetes and hyperglycemic states with SARS-CoV-2 infection. These reports were either from patients with diabetes as a previous underlying disease or who developed a hyperglycemic state during admission, mainly precipitated by the infection. However, there are still few studies showing the metabolic profile of these patients focusing on diabetic ketoacidosis occurrence and its consequences to COVID-19 management and the prognosis on patient’s care (Table 1).
Table 1.
Retrospective review of COVID-19 diagnosed patients with DKA
| Type of Study | Sex | Age | CC | DM | pH and/or ketones | Glucose and/or HbA1C | Ferritin | D-Dimer | Other Findings | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective cross-sectional study | M and F | 65–75 | 87 Patients with COVID-19 and diabetes | Yes | 8 Patients with DKApH ND | ND | ND | ND | ND | ND | Patients with DKA more likely to survive (87.1%) compared with patients without DKA (50.6%) | Alkundi et al. (20) |
| Case report | M | 23 | Lethargy, nauseas, body aches, fever, dry cough, decreased level of consciousness | Yes | pH 7.0 | 1,384 mg/dL | 18,431 ng/mL | >11,000 ng/mL | Multifocal pneumonia subcortical hemorrhage | AzithromycinHCQ | No recovery followed by intubation, hypotension, and fulminant acute respiratory distress syndrome | Cavalcanti et al. (22) |
| Case report | M | 37 | Fever, vomiting, polydipsia, and polyuria | Diagnosed during admission | pH 7.28 ketones 6.4 mmol/L | HbA1C 14.2% | ND | ND | Hyperglycemia, high anion gap metabolic acidosis, and ketonemia | 24-h Intravenous insulin infusion + subcutaneous insulin therapy | Recovered | Chee et al. (23) |
| Case report | M | 57 | Worsening shortness of breath, fatigue, intermittent cough | Diagnosed during admission (new-onset diabetes) | 7.193 urine ketones >160 mg/dL | 436 mg/dL | 1,763 ng/mL | 410 ng/mL | Hyperglycemia, anion gap metabolic acidosis, and ketonuria | 2 L normal saline and insulin drip at 0.1 unit/kg of ideal body weight/hour | Recovered leaving hospital on day 5 | Heaney et al. (24) |
| Retrospective review | ND | 40 | 4 Diabetic patients with DKA out of 218 patients admitted to hospital for COVID-19 | No | 7.12 | 19 mmol/L | ND | Persistently elevated capillary ketones, metabolic acidosis, hypoxemic respiratory failure | Intravenous insulin, critical care (venovenous hemofiltration, mechanical invasive ventilation) | 2 Patients not recovered and 1 remained in intensive care for 30 days | Goldman et al. (25) | |
| ND | 42 | Yes | 7.1 | 20 mmol/L | ||||||||
| ND | 59 | Yes | 7.23 | 26 mmol/L | ||||||||
| ND | 82 | Yes | 7.27 | 22 mmol/L | ||||||||
| Type of Study | Sex | Age | CC | DM | pH and/or ketones | Glucose and/or HbA1C | Ferritin | D-dimer | Other findings | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | M | 59 | General weakness, polydipsia, polyuria, mild dyspnea for 4 days | Yes | Plasma ketone body 4.9 mmol/L metabolic acidosis | 655 mg/dL HbA1C 11.4% | ND | ND | Hypertension, tachypnea, oxygen saturation at 94% | Lopinavir/ritonavir, empirical antibiotics, insulin, mechanical ventilation, extracorporeal membrane oxygenation, renal replacement therapy | No recovery from respiratory failure and hemodynamic instability | Kim et al. (26) |
| Case report | M | 52 | Severe polypnea, myalgia, diarrhea, and fever over 38°C lasting for 4 days | Yes T1DM | 7.48 on admission | 250–270 mg/dL HbA1C at 6.9%–7.2% past 2 yr | ND | ND | Euglycemic DKA, bilateral pneumonia, polypnea, dyspnea, and altered consciousness, 89% oxygen saturation, elevated anion gap, decreased bicarbonates | Empagliflozin discontinued, intravenous fluids administration, and intravenous insulin infusion | Pneumonia evolved into and ARDS and mechanical ventilation support was required. Pulmonary embolism was observed. Patient left ICU after 6 wk | Oriot and Hermans (27) |
| 7.25 ICUUrine Ketones (3+/4+) | ||||||||||||
| Retrospective cohort study | M and F | Median age 56 yr | 129 Patients with COVID-19 and diabetes 15 patients with ketosis fever (10), cough (7), fatigue (7), chest tightness (5), headache (1), nausea and vomiting (3), shortness of breath (3) |
Yes | 3 Patients developed DKA pH 6.86–7.32 Urine Ketone +++ Ketone body 1–2 mmol/L Lactate 0.7–1.7 mmol/L |
Glucose 9.2–16.6 mmol/L HbA1C at 7.6%–12.3% |
ND | 0.5–2.6 μg/mL | Decreased levels of bicarbonates, lymphocytes and albumin, unilateral (3) and bilateral (12) pneumonia, acute liver injury (2), septic shock (1), acute respiratory distress syndrome (6) | Antibiotics (15), antiviral drugs (14), hormones (10), immunoglobulin (4), invasive mechanical ventilation (4), noninvasive mechanical ventilation (4) | From the 15 patients with diabetes and ketosis, 10 were rehabilitated with hospital discharge and 5 died, of which 1 had developed DKA | Li et al. (7) |
| Type of Study | Sex | Age | CC | DM | pH and/or ketones | Glucose and/or HbA1C | Ferritin | D-dimer | Other findings | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | F | 53 | Sore throat, diarrhea, fever, and progressive dyspnea | Yes | 7.41 on admission | 151 mg/dLHbA1C 7.5% on admission | 440 ng/mL on admission | ND | On admission: tachypneic, oxygen saturation 78%, acanthosis nigricans, central adiposity, BMI 33 kg/m² | On admission: azithromycin and HCQ. Oral hypoglycemics discontinued, correctional insulin treatment for glycemic control | ND | Palermo et al. (5) |
| 7.24 24-h postadmission | 192 mg/dL 24-h postadmission |
24-h postadmission: elevated anion gap, bicarbonate 15 mmol/L, venous blood pH 7.24, and lactic acid 1.3 mmol/L | 24-h postadmission: intravenous insulin with dextrose support for DKA treatment | |||||||||
| M | 45 | Fatigue, symptomatic hyperglycemia (polyuria, polydipsia) | Diagnosed during admission (new-onset diabetes) | 7.18 | 599 mg/dL HbA1C 12.6% | ND | ND | Hypertensive, afebrile, hypoxemic respiratory failure, oxygen saturation 83%, BMI 28 kg/m², bicarbonate 15 mmol/L, elevated anion gap, urinalysis showed 2+ ketones and 3+ glucose; β-hydroxybutyrate >9 mmol/L, lactic acid 2.4 mmol/L | 6 L of supplemental oxygen and treatment for new-onset diabetes | ND | ||
| Type of Study | Sex | Age | CC | DM | pH and/or ketones | Glucose and/or HbA1C | Ferritin | D-dimer | Other findings | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | F | 4 | Somnolence and polypnea | Diagnosed during admission (new-onset T1DM) | ND | HbA1C 11.8%Glucose 390 mg/dL | 92 ng/mL | 0.49 μgFEU/mL | Hyponatremia at 128 mEq/L and a low alkaline reserve at 2 mmol/LUrine strips: sugar (3+/4+) and ketones (3+/4+) | Rehydration and intravenous insulin therapy with correction of hyponatremia and close clinical monitoring | Discharged 8 days after hospitalization with a diagnosis of new-onset type 1 diabetes | Chekhlabi et al. (28) |
| M | 7 | Fatigue, polydipsia, polyuria, and weight loss of 3 kg and complicated by vomiting, abdominal pain, and severe polypnea | Diagnosed during admission (new-onset T1DM) | ND | HbA1C 10.3%Glucose 423 mg/dL | 255 ng/mL | 0.36 μgFEU/mL | Hyponatremia at 130 mEq/L and a low alkaline reserve at 6.8 mmol/LUrine analysis with large amount of sugar and ketone | Discharged 10 days after hospitalization with a diagnosis of new-onset type 1 diabetes | |||
| Case report | M | 30 | General weakness, fever, loss of taste, and mild dyspnea | Diagnosed during admission | pH 7.07 Urine Ketones |
HbA1C 9.6%Glucose 555 mg/dL | 817 ng/mL | 1.86 mg/L | Tachypneic, oxygen saturation 90%, temperature 100°F; HCO3− 6.1 mmol/L | Both patients were treated with intravenous fluid, intravenous insulin infusion with monitoring of serum electrolytes. Remdesivir, empirical antibiotics, and other symptomatic respiratory treatment for COVID-19. Enoxaparin due to elevated D-dimer levels | Both patients were discharged in stable condition | Reddy et al. (29) |
| M | 60 | Inability to move the left upper limb associated with weakness of left lower limb | Diagnosed during admission | pH 7.30 Urine Ketones |
HbA1C 12.6%Glucose 582 mg/dL | 135 ng/mL | 0.88 mg/L | Tachypneic, oxygen saturation 92%; body temperature 99°F; HCO3− 13 mmol/L; acute cerebrovascular accident | ||||
ARDS, acute respiratory distress syndrome; BMI, body mass index; CC, chief complaint; DKA, diabetic ketoacidosis; DM, diabetes mellitus; T1DM, type 1 diabetes mellitus; HbA1C, glycated hemoglobin; HCQ, hydroxychloroquine; ICU, intensive care unit; ND, not described.
Chee and collaborators (23) reported a case of a previously healthy 37-yr-old male patient who was admitted with complaints of fever, polydipsia, polyuria, and vomiting. The patient was diagnosed with COVID-19 and diabetes and further presented intense diabetic ketoacidosis. Because DKA results from insulin deficiency and increased counter-regulatory responses that favor ketone production, the entry of SARS-CoV-2 virus into β-pancreatic cells may be a risk factor to its occurrence. The virus impairs pancreatic cells function besides reducing the expression of ACE2, which disrupts insulin secretion by angiotensin 2. Therefore, according to Chee et al. (23), this represents the main mechanism by which COVID-19 may precipitate the development of diabetic ketoacidosis in previously healthy and controlled patients.
In a retrospective study by Goldman et al. (25) with 218 patients with COVID-19, it was observed that four of these met the diagnostic criteria for diabetic ketoacidosis, including pH <7.3 and/or bicarbonate <15 mmol/L, blood glucose >11 mmol/L, and ketonemia ≥ 3.0 mmol/L. These four patients had a persistently high rate of capillary ketones even after 24 h of constant intravenous insulin treatment. Of these, two patients required intensive care: one because of continuous venous hemofiltration due to refractory severe metabolic acidosis and another for invasive mechanical ventilation because of the management of hypoxemic respiratory failure. Both patients died and another remained in intensive care with posthospitalization follow-up for at least 30 days. Although the study presents a small single-center data set, it reinforces the need for attention to diabetic ketoacidosis in patients with COVID-19, whether for patients previously diagnosed with diabetes or for those whose DKA is precipitated by COVID-19. This way, metabolic management should be optimized, severe and treatment-resistant DKA better managed, and the outcome of this type of patient will be much more effective.
Diabetic ketoacidosis is a risk factor for the development of sequelae associated with COVID-19, not only due to changes in plasma glucose levels but also because it influences other pathologies. For instance, Cavalcanti and collaborators (22) described a case report of a 23-yr-old male patient who presented subcortical hemorrhage after infection by SARS-CoV-2 that may have been an outcome from complications associated with diabetic ketoacidosis (22).
In addition, new case reports have highlighted the need and importance of careful evaluation of patients with high blood glucose levels and without a history of diabetes. There is a real possibility of developing diabetes mellitus and diabetic ketoacidosis, especially in the context of concomitant COVID-19. Heaney and collaborators (24) reported a case of a 57-yr-old male patient with no previous history of diabetes who was admitted to the hospital with COVID-19. The diagnosis was confirmed by laboratory examination and this patient was both diagnosed with diabetes and diabetic ketoacidosis, precipitated by COVID-19 (24).
Even though DKA occurs mainly in critical hospitalized patients resulting in worst outcomes, there are reports of it in patients with new-onset diabetes upon COVID infection. Chekhlabi and colleagues (28) reported two cases of children diagnosed with COVID-19 that developed symptoms suggestive of diabetic ketoacidosis upon hospital admission. One child presented with acidosis and urinary ketones and the other had fatigue, polydipsia, polyuria, and urine ketones. The laboratories showed both hyperglycemia and HbA1c of 11.8% and 10.3%, respectively. They received intravenous rehydration followed by intravenous insulin therapy, which was subsequently replaced by subcutaneous insulin (as basal insulin) and Novorapid postmeal (28). Also, Reddy and colleagues (29) described two cases of adult patients that presented with hyperglycemia upon admission with no prior history of diabetes. The first patient, 30-yr-old, presented with general weakness, fever, loss of taste, and mild dyspnea with an initial plasma glucose of 555 mg/dL (HbA1c 9.6%) and metabolic acidosis (pH 7.07 and HCO3− 6.1 mmol/L) with urine ketones present. He was managed with intravenous fluid replacement followed by intravenous insulin infusion. DKA resolved the second day and subcutaneous insulin was initiated. The second case included a 60-yr-old man with hypertension. He presented with inability to move the left upper limb associated with weakness of left lower limb, not able to walk. Upon admission, blood glucose was 582 mg/dL and HbA1c was 12.6%, pH 7.30, and HCO3− 13 mmol/L, with urine ketones present. He was managed with intravenous fluid and insulin infusion. Computer tomography (CT) of the head revealed acute cerebrovascular accident. He was managed as per neurology guidance and, later, was discharged in stable condition and education regarding the management of stroke and diabetes at home (29). There is an issue regarding the type of diabetes associated with DKA in new-onset diabetes, especially in patients with no previous history of DM, which would indicate type 1 diabetes mellitus (T1DM). However, Kuchay and colleagues (30) followed three patients with new-onset diabetes presenting with DKA, who had a good glycemic control on metformin or a combination with dipeptidyl peptidase-4 (DPP-4) 4 inhibitor therapy, a characteristic scenario of typical new-onset type 2 diabetes, revealing these patients had developed type 2 diabetes mellitus (T2DM) upon COVID-19 infection.
In addition to T2DM, there have been some reports of COVID-19 precipitating DKA in patients with new-onset T1DM. In the United States, a multicenter study with 64 patients with T1DM including 33 COVID-19-positive patients and 31 COVID-19-like patients revealed that in both groups the most prevalent symptom was high blood glucose and the most adverse outcome was DKA (31). In Germany, there was a twofold increase of DKA in children and adolescents diagnosed with diabetes during COVID-19 pandemic. During the first 2 mo of the pandemics, 238 out of 532 children and adolescents with newly diagnosed T1DM presented with DKA, a higher frequency compared with the previous year (32). Finally, in UK, a recent multicenter study following 30 children with T1DM revealed 21 had DKA, from which 50% were severe DKA. Among them, five children were positive (2 SARS-CoV-2 PCR positive and 3 SARS-CoV-2 IgG positive) suffering from severe DKA (33). Therefore, COVID-19 infection may disturb glucose metabolism in both new-onset T1DM and T2DM resulting in severe consequences to patients.
Long COVID-19 syndrome refers to survivors of the disease who present some clinical manifestation within 4 mo from the onset of the symptoms. Emerging evidence shows that in the postacute phase of COVID-19, the so-called long COVID, we can observe transient hyperglycemia, new-onset diabetes, and even diabetic ketoacidosis; however, new prospective studies are needed to evaluate and follow-up symptoms and possible sequelae of patients recovering from COVID-19. Therefore, it is essential to screen patients with COVID-19 for new-onset diabetes (NOD) during the acute phase and after recovery from the disease (34–36).
In the context of COVID-19 outbreak, the appropriate use of medical resources is increasingly limited. This represents an aggravation in the health of individuals with diabetes because COVID-19 can prevent glycemic control and precipitate hyperglycemic crises, which are significantly related to increased morbidity and mortality in these patients resulting in catastrophic outcomes (26). Kim and colleagues (26) discussed in their case report the need for aggressive and appropriate management in patients with diabetes who present a hyperglycemic crisis precipitated by COVID-19, emphasizing the need for a rapid adoption of policies and strategies for the effective distribution of medical resources to prevent future deaths due to chronic diseases in face of COVID-19 (26).
Here, we briefly reviewed the main case reports and retrospective studies published in the past few months correlating COVID-19 and DKA. It is necessary, therefore, to emphasize the need for an immediate and effective approach and management of patients with blood glucose changes infected by SARS-CoV, especially diabetics, at the time of hospitalization. Such measures can prevent fatal outcomes, especially those resulting from complications associated with diabetic ketoacidosis precipitated by COVID-19. The appropriate hospitalization management information gathered here will be critical to the medical community in the months ahead.
Management
The COVID-19 pandemic presents new challenges for the hospital health team (HHT). Such professionals have taken care of the infected patients with diabetes, which represent more than 20% of the critically ill patients in the intensive care units (37). Hyperglycemia increases the risk of infections, alters the function of leukocytes, prolongs hospital stay, increases the virulence of some pathogens, and also increases the risk of cardiac arrhythmias and the mortality rate (38, 39). The implementation of protocols designed to control the patients’ glucose levels should have the ability to reduce these adverse results (39, 40).
Although optimizing glycemic control to reduce the risk of complications from COVID-19 is essential, specific considerations about the kind of treatment are necessary during the pandemic. Adequate glycemic management contributes to the reduction of adverse clinical outcomes from such acute disease, but it also requires intensive interactions with frequent bedside glucose monitoring, intravenous and subcutaneous administration of insulin, as well as rapid intervention for hypoglycemic events. Therefore, it is essential to treat hyperglycemia presented by hospitalized patients as protecting health professionals, especially at places where there is a lack of available personal protective equipment and employees (5).
The strategies suggested to limit the healthcare workers’ exposure when taking care of the patients with COVID-19 and diabetes, within the hospital, include minimizing the use of venous insulin VI infusions to critically ill patients, trying to treat diabetic ketoacidosis/hyperosmolar hyperglycemic state (DKA/HHS) from mild to moderate with subcutaneous insulin (SQ) regimen, and decrease the frequency of capillary glycemia (POC-BG) checking in patients on subcutaneous insulin (SQ) regimens and in those on VI insulin infusions when infusion rates are stable, using continuous remote glucose monitoring (CGM) devices. The role of diabetes self-management done by selected patients with diabetes in the hospital has gained renewed interest (41, 42).
At the moment of hospital admission, all patients should measure capillary blood glucose, regardless of the diagnosis of diabetes, since hyperglycemia increases mortality and treating it can influence the prognosis of COVID-19. Many inpatients are unaware of having diabetes. The SARS-Cov-2 infection itself provides new cases of diabetes, as well as DKA or HHS in nondiabetic patients. The glycated hemoglobin (A1c) should be verified if it has not been checked in the past 3 mo. Insulin is the appropriate medication for critical inpatients. Most of the algorithms for treating in-hospital diabetes due to COVID-19 advice to suspend all oral hypoglycemic agents and maintain only insulin (5).
Insulin is the appropriate treatment for patients hospitalized with severe forms of COVID-19 due to its security and anti-inflammatory potential. However, patients with DM2 and COVID-19 using insulin have shown a worse prognosis, generally attributed to the severity of diabetes in these patients. As previously described in experimental models, insulin can increase airway reactivity and pulmonary resistance (43). Hyperinsulinemia associated with obesity and diabetes can impair airway reactivity, and the administration of high doses of insulin can worsen this effect. Nonetheless, in noncritically inpatient the association of insulin with oral antihyperglycemic agents, in order to reduce excessive insulin doses, is possible to attenuate the airway reactivity.
The beneficial potential of drugs used to treat patients with diabetes during COVID-19 in noncritically inpatient is currently being discussed, emphasizing the role of metformin and iDPP4. Retrospective studies have confirmed a reduction in mortality rates in metformin users compared with nonusers in patients with DM2 hospitalized for COVID-19. Care must be taken when interpreting these observational findings, as only randomized controlled trials (RCTs) can provide definitive conclusions (44, 45). Metformin, in addition to increasing insulin sensitivity, may be beneficial in the treatment of COVID-19, as it has molecular pleiotropic effects, which can lead to attenuation of complications by COVID-19. Among the potential beneficial mechanisms, we highlight the decrease in insulin resistance, reduction of some inflammatory cytokines such as IL-6 and TNF-α, modulation of the angiotensin 2 receptor-converting enzyme (ACE2), and better neutrophils to lymphocytes ratio (46).
On the other hand, in the case of hospitalized patients with severe symptoms of COVID-19 and underlying diseases, the possibility of adverse effects of metformin such as lactic acidosis should be taken into account (46). Current clinical evidence supports continued metformin treatment in individuals with mild to moderate COVID-19 and pre-existing T2DM, but acidosis and renal function should be carefully monitored in individuals with severe COVID-19 (44, 45, 47). Therefore, metformin may be a beneficial adjuvant therapy for patients in the acute, chronic, and even recovery phases of COVID-19 (45). However, it should be noted that metformin is not encouraged for use in critically ill patients.
DPP-4 inhibitors are a group of drugs that are associated with many advantages, even in severe cases of COVID-19, as they are well tolerated, can be used alone or in combination with insulin, regardless of renal function, and present a low risk of hypoglycemia. Also, experimental studies have shown that these drugs can reduce the inflammatory response (45). A recent retrospective multicenter study showed that treatment with the DPP-4 inhibitor sitagliptin during hospitalization for COVID-19 was associated with decreased mortality and improved clinical outcomes compared with standard care in patients with T2DM (48). Thus, we can consider recommending the broader use of DPP-4 inhibitors in patients with diabetes hospitalized with severe COVID-19 alone or associated with insulin therapy (45).
For all degrees of severity of DKA, the therapeutic triad is the same: hydration, electrolyte replacement, and insulin therapy. However, the intensity of the treatment, the management of insulin therapy, and the treatment site depend on the severity of DKA and the patient’s clinical conditions.
The treatment of DKA, therefore, must be individualized. To distinguish patients with mild or moderate DKA from the ones with severe DKA is an aspect that must be taken into account. The first decision to be considered is the need for continuous insulin infusion (CVI). Those with severe DKA require admission to the intensive care unit (ICU) with continuous infusion of venous insulin (CVI), respiratory support, and cardiac monitoring (5).
COVID-19 approach: severe DKA.
In patients with COVID-19 and DKA in critical condition, the glycemic target is between 140 and 180 mg/dL (49). To achieve this goal, in those with severe DKA, HHS, or combined DKA-HHS, the use of regular insulin in a continuous infusion pump of intravenous insulin (CVI) in the dose 0.1 IU/kg/h, is advisable, until the resolution of acidosis with blood glucose monitoring every 1–2 h (49). In the current scenario, intense monitoring of patients becomes extremely risky. To minimize the time of the nursing staff at the bedside with VI insulin infusion protocols, some hospitals reduced the frequency of glycemic measurements to every 2–4 h, when the infusion rates remain stable (50). Likewise, the use of continuous glucose monitoring devices (GGM) during the COVID-19 pandemic period has been used to ensure the safety of the hospital health team (HHT) (5, 37).
COVID-19 approach: mild to moderate DKA/HHS.
On the other hand, in cases of mild to moderate DKA/HHS, the rapid subcutaneous insulin (SC) every 3–4 h can be initially considered (42). Those who are not on vasopressors, parenteral nutrition, or high steroid doses may use weight-based subcutaneous insulin instead of intravenous dosing and so decrease blood glucose checking to every 4–6 h. The implementation of adapted protocols based on previous studies for therapy with SC insulin treatment in patients with mild to moderate DKA (39) to decrease the interaction of the health team with patients, as well as the use of ICU, has been proposed in several centers during the pandemic (37, 50).
Final considerations of DKA treatment associated with COVID-19.
In patients with COVID-19 and severe DKA, high levels of inflammatory markers associated with these two pathologies lead to a state of insulin resistance and result in an increase of insulin needs (5). There are reports of an above-normal increase (up to 4 units/kg/day) of insulin requirement during treatment of COVID-19 (37). The concomitant use of vasopressors or corticosteroids can also significantly impact insulin requirements over time (5). Also, great variability in insulin sensitivity is described over the course of the disease, regardless of glucocorticoid therapy. Patients with preexisting or acute chronic kidney disease as part of COVID-19 infection may be particularly sensitive to insulin and the risk of hypoglycemia. This requires attention to abrupt changes in blood glucose measurements with the need for continuous adjustments of insulin therapy (37).
The approach and the considerations of euglycemic ketoacidosis by glucose-sodium cotransporter 2 (SGLT2) inhibitors deserve special consideration in times of the COVID-19 pandemic. This class of drugs presents a risk of dehydration, diabetic euglycemic ketoacidosis (euDKA), and genitourinary infections. It is likely that it is a combination of factors with volume depletion and decreased availability of glucose at tissue level playing important roles. Treatment includes the same triad approach as DKA, but with the clear distinction that dextrose-containing fluids are needed as an initial step, rather than added later as glucose levels decrease. The other key point in the treatment is that glycosuria can persist for days (51), requiring prolonged fluid replacement. Therefore, these drugs should be discontinued in case of suspicion or confirmation of COVID-19. Patients need to be aware of the risks of this medication. The use of ketone tapes at home can identify ketosis and prevent worsening of the condition (5, 50).
Hospital discharge is an important time to review aspects of diabetes education and training. They are an essential part of treatment as well as of the discharge planning. DKA is a predictable complication, but unfortunately, many patients with diabetes hospitalized with COVID-19 are not informed about self-management of DM (52). Technology and education in diabetes during the COVID-19 pandemic are fundamental weapons in the prevention of DKA (5, 50).
Diabetes and Technology
The COVID-19 pandemic and the resulting social isolation raised the importance of establishing alternative forms of health care and specifically the ones that could occur remotely. With the need to avoid exposure to SARS-CoV-2, many patients, in different contexts, can end up without proper assistance. Thus, telemedicine can be considered as a way of maintaining contact between the doctor and the patient.
Individuals already diagnosed with DM, using insulin, for example, are at increased risk of developing DKA if treatment is interrupted, or if they are left without proper monitoring (15). Patients newly diagnosed with T1DM, who presented with DKA, and need regular follow-up after hospital discharge, can also benefit from telemedicine so that appropriate treatment adjustment is possible during the pandemic period (53). The same benefit is seen in diabetics who, due to COVID-19 infection, may decompensate, increasing the risk of ketoacidosis (7).
Technology also offers us devices that monitor blood glucose continuously—CGMs, which even remotely can assist in patient monitoring, thanks to cloud data sharing systems. Despite being an extremely useful resource, as it allows the medical team to access their patients’ blood glucose in real time even when they are at home, it is still inaccessible to most of the population. On the other hand, capillary blood glucose measured manually by the patient, who reports it periodically during consultations with the doctor, is the most consistent way with the reality of health systems and can also be used as a mechanism for glycemic surveillance (54).
CONCLUSIONS
There are increasing reports of diabetes as one of the main risk factors for COVID-19 complications. On the other hand, COVID-19 increases the risk of diabetic ketoacidosis. These conditions may even represent, according to some studies, a poor prognosis, which can lead to an increased mortality rate. The precipitation mechanisms and the long-term effects of the interaction between SARS-CoV-2 and DKA are not yet conclusive. Thus, the follow-up of these patients must be done during a long-term period to check for late manifestations of the condition and also to consider the new-onset diabetes as a clinical metabolic sequela of SARS-CoV-2 infection. The current treatment of ketoacidosis may lead to contamination in the context of COVID-19; therefore, new approaches have been developed to prevent the spread of disease.
ETHICAL APPROVALS
This review is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
DISCLAIMERS
All authors have fully contributed to the review and take responsibility for the integrity of the work giving their approval for this version to be published.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.O.d.S.-F., C.H.M.d.C., J.C.W.G., N.S.S., L.d.M.L.S., L.P.d.M., N.G.R., T.L.d.S., S.C., and E.C.Y. conceived and designed research; C.O.d.S.-F. drafted manuscript; C.O.d.S.-F., C.H.M.d.C., J.C.W.G., N.S.S., L.d.M.L.S., L.P.d.M., N.G.R., T.L.d.S., S.C., and E.C.Y. edited and revised manuscript; C.O.d.S.-F., C.H.M.d.C., J.C.W.G., N.S.S., L.d.M.L.S., L.P.d.M., N.G.R., T.L.d.S., S.C., and E.C.Y. approved final version of manuscript.
APPENDIX: SEARCH STRATEGIES
PubMed
(“Coronavirus Infections” [Title/Abstract] OR “Infection, Coronavirus” [Title/Abstract] OR “Infections, Coronavirus” [Title/Abstract] OR “Middle East Respiratory Syndrome” [Title/Abstract] OR “MERS (Middle East Respiratory Syndrome)” [Title/Abstract] OR “COVID 19” [Title/Abstract] OR “COVID-19” [Title/Abstract] OR “19 COVID”) [Title/Abstract] AND (“Diabetic Ketoacidosis” [Title/Abstract] OR “Diabetic Acidosis” [Title/Abstract] OR “Ketoacidosis, Diabetic” [Title/Abstract] OR “Diabetic Ketoacidoses” [Title/Abstract] OR “Ketoacidoses, Diabetic” [Title/Abstract] OR “Ketosis, Diabetic” [Title/Abstract] OR “Acidosis, Diabetic” [Title/Abstract] OR “Acidoses, Diabetic” [Title/Abstract] OR “Diabetic Acidoses” [Title/Abstract] OR “Diabetic Ketosis” [Title/Abstract] OR “Diabetic Ketoses” [Title/Abstract] OR “Ketoses, Diabetic”) [Title/Abstract] Filters: in the last 1 year, English
Scopus
TITLE-ABS-KEY (“Coronavirus Infections” OR “Infection, Coronavirus” OR “Infections, Coronavirus” OR “Middle East Respiratory Syndrome” OR “MERS (Middle East Respiratory Syndrome)” OR “COVID 19” OR “COVID-19” OR “19 COVID”) AND (“Diabetic Ketoacidosis” OR “Diabetic Acidosis” OR “Ketoacidosis, Diabetic” OR “Diabetic Ketoacidoses” OR “Ketoacidoses, Diabetic” OR “Ketosis, Diabetic” OR “Acidosis, Diabetic” OR “Acidoses, Diabetic” OR “Diabetic Acidoses” OR “Diabetic Ketosis” OR “Diabetic Ketoses” OR “Ketoses, Diabetic”) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”) ) AND (LIMIT-TO (PUBYEAR, 2020) ) AND (LIMIT-TO (LANGUAGE, “English”) )
Web of Science—Title
((TI=(“Coronavirus Infections” OR “Infection, Coronavirus” OR “Infections, Coronavirus” OR “Middle East Respiratory Syndrome” OR “MERS (Middle East Respiratory Syndrome)” OR “COVID 19” OR “COVID-19” OR “19 COVID”) AND TI=(“Diabetic Ketoacidosis” OR “Diabetic Acidosis” OR “Ketoacidosis, Diabetic” OR “Diabetic Ketoacidoses” OR “Ketoacidoses, Diabetic” OR “Ketosis, Diabetic” OR “Acidosis, Diabetic” OR “Acidoses, Diabetic” OR “Diabetic Acidoses” OR “Diabetic Ketosis” OR “Diabetic Ketoses” OR “Ketoses, Diabetic”))) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article OR Review) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan=2019-2020
Web of Science—Abstract
((AB=(“Coronavirus Infections” OR “Infection, Coronavirus” OR “Infections, Coronavirus” OR “Middle East Respiratory Syndrome” OR “MERS (Middle East Respiratory Syndrome)” OR “COVID 19” OR “COVID-19” OR “19 COVID”) AND AB=(“Diabetic Ketoacidosis” OR “Diabetic Acidosis” OR “Ketoacidosis, Diabetic” OR “Diabetic Ketoacidoses” OR “Ketoacidoses, Diabetic” OR “Ketosis, Diabetic” OR “Acidosis, Diabetic” OR “Acidoses, Diabetic” OR “Diabetic Acidoses” OR “Diabetic Ketosis” OR “Diabetic Ketoses” OR “Ketoses, Diabetic”))) AND LANGUAGE: (English) AND DOCUMENT TYPES: (Article OR Review) Timespan: 2019-2020. Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI.
REFERENCES
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease (COVID-19) Pandemic (Online). https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [2021 Jan 27].
- 3.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging (Albany NY) 12: 6049–6057, 2020. doi: 10.18632/AGING.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of COVID-19 in New York city. N Engl J Med 382: 2372–2374, 2020. doi: 10.1056/nejmc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palermo NE, Sadhu AR, McDonnell ME. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab 105: 2819–2829, 2020. doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuschieri S, Grech S. COVID-19 and diabetes: the why, the what and the how. J Diabetes Complications 34: 107637, 2020. doi: 10.1016/j.jdiacomp.2020.107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab 22: 1935–1941, 2020. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract 162: 108142, 2020. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim S, Bae JH, Kwon H-S, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 17: 11–30, 2021. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr 14: 303–310, 2020. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism 65: 507–521, 2016. doi: 10.1016/j.metabol.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Kitabchi AE, Fisher JN. Hyperglycemic crises: diabetic ketoacidosis (DKA) and hyperglycemic, hyperosmolar state (HHS). In: Acute Endocrinol: From Cause to Consequence, edited by Van den Berghe G. Totowa, NJ: Humana Press, 2008, p. 119–147. doi: 10.1007/978-1-60327-177-6_6. [DOI] [Google Scholar]
- 13.Dhatariya KK, Umpierrez GE. Guidelines for management of diabetic ketoacidosis: time to revise? Lancet Diabetes Endocrinol 5: 321–323, 2017. doi: 10.1016/S2213-8587(17)30093-1. [DOI] [PubMed] [Google Scholar]
- 14.Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis: a review. Curr Diabetes Rev 13: 315–321, 2017. doi: 10.2174/1573399812666160421121307.[27097605] [DOI] [PubMed] [Google Scholar]
- 15.Munro JF, Campbell IW, McCuish AC, Duncan LJP. Euglycaemic diabetic ketoacidosis. Br Med J 2: 578–580, 1973. doi: 10.1136/bmj.2.5866.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, Wall BM. Management of hyperglycemic crises in patients with diabetes. Diabetes Care 24: 131–153, 2001. doi: 10.2337/diacare.24.1.131. [DOI] [PubMed] [Google Scholar]
- 17.Chamorro-Pareja N, Parthasarathy S, Annam J, Hoffman J, Coyle C, Kishore P. Letter to the editor: unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metabolism 110: 154301, 2020. doi: 10.1016/j.metabol.2020.154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sathish T, Tapp RJ, Cooper ME, Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab 47: 101204, 2021. doi: 10.1016/J.DIABET.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47: 193–199, 2010. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkundi A, Mahmoud I, Musa A, Naveed S, Alshawwaf M. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: a retrospective single centre study. Diabetes Res Clin Pract 165: 108263, 2020. doi: 10.1016/j.diabres.2020.108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquel FJ, Tsegka K, Wang H, Cardona S, Galindo RJ, Fayfman M, Davis G, Vellanki P, Migdal A, Gujral U, Narayan KMV, Umpierrez GE. Clinical outcomes in patients with isolated or combined diabetic ketoacidosis and hyperosmolar hyperglycemic state: a retrospective, hospital-based cohort study. Diabetes Care 43: 349–357, 2020. doi: 10.2337/dc19-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, Nossek E, Torres J, Jain R, Riina HA, Radmanesh A, Nelson PK. Cerebral venous thrombosis associated with COVID-19. AJNR Am J Neuroradiol 41: 1370–1376, 2020. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by COVID-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract 164: 108166, 2020. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaney AI, Griffin GD, Simon EL. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med 38: 2491.e3–2491.e4, 2020. doi: 10.1016/j.ajem.2020.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman N, Fink D, Cai J, Lee Y-N, Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract 166: 108291, 2020. doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Acute hyperglycemic crises with coronavirus disease-19: Case reports. Diabetes Metab J 44: 349–353, 2020. doi: 10.4093/DMJ.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oriot P, Hermans MP. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case report and review of the literature. Acta Clin Belg 16: 1–5, 2020. doi: 10.1080/17843286.2020.1780390. [DOI] [PubMed] [Google Scholar]
- 28.Chekhlabi N, Haoudar A, Echcharii N, Ettair S, Dini N. New-onset diabetes with ketoacidosis precipitated by COVID-19 in children: a report of two cases. Case Rep Pediatr 2021: 5545258, 2021. doi: 10.1155/2021/5545258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: A report of two cases and review of literature. Diabetes Metab Syndr 14: 1459–1462, 2020. doi: 10.1016/J.DSX.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuchay MS, Reddy PK, Gagneja S, Mathew A, Mishra SK. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Diabetes Metab Syndr 14: 2039–2041, 2020. doi: 10.1016/J.DSX.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care 43: e83–e85, 2020. doi: 10.2337/DC20-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, Holl RW. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA 324: 801–804, 2020. doi: 10.1001/JAMA.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, Kwong RMW, Kumar P, Logan KM. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care 43: e170–e171, 2020. doi: 10.2337/DC20-1551. [DOI] [PubMed] [Google Scholar]
- 34.The Lancet. Facing up to long COVID. Lancet 396: 1861, 2020. doi: 10.1016/S0140-6736(20)32662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med 27: 601–615, 2021. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: Causality or coincidence? A report of three cases. J Med Virol 93: 1150–1153, 2021. doi: 10.1002/JMV.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korytkowski M, Antinori-Lent K, Drincic A, Hirsch IB, McDonnell ME, Rushakoff R. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J Clin Endocrinol Metab 105: 3076–3087, 2020. doi: 10.1210/clinem/dgaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 32: 1119–1131, 2009. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 30: 2181–2186, 2007. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 40.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med 345: 1359–1367, 2001. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association. Diabetes care in the hospital: standards of medical care in diabetes—2020. Diabetes Care 43, Suppl 1: S193–S202, 2020. doi: 10.2337/dc20-S015.[31862758] [DOI] [PubMed] [Google Scholar]
- 42.Aljehani FA, Funke K, Hermayer KL. Inpatient diabetes and hyperglycemia management protocol in the COVID-19 era. Am J Med Sci 360: 423–426, 2020. doi: 10.1016/j.amjms.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leiria LOS, Arantes-Costa FM, Calixto MC, Alexandre EC, Moura RF, Folli F, Prado CM, Prado MA, Prado VF, Velloso LA, Donato J Jr, Antunes E, Martins MA, Saad MJA. Increased airway reactivity and hyperinsulinemia in obese mice are linked by ERK signaling in brain stem cholinergic neurons. Cell Rep 11: 934–943, 2015. doi: 10.1016/J.CELREP.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol (Lausanne) 11: 600439, 2021. doi: 10.3389/FENDO.2020.600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos A, Magro DO, Evangelista-Poderoso R, Saad MJA. Diabetes, obesity, and insulin resistance in COVID-19: molecular interrelationship and therapeutic implications. Diabetol Metab Syndr 13: 23, 2021. doi: 10.1186/S13098-021-00639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zangiabadian M, Nejadghaderi SA, Zahmatkesh MM, Hajikhani B, Mirsaeidi M, Nasiri MJ. The efficacy and potential mechanisms of metformin in the treatment of COVID-19 in the diabetics: a systematic review. Front Endocrinol (Lausanne) 12: 645194, 2021. doi: 10.3389/FENDO.2021.645194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheen AJ. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab 46: 423–426, 2020. doi: 10.1016/J.DIABET.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solerte SB, D’Addio F, Trevisan R, Lovati E, Rossi A, Pastore I, Dell’Acqua M, Ippolito E, Scaranna C, Bellante R, Galliani S, Dodesini AR, Lepore G, Geni F, Fiorina RM, Catena E, Corsico A, Colombo R, Mirani M, De Riva C, Oleandri SE, Abdi R, Bonventre JV, Rusconi S, Folli F, Di Sabatino A, Zuccotti G, Galli M, Fiorina P. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care 43: 2999–3006, 2020. doi: 10.2337/DC20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasquel FJ, Umpierrez GE. Individualizing inpatient diabetes management during the coronavirus disease 2019 pandemic. J Diabetes Sci Technol 14: 705–707, 2020. doi: 10.1177/1932296820923045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou K, Al-Jaghbeer MJ, Lansang MC. Hyperglycemia management in hospitalized patients with COVID-19. Cleve Clin J Med, 2020. doi: 10.3949/ccjm.87a.ccc012. [DOI] [PubMed] [Google Scholar]
- 51.Alhassan S, Rudoni M, Alfonso‐Jaume MA, Jaume JC. Protracted glycosuria after discontinuation of sodium‐glucose cotransporter 2 inhibitors: implications for weekly dosing and extended risk of euglycemic diabetes ketoacidosis. J Diabetes 11: 410–411, 2019. doi: 10.1111/1753-0407.12885. [DOI] [PubMed] [Google Scholar]
- 52.Donihi AC. Practical recommendations for transitioning patients with type 2 diabetes from hospital to home. Curr Diab Rep 17: 52, 2017. doi: 10.1007/s11892-017-0876-1. [DOI] [PubMed] [Google Scholar]
- 53.Garg SK, Rodbard D, Hirsch IB, Forlenza GP. Managing new-onset type 1 diabetes during the COVID-19 pandemic: challenges and opportunities. Diabetes Technol Ther 22: 431–439, 2020. doi: 10.1089/dia.2020.0161. [DOI] [PubMed] [Google Scholar]
- 54.Peters AL, Garg SK. The silver lining to COVID-19: avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technol Ther 22: 449–453, 2020. doi: 10.1089/dia.2020.0187. [DOI] [PubMed] [Google Scholar]


