Abstract
Background
The coexistence of sarcopenia and dementia in aging populations is not uncommon, and they may share common risk factors and pathophysiological pathways. This study aimed to evaluate the relationship between brain atrophy and low lean mass in the elderly with impaired cognitive function.
Methods
This cross-sectional study included 168 elderly patients who visited the multi-disciplinary dementia outpatient clinic at Kaohsiung Chang Gung Memorial Hospital for memory issues, between 2017 and 2019. The body composition was assessed by dual energy X-ray absorptiometry (DEXA) and CT based skeletal muscle index including L3 skeletal muscle index (L3SMI) and masseter muscle mass index (MSMI). The brain atrophy assessment was measured by CT based visual rating scale. Possible predictors of low lean mass in the elderly with cognitive impairement were identified by binary logistic regression. ROC curves were generated from binary logistic regression.
Results
Among the 81 participants, 43 (53%) remained at a normal appendicular skeletal muscle index (ASMI), whereas 38 (47%) showed low ASMI. Compared with the normal ASMI group, subjects with low ASMI exhibited significantly lower BMI, L3SMI, and MSMI (all p < 0.05), and showed significant brain atrophy as assessed by visual rating scale (p < 0.001). The accuracy of predictive models for low ASMI in the elderly with cognitive impairment were 0.875, (Area under curve (AUC) = 0.926, 95% confidence interval [CI] 0.844–0.972) in model 1 (combination of BMI, GCA and L3SMI) and 0.885, (Area under curve (AUC) = 0.931, [CI] 0.857–0.979) in model 2 (combination of BMI, GCA and MSMI).
Conclusions
Global cortical atrophy and body mass index combined with either L3 skeletal muscle index or masseter skeletal muscle index can predict low lean mass in the elderly with cognitive impairment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02626-8.
Keywords: Sarcopenia, Body composition, Cognitive impairement, Dementia, Brain atrophy, Computed tomography imaging
Introduction
Dementia is characteristic of progressive cognitive decline involving one or more of the cognitive domains. As the population ages, the number of patients with dementia also increases, resulting in extensive burdens on personal health, familial relationships, economies, and societies in general [1]. There is growing evidence indicating a complex interrelationship between dementia and risk factors and certain non-communicable diseases, including physical inactivity, malnutrition, smoking, alcohol use, mild cognitive impairment, lack of anabolic hormones, persistent inflammatory reactions, diabetes, depression, and hypertension [2]. In aging populations, some of the known risks may present simultaneously, such as cognitive decline, physical inactivity, malnutrition, and sarcopenia, the amalgamation of which may accelerate the brain’s aging process [3, 4]. Clarifying the potential links and interactions by using available tools of measurement could help to prevent or alleviate adverse outcomes in aging/super-aged societies.
The prevalence of sarcopenia is significantly higher in individuals with cognitive impairment/dementia [5]. Furthermore, recent studies have identified it as a significant risk factor for cognitive deterioration [6]. Sarcopenia is associated with increased rates of functional impairment, disability, falls, and frailty, resulting in adverse health events [7, 8]. The coexistence of sarcopenia, cognitive impairment, and brain atrophy in aging populations may indicate shared common risk factors and pathophysiological pathways [8]. A recent study reported that physical frailty is associated with a longitudinal decline in global cognitive function in non-demented older adults, while cognitive impairment could accelerate physical impairment and negative outcomes in older persons [9]. Other studies suggest sarcopenia and low muscle mass are linked to cognitive impairment/dementia and brain atrophy [10–13]. However, evidence of an association between sarcopenia/low muscle mass and cognitive impairment/dementia among the elderly remains inconsistent and lacking [14]. Although several brain-muscle axes have been proposed, the precise mechanism remains unclear and controversial [15, 16]. Accumulating evidence indicates that non-pharmacological treatments such as optimized nutrition and physical activity can be valuable countermeasures to both sarcopenia/low muscle mass and dementia/mild cognitive impairment (MCI) in terms of treatment and prevention [17–22]. Therefore, understanding the relationship between sarcopenia and dementia, and the early detection of risk factors and signs, are essential for early intervention in order to maintain or decrease the rate of disease progression.
Currently, dual energy X-ray absorptiometry (DEXA) is considered the gold standard for the diagnosis of sarcopenia, and is recommended by the Asian Working Group for Sarcopenia (AWGS) 2019 [23, 24]. In addition, quantification of skeletal muscle and masseter muscle by using CT could effectively represent nutritional status and physical activity [25, 26]. Meanwhile, CT is also becoming a useful imaging tool for dementia assessment. Although MRI is more precise in the evaluation of brain volume, several reliable measurements using CT study including visual rating scales for assessing brain atrophy in aging and neurodegenerative diseases are now widely applied [27–32]. Several studies have also shown a significant correlation between whole-body muscle, lumbar skeletal muscle, and masseter muscle [33–38]. However, the association between sarcopenia and brain volume-related cognitive impairment remains unclear. Revealing the possible common risk factors, as well as potential relationships and pathophysiology may be helpful for the early detection and prevention of adverse events in aging societies.
In this study, we aimed to determine: 1) The possible risk factors and relationship between sarcopenia/low muscle mass and cognitive function impairment; 2) The association between body composition and brain atrophy; 3) To establish a model to accurately predict sarcopenia/low muscle mass in elderly Taiwanese with impaired cognitive function, which may be helpful for early risk detection to facilitate non-pharmacological intervention, improve quality of life, and reduce overall healthcare costs.
Materials and methods
Subjects
This cross-sectional study was conducted during the period of 2017 to 2019. All study participants visited the multi-disciplinary dementia outpatient clinic at Kaohsiung Chang Gung Memorial Hospital complaining of memory issues, accompanied by their family member(s). The demographic data and family history were recorded, and physical and neurological examinations were performed. All participants were evaluated by the consensus of a panel composed of neurologists, psychiatrists, and neuropsychologists to determine the presence or absence of dementia and its severity using the Clinical Dementia Rating (CDR). All participants consented to undergo cerebral and abdominal CT and dual-energy x-ray absorptiometry (DEXA). The study participants were selected according to the following criteria: age ≥ 65 years, CDR ≥ 0.5, no difficulty performing basic activities of daily living (ADL), no active treatment for cancer in the prior 3 years, and only cases with no hematoma, brain tumor, acute stroke, or post-infarction encephalomalacia resulting in mass effect or asymmetry affecting the ventricular system shape or subarachnoid space volume [39]. Patients with history of other neurologic or psychiatric illness, psychotropic medication usage, or substance abuse were excluded. Participants were divided into two groups (normal and low ASMI groups) according to the cut-off values (< 7.0 kg/m2 in men, and < 5.4 kg/m2 in women) for ASMI by AWGS 2019 reference. (See Supplemental Fig. 1) [23].
Measurements of hand grip strength and gait speed
Hand grip strength (kg) was measured using a digital hand dynamometer. The grip strength of each hand was assessed twice; the maximal value of each hand was averaged as the final estimate of hand grip strength for analysis. The gait speed (m/s) was measured using a 6-m walking test at the usual pace.
Head computed tomography (CT) data acquisition
All patients were in the supine position, with unenhanced CT scans performed of the vertex to base of skull using 256-row helical scanners with slice thickness of 5 mm, 120 kV, 225mAs. The scan angle was determined by the orbitomeatal line.
Visual rating of cerebral atrophy
Visual rating of clinical brain images was performed independently by three physicians, including a 2nd-year radiology resident (YTC) and two neuroimaging specialists (CCY and WCL), blind to all clinical information, using a 4-point global cortical atrophy (GCA) scale (See Supplement 1, Additional file 1 [40, 41]). A definite score would be discussed and assigned by three raters only when disputable results occurred.
Brain atrophy parameters of cerebral atrophy
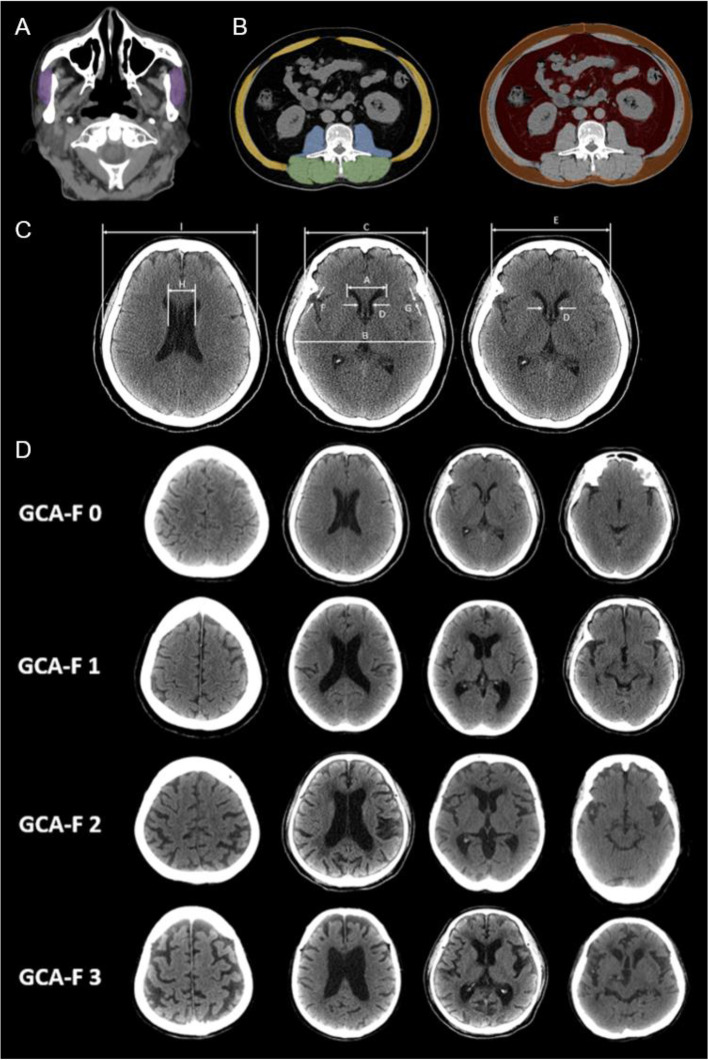
The brain atrophy parameters were measured on the CT scans, based on the commonly used method described by Meese [42], including Evans index (EI), Frontal horn index (FHI), Bicaudate ratio (BCR), Sylvian fissure ratio (SFR), Schiersmann index, and Huckman number (Fig. 1). Every parameter was measured twice, and the mean value was calculated to increase accuracy and limit the “partial volume” effect [39].
Fig. 1.
CT image analysis of body composition and brain atrophy. A. Head CT analysis of masseter muscle area. (Purple area: masseter muscle). B. Abdominal CT analysis of skeletal muscle area at third lumbar vertebral level. (Yellow area: abdominal wall muscle; Blue area: psoas muscle; Green area: paraspinal muscle; Orange area: subcutaneous adipose tissue; Red area: visceral adipose tissue). C. Measurements of brain atrophy parameters: A, B, C, D, E, F, G, H, I, in head CT images. Evans index (EI): ratio of maximum width of the frontal horns of the lateral ventricles (A) to the maximal internal diameter of the skull; Frontal horn index (FHI): ratio of the greatest external diameter of the frontal bone (C) to the greatest distance between the frontal horns at the same line (A); Bicaudate ratio (BCR): ratio of the maximum caudate nuclei distance (D) to distance between inner tables of skull at the same line (E); (B) at the same level; Sylvian fissure ratio (SFR): sum of bilateral width of the insular cisterns (in mm) (F)(G); Schiersmann index: ratio of the lateral ventricles greatest distance at the level of cella media (H) to distance between outer tables of skull (I) at the same line; Huckman number: sum of maximum width of the frontal horns of the lateral ventricles (in mm) (A) and the maximum caudate nuclei distance (in mm) (D). D. Examples of frontal cortical atrophy (F-GCA) scale. GCA 0: normal sulci and ventricle; GCA 1: slight widening of sulci with mild ventricular enlargement; GCA 2: gyral volume loss with moderate ventricular enlargement; GCA 3: pronounced widening of sulci with severe volume loss (knife blade atrophy) and severe ventricular enlargement
Head CT analysis of masseter cross-sectional area
Bilateral masseter cross-sectional area was quantified by evaluating axial measurements 2 cm below the zygomatic arch, as previously demonstrated in a study by Hu et al. Manual region of interest (ROI) was performed twice by primary author (YTC) under the supervision of a neuroradiologist with 3 years’ experience and the mean value was calculated. The average area of both masseter muscles was used for analysis (Fig. 1) [36, 43].
Abdominal computed tomography (CT) data acquisition
All patients were in the supine position with unenhanced CT scans performed of the lower thorax to the pelvis using 256-row helical scanners, with slice thickness of 5 mm, 120 kV, 100–200 mAs.
Abdominal CT analysis of body composition
Three consecutive slices at the L3 vertebral body level were included, and measurements including area and attenuation of skeletal muscle and adipose tissue were averaged over these three images, as previously reported (See Supplement 2, Additional file 1 [44]) (Fig. 1). All measurements were taken by a 3rd-year radiology resident under the supervision of a musculoskeletal radiologist with 6 years’ experience [44].
Dual energy x-ray absorptiometry (DEXA) examinations
Dual energy x-ray absorptiometry (DEXA) was used to estimate muscle mass. Subjects were clinically classified as low appendicular skeletal muscle mass (ASMI) according to appendicular skeletal muscle index (ASMI; ASM/height2) < 7.0 kg/m2 for men and < 5.4 kg/m2 for women, as recommended by the Asian Working Group for Sarcopenia (AWGS). In total, 38 low ASMI and 43 normal ASMI subjects participated in the study.
Statistical analyses
Analyses were conducted using SPSS software (SPSS V.23, Chicago, IL, USA) and Stata software (Stata V.16.0). The demographic data, including age, sex, and CDR data were compared among the study groups using the 2-sample Student t-test, Pearson chi-square test, and Fisher’s exact test, and were reported as mean ± standard deviation (SD). The significance of differences in other demographic data, brain atrophy severity, and the body composition of the adipose tissue and muscle area were analyzed by analysis of covariance (ANCOVA) with the participant’s age and sex as covariates. Partial correlation analysis was performed with age and sex adjustments to determine associations among the body composition and brain atrophy visual rating scale with low ASMI. The threshold for statistical significance was set at p < 0.05. Factors associated with ASMI were analyzed using univariate logistic regression analysis, with the statistical significance set at p < 0.1. The significant factors in the univariate analysis were further analyzed using stepwise selection method with entry testing of binary logistic regression, adjusting for age and sex, to identify the predictors and calculate the odds ratios (OR) with 95% confidential intervals (95% CI). The ROC curves were constructed using the predictions (i.e., BMI, GCA, L3SMI, MSMI, model 1 (combination of BMI, GCA and L3SMI), and model 2 (combination of BMI, GCA and MSMI)) from the stepwise binary logistic regression model. Finally, Stata software was used to compare the two ROC curves with the statistical significance set at p < 0.05.
Results
Participants were given an Institutional Review Board-approved study information sheet explaining the project objectives, and inclusion and exclusion criteria. Participants with age ≥ 65 years, and CDR ≥ 0.5 were included in our study. The exclusion criteria included age < 65 years, CDR = 0, having difficulty performing basic activities of daily living, under treatment for cancer in the prior 3 years, brain CT showed an organic brain lesion such as hematoma, brain tumor, acute stroke, or post-infarction encephalomalacia resulting in mass effect or asymmetry affecting the ventricular system shape or subarachnoid space volume, and missing data involving abdominal and brain CT, DEXA, demographic data and CDR [23]. Of 168 patients, 81 patients met the entry criteria to participate in the study and were divided into two groups (normal and low ASMI groups) according to the cut-off values for ASMI by AWGS 2019 reference. (See Supplemental Fig. 1).
Demographic and clinical characteristics
The demographic and clinical data of the participants are shown in Table 1. The low ASMI and normal ASMI groups had similar mean age, gender distribution, and clinical dementia rating (CDR) scale level (age: p = 0.13; gender: p = 0.18; CDR: p = 1.00). There were no significant group differences in body height, grip strength, or gait speed (height: p = 0.10; grip strength: p = 0.73; gait speed: p = 0.78).
Table 1.
Demographic characteristics
| Low ASMI (n = 38)3 | Normal ASMI (n = 43)3 | p4 | |
|---|---|---|---|
| Clinical demographic data1 | |||
| Age (years) | 77.6 ± 4.8 | 75.6 ± 6.7 | 0.128 |
| Gender (%) | |||
| Male | 17 | 13 | 0.177 |
| Female | 21 | 30 | |
| Height (cm) | 155.6 ± 8.7 | 152.2 ± 7.6 | 0.101 |
| Body weight (kg) | 54.1 ± 8.6 | 60.8 ± 10.6 | < 0.001* |
| BMI (kg/m2) | 22.3 ± 3.0 | 26.2 ± 4.0 | < 0.001* |
| Grip strength (kg) | 21.5 ± 8.9 | 20.6 ± 7.3 | 0.731 |
| Gait speed (m/s) | 0.9 ± 0.4 | 1.0 ± 0.3 | 0.777 |
| CDR | |||
| 0.5 | 31 | 36 | 1.000 |
| 1 | 5 | 5 | |
| 2 | 2 | 2 | |
| Assessments of brain atrophy2 | |||
| Visual rating scales | |||
| GCA | 26 ± 4 | 20 ± 4 | < 0.001* |
| Brain atrophy index | |||
| EI | 0.35 ± 0.04 | 0.35 ± 0.04 | 0.439 |
| FHI | 0.28 ± 0.03 | 0.28 ± 0.03 | 0.247 |
| BCR | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.525 |
| SFR | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.734 |
| Schiersmann index | 0.29 ± 0.04 | 0.28 ± 0.04 | 0.652 |
| Huckman index | 57.50 ± 8.46 | 56.10 ± 8.20 | 0.884 |
| Assessments of body composition2 | |||
| Dual energy x-ray absorptiometry | |||
| ASMI (kg/m2) | 5.54 ± 0.77 | 6.50 ± 0.83 | < 0.001* |
| Cross-sectional CT image at the L3 vertebral body level | |||
| Adipose tissue index (cm2/m2) | |||
| Visceral adipose tissue | 48.4 ± 28.5 | 63.0 ± 29.3 | 0.008* |
| Subcutaneous adipose tissue | 29.4 ± 20.3 | 51.9 ± 36.8 | 0.001* |
| Skeletal muscle index (cm2/m2) | |||
| Abdominal wall muscle | 16.1 ± 3.3 | 19.2 ± 3.9 | < 0.001* |
| Paraspinal muscle | 14.8 ± 2.7 | 17.1 ± 2.9 | < 0.001* |
| Psoas muscle | 4.7 ± 1.6 | 5.3 ± 1.7 | 0.041* |
| Total muscle | 35.5 ± 5.6 | 41.7 ± 6.6 | < 0.001* |
| Assessments of body composition2 | |||
| Cross-sectional CT image at the L3 vertebral body level | |||
| Attenuation (HU) | |||
| Visceral adipose tissue | −82.4 ± 15.5 | −88.9 ± 12.2 | 0.053 |
| Subcutaneous adipose tissue | −108.9 ± 6.5 | − 108.7 ± 5.5 | 0.794 |
| Abdominal wall muscle | 21.2 ± 9.7 | 21.3 ± 8.8 | 0.933 |
| Paraspinal muscle | 32.6 ± 6.6 | 30.5 ± 8.6 | 0.074 |
| Psoas muscle | 39.8 ± 4.3 | 41.0 ± 4.7 | 0.283 |
| Total muscle | 28.4 ± 7.2 | 27.7 ± 7.6 | 0.416 |
| Cross- sectional CT image at 2 cm below the zygomatic arch | |||
| Masseter muscle index | 118.2 ± 25.4 | 152.75 ± 27.07 | < 0.001* |
Demographic data, cognitive function level, and assessments of brain atrophy and body composition in elderly patients with low ASMI and normal ASMI subjects
Abbreviations: ASMI Appendicular skeletal muscle index, BCR Bicaudate ratio, BMI Body Mass Index, CDR Clinical dementia rating scale, EI Evans index, FHI Frontal horn index, GCA Global cortical atrophy, SFR Sylvian fissure ratio
1Sex data were compared by Pearson chi-square test. CDR data were compared by Fisher’s exact test. Age data were compared by independent t test. The others demographic data were compared by analysis of covariance (ANCOVA) after controlling for age and sex
2Visual rating scales, brain atrophy index, and cross-sectional CT image data were compared by ANCOVA after controlling for age and sex
3Data are presented as mean ± standard deviation; 4* p < 0.05
Assessments of brain atrophy
Table 1 demonstrates the significant differences between the two groups in terms of the Global cortical atrophy (GCA) scale (Low ASMI: 26 ± 4; Normal ASMI: 20 ± 4; p < 0.001), after controlling for sex and age. No statistically significant differences were found in other brain atrophy parameters, including the Evans index (p = 0.44), Frontal horn index (p = 0.25), Bicaudate ratio (p = 0.53), Sylvian fissure ratio (p = 0.73), Schiersmann index (p = 0.65), and Huckman index (p = 0.88) between the two groups.
Assessments of body composition
The skeletal muscle index (SMI) and adipose tissue index (ATI) were compared between the two groups (Table 1). Significant differences between the two groups were identified in the SMI of the abdominal muscle (Low ASMI: 16.1 ± 3.3; Normal ASMI: Mean:19.2 ± 3.9; p < 0.001), paraspinal muscle (Low ASMI: 14.8 ± 2.7; Normal ASMI: 17.1 ± 2.9; p < 0.001), psoas muscle (Low ASMI: 4.7 ± 1.6; Normal ASMI: 5.3 ± 1.7; p = 0.041), total abdominal muscle (Low ASMI: 35.5 ± 5.6; Normal ASMI: 41.7 ± 6.6; p < 0.001), and masseter muscle (Low ASMI: 118.2 ± 25.4; Normal ASMI: 152.8 ± 27.1; p < 0.001), as well as the ATI of the visceral adipose tissue (VAT) (Low ASMI: 48.4 ± 28.5; Normal ASMI: 63.0 ± 29.3; p = 0.008) and subcutaneous adipose tissue (SAT) (Low ASMI: 29.4 ± 20.3; Normal ASMI: 51.9 ± 36.8; p = 0.001), after controlling for age and sex. There were no significant differences in attenuation of the skeletal muscle or adipose tissue between the two groups.
Associations between ASMI and body composition parameter and visual rating scale
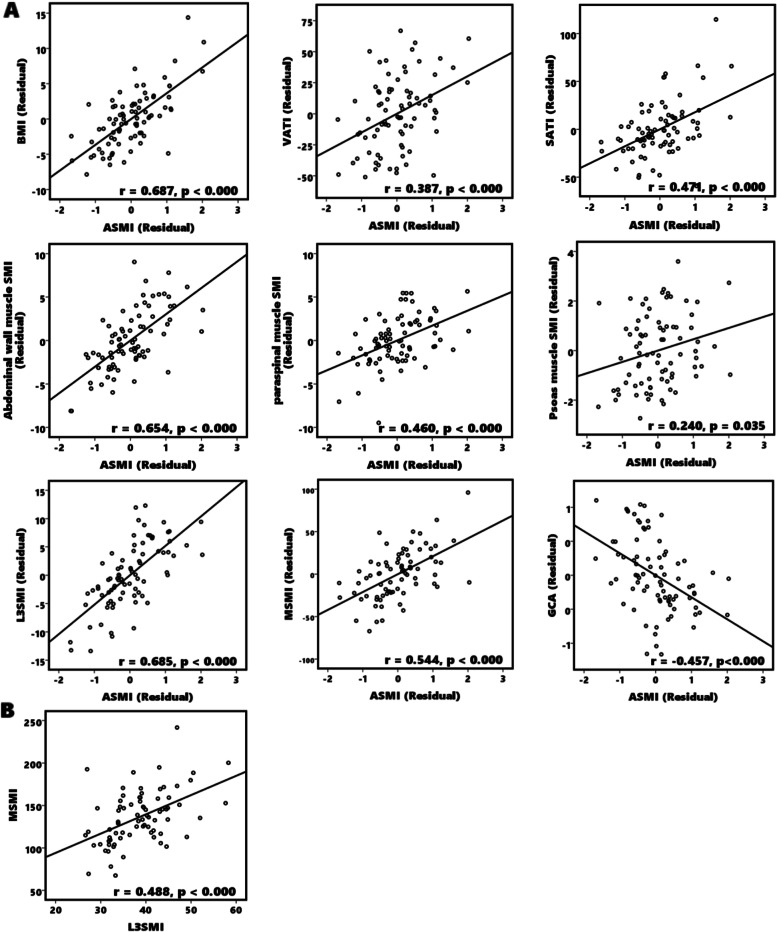
Figure 2 outlines the correlations between ASMI and BMI, skeletal muscle index, adipose tissue index, and the GCA by using a partial correlation analysis controlling for age and sex. A higher ASMI was positively correlated with higher BMI (correlation confident(r) = 0.687, p < 0.001), visceral adipose tissue index (r = 0.387, p < 0.001), subcutaneous adipose tissue index (r = 0.471, p < 0.001), abdominal muscle index (r = 0.654, p < 0.001), paraspinal muscle index (r = 0.460, p < 0.001), psoas muscle index (r = 0.240, p < 0.035), total abdominal muscle index (r = 0.685, p < 0.001), and masseter muscle index (r = 0.551, p < 0.001). The higher ASMI was negatively correlated with a higher global cortical atrophy scale (r = − 0.457, p < 0.001). The correlation between L3SMI and MSMI was also investigated, revealing that a higher L3SMI was positively correlated with higher MSMI (r = 0.488, p < 0.001).
Fig. 2.
Associations between ASMI and body composition parameter and visual rating scale. The Partial correlation was conducted after controlling for age and sex variables. A A higher ASMI was positively correlated with higher BMI, L3 level ATI, L3 level SMI, and MSMI and negatively correlated with a higher GCA. B A higher L3SMI was positively correlated with higher MSMI. Abbreviations: ASMI, Appendicular skeletal muscle index; ATI, Adipose tissue index; BMI, Body mass index; GCA, Global cortical atrophy; L3SMI, L3 skeletal muscle index; MSMI, Masseter skeletal muscle index, SMI, Skeletal muscle mass index
Univariate analysis and logistic regression analysis
The results of the univariate analysis and multivariate analysis are presented in Table 2. A total of 6 variables (BMI, VATI, SATI, L3SMI, MSMI and GCA) showing a statistically significant odds ratio (p < 0.10) were entered into a stepwise binary regression model for the prediction of low ASMI. After adjusting for age and sex, BMI (p = 0.049, OR = 0.748), L3SMI (p = 0.003, OR = 0.705) and GCA (p = 0.002, OR = 1.50) were associated with a low ASMI. Considering the correlation between L3SMI and MSMI, we performed the binary logistic regression without the L3SMI variable, with the results revealing that BMI (p = 0.013, OR = 0.718), MSMI (p = 0.010, OR = 0.960) and GCA (p = 0.001, OR = 1.44) were associated with a low ASMI.
Table 2.
Univariate analysis and multivariate analysis of factors associated with low lean mass
| Univariate Analysis | Multivariate Analysis1 | Multivariate Analysis without L3SMI1 | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p value2 | OR (95% CI) | p value3 | OR (95% CI) | p value3 |
| Age | 1.062 (0.983, 1.147) | 0.130 | ||||
| Sex | 1.868 (0.750, 4.652) | 0.179 | ||||
| BMI | 0.702 (0.587, 0.841) | < 0.001* | 0.748 (0.560, 0.999) | 0.049* | 0.718 (0.554, 0.932) | 0.013* |
| VATI | 0.983 (0.968, 0.999) | 0.042* | ||||
| SATI | 0.971 (0.952, 0.991) | 0.004* | ||||
| L3SMI | 0.843 (0.769, 0.924) | < 0.001* | 0.705 (0.562, 0.885) | 0.003* | ||
| MSMI | 0.946 (0.921, 0.971) | < 0.001* | 0.960 (0.932, 0.990) | 0.010* | ||
| GCA | 1.399 (1.198, 1.633) | < 0.001* | 1.496 (1.153, 1.941) | 0.002* | 1.442 (1.153, 1.802) | 0.001* |
Abbreviations: ASMI Appendicular skeletal muscle index, BMI Body mass index, CI Confidence interval, GCA Global cortical atrophy, L3SMI L3 skeletal muscle index, MSMI Masseter skeletal muscle index, OR Odds ratio, SATI Subcutaneous adipose tissue index, SMI Skeletal muscle index, VATI Visceral adipose tissue index
1The multivariate analysis was conducted after controlling for age and sex
2Univariate Analysis: * p < 0.1
3Multivariate Analysis: * p < 0.05
Diagnostic value of BMI, GCA, and skeletal muscle index for predicting low lean mass
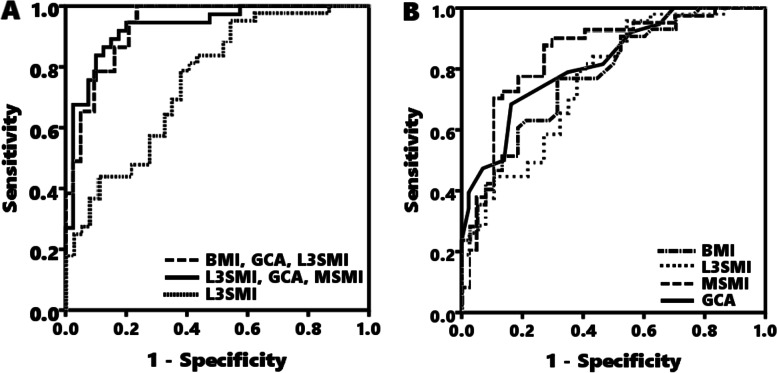
The ROC curves were constructed using the predictions from the logistic regression models. The sensitivity, specificity, positive predictive values, negative predictive values, positive likelihood ratio, negative likelihood ratio, standard error, and area under the curve of BMI, GCA, L3SMI, MSMI for model 1 (combination of BMI, GCA and L3SMI), and model 2 (combination of BMI, GCA and MSMI) are shown in Table 3. The AUC of model 1 (AUC: 0.926; accuracy: 0.875; [CI] 0.844–0.972) was significantly greater than BMI, GCA, and L3SMI individually (AUC: 0.777, 0.820, and 0.757; [CI] 0.672–0.863, 0.713–0.892, and 0.654–0.851; Bonferroni-adjusted p-value: 0.004, 0.020, and 0.002 respectively). The AUC of model 2 (AUC: 0.931; accuracy: 0.885; [CI] 0.857–0.979) was significantly greater than BMI, GCA, L3SMI, and MSMI individually (AUC: 0.777, 0.820, 0.757, and 0.827; [CI] 0.672–0.863, 0.713–0.892, 0.654–0.851, and 0.732–0.908; Bonferroni-adjusted p-value: 0.008, 0.048, 0.035, 0.014 respectively)(Table 3) (Fig. 3) (See Supplemental Table 1, Additional file 2). There was no significant difference between model 1 and model 2 in the AUC (Bonferroni-adjusted p-value = 1.000). The pairwise comparison of ROC curves are shown in Supplemental Table 1. There was no significant difference between BMI, GCA, L3SMI, and MSMI in the AUC. (See Supplemental Table 1, Additional file 2).
Table 3.
Diagnostic value of BMI, GCA, and skeletal muscle index for predicting low lean mass
| Sensitivity, Specificity, Positive and Negative Predictive Values and Receiver Operating Curve Model of the BMI, GCA, L3SMI, MSMI and combination of BMI, GCA and skeletal muscle index (either L3SMI or MSMI). | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable1 | Sensitivity | Specificity | PPV | NPV | +LR | -LR | Accuracy | SE | AUC (95%CI) |
| BMI | 0.790 | 0.628 | 0.722 | 0.733 | 2.121 | 0.335 | 0.704 | 0.051 | 0.777 (0.672, 0.863) |
| GCA | 0.684 | 0.837 | 0.788 | 0.750 | 4.203 | 0.377 | 0.765 | 0.046 | 0.820 (0.713, 0.892) |
| L3SMI | 0.622 | 0.791 | 0.719 | 0.708 | 2.970 | 0.479 | 0.713 | 0.054 | 0.757 (0.654, 0.851) |
| MSMI | 0.865 | 0.707 | 0.844 | 0.778 | 2.955 | 0.191 | 0.782 | 0.049 | 0.827 (0.732, 0.908) |
| Model 1a | 0.919 | 0.837 | 0.829 | 0.923 | 5.645 | 0.097 | 0.875 | 0.028 | 0.926 (0.844, 0.972) |
| Model 2b | 0.919 | 0.830 | 0.853 | 0.917 | 6.279 | 0.095 | 0.885 | 0.029 | 0.931 (0.857,0.979) |
Abbreviations: AUC Area under the curve, BMI Body mass index, GCA Global cortical atrophy, L3SMI L3 skeletal muscle index, MSMI Masseter skeletal muscle index, NPV Negative predictive values, PPV Positive predictive values, SE Standard error, +LR Positive likelihood ratio, −LR Negative likelihood ratio
1Significantly different with all variables (p < 0.05)
aModel 1: combination of BMI, GCA and L3SMI
bModel 2: combination of BMI, GCA and MSMI
Fig. 3.
Receiver operating characteristic (ROC) curve for predicting low lean mass in cognitive impaired elderly. The ROC curves of BMI, GCA, L3SMI, MSMI, model 1 (combination of BMI, GCA, and L3SMI), and model 2 (combination of BMI, GCA, and MSMI) for predicting low lean mass. A The AUC of model 1 and model 2 was significantly greater than L3SMI. B There was no significant difference between BMI, GCA, L3SMI, and MSMI in the AUC. (Table 3) (See Supplemental Table 1, Additional file 2). Abbreviations: BMI, Body mass index; GCA, Global cortical atrophy; L3SMI, L3 skeletal muscle index; MSMI, Masseter skeletal muscle index
Discussion
Currently, brain CT is widely used in dementia workups as the first step brain diagnostic imaging method due to its faster acquisition speed, cost-effectiveness, availability, and ability to exclude other pathologies [27]. Additional information, such as masseter muscle mass and brain atrophy, can also be obtained by non-enhanced brain CT. Several reliable methods, including visual rating scale (GCA), brain atrophy parameters (EI, FHI, BCR, SFR, Schiersmann index, and Huckman number), voxel-based morphometry (VBM), and region of interest (ROI) analyses have been used in previous studies to assess brain atrophy/neurodegenerative disease. Although VBM and ROI analyses are often considered more accurate methods, they require prohibitively expensive techniques and specialized hardware. The global cortical atrophy (GCA) scale not only reflects cortical atrophy itself but also the degree of sulcal and ventricular dilatation secondary to atrophy of the white matter. Previous studies have demonstrated that the GCA scale can be evaluated using CT with substantial agreement, as compared to MRI [41, 45]. Due to its efficiency, accuracy, and ease to perform, we recommend using the GCA scale in radiology reports for early detection of the risk of lean mass loss to facilitate early intervention.
Clinically, some cognitively impaired elderly with low ASMI do not exhibit significant declines in physical performance, while others are unaware of their reduced physical activity. Nonetheless, early detection of undiagnosed sarcopenia/low muscle mass is important. Primary intervention, which includes resistance training and nutritional supplementation, is crucial. The DEXA evaluation is presently considered the gold standard technique both in research and in clinical practice for the assessment of muscle mass [46]. Several studies have shown a significant association between the CT-based cross-section muscle mass index, including MSMI and L3SMI, and the ASMI [35, 37]. .Its application for predicting morbidities in certain conditions (cirrhosis, pulmonary disease, severe traumatic brain injury, etc.) has also been reported, and is considered a marker of sarcopenia [47–50]. .However, its representative efficacy for determining overall body muscle mass remains debatable [51]. In our study, there was a significant correlation between both MSMI and L3SMI and the ASMI. The body mass index (BMI) has been used for evaluating nutritional status; as such, a lower BMI has been commonly recognized as a marker for poor nutrition status [52]. Of note, the association between late-life BMI and dementia is still inconclusive. Some studies have identified late-life obesity (or high BMI) as a protective state for dementia, commonly termed the “obesity paradox” [53–55]. In our study, a lower BMI is independently associated with lean body mass loss in cognitively impaired elderly, which may reflect poor nutrition and physical activity [56]. In addition, our study noted a positive correlation between brain volume and body muscle mass, as also demonstrated in a previous study [16]. The degree of brain atrophy in the low ASMI group was significantly higher than that of the normal ASMI group, suggesting that brain atrophy and muscle wasting may occur concurrently. One previous study has also revealed a strong relationship between lean mass and white matter volume in participants both with and without dementia [11].
Our logistic regression results showed that several body composition and brain atrophy parameters (BMI, L3SMI, MSMI, and GCA) were predictive of low ASMI independently of other possible confounders. However, none of these attained optimal cutoff values, with both sensitivity and specificity at > 80%, resulting in an AUC < 0.85. The combination of the BMI, GCA score, and one of SMI (L3SMI (model 1) or MSMI (model 2)) into a single risk score enhanced the predictive accuracy of low ASMI, with both sensitivity and specificity at > 80% and AUC > 0.9, and showed significantly better predictive accuracy than BMI, GCA, L3SMI, and MSMI individually. No statistically significant difference was found between the two models in predicting low ASMI, indicating that MSMI could serve as an alternative marker for body muscle mass loss.
Recent studies have also demonstrated that masseter muscle mass is a simple and effective tool for assessing sarcopenia in elderly populations [34–36, 50]. Additionally, it is regarded as an effective predictor of early mortality following traumatic brain injury in the elderly [34, 36, 50]. For this reason, we suggest using MSMI as part of a muscle wasting assessment as it provides equally valid results as L3SMI, and is more accessible and easier to perform due to the lack of specialized software or arduous image analysis. Furthermore, a clinician can concurrently obtain reliable information regarding brain atrophy and skeletal muscle loss in a simple, non-enhanced brain CT scan.
Previous studies have suggested that the mechanism of the brain-muscle axis remains unclear, and is considered to be multifactorial [15, 16]. In the present study, brain volume is positively correlated with body lean mass. Masseter muscle mass loss may limit the action of mastication, leading to poor nutrient intake and malnutrition. Malnutrition may further aggravate the loss of muscle mass, resulting in poor muscle strength and reduced physical activity. Abdominal muscle wasting may reflect a deficiency in core stability, low physical activity, and unsteadiness which collectively increase the risk of adverse events, including falls. In addition, muscle wasting may further affect brain volume by impairing certain physiological functions, such as glucose regulation, hormone production, and cellular communication, which can also regulate neuroinflammatory responses and lead to neuroendocrine dysregulation and brain atrophy [57, 58]. Recent evidence shows that adequate physical activity not only enhances muscle mass and strength, but also benefits cognitive function and brain volume in the elderly with cognitive deficits [59–62]. Several pathophysiology pathways of neurologic insults, affecting brain atrophy and muscle wasting simultaneously, have been demonstrated in previous studies, such as primary reward system deficit, visuomotor control failure, and gait and balance associated white matter alterations [63–67]. Furthermore, any condition that causes inflammation not only increases muscle catabolism and impairs myogenesis leading to muscle wasting, but also alters blood-brain barrier functions and reduces neural plasticity, resulting in neurodegenerative diseases and brain atrophy [68–71]. Chronic illnesses and other conditions leading to prolonged inactivity, inadequate nutrient intake, and poor nutrient absorption, may also result in both muscle wasting and neurodegeneration [72]. As such, early detection and intervention for elderly patients with a higher risk of brain atrophy and muscle wasting is necessary.
Several limitations to our study should indeed be mentioned. First, the population of our study consisted only of cognitively impaired elderly recruited from a single medical center, composing a relatively small sample size, thus limiting the generalizability of the results. Second, the study cannot infer causality between brain volume and body composition as the temporal sequence cannot be established in a cross-sectional study. To achieve this, further long-term longitudinal study is necessary. Third, measurement of masseter cross-sectional area was performed twice by a trained investigator manually to reduce the partial volume effect; however, the possibility of intra-examiner errors cannot be completely excluded. Several studies have documented automatic or semi-automatic segmentation methods in masseter muscle area measurement, while manual segmentation, offers more convenience and availability for clinical physicians. In addition, future research should involve a multi-center prospective analysis, and include additional patient characteristics, such as dental status, nutrition status, and inflammatory biomarkers which may affect both muscle mass and brain atrophy, and provide more conclusive evidence regarding brain atrophy. Finally, further research is needed to provide evidence of applicability in other population groups, such as the elderly with normal cognitive function, the elderly with known neurodegenerative diseases, and cerebral atrophy in younger persons.
In conclusion, we herein demonstrate a significant association between low lean mass and brain volume loss in elderly Taiwanese with cognitive deficiency. The combination of BMI, GCA, and SMI, either L3SMI or MSMI, achieved good predictive accuracy for low ASMI. Early detection of such patients is necessary to facilitate further treatment and prevent adverse events, thereby improving quality of life in the elderly. Further clarification of the underlying mechanisms of muscle wasting, and future studies involving additional parameters and various conditions are required.
Supplementary Information
Additional file 1 : Supplement 1. Visual rating of global cortical atrophy. Supplement 2. Abdominal CT analysis of body composition at L3 level.
Additional file 2 : Supplemental Table 1. Pairwise comparison of ROC curves between BMI, GCA, L3SMI, MSMI, Model1 and Model 2.
Additional file 3 : Supplemental Figure 1. Participant flow charts.
Acknowledgments
We thank the research participants and the research staff involving in this study. This article has not been previously published in whole or in part, in any language, except as an abstract.
Abbreviations
- ADL
Activities of daily living
- ANCOVA
Analysis of covariance
- ASMI
Appendicular skeletal muscle index
- ATI
Adipose tissue index
- AUC
Area under the Curve
- AWGS
Asian Working Group for Sarcopenia
- BCR
Bicaudate ratio
- BMI
Body mass index
- CCY
Chiun Chieh Yu
- CDR
Clinical Dementia Rating
- CI
Confidential intervals
- CRP
C-Reactive protein
- CT
Computed tomography
- DEXA
Dual energy X-ray absorptiometry
- EI
Evans index
- FHI
Frontal horn index
- GCA
Global cortical atrophy
- L3SMI
L3 skeletal muscle index
- MCI
Mild cognitive impairment
- MRI
Magnetic resonance imaging
- MSMI
Masseter muscle mass index
- OR
Odds ratios
- ROC
Receiver operating characteristic
- ROI
Region of interest
- SAT
Subcutaneous adipose tissue
- SD
Standard deviation
- SFR
Sylvian fissure ratio
- SPSS
Statistical Product and Service Solutions
- SMI
Skeletal muscle mass index
- VAT
Visceral adipose tissue
- VBM
Voxel-based morphometry
- WCL
Wei-Che Lin
- YTC
Yun Ting Chen
Authors’ contributions
YTC, CCY, SHC, NCC and WCL designed the research methods and protocol; YCL and NCC provided essential materials; YTC, CCY, YYL, NCC, and WCL conducted research; YTC and WCL analyzed data; and YTC, NCC, and WCL wrote the paper. WCL had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This article was supported by a grant from the National Science Council of Taiwan (MOST 108-2314-B-182A-014-MY3(NMRPG8J6023)) and the Kaohsiung Chang Gung Memorial Hospital Reach Program (CORPG8L0071). The funders had no role in research design, collection, analysis, and interpretation of data and manuscript writing.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to patient privacy and Institutional Review Boards (IRB) disapproval, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Memorial Hospital. The patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nai-Ching Chen and Wei-Che Lin contributed equally to this work and share the role of corresponding author.
Contributor Information
Nai-Ching Chen, Email: m0124@cgmh.org.tw.
Wei-Che Lin, Email: alex@cgmh.org.tw.
References
- 1.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH . Global action plan on the public health response to dementia 2017–2025. 2017. [Google Scholar]
- 3.Huang CY, Hwang AC, Liu LK, Lee WJ, Chen LY, Peng LN, Lin MH, Chen LK. Association of dynapenia, sarcopenia, and cognitive impairment among community-dwelling older Taiwanese. Rejuvenation Res. 2016;19:71–78. doi: 10.1089/rej.2015.1710. [DOI] [PubMed] [Google Scholar]
- 4.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: a systematic review and meta-analysis. Exp Gerontol. 2020;131:110801. doi: 10.1016/j.exger.2019.110801. [DOI] [PubMed] [Google Scholar]
- 6.Nishiguchi S, Yamada M, Shirooka H, Nozaki Y, Fukutani N, Tashiro Y, Hirata H, Yamaguchi M, Tasaka S, Matsushita T, et al. Sarcopenia as a risk factor for cognitive deterioration in community-dwelling older adults: a 1-year prospective study. J Am Med Dir Assoc. 2016;17(372):e375–e378. doi: 10.1016/j.jamda.2015.12.096. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I. Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim M, Won CW. Sarcopenia is associated with cognitive impairment mainly due to slow gait speed: results from the Korean frailty and aging cohort study (KFACS) Int J Environ Res Public Health. 2019;16:1491. doi: 10.3390/ijerph16091491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauretani F, Maggio M, Ticinesi A, Tana C, Prati B, Gionti L, Nouvenne A, Meschi T. Muscle weakness, cognitive impairment and their interaction on altered balance in elderly outpatients: results from the TRIP observational study. Clin Interv Aging. 2018;13:1437–1443. doi: 10.2147/CIA.S165085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson HH, Cesari M, Kritchevsky SB, Penninx BW, Fried LP, Guralnik JM, Williamson JD. Predictors of combined cognitive and physical decline. J Am Geriatr Soc. 2005;53:1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- 11.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67:428–433. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nourhashémi F, Andrieu S, Gillette-Guyonnet S, Reynish E, Albarède JL, Grandjean H, Vellas B. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc. 2002;50:1796–1801. doi: 10.1046/j.1532-5415.2002.50507.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohta Y, Nomura E, Hatanaka N, Osakada Y, Matsumoto N, Sasaki R, Tsunoda K, Takemoto M, Tadokoro K, Hishikawa N, et al. Female dominant association of sarcopenia and physical frailty in mild cognitive impairment and Alzheimer's disease. J Clin Neurosci. 2019;70:96–101. doi: 10.1016/j.jocn.2019.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Noh H-M, Oh S, Song HJ, Lee EY, Jeong J-Y, Ryu O-H, Hong K-S, Kim D-H. Relationships between cognitive function and body composition among community-dwelling older adults: a cross-sectional study. BMC Geriatr. 2017;17:259. doi: 10.1186/s12877-017-0651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon Y, Choi YJ, Kim JO, Han SH. Muscle profile and cognition in patients with Alzheimer's disease dementia. Neurol Sci. 2018;39:1861–1866. doi: 10.1007/s10072-018-3505-0. [DOI] [PubMed] [Google Scholar]
- 16.Kilgour AH, Todd OM, Starr JM. A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatr. 2014;14:85. doi: 10.1186/1471-2318-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pillard F, Laoudj-Chenivesse D, Carnac G, Mercier J, Rami J, Rivière D, Rolland Y. Physical activity and sarcopenia. Clin Geriatr Med. 2011;27:449–470. doi: 10.1016/j.cger.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Robinson S, Cooper C, Aihie Sayer A. Nutrition and sarcopenia: a review of the evidence and implications for preventive strategies. J Aging Res. 2012;2012:510801. doi: 10.1155/2012/510801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367:31–37. doi: 10.1111/nyas.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlachos GS, Scarmeas N. Dietary interventions in mild cognitive impairment and dementia. Dialogues Clin Neurosci. 2019;21:69–82. doi: 10.31887/DCNS.2019.21.1/nscarmeas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouloutbani K, Karteroliotis K, Politis A. The effect of physical activity on dementia. Psychiatriki. 2019;30:142–155. doi: 10.22365/jpsych.2019.302.142. [DOI] [PubMed] [Google Scholar]
- 22.Tabei KI, Satoh M, Ogawa JI, Tokita T, Nakaguchi N, Nakao K, Kida H, Tomimoto H. Cognitive function and brain atrophy predict non-pharmacological efficacy in dementia: the Mihama-Kiho scan Project2. Front Aging Neurosci. 2018;10:87. doi: 10.3389/fnagi.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307.e302. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, Maggi S, Dennison E, Al-Daghri NM, Allepaerts S, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9:269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh DD, Ortiz-Reyes LA, Quraishi SA, Chokengarmwong N, Avery L, Kaafarani HMA, Lee J, Fagenholz P, Chang Y, DeMoya M, Velmahos G. Early nutritional inadequacy is associated with psoas muscle deterioration and worse clinical outcomes in critically ill surgical patients. J Crit Care. 2018;45:7–13. doi: 10.1016/j.jcrc.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Hwang Y, Lee YH, Cho DH, Kim M, Lee DS, Cho HJ. Applicability of the masseter muscle as a nutritional biomarker. Medicine (Baltimore) 2020;99:e19069. doi: 10.1097/MD.0000000000019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adduru V, Baum SA, Zhang C, Helguera M, Zand R, Lichtenstein M, Griessenauer CJ, Michael AM. A method to estimate brain volume from head CT images and application to detect brain atrophy in Alzheimer disease. Am J Neuroradiol. 2020;41:224–230. doi: 10.3174/ajnr.A6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephen R, Liu Y, Ngandu T, Antikainen R, Hulkkonen J, Koikkalainen J, Kemppainen N, Lötjönen J, Levälahti E, Parkkola R, et al. Brain volumes and cortical thickness on MRI in the Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER) Alzheimers Res Ther. 2019;11:53. doi: 10.1186/s13195-019-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury R, Ghossoub E. Diagnostic biomarkers of Alzheimer’s disease: a state-of-the-art review. Biomark Neuropsych. 2019;1:100005. [Google Scholar]
- 30.Young PNE, Estarellas M, Coomans E, Srikrishna M, Beaumont H, Maass A, Venkataraman AV, Lissaman R, Jiménez D, Betts MJ, et al. Imaging biomarkers in neurodegeneration: current and future practices. Alzheimers Res Ther. 2020;12:49. doi: 10.1186/s13195-020-00612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Fu Y, Zeng YF, Hu JP, Lin XZ, Cai NQ, Weng Q, Zhao YJ, Lin Y, Cao DR, Wang N. Six visual rating scales as a biomarker for monitoring atrophied brain volume in Parkinson's disease. Aging Dis. 2020;11:1082–1090. doi: 10.14336/AD.2019.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aljondi R, Szoeke C, Steward C, Yates P, Desmond P. A decade of changes in brain volume and cognition. Brain imaging Behav. 2019;13:554–563. doi: 10.1007/s11682-018-9887-z. [DOI] [PubMed] [Google Scholar]
- 33.Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KM, de Bree R, Leenen LP, Grolman W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016;62:28–33. doi: 10.1016/j.oraloncology.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Tanabe C, Reed MJ, Pham TN, Penn K, Bentov I, Kaplan SJ. Association of Brain Atrophy and Masseter Sarcopenia with 1-year mortality in older trauma patients. JAMA Surg. 2019;154:716–723. doi: 10.1001/jamasurg.2019.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umeki K, Watanabe Y, Hirano H, Edahiro A, Ohara Y, Yoshida H, Obuchi S, Kawai H, Murakami M, Takagi D, et al. The relationship between masseter muscle thickness and appendicular skeletal muscle mass in Japanese community-dwelling elders: a cross-sectional study. Arch Gerontol Geriatr. 2018;78:18–22. doi: 10.1016/j.archger.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Wallace JD, Calvo RY, Lewis PR, Brill JB, Shackford SR, Sise MJ, Sise CB, Bansal V. Sarcopenia as a predictor of mortality in elderly blunt trauma patients: comparing the masseter to the psoas using computed tomography. J Trauma Acute Care Surg. 2017;82:65–72. doi: 10.1097/TA.0000000000001297. [DOI] [PubMed] [Google Scholar]
- 37.Portal D, Hofstetter L, Eshed I, Dan-Lantsman C, Sella T, Urban D, Onn A, Bar J, Segal G. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag Res. 2019;11:2579–2588. doi: 10.2147/CMAR.S195869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 1985;2004(97):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 39.Chrzan R, Gleń A, Bryll A, Urbanik A. Computed tomography assessment of brain atrophy in centenarians. Int J Environ Res Public Health. 2019;16(19). [DOI] [PMC free article] [PubMed]
- 40.Harper L, Barkhof F, Fox NC, Schott JM. Using visual rating to diagnose dementia: a critical evaluation of MRI atrophy scales. J Neurol Neurosurg Psychiatry. 2015;86:1225–1233. doi: 10.1136/jnnp-2014-310090. [DOI] [PubMed] [Google Scholar]
- 41.Wahlund LO, Westman E, van Westen D, Wallin A, Shams S, Cavallin L, Larsson EM. Imaging biomarkers of dementia: recommended visual rating scales with teaching cases. Insights Imaging. 2017;8:79–90. doi: 10.1007/s13244-016-0521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meese W, Kluge W, Grumme T, Hopfenmüller W. CT evaluation of the CSF spaces of healthy persons. Neuroradiology. 1980;19:131–136. doi: 10.1007/BF00342387. [DOI] [PubMed] [Google Scholar]
- 43.Hu P, Uhlich R, White J, Kerby J, Bosarge P. Sarcopenia measured using masseter area predicts early mortality following severe traumatic brain injury. J Neurotrauma. 2018;35:2400–2406. doi: 10.1089/neu.2017.5422. [DOI] [PubMed] [Google Scholar]
- 44.Deng C-Y, Lin Y-C, Wu JS, Cheung Y-C, Fan C-W, Yeh K-Y, McMahon CJ. Progressive sarcopenia in patients with colorectal cancer predicts survival. AJR Am J Roentgenol. 2018;210:526–532. doi: 10.2214/AJR.17.18020. [DOI] [PubMed] [Google Scholar]
- 45.Wattjes MP, Henneman WJ, van der Flier WM, de Vries O, Träber F, Geurts JJ, Scheltens P, Vrenken H, Barkhof F. Diagnostic imaging of patients in a memory clinic: comparison of MR imaging and 64-detector row CT. Radiology. 2009;253:174–183. doi: 10.1148/radiol.2531082262. [DOI] [PubMed] [Google Scholar]
- 46.Martone AM, Marzetti E, Calvani R, Picca A, Tosato M, Bernabei R, Landi F. Assessment of sarcopenia: from clinical practice to research. J Gerontol Geriatr. 2019;67:39–45. [Google Scholar]
- 47.Gu DH, Kim MY, Seo YS, Kim SG, Lee HA, Kim TH, Jung YK, Kandemir A, Kim JH, An H, et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319–330. doi: 10.3350/cmh.2017.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelmann C, Schob S, Nonnenmacher I, Werlich L, Aehling N, Ullrich S, Kaiser T, Krohn S, Herber A, Sucher R, et al. Loss of paraspinal muscle mass is a gender-specific consequence of cirrhosis that predicts complications and death. Aliment Pharmacol Ther. 2018;48:1271–1281. doi: 10.1111/apt.15026. [DOI] [PubMed] [Google Scholar]
- 49.Choe EK, Lee Y, Kang HY, Choi SH, Kim JS. Association between CT-Measured Abdominal Skeletal Muscle Mass and Pulmonary Function. J Clin Med. 2019;8(5). [DOI] [PMC free article] [PubMed]
- 50.Uhlich R, Hu P. Sarcopenia diagnosed using masseter muscle area predictive of early mortality following severe traumatic brain injury. Neural Regen Res. 2018;13:2089–2090. doi: 10.4103/1673-5374.241451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi: 10.21037/tgh.2019.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pegueroles J, Jiménez A, Vilaplana E, Montal V, Carmona-Iragui M, Pané A, Alcolea D, Videla L, Casajoana A, Clarimón J, et al. Obesity and Alzheimer's disease, does the obesity paradox really exist? A magnetic resonance imaging study. Oncotarget. 2018;9:34691–34698. doi: 10.18632/oncotarget.26162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.García-Ptacek S, Faxén-Irving G, Cermáková P, Eriksdotter M, Religa D. Body mass index in dementia. Eur J Clin Nutr. 2014;68:1204–1209. doi: 10.1038/ejcn.2014.199. [DOI] [PubMed] [Google Scholar]
- 56.Lee I, Cho J, Hong H, Jin Y, Kim D, Kang H. Sarcopenia is associated with cognitive impairment and depression in elderly Korean women. Iran J Public Health. 2018;47:327–334. [PMC free article] [PubMed] [Google Scholar]
- 57.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 58.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. 2019;51:1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291:E1003–E1008. doi: 10.1152/ajpendo.00100.2006. [DOI] [PubMed] [Google Scholar]
- 61.Yilmaz C, Karali K, Fodelianaki G, Gravanis A, Chavakis T, Charalampopoulos I, Alexaki VI. Neurosteroids as regulators of neuroinflammation. Front Neuroendocrinol. 2019;55:100788. doi: 10.1016/j.yfrne.2019.100788. [DOI] [PubMed] [Google Scholar]
- 62.Pan X, Wu X, Kaminga AC, Wen SW, Liu A. Dehydroepiandrosterone and Dehydroepiandrosterone Sulfate in Alzheimer's Disease: A Systematic Review and Meta-Analysis. Frontiers in Aging Neuroscience. 2019;11(61). [DOI] [PMC free article] [PubMed]
- 63.Kwon YN, Yoon SS. Sarcopenia: neurological point of view. J Bone Metab. 2017;24:83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwan P. Sarcopenia, a neurogenic syndrome? J Aging Res. 2013;2013:791679. doi: 10.1155/2013/791679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bherer L, Erickson KI, Liu-Ambrose T. Physical exercise and brain functions in older adults. J Aging Res. 2013;2013:197326. doi: 10.1155/2013/197326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perry DC, Kramer JH. Reward processing in neurodegenerative disease. Neurocase. 2015;21:120–133. doi: 10.1080/13554794.2013.873063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo X, Steen B, Matousek M, Andreasson LA, Larsson L, Palsson S, Sundh V, Skoog I. A population-based study on brain atrophy and motor performance in elderly women. J Gerontol: Ser A. 2001;56:M633–M637. doi: 10.1093/gerona/56.10.m633. [DOI] [PubMed] [Google Scholar]
- 68.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rong Y-D, Bian A-L, Hu H-Y, Ma Y, Zhou X-Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018;18:308. doi: 10.1186/s12877-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon HS, Koh S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translat Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janowitz D, Habes M, Toledo JB, Hannemann A, Frenzel S, Terock J, Davatzikos C, Hoffmann W, Grabe HJ. Inflammatory markers and imaging patterns of advanced brain aging in the general population. Brain Imaging Behav. 2020;14:1108–1117. doi: 10.1007/s11682-019-00058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pasco JA, Williams LJ, Jacka FN, Stupka N, Brennan-Olsen SL, Holloway KL, Berk M. Sarcopenia and the common mental disorders: a potential regulatory role of skeletal muscle on brain function? Curr Osteoporos Rep. 2015;13:351–357. doi: 10.1007/s11914-015-0279-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 : Supplement 1. Visual rating of global cortical atrophy. Supplement 2. Abdominal CT analysis of body composition at L3 level.
Additional file 2 : Supplemental Table 1. Pairwise comparison of ROC curves between BMI, GCA, L3SMI, MSMI, Model1 and Model 2.
Additional file 3 : Supplemental Figure 1. Participant flow charts.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to patient privacy and Institutional Review Boards (IRB) disapproval, but are available from the corresponding author on reasonable request.