Abstract
The International Subarachnoid Aneurysm Trial (ISAT) showed superiority for endovascular treatment of ruptured aneurysms and technology has since moved on rapidly. Many approaches and technology now exist for the endovascular management of ruptured and unruptured intracranial aneurysms, which reflects their varied nature – there is no one-size-fits-all technique. We aim to provide an overview of the various classes of device and the major developments over the past decade. Coiling is the oldest of the technology and continues to demonstrate high levels of occlusion and acceptable risks, making it the default treatment choice, particularly in the acutely ruptured aneurysm setting. Advances on coiling include the use of adjuncts such as balloons, stents and fully retrievable temporary neck-bridging devices, which have facilitated the treatment of more complex aneurysms. Flow divertors have also revolutionised complex aneurysm treatment with small added risk in acute aneurysm treatment and seek to remodel the aneurysm–vessel interface without accessing the aneurysm sac. The latest development and most promising avenue appears to be intrasaccular flow disrupting devices like WEB, Contour and Neqstent that provide excellent opportunities to treat wide neck complex aneurysm with minimal mortality and morbidity and good occlusion rates and may in future replace a significant number of stent-assisted coiling too.
Introduction
Endovascular treatment of aneurysms has changed since the International Subarachnoid Aneurysm Trial (ISAT) reported superior outcomes with coiling compared to surgery for ruptured aneurysms.1 Since coiling’s introduction, recanalization and retreatment have been a concern.2 Key factors include aneurysm location, diameter and neck size.3 Balloon-assisted coiling (BAC) was one of the first major developments in technique that remains popular and prospective, multicentre evidence, for example, Analysis of Recanalization after Endovascular Treatment of Intracranial Aneurysm (ARETA) support the popularity, safety and feasibility of coiling.4,5 BAC offers higher packing density and control of proximal vessel flow.6 In this review, however, we provide an overview of more recent technology advances since ISAT, specifically the use of temporary and permanent stent-assisted coiling (SAC), flow divertors (FD) and intrasaccular flow disruptors (ISFD, and the reader is directed to other articles which describe earlier reviews on this topic.3
Stent-assisted coiling
SAC facilitates treatment of more complex aneurysms. A variety of techniques exist: the “coil-through” technique; the “jailing” technique ; dual-stenting for wide-necked bifurcation aneurysms (WNBA) – typically used in “X” or “Y” configurations to preserve flow in the parent and branch vessels; temporary stenting – retrievable stents are used to support coiling and then removed.7 This section will firstly discuss the temporary stent-like bridging devices and then discuss permanent SAC – an in-depth discussion of SAC can be found in other reviews.7 An overview of the evidence discussed is provided in Table 1.
Table 1.
Overview of stent-assisted coiling devices and main evidence discussed
| Device | Study (Author, year) | Patients, aneurysms | Ruptured aneurysms? | Occlusion rate | Morbidity; mortality (%) |
|---|---|---|---|---|---|
| Comaneci | Sirakov et al8 | 29, 29 | Ruptured | 97% complete at 3 months | 0; 3.44 |
| Cascade | Sirakov et al8 | 12, 12 | Ruptured | 75% complete at end-of-procedure | 0; 0 |
| Tomasello et al9 | 15, 15 | Mostly unruptured | 83.3% complete at 6 months | 0a; 0 | |
| Meta-analysis: SAC vs coiling | Zhang et al10 | 1758, 1857 | Ruptured | SAC: 73.4% at 9b months (61% coiling) | SAC: 20.2% (13.1% coiling)c; SAC: 6.3% (6.2% coiling) |
| Meta-analysis: SAC vs coiling | Zhao et al11 | 2446, 2556 | Mostly unruptured | SAC: 73% complete or near complete (80.1% coiling) at 6 months or later. | SAC: 2.8% (1.9% coiling); SAC: 1 (1.1% coiling) |
| Neuroform Atlas | Jankowitz et al12 | 30, 30 | Unruptured | 92.6% complete at 12 months | 3.3, 0 |
| Ciccio et al13 | 55, 55 | Mostly unruptured | 87% complete at 16 months | 12.7, 0 | |
| Pranata et al14, meta-analysis | 557, 568 | Mostly unruptured | 80% complete at 9 months | 6c, NR | |
| Acclino | Kabbasch et al15 | 14, 14 | Mostly unruptured | 57% complete at end-of-procedure | 7, 0 |
| LVIS and LVIS Jr | Fiorella et al16 | 153, 153 | Mostly unruptured | 79.1% complete at 12 months | 3.9d, 2d |
| LVIS EVO | Vollherbst et al17 | 57, 59 | Mostly unruptured | 54.2% complete at end-of-procedure | 6.8, 0 |
| Poncyljusz et al18 | 30, 35 | Mostly unruptured | 100% complete at end-of-procedure | 3c, 3 | |
| LEO | Sedat et al19 | 156, 158 | Unruptured | 85% complete at 36 months | 9.15, 0 |
NR, Not reported; SAC, Stent-assisted coiling.
No procedural complications reported. 4/5 patients with ruptured aneurysms were discharged with mRS of 2–3.
Mean follow-up time calculated from result tables in the study.
Peri-operative complication rate is reported.
Morbidity = number of patients with permanent neurological morbidity related to ipsilateral stroke and mortality = neurological death during study.
Bridging stent devices
Temporary neck-bridging devices
Temporary neck bridging devices, for example, Comaneci (Rapid Medical, Israel) and Cascade (Perflow Medical Ltd, Netanya, Israel) are retrievable stent-like devices and stabilise the coil mass but do not occlude the flow within the parent vessel. In older patients, flow arrest with BAC may risk neurological deficit, whereas Comaneci or Cascade causes flow restriction.
The Comaneci is a radio-opaque compliant mesh made from 12 nitinol wires with a flexible tip (Figure 1), whereas the Cascade is a braided net-like structure made from 42 interwoven nitinol and platinum wires. Both are retrieved and, therefore, do not require dual-antiplatelets. Cascade has a higher cell density and small cell size (0.3 mm2) with less risk of coil entanglement, or protrusion and also aneurysmal flow reduction.9
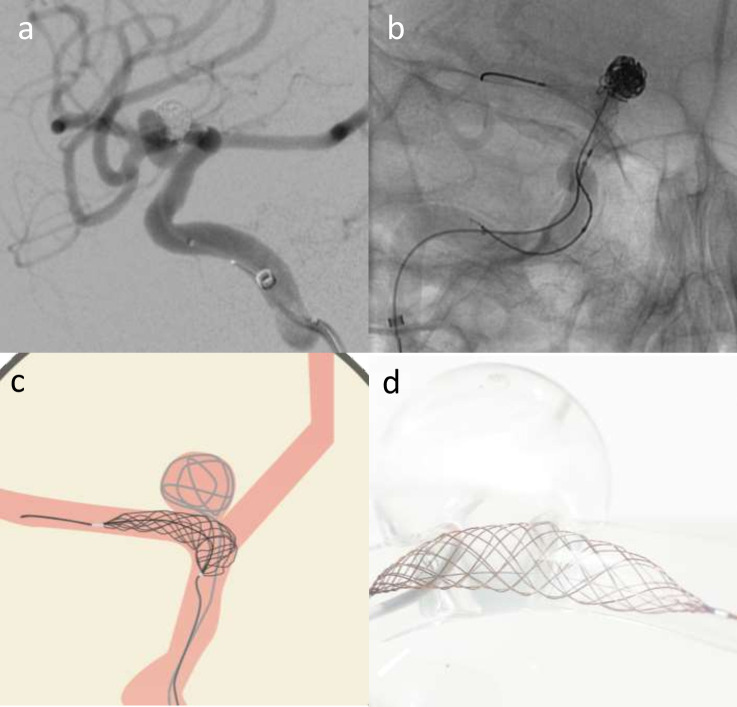
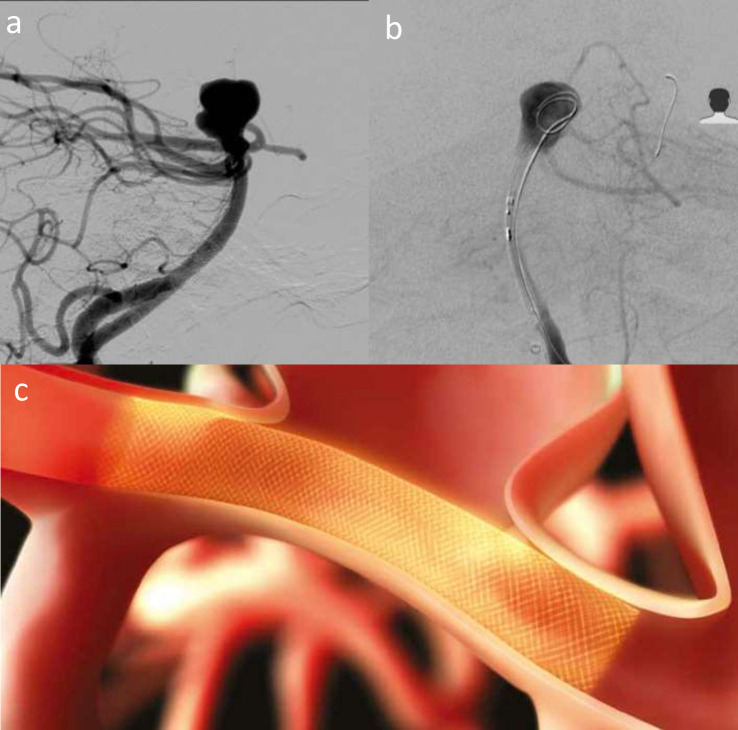
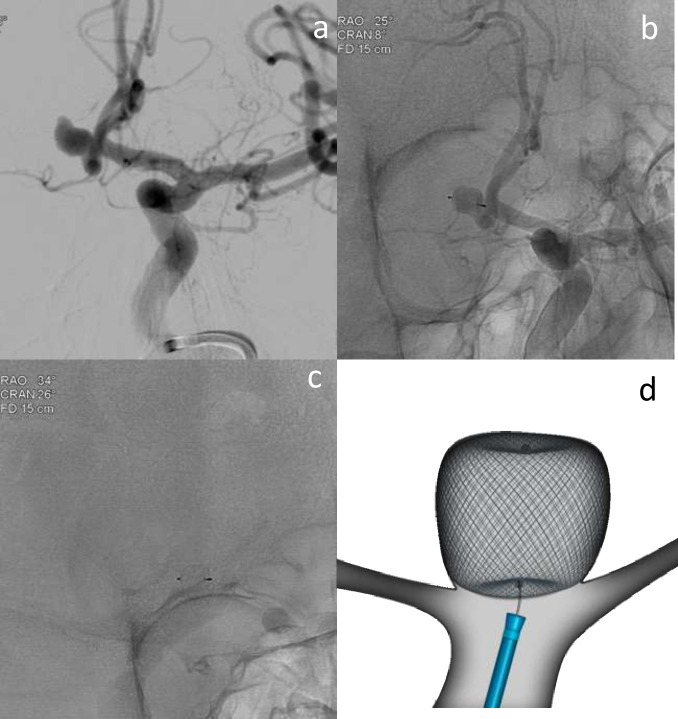
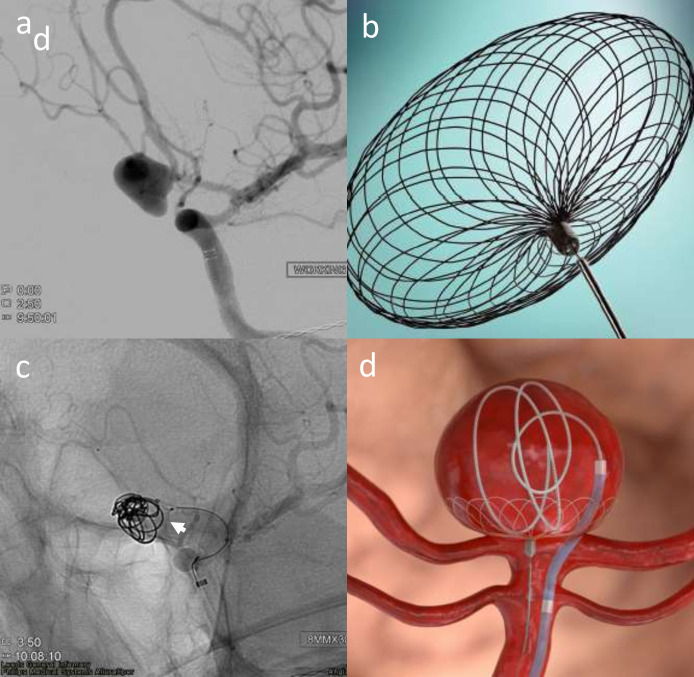
Figure 1.
Elective re-treatment of a right carotico-ophthalmic aneurysm (a), previously treated with balloon-assisted coiling. Angiography image showing recurrence treated with Comaneci-assisted coiling (b). Illustration demonstrating Comaneci-assisted coiling (c) and a image of the deployed comaneci device (1d) – images provided courtesy of Rapid-Medical.
Retrospective series with Comaneci in 18 unruptured ICA aneurysms reported a symptomatic carotid occlusion that may have been device related. Short follow-up (3 months) demonstrated 100% adequate occlusion.8 12 patients with ruptured aneurysms treated with Cascade had 75% immediate complete occlusion.20 No periprocedural clinical complications were reported, but no follow-up data are available. Multicentre experience of Cascade (n = 15) reported 100% adequate occlusion rate, with no thrombotic complications at 6 months.9
Permanent stent-devices
Comparable occlusion rates have been supported in meta-analysis with the use of permanent devices for SAC. In 1408 ruptured aneurysms, there was lower immediate (54.3% SAC vs 64.2%) but higher delayed occlusion rates (73.4% SAC vs 61.0%) and lower recurrence rate (4.8 SAC vs 16.6%).10 SAC was associated with higher peri-operative complications (20.2 SAC vs 13.1%, p = 0.466). Earlier meta-analysis (n = 2446) did not show any inferiority of SAC with lower overall permanent morbidity rate.11 There was no significant difference in the long-term adequate occlusion rate (73.0 SAC vs 80.1% non-SAC).
SAC is associated with higher risks for treating ruptured aneurysms.21,22 Dual-antiplatelet treatment is advised in SAC to reduce the risk of in-stent thrombosis and thrombotic complications. A meta-analysis including 1408 ruptured aneurysms confirmed a higher periprocedural complication rate in SAC (20.2% vs 13.1%).10
First-generation stents, Neuroform and Neuroform EZ/EZ3 (Stryker) with open cell-cell design, and second-generation closed cell stents, Enterprise (Codman Neurovascular, Raynham, Massachusetts, USA) and Solitaire (ev3/Covidien, Irvine, California, USA) are laser cut. Newer generations of laser-cut stents include the third-generation Neuroform Atlas stent and the low-profile Acclino microstent (Acandis, Pfozheim, Germany). Third-generation-braided stents, include low-profile visualised intraluminal support (LVIS), LVIS Jr and LVIS EVO (Microvention/Terumo, Tustin, California, USA) and the LEO family including LEO + and baby Leo (BALT Extrusion, Montmorency, France). Laser-cut stents offer easier deployment, and braided stents offer the potential for better wall apposition, particularly in curved vessels and in significant vessel diameter discrepancy and also reduced radial forces exerted on the device, which may reduce migration. Braided stents have shown high occlusion rates in recent meta-analysis (n = 1426) with 88% complete occlusion and 7% treatment-related complication rates.23
Newer stents also feature a hybrid cell design, offering a compromise between open-cell (lower radial stiffness and easier navigation) and closed-cell design (less porous and promote aneurysm thrombosis through a flow-diversion effect).7 Examples include LVIS, Neuroform Atlas and LEO family.
Laser-cut technology
Neuroform atlas
The neuroform atlas (Figure 2) is the latest generation of the self-expandable nitinol neuroform stents, featuring a hybrid cell design, deliverable through 0.0165 inch catheters.12 A prospective study (n = 30) reported 92.6% 12 months complete occlusion rate. One patient had over 50% parent artery stenosis and one suffered ipsilateral stroke but recovered without deficit.12 A series of 55 unruptured bifurcation aneurysms treated in X or Y configuration, reported 95% adequate occlusion at 16 months and 12.7% symptomatic complication rates.13 Meta-analysis reported 86% immediate and 93% 9 month occlusion and a 6% periprocedure complication rate in wide-neck aneurysms.14
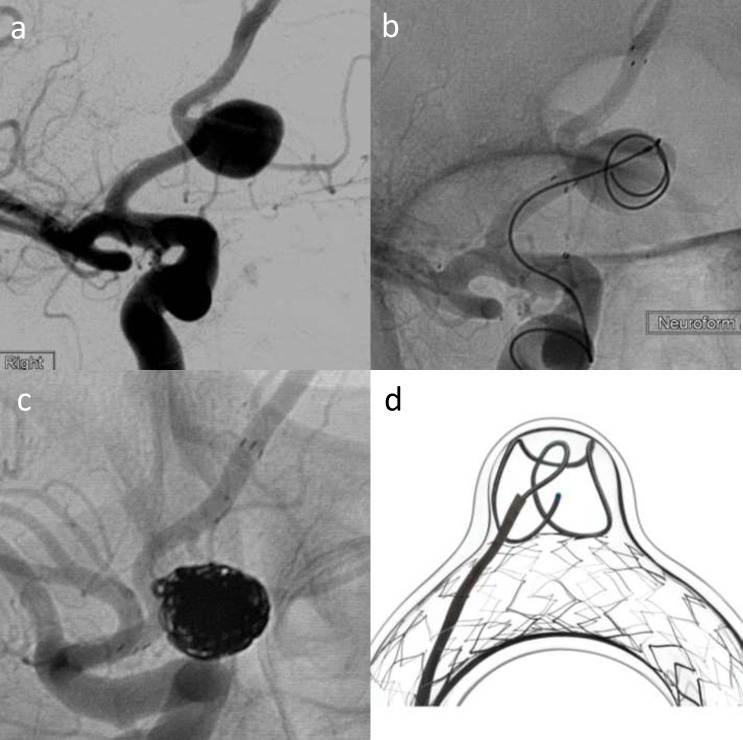
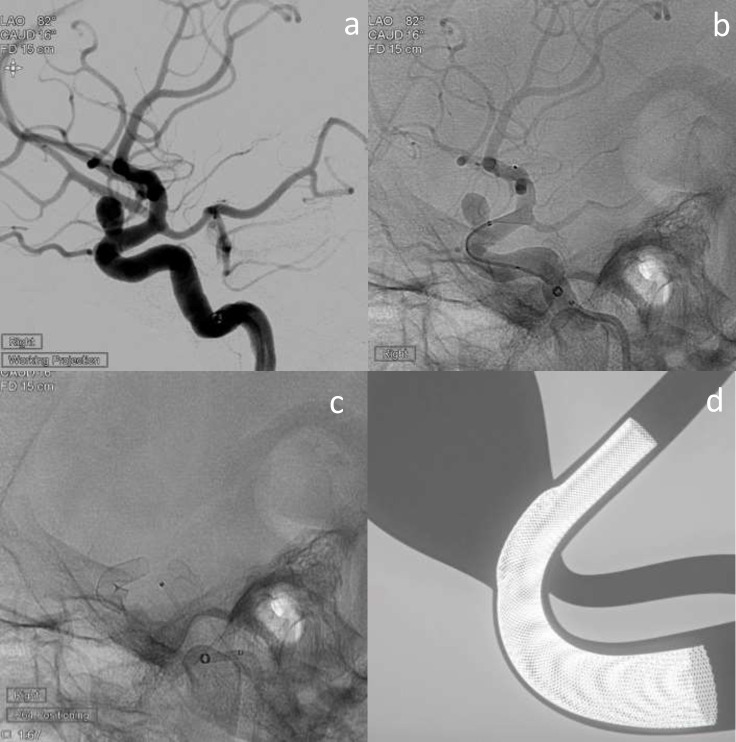
Figure 2.
Elective treatment of a wide-neck Acom aneurysm (2a) with Neuroform atlas stent-assisted coiling using a combination of Target 360 Soft, Axium Prime and Kaneka coils (b–d). (b) shows deployment of the first coil with stent across the neck, and 2c shows the final coil mass with stent in situ. (d) is a schematic image illustrating Neuroform atlas-assisted coiling in a model vessel and aneurysm – courtesy of Stryker Neurovascular.
Acclino microstent
The Acclino stent can be delivered by 0.0165 in microcatheters and is resheathable up to 90%. Early evidence (n = 14) reported 86% adequate immediate occlusion.15 One case of in-stent thrombus resolved without any permanent deficit.
Braided stents
LVIS and LVIS Jr
LVIS and LVIS Jr are closed cell, re-sheathable and retrievable, and have smaller pores than other non-braided stents.24 A prospective series reported that more than 90% occlusion rate at 12 months was 95%, and 5.2% primary safety endpoint (stroke or death within 30 days, ipsilateral stroke or neurological death within 12 months) rate.16
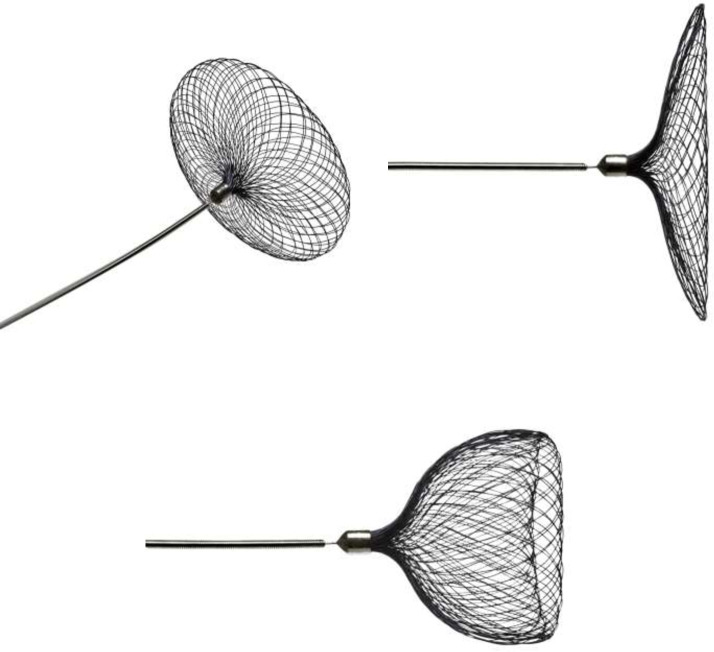
LVIS evo
LVIS EVO is braided, offers enhanced visibility and smaller cells to LVIS Jr (Figures 3 and 4). Multicentre data on (n = 57) reported 64.4% adequate occlusion and 12% periprocedural complication rates.17 Other series have reported 100% immediate complete occlusion, with one case of procedure-related thrombotic complication.18 The EVO stent is much easier to deploy and detach compared to baby leo that can be sometimes tricky to detach because of its proximal hook. The braided stents also offer some degree of flow diversion as some people would call the light flow divertors.
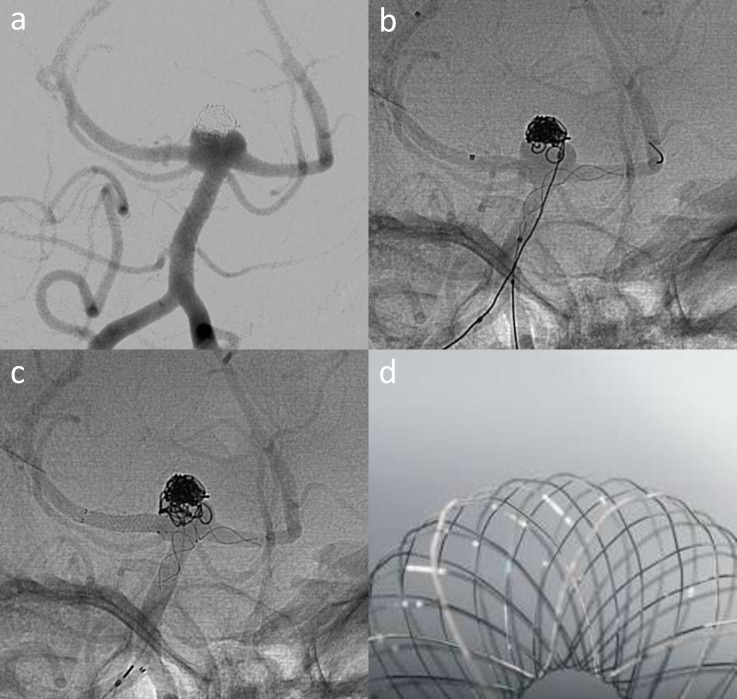
Figure 3.
LVIS EVO stent shown fully opened. Image provided courtesy of Microvention/Terumo.
Figure 4.
Elective treatment of a recurrent basilar tip aneurysm (a) that was previously treated with balloon-assisted coiling. T-stenting performed with Baby Leo deployed from the left P1 to the basilar (b) and LVIS EVO across the right P1 (c). A image of the Baby Leo device illustrates the stent cells that support the coil mass – image courtesy of Sela Medical.
LEO family
The braided stents offer some degree of light flow diversion. LEO plus is open-cell with high radial force, improved wall apposition, and resheathable up to 90%.7 A retrospective study (n = 153) reported 98% adequate occlusion rates at 36 months.19 The LEO baby (Figure 4) is low-profile, self-expandable, retrievable up to 95% and has a hybrid sliding-strut design for better wall apposition.25 Meta-analysis reported 93% immediate, 6-month adequate occlusion and 5.7% recurrence rates.26
Flow divertors
Flow diverters
In the following section, flow diverters will be discussed – readers are guided to other reviews of flow diverters for an in-depth discussion solely of this technology.27,28 An overview of the evidence discussed can be found in Table 2. Flow diverting (FD) stents have low porosity, reducing flow within the sac, which promotes thrombosis over hours to days.24 A new endothelial lining grows over the stent, (“neoendothelialisation”) and remodelling of the vessel resorps the aneurysm with neointimal coverage.3,24 FD treats the weakened arterial wall and the aneurysm should be excluded from the circulation without directly accessing the sac.27
Table 2.
Overview of flow diverter devices and main evidence discussed
| Device | Study (first Author, year) | Patients, aneurysms | Ruptured aneurysms? | Occlusion rate | Morbidity; mortality (%) |
|---|---|---|---|---|---|
| PED | Nelson et al29 | 31, 31 | Unruptured | 93.3% complete at 6 months | 6.5, 0 |
| Becske et al30 | 108, 108 | Unruptured | 73.6% complete at 6 months without major stenosis or adjunctive coils | 2.8, 2.8 | |
| Becske et al31 | 74, 76 | Unruptured | 93.4% complete at 3 years | 2.7a, 0 | |
| Kallmes et al32 | 191, 207 | Unruptured | 74.8% complete at 8 months | 6.8, 1.6 | |
| Kallmes et al33 | 1092, 1221 | Mixture ruptured and unruptured | 75% complete at 6 months, 85.5% at 1 year, 93.4% at 3 years, 95.2% at 5 years | 5.7, 3.3b | |
| Hanel et al34 | 141, 141 | Small to medium unruptured | 81.9% complete at 12 months | 1.4, 0.7 | |
| Griessenauer et al35 | 129, 131 | Ruptured and unruptured posterior circulation including dissecting aneurysms | 66.4% complete at 11 months | 24.8c, 11.2 | |
| PED Shield | Manning et al36 | 14, 14 | Acutely ruptured | 85.7% near-complete at end-of-procedure | 7.1, 7.1 |
| Trivelato et al37 | 151, 182 | Mostly unruptured | 79.7% complete at 6 months, 85.3% at 12 months | 6, 0.7 | |
| Surpass | Meyers et al38 | 180, 180 | Unruptured or not acutely ruptured | 62.8% complete at 12 months without significant stenosis or retreatment | 8.3, 2.2 |
| Taschner et al39 | 53, 53 | Mostly unruptured posterior circulation | 66% complete at 11 months | 9.6, 17 | |
| FRED | Pierot et al40 | 103, 103 | Unruptured | 73.3% complete at 12 months | 2.9, 1.9 |
| Killer-Oberpfalzer et al41 | 579, 531 | Mixture unruptured and ruptured | 69.2 complete at 6 months, 91.3% at 1 year | 14, 1.5 | |
| p48_HPC | Aguilar-Perez et al42 | 8, 8 | Acutely ruptured | 83% complete at 6 months | 37.5d, 25 |
| p64_HPC | Petrov et al43 | 29, 46 | Unruptured | 85% complete at 6 months | 0, 0e |
Same cohort as Becske 2013, with long-term outcome for 74 patients. 2/74 patients developed non-permanent neurological symptoms in addition to the morbidity reported for the initial study.
Neurological mortality rate is quoted.
Major and minor complication rate combined.
3/8 patients had mRS > 2 on follow-up.
No permanent neurological morbidity or mortality.
There is risk of thromboembolism from the FD stent, and the risk of dual antiplatelets. Other risks are delayed rupture, remote haemorrhage, and delayed ipsilateral parenchymal haemorrhage.3,44–48 Delayed ruptures have predominantly been reported with giant aneurysms, which have a higher bleeding risk and are difficult to manage by other approaches.47,49 Several suggested mechanisms for delayed ipsilateral haemorrhage include the transformation of ischaemic injury, modified blood pressure, and the use of dual antiplatelets.3 Coverage of collateral branches is also a concern.50,51
The first devices were the Pipeline Embolisation Device (PED, Medtronic, Irvine, California, USA) and Silk (Balt). Initial uses included giant, wide-neck and fusiform internal carotid artery (ICA) aneurysms or recanalized aneurysms.52–57 The PED for the Intracranial Treatment of Aneurysm (PITA) and the PED for Uncoilable or Failed Aneurysms (PUFS) trials reported good efficacy and safety profile (5.6–6.5% ischaemic complication rate): 93.3% 6 month occlusion rate in PITA and 73.6% complete occlusion without major stenosis or use of adjunctive coils in PUFS at 6 months.29,30 3-year follow-up shows 92.1% complete occlusion rate and no delayed haemorrhage.31 Observational registry of PED has confirmed low complication rates from ophthalmic artery coverage.32,33,58 Pooled analysis (n = 1092) showed 85.5% 1 year occlusion, 4% major ipsilateral stroke and 2% haemorrhage rates and 7% major neurological morbidity and mortality rate.33 A retrospective review of 74 patients with para-ophthalmic aneurysms reported 88.9% 32 month complete occlusion rate, and a 1.4% morbidity rate.59 Compared to some series of surgically treated paraclinoid and para-ophthalmic aneurysms, there have been reports of 29% overall morbidity rate with a surgery.60
Other devices
Other devices include Surpass (Stryker) (Figure 5), Flow-Redirection Endoluminal Device (FRED and FRED Jr, Microvention, Tustin, CA) and Derivo (Acandis GmbH, Pforzheim, Germany). The Surpass Intracranial Aneurysm Embolization System Pivotal Trial to Treat Large or Giant Wide Neck Aneurysms (SCENT) (n = 180) reported 62.8% 12-month primary efficacy (complete occlusion, no parent artery stenosis >50% and no retreatment) and 8% major adverse event rates (major ipsilateral stroke or neurological death).38 It also included 21% posterior communicating (PCom) artery aneurysms (21.1%) – significant given concerns about side-branch coverage.50
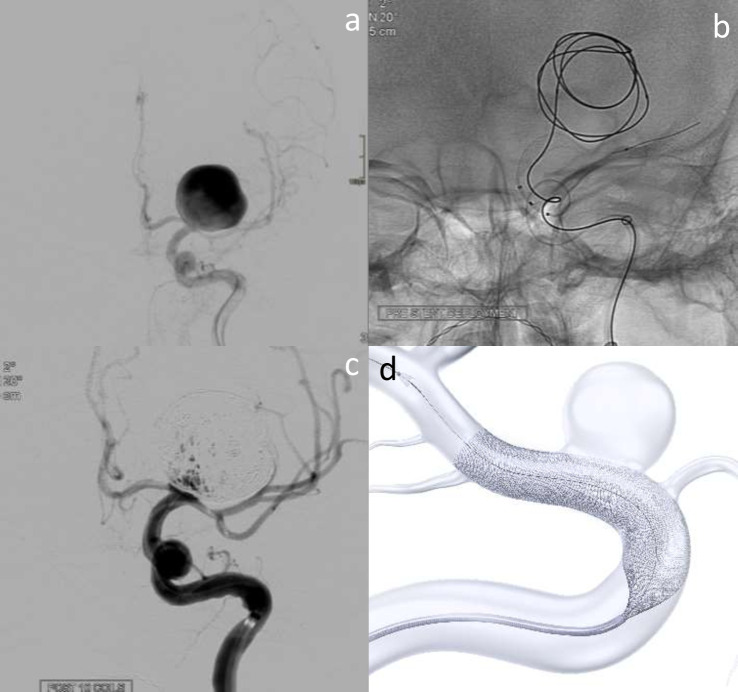
Figure 5.
Surpass Evolve assisted coiling of giant left ICA termination aneurysm. a shows a giant terminal ICA aneurysm on the left. b demonstrates coil deployment into the aneurysm, with Surpass Evolve stent placed in the terminal ICA, but not yet deployed. 5c shows small volume filling after several coils have been deployed. d is a schematic image of the stent during deployment – courtesy of Stryker Neurovascular.
The Safety and efficacy analysis of FRED embolic device in aneurysm treatment (SAFE) study (n = 103) reported 81% 1-year adequate occlusion, 1.9% mortality and 2.9% morbidity rates.40 The European registry study of FRED system use (n = 531) demonstrated progressive aneurysm occlusion – 20% 90-day complete occlusion increased to 95.3% at 1 year.41 There was a 14% adverse event and complication rate including 1.5% mortality rate.
Beyond large ICA aneurysms
The Prospective Study on Embolization of Intracranial Aneurysms with the Pipeline Device (PREMIER) showed efficacy in small-to-medium aneurysms – 76.8% complete 1-year occlusion and 2.1% primary safety event rates (major stroke or neurological death).34 Meta-analyses of FD in anterior communicating artery (AcomA) and middle cerebral artery (MCA) aneurysms reported MCA aneurysms had overall adequate occlusion rate of 78.7% and AcomA aneurysms had 87.4% long-term adequate occlusion.61,62 However, there was 20.7% treatment-related complication rate for MCA aneurysms and 8.6% overall complication rate for AcomA aneurysms.
Treatment of posterior circulation aneurysms has been controversial given perforators and lack of collateralisation. Early data in giant or large fusiform vertebrobasilar aneurysms suggest high mortality rate, albeit based on very small numbers.63 Larger series demonstrated 27% overall morbidity and mortality rate but suggested that careful patient selection may reduce this.39 Another series (n = 129) with dissecting, fusiform and saccular aneurysms reported 78.1% complete 11 month occlusion rate.35 However, 22.5% thrombotic complication and 11.2% mortality rate was observed. Current evidence suggests that the patient’s anatomy needs to be favourable and that careful patient selection is required.
Ruptured aneurysms and FD
FD use has been described in ruptured aneurysms, particularly with promising occlusion in difficult to treat lesions – blister, dissecting or fusiform lesions aneurysms.64 A small series of ruptured aneurysms treated with Pipeline Shield and single antiplatelet reported 86% near-complete 7 day occlusion rate, although the majority of these patients also had adjunctive coiling.36 Complications (7.1% treatment related morbidity and 7.1% mortality) were associated with post-operative heparin infusion. A meta-analysis of FD in 223 ruptured aneurysms reported a 89% adequate 10 month occlusion, 8% ischaemic/thrombotic, 7% haemorrhage and 4% rebleed rates.65 Comparision with other endovascular treatments is difficult, given that these were likely unsuitable for standard coiling. It remains to be seen whether FD stents can be safely used alongside coiling in the acute SAH setting, or whether newer intrasaccular devices will fill this role.
Coated FD
Hydrophilic stent coatings could lead to improved outcomes but more safety data are required and there isn’t enough evidence to support single antiplatelet use. Hydrophilic coatings reduce platelet adherence – the Pipeline Flex Embolization device with Shield Technology (Pipeline Shield, Medtronic) has a phosphorylcholine surface modification that reduces thrombogenicity (Figure 6), and it has an improved delivery system.66,67 A prospective study (151 patients; 182 aneurysms) reported a 3.3% ischaemic complication, 7.3% periprocedural complication and 85% 12-month complete occlusion rates.37
Figure 6.
Acute admission with lumbar puncture positive SAH. Wide-neck basilar aneurysm (a) treated with Pipeline Embolisation Device with Shield Technology delivered to left P1 (b) and across the aneurysm neck. Schematic illustration (c) of PED shield across an aneurysm neck illustrates the device position – image courtesy of Medtronic.
The p48_HPC and p64_HPC (Figure 7) FD (phenox, Bochum, Germany) are novel-coated devices with a glycan-based surface hydrophilic polymer coating (pHPC).68 They are made of a 48 and 64 nitinol wire braid and are compatible with 0.021-inch and 0.027-inch inner diameter microcatheters. p48_HPC is designed for 1.75–3 mm diameter vessels and p64_HPC for 2.5–5 mm. A small series (n = 8) treated with p48_ HPC, shortly after acute SAH, and with single antiplatelet therapy reported 5/6 complete occlusion at follow-up.42 50% had intraprocedural thrombus formation inside or close to the FD, and two patients had non-occlusive thrombus inside the FD at 5 days. A series (n = 29) of p64_HPC with dual antiplatelets reported 85% 6-month adequate occlusion.43 A small series of p48_HPC or p64_HPC treatment (n = 7) with acutely ruptured aneurysms and single antiplatelet therapy reported no rebleeding or stent thrombosis but only 43% had adequate occlusion.69 There remain questions over the safety of their use with single antiplatelet agents, particularly in acute rupture, and there is insufficient evidence to support the use of single antiplatelets routinely.
Figure 7.
Elective treatment of a right carotico-ophthalmic aneurysm (a) with p64 HPC-coated flow diverter. b shows the initial opening of the flow diverter beyond the aneurysm neck, and c shows the flow diverter in situ and fully deployed. d is a schematic representation of the p64 device in situ across the aneurysm neck – image courtesy of Phenox.
Intrasaccular flow disruptors
Intrasaccular flow disrupting devices (ISFDs), which are designed to sit within the sac or neck of WNBA, promote intraaneurysm thrombosis and remodel the aneurysm-parent vessel interface.70 WNBAs remain a difficult group of patients to treat by endovascular or surgical techniques and ISFDs represent an attractive solution in acute rupture, as dual-antiplatelets are not necessary.71 An overview of the evidence discussed can be found in Table 3.
Table 3.
Overview of intrasaccular flow disrupting devices and main evidence discussed
| Device | Study (first Author, year) | Patients, aneurysms | Ruptured aneurysms? | Occlusion rate | Morbidity; mortality (%) |
|---|---|---|---|---|---|
| Meta-analysis: pCONus | Sorenson et al72 | 201, 203 | Mostly unruptured | 60% complete at 10 months | 7, 0 |
| pCONus HPC | Perez et al73 | 15, 15 | Ruptured | 0% complete at 5 months | 26.7a, 5.9 |
| pCANvas | Lylyk et al74 | 17, 17 | Unruptured | 31% complete at 6 months | 0, 0 |
| second generation eCLIPs | Chiu et al73 | 25, 25 | Mostly unruptured | 33% complete at 8 months | 36b, 8 |
| WEB-DL, SL, SLS | Lawson et al75 | 22, 25 | Mostly unruptured | 36.4% complete at 3 months | 22.7c, 0 |
| Lawson et al76 | 109, 112 | Mixture ruptured and unruptured | NR | 6, 5 | |
| Kabbasch et al77 | 66, 66 | Mostly unruptured | 83.3% complete at 6 months | 12.1, 0 | |
| Fiorella et al78, Arthur et al79 | 150, 150 | Mostly unruptured | 53.8% complete at 12 months | 0.7, 0 | |
| Pierot et al80 | 168, 169 | Mostly unruptured | 51.2% complete at 2 years | 1.4, 0.7 | |
| van Rooij et al 201781 | 100, 100 | Ruptured | 73% complete at 3 months | 3, 1 | |
| WEB 17 | Goertz et al82 | 38, 38 | Mixtured ruptured and unruptured | 57.9% complete at end-of-procedure | 5.3d, 0 |
| Contour | Akhunbay-Fudge 202083 | 11, 11 | Unruptured | 55.6% complete at 12 months | 0, 0 |
NR, Not reported.
Morbidity rate is not reported – procedure or device-related complication (including asymptomatic) rate used instead.
9/25 patients with reported peri-procedural complications.
Morbidity rate not reported – 5/22 ischaemia and new symptoms post-WEB.
Thrombotic complication rate.
Stent-like devices pCONus and pCANvas
Stent-like devices with a distal crown of “petals”, with or without an impermeable membrane, include pCONus 1, pCONus two and pCANvas (Phenox, Bochum, Germany). Figure 8 illustrates how the pCONus two device supports coiling of bifurcation aneurysms. The distal end is placed within the neck, providing stability for coils, maintaining vessel patency, and it reduces the intra-aneurysmal flow.84 Current use is limited as there is a need for dual antiplatelets with non-coated devices and literature shows lower occlusion rates. A systematic review (n = 201) reported 60% long-term occlusion and 14% retreatment rates with pCONus.72 pCANvas, a third-generation device that is no longer available, with an impermeable distal membrane, has shown high rates of persistent aneurysm filling and retreatment rates (7 of 17 patients treated) on mid-term follow-up.74 The hydrophilic polymer-coated (HPC) pCONus HPC devices are intended for single antiplatelets in acutely ruptured aneurysms.85,86 Retrospective data (n = 15) with acutely ruptured aneurysms treated with pCONus HPC and single antiplatelet reported 66.6% immediate adequate occlusion and 46% neck remnant and 54% 5month persistent filling rates.86
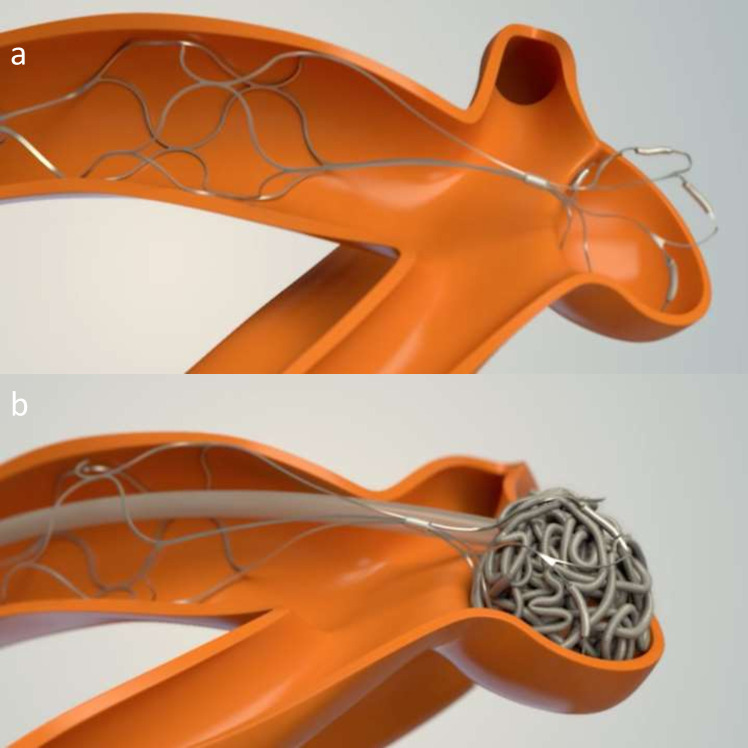
Figure 8.
pCONus two device mechanism illustrated through schematic images. a shows the device placed in the parent vessel and petals sited within the aneurysm neck and sac. b shows the pCONus two assisted coiling of the aneurysm.
The Endovascular Clip System (eCLIPs) eCLIPs (Evasc Medical Systems Corp, Vancouver, Canada) is a self-expanding nitinol non-circumferential stent-like device (Figure 9) that has flow diversion properties,87 designed to treat WNBA.73 There are two sections: the anchor, placed into one branch and designed to conform to the wall just besides the aneurysm neck, and leaf segment that crosses the aneurysm neck and has moveable ribs to allow the passage of a coil delivery microcatheter. The branch vessels are not jailed and the leaf segment allows neointimal growth, and has flow diversion properties.88 Second-generation eCLIPs has shown 81% adequate occlusion rate at follow-up (median 8 months).73
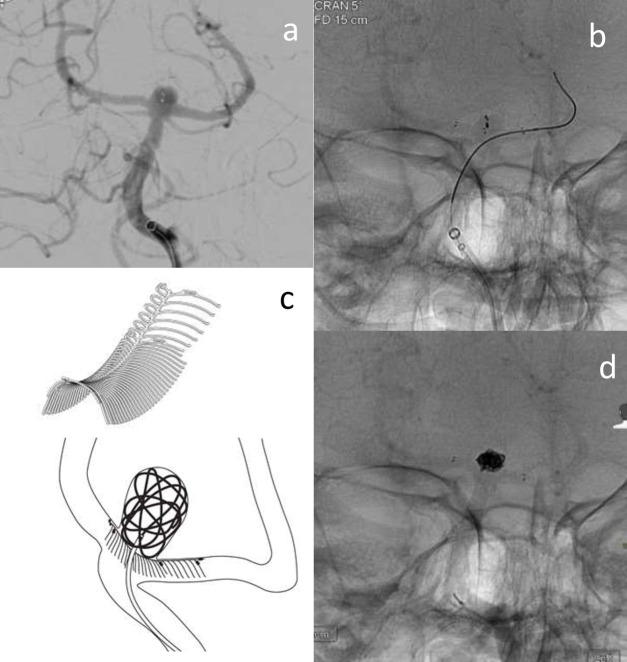
Figure 9.
E clips deployed into both PCA’s and across the aneurysm neck to facilitate coil placement into a basilar tip aneurysm recurrence (previously treated with WEB). a shows the recurrent basilar tip aneurysm with previous WEB device visible. b shows microcatheter in the left PCA and a deployed eCLIPs device placed across the aneurysm neck. c is a schematic drawing and image of the eCLIPs device showing how it supports coiling of a bifurcation aneurysm – courtesy of Evasc Medical Systems. 9d shows the final coil mass, deployed through the eCLIPs device.
Woven EndoBridge
The Woven EndoBridge (WEB) device (Sequent Medical Inc, Aliso Viejo, CA, USA) was the first major ISFD (Figure 10). The first-generation the WEB-DL (dual layer) is a self-expanding mesh composed of 2 nitinol-braided layers. Subsequent iterations include single-layer (WEB-SL) and single-layer spherical (WEB-SLS), and devices offering better visibility (Enhanced Visualisation, EV) and delivery through smaller microcatheters (WEB 15 and 17).
Figure 10.
WEB placement in an acutely ruptured complex Acom aneurysm with superiorly located rupture point (a). b and c show the deployed WEB device within the aneurysm sac. d is a schematic diagram that shows how the WEB device sits within the aneurysm sac – courtesy of Microvention.
Multiple studies and systematic reviews demonstrate its safety and efficacy. UK data (n = 109) in mostly unruptured aneurysms used a mixture of WEB DL, SL and SLS.76 15.6% thromboembolic complication, 6% 3-month follow-up morbidity and 5% mortality rates were reported. A single-centre UK experience with WEB treatment (n = 25) reported 73% adequate 3-month occlusion.75 Matched case-control study of WEB and SAC demonstrated a lower complication rate in the use of WEB (12.1 vs 21.2%, p = 0.03) with similar adequate occlusion rates (93.9% in both).77
The WEB Intrasaccular Therapy (WEB-IT) study was a US prospective, multicentre study (n = 150), mostly with unruptured aneurysms.78,79 1-year complete and adequate occlusion rates were 53.8 and 84.6%, respectively. 0.7% primary safety endpoint (delayed ipsilateral parenchymal haemorrhage within 30 days) and 5% minor ischaemic stroke rates were shown. The 2 WEB Clinical Assessment of Intrasaccular Aneurysm Therapy (WEBCAST and WEBCAST 2) studies of WEB-DL, and the SL-EV and SLS-EV devices, respectively, and the French Observatory study included WEB-DL, SL and SLS devices.89–91 For 168 patients, 2-year safety and efficacy data reports overall 1.4% procedural morbidity, 0.7% mortality, and 81% adequate occlusion rates with no associated with bleeding/rebleeding.80 13.4% had worsened occlusion compared to 1-year occlusion.
Recent experiences are mixed with lower occlusion rates and compaction of device. The authors personal experience in WEB is considerable and has shown good occlusion rates consistent with the trials. This may be related to lots of experience using the device which is critical. There has been some suggestion that the new 17 system WEB is softer and prone to recurrences, but this is still not proven, and current evidence suggests that there are similar occlusion rates compared with predecessor WEB devices in small aneurysms. A retrospective study of 38 aneurysms treated with WEB 17, and 70 treated with earlier WEB devices reported lower failure rate (0 vs 10.3%, p = 0.05) and lower thrombotic complication rate (5.3 vs 14.3%, p = 0.002) with the WEB 17.82 The immediate occlusion rates did not differ between groups significantly (57.9% WEB 17 vs 54.3% predecessor, p = 0.21).
WEB treatment of partially thrombosed aneurysms in the literature includes a small series performed in our institution for patients that were neither suitable for surgical treatment or flow diversion.92 There was early recurrence and displacement of the WEB when used exclusively and it may still be effective when used in conjunction with other devices, but further evidence is required.
WEB and ruptured aneurysms
There has been a progressive increase in use of WEB in acute ruptured aneurysms.93
A single-centre study illustrated feasibility, with no re-bleeding in the patients who survived to discharge, and 73% 3-month complete occlusion rate.81 The Clinical Assessment of WEB Device in Ruptured Aneurysms (CLARYS) trial (n = 60) was designed to address this question and early results seem promising – 0% rebleed, 1.7% mortality and 15% morbidity rates (due to the presentation, vasospasm and hydrocephalus).94 A recent systematic review (n = 398) reported 95.8–100% technical success rate, 9.8% thrombotic complication rate, 0–16.6% intra-operative rupture rate, 0–15% treatment related morbidity and mortality and adequate occlusion rate of 71–96%.93
Many difficulties have been reported, with one of the main technical difficulties being adequate sizing. One approach is to oversize the diameter by 1 mm and undersize the height by 1 mm relative to the corresponding measurements of the target aneurysm. A small analysis showed a higher proportion of adequately occluded aneurysms in the over-sized group (92.9% vs 70%, p = 0.1).95 Other issues with noted in our institutional use of WEB include the learning curve with the device and the Via delivery system catheter and therefore meaning that user experience can have a great impact on the results.83
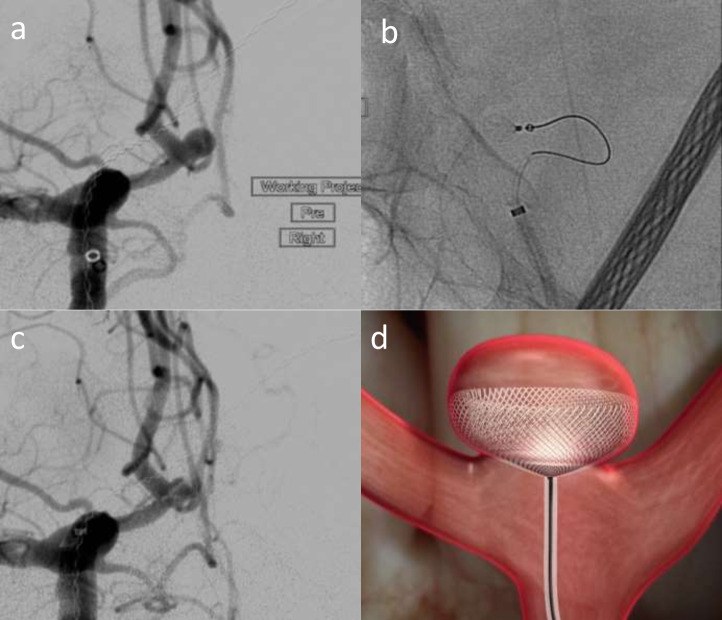
Contour
The Contour Neurovascular System (CNS, Cerus Endovascular Inc, Fremont, CA, USA) is a self-expanding ISFD and flow diverter (Figures 11–13), specifically targeting the aneurysm neck, consisting of a double-layer nitinol memory mesh that forms a “Chalice”-like shape that conforms to the neck when deployed. It can be used to treat complex aneurysm shapes unlike with WEB. Its currently available in Europe, and early experience has shown promise: single-centre series (n = 11) reported 55.6% complete occlusion and 44.5% neck remnant rate (100% adequate occlusion) at 1 year. 2/11 experienced thrombotic complications without permanent neurological disability or death.83 The device is easier to size than the WEB, only the largest aneurysm diameter and neck measurements require assessment – the height, morphology and neck play a role in the suitability and sizing of the WEB. The CERUS trial has completed its enrolment and follow-up. Results are promising with higher occlusion rates than WEB with similar safety [Personal communication]. Albeit a smaller study, many of these aneurysms in this study were quite challenging. CNS is easier to size with more predictable and controlled deployment in aneurysms. CNS starts to open immediately upon exiting the microcatheter, with no delay or “popping” open as exhibited by WEB and it does not need to engage with the vulnerable dome, reducing the likelihood of perforation during deployment. Any 021/027 catheter can be used. It appears to reconstruct the neck and because of the braiding there is a highly effective flow diversion in the neck of the aneurysm.
Figure 11.
Images of the Cerus Contour Neurovascular System. Top row shows the device in the open form. Bottom row shows the device in the deployed device in the “Chalice” shape that conforms to the aneurysm neck. Images courtesy of Cerus.
Figure 12.
Elective right Acom aneurysm (a) treated with the Contour Neurovascular System (CNS). b shows deployment of the CNS before it is detached. c shows the device in situ across the neck with contrast stasis. d is a schematic figure that shows the device sat in the aneurysm neck – courtesy of Cerus.
Figure 13.
Previously coiled Acom aneurysm following acute rupture and SAH. Additional unruptued left terminal ICA aneurysm treated with Contour Neurovascular System (CNS) (13a). b shows deployment of the CNS before detachment. c shows the device in-situ across the aneurysm neck. d is a schematic image of the CNS post-deployment with contrast stasis within the the aneurysm – courtesy of Cerus.
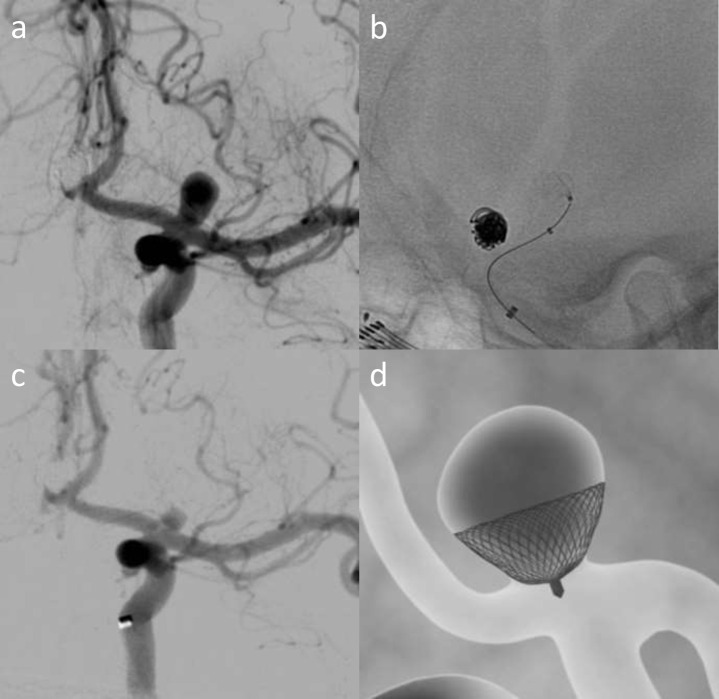
Neqstent device
The Neqstent Coil-Assisted Flow Diverter (Cerus) is an adjunctive flow diverting device that is also available in Europe and concurrently undergoing clinical evaluation, and also targets the neck of the aneurysm (Figure 14). Once placed across the neck, a coiling microcatheter can pass through its mesh or can be jailed to secure the aneurysm. Both CNS and Neqstent devices represent promising avenues, particularly Neqstent in the treatment of acutely ruptured wide-neck and WNBA’s and the clinical outcome data are greatly anticipated. Early experiences of the use of the Neqstent are consistent with CNS: easy to use and simple to size, only requiring the aneurysm neck to be considered. WEB, Contour and Neqstent are complimentary devices, and will probably be used in more than 50% of all saccular aneurysms in the future and may replace SAC in many complex aneurysms. New generation devices take the art out of coiling and offer more prescriptive therapeutic solutions. Both these devices are easy to use and are anticipated to show similar if not better efficacy than WEB with low mortality and morbidity in future trials.
Figure 14.
Acute Grade 1 SAH from left Acom aneurysm (a) treated with Neqstent-assisted coiling. b is a picture of the Neqstent device which sits across the neck and supports coiling and c shows deployment of the Neqstent device (arrowhead) with second microcatheter delivering coils into the aneurysm sac. d is a schematic figure illustrating how the Neqstent supports coiling of bifurcation aneurysms. Images courtesy of Cerus.
Conclusions
Many approaches exist for the management of ruptured and unruptured intracranial aneurysms, which reflects their varied nature – there is no one-size-fits-all technique. We have provided an overview of the classes of device and the developments that have occurred in the last decade. Newer devices are accruing more evidence on their safety and efficacy and will continue to grow in popularity. Coiling, however, is the oldest of the technology and continues to demonstrate high levels of occlusion and acceptable risks, making it the default treatment choice, particularly in the setting of the acutely ruptured aneurysm that needs immediate security from re-bleeding. Flow divertors have revolutionised complex aneurysm treatment with small added risk in acute aneurysm treatment. Intrasaccular devices like WEB, Contour and Neqstent provide excellent opportunities to treat wide-neck complex aneurysm with minimal mortality and morbidity and good occlusion rates and may in future replace a significant number of stent assisted coiling too.
Contributor Information
Kavi Fatania, Email: Kavi.fatania@nhs.net.
Dr Tufail Patankar, Email: tufail.patankar@nhs.net.
REFERENCES
- 1.Molyneux AJ, Kerr RSC, Yu L-M, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–17. doi: 10.1016/S0140-6736(05)67214-5 [DOI] [PubMed] [Google Scholar]
- 2.Ferns SP, Sprengers MES, van Rooij WJ, Rinkel GJE, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 2009; 40: e523-9. doi: 10.1161/STROKEAHA.109.553099 [DOI] [PubMed] [Google Scholar]
- 3.Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke 2013; 44: 2046–54. doi: 10.1161/STROKEAHA.113.000733 [DOI] [PubMed] [Google Scholar]
- 4.Pierot L, Barbe C, Ferré J-C, Cognard C, Soize S, White P, et al. Patient and aneurysm factors associated with aneurysm rupture in the population of the ARETA study. J Neuroradiol 2020; 47: 292–300. doi: 10.1016/j.neurad.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 5.Pierot L, Barbe C, Herbreteau D, Gauvrit J-Y, Januel A-C, Bala F, et al. Rebleeding and bleeding in the year following intracranial aneurysm coiling: analysis of a large prospective multicenter cohort of 1140 patients-Analysis of recanalization after endovascular treatment of intracranial aneurysm (ARETA) study. J Neurointerv Surg 2020; 12: 1219–25. doi: 10.1136/neurintsurg-2020-015971 [DOI] [PubMed] [Google Scholar]
- 6.Santillan A, Gobin YP, Greenberg ED, Leng LZ, Riina HA, Stieg PE, et al. Intraprocedural aneurysmal rupture during coil embolization of brain aneurysms: role of balloon-assisted coiling. AJNR Am J Neuroradiol 2012; 33: 2017–21. doi: 10.3174/ajnr.A3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oushy S, Rinaldo L, Brinjikji W, Cloft H, Lanzino G. Recent advances in stent-assisted coiling of cerebral aneurysms. Expert Rev Med Devices 2020; 17: 519–32. doi: 10.1080/17434440.2020.1778463 [DOI] [PubMed] [Google Scholar]
- 8.Sirakov S, Sirakov A, Hristov H, Minkin K, Penkov M, Karakostov V. Early experience with a temporary bridging device (Comaneci) in the endovascular treatment of ruptured wide neck aneurysms. J Neurointerv Surg 2018; 10: 978–82. doi: 10.1136/neurintsurg-2017-013641 [DOI] [PubMed] [Google Scholar]
- 9.Tomasello A, Hernandez D, Gramegna LL, Aixut S, Barranco Pons R, Jansen O, et al. Early experience with a novel net temporary bridging device (cascade) to assist endovascular coil embolization of intracranial aneurysms. J Neurosurg 2021; 134: 591–9. doi: 10.3171/2019.11.JNS192477 [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Zuo Q, Tang H, Xue G, Yang P, Zhao R, et al. Stent assisted coiling versus non-stent assisted coiling for the management of ruptured intracranial aneurysms: a meta-analysis and systematic review. J Neurointerv Surg 2019; 11: 489–96. doi: 10.1136/neurintsurg-2018-014388 [DOI] [PubMed] [Google Scholar]
- 11.Zhao B, Yin R, Lanzino G, Kallmes DF, Cloft HJ, Brinjikji W. Endovascular coiling of Wide-Neck and Wide-Neck bifurcation aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2016; 37: 1700–5. doi: 10.3174/ajnr.A4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankowitz BT, Hanel R, Jadhav AP, Loy DN, Frei D, Siddiqui AH, et al. Neuroform atlas stent system for the treatment of intracranial aneurysm: primary results of the atlas humanitarian device exemption cohort. J Neurointerv Surg 2019; 11: 801–6. doi: 10.1136/neurintsurg-2018-014455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccio G, Robert T, Smajda S, Fahed R, Desilles JP, Redjem H, et al. Double stent assisted coiling of intracranial bifurcation aneurysms in Y and X configurations with the Neuroform atlas stent: immediate and mid term angiographic and clinical follow-up. J Neurointerv Surg 2019; 11: 1239–42. doi: 10.1136/neurintsurg-2019-015175 [DOI] [PubMed] [Google Scholar]
- 14.Pranata R, Yonas E, Deka H, Vania R, July J. Stent-assisted coiling of intracranial aneurysms using a Nitinol-based stent (Neuroform atlas): a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2020; 43: 1049–61. doi: 10.1007/s00270-020-02502-9 [DOI] [PubMed] [Google Scholar]
- 15.Kabbasch C, Liebig T, Faymonville A, Dorn F, Mpotsaris A. Initial clinical experience with a new self-expanding nitinol Microstent for the treatment of Wide-Neck intracranial cerebral aneurysms: the Acandis Acclino stent. J Vasc Interv Neurol 2015; 8: 1–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorella D, Boulos A, Turk AS, Siddiqui AH, Arthur AS, Diaz O, et al. The safety and effectiveness of the LVIS stent system for the treatment of wide-necked cerebral aneurysms: final results of the pivotal us LVIS trial. J Neurointerv Surg 2019; 11: 357–61. doi: 10.1136/neurintsurg-2018-014309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollherbst DF, Berlis A, Maurer C, Behrens L, Sirakov S, Sirakov A, et al. Periprocedural safety and feasibility of the new LVIS EVO device for stent-assisted coiling of intracranial aneurysms: an observational multicenter study. AJNR Am J Neuroradiol 2021; 42: 319–26. doi: 10.3174/ajnr.A6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poncyljusz W, Kubiak K. Initial experience with LVIS EVO stents for the treatment of intracranial aneurysms. J Clin Med 2020; 9: 3966. doi: 10.3390/jcm9123966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedat J, Chau Y, Gaudart J, Sachet M, Beuil S, Lonjon M. Stent-assisted coiling of intracranial aneurysms using Leo stents: long-term follow-up in 153 patients. Neuroradiology 2018; 60: 211–9. doi: 10.1007/s00234-017-1965-1 [DOI] [PubMed] [Google Scholar]
- 20.Sirakov S, Sirakov A, Minkin K, Karakostov V, Raychev R. Early clinical experience with cascade: a novel temporary neck bridging device for embolization of intracranial aneurysms. J Neurointerv Surg 2020; 12: 303–7. doi: 10.1136/neurintsurg-2019-015338 [DOI] [PubMed] [Google Scholar]
- 21.Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013; 44: 1348–53. doi: 10.1161/STROKEAHA.111.000641 [DOI] [PubMed] [Google Scholar]
- 22.Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 2010; 41: 110–5. doi: 10.1161/STROKEAHA.109.558114 [DOI] [PubMed] [Google Scholar]
- 23.Cagnazzo F, Cappucci M, Lefevre P-H, Dargazanli C, Gascou G, Morganti R, et al. Treatment of intracranial aneurysms with Self-Expandable braided stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2018; 39: 2064–9. doi: 10.3174/ajnr.A5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dholakia R, Sadasivan C, Fiorella DJ, Woo HH, Lieber BB. Hemodynamics of flow diverters. J Biomech Eng 2017; 139: 1. doi: 10.1115/1.4034932 [DOI] [PubMed] [Google Scholar]
- 25.Akmangit I, Aydin K, Sencer S, Topcuoglu OM, Topcuoglu ED, Daglioglu E, et al. Dual stenting using low-profile Leo baby stents for the endovascular management of challenging intracranial aneurysms. AJNR Am J Neuroradiol 2015; 36: 323–9. doi: 10.3174/ajnr.A4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S-Y, Oh J-S, Oh H-J, Yoon S-M, Bae H-G. Safety and efficacy of low-profile, self-expandable stents for treatment of intracranial aneurysms: initial and midterm results - a systematic review and meta-analysis. Interv Neurol 2017; 6(3–4): 170–82. doi: 10.1159/000471890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos JK, Cheaney Ii B, Lien BV, Zarrin DA, Vo CD, Colby GP, et al. Advances in endovascular aneurysm management: flow modulation techniques with braided mesh devices. Stroke Vasc Neurol 2020; 5: 1–13. doi: 10.1136/svn-2020-000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu AHY, Phillips TJ. Future directions of flow Diverter therapy. Neurosurgery 2020; 86(Suppl 1): S106–16. doi: 10.1093/neuros/nyz343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011; 32: 34–40. doi: 10.3174/ajnr.A2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–68. doi: 10.1148/radiol.13120099 [DOI] [PubMed] [Google Scholar]
- 31.Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg 2017; 127: 81–8. doi: 10.3171/2015.6.JNS15311 [DOI] [PubMed] [Google Scholar]
- 32.Kallmes DF, Brinjikji W, Boccardi E, Ciceri E, Diaz O, Tawk R, et al. Aneurysm study of pipeline in an observational registry (ASPIRe. Interv Neurol 2016; 5(1–2): 89–99. doi: 10.1159/000446503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P, et al. Safety and efficacy of the pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg 2017; 127: 775–80. doi: 10.3171/2016.8.JNS16467 [DOI] [PubMed] [Google Scholar]
- 34.Hanel RA, Kallmes DF, Lopes DK, Nelson PK, Siddiqui A, Jabbour P, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the premier study 1 year results. J Neurointerv Surg 2020; 12: 62–6. doi: 10.1136/neurintsurg-2019-015091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griessenauer CJ, Ogilvy CS, Adeeb N, Dmytriw AA, Foreman PM, Shallwani H, et al. Pipeline embolization of posterior circulation aneurysms: a multicenter study of 131 aneurysms. J Neurosurg 2019; 130: 923–35. doi: 10.3171/2017.9.JNS171376 [DOI] [PubMed] [Google Scholar]
- 36.Manning NW, Cheung A, Phillips TJ, Wenderoth JD. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J Neurointerv Surg 2019; 11: 694–8. doi: 10.1136/neurintsurg-2018-014363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trivelato FP, Wajnberg E, Rezende MTS, Ulhôa AC, Piske RL, Abud TG, et al. Safety and effectiveness of the pipeline flex embolization device with shield technology for the treatment of intracranial aneurysms: midterm results from a multicenter study. Neurosurgery 2020; 87: 104–11. doi: 10.1093/neuros/nyz356 [DOI] [PubMed] [Google Scholar]
- 38.Meyers PM, Coon AL, Kan PT, Wakhloo AK, Hanel RA. Scent trial. Stroke 2019; 50: 1473–9. doi: 10.1161/STROKEAHA.118.024135 [DOI] [PubMed] [Google Scholar]
- 39.Taschner CA, Vedantham S, de Vries J, Biondi A, Boogaarts J, Sakai N, et al. Surpass flow Diverter for treatment of posterior circulation aneurysms. AJNR Am J Neuroradiol 2017; 38: 582–9. doi: 10.3174/ajnr.A5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierot L, Spelle L, Berge J, Januel A-C, Herbreteau D, Aggour M, et al. Safe study (safety and efficacy analysis of Fred embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg 2019; 11: 184–9. doi: 10.1136/neurintsurg-2018-014261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Killer-Oberpfalzer M, Kocer N, Griessenauer CJ, Janssen H, Engelhorn T, Holtmannspötter M, et al. European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: the European flow-redirection intraluminal device study. AJNR Am J Neuroradiol 2018; 39: 841–7. doi: 10.3174/ajnr.A5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilar-Perez M, Hellstern V, AlMatter M, Wendl C, Bäzner H, Ganslandt O, et al. The p48 flow modulation device with hydrophilic polymer coating (HPC) for the treatment of acutely ruptured aneurysms: early clinical experience using single antiplatelet therapy. Cardiovasc Intervent Radiol 2020; 43: 740–8. doi: 10.1007/s00270-020-02418-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrov A, Rentsenkhuu G, Nota B, Ganzorig E, Regzengombo B, Jagusch S, et al. Initial experience with the novel p64MW HPC flow diverter from a cohort study in unruptured anterior circulation aneurysms under dual antiplatelet medication. Interv Neuroradiol 2021; 27: 42-50. doi: 10.1177/1591019920939845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turowski B, Macht S, Kulcsár Z, Hänggi D, Stummer W. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): do we need to rethink our concepts? Neuroradiology 2011; 53: 37–41. doi: 10.1007/s00234-010-0676-7 [DOI] [PubMed] [Google Scholar]
- 45.Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol 2011; 32: 27–33. doi: 10.3174/ajnr.A2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulcsár Z, Houdart E, Bonafé A, Parker G, Millar J, Goddard AJP, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011; 32: 20–5. doi: 10.3174/ajnr.A2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulcsár Z, Szikora I. The ESMINT retrospective analysis of delayed aneurysm ruptures after flow diversion (radar) study. EJMINT 2012; 2012: 1244000088. [Google Scholar]
- 48.Cruz JP, Chow M, O'Kelly C, Marotta B, Spears J, Montanera W, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol 2012; 33: 603–8. doi: 10.3174/ajnr.A3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafe A, Cekirge S. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. Ajnr Am J Neuroradiol 2015; 36: 108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhogal P, Ganslandt O, Bäzner H, Henkes H, Pérez MA. The fate of side branches covered by flow Diverters-Results from 140 patients. World Neurosurg 2017; 103: 789–98. doi: 10.1016/j.wneu.2017.04.092 [DOI] [PubMed] [Google Scholar]
- 51.Cagnazzo F, Lefevre P-H, Mantilla D, Rouchaud A, Morganti R, Perrini P, et al. Patency of the supraclinoid internal carotid artery branches after flow diversion treatment. A meta-analysis. J Neuroradiol 2019; 46: 9–14. doi: 10.1016/j.neurad.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 52.Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery 2009; 64: 632–42. doi: 10.1227/01.NEU.0000339109.98070.65 [DOI] [PubMed] [Google Scholar]
- 53.Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol 2010; 31: 1139–47. doi: 10.3174/ajnr.A2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One 2010; 5: 2. doi: 10.1371/journal.pone.0012492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lubicz B, Collignon L, Raphaeli G, Pruvo J-P, Bruneau M, De Witte O, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke 2010; 41: 2247–53. doi: 10.1161/STROKEAHA.110.589911 [DOI] [PubMed] [Google Scholar]
- 56.Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 2012; 33: 1150–5. doi: 10.3174/ajnr.A2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–15. doi: 10.3174/ajnr.A4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahlein DH, Fouladvand M, Becske T, Saatci I, McDougall CG, Szikora I, et al. Neuroophthalmological outcomes associated with use of the pipeline embolization device: analysis of the PUFS trial results. J Neurosurg 2015; 123: 897–905. doi: 10.3171/2014.12.JNS141777 [DOI] [PubMed] [Google Scholar]
- 59.Bhogal P, Hellstern V, Bäzner H, Ganslandt O, Henkes H, Aguilar Pérez M, Pérez MA. The use of flow diverting stents to treat para-ophthalmic aneurysms. Front Neurol 2017; 8(AUG): 381. doi: 10.3389/fneur.2017.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery 2001; 48: 78–90. doi: 10.1097/00006123-200101000-00014 [DOI] [PubMed] [Google Scholar]
- 61.Cagnazzo F, Mantilla D, Lefevre P-H, Dargazanli C, Gascou G, Costalat V. Treatment of middle cerebral artery aneurysms with Flow-Diverter stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2017; 38: 2289–94. doi: 10.3174/ajnr.A5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cagnazzo F, Limbucci N, Nappini S, Renieri L, Rosi A, Laiso A, et al. Flow-Diversion treatment of unruptured saccular anterior communicating artery aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2019; 40: 497–502. doi: 10.3174/ajnr.A5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siddiqui AH, Abla AA, Kan P, Dumont TM, Jahshan S, Britz GW, et al. Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg 2012; 116(June): 1258–66. doi: 10.3171/2012.2.JNS111942 [DOI] [PubMed] [Google Scholar]
- 64.Chancellor B, Raz E, Shapiro M, Tanweer O, Nossek E, Riina HA, et al. Flow diversion for intracranial aneurysm treatment: trials involving flow Diverters and long-term outcomes. Neurosurgery 2020; 86(Suppl 1): S36–45. doi: 10.1093/neuros/nyz345 [DOI] [PubMed] [Google Scholar]
- 65.Cagnazzo F, di Carlo DT, Cappucci M, Lefevre P-H, Costalat V, Perrini P. Acutely ruptured intracranial aneurysms treated with Flow-Diverter stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2018; 39: 1669–75. doi: 10.3174/ajnr.A5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lenz-Habijan T, Bhogal P, Peters M, Bufe A, Martinez Moreno R, Bannewitz C, et al. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: initial results in vitro. Cardiovasc Intervent Radiol 2018; 41: 1779–85. doi: 10.1007/s00270-018-2036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Girdhar G, Li J, Kostousov L, Wainwright J, Chandler WL. In-Vitro thrombogenicity assessment of flow diversion and aneurysm bridging devices. J Thromb Thrombolysis 2015; 40: 437–43. doi: 10.1007/s11239-015-1228-0 [DOI] [PubMed] [Google Scholar]
- 68.Colgan F, Aguilar Pérez M, Hellstern V, Reinhard M, Krämer S, Bäzner H. Vertebral artery aneurysm: ruptured dissecting aneurysm, implantation of Telescoping p48MW HPC flow Diverter stents under antiaggregation with ASA only. Aneurysm Caseb 2020;: 1081–95. doi: 10.1007/978-3-319-70267-4_80-1 [DOI] [Google Scholar]
- 69.Guzzardi G, Galbiati A, Stanca C, Del Sette B, Paladini A, Cossandi C, et al. Flow diverter stents with hydrophilic polymer coating for the treatment of acutely ruptured aneurysms using single antiplatelet therapy: preliminary experience. Interv Neuroradiol 2020; 26: 525–31. doi: 10.1177/1591019920950878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhogal P, Udani S, Cognard C, Piotin M, Brouwer P, Sourour N-A, et al. Endosaccular flow disruption: where are we now? J Neurointerv Surg 2019; 11: 1024–5. doi: 10.1136/neurintsurg-2018-014623 [DOI] [PubMed] [Google Scholar]
- 71.Fiorella D, Arthur AS, Chiacchierini R, Emery E, Molyneux A, Pierot L. How safe and effective are existing treatments for wide-necked bifurcation aneurysms? Literature-based objective performance criteria for safety and effectiveness. J Neurointerv Surg 2017; 9: 1197–201. doi: 10.1136/neurintsurg-2017-013223 [DOI] [PubMed] [Google Scholar]
- 72.Sorenson TJ, Iacobucci M, Murad MH, Spelle L, Moret J, Lanzino G. The pCONUS bifurcation aneurysm implants for endovascular treatment of adults with intracranial aneurysms: a systematic review and meta-analysis. Surg Neurol Int 2019; 10: 24. doi: 10.4103/sni.sni_297_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiu AH, De Vries J, O'Kelly CJ, Riina H, McDougall I, Tippett J, et al. The second-generation eCLIPs endovascular clip system: initial experience. J Neurosurg 2018; 128: 482–9. doi: 10.3171/2016.10.JNS161731 [DOI] [PubMed] [Google Scholar]
- 74.Lylyk P, Chudyk J, Bleise C, Henkes H, Bhogal P. Treatment of Wide-Necked Bifurcation Aneurysms : Initial Results with the pCANvas Neck Bridging Device. Clin Neuroradiol 2019; 29: 467–77. doi: 10.1007/s00062-018-0680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lawson A, Goddard T, Ross S, Tyagi A, Deniz K, Patankar T. Endovascular treatment of cerebral aneurysms using the Woven EndoBridge technique in a single center: preliminary results. J Neurosurg 2017; 126: 17–28. doi: 10.3171/2015.4.JNS142456 [DOI] [PubMed] [Google Scholar]
- 76.Lawson A, Molyneux A, Sellar R, Lamin S, Thomas A, Gholkar A, et al. Safety results from the treatment of 109 cerebral aneurysms using the Woven EndoBridge technique: preliminary results in the United Kingdom. J Neurosurg 2018; 128: 144–53. doi: 10.3171/2016.9.JNS152849 [DOI] [PubMed] [Google Scholar]
- 77.Kabbasch C, Goertz L, Siebert E, Herzberg M, Borggrefe J, Krischek B, et al. Web embolization versus stent-assisted coiling: comparison of complication rates and angiographic outcomes. J Neurointerv Surg 2019; 11: 812–6. doi: 10.1136/neurintsurg-2018-014555 [DOI] [PubMed] [Google Scholar]
- 78.Fiorella D, Molyneux A, Coon A, Szikora I, Saatci I, Baltacioglu F, et al. Demographic, procedural and 30-day safety results from the web Intra-saccular therapy study (WEB-IT. J Neurointerv Surg 2017; 9: 1191–6. doi: 10.1136/neurintsurg-2016-012841 [DOI] [PubMed] [Google Scholar]
- 79.Arthur AS, Molyneux A, Coon AL, Saatci I, Szikora I, Baltacioglu F, et al. The safety and effectiveness of the Woven EndoBridge (web) system for the treatment of wide-necked bifurcation aneurysms: final 12-month results of the pivotal web intrasaccular therapy (WEB-IT) study. J Neurointerv Surg 2019; 11: 924–30. doi: 10.1136/neurintsurg-2019-014815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierot L, Moret J, Barreau X, Szikora I, Herbreteau D, Turjman F, et al. Aneurysm treatment with woven endobridge in the cumulative population of 3 prospective, multicenter series: 2-year follow-up. Neurosurgery 2020; 87: 357–67. doi: 10.1093/neuros/nyz557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Rooij SBT, van Rooij WJ, Peluso JP, Sluzewski M, Bechan RS, Kortman HG, et al. Web treatment of ruptured intracranial aneurysms: a single-center cohort of 100 patients. AJNR Am J Neuroradiol 2017; 38: 2282–7. doi: 10.3174/ajnr.A5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goertz L, Liebig T, Siebert E, Herzberg M, Pennig L, Schlamann M, et al. Low-Profile Intra-Aneurysmal flow disruptor web 17 versus web predecessor systems for treatment of small intracranial aneurysms: comparative analysis of procedural safety and feasibility. AJNR Am J Neuroradiol 2019; 40: 1766–72. doi: 10.3174/ajnr.A6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akhunbay-Fudge CY, Deniz K, Tyagi AK, Patankar T. Endovascular treatment of wide-necked intracranial aneurysms using the novel contour neurovascular system: a single-center safety and feasibility study. J Neurointerv Surg 2020; 12: 987–92. doi: 10.1136/neurintsurg-2019-015628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pérez MA, Henkes H, Bouillot P, Brina O, Slater L-A, Pereira VM. Intra-aneurysmal hemodynamics: evaluation of pCONus and pCANvas bifurcation aneurysm devices using DSA optical flow imaging. J Neurointerv Surg 2016; 8: 1197–201. doi: 10.1136/neurintsurg-2015-011927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhogal P, Lenz-Habijan T, Bannewitz C, Hannes R, Monstadt H, Simgen A, et al. The pCONUS HPC: 30-day and 180-Day in vivo biocompatibility results. Cardiovasc Intervent Radiol 2019; 42: 1008–15. doi: 10.1007/s00270-019-02202-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aguilar Perez M, AlMatter M, Hellstern V, Wendl C, Ganslandt O, Bäzner H, et al. Use of the pCONus HPC as an adjunct to coil occlusion of acutely ruptured aneurysms: early clinical experience using single antiplatelet therapy. J Neurointerv Surg 2020; 12: 862–8. doi: 10.1136/neurintsurg-2019-015746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.G. Yazdi S, Mercier D, Bernard R, Tynan A, Ricci DR. Particle image velocimetry measurements of the flow-diverting effects of a new generation of the eclips implant for the treatment of intracranial bifurcation aneurysms. Appl Sci 2020; 10: 8639–13. doi: 10.3390/app10238639 [DOI] [Google Scholar]
- 88.Peach TW, Ricci D, Ventikos Y. A virtual comparison of the eCLIPs device and conventional Flow-Diverters as treatment for cerebral bifurcation aneurysms. Cardiovasc Eng Technol 2019; 10: 508–19. doi: 10.1007/s13239-019-00424-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pierot L, Costalat V, Moret J, Szikora I, Klisch J, Herbreteau D, et al. Safety and efficacy of aneurysm treatment with web: results of the WEBCAST study. J Neurosurg 2016; 124: 1250–6. doi: 10.3171/2015.2.JNS142634 [DOI] [PubMed] [Google Scholar]
- 90.Pierot L, Gubucz I, Buhk JH, Holtmannspötter M, Herbreteau D, Stockx L, et al. Safety and efficacy of aneurysm treatment with the web: results of the WEBCAST 2 study. AJNR Am J Neuroradiol 2017; 38: 1151–5. doi: 10.3174/ajnr.A5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierot L, Moret J, Turjman F, Herbreteau D, Raoult H, Barreau X, et al. Web treatment of intracranial aneurysms: feasibility, complications, and 1-Month safety results with the web DL and web SL/SLS in the French Observatory. AJNR Am J Neuroradiol 2015; 36: 922–7. doi: 10.3174/ajnr.A4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anil G, Goddard AJP, Ross SM, Deniz K, Patankar T. Web in partially thrombosed intracranial aneurysms: a word of caution. AJNR Am J Neuroradiol 2016; 37: 892–6. doi: 10.3174/ajnr.A4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crinnion W, Bhogal P, Makalanda HLD, Wong K, Arthur A, Cognard C, et al. The Woven Endobridge as a treatment for acutely ruptured aneurysms: a review of the literature. Interv Neuroradiol 2021;: 1591019921991397. doi: 10.1177/1591019921991397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spelle L, Herbreteau D, Narata A, Bibi R, Ikka L, Mihalea C. CLARYS: clinical assessment of WEB® device in ruptured aneurysms: 1-month clinical results. J NeuroIntervent Surg 2018; 10: A139. [Google Scholar]
- 95.Herbreteau D, Bibi R, Narata AP, Janot K, Papagiannaki C, Soize S, et al. Are anatomic results influenced by web shape modification? analysis in a prospective, single-center series of 39 patients with aneurysms treated with the web. AJNR Am J Neuroradiol 2016; 37: 2280–6. doi: 10.3174/ajnr.A4918 [DOI] [PMC free article] [PubMed] [Google Scholar]