Abstract
Objective:
To investigate the incidence and risk factors for liver abscess formation after treatment with drug-eluting bead chemoembolization (DEB-TACE) in patients with metastatic hepatic tumors (MHT).
Methods:
The current study is a retrospective analysis of the clinical data of 137 patients with metastatic hepatic tumors who received DEB-TACE treatment in our institute (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology) between June 2015 and September 2020. Patients were evaluated for the presence or absence of post-DEB-TACE liver abscess. Univariate and multivariate analyses were used to identify risk factors for liver abscess formation.
Results:
The incidence of liver abscess formation after the DEB-TACE procedure was 8.76% per patient and 5.53% per procedure. Univariate analysis showed that larger maximum tumor diameter (p = 0.004), Grade 1 artery occlusion (p < 0.001) and systemic chemotherapy within 3 months before the DEB-TACE procedure (p < 0.001) were all associated with liver abscess formation. However, only systemic chemotherapy within 3 months before the DEB-TACE procedure (OR 5.49; 95% CI 0.34–13.54; p < 0.001) was identified by multivariate analysis to be an independent risk factor.
Conclusions:
Tumor size, Grade 1 artery occlusion and recent systemic chemotherapy may all be associated with increased risk of liver abscess formation following DEB-TACE treatment in patients with metastatic hepatic tumors.
Advances in knowledge:
Identification of risk factors for liver abscess formation following DEB-TACE in patients with MHT. These findings suggest the need for caution and consideration of the aforementioned risk factors on the part of interventional radiologists when designing DEB-TACE strategies and performing post-procedure patient management.
Introduction
Drug-eluting bead chemoembolization (DEB-TACE) is considered to have utility as an optional therapeutic strategy for the treatment of liver tumors, especially for hypovascular liver metastasis.1,2 There are some advantages of DEB-TACE relative to conventional TACE. The symmetrical and uniform shape and size of the embolic beads results in more efficient embolization of the tumor supply. In addition, slow and continuous release of anti tumor drugs is promoted, increasing drug concentration and improving the control over tumor recurrence.1,3
Despite being minimally invasive, TACE can cause severe complications, including hepatic failure, liver abscess and biloma formation.4,5 Liver abscess formation is a relatively rare complication with an incidence rate between 0.1 and 4.5% in large-scale studies of patients with hepatic malignancies who received TACE.6–9 However, it is a severe complication with a reported mortality rate of up to 50%.9 Previous studies on liver abscess formation are largely limited to patient cohorts with primary hepatocellular carcinoma and there are few reports concerning metastatic hepatic tumors (MHT). Moreover, most reports refer to conventional chemoembolization which differs significantly from DEB-TACE. Reports indicate that DEB-TACE is associated with a high tumor necrosis rate and may stimulate pathophysiological inflammatory signals more strongly than conventional TACE.10,11 Any impact of these differences on liver abscess formation remains unclear.
To the best of our knowledge, little information regarding liver abscess formation following DEB-TACE in patients with MHT is available and no investigation of risk factors has been performed. Therefore, the purpose of the current study was to investigate frequency and risk factors for liver abscess formation in MHT patients who had undergone the DEB-TACE procedure.
Methods and materials
This study was approved by the institutional review board at the corresponding author’s institution. The requirement for informed consent was waived as the study involved a retrospective review of medical records and images.
Patient cohort
Medical records for patients diagnosed with MHT who underwent a DEB-TACE procedure in our institute between June 2015 and September 2020 were reviewed. Diagnosis of liver metastasis was made based on the following criteria: (1) extra hepatic primary malignancy shown by histological examination; (2) elevated plasma concentrations of tumor biomarkers; (3) consistent findings of liver mass shown by imaging. Data, including gender, age, medical history (including diabetes mellitus, chronic liver disease, bilioenteric anastomosis, use of prophylactic antibiotics and systemic chemotherapy prior to DEB-TACE procedure), Eastern Cooperative Oncology Group (ECOG) score, liver function (Child-Pugh classification), laboratory test for white blood cells, primary tumors, tumor numbers, maximum tumor diameter, portal vein thrombosis, selectivity of embolization and degree of artery occlusion were collated.
The degree of embolization selectivity was categorized according to the classification of Woo et al9: lobar, segmental or sub segmental. The degree of artery occlusion was graded according to the method of Miyayama et al with slight modifications12: 0: obvious blood flow slowing without achievement of arterial stasis; 1: achievement of arterial stasis.
DEB-TACE procedure
Informed consent was received from all patients before each DEB-TACE procedure. The procedure was performed as follows: (1) a 2.7F microcatheter was placed in the tumor feeding arteries as precisely as possible; (2) drug-eluting beads, CalliSpheres® (Jiangsu Hengrui Medicine Co., Ltd., China), 100–300 micrometers in diameter in non-ionic contrast medium (each vial was loaded with 60 mg of a doxorubicin solution or 80 mg of an irinotecan solution), were injected until slowing or stasis of blood flow in the tumor feeding arteries could be observed. No other embolic agents were administered. Patients were assessed at intervals for efficacy and treatment-related complications. In the absence of a complete response, based on modified Response Evaluation Criteria in Solid Tumours, further DEB-TACE sessions were performed dependent on the patient’s tolerance of the procedure.
Diagnosis of liver abscess
Patients' medical records were reviewed from Day 1 to at least 1 month after the procedure to identify the occurrence of liver abscess. Liver abscess was defined as follows: (1) CT images showing hypoattenuating lesion or MRI showing long T1 and long T2 lesion with gas-fluid level or a typical enhancement pattern (peripheral rim enhancement); (2) clinical presentation or laboratory tests meeting any of the following conditions: blood or pus culture revealing bacteria; a purulent pus from percutaneous drainage; body temperature higher than 38.5°C for at least 5 days with leukocyte count above 12 × 109 l−1 without any other cause.
Statistical analysis
Continuous data are presented as median (range). The Kolmogorov–Smirnov test was used to assess normal distribution of data. Normally distributed continuous variables were compared between groups using the independent samples t-test and those with abnormal distribution were compared using the Mann–Whitney U test. Categorical variables are presented as numbers (percentages). Differences in categorical data were compared using the Pearson X2 test or Fisher’s exact test.
Variables with significant differences in univariate analysis were analyzed by multivariate analysis using the Firth maximum likelihood estimation to identify independent risk factors. Statistical analyses were performed using Rstudio 1.4 (JJ Allaire, Boston, MA). A p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 137 patients (73 males and 64 females; median age: 58 years; range: 28–81 years) who had undergone 217 DEB-TACE procedures were enrolled. Of these, 131 (95.6%) had failed to respond to systemic chemotherapy (49 failed to respond to one line, and 82 failed to respond to two lines). The remaining six patients (4.4%) had refused systemic chemotherapy and accepted DEB-TACE as their first-line treatment after sufficient consultation. Clinical and imaging characteristics for all patients are summarized in Table 1. The most common type of primary tumor was colorectal cancer (CRC; n = 63; 46.0%), followed by gastric cancer (GC; n = 24; 17.5%), lung cancer (LC; n = 19; 13.9%), breast cancer (BC; n = 16; 11.7%), nasopharyngeal carcinoma (NPC; n = 10; 7.3%), neuroendocrine neoplasm (NEN; n = 4; 2.9%) and pancreatic carcinoma (PC; n = 1; 0.7%). Five (3.6%) patients had comorbidities of bilioenteric anastomosis and 10 (7.3%) had chronic liver disease. Diabetes mellitus was reported in 8 (5.8%) patients. 40 (29.2%) patients had one or two tumors and the remainder had multiple tumors (three or more). The median maximum tumor diameter was 5.2 cm (range: 3–11 cm).
Table 1.
Patients’ baseline characteristics divided according to liver abscess
| Characteristics | Total (n = 137) | LA (n = 12) | Non-LA (n = 125) |
|---|---|---|---|
| Age (year), median (range) | 58 (28–81) | 55 (39–73) | 59 (28–81) |
| Male gender, n (%) | 73 (53.3%) | 7 (58.3%) | 66 (52.8%) |
| Comorbilities | |||
| Diabetes mellitus, n (%) | 8 (5.8%) | 2 (16.7%) | 6 (4.8%) |
| Chronic liver disease, n (%) | 10 (7.3%) | 3 (25%) | 7 (5.6%) |
| Biliaryenteric anastomosis, n (%) | 5 (3.6%) | 1 (8.3%) | 4 (3.2%) |
| ECOG score | |||
| 0 | 123 (89.8%) | 10 (83.3%) | 113 (90.4%) |
| 1 | 14 (10.2%) | 2 (16.7%) | 12 (9.6%) |
| Child-Pugh classification | |||
| Child-Pugh A, n (%) | 128 (93.4%) | 10 (83.3%) | 118 (94.4%) |
| Child-Pugh B, n (%) | 9 (6.6%) | 2 (16.7%) | 7 (5.6%) |
| WBC (×109/L ), median (range) | 6.4 (2.9–10.7) | 6.1 (4.9–9.2) | 6.5 (2.9–10.7) |
| Primary tumor | |||
| Colorectal cancer, n (%) | 63 (46.0%) | 5 (41.7%) | 58 (46.4%) |
| Gastric cancer, n (%) | 24 (17.5%) | 3 (25.0%) | 21 (16.8%) |
| Lung cancer, n (%) | 19 (13.9%) | 2 (16.7%) | 17 (13.6%) |
| Breast cancer, n (%) | 16 (11.7%) | 0 | 16 (12.8%) |
| Nasopharyngeal carcinoma, n (%) | 10 (7.3%) | 1 (8.3%) | 9 (7.2%) |
| Neuroendocrine neoplasm, n (%) | 4 (2.9%) | 0 | 4 (3.2%) |
| Pancreas carcinoma, n (%) | 1 (0.7%) | 1 (8.3%) | 0 |
| Tumor number | |||
| 1, n (%) | 11 (8.0%) | 1 (8.3%) | 10 (8.0%) |
| 2, n (%) | 29 (21.2%) | 4 (33.3%) | 25 (20.0%) |
| 3 or more, n (%) | 97 (70.8%) | 7 (58.3%) | 90 (72.0%) |
| MTD (cm), median (range) | 5.2 (3–11) | 6.7 (4–9) | 4.9 (3–11) |
| Portal vein thrombosis | |||
| No, n (%) | 129 (94.2%) | 10 (83.3%) | 119 (95.2%) |
| Branch, n (%) | 7 (5.1%) | 2 (16.7%) | 5 (4.0%) |
| Main, n (%) | 1 (0.7%) | 0 | 1 (0.8%) |
| Antibiotic prophylaxis | |||
| No, n (%) | 129 (94.2%) | 11 (91.7%) | 118 (94.4%) |
| Yes, n (%) | 8 (5.8%) | 1 (8.3%) | 7 (5.6%) |
| SC before DEB-TACE | |||
| No, n (%) | 6 (4.4%) | 1 (8.3%) | 5 (4.0%) |
| Within 3 months, n (%) | 9 (6.6%) | 6 (50.0%) | 3 (2.4%) |
| More than 3 months, n (%) | 122 (89.1%) | 5 (41.7%) | 117 (93.6%) |
| Selectivity of embolization | |||
| Lobar, n (%) | 4 (2.9%) | 1 (8.3%) | 3 (2.4%) |
| Segmental, n (%) | 22 (16.1%) | 3 (25.0%) | 19 (15.2%) |
| Sub segmental, n (%) | 111 (81%) | 8 (66.7%) | 103 (82.4%) |
| Degree of artery occlusion | |||
| Grade 0, n (%) | 124 (90.5%) | 2 (16.7%) | 122 (97.6%) |
| Grade 1, n (%) | 13 (9.5%) | 10 (83.3%) | 3 (2.4%) |
LA, liver abscess; WBC, white blood cells; MTD, maximum tumor diameter; SC, systemic chemotherapy; DEB-TACE, drug-eluting bead chemoembolization.
Liver abscess formation
Liver abscesses developed in 12 of the 137 patients (217 procedures) in the cohort, giving an incidence rate of 8.76% per patient and 5.53% per procedure. Clinical characteristics of these patients are summarized in Table 2. The most common symptom of patients with liver abscess was hepatalgia (100%), followed by fever (95%), chill (82%), fatigue (73%) and nausea (64%). A mean period of 4.7 days (range: 2–12 days) elapsed between the DEB-TACE procedure and the confirmed diagnosis of liver abscess. Blood bacterial culture returned positive results for five patients (41.67%). The pathogenic microorganisms responsible were Escherichia coli (n = 3), Klebsiella pneumonia (n = 1) and Citrobacter freundii (n = 1). Pus bacterial culture returned positive results for 11 patients (91.67%). The pathogenic microorganisms responsible were Escherichia coli (n = 6), Klebsiella pneumonia (n = 2), Citrobacter freundii (n = 2) and Enterobacter cloacaeand (n = 1). Positive bacterial culture results for both blood and pus were observed in ive patients. The pathogenic microorganisms were Escherichia coli (n = 3), Klebsiella pneumonia (n = 1) and Citrobacter freundii (n = 1).
Table 2.
Characteristics of patients with liver abscess formation after DEB-TACE (n = 12)
| Age (year) | Gender | Comorbidities | SC before procedure | Child-Pugh classification | ECOG Score | Leukocyte count* | Primary tumors | Tumor number | MTD (cm) | PVTT | Degree of artery occlusion | Selectivity of embolization | Prophylactic antibiotics | Blood bacterial culture | Pus bacterial culture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 | M | N/A | N/A | A | 0 | 5.6 | CRC | 2 | 6.4 | No | 1 | Sub segmental | No | – | E.coli |
| 58 | M | N/A | >=3 m | A | 0 | 4.9 | GC | 2 | 3.8 | No | 1 | Sub- segmental | No | – | N/A |
| 47 | F | BA | >=3 m | A | 0 | 5.3 | GC | >=3 | 5.4 | No | 1 | Sub- segmental | No | E.coli | E.coli |
| 73 | M | CLD | <3 m | A | 0 | 6.7 | PC | 1 | 7.1 | No | 1 | Segmental | No | Citrobacter freundii | Citrobacter freundii |
| 54 | F | DM | <3 m | A | 0 | 5.1 | CRC | >=3 | 5.7 | Yes | 1 | Sub- segmental | No | E.coli | E.coli |
| 39 | M | N/A | <3 m | A | 1 | 9.2 | CRC | >=3 | 9.2 | No | 0 | Segmental | Yes | – | E.coli |
| 71 | M | N/A | >=3 m | A | 0 | 7.4 | NPC | >=3 | 5.9 | No | 1 | Lobar | No | – | E.coli |
| 55 | F | N/A | >=3 m | A | 0 | 6.3 | LC | >=3 | 6.9 | Yes | 1 | Sub- segmental | No | Klebsiella pneumonia | Klebsiella pneumonia |
| 62 | M | CLD | >=3 m | A | 1 | 5.9 | LC | >=3 | 8.1 | No | 1 | Segmental | No | E.coli | E.coli |
| 49 | F | N/A | >=3 m | B | 0 | 5.5 | CRC | 2 | 5.3 | No | 1 | Sub- segmental | No | – | Enterobacter cloacaeand |
| 68 | M | DM +CLD | <3 m | A | 0 | 6.2 | GC | >=3 | 7.2 | No | 1 | Sub- segmental | No | – | Klebsiella pneumonia |
| 52 | F | N/A | <3 m | A | 0 | 6.8 | CRC | 2 | 8.7 | No | 0 | Sub- segmental | No | – | Citrobacter freundii |
BA, bilioenteric anastomosis; CLD, chronic liver disease; DM, diabetes mellitus; E. coli, Escherichia coli; MTD, maximum tumor diameter; PVTT, portal vein tumor thrombosis; SC, systemic chemotherapy.
Notes: *×109 l−1.
Univariate analysis of all variables was performed. The occurrence of larger maximum tumor diameter, systemic chemotherapy within 3 months before DEB-TACE procedure and Grade 1 artery occlusion were statistically different between patients with liver abscess formation and those without (Table 3). Multivariate analysis using the Firth maximum likelihood estimation showed that only systemic chemotherapy within 3 months before DEB-TACE procedure (OR 5.49; 95% CI 0.34–13.54; p < 0.001) constituted an independent risk factor for liver abscess formation.
Table 3.
Relation between predisposing variables and liver abscess formation in all patients (n = 137)
| Variables | Liver abscess with predisposing factor | P (Univariate) | P (Multivariate) | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Older patienta | 7/53 | 5/72 | 0.366 | |||
| Male gender, n (%) | 5/59 | 7/66 | 0.714 | |||
| Comorbidities | ||||||
| Diabetes mellitus, n (%) | 10/119 | 2/6 | 0.147 | |||
| Chronic liver disease, n (%) | 9/118 | 3/7 | 0.059 | |||
| Biliaryenteric anastomosis, n (%) | 11/121 | 1/4 | 0.372 | |||
| ECOG score (1), n (%) | 10/113 | 2/12 | 0.353 | |||
| Child-Pugh Class B, n (%) | 10/118 | 2/7 | 0.179 | |||
| Leukopenia, n (%) | 12/115 | 0/10 | 0.600 | |||
| Three or more tumors, n (%) | 5/35 | 7/90 | 0.462 | |||
| Larger MTDb, n (%) | 1/65 | 11/60 | 0.004 | |||
| PVTT, n (%) | 10/119 | 2/6 | 0.186 | |||
| Antibiotic prophylaxis, n (%) | 11/118 | 1/7 | 0.529 | |||
| SC within 3 m before DEB-TACE, n (%) | 6/122 | 6/3 | <0.001 | <0.001 | 5.49 | 0.34–13.54 |
| Sub segmental embolization, n (%) | 4/22 | 8/103 | 0.241 | |||
| Grade 1 artery occlusion, n (%) | 2/122 | 10/3 | <0.001 | |||
Older patient was defined as age above the median.
Larger MTD was defined as maximum tumor diameter above the median.
Patient outcomes
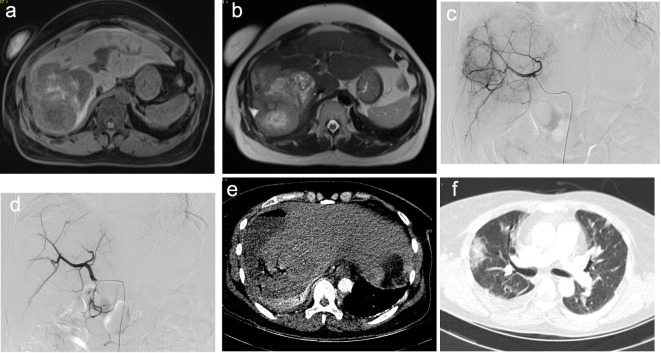
All patients in whom abscess was confirmed were treated with parenteral antibiotics either empirically or according to available bacterial culture results. Percutaneous catheter drainage (PTCD) was performed in eight patients and percutaneous aspiration in three. Two patients died as a result of the rapid progression of the abscess to disseminated intravascular coagulation (DIC), 1–2 days after diagnosis of abscess. One patient died from respiratory failure due to combined pulmonary infection 15 days after diagnosis (Figure 1). The mortality rate was 25% and the duration of hospitalization was 8 days (range: 3–15 days).
Figure 1.
A 55-year-old female patient with multiple MHTs originating from lung cancer who underwent the DEB-TACE procedure. 5 days after the operation, the patient complained of fever, chill and hepatalgia. A blood test showed elevated white blood cells (18.7 × 109 l−1). Abdominal CT showed a ruptured liver abscess and percutaneous liver abscess drainage was performed immediately. Bacterial culture from blood and pus was positive for Klebsiella pneumonia. Despite intravenous administration of appropriate antibiotics, the patient died of respiratory failure due to severe pulmonary infection. (a) (T1WI) and (b) (T2WI) Pre-operative MRI showed multiple lesions in the liver. (c) DSA imaging of the liver metastasis. (d) DSA imaging after embolization with the achievement of blood flow stasis. (e) CT imaging showed liver abscess with low-intensity and gas-fluid level inside the lesion, accompanied by rupture into the capsule of the liver. (f) CT imaging showed pulmonary infection. DSA, digital subtraction angiography; MHT, metastatic hepatic tumor.
Discussion
There have been several reports of higher rates of liver abscess formation following chemoembolization/embolization (TACE/TAE) procedures in patients with MHT compared with those with hepatocellular carcinoma (HCC). Song et al reported an overall incidence of 0.2% in 2439 patients while the incidence was 2.6% in patients with metastatic tumors.6 Furthermore, Lv et al demonstrated a significantly higher incidence of liver abscess in patients with MHT than in those with HCC (1.54% vs 0.32%).13 The current study reports data which indicate a higher incidence of liver abscess formation (8.76% per patient and 5.53% per procedure) than those previously reported. Differences in the embolic agents used for the TACE procedures may account for this disparity.
The current study enrolled a cohort of patients for whom the embolic agent used in the DEB-TACE procedure was a permanent, heterogeneous drug-eluting bead. Such agents have been associated with a higher necrosis rate and with greater pathophysiological inflammatory responses by comparison with conventional iodized-oil TACE procedures. There have also been suggestions of increased risk of liver and biliary injuries following use of drug-eluting beads which may be risk factors for liver abscess formation.10,14,15 Blood supply to the biliary duct depends on arterial blood flow in contrast with the double blood supply to the liver parenchyma. HCC usually develops on a background of cirrhosis and portal hypertension, which may promote the development of a more complex arterial network to supply the biliary ductal system.16 However, most MHTs lack this protective effect. These anatomical and pathological characteristics make the biliary duct in MHTs more susceptible to the ischemic injury caused by artery occlusion. This observation may partially explain why biliary injury following TACE occurred more frequently for patients with MHTs than for those with HCC. Inconsistencies in the performance of DEB-TACE techniques for MTHs, particularly concerning the degree and selectivity of embolization, characterize the literature concerning this treatment.1,17 Many authors recommend a lobar treatment and avoiding arterial stasis for colorectal cancer liver metastases (CRCLM).18,19 A recent study demonstrated the association between the branch level of embolization and the severity of post-DEB-TACE syndrome in CRCLM patients.17 Only a small proportion of the patients included in the current study received lobar embolization (2.9%) or Grade 1 artery occlusion (9.5%). Univariate analysis showed that Grade 1 artery occlusion was associated with liver abscess formation, although this was not confirmed by multivariate analysis. Further large scale and well-designed studies are required to investigate any association between the degree and selectivity of embolization and the rate of abscess formation.
Given the small sample size of the present patient cohort, a multivariable analysis using the Firth maximum likelihood estimation was performed to identify independent risk factors for liver abscess formation. The results showed that only systemic chemotherapy within 3 months before the DEB-TACE procedure constituted an independent risk factor. Systemic chemotherapy is widely used to treat malignancies, especially in patients with CRC.20,21 Potential side-effects, such as intestinal mucositis along the entire gastrointestinal tract, leave mucosal tissue open to infection and ulceration.22,23 Moreover, systemic chemotherapy (e.g. 5-FU chemotherapy) promotes an imbalance in gut microbes.24,25 MHTs have a better blood supply from the portal vein than primary liver cancers and blood continues to be supplied after the TACE procedure.26,27 We consider that the imbalance in gut microbes plus intestinal mucositis caused by systemic chemotherapy might increase the opportunity of infection brought by blood flow in the portal vein. This inference was supported by results of blood and pus culture which showed that the most common pathogens were Enterobacteriaceae. However, further studies are required to elucidate the underlying mechanisms.
Bilioenteric anastomosis has also been recognized as a risk factor for liver abscess formation due to the lack of the duodenal extensor muscle in preventing bacterial flow from the intestine into the bile duct.28–31 Woo et al reported a high incidence of liver abscess after TACE among patients with bilioenteric anastomoses (26.2% per procedure and 48.0% per patient) and the use of prophylactic antibiotics did not significantly decrease the incidence.9 The current study included 5 patients with a history of bilioenteric anastomosis. These patients had normal preoperative blood cell tests and no evidence of infection. None was treated with prophylactic antibiotics in the peri-operative period. Of these patients, one (1/5; 20%) developed a liver abscess 3 days after TACE and died of DIC 2 days later. The others remained stable except for post-embolization syndrome. Further extensive studies are required to establish the relationship between bilioenteric anastomosis and post-TACE liver abscess formation plus any role of prophylactic antibiotics.
The limitations inherent to any retrospective study apply to the current findings. Firstly, this study was conducted in a single tertiary hospital over a long period and data collection and selection bias may have occurred. Secondly, there was no standardization of patient treatment which may generate bias in data analysis. In addition, our results showed that systemic chemotherapy within 3 months before the DEB-TACE procedure was an independent risk factor. However, the current study lacked the scope for investigation of which chemotherapy agent(s) may be responsible for this finding.
In conclusion, liver abscess formation was not a rare complication following the DEB-TACE procedure in patients with MHTs. Larger tumor size, systemic chemotherapy within 3 months before DEB-TACE procedure and Grade 1 artery occlusion were all associated with liver abscess formation. These findings suggest that caution should be exercised by interventional radiologists when designing DEB-TACE strategies. Attentive follow-up is required to detect and manage this severe complication in patients with the aforementioned risk factors.
Footnotes
Funding: This work was granted by Medical Scientific Research Project of Wuhan Municipal Heal Commission (No.WX20A08).
The authors Tianhe Ye and Peng Zhu contributed equally to the work.
Tianhe Ye and Peng Zhu have contributed equally to this study and should be considered as co-first authors.
Contributors: All the authors read and approved the final manuscript and agreed to submit the manuscript to BRITISH JOURNAL OF RADIOLOGY. The authors claim that none of the material in the manuscript has been published or is under consideration for publication elsewhere.
Contributor Information
Tianhe Ye, Email: xinwu0401@aliyun.com.
Peng Zhu, Email: 68348771@qq.com.
Zhiping Liu, Email: 17379346@qq.com.
Qianqian Ren, Email: 45194133@qq.com.
Chuansheng Zheng, Email: chuansheng_zheng@sina.com.
Xiangwen Xia, Email: xiangwen_xia@hust.edu.cn.
REFERENCES
- 1.Xu H, Min X, Ren Y, Yang L, Liu F. Comparative study of drug-eluting beads versus conventional transarterial chemoembolization for treating peculiar anatomical sites of gastric cancer liver metastasis. Med Sci Monit 2020; 26: e922988. doi: 10.12659/MSM.922988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogl TJ, Marko C, Langenbach MC, Naguib NNN, Filmann N, Hammerstingl R, et al. Transarterial chemoembolization of colorectal cancer liver metastasis: improved tumor response by DSM-TACE versus conventional TACE, a prospective, randomized, single-center trial. Eur Radiol 2021; 31: 2242–51. doi: 10.1007/s00330-020-07253-2 [DOI] [PubMed] [Google Scholar]
- 3.Malagari K, Iezzi R, Goldberg SN, Bilbao JI, Sami A, Akhan O, et al. The ten commandments of chemoembolization: expert discussion and report from Mediterranean Interventional Oncology (MIOLive) congress 2017. Eur Rev Med Pharmacol Sci 2018; 22: 372–81. doi: 10.26355/eurrev_201801_14184 [DOI] [PubMed] [Google Scholar]
- 4.Tu J, Jia Z, Ying X, Zhang D, Li S, Tian F, et al. The incidence and outcome of major complication following conventional TAE/TACE for hepatocellular carcinoma. Medicine 2016; 95: e5606. doi: 10.1097/MD.0000000000005606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massmann A, Rodt T, Marquardt S, Seidel R, Thomas K, Wacker F, et al. Transarterial chemoembolization (TACE) for colorectal liver metastases-current status and critical review. Langenbecks Arch Surg 2015; 400: 641–59. doi: 10.1007/s00423-015-1308-9 [DOI] [PubMed] [Google Scholar]
- 6.Song SY, Chung JW, Han JK, Lim HG, Koh YH, Park JH, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol 2001; 12: 313–20. doi: 10.1016/S1051-0443(07)61910-1 [DOI] [PubMed] [Google Scholar]
- 7.Arslan M, Degirmencioglu S. Liver abscesses after transcatheter arterial embolization. J Int Med Res 2019; 47: 1124–30. doi: 10.1177/0300060518816875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong G-Y, Changchien C-S, Lee C-M, Wang J-H, Tung H-D, Chuah S-K, et al. Liver abscess complicating transcatheter arterial embolization: a rare but serious complication. A retrospective study after 3878 procedures. Eur J Gastroenterol Hepatol 2004; 16: 737–42. doi: 10.1097/01.meg.0000108361.41221.8c [DOI] [PubMed] [Google Scholar]
- 9.Woo S, Chung JW, Hur S, Joo S-M, Kim H-C, Jae HJ, et al. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR Am J Roentgenol 2013; 200: 1370–7. doi: 10.2214/AJR.12.9630 [DOI] [PubMed] [Google Scholar]
- 10.Bhagat N, Reyes DK, Lin M, Kamel I, Pawlik TM, Frangakis C, et al. Phase II study of chemoembolization with drug-eluting beads in patients with hepatic neuroendocrine metastases: high incidence of biliary injury. Cardiovasc Intervent Radiol 2013; 36: 449–59. doi: 10.1007/s00270-012-0424-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiu B, Deschamps F, Aho S, Munck F, Dromain C, Boige V, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol 2012; 56: 609–17. doi: 10.1016/j.jhep.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 12.Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 2007; 18: 365–76. doi: 10.1016/j.jvir.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 13.WF L, Lu D, YS H, Xiao JK, Zhou CZ, Cheng DL. Liver abscess formation following transarterial chemoembolization: clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage. Medicine 2016; 95: e3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Chung JW, Lee K-H, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol 2017; 28: 502–12. doi: 10.1016/j.jvir.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Nishiofuku H, Maeda S, Masada T, Anai H, Sakaguchi H, et al. Repeated bland-TAE using small microspheres injected via an implantable port-catheter system for liver metastases: an initial experience. Cardiovasc Intervent Radiol 2014; 37: 493–7. doi: 10.1007/s00270-013-0691-2 [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto I, Iwanaga S, Nagaoki K, Matsuoka Y, Ashizawa K, Uetani M, et al. Intrahepatic biloma formation (bile duct necrosis) after transcatheter arterial chemoembolization. AJR Am J Roentgenol 2003; 181: 79–87. doi: 10.2214/ajr.181.1.1810079 [DOI] [PubMed] [Google Scholar]
- 17.Szemitko M, Golubinska-Szemitko E, Wilk-Milczarek E, Falkowski A. Side effect/complication risk related to injection branch level of chemoembolization in treatment of metastatic liver lesions from colorectal cancer. J Clin Med 2020; 10: 121: E121. doi: 10.3390/jcm10010121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichler K, Zangos S, Mack MG, Hammerstingl R, Gruber-Rouh T, Gallus C, et al. First human study in treatment of unresectable liver metastases from colorectal cancer with irinotecan-loaded beads (DEBIRI). Int J Oncol 2012; 41: 1213–20. doi: 10.3892/ijo.2012.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lencioni R, Aliberti C, de Baere T, Garcia-Monaco R, Narayanan G, O'Grady E, et al. Transarterial treatment of colorectal cancer liver metastases with irinotecan-loaded drug-eluting beads: technical recommendations. J Vasc Interv Radiol 2014; 25: 365–9. doi: 10.1016/j.jvir.2013.11.027 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H. Ann Oncol 2014; 25: 1032–8. doi: 10.1093/annonc/mdu100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin J, Petrillo A, Smyth EC, Shaida N, Khwaja S, Cheow HK, et al. Colorectal liver metastases: current management and future perspectives. World J Clin Oncol 2020; 11: 761–808. doi: 10.5306/wjco.v11.i10.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 2014; 40: 409–21. doi: 10.1111/apt.12878 [DOI] [PubMed] [Google Scholar]
- 23.Hueso T, Ekpe K, Mayeur C, Gatse A, Joncquel-Chevallier Curt M, Gricourt G, et al. Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: the role of mucosal strengthening. Gut Microbes 2020; 12: 1800897. doi: 10.1080/19490976.2020.1800897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sougiannis AT, VanderVeen BN, Enos RT, Velazquez KT, Bader JE, Carson M, et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav Immun 2019; 80: 44–55. doi: 10.1016/j.bbi.2019.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karin M, Jobin C, Balkwill F. Chemotherapy, immunity and microbiota-a new triumvirate? Nat Med 2014; 20: 126–7. doi: 10.1038/nm.3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Sharif E, Simoneau E, Hassanain M. Portal vein embolization effect on colorectal cancer liver metastasis progression: lessons learned. World J Clin Oncol 2015; 6: 142–6. doi: 10.5306/wjco.v6.i5.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zattar-Ramos LC, Bezerra RO, Siqueira LT, Marques DT, Menezes MR, Herman P, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in colorectal liver metastasis: the radiologist's perspective. Abdom Radiol 2016; 41: 2150–60. doi: 10.1007/s00261-016-0832-6 [DOI] [PubMed] [Google Scholar]
- 28.Yu M-A, Liang P, Yu X-L, Cheng Z-G, Han Z-Y, Liu F-Y, et al. Liver abscess as a complication of microwave ablation for liver metastatic cholangiocarcinoma after bilioenteric anastomosis. Int J Hyperthermia 2011; 27: 503–9. doi: 10.3109/02656736.2011.555876 [DOI] [PubMed] [Google Scholar]
- 29.Lv W-F, Lu D, He Y-S, Xiao J-K, Zhou C-Z, Cheng D-L. Liver abscess formation following transarterial chemoembolization: clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage. Medicine 2016; 95: e3503. doi: 10.1097/MD.0000000000003503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi S-H, Zhai Z-L, Zheng S-S. Pyogenic liver abscess of biliary origin: the existing problems and their strategies. Semin Liver Dis 2018; 38: 270–83. doi: 10.1055/s-0038-1661363 [DOI] [PubMed] [Google Scholar]
- 31.Yu M-an, Liang P, Yu X-ling, Cheng Z-gang, Han Z-yu, Liu F-yi, et al. Complications and clinical outcomes of microwave ablation for liver metastatic cholangiocarcinoma after bilioenteric anastomosis. Zhonghua Yi Xue Za Zhi 2013; 93: 516–9. [PubMed] [Google Scholar]