Abstract
Objectives:
The use of stereotactic body radiotherapy (SBRT) to treat ultra-central lung tumours remains more controversial than for peripheral and central tumours. Our objective was to assess toxicities, local control (LC) rate and survival data in patients with ultra-central lung tumours treated with SBRT.
Methods:
We conducted a retrospective and monocentric study about 74 patients with an ultra-central lung tumour, consecutively treated between 2012 and 2018. Ultra-central tumours were defined as tumours whose planning target volume overlapped one of the following organs at risk (OARs): the trachea, right and left main bronchi, intermediate bronchus, lobe bronchi, oesophagus, heart.
Results:
Median follow-up was 25 months. Two patients (2.7%) showed Grade 3 toxicity. No Grade 4 or 5 toxicity was observed. 11% of patients experienced primary local relapse. LC rate was 96.7% at 1 year and 87.6% at 2 years. Median progression free survival was 12 months. Median overall survival was 31 months.
Conclusion:
SBRT for ultra-central tumours remains safe and effective as long as protecting organs at risk is treatment-planning priority.
Advances in knowledge:
The present study is one of the rare to describe exclusively ultra-central tumours through real-life observational case reports. Globally, literature analysis reveals a large heterogeneity in ultra-central lung tumours definition, prescribed dose, number of fractions. In our study, patients treated with SBRT for ultra-central lung tumours experienced few Grade 3 toxicities (2.7%) and no Grade 4 or 5 toxicities, due to the highest compliance with dose constraints to OARs. LC remained efficient.
Introduction
Stereotactic body radiotherapy (SBRT) has become the standard of care for inoperable, early-stage non-small cell lung cancers (NSCLCs).1–3 It is also used for inoperable pulmonary metastases in case of controlled oligometastatic disease.4 SBRT definitely has a positive efficacy/toxicity ratio for peripheral lung tumours.5 Yet, this was counterbalanced by a prospective study by Timmerman et al showing that using the same 3-fraction schedule for central tumours caused more toxicities than for peripheral tumours. Indeed, 2 years’ survival with no grade ≥3 toxicity was 54% in the central tumours group vs 83% in the peripheral tumours group.6 Importantly, the RTOG 0813 trial defined central lesions as tumours contacting or overlapping a 2 cm zone around the proximal bronchial tree (carina, right and left main bronchi, intermediate bronchus and lobe bronchi) as well as tumours directly adjacent to the mediastinal pleura. In case of a 5-fractions dose escalation, 12 Gy/fraction was the highest tolerated dose and grade ≥3 toxicity was likely to be 7.2%. Local control (LC) rate at 2 years was 87.9% in the 12 Gy/fraction group.7 Treatment in five fractions seems to be better adapted to central lesions regarding toxicity and efficacy.
The use of SBRT for ultra-central tumours remains more controversial than for peripheral and central tumours. Some studies pointed out a higher risk of toxicity and a lower LC rate,8,9 mainly due to the proximity of organs at risk (OARs). Moreover, the definition of ultra-central tumours fails to win unanimous support10–12 as about 12 definitions can be found in the literature (tumour or planning target volume (PTV) directly contacting the proximal bronchial tree and even the trachea, oesophagus, pulmonary vessels and heart). In 2015, Chaudhuri et al were the first to specifically study ultra-central tumours. They defined them as tumours directly overlapping the trachea or the proximal bronchial tree.10 For other studies, they are tumours contacting the proximal bronchial tree only.9,13 Conversely, other reports add tumours whose PTV contact with the oesophagus or the pulmonary vein or artery.12,14 Finally, some authors include tumours contacting with the pericardium or the heart.15
The present study is one the rare studies exclusively dedicated to ultra-central locations. The main objectives of the study were to assess grade ≥3 toxicities, LC rate, survival data and dosimetric considerations.
Methods and materials
Patients’ characteristics
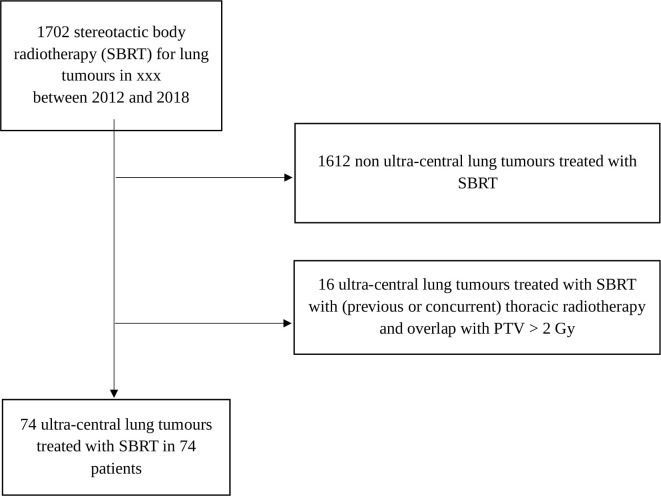
We carried out a retrospective study about all consecutive patients with an ultra-central pulmonary tumour. All patients were treated with SBRT in a unique Cancer Centre (Léon Bérard Cancer Centre Radiotherapy Department, Lyon, France) between January 2012 and December 2018. Patients’ selection is detailed in the flow chart (Figure 1). The study was registered in compliance with the General Data Protection Regulation (GDPR) under the number R201-004-045. All patients provided informed consent. Patients treated with SBRT for any ultra-central lesion were included – whether primary or not, parenchymal or nodal (mediastinal or hilar). Patients were accrued only in case of biopsy-confirmed disease or in case of hypermetabolic suspiciously growing lesion visible on the positron emission tomography (PET) scan.
Figure 1.
Flow chart. PTV, planning target volume; SBRT, stereotactic body radiotherapy.
Tumour location definition
Ultra-central tumours were defined as tumours whose PTV overlapped one of the following OARs: the trachea, right and left main bronchi, intermediate bronchus, lobe bronchi, oesophagus, heart. We did not include mediastinal vessels as their dose constraints are easily respectable unlike those to the main bronchi and oesophagus.
SBRT technique
Machines
Depending on machine implementation, treatment was delivered on three different machines namely CyberKnife® (Accuray Sunnyvale), Synergy® (Elekta, Stockholm, Sweden) and Versa HD® (Elekta, Stockholm, Sweden). The possibility or not for the Cyberknife® to track the tumour motion real-time because of its location and size justified the change of machine. Thus, some treatments were delivered on different machines.
Treatment planning
Simulation CT scans were performed on a Philips Brillance Big Bore® (Philips Healthcare, Cleveland, OH). A 4DCT scan associated with respiration was obtained for parenchymal lesions, without intravenous contrast injection. A 3DCT scan was obtained after i.v. injection for mediastinal and hilar tumours with a 2 mm slice thickness. Radiation total dose was delivered in 5–10 fractions in 4.5–10 Gy per fraction. Each fraction was delivered every 2 days.
PTV was usually obtained after a 5 mm margin was added to the GTV or ITV. Delineated OARs were the trachea, right and left main bronchi, intermediate bronchus, lobe bronchi, lungs, oesophagus, heart (pericardium included) and the medullary canal. Isodose covering PTV (often 80% isodose) was used for the treatment prescription. OAR constraints were the same as those from Timmerman et al. in 2008,16 EORTC LungTech17 and RTOG 08137 trials. When necessary, PTV was underdosed to protect OARs. Dosimetric parameters for each OAR were maximal dose (Dmax) except for homolateral lung for which it was median dose (Dmed).
Follow-up
Patients were followed every 3 months for the first 2 years and every 6 months thereafter. The radiation oncologists graded radiation induced toxicities using the Common Terminology Criteria for Adverse Events (CTCAE), v. 4.0. Regarding radiation pneumonitis, only symptomatic pneumonitis were taken into account. Toxicities occurring between 0 and 90 days after radiotherapy were considered as acute. Those occurring after 90 days were regarded as late toxicities. Patients were assessed by chest, abdomen and pelvis CT scan and brain imaging (MRI or CT). PET CT scan was optional. Tumour response was evaluated according to the RECIST criteria (Response Evaluation Criteria in Solid Tumours, v. 1.1). Local relapse was defined as recurrence in the irradiated site and regional relapse as mediastinal and ipsilateral lung recurrence.
Statistical analysis
Descriptive statistics were calculated and presented as frequency, percentage and median values (minimum, maximum). Follow-up duration was calculated from the first day of SBRT and the most recent follow-up or death. The events of interest to calculate LC and progression free survival (PFS) rates were the date of the first recurrence (local on the irradiated site, regional, metastatic) and the date of the first metastatic recurrence (in case of non-primary metastatic relapse). Last follow-up update was May 2020. Estimates for overall survival (OS), PFS and LC rates were calculated using the Kaplan–Meier method and univariate analysis used the Log-rank test. For the statistical analysis, some continuous variables (biologically effective dose with α/β = 10 (BED10)) were determined by significant clinical data. Fisher’s exact test was carried out for univariate analyses of qualitative variables using contingency tables.
In order to compare data about the different treatment plans, tumours BED10 were calculated to compare doses in different fractionations. Dosimetric parameters for target volumes were the different BED10: minimum (Dmin), maximum (Dmax), median, (Dmed), received by 1% of the PTV (D1%) and received by 98% of the PTV (D98%), as well as the average between D1% and D98%. The 2 Gy per fraction equivalent dose (EQD2) for OAR was calculated.
Data were analysed using a SPSS v. 20.0 software. p-value < 0.05 was considered to represent statistical significance.
Results
Patient and tumour characteristics
74 patients with ultra-central lung tumours treated with SBRT were retrospectively studied. They accounted for 4.4% of the 1702 lung SBRT treated in our centre. Patient and tumour characteristics are provided in Table 1. Patient median age was 69 years old, 46% of them were at least 70 years old.
Table 1.
Patient and tumour characteristics
| Patient and tumour characteristics | Number of patients (%) or median value (min-max) |
|---|---|
| Age at SBRT (years) | 69 (19–90) |
| Gender | |
| Female | 28 (37.8%) |
| Male | 46 (62.2%) |
| ECOG performance status | |
| 0/1/2/3/NA | 20 (27%) / 31 (41.9%) / 7 (9.5%) / 1 (1.4%) / 15 (20.3%) |
| Previous lung surgery | 20 (27%) |
| One lung only | 2 (2.7%) |
| Other thoracic radiation with <2 Gy overlap | |
| Previous | 9 (12.2%) |
| Concurrent | 6 (8.1%) |
| Type of primary cancer | |
| NSCLC | 37 (50%) |
| ADK/SCC/other histology | 21 (28.4%) / 14 (18.9%) / 2 (2.7%) |
| No histology | 18 (24.3%) |
| Sarcoma | 7 (9.5%) |
| Colorectal cancer | 4 (5.4%) |
| Kidney cancer | 3 (4.1%) |
| Thyroid cancer | 2 (2.7%) |
| Breast cancer | 1 (1.4%) |
| Prostate cancer | 1 (1.4%) |
| Pleural mesothelioma | 1 (1.4%) |
| Stage at time of treatment | |
| Localised disease | 39 (52.7%) |
| Metastatic disease | 30 (40.5%) |
| Localised relapse | 5 (6.8%) |
| Location of irradiated tumour | |
| Parenchymal tumour | 67 (90.5%) |
| Lymph node | 6 (8.1%) |
| Pleural tumour | 1 (1.4%) |
ADK: adenocarcinoma; ECOG: eastern cooperative oncology group; NA: not applicable; NSCLC: non-small cell lung cancer;SBRT: stereotactic body radiotherapy; SCC: squamous cell carcinoma.
Treatments
Treatment characteristics are summarised in Table 2. PTV overlapped the right and left main bronchi or intermediate bronchus or lobe bronchi (50% of cases), the heart (40.5%), the trachea (13.5%) and the oesophagus (10.8%).
Table 2.
Treatment characteristics
| Treatment characteristics | Number of patients (%) or median value (min-max) |
|---|---|
| Planning, days | 10 (5–25) |
| Ultra-central organs at risk overlapped by PTV | |
| Trachea | 10 (13.5%) |
| Right and left main bronchi | 12 (16.2%) |
| Intermediate bronchus and lobe bronchi | 25 (33.8%) |
| Oesophagus | 8 (10.8%) |
| Heart | 30 (40.5%) |
| Number of fractions | 5 (5–10) |
| 5 | 51 (68.9%) |
| 6 | 6 (8.1%) |
| 7 | 1 (1.4%) |
| 8 | 9 (12.2%) |
| 10 | 7 (9.5%) |
| Dose per fraction, Gy | 8 (4.5–10) |
| ≤6 Gy | 18 (24.3%) |
| From 7 to 8 Gy | 23 (31.1%) |
| ≥9 Gy | 33 (44.6%) |
| BED10 prescribed dose, Gy | 82 (28–105) |
| BED10 prescribed dose ≥100 Gy | 28 (37.8%) |
| BED10 prescribed dose <100 Gy | 46 (62.2%) |
| Prescription isodose, % | 80 (78–95) |
| 78–82% | 69 (93.2%) |
| 90–95% | 5 (6.8%) |
| GTV, mL | 18.3 (1–108) |
| PTV, mL | 51.6 (6.6–243) |
BED10: biologically effective dose with α/β = 10; GTV: Gross tumour volume;PTV: planning target volume.
Treatments were performed on the CyberKnife®, the VersaHD® and the Synergy® for 52 patients (70.3%), 14 patients (18.9%) and 8 patients (10.8%), respectively. Several fractionation schedules were prescribed. Most treatments were performed in 5 fractions (68.9%) with 10 Gy per fraction for 47.1%, 9 or 9.5 Gy per fraction for 17.7%, 8 Gy per fraction for 25.5% and 5.5 to 7.6 Gy per fraction for 9.8%. 12.1% of patients were treated in 8 fractions, with 7.5 Gy per fraction for 50% and 5 to 6 Gy for 50%. 9.5% of patients were treated in 10 fractions of 4.5 to 5 Gy. 8.1% of treatments required 6 fractions of 5.7 to 7.5 Gy, and 1.4% of treatments required 7 fractions of 6 Gy. 50 Gy in 5 fractions of 10 Gy was the fractionation schedule the mostly used (32.4%). Median prescribed BED10 was 82 Gy and BED10 was ≥100 Gy in 37.8% of treatments. Prescription isodose ranged from 78 to 82% for most treatments (93.2%).
Dosimetry
Dosimetric parameters are listed in Table 3. Median GTV BED10 was 99 Gy.
Table 3.
Dosimetry
| Dosimetric parameters | Median value (min-max) |
|---|---|
| GTV Dave (BED10), Gy | 99 (54–139) |
| GTV Dmed (BED10), Gy | 99 (53–140) |
| GTV Dmin (BED10), Gy | 65 (39–120) |
| GTV Dmax (BED10), Gy | 112 (59–151) |
| D98% PTV (BED10), Gy | 64 (28–98) |
| D1% PTV (BED10), Gy | 110 (58–149) |
| Dave PTV (between D1% and D98%) (BED10), Gy | 86 (51–121) |
| Dave PTV (between D1% and prescribed dose) (BED10), Gy | 96 (50–126) |
| PTV coverage, % | 87 (40–100) |
| Paddick conformity index | 0,72 (0.1–0.89) |
| Dmax trachea (EQD2 3/α = 3), Gy | 8 (0–155) |
| Dmax bronchi (EQD2 3/α = 3), Gy | 65 (0–188) |
| Dmax eosophagus (EQD2 3/α = 3), Gy | 22 (2–91) |
| Dmax heart (EQD2 3/α = 3), Gy | 65 (0–173) |
| Dave homolateral lung (EQD2 3/α = 3), Gy | 6 (1–24) |
BED10: biologically effective dose with α/β = 10; D1%: dose received by 1% of PTV; D98%: dose received by 98% of PTV; D: dose; Dave: average dose; Dmax: maximum dose; Dmed: median dose; Dmin: minimum dose; EQD2: 2 Gy per fraction equivalent dose;GTV: gross tumour volume; PTV: planning target volume.
Toxicities
Acute toxicities are described in Table 4. 44 patients out of 74 (59.5%) experienced no toxicity. 32 Grade 1 toxicities were reported. Two patients (2.7%) had Grade 3 toxicities: 1 pneumonitis and 1 oesophagitis. Clinical cases corresponding to these toxicities are detailed in Table 5. No patient experienced Grade 4 or 5 toxicities. Fatigue was the most common toxicity: 27.1% (n = 20) of patients (Grade o1 and/or 2). Median time to acute toxicities occurrence was 11 days (range 5–81).
Table 4.
Acute toxicities
| Acute toxicities | Grade 1 | Grade 2 | Grade 3 | |||
|---|---|---|---|---|---|---|
| Anorexia | 1 | 1.4% | 0 | 0% | 0 | 0% |
| Fatigue | 17 | 23.0% | 3 | 4.1% | 0 | 0% |
| Lung toxicities | ||||||
| Cough | 8 | 10.9% | 1 | 1.4% | 0 | 0% |
| Dyspnea | 4 | 5.4% | 0 | 0% | 0 | 0% |
| Hemoptysis | 1 | 1.4% | 0 | 0% | 0 | 0% |
| Pneumonitis | 0 | 0% | 0 | 0% | 1 | 1.4% |
| Oesophagitis | 1 | 1.4% | 2 | 2.7% | 1 | 1.4% |
Table 5.
Grade three acute toxicities
| Characteristics | Oesophagitis | Pneumonitis |
|---|---|---|
| History | 61 years old, no previous radiotherapy or thoracic surgery, no diabetes | 83 years old, no previous radiotherapy or thoracic surgery, no diabetes |
| Irradiated tumour | Parenchymal primary tumour (adenocarcinoma) | Parenchymal primary tumour (no histology) |
| Prescribed dose | 50 Gy in 5 fractions on 80% isodose, BED10 = 100 Gy | 40 Gy in 5 fractions on 80% isodose, BED10 = 72 Gy |
| OAR | Oesophagus | Heart |
| Dosimetry | Dmax oesophagus = 64,1 Gy | Dmax heart = 86 Gy; Dave homolateral lung = 4,6 Gy |
| PTV coverage | 94% | 92% |
| Paddick conformity index | 0.79 | 0.79 |
| PTV, mL | 53.5 | 42.7 |
| Planning, days | 11 | 7 |
| Time to Grade 3 toxicities, days | 11 | 42 |
BED10, biologically effective dose with α/β = 10; D, dose; Dave, average dose; Dmax, maximum dose; GTV, gross tumour volume; OAR, organ at risk; PTV, planning target volume.
Four acute oesophagitis were reported; PTV overlapped or contacted with either oesophagus (3 patients) or trachea (1 patient) in all those cases. When PTV overlapped or contacted with oesophagus, oesophagitis were recurrent (p = 0.007) and of higher grade (p = 0.004). Oesophagitis were also more common when Dmax to oesophagus was >50 Gy in EQD2 (α/β = 3) (p = 0.005). A Dmax to oesophagus in EQD2 (α/β = 3)>64 Gy (Dmax accepted in LungTech trial17) was linked with oesophagitis (p = 0.009) and with higher-grade oesophageal toxicity (p = 0.005). Only one Grade 3 oesophagitis was reported, 11 days after the start of SBRT: PTV overlapped with oesophagus and Dmax oesophagus was 64.1 Gy (EQD2) for a prescribed dose of 50 Gy in 5 fractions on 80% isodose line.
Patients whose PTV overlapped or contacted with the heart experienced more acute lung toxicities (p = 0.014) and more coughing (p = 0.024). There was no correlation between dosimetric parameters regarding the heart, bronchi, trachea, ipsilateral lung and acute lung toxicities. There was only one Grade 3 lung toxicity, which could be radiotherapy-induced. Indeed, the patient experienced some symptoms of pneumonitis but no fever or inflammatory syndrome. Besides, images showed possible radiation-induced lung injury. It appeared 42 days post-radiation in an 83-year-old patient with a medical history of chronic respiratory failure requiring home oxygen. He had to be hospitalised and fully recovered from pneumonia.
Grade 1 dermatitis was the sole reported late toxicity (1.4%), it was observed 10 months after SBRT start.
Efficacy
Median follow-up was 25 months (range: 3–86). At last follow-up, 31.1% (n = 23) of the patients were alive and 5.4% (n = 4) of the patients were lost to follow-up.
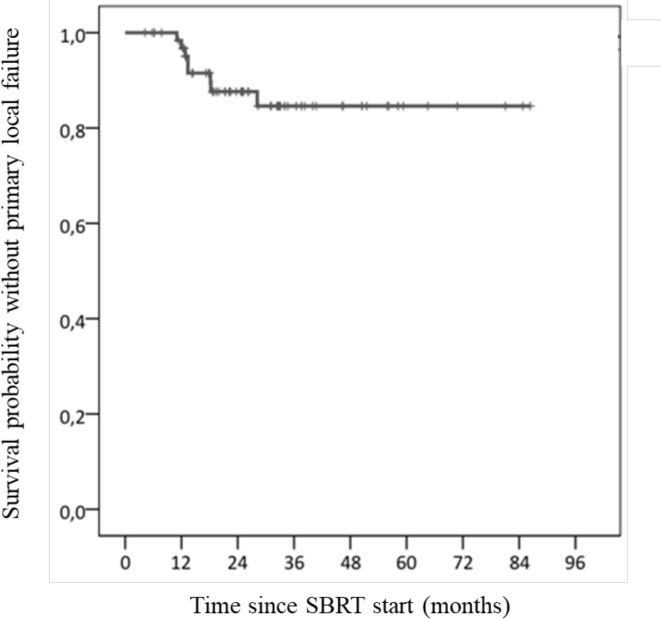
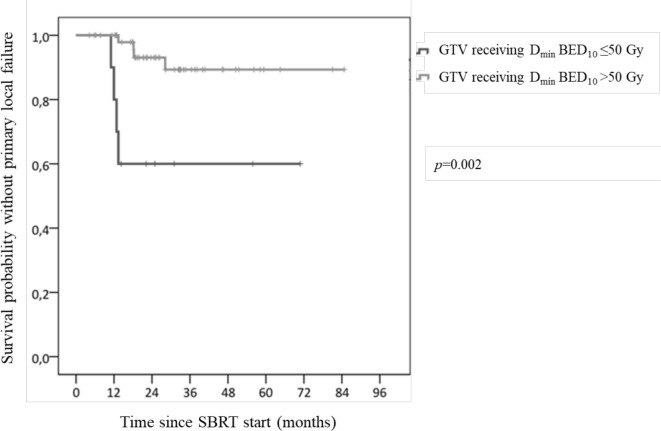
At a 7 month median time (range: 1–17), 8 patients (10.8%) had primary local relapse. They first relapsed in the irradiated site either before or concurrently to regional and/or metastatic recurrence. Four patients (5.4%) had an isolated primary local relapse and four others experienced local and regional and/or metastatic primary relapse. At 1 and 2 years, primary local control rates were 96.7 and 87.6%, respectively (Figure 2). LC rate did not differ between patients with localised cancer and those with metastatic cancer (or localised relapse). The type of OAR overlapping with PTV made no difference. Patients treated with PTV prescribed BED10 <50 Gy experienced more primary local relapse (p = 0.001). When treatment was delivered with PTV prescribed BED10 at 60, 70, 90 or 100 Gy, no difference was evidenced. Local recurrences were more common with GTV receiving Dmin BED10 ≤50 Gy (p = 0.002) (Figure 3). No correlation was found between LC rate and the following doses to GTV (BED10): mean, median and maximum doses. Similarly, no correlation was observed between LC rate and dosimetry doses to PTV (BED10): D1%, D98%, Dave (between D98% and D1%) or between LC rate and PTV coverage, GTV volume, and PTV volume. Regarding the four isolated local primary relapses, they were correlated with a Paddick conformity index ≤0.8 (p = 0.042).
Figure 2.
Local control rate. SBRT, stereotactic body radiotherapy.
Figure 3.
Local control rate separately plotted for Dmin BED10 received by GTV. BED, biologically effectve dose; GTV, gross tumour volume; SBRT, stereotactic body radiotherapy.
12 patients (16.2%) presented with primary regional recurrence, 8 (66.7%) of them had an isolated regional recurrence. The risk of regional recurrence was higher in patients with localised relapse than in those with localised or metastatic disease (p = 0.007). The risk of regional recurrence was higher if local relapse occurred during follow-up (p = 0.004). 41 patients (55.4%) had metastatic recurrence. For 32 of them (78%), it was their first recurrence after SBRT whereas for the 9 others (22%) it occurred after local and/or regional relapse.
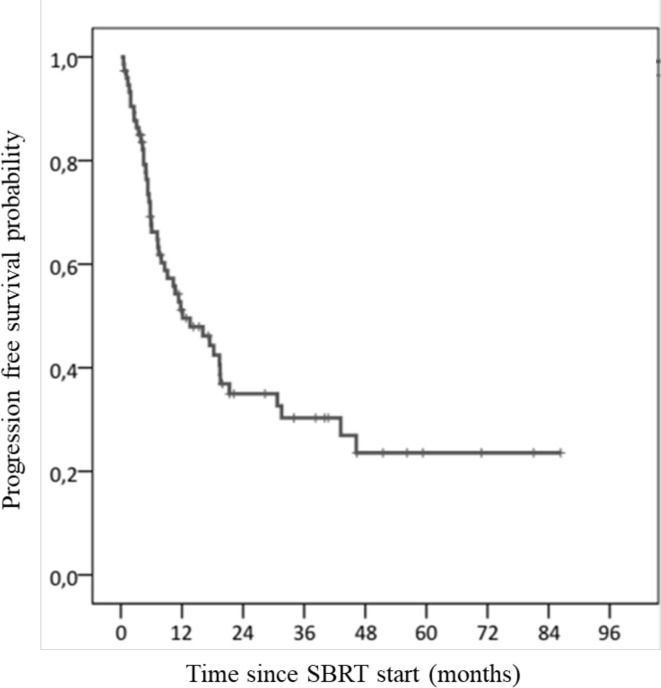
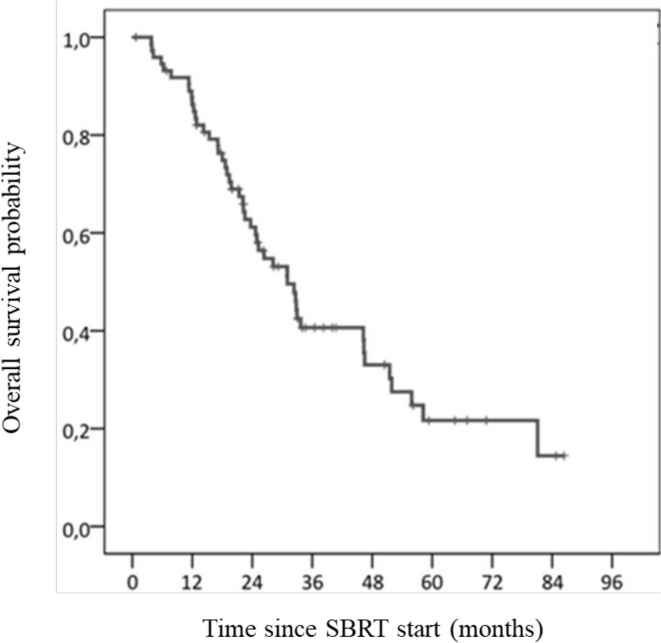
Median PFS was 12 months (95% CI: 5–20). PFS at 1 and 2 years were 51.2% (95% CI: 45.2–57.2) and 35% (95% CI: 28.9–41.1), respectively (Figure 4). By the end of the study, 47 patients had relapsed (63.5%). Median OS was 31 months (95% CI: 23–39). OS were 86.2% (95% CI: 82.1–90.3) at 1 year and 61.2% (95% CI: 55.3–67.1) at 2 years (Figure 5). Median age of death was 70 years old (range: 20–93). There was no difference between the median OS of patients with primary localised tumour (32 months) (95% CI: 19–46) and patients with localised recurrence or metastases (31 months) (95% CI: 25–37). Patients with metastatic relapse after SBRT had a lower OS (p = 0.044).
Figure 4.
Progression free survival rate. SBRT, stereotactic body radiotherapy.
Figure 5.
Overall survival rate. SBRT, stereotactic body radiotherapy.
Discussion
The present study is one of the rare to describe exclusively ultra-central tumours through real-life observational case reports. The 74 patients who were included represent a very low number of patients (4.4%) in view of the 1702 SBRT procedures for lung tumours in our Cancer Centre. In literature, dedicated data remain scarce and insufficiently reported. Few patients experienced grade ≥3 toxicities (2.7%) and none had Grade 5. LC was 96.7% at 1 year and 87.6% at 2 years. Median OS was 31 months.
As this kind of location remains uncommon, few significant articles can be found in literature. All the studies about ultra-central lung tumours treated with SBRT are retrospective and include no more than 100 patients. Globally, literature analysis reveals a large heterogeneity regarding ultra-central lung tumours definition, prescribed dose, number of fractions. Indeed, the prescribed dose was usually either 50 Gy or 60 Gy, delivered with 5 to 15 fractions of 5 to 12 Gy.10,13,18 The BED10 ranged from 75 to 132 Gy. The most commonly described SBRT schedule for ultra-central tumours was 60 Gy in 8 fractions of 7.5 Gy.14,15 In our study, we mostly used five fractions (68.9% of treatments) of 10 Gy so 50 Gy were performed on the whole (32.4%). When OAR dose constraints could not be respected, the radiation oncologist reduced the dose per fraction or even shifted from 5 to for 8 (12.2% of cases) or 10 (9.5%) fractions.
In the present study, 2.7% (n = 2) of patients experienced grade ≥3 toxicities, without toxicity-related death. BED10 was <100 Gy in 62.2% of patients. Yang et al and Cong et al obtained similar results for ultra-central SBRT with less than 5% of severe toxicities.15,19 Other studies about ultra-central SBRT found more grade ≥3 and Grade 5 toxicities. Wang et al found 22% of grade ≥3 and 11% of Grade 5, with 10% of patients who received antiangiogenic agents within 90 days of SBRT.20,21 In Tekatli et al trial, 38% of patients had grade ≥3 toxicities and 21% had Grade 5.17 Conversely, Mazzola et al. – who used what they called “simultaneous integrated protection” (SIP) that is to say intensity-modulated prescription dose to nearby OARs – reported fewer toxicities. Indeed, out of the 40 patients included in their study, 5 and 7% experienced acute and late toxicities, respectively.22 The fact that reported toxicity in literature is much higher than the one presented in the present study can be explained by the decision to strictly comply with the dose constraints.
5% of oesophageal toxicities were observed in our study. It was statistically significant when PTV overlapped with oesophagus volume. When Dmax to oesophagus was >64 Gy (EQD2) – a higher constraint than in the LungTech Trial17 – the risk of oesophagitis was higher as well as its grade. Duijm et al showed that meeting with usual dose constraints to oesophagus for central SBRT induced a low risk of high grade toxicity.23
The low rate of radiation-induced pneumonitis could be explained by an ipsilateral lung Dave <9.14 Gy (6 Gy in the present study).24 Moreover, we only focused on pneumonitis symptoms because radiological imaging was less clinically significant. The rate of lung toxicity was higher when PTV overlapped the heart. In a large cohort of NSCLC patients, Tucker et al found no evidence that incidental heart exposure during standard fractionation radiotherapy had a noticeable impact on the occurrence of moderate or severe radiation-induced pneumonitis.25 However, Wong et al showed that bilateral ventricles Dmax was associated with poorer survival (no cancer-related death).26
Tumours contacting the trachea and/or the proximal bronchial tree should be regarded as ultra-central tumours and thus, dose fractionation should be adapted to avoid potential severe toxicities such as stenosis, necrosis, fistula, pulmonary haemorrhage and even pneumonitis.21 Nevertheless, tumours whose PTV overlapped oesophagus (One Grade 3 oesophagitis in our study) should also be taken into account, even if some trials decided against it.13,27 Moreover, further investigations are required to consider tumours contacting the heart as ultra-central tumours. Thus, limiting the definition of ultra-central tumours to those contacting the trachea and/or the proximal bronchial tree may not be satisfactory to us.
In the present study, with a median prescribed BED10 = 82 Gy and a prescribed BED10 <100 Gy for 62.2% of the lesions, a low LC rate could be expected, according to studies published about 10 years ago.28,29 Moreover, the median PTV coverage was 87% and the median Paddick conformity index was 0.72. Yet, we obtained a LC rate of 96.7% at 1 year and 87.6% at 2 years; 10.8% of patients had a primary local relapse. Similar results were reported by Yang et al and in the meta-analysis by Chen et al..15,30 In the present study, a prescribed BED10 <50 Gy was correlated with an increasing risk of local recurrence. Loi et al found BED10 >75 Gy was associated with higher LC rate.14 In a modelling study, Klement et al showed that in lung SBRT, the following BED10 of PTV were predictive of LC rate: D1%, prescribed dose.31 Such a link was not found in our study but Klement’s study was not specifically about ultra-central tumours.
Conclusion
To sum up, in the present study, patients treated with SBRT for ultra-central lung tumours experienced few Grade 3 toxicities (2.7%) and no Grade 4 or 5 toxicities, due to the highest compliance with dose constraints to OAR. In terms of LC rate, SBRT remained efficient. Nevertheless, prospective data are lacking. Yet, the Phase 3 SUNSET trial is currently ongoing and aims at determining safety of SBRT with a dose escalation design (starting dose 60 Gy in 8 fractions and other levels with 5, 6, 10 fractions).32 As long as the results of ongoing prospective trial have not been published, SBRT for ultra-central tumours should be performed with caution.
Contributor Information
Elodie Guillaume, Email: Elodiie42@live.fr.
Ronan Tanguy, Email: ronan.tangy@lyon.unicancer.fr.
Myriam Ayadi, Email: myriam.ayadi@lyon.unicancer.fr.
Line Claude, Email: line.claude@lyon.unicancer.fr.
Sandrine Sotton, Email: sandrinesotton42@gmail.com.
Coralie Moncharmont, Email: coralie.moncharmont@lyon.unicancer.fr.
Nicolas Magné, Email: nicolas.magne@icloire.fr.
Isabelle Martel-Lafay, Email: isabelle.martel-lafay@lyon.unicancer.fr.
REFERENCES
- 1.Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol 2017; 7(no 5): 295–301. doi: 10.1016/j.prro.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 2.Falkson CB, Vella ET, Yu E, El-Mallah M, Mackenzie R, Ellis PM, et al. Guideline for radiotherapy with curative intent in patients with early-stage medically inoperable non-small-cell lung cancer. Curr Oncol 2017; 24: 44–9. doi: 10.3747/co.24.3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. doi: 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodeweges JE, Klinkenberg TJ, Ubbels JF, Groen HJM, Langendijk JA, Widder J. Long-Term outcome of surgery or stereotactic radiotherapy for lung Oligometastases. J Thorac Oncol 2017; 12: 1442–5. doi: 10.1016/j.jtho.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 5.Guckenberger M, Andratschke N, Dieckmann K, Hoogeman MS, Hoyer M, Hurkmans C, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol 2017; 124: 11–17. doi: 10.1016/j.radonc.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006; 24: 4833–9. doi: 10.1200/JCO.2006.07.5937 [DOI] [PubMed] [Google Scholar]
- 7.Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, et al. Safety and efficacy of a Five-Fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG Oncology/RTOG 0813 trial. J Clin Oncol 2019; 37: 1316–25. doi: 10.1200/JCO.18.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly M, Novak J, Monjazeb A. P2.05-056 safety of stereotactic body radiotherapy for central, ultracentral, and paramediastinal lung tumors. Journal of Thoracic Oncology 2017; 12: S1066. doi: 10.1016/j.jtho.2016.11.1491 [DOI] [Google Scholar]
- 9.Meng M-B, Wang H-H, Zaorsky NG, Sun B-S, Zhu L, Song Y-C, et al. Risk-adapted stereotactic body radiation therapy for central and ultra-central early-stage inoperable non-small cell lung cancer. Cancer Sci 2019; 110: 3553–64. doi: 10.1111/cas.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri AA, Tang C, Binkley MS, Jin M, Wynne JF, von Eyben R, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015; 89: 50–6. doi: 10.1016/j.lungcan.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Tekatli H, Haasbeek N, Dahele M, De Haan P, Verbakel W, Bongers E, et al. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with "Ultracentral" Non-Small Cell Lung Cancer. J Thorac Oncol 2016; 11: 1081–9. doi: 10.1016/j.jtho.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 12.Raman S, Yau V, Pineda S, Le LW, Lau A, Bezjak A, et al. Ultracentral tumors treated with stereotactic body radiotherapy: single-institution experience. Clin Lung Cancer 2018; 19: e803–10. doi: 10.1016/j.cllc.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Murrell DH, Laba JM, Erickson A, Millman B, Palma DA, Louie AV. Stereotactic ablative radiotherapy for ultra-central lung tumors: prioritize target coverage or organs at risk? Radiation Oncology 2018; 13. doi: 10.1186/s13014-018-1001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loi M, Franceschini D, Dominici L, Franzese C, Chiola I, Comito T, et al. Stereotactic radiotherapy for ultra-central lung oligometastases in non-small-cell lung cancer. Cancers 2020; 12: 885. doi: 10.3390/cancers12040885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Cui J, Zhao J, You J, Yu R, Yu H, et al. Stereotactic ablative radiotherapy of 60 Gy in eight fractions is safe for ultracentral non-small cell lung cancer. Thorac Cancer 2020; 11: 754–61. doi: 10.1111/1759-7714.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol 2008; 18: 215–22. doi: 10.1016/j.semradonc.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 17.Adebahr S, Collette S, Shash E, Lambrecht M, Le Pechoux C, Faivre-Finn C, et al. LungTech, an EORTC phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol 2015; 88: 20150036. doi: 10.1259/bjr.20150036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen KNB, Hause DJ, Novak J, Monjazeb AM, Daly ME. Tumor control and toxicity after SBRT for ultracentral, central, and paramediastinal lung tumors. Pract Radiat Oncol 2019; 9: e196–202. doi: 10.1016/j.prro.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong Y, Sun B, Wang J, Meng X, Xuan L, Zhang J, et al. Outcomes and toxicity of stereotactic body radiation therapy for advanced stage ultra-central non-small cell lung cancer. Thorac Cancer 2019; 10: 1567–75. doi: 10.1111/1759-7714.13105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Rimner A, Gelblum DY, Flynn J, Jackson A, Yorke E, et al. Analysis of toxic effects with antiangiogenic agents plus stereotactic body radiation in Ultracentral lung tumors. JAMA Oncol 2019; 5: 737. doi: 10.1001/jamaoncol.2019.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Rimner A, Gelblum DY, Dick-Godfrey R, McKnight D, Torres D, et al. Analysis of pneumonitis and esophageal injury after stereotactic body radiation therapy for ultra-central lung tumors. Lung Cancer 2020; 147: 45–8. doi: 10.1016/j.lungcan.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzola R, Ruggieri R, Figlia V, Rigo M, Giaj Levra N, Ricchetti F, et al. Stereotactic body radiotherapy of central lung malignancies using a simultaneous integrated protection approach. Strahlenther Onkol 2019; 195: 719–24. doi: 10.1007/s00066-018-01419-0 [DOI] [PubMed] [Google Scholar]
- 23.Duijm M, van der Voort van Zyp NC, van de Vaart P, Oomen-de Hoop E, Mast ME, Hoogeman MS, et al. Predicting high-grade esophagus toxicity after treating central lung tumors with stereotactic radiation therapy using a normal tissue complication probability model. Int J Radiat Oncol Biol Phys 2020; 106: 73–81. doi: 10.1016/j.ijrobp.2019.08.059 [DOI] [PubMed] [Google Scholar]
- 24.Chang JY, Liu H, Balter P, Komaki R, Liao Z, Welsh J, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiat Oncol 2012; 7: 152. doi: 10.1186/1748-717X-7-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tucker SL, Liao Z, Dinh J, Bian SX, Mohan R, Martel MK, et al. Is there an impact of heart exposure on the incidence of radiation pneumonitis? analysis of data from a large clinical cohort. Acta Oncol 2014; 53: 590–6. doi: 10.3109/0284186X.2013.831185 [DOI] [PubMed] [Google Scholar]
- 26.Wong OY, Yau V, Kang J, Glick D, Lindsay P, Le LW, et al. Survival impact of cardiac dose following lung stereotactic body radiotherapy. Clin Lung Cancer 2018; 19: e241–6. doi: 10.1016/j.cllc.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Wang H-H, Zaorsky NG, Meng M-B, Zeng X-L, Deng L, Song Y-C, et al. Stereotactic radiation therapy for oligometastases or oligorecurrence within mediastinal lymph nodes. Oncotarget 2016; 7: 18135–45. doi: 10.18632/oncotarget.7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen NP, Garland L, Welsh J, Hamilton R, Cohen D, Vinh-Hung V. Can stereotactic fractionated radiation therapy become the standard of care for early stage non-small cell lung carcinoma. Cancer Treat Rev 2008; 34: 719–27. doi: 10.1016/j.ctrv.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 29.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2: S94–100. doi: 10.1097/JTO.0b013e318074de34 [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Laba JM, Zayed S, Boldt RG, Palma DA, Louie AV. Safety and effectiveness of stereotactic ablative radiotherapy for ultra-central lung lesions: a systematic review. J Thorac Oncol 2019; 14: 1332–42. doi: 10.1016/j.jtho.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 31.Klement RJ, Sonke J-J, Allgäuer M, Andratschke N, Appold S, Belderbos J, et al. Correlating dose variables with local tumor control in stereotactic body radiation therapy for early-stage non-small cell lung cancer: a modeling study on 1500 individual treatments. Int J Radiat Oncol Biol Phys 2020; 107: 579–86. doi: 10.1016/j.ijrobp.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 32.Giuliani M, Mathew AS, Bahig H, Bratman SV, Filion E, Glick D, et al. SUNSET: stereotactic radiation for ultracentral non-small-cell lung cancer-a safety and efficacy trial. Clin Lung Cancer 2018; 19: e529–32. doi: 10.1016/j.cllc.2018.04.001 [DOI] [PubMed] [Google Scholar]