Abstract
Objectives
Adrenal adenoma with myelolipomatous degeneration (AMD) is a rarely reported and often overlooked entity. The aim of this study is to improve understanding of these lesions by characterizing the imaging findings with pathologic and clinical correlation.
Methods
In the largest series to date, we report 11 nodules in 11 patients confirmed with a pathologic diagnosis of AMD. The available cross-sectional imaging and histopathologic features were reviewed by two radiologists and two pathologists, respectively. Clinical and laboratory data for each patient were obtained from the electronic medical records, when available.
Results
All 11 patients had a CT prior to resection or biopsy of the adrenal nodule, with five having received an adrenal mass protocol study. An MRI was available in three patients. The median size of the nodules on imaging was 4.5 cm (range 2.8–8.7) and all but one had macroscopic fat. The largest focus of macroscopic fat had a median size of 0.7 cm (range 0.2–1.6) and on average was 14.4% the size of the tumor, using greatest dimensions. Four (36.4%) patients had a diagnosis of Cushing syndrome prior to nodule resection.
Conclusions
Not all adrenal nodules with macroscopic fat on imaging are pure myelolipomas. An AMD should be considered, especially if the foci of fat are small and other features of an adenoma are present. Some may also be associated with Cushing syndrome.
Advances in knowledge:
Myelolipomatous degeneration within an adrenal adenoma has only rarely been previously reported with very few reports emphasizing the imaging features. There may be an association with cortisol hypersecretion and improved recognition of this entity could lead to changes in clinical management.
Introduction
Adrenal adenomas are the most common adrenal cortical tumors, seen in 2.3% of individuals at autopsy.1 They account for around 80% of all incidentally discovered adrenal lesions on routine imaging, also called “adrenal incidentalomas” when greater than 1 cm.2 Adenomas, and the majority of all adrenal incidentalomas, are benign lesions; However, diagnosis is important to exclude malignancy. Adrenal adenomas are usually non-functioning hormonally, but autonomously secrete cortisol around 12% of the time, which can cause Cushing syndrome.2
Approximately 70% of adrenal adenomas are lipid-rich, containing significant intracytoplasmic fat. The presence of microscopic fat results in a lower attenuation and, by utilizing a cutoff of 10 Hounsfield units (HU) on unenhanced CT, there is a sensitivity and specificity of 71 and 98% for an adenoma, respectively.3 For others, including lipid-poor adenomas, an MRI or adrenal mass protocol CT may be required. A contrast-enhanced CT relies on the washout pattern applying either absolute washout or relative washout. An absolute washout of ≥60 HU has been shown to have a high sensitivity of 86–88% and specificity of 92–96% in diagnosing adrenal adenoma.4,5 Meanwhile, a relative washout cutoff of ≥40 HU has a sensitivity and specificity of 96 and 100%, respectively.4
Like unenhanced CT, MRI evaluation relies heavily on the presence of microscopic fat for diagnosis. However, MRI with chemical shift imaging is more accurate than unenhanced CT, with 92% of all adenomas demonstrated by Israel et al to show signal drop on out-of-phase imaging >16.5%.6
Macroscopic fat, as opposed to microscopic lipid, within an adrenal nodule has historically been considered diagnostic for a benign adrenal myelolipoma. However, there have been several reported cases of myelolipomas mixed with adenomas, many of which contained macroscopic fat on CT images. These lesions have been described under various names, such as myelolipomatous adenoma, and pathologically either as two coexisting entities or as an adenoma undergoing myelolipomatous metaplasia. The latter is likely more accurate given the presumed origin of myelolipomatous elements as a metaplastic response to a stimulus such as hemorrhage or stress. Thus, we refer to these lesions as adenomas with myelolipomatous degeneration (AMD).7,8 It is also important that a distinction be made between these and so-called adrenal collision tumors, which are two adjacent, but pathologically different, lesions in the same adrenal gland without histologic mixing.
Myelolipomas are benign adrenal masses composed of hematopoietic tissue and mature adipocytes. They may contain hemorrhage or calcification and, like adenomas, are often discovered incidentally. However, unlike adenomas, myelolipomas are almost always non-functioning.7 In the few reported cases of AMDs, a large percentage have described an association with clinical or subclinical Cushing syndrome, more than would be expected for either alone. Therefore, in the following series of 11 patients, we attempt to study the imaging features of AMD with radiology and pathology correlation, and we also examine the clinical context and laboratory values, when available, for signs of cortisol hypersecretion.
Methods and materials
Patient selection
This is a single institution, retrospective study performed at The University of Texas MD Anderson Cancer Center. The institutional review board approved this analysis, which was compliant with the Health Insurance Portability and Accountability Act, and waiver of patient consent was obtained. A search was performed in both pathology and radiology databases to identify potential subjects. Patients were included if they had a pathologically proven adrenal adenoma with a myelolipomatous component and a CT and/or MRI available prior to pathologic diagnosis. 11 nodules in 11 patients met the inclusion criteria of having cross-sectional imaging available prior to resection and available pathology confirming AMD. Although one patient had bilateral adrenal adenomas with macroscopic fat, only one nodule was resected and had pathology available to meet the inclusion criteria. Demographic information including age, sex, and clinical history was obtained from the electronic medical records.
Radiology
The CT and MRI examinations were performed prior to resection and showed the entire extent of the nodule. All CTs were contrast-enhanced and included an unenhanced series. When available, the adrenal mass protocol comprised a pre-contrast phase, portal venous phase, and a 15-min delay. MRI examinations included, at minimum, in-phase/out-of-phase chemical shift, T1-weighted pre-contrast, and T1-weighted pre-contrast fat-saturated sequences. Absolute washout on CT was calculated by the formula [venous phase attenuation – delayed phase attenuation]/[venous phase attenuation – unenhanced phase attenuation] with ≥60% used as the cutoff for determining washout. Relative washout was not calculated since all qualifying studies had a pre-contrast phase. To calculate the presence of microscopic fat on MRI, signal drop from in-phase to out-of-phase imaging with a cutoff of >16.5% was performed by using the formula [signal intensity in-phase – signal intensity out-of-phase]/signal intensity in-phase. Images were evaluated and measurements provided by two fellowship trained abdominal radiologists, in consensus.
Pathology
Pathology was reviewed for all cases by two experienced pathologists, who reached consensus. Confirmation was made of myelolipomatous elements intermixed within the adrenal adenoma. All patients and nodules had complete pathologic specimens from surgical resection.
Results
There were 11 collected cases in 11 patients (four males, seven females) in the study population, summarized in Table 1. The median age was 59, with a range of 47–84 years of age. Several patients (n = 4) had a history of malignancy with discovery of the adrenal nodule during staging. The remaining adenomas were discovered incidentally during a CT performed for another purpose. Four (36.4%) patients had a diagnosis of Cushing syndrome prior to nodule resection, two of which was subclinical and based on laboratory data. The remainder of the patients did not have testing performed at our institution or a known clinical history of Cushing syndrome prior to resection. None of the patients had a diagnosis of laboratory evidence suggesting hyperaldosteronism.
Table 1.
Clinical characteristics
| Patient | Age | Sex | Clinical History | Diagnosis of Cushing syndrome |
|---|---|---|---|---|
| 1 | 62 | F | Adrenal incidentaloma | Yes |
| 2 | 53 | F | Neuroendocrine tumor | No |
| 3 | 47 | M | Ruptured appendicitis | No |
| 4 | 53 | M | Renal Cell Carcinoma | No |
| 5 | 52 | M | Adrenal incidentaloma | No |
| 6 | 60 | F | Adrenal incidentaloma | No |
| 7 | 52 | F | Adrenal incidentaloma | Yes; subclinical |
| 8 | 72 | F | Adrenal incidentaloma | No |
| 9 | 70 | M | Adrenal incidentaloma | No |
| 10 | 84 | F | Bladder cancer | Yes |
| 11 | 59 | F | Colorectal cancer | Yes; subclinical |
Radiology
In all patients, a CT was acquired prior to adrenal mass resection, with 5/11 patients receiving a complete adrenal mass protocol. In addition, three also had an MRI of the abdomen. Five of the nodules had imaging findings suggestive of an adenoma such as absolute washout ≥60%, pre-contrast attenuation of ≤10 HU, and/or signal drop of >16.5%. Macroscopic fat was seen in all but one nodule on imaging with the typical appearance being a single small focus or a few small foci of fat (Figures 1–2). In some nodules, the fat was larger or more widespread, but they were in the minority (Figure 3). The median size of the largest focus of fat was 0.7 cm (range 0.2–1.6), while the median size of the adrenal nodules on CT was 4.5 cm (range 2.8–8.7), when measured in the greatest dimension. Therefore, on average, the largest focus of fat was 14.4% the size of the tumor. Calcification was present in 6/11 (54.5%) of the adrenal nodules on imaging Table 2.
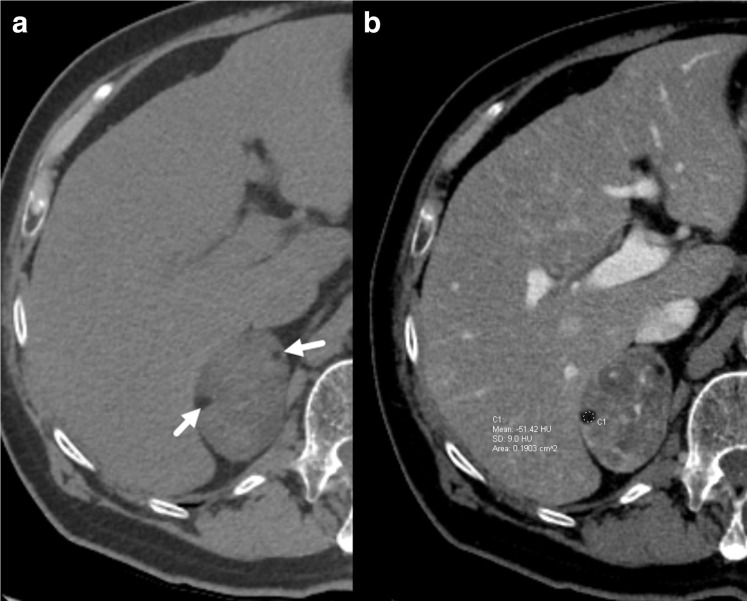
Figure 1.
62-year-old female with a clinical diagnosis of Cushing’s syndrome. Axial unenhanced CT (a) demonstrates a large right adrenal nodule containing small foci of fat attenuation (arrows). Postcontrast imaging (b) demonstrates heterogeneous nodule enhancement with a Hounsfield unit (HU) of −51 in one of the foci confirming macroscopic fat. The nodule was later pathologically confirmed to be an adrenal adenoma with foci of myelolipomatous degeneration.
Figure 2.
68-year-old male with neuroendocrine pancreatic tumor and an incidentally discovered 4.2 cm left adrenal nodule. Axial CT slice of the abdomen with contrast in the late arterial phase shows a left adrenal nodule with a solitary small (7 mm) focus of fat (−97 HU) (arrow). A focus of myelolipomatous degeneration was seen within an adrenal adenoma pathologically.
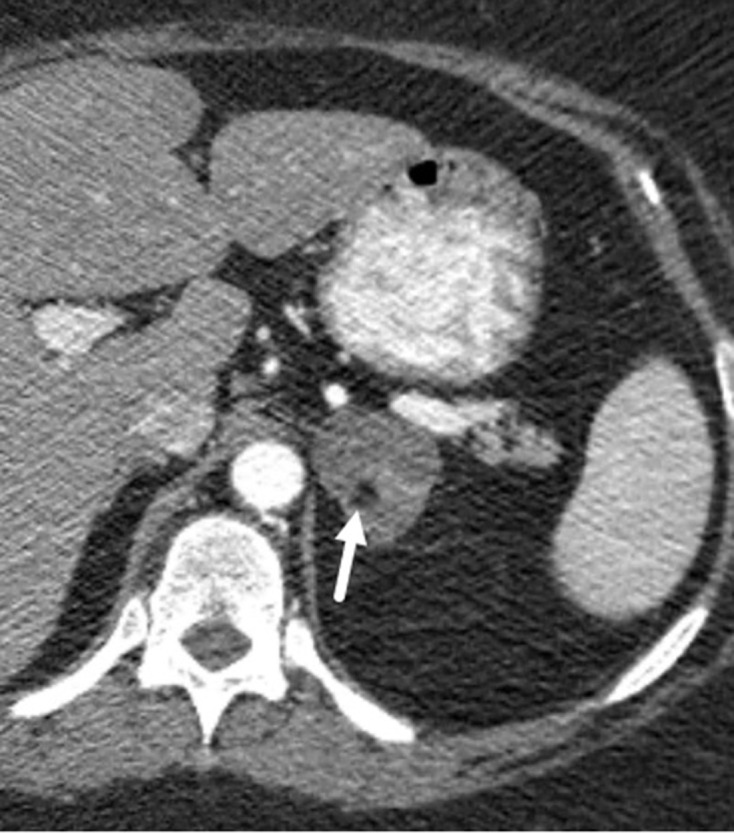
Figure 3.
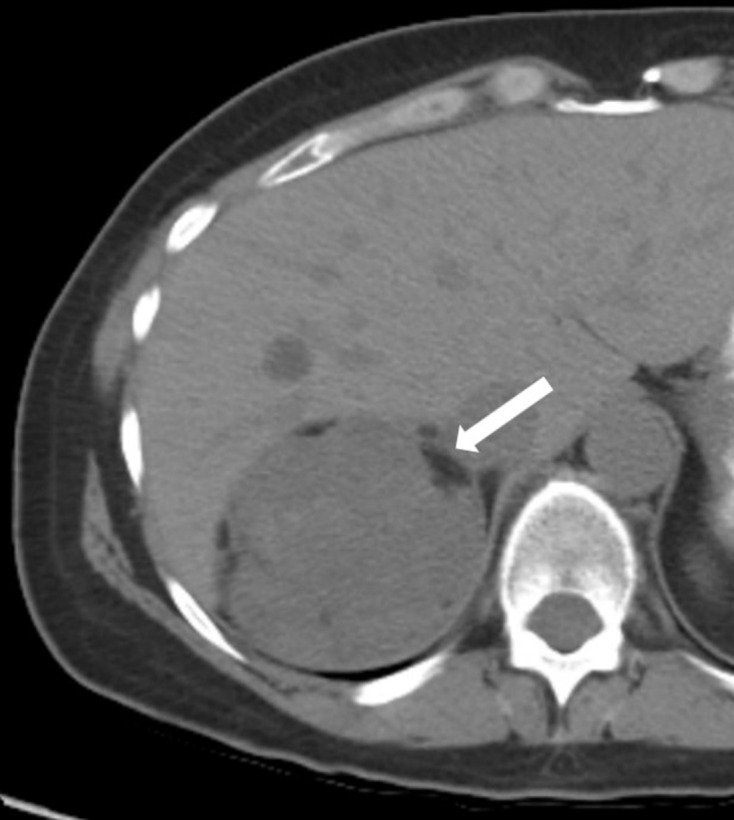
58-year-old female with a right adrenal nodule incidentally discovered on a CT obtained for abdominal pain. Axial unenhanced CT shows a 9.2 cm right adrenal nodule exerting mass effect on the liver with several foci of macroscopic fat (−35 HU in one locule; not shown) around the periphery (arrow). Pathology after resection revealed several foci of myelolipomatous degeneration in an adrenal adenoma.
Table 2.
Characteristics of adrenal nodules
| Patient | Pathology size | Size on CT | Gross imaging appearance | Largest macroscopic fat focus on imaging | Pre-contrast HU ≤ 10 | Absolute washout ≥60% | MRI Signal Drop > 16.5% | Calcification |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.5 cm | 7.7 cm | Heterogeneous | 1.6 cm | No | – | – | Yes |
| 2 | 3.2 cm | 3.8 cm | Heterogeneous | 0.7 cm | Yes | – | – | Yes |
| 3 | 7 cm | 4.5 cm | Homogeneous | 0.7 cm | No | Yes | – | No |
| 4 | 4 cm | 4.2 cm | Homogeneous | 0.2 cm | Yes | – | – | No |
| 5 | 5 cm | 4.5 cm | Heterogeneous | 0.6 cm | No | No | – | No |
| 6 | 9.2 cm | 8.7 cm | Heterogeneous | 1.1 cm | No | No | – | Yes |
| 7 | 8.5 cm | 3.9 cm | Homogeneous | 0.5 cm | Yes | Yes | Yes | No |
| 8 | 5.2 cm | 5.1 cm | Heterogeneous | 0.2 cm | Yes | – | Yes | Yes |
| 9 | 6.1 cm | 5.8 cm | Heterogeneous | No fat seen | No | – | No | Yes |
| 10 | 3.5 cm | 7 cm | Heterogeneous | 1.0 cm | No | – | – | Yes |
| 11 | 2 cm | 2.8 cm | Homogeneous | 0.6 cm | No | Yes | – | No |
Pathology
Complete surgical resection of the adrenal adenoma was done in all 11 cases. Among these resected nodules, 4/11 (36.4%) had internal hemorrhage identified on pathology. The median reported size was 5.2 cm, compared to a median size of 4.5 cm on CT for these same nodules. All adenomas had at least one focus of myelolipomatous degeneration, although some contained several foci (Figure 4).
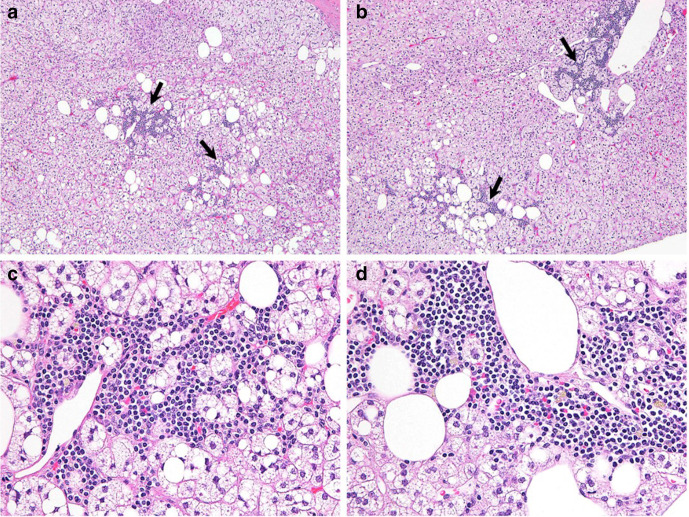
Figure 4.
Adrenal adenoma with myelolipomatous component. (a, b) Areas of myelolipomatous component (arrows) within adrenal adenoma (original magnification ×40). (c, d) High power views of myelolipomatous component with hematopoietic cells (original magnification ×200).
Discussion
To the best of our knowledge, this is the largest series to date reporting on this entity. Based solely on literature review, it appears to be rare, with our search yielding 16 prior individual cases among 11 reports Table 3.9–19 While perhaps this is a rare lesion, it may be underreported or underrecognized given the lack of formal classification and nomenclature. To compound this, macroscopic fat in an adrenal nodule on imaging is often thought to be diagnostic of a benign, and usually asymptomatic, myelolipoma, thus they are infrequently biopsied or resected. We show that this is not necessarily true. Additionally, it is important to note that an entirely different adrenal adenoma has also been reported containing only mature macroscopic fat but without the myelolipomatous elements, so-called lipoadenomas or adenolipomas.
Table 3.
Prior reports of adrenal adenomas with myelolipomatous degeneration
| Reporting author | Year | Total cases | Number with cortisol hypersecretion |
|---|---|---|---|
| Weiner et al9 | 1981 | 1 | 0 |
| Goetz et al10 | 1994 | 5 | 5 |
| Pasimeni et al11 | 2000 | 1 | 0 |
| Vrezas et al12 | 2003 | 1 | 1 |
| Manassero et al13 | 2004 | 1 | 0 |
| Armand et al14 | 2004 | 1 | 1 |
| Matsuda et al15 | 2004 | 1 | 1 |
| Ong et al16 | 2007 | 1 | 0 |
| Lamas et al17 | 2009 | 2 | 2 |
| Gurbuz et al18 | 2013 | 1 | 0 |
| Corpas Jiménez et al19 | 2014 | 1 | 1 |
| Total: | 16 | 11 |
In our experience, the fat component in these lesions makes up a very small percentage of the overall volume. The largest focus of fat in each lesion was subjectively identified and measured on CT or MRI, which on average made up 14.4% of the lesion in size, using the largest measurement. While frequently the adenoma contained multiple foci of macroscopic fat, if combined the total percentage of fat content is unlikely to be significantly higher. This is a relative limitation and could be corrected using multiplanar or 3D volumetric measurements of all macroscopic fat foci.
Adenomas are infrequently functional, secreting cortisol autonomously around 12% of the time or aldosterone in 2% of cases.2,20 In contrast, myelolipomas are almost always non-functional, with only a few reports of hypersecretion. In our study, four patients had findings of cortisol hypersecretion, two with clinical manifestations and two without. This was a relatively small and heterogenous sampling but around 36% of patients had findings of autonomous secretion, possibly even higher if all patients had been tested. This is more than is expected to be seen in adrenal adenomas or myelolipomas alone; However, it is consistent with previous reports of AMD. Of the 16 cases we compiled from the English and Spanish literature, 11 (68.8%) had evidence of cortisol hypersecretion and Cushing syndrome. Additionally, one of the reports described a hyperfunctioning nodule causing Conn syndrome from aldosterone hypersecretion.11 A smaller percentage of our nodules had documented cortisol hypersecretion, but this could be from a number of things. Not all patients were tested so perhaps more individuals had subclinical Cushing syndrome or had testing at an outside facility. Publication and selection biases may also play a role in previous reports, selecting cases with evidence of hormonal hypersecretion.
Adrenal adenomas can often be reliably differentiated on imaging and therefore are infrequently resected. However, atypical features may be present, such as hemorrhage, necrosis, or large size, which raises the suspicion for malignancy. Size is particularly important as it has been previously demonstrated that nodule size >4 cm on imaging may have a 70% chance of malignancy and that rises to 85% in nodules > 6 cm.21,22 Although the reason for resection was not clearly documented in all cases, most were removed either because of large size >4 cm or due to Cushing syndrome from a suspected hyperfunction nodule, although many otherwise had imaging features suggestive of adenoma. Five patients had an adrenal mass protocol CT, of which three had ≥60% absolutely washout, suggesting with high probability the diagnosis of adrenal adenoma. Four nodules demonstrated an attenuation of ≤10 HU prior to contrast, including in two patients that also had an adrenal mass protocol CT and/or MRI, which suggests a diagnosis of adenoma as well. In the three patients who had MRI, two nodules showed signal drop from in-phase to out-of-phase imaging >16.5% which suggests a diagnosis of adenoma based on microscopic fat content (Figure 5). Interestingly, one patient who did not have signal drop >16.5%, also lacked identifiable macroscopic on CT or MRI, despite multiple foci of myelolipomatous degeneration on pathology. Given the retrospective nature of the study, an adrenal mass protocol was unable to be obtained in all patients prior to resection. However, additional imaging may have been deemed unnecessary in those patients who would have undergone nodule resection regardless, based on size, nodule features, or clinical characteristics.
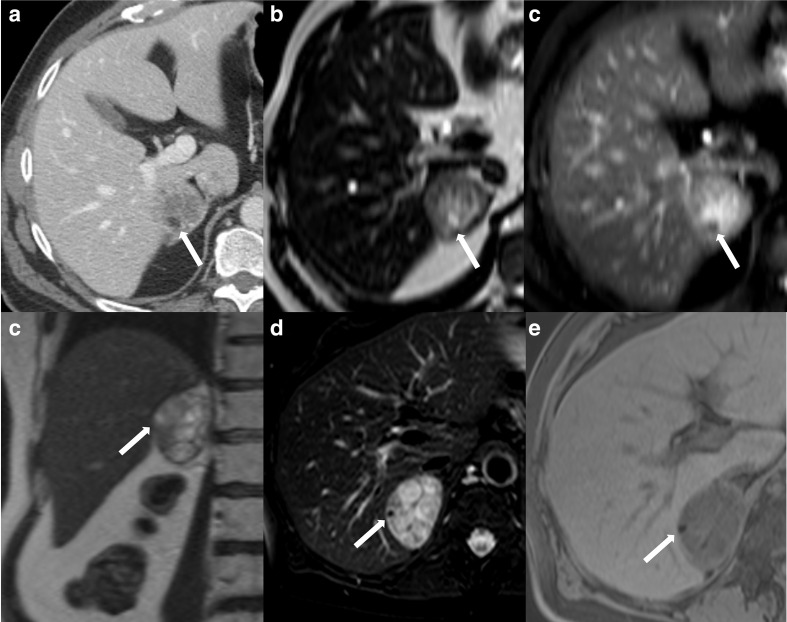
Figure 5.
52-year-old female with an incidentally discovered right adrenal nodule and subclinical Cushing syndrome. Contrast-enhanced axial CT through the upper abdomen (a) demonstrates a right adrenal nodule containing a tiny focus of macroscopic fat (arrow). Axial MRI T2-weighted (b) and T2-weighted fat-saturated (c) images show the same focus (arrow) lose signal with fat saturation, confirming the presence of macroscopic fat. An additional focus of fat seen on the coronal T2-weighted image (d) also shows loss of signal on fat-saturated T2-weighted (e) and fat-saturated T1-weighted (f) sequences. The nodule was pathologically proven as an adrenal adenoma with foci of myelolipomatous degeneration.
Conclusions
Not all adrenal masses with macroscopic fat on imaging are pure myelolipomas. We document 11 cases of pathologically confirmed AMDs, 10 of which had identifiable macroscopic fat on imaging. These foci of fat appear to make up a relatively small percentage of the overall size of adrenal nodule and further investigation may be required to see if there is an association between the fat content and nodule type. Finally, there appears to be a high incidence of cortisol hypersecretion and Cushing syndrome in these nodules, much higher than would be expected for adrenal adenomas or myelolipomas alone. This is consistent with prior literature and should prompt the consideration of Cushing syndrome or hormonal hypersecretion when macroscopic fat is found within an adrenal adenoma.
Contributor Information
Jeffrey Guccione, Email: jguccio@stanford.edu.
Moataz Soliman, Email: moataz_as20@hotmail.com.
Miao Zhang, Email: MZhang8@mdanderson.org.
Mouhammed Amir Habra, Email: MAHabra@mdanderson.org.
Katrina Collins, Email: katcoll@iu.edu.
Jianping Zhao, Email: JZhao9@mdanderson.org.
Khaled M Elsayes, Email: kmelsayes@mdanderson.org.
REFERENCES
- 1.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 2003; 149: 273–85. doi: 10.1530/eje.0.1490273 [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. European Journal of Endocrinology 2016; 175: G1–34. doi: 10.1530/EJE-16-0467 [DOI] [PubMed] [Google Scholar]
- 3.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 1998; 171: 201–4. doi: 10.2214/ajr.171.1.9648789 [DOI] [PubMed] [Google Scholar]
- 4.Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology 2002; 222: 629–33. doi: 10.1148/radiol.2223010766 [DOI] [PubMed] [Google Scholar]
- 5.Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F. Ct time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol 1998; 170: 747–52. doi: 10.2214/ajr.170.3.9490968 [DOI] [PubMed] [Google Scholar]
- 6.Israel GM, Korobkin M, Wang C, Hecht EN, Krinsky GA. Comparison of unenhanced CT and chemical shift MRI in evaluating lipid-rich adrenal adenomas. AJR Am J Roentgenol 2004; 183: 215–9. doi: 10.2214/ajr.183.1.1830215 [DOI] [PubMed] [Google Scholar]
- 7.Lam AK-yin, Lam AK. Lipomatous tumours in adrenal gland: who updates and clinical implications. Endocr Relat Cancer 2017; 24: R65–79. doi: 10.1530/ERC-16-0564 [DOI] [PubMed] [Google Scholar]
- 8.Elbanan MG, Javadi S, Ganeshan D, Habra MA, Rao Korivi B, Faria SC, et al. Adrenal cortical adenoma: current update, imaging features, atypical findings, and mimics. Abdom Radiol 2020; 45: 905–16. doi: 10.1007/s00261-019-02215-9 [DOI] [PubMed] [Google Scholar]
- 9.Weiner SN, Bernstein RG, Lowy S, Karp H. Combined adrenal adenoma and myelolipoma. J Comput Assist Tomogr 1981; 5: 440–2. doi: 10.1097/00004728-198106000-00027 [DOI] [PubMed] [Google Scholar]
- 10.Goetz SP, Niemann TH, Robinson RA, Cohen MB. Hematopoietic elements associated with adrenal glands. A study of the spectrum of change in nine cases. Arch Pathol Lab Med 1994; 118: 895–6. [PubMed] [Google Scholar]
- 11.Pasimeni G, Rossi F, Ragazzo M, Guerrini L, Markouizou A, Santiemma V. Adrenal adenoma and myelolipoma in an elderly patient with Conn’s syndrome. Recenti Prog Med 2000; 91: 116–8. [PubMed] [Google Scholar]
- 12.Vrezas I, Wentworth P, Bornstein SR. Myelolipomatous Foci in an Adrenal Adenoma Causing Cushing’s Syndrome? Endocr Res 2003; 29: 67–71. doi: 10.1081/ERC-120018677 [DOI] [PubMed] [Google Scholar]
- 13.Manassero F, Pomara G, Rappa F, Cuttano MG, Crisci A, Selli C. Adrenal myelolipoma associated with adenoma. International Journal of Urology 2004; 11: 326–8. doi: 10.1111/j.1442-2042.2004.00793.x [DOI] [PubMed] [Google Scholar]
- 14.Armand R, Cappola AR, Horenstein RB, Drachenberg CB, Sasano H, Papadimitriou JC. Adrenal cortical adenoma with excess black pigment deposition, combined with myelolipoma and clinical cushing’s syndrome: a case report and review of the literature. Int J Surg Pathol 2004; 12: 57–61. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda T, Abe H, Takase M, Arakawa A, Matsumoto T, Fujime M, et al. Case of combined adrenal cortical adenoma and myelolipoma. Pathol Int 2004; 54: 725–9. doi: 10.1111/j.1440-1827.2004.01686.x [DOI] [PubMed] [Google Scholar]
- 16.Ong K, Tan KB, Putti TC. Myelolipoma within a non-functional adrenal cortical adenoma. Singapore Med J 2007; 48: e200–2. [PubMed] [Google Scholar]
- 17.Lamas C, López L, Lozano E, Atienzar M, Ruiz-Mondéjar R, Alfaro J, et al. Myelolipomatous Adrenal Masses Causing Cushing’s Syndrome. Exp Clin Endocrinol Diabetes 2009; 117: 440–5. doi: 10.1055/s-0029-1202274 [DOI] [PubMed] [Google Scholar]
- 18.Gurbuz E, Sayar H, Bakaris S, Inci MF. Adrenal myelolipoma’s connection with adenoma in the same adrenal gland. Case Reports 2013; 2013(may20 1): bcr2013008925. doi: 10.1136/bcr-2013-008925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corpas Jiménez MS, Ortega Salas R, Tenorio Jiménez C, Molina Puerta MJ. Myelolipoma associated with adrenocortical adenoma: an unusual cause of Cushing’s syndrome. Endocrinología y Nutrición 2014; 61: e7–9. [DOI] [PubMed] [Google Scholar]
- 20.Arnold DT, Reed JB, Burt K. Evaluation and management of the incidental adrenal mass. Baylor University Medical Center Proceedings 2003; 16: 7–12. doi: 10.1080/08998280.2003.11927882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med 2007; 356: 2005. [DOI] [PubMed] [Google Scholar]
- 22.Blake MA, Holalkere N-S, Boland GW. Imaging techniques for adrenal lesion characterization. Radiol Clin North Am 2008; 46: 65–78. doi: 10.1016/j.rcl.2008.01.003 [DOI] [PubMed] [Google Scholar]