Highlights
-
•
Antidepressant gender disparity less in type 2 diabetes than general population
-
•
No evidence of ethnic disparity in antidepressant prescribing in type 2 diabetes
-
•
No evidence of disparity in antidepressant prescribing for insulin users
-
•
Considerable evidence gaps for antidepressant prescribing in type 2 diabetes
Keywords: Meta-analysis, Antidepressant, Depression, Type 2 diabetes, Prevalence, Risk factors
Summary
Background
Treatment guidelines do not provide specific recommendations for antidepressant prescribing in people with type 2 diabetes mellitus (T2DM). It is important to understand the prevalence of antidepressant prescribing and associated patient characteristics, to recognise safety issues or inequalities related to treatment access.
Methods and Results
Seven databases were searched using terms related to depression, T2DM and antidepressant medication. From 14,389 reports retrieved, 9 met inclusion criteria. The prevalence of antidepressant prescribing varied considerably between studies from 18% to 87%. Where meta-analyses were possible, the pooled odds ratio for receiving an antidepressant were 1.52 (95% confidence intervals (CIs) 1.28 – 1.82) in women compared to men, 0.53 (95% CIs 0.23-1.20%) in Black and Ethnic Minorities compared to White ethnicity and 1.29 (95% CIs 0.92-1.80) in insulin users compared to individuals with non-insulin controlled T2DM.
Conclusions
Antidepressant prescribing is more common in women with T2DM compared to men, however, the difference is less than in the general population. Insulin users, representing individuals with more advanced T2DM, were as likely to be prescribed antidepressants as those who did not use insulin. There is a gap in the literature concerning which antidepressant agents are being prescribed, and alongside which concurrent medications and comorbidities.
1. Introduction
There is substantial evidence showing increased prevalence rates of depression in type 2 diabetes (T2DM) [1]. Depression can be linked to worsened self-care in diabetes [2,3] and has been shown to decrease adherence to diabetic treatments [4]. Furthermore, depression has been shown to be associated with poor glycaemic control [5] and the development of diabetic complications [6]. Thus, the successful treatment of depression in people with comorbid depression and T2DM can be important to improving both physical and mental health.
National and international healthcare guidelines [7], [8], [9], [10] recommend antidepressant medication as a treatment option for people with moderate to severe depression. A number of antidepressants have been shown to be effective in the general population but differ substantially in terms of side-effects [11], [12], [13]. Guidelines addressing the treatment of depression in people with physical long-term conditions are non-specific and limited [14]. A review of clinical guidelines with respect to multimorbidity notes the difficulties of applying current guidelines to patients with multiple conditions [15].
A 2012 Cochrane review [16] of randomised-controlled trials (RCTs), and a 2016 systematic review including both RCTs and observational studies [17], both found antidepressant medication to have moderate effects on decreasing depressive symptoms in individuals with T2DM and small effects on improving glycaemic control. However, follow-up periods were limited, with no medium-long term evidence available regarding safety or effectiveness. It is known that a number of commonly prescribed antidepressants cause side-effects that potentially exacerbate T2DM and/or its complications, such as weight gain [18], cardiac complications, arthralgia, gastrointestinal disturbances, sexual disfunction and visual impairment [19]. Furthermore, selective serotonin re-uptake inhibitors, which are recommended as first-line antidepressant choice [14] are cautioned for use in people with diabetes mellitus [19].
Polypharmacy is common in T2DM, given the number of metabolic factors that need to be controlled, in addition to the number of potential complications that need management [20]. The addition of one or more antidepressants adds to the potential burden and risks associated with polypharmacy. Patients with multimorbidity taking multiple medications are more likely to experience medication side-effects [21], medication interactions [22], have impaired medication adherence [23] and reduced quality of life [24]. There are no studies to the authors’ knowledge that evaluate the prescribing of antidepressants in the context of the wider diabetic pharmacological regimen.
While there is a heightened need to successfully treat depression in people with T2DM, the lack of clarity from treatment guidelines, lack of medium-long-term evidence supporting antidepressant safety and effectiveness, and increased relevance of side effects and risks from polypharmacy, makes for difficult prescribing decisions. It is important to understand current trends in antidepressant prescribing in this group, including the patient characteristics associated with antidepressant treatment. This could highlight safety issues, or alternatively, the undertreatment of depression in patients with certain characteristics.
There is consistent evidence in the general population showing variation in the prevalence of antidepressant prescribing by ethnicity, gender and socioeconomic status [25], [26], [27]. These characteristics are all associated with variation in outcomes in T2DM and its associated complications, such as CVD and renal disease [28], [29], [30]. The sociodemographic treatment gap for antidepressants has the potential to contribute to worsening physical health, in groups of individuals who are already at increased risk. Conversely, there is evidence showing increased rates of antidepressant prescribing in individuals aged 60+ [25] who may be more susceptible to antidepressants side-effects [31] that can exacerbate T2DM and its complications. Therefore, additional caution may need to be exercised when prescribing in this group. Thus, it is important to know whether the sociodemographic differences seen in the general population with depression exist in individuals with comorbid depression and T2DM.
To our knowledge there is no systematic review at present investigating antidepressant prescribing trends in adults with comorbid depression and T2DM. Our objectives were, in adults with comorbid depression and T2DM:
-
1
To determine the prevalence of antidepressant prescribing
-
2
To determine patient characteristics associated with antidepressant prescribing
-
3
To determine the patient characteristics associated with the prescription of specific antidepressant agents
2. Methods
We performed a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [32] and registered the protocol on PROSPERO prior to the commencement of screening (CRD42020182581).
2.1. Study inclusion criteria
2.1.1. Types of study
We included observational studies from routinely collected data, registry studies, cohort studies, case-control studies and cross-sectional studies. We excluded randomised controlled trials, quasi-experimental trials where patients receive an antidepressant as part of the study, systematic reviews, case studies/reports, editorials, letters and opinion pieces.
2.1.2. Participants
We included studies investigating adults (18+ years) with comorbid depression and T2DM.
Depression could be identified by clinician diagnosis, medical records, standardised interviews, or self‐reports. Where diagnostic criteria were not available, we used the authors’ definition of depression, provided depression was explicitly stated for all participants or the subgroup being used for analysis. We did not include studies where definition of depression was the prescription of an antidepressant alone.
Type 2 diabetes could be defined as an in-study clinician diagnosis, medical records, or self‐reports. The type of diabetes should have been explicitly verified as type 2, and we did not include studies where the diabetes type was ambiguous or mixed. If necessary, we accepted the authors’ definition of type 2 diabetes.
We excluded participants with mental disorders other than depression, e.g. schizophrenia or bipolar disorder.
2.1.3. Outcome
Our primary outcome was the prescription of any antidepressant medication. We included antidepressant prescriptions defined through self-report, prescription records or clinician report. We excluded antidepressant prescriptions explicitly indicated for conditions other than depression.
Our secondary outcome was the individual antidepressant medication or medication class.
2.1.4. Comparison
Our comparison group for the primary outcome was no antidepressant treatment. For the secondary outcome a comparison was made between different antidepressant classes.
2.1.5. Exposures
We did not limit the potential patient characteristics that could be associated with antidepressant prescription: these included sociodemographic factors, depression severity, glycaemic control, comorbidities and polypharmacy.
2.2. Search strategy
We used the following sources from inception to 10-May-2021 for the identification of studies:
-
•
MEDLINE
-
•
EMBASE
-
•
Scopus
-
•
CINAHL Plus
-
•
Web of Science
-
•
PsychInfo
-
•
PsycExtra
-
•
Open Grey
Databases were searched using Medical Subject Headings (MeSH) and keywords for: depression AND type 2 diabetes AND antidepressant.
Articles in the following languages were eligible for inclusion: English, French, Spanish, Italian, Portuguese, Greek.
In addition, we reviewed references of all studies screened at full text stage and all relevant systematic reviews found during the search.
2.3. Data collection and analysis
2.3.1. Screening and extraction
The first (AJ) and third (EF) authors independently screened all titles and abstracts against the eligibility criteria. The first (AJ) and second (LM) review authors independently carried out full-text screening of studies that potentially met our inclusion criteria.
We included studies that used data from the same dataset, if they reported a unique outcome/exposure. If more than one study reported
the same outcome/exposure, we included the study with the most generalisable population for that outcome, larger sample size and lower risk of bias.
The first review author (AJ) carried out data extraction from included articles and data extraction tables were be checked by the second review author (LM).
Any disagreement was resolved through consensus.
2.3.2. Assessment of risk of bias
The first and second review authors (AJ, LM) used an adapted version of the Newcastle-Ottawa Scale [33] to independently assess the risk of bias for each study. We adapted the scale by combining criteria from both the cohort and case-control scales, to be appropriate for the design of the included studies.
Disagreement was resolved by consensus, or by consulting an additional reviewer (JH) when this was not possible. We decided not to exclude studies with higher risk of bias from the meta-analysis due to the strict inclusion criteria of the review; however, risk of bias was considered in the interpretation of the results.
2.3.2. Measures of effect
We calculated prevalence estimates based on the percentage of eligible participants who were reported as having been prescribed an antidepressant, and 95% CIs were calculated. For categorical exposures we extracted the odds ratio (OR) and 95% CIs; for continuous exposures we extracted the mean difference (MD) and standard deviation (SD). Where measures of effect were not provided, if possible, they were calculated from the data provided. For a comparison between different antidepressant classes, selective-serotonin reuptake inhibitors (SSRIs) were used as a reference category.
2.3.3. Assessment of heterogeneity
We assessed statistical heterogeneity by visual inspection of forest plots and by the I2 statistic quantifying inconsistency across studies, where I2 > 75% indicates considerable inconsistency [34].
2.3.4. Data synthesis
Where three or more studies that were sufficiently similar reported the same outcome/exposure of interest, we conducted random effects meta-analyses for each exposure to calculate a pooled effect size with 95% confidence intervals. Otherwise, we synthesised the results narratively.
3. Results
3.1. Description of studies
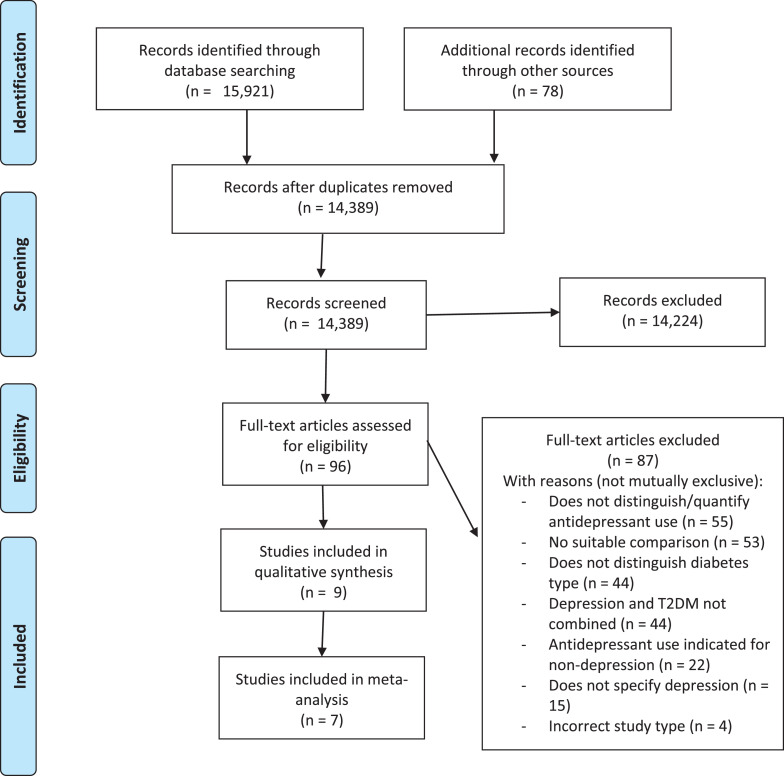
Our search yielded 14,389 unique abstracts, of which we assessed 96 full-text articles for eligibility, and selected nine studies for inclusion in the review [35], [36], [37], [38], [39], [40], [41], [42], [43]. Three studies used the same NHANES survey data [44], and so, are expected to have significant duplication of participants: Binsalah 2018 was chosen for inclusion for the primary outcome, however, did not report data on individual antidepressant medications or classes, and so, Perez 2017 and Wang 2016 were included for this outcome, each reporting on a different exposure. This resulted in at least 13,674 participants included overall (dependent on the extent of duplication between NHANES studies). A PRISMA flowchart showing study selection can be seen in Figure 1. All participants were community dwelling, with average ages ranging from 45 – 64. A summary of study characteristics and exposures can be seen in Table 1.
Figure 1.
PRISMA Flow Diagram of study selection process
Table 1.
Study characteristics
| Reference | Study Design/Setting | Study Population | Study Aim | Study Size | Country | Average Age | Female % | Ethnicity | Education | Depression severity | Insulin dependent % | Case definition | Identification of outcome | Exposures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binsalah 2018 [35] | NHANES[44] cross-sectional survey; home interviews and research centre assessment | Community dwelling | To evaluate "the association of antidepressant use with healthcare utilization in a nationally representative sample of patients with type 2 diabetes and mild to severe depressive symptoms in the United States population" | 955 | USA | 58 ± (12-13) | 66.1 | Black (17.8%); hispanic (17.1%); other (6.5%); white (58.6%) | > high school 10.8% | Mild (60.6%); moderate-severe (39.4%) | 20.0% | PHQ-9 [45]; self-reported T2DM | Self-report | Age; gender; ethnicity; education; insurance; depression severity; diabetes duration; glycaemic control; insulin use; oral antidiabetics; macrovascular/ microvascular complications |
| Brieler 2016 [36] | Retrospective cohort study; electronic medical records | Community dwelling; urban location | To determine whether the use of antidepressant medication is associated with glycaemic control in depressed patients with T2DM | 265 | USA | 61-62 ± (11-12) | 72.8 | White (51.3%) | Not available | Not available | 40.4% | ICD codes | Prescription | Age; gender; ethnicity; glycaemic control; insulin use; oral antidiabetics; hyperlipidemia; hypertension; obesity; vascular disease |
| Chen 2011 [37] | Secondary analysis of programme evaluation; data recorded by diabetes educator | Community dwelling; in diabetes education programme | To examine the effects of depression and antidepressant use on goal setting and barrier identification in patients with type 2 diabetes" | 271 | USA | 55-57 | 72 | BAME (14%); white (85.2%) [study reporting is missing 0.8%] | < high school 10%; > high school 41% | Not available | 29.5% | Self-reported depression; participation in T2DM education programme | Documented by diabetes educator | Age; gender; ethnicity; education; diabetes duration; glycaemic control; insulin use; BMI; hypertension; heart disease; neuropathy; renal disease; retinopathy; sexual disfunction |
| Higgins 2007 [38] | Retrospective cohort study; electronic medical records | Community dwelling military veterens | To examine the association of heart disease with depression and the impact of treatment with anti-depressants on this association in older males with T2DM | 691 | USA | 42% > 60 | 0 | White (85.2%) | Not available | Not available | Not available | ICD codes | Prescription | None |
| Perez 2017 [39] | NHANES[44] cross-sectional survey; home interviews and research centre assessment | Community dwelling adults; white, black and hispanic | To determine antidepressant use among Mexican Americans and non-Hispanic blacks and whites with T2DM and depressive symptoms" | 560 | USA | 45-64 | 65.6 | Black (22.2%); hispanic (11.5%); white (66.3%) | < high school 32.8%; > high school 42.3% | Mild (59.9%); moderate-severe (40.1%) | Not available | PHQ-9 [45]; self-reported T2DM | Self-report | Ethnicity |
| Shrestha 2013 [40] | Cross-sectional; insurance claimes | Community dwelling; with employee insurance; not taking insulin | To estimate excess medical expenditures associated with major depressive disorder among working-age adults diagnosed with diabetes | 10,881 | USA | 51.3-52.2 | 56.7 | Not available | Not available | Not available | Not available | Primary/secondary inpatient or outpatient encounters | Prescription | Age; gender; cerebrovascular disease; heart failure; liver disease; myocardial infarction; renal disease |
| Wagner 2009 [41] | Cross-sectional; phone interviews and research centre assessement | Community dwelling; with private insurance; urban location; | "To compare rates of discussion and treatment for depression among African Americans and Whites with diabetes" | 56 | USA | 55.7 ± 7.2 | 56.6 | White (40%) | Not available | Mean PHQ-9 = 11 | 55.4% | PHQ-9 [45]; ICD code or prescription for T2DM | Unclear - possible self-report | Ethnicity |
| Wang 2016 [42] | NHANES[44] cross-sectional survey; home interviews and research centre assessment | Community dwelling | To provide an updated, population-based estimate for the prevalence of depression in people with T2DM" | 625 | USA | 50-64 | 66.5 | Black (18.5%); hispanic (21.4%); other (17.0%); white (57.4%) | < high school 36.7%; > high school 39.0% | Mild (59.0%); moderate-severe | 25.1% | PHQ-9 [45]; self-reported T2DM | Self-report | Depression severity |
| Whitworth 2017 [43] | Prospective cohort study; research centre assessment | Community dwelling | To describe the long‐term trajectories of depression symptom severity in people with T2DM, and to identify predictors and associates of these trajectories | 178 | Australia | 58-63 ± (11-12) | 56.7 | White (51.1%) | < high school 13.6% | Mild (49.4%); moderate-severe (50.6%) | 29.7% | PHQ-9 [45]; clinical diagnosis | Unclear - possible self-report | Depression severity |
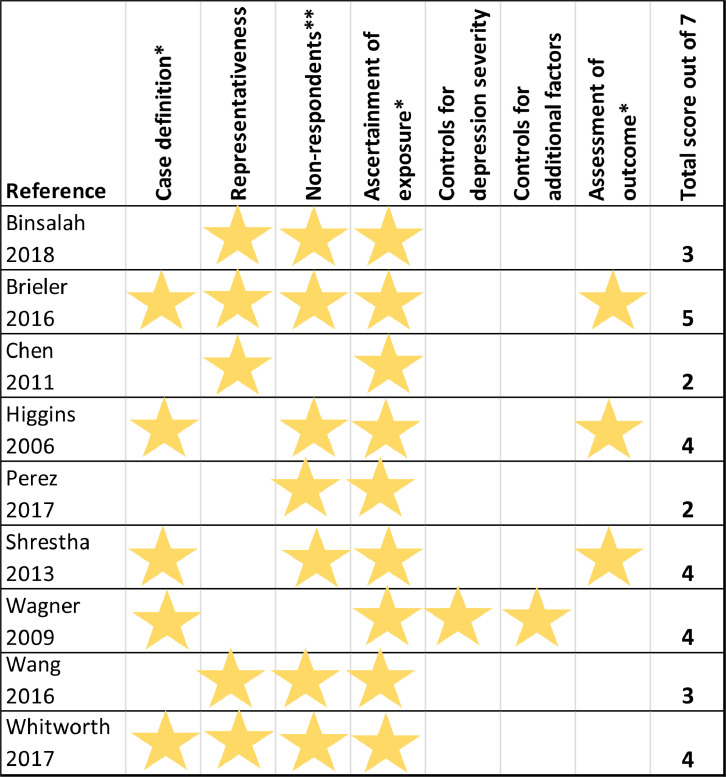
Risk of bias scores ranged from 2 to 5 out of 7, with 0 being highest risk of bias. A risk of bias summary for all included studies is shown in Figure 2.
Figure 2.
Risk of Bias Summary
Stars represent 1 point towards the total score, where studies met the criteria to have low risk of bias in each category. The higher the total score, the lower the risk of bias.
*Case definition, ascertainment of exposure and assessment of outcome all accepted in-study clinician diagnosis, validated questionnaires, medical records or prescriptions as meeting the criteria for low risk of bias. Self-report or no description did not meet the criteria for low risk of bias.
**Studies were considered to meet the criteria for low risk of bias if they made a reasonable attempt to manage non-respondents and described this
3.2. Overall prevalence of antidepressant prescribing
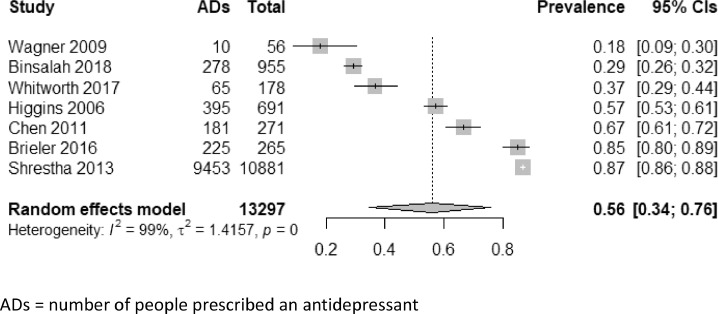
There was considerable variation in the prevalence of antidepressant prescribing across studies, which ranged from 18% - 87%. The pooled prevalence estimate from the random effects meta-analysis was 56% (95% CIs 34 – 76%), however, statistical heterogeneity was considerable, at I2 = 99% (Figure 3). Sensitivity analyses were performed according to study design, case and outcome identification, population setting, country and year. Three studies identified depression through clinical coding in health or insurance records – two of these studies had the highest prevalence rates of 85% and 87% [36,40]; the third had a relatively high prevalence rate of 0.57, however, this was recorded in a population of all-male military veterans [38]. The three studies with the lowest prevalence rates, ranging from 18% to 37% all identified depression in the community as part of the research study [35,41,43]. When combined in meta-analysis, these studies gave a pooled prevalence estimate of 29% (23% – 37%) and I2 of 62%.
Figure 3.
Forest plot and results of random effects model meta-analysis for prevalence of antidepressant prescribing
ADs = number of people prescribed an antidepressant
3.4. Depression severity
Two studies reported the prevalence of antidepressant prescribing across depression severities [35,43]. Only one of the studies showed evidence of a difference in antidepressant prescribing, with an OR of 2.30 (95% CIs 1.73-3.06) in moderate-severe compared to mild depression [35].
Only one study compared the prescription of different antidepressant classes by depression severity [42]. When comparing each antidepressant class to SSRIs there was no evidence of a difference between mild and moderate-to-severe depression.
3.5. Clinical features of diabetes
Two studies reported diabetes duration for participants prescribed antidepressants compared to those not, with no evidence of a difference [35,37].
Three studies reported the difference in glycaemic control for participants who were prescribed antidepressants compared to those not, with no evidence of a difference [35], [36], [37]. The heterogeneity of measures used meant that meta-analysis could not be performed.
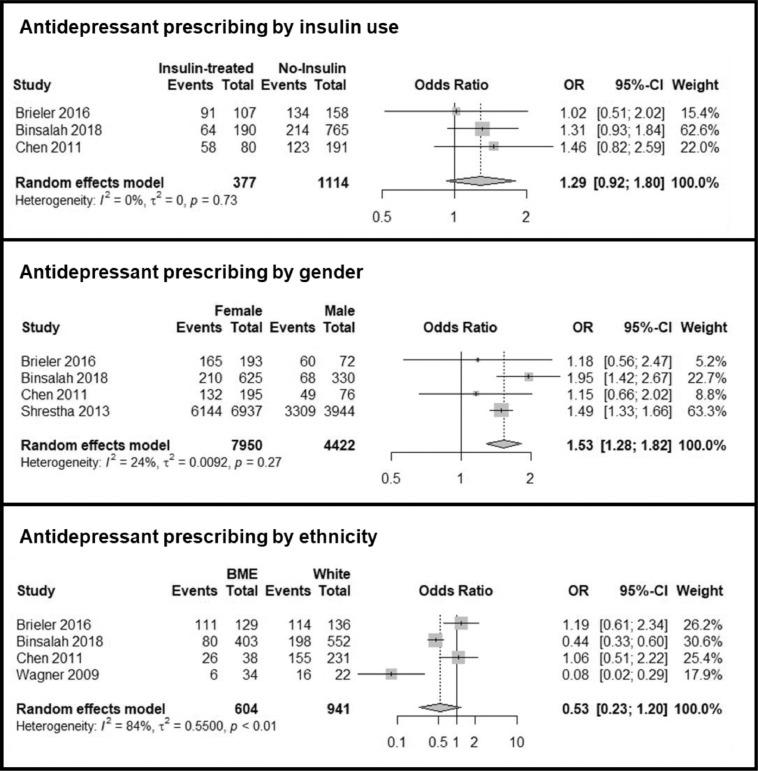
Three studies reported the prevalence antidepressant use in participants who were treated with insulin, compared to those who were not [35], [36], [37]. None of the studies showed evidence of a difference in the odds of being prescribed an antidepressant in participants with insulin controlled T2DM, with a pooled OR estimate of 1.29 (95% CIs 0.92-1.80) (Figure 4). There was no evidence of statistical heterogeneity between studies (I2 of 0%).
Figure 4.
Forest Plots and results of meta-analysis for the odds ratio of being prescribed an antidepressant, comparing different exposures.
One study reported antidepressant prevalence in those prescribed oral antidiabetic medication compared to those not prescribed any antidiabetic medication, with no evidence of a difference [36].
3.6. Comorbidities and polypharmacy
Four studies reported antidepressant prevalence across different comorbidities, listed in Table 1 [35], [36], [37],40]. Due to the difference in comorbidities reported, it was not possible to synthesise these in meta-analysis. None of the studies showed evidence of a difference in antidepressant prescribing across any comorbidity.
No studies reported if there were differences in antidepressant prescribing because of other medications prescribed, other than antidiabetic medications described above.
3.7. Demographic factors
Four studies reported on the age of participants who were prescribed antidepressants compared to those who were not, with no statistical evidence of a difference [35], [36], [37],40]. The maximum difference in median was 2 years. Insufficient information was available to perform a meta-analysis.
Four studies reported the prevalence of antidepressant prescription by gender [35], [36], [37],40]. All four studies found increased rates of antidepressant prescription in women, with a pooled OR estimate of 1.53 (95% CIs 1.28-1.82%) compared to men (Figure 4). There was low heterogeneity between studies with an I2 of 24%.
Four studies reported the prevalence of antidepressant prescribing in Black and Ethnic Minorities compared to White ethnicity [35], [36], [37],41]. However, statistical inconsistency was high, at I2 = 84%, and the pooled OR estimate of 0.53 (95% CIs 0.23-1.20%) did not show evidence of a difference between ethnicities (Figure 4).
Only one study compared the prescription of different antidepressant classes between ethnicity groups [39]. When comparing each antidepressant class to SSRIs there was no evidence of a difference between ethnicities.
Two studies reported the prevalence of antidepressant prescription by educational status, with no evidence of a difference [35,37].
One study reported the prevalence of antidepressant prescribing by insurance status, with an odds ratio of 0.43 (95% CIs 0.27 – 0.68) for those without private medical insurance, compared to those with private insurance [35].
4. DISCUSSION
4.1. Summary of results
This systematic review investigated the prevalence of antidepressant prescribing in adults with comorbid depression and T2DM, and its association with sociodemographic factors, depression severity, clinical features of diabetes, other comorbidities and polypharmacy
However, The two highest prevalence rates of antidepressant prescribing were from studies where depression was identified through clinical coding, whereas the lowest three prevalence rates were in community screened populations. Populations with depression identified through clinical coding may have more severe depression than those identified through community screening, as they may be more likely to access care [46]. Additionally, the decision to prescribe may drive the coding of depression diagnoses resulting in a tendency to see falsely high prevalence rates of prescribing using these data sources [47,48]. Thus, the prevalence estimate of 29% from the studies which identified depression through community screening, may be more representative.
An increase in antidepressant prescribing from mild to moderate-to-severe depression categories might be expected considering treatment guidelines recommend antidepressants only when depression severity is moderate-to-severe [7,8], however, this was only the case in one study. The two studies that did report antidepressant prescribing by depression severity were from different countries (USA and Australia) which may suggest a difference in prescribing practices between countries [26,27].
As no studies reported the gender difference in antidepressant prescribing adjusted for depression severity, it is not clear whether this indicates more severe depression in women, or whether more women are likely to be offered and/or accept pharmacological treatment for depression. This result is considerably lower than might be expected from the relative prevalence of antidepressant-prescribed individuals in men and women in the general population, which a large international study [27] reported as being almost double for women compared to men. This may suggest reduced gender inequalities in access to antidepressant treatment for men with comorbid depression and T2DM compared to those without diabetes, perhaps due to the increased contact with healthcare services for people with diabetes of both genders compared to those without diabetes [49].
Evidence for the association between White ethnicity and antidepressant prescription was inconclusive due to statistical heterogeneity. There is consistent evidence in the general population of a positive association between white ethnicity and increased rates of antidepressant prescribing [25], [26], [27],50]. The variation from findings in the general population, was introduced in this review by two studies that showed no evidence of an association between white ethnicity and antidepressant prescription. A large survey across 27 European countries [26] found that a key factors in the variation in antidepressant prescribing rates were cultural factors concerning beliefs and attitudes about mental health. While all studies considered in this review for ethnic disparity in antidepressant prescription were from the USA, the population and culture of the USA is varied [51], and so different attitudes could be apparent in the different populations included in these studies. On the other hand, again, the increased contact with healthcare services experienced by people with diabetes could have a positive effect on access to antidepressant treatment – indeed, the two studies with no evidence of ethnicity association were conducted in patients identified through ambulatory care or in receipt of diabetic education intervention, while the other two studies showing a higher association for white ethnicity recruited patients from outside a care setting.
While there was limited evidence concerning socio-economic status, one study reported increased rates of antidepressant prescribing in participants with insurance coverage. This suggests that there may be social inequalities in prescribing in countries where healthcare is paid for or relies on insurance that may not be available to groups who have lower socio-economic status [26].
Higher odds of antidepressant prescribing may have been expected in insulin users compared to participants not using insulin, as a 2006 meta-analysis [52] showed that insulin use was associated with increased depression symptoms in T2DM. Insulin users represent participants with more complex T2DM, who may be more likely to have increased contact time with healthcare services [49], which could provide increased opportunity to discuss antidepressant treatment. Again, however, this was not found to be the case.
4.2. Strengths and limitations
This is the first systematic review to our knowledge that investigates the prevalence and characteristics of antidepressant prescribing in adults with comorbid depression and T2DM. This review provides evidence from the first known studies to investigate patient characteristics associated with antidepressant prescription in this population.
The search terms used by the review were broad, searching seven databases to provide a wide range of coverage; this resulted in a large number (14,389) of references being screened. As the review was also exploratory, the range of potential characteristics was kept open, this enabled the review to identify evidence of a broad range of patient characteristics associated with antidepressant prescription.
Although the search terms were broad, the inclusion/exclusion criteria were strict. We only included studies that specifically identified patients as having depression, excluding those that made this assumption based on antidepressant prescription. Antidepressants can be used for conditions other than depression, such as neuropathic pain (particularly relevant to this population), sleep disorders or anxiety, and so antidepressant prescribing was not considered, for the purpose of this review, to be a confirmation of depression. Furthermore, as antidepressant prescription was the outcome of interest, this would not have been appropriate for participant inclusion criteria.
The review excluded any studies (n=22) where antidepressants were explicitly prescribed for a condition other than depression, however, while participants in the included studies met our criteria for depression, the indication of the antidepressant prescription was not always known (the exceptions being studies using NHANES data and Chen 2011). For individuals with T2DM, this is particularly relevant as a number of antidepressants could be indicated for diabetic neuropathic pain, as well as other comorbidities: This could have influenced the results for insulin use, because, as described above, insulin users have more complex disease and so are more likely to experience neuropathic pain which could lead to non-depression related prescription of antidepressants. However, this only applies to one of the studies included in our meta-analysis. Furthermore, there was no evidence of an association between insulin use or additional comorbidities and increased antidepressant prevalence.
For each exposure, only a small number of studies were identified with a maximum of four being suitable for each meta-analysis. Both meta-analyses covered a reasonable number of participants: 1,483 (insulin use) and 12,372 (gender). Despite the reasonable sample sizes, caution should be exercised when drawing conclusions from the results, taking into consideration the limited generalisability and high risk of bias of the included studies. No language restrictions were put on the literature searches and no studies were excluded based on language. However, search terms were in the English language and the databases searched primarily contain research in European languages, therefore this review may not have identified studies outside these limits.
All included studies, with one exception, were conducted in the USA. The USA operates an insurance-based healthcare system, where it has been shown that increased insurance coverage increases access to healthcare treatment [53]. The nature of an insurance-based health system is likely to impact sociodemographic inequalities [53]. The conclusions of this review, therefore, should be treated with caution, especially with regards to applicability to other types of healthcare system.
4.3. Implications
Effective treatment of depression is important in individuals with comorbid depression and T2DM. However, there was considerable variation in the prevalence of antidepressant prescribing between studies, with prevalence rates in the community considerably lower than in clinical populations. Female gender was the only predictor of antidepressant prescription for which we were able to find evidence from meta-analysis in this review, suggesting either that women with comorbid depression and T2DM are more likely to have depression requiring treatment than men, or that men are more likely to be undertreated. It is important to note, however, that the gender disparity for antidepressant prescribing is less than in the general population. White ethnicity was also not found to be associated with increased prevalence of antidepressant prescribing, while it is in the general population. This review has highlighted the gap in our knowledge about which antidepressant agents are being used in adults with comorbid depression and T2DM, and alongside which concurrent medications and comorbidities. Thus, we are unaware of the extent to which such individuals are at risk from the risks associated with polypharmacy and other adverse effects. With a lack of evidence to support the medium-long term use of antidepressants in individuals with T2DM, care should be taken when prescribing antidepressants in more complex patients, who have higher risks of potential adverse events. There is an urgent need for longitudinal studies to inform guidelines on the long-term safety and effectiveness of antidepressant prescription in persons with comorbid depression and T2DM.
Funding
This report is independent research funded by the National Institute for Health Research ARC North Thames. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Annie Jeffery, Email: annie.jeffery.09@ucl.ac.uk.
Lucy Maconick, Email: l.maconick@ucl.ac.uk.
Emma Francis, Email: emma.francis.19@ucl.ac.uk.
Kate Walters, Email: k.walters@ucl.ac.uk.
Ian C.K. Wong, Email: i.wong@ucl.ac.uk, wongick@hku.hk.
David Osborn, Email: d.osborn@ucl.ac.uk.
Joseph F. Hayes, Email: joseph.hayes@ucl.ac.uk.
References
- 1.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001 Jun;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 2.Weyerer S. Physical inactivity and depression in the community. Evidence from the Upper Bavarian Field Study. Int J Sports Med. 1992 Aug;13(6):492–496. doi: 10.1055/s-2007-1021304. [DOI] [PubMed] [Google Scholar]
- 3.McMartin SE, Jacka FN, Colman I. The association between fruit and vegetable consumption and mental health disorders: evidence from five waves of a national survey of Canadians. Prev Med. 2013 Mar;56(3-4):225–230. doi: 10.1016/j.ypmed.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008 Dec;31(12):2398-403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed]
- 5.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000 Jul;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 6.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001 Jul-Aug;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 7.The National Institute for Health and Care Excellence. Depression in adults: recognition and management, https://www.nice.org.uk/guidance/cg90/chapter/Recommendations#step-1-recognition-assessment-and-initial-management; 2009 [accessed 18 July 2021]. [PubMed]
- 8.World Health Organisation. mhGAP Intervention Guide. https://apps.who.int/iris/bitstream/handle/10665/250239/9789241549790-eng.pdf?sequence=1&isAllowed=y; 2016 [accessed 18 July 2021].
- 9.American Psychological Association. Clinical Practice Guideline for the Treatment of Depression Across Three Age Cohorts, https://www.apa.org/depression-guideline/guideline.pdf; 2019 [accessed 18 July 2021].
- 10.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R, CANMAT Depression Work Group Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry. 2016 Sep;61(9):540–560. doi: 10.1177/0706743716659417. Epub 2016 Aug 2. Erratum in: Can J Psychiatry. 2017 May;62(5):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD003012.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, Kerse N, Macgillivray S. Antidepressants versus placebo for depression in primary care. Cochrane Database Syst Rev. 2009 Jul 8;(3) doi: 10.1002/14651858.CD007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayner L, Price A, Evans A, Valsraj K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev. 2010 Mar 17;(3):CD007503. doi: 10.1002/14651858.CD007503.pub2. [DOI] [PubMed]
- 14.National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: recognition and management, https://www.nice.org.uk/guidance/cg91; 2009 [accessed 18 July 2021].
- 15.Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, Alderson P, Thompson A, Payne K, Guthrie B. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015 Mar 11;350 doi: 10.1136/bmj.h949. h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev. 2012 Dec 12;12 doi: 10.1002/14651858.CD008381.pub2. [DOI] [PubMed] [Google Scholar]
- 17.van der Feltz-Cornelis CM, Nuyen J, Stoop C, Chan J, Jacobson AM, Katon W, Snoek F, Sartorius N. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2010 Jul-Aug;32(4):380–395. doi: 10.1016/j.genhosppsych.2010.03.011. Epub 2010 May 15. Erratum in: Gen Hosp Psychiatry. 2010 Nov-Dec;32(6):645. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence. Mirtazapine, https://bnf.nice.org.uk/drug/mirtazapine.html; 2021 [accessed 18 July 2021].
- 19.National Institute for Health and Care Excellence. Sertraline, https://bnf.nice.org.uk/drug/sertraline.html; 2021 [accessed 18 July 2021].
- 20.National Institute for Health and Care Excellence. Diabetes - type 2: Scenario: Management – adults, https://cks.nice.org.uk/topics/diabetes-type-2/management/management-adults/; 2021 [accessed 18 July 2021].
- 21.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004 Jul 3;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med. 2015 Apr 7;13:74. doi: 10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence, www.nice.org.uk/CG76; 2009 [accessed 18 July 2021]. [PubMed]
- 24.The King's Fund. Polypharmacy and Medicines Optimisation, https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/polypharmacy-and-medicines-optimisation-kingsfund-nov13.pdf; 2013 [accessed 18 July 2021].
- 25.Pratt LA, Brody DJ, Gu Q. Antidepressant Use Among Persons Aged 12 and Over:United States,2011-2014. NCHS Data Brief. 2017 Aug;(283):1–8. [PubMed] [Google Scholar]
- 26.Lewer D, O'Reilly C, Mojtabai R, Evans-Lacko S. Antidepressant use in 27 European countries: associations with sociodemographic, cultural and economic factors. Br J Psychiatry. 2015 Sep;207(3):221–226. doi: 10.1192/bjp.bp.114.156786. Epub 2015 Jul 9. [DOI] [PubMed] [Google Scholar]
- 27.Abbing-Karahagopian V, Huerta C, Souverein PC, de Abajo F, Leufkens HG, Slattery J, Alvarez Y, Miret M, Gil M, Oliva B, Hesse U, Requena G, de Vries F, Rottenkolber M, Schmiedl S, Reynolds R, Schlienger RG, de Groot MC, Klungel OH, van Staa TP, van Dijk L, Egberts AC, Gardarsdottir H, De Bruin ML. Antidepressant prescribing in five European countries: application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur J Clin Pharmacol. 2014 Jul;70(7):849–857. doi: 10.1007/s00228-014-1676-z. [DOI] [PubMed] [Google Scholar]
- 28.Julin B, Willers C, Leksell J, Lindgren P, Looström Muth K, Svensson AM, Lilja M, Dahlström T. Association between sociodemographic determinants and health outcomes in individuals with type 2 diabetes in Sweden. Diabetes Metab Res Rev. 2018 May;34(4):e2984. doi: 10.1002/dmrr.2984. Epub 2018 Mar 6. [DOI] [PubMed] [Google Scholar]
- 29.Walker RJ, Strom Williams J, Egede LE. Influence of Race, Ethnicity and Social Determinants of Health on Diabetes Outcomes. Am J Med Sci. 2016 Apr;351(4):366–373. doi: 10.1016/j.amjms.2016.01.008. doi: 10.1016/j.amjms.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016 Jun;37(3):278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeVane CL, Pollock BG. Pharmacokinetic considerations of antidepressant use in the elderly. J Clin Psychiatry. 1999;60(Suppl 20):38–44. [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp; [accessed 18 July 2021].
- 34.Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002 Jan;7(1):51–61. doi: 10.1258/1355819021927674. [DOI] [PubMed] [Google Scholar]
- 35.Binsaleh AY, Perez A, Popovici I, Rabionet SE. Impact of Antidepressant Use on Healthcare Utilization among Individuals with Type 2 Diabetes and Depression Symptoms in the United States: Sociodemographic, Clinical, and Behavioral Factors Matter. Int J Environ Res Public Health. 2018 Sep 1;15(9):1904. doi: 10.3390/ijerph15091904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brieler JA, Lustman PJ, Scherrer JF, Salas J, Schneider FD. Antidepressant medication use and glycaemic control in co-morbid type 2 diabetes and depression. Fam Pract. 2016 Feb;33(1):30–36. doi: 10.1093/fampra/cmv100. [DOI] [PubMed] [Google Scholar]
- 37.Chen HY, Ruppert K, Charron-Prochownik D, Noullet WV, Zgibor JC. Effects of depression and antidepressant use on goal setting and barrier identification among patients with type 2 diabetes. Diabetes Educ. 2011 May-Jun;37(3):370–380. doi: 10.1177/0145721711400662. [DOI] [PubMed] [Google Scholar]
- 38.Higgins TS, Jr, Ritchie CS, Stetson BA, Burke JD, Looney SW. An examination of the moderating effect of treatment with anti-depressants on the association of heart disease with depression in males with type 2 diabetes attending a Veterans Affairs Medical Center. Diabetes Res Clin Pract. 2007 Feb;75(2):220–228. doi: 10.1016/j.diabres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Perez A, Cabrera P, Gutierrez C, Valdes J. A Comparison of the Use of Antidepressant Treatment Between Non-Hispanic Black and White and Mexican American Adults With Type 2 Diabetes in the United States. Diabetes Educ. 2017 Apr;43(2):171–179. doi: 10.1177/0145721717697191. [DOI] [PubMed] [Google Scholar]
- 40.Shrestha SS, Zhang P, Li R, Thompson TJ, Chapman DP, Barker L. Medical expenditures associated with major depressive disorder among privately insured working-age adults with diagnosed diabetes in the United States, 2008. Diabetes Res Clin Pract. 2013 Apr;100(1):102–110. doi: 10.1016/j.diabres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner JA, Perkins DW, Piette JD, Lipton B, Aikens JE. Racial differences in the discussion and treatment of depressive symptoms accompanying type 2 diabetes. Diabetes Res Clin Pract. 2009 Nov;86(2):111–116. doi: 10.1016/j.diabres.2009.08.004. doi: 10.1016/j.diabres.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Lopez JM, Bolge SC, Zhu VJ, Stang PE. Depression among people with type 2 diabetes mellitus, US National Health and Nutrition Examination Survey (NHANES), 2005-2012. BMC Psychiatry. 2016 Apr 5;16:88. doi: 10.1186/s12888-016-0800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitworth SR, Bruce DG, Starkstein SE, Davis WA, Davis TME, Skinner TC, Bucks RS. Depression symptoms are persistent in Type 2 diabetes: risk factors and outcomes of 5-year depression trajectories using latent class growth analysis. Diabet Med. 2017 Aug;34(8):1108–1115. doi: 10.1111/dme.13372. [DOI] [PubMed] [Google Scholar]
- 44.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2. 2013 Sep;(161):1–24. [PubMed] [Google Scholar]
- 45.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howren A, Aviña-Zubieta JA, Puyat JH, Esdaile JM, Da Costa D, De Vera MA. Defining Depression and Anxiety in Individuals With Rheumatic Diseases Using Administrative Health Databases: A Systematic Review. Arthritis Care Res (Hoboken) 2020 Feb;72(2):243–255. doi: 10.1002/acr.24048. [DOI] [PubMed] [Google Scholar]
- 47.Konrad R, Zhang W, Bjarndóttir M, Proaño R. Key considerations when using health insurance claims data in advanced data analyses: an experience report. Health Syst (Basingstoke) 2019 Mar 1;9(4):317–325. doi: 10.1080/20476965.2019.1581433.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldberg RJ, Oxman TE. Billing for the Evaluation and Treatment of Adult Depression by the Primary Care Clinician. Prim Care Companion J Clin Psychiatry. 2004;6(1):21–26. doi: 10.4088/pcc.v06n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni Y, Liu S, Li J, Li S, Dong T. Patient-perceived service needs and health care utilization in people with type 2 diabetes: A multicenter cross-sectional study. Medicine (Baltimore) 2020 May 22;99(21):e20322. doi: 10.1097/MD.0000000000020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009 Aug;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 51.Franks P, Gold MR, Fiscella K. Sociodemographics, self-rated health, and mortality in the US. Soc Sci Med. 2003 Jun;56(12):2505–2514. doi: 10.1016/s0277-9536(02)00281-2. [DOI] [PubMed] [Google Scholar]
- 52.Bai X, Liu Z, Li Z, Yan D. The association between insulin therapy and depression in patients with type 2 diabetes mellitus: a meta-analysis. BMJ Open. 2018 Nov 28;8(11) doi: 10.1136/bmjopen-2017-020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Healthcare in America, https://www.cdc.gov/nchs/data/misc/healthcare.pdf; 2003 [accessed 18 July 2021].