Abstract
The Lactate Shuttle hypothesis is supported by a variety of techniques including mass spectrometry analytics following infusion of carbon-labeled isotopic tracers. However, there has been controversy over whether lactate tracers measure lactate (L) or pyruvate (P) turnover. Here, we review the analytical errors, use of inappropriate tissue and animal models, failure to consider L and P pool sizes in modeling results, inappropriate tracer and blood sampling sites, and failure to anticipate roles of heart and lung parenchyma on L⇔P interactions. With support from magnetic resonance spectroscopy (MRS) and immunocytochemistry, we conclude that carbon-labeled lactate tracers can be used to quantitate lactate fluxes.
Keywords: energy-substrate partitioning, exercise, glycolysis, isotope tracers, oxidative metabolism
INTRODUCTION
Recognition of the diverse roles of lactate (L) in intermediary metabolism is growing (1–5). No longer considered to be a byproduct of oxygen-limited metabolism, it is widely acknowledged that lactate functions as follows: a body energy substrate in skeletal (6) and cardiac muscle (7, 8) and brain tissues (9, 10); a mitochondrial energy substrate (1, 11–13); a conveyor of carbohydrate carbon following digestion (14–16); a gluconeogenic precursor (17–19); a signaling molecule working through redox control (20), HCAR-1 (GPR81) binding (20–22), and TGF-β (5); and regulator of gene expression by lactylation of histones (23). The overarching Lactate Shuttle hypothesis of lactate metabolism (24–26) was developed, and has been supported by a variety of experimental approaches including: measurements of tissue metabolite levels (27, 28), arterial-venous difference and blood flow measurements (6), mass spectrometric measurements of tissue oxygenation during net lactate release (29), magnetic resonance spectroscopy to assess muscle metabolite turnover in situ (30), and cell fractionation with immunocoprecipitation and immunohistochemistry identifying the presence of a mitochondrial lactate oxidation complex (31, 32). As well, support of the Lactate Shuttle hypothesis came from studies using carbon-labeled radiotracer (14C) (33–36) and stable (13C) isotopic tracers (6, 37–40). However, there has been controversy over whether lactate tracers measure lactate (L) or pyruvate (P) turnover, and, therefore, total carbohydrate flux and oxidation (41, 42). Resolution of the controversy is important because it informs about regulation of metabolic rate and energy-substrate partitioning (43). In this paper, as part of the thesis that L is far more important than P in the regulation of metabolism, the use of carbon-labeled lactate tracers to quantitate blood and tissue lactate fluxes is evaluated.
RESULTS AND DISCUSSION
Apparent Equilibria, the [L]/[P]
Issues related to L/P (lactate⇔pyruvate) equilibration will be sequentially discussed in terms of L and P chemical concentrations (L/P) and isotopic enrichments [LIE]/[PIE]. However, it should be prefaced that outside of well-defined conditions, the L/P ratio in blood has little or no meaning for cellular metabolic regulation by redox state (44, 45). Hence, the analysis of blood sampled during experiments with poorly defined conditions, with uncertain contributions from many undefined dehydrogenases or oxidoreductases in diverse tissues, with significant dilution and uncertain admixture along with inappropriate sampling cannot yield meaningful interpretation of L to P equilibration or cellular redox state. Hence, the fractious assertion that blood L/P ratios having physiological meaning stands well beyond the question of metabolic “equilibrium” and a purported role in metabolic regulation (44).
The equilibrium constant (Keq) for lactate dehydrogenase (LDH) is reported to be 1,000 (46). In vivo, the concentration ratio ([L]/[P]) is nominally 10 in arterial blood, can rise more than an order of magnitude in the venous effluent of working muscle (47), but does not approach the value of 103. Notwithstanding the thermodynamics of equilibria determined under standard conditions, investigators in the tracer metabolism field have endeavored to assign meaning of blood L/P. For instance, L and P have been considered to be “in equilibrium” if their chemical concentrations in a blood sample are constant, an assumption being that the flux of lactate to pyruvate is equal to the reverse flux. However, as described in Apparent Equilibria, simplifying thinking to considerations to the [L]/[P], or the respective isotopic enrichments ([LIE]/[PIE]), is fraught with difficulties as described in Interpretation of the [LIE]/[PIE]. Importantly, the chemical levels and isotopic enrichments of L and P in blood after 13C-tracer administration need not be, and seldom are either constant or equivalent over the dynamic physiological range (47).
Despite admonitions of some that the L/P is informative of cell redox (45), the relationship between L and P levels in blood and other tissue has long been of interest as this ratio was thought to reflect adequacy of tissue oxygenation and cell redox with “excess lactate” being related to the “Oxygen Debt” following exercise (48) and in other conditions such as hypoxemia from breathing low-O2 gases (49). That cell redox is related to the L/P and other redox pairs is a persistent concept in physiology (20) and medicine to the extent that the L/P is sometimes taken as a measure of tissue oxygenation, even if no evidence of the latter is apparent (50, 51).
Although arguably informative of cell redox, measurement of the L/P as a measure of cell redox is unsatisfying because it is labile at the tissue site of measurement and varies among tissue compartments. As well, the L/P in blood changes during a circulatory passage, being different in arterial compared with venous blood, because of tissue metabolism (production and utilization) and the activities of lactate dehydrogenase (LDH) and monocarboxylate transporters (MCTs) in blood and tissue (heart and lung) parenchyma (52, 53).
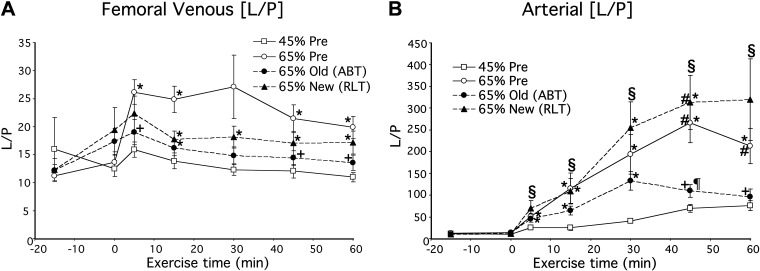
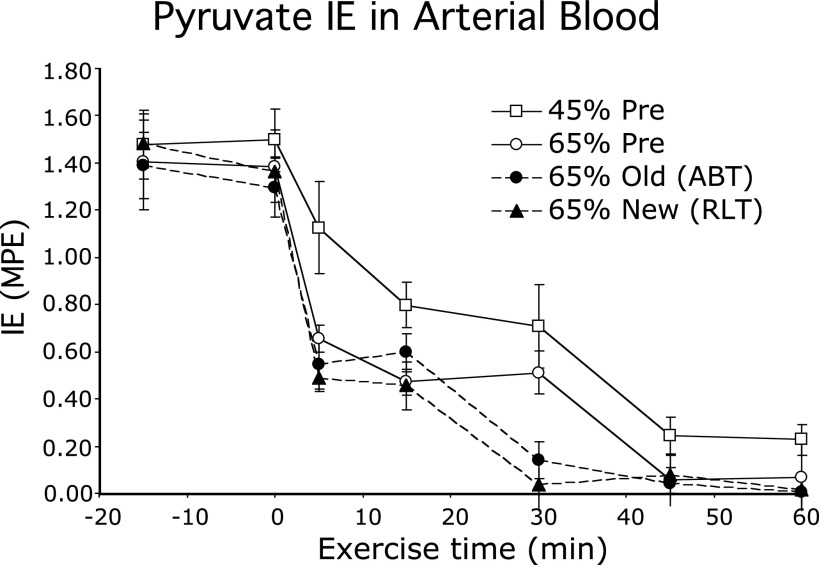
Evidence for the presence of a transpulmonary lactate shuttle was first observed by Henderson et al. (47) in which lactate and pyruvate concentrations and isotopic enrichments (IEs) across resting and working human skeletal muscle were determined after primed-continuous infusions of [3-13C]lactate. At rest, the L/P in venous effluent of resting muscle was 10–15 and increased significantly to 20–25 during exercise (Fig. 1A). In contrast, the L/P in arterial blood was <10 during rest and increased into the range of 100–350 with some individual values of 500 during hard (65% V̇o2max) exercise after training (Fig. 1B) (47). At the same time, pyruvate isotopic enrichment [in mole percent excess (MPE)] in arterial blood from infused tracer lactate fell from 1.4–1.6 to 0.30 or less during exercise (Fig. 2). Hence, in the course of blood leaving a resting or working muscle bed and reaching the arterial circulation, a massive pyruvate to lactate conversion occurred; events in the blood and pulmonary circulation were implicated by results of Henderson et al. (47), but were unanticipated and missed by others (3, 41, 42, 54, 55). Subsequently, using an anesthetized rodent (rat) model, Johnson et al. (52, 53) verified the presence of the transpulmonary lactate shuttle, vide infra.
Figure 1.
Lactate-to-pyruvate (L/P) concentration ratio in femoral venous (A) and arterial blood (B) of men during rest and exercise before and after 2-mo of supervised endurance training. Values are means ± SE; n = 7–9 men. Exercise conditions are: Pre, before training; Post, after training; 45% Pre, 45% pretraining peak oxygen consumption (V̇o2peak); 65% Pre, 65% pretraining V̇o2peak; 65% Old (ABT), 65% old V̇o2peak peak post-training; 65% New (RLT), 65% post-training V̇o2peak. *Significantly different from 45% Pre, P < 0.05; +significantly different from 65% Pre, P < 0.05, #significantly different from 65% Old (ABT), P < 0.05; ¶significantly different from 65% New (RLT), P < 0.05. From Ref. 47.
Figure 2.
Isotopic enrichment (IE) in mole percent excess (MPE) of [3-13C]pyruvate in arterial blood of men during rest and exercise; data obtained simultaneously with those in Fig. 1. Values are means ± SE; n = 8 or 9. Exercise conditions are described in the legend to Fig. 1. From Ref. 47.
Apparent Equilibria, the [Lie]/[Pie]
Although lactate and pyruvate have been considered to be in equilibrium if their concentrations are constant and if the assumption of equivalence in forward and reverse fluxes across LDH are valid, the measurement gives no appreciation of the rates of those fluxes. Hence, investigators have utilized carbon-labeled tracers to assess the extent of lactate-pyruvate cycling. This issue is relevant because numerous investigators have utilized carbon-labeled isotope tracers to assess lactate turnover and oxidation rates in humans (39, 56–59) and other mammals (33–36).
Interpretation of the [Lie]/[Pie]
Concern about the use of carbon-labeled tracers arose when Wolfe et al. determined L/P interactions in anesthetized dogs while infusing combinations of [3-13C]lactate and alanine into the left ventricle and sampling from the right ventricles (42). Wolfe et al. observed that pyruvate enrichment was 91% of the lactate enrichment, and alanine enrichment was 81% of the pyruvate enrichment and 72% of the lactate enrichment. They concluded that lactate kinetics as traditionally determined using carbon-labeled tracers more “accurately reflect whole body pyruvate kinetics.” Subsequently, in the Wolfe Lab Romijn et al. spiked human blood with [1-13C]lactate or -pyruvate and observed L⇔P over time ex vivo (41). In this case, the choice of using 1-13C-tracers was appropriate because 1C is lost in the conversion of pyruvate to acetate in the tricarboxylic acid cycle (TCA). Because erythrocytes do not contain a mitochondrial reticulum, and are purely glycolytic expressing high levels of LDH, results of Romijn et al. were assumed to represent L⇔P interconversion in the cytosol of erythrocytes. They found that pyruvate and lactate tracer-to-tracee ratios equilibrated rapidly in whole blood, whereas as expected there was no isotopic exchange in plasma ex vivo. Results implicated the effects of erythrocytes known to have high levels of erythrocyte MCT (60) and LDH expression (61). Following on the earlier report of Wolfe et al. (42), Romijn et al. concluded that there is a very rapid interconversion between lactate and pyruvate in blood that has to be considered in the interpretation of in vivo tracer studies.
Neglecting experimental errors in determining L and P isotopic enrichments that have not been replicated in animal models or humans, vide infra, there are multiple problems with those and similar reports related to anatomy, physiology, and metabolism (41, 42, 62).
In Wolfe et al. (42), 13C-labeled tracers were infused on the arterial side of the systemic circulation, but investigators had no measures of downstream arterial lactate concentrations or isotopic enrichments, the input functions to all body tissues. Furthermore, they did not measure mixed venous L or P concentrations; hence, they had no measures of the quantities of L or P tracers presented to or released from various tissues. Minimally, the quantity of lactate tracer in arterial blood probably exceeded that of pyruvate tracer by an order of magnitude, or two as shown by Henderson et al. (47).
As well, by sampling on the right atrium, as opposed to the pulmonary artery (generally accepted as the site for sampling of “mixed venous blood”) (63), Wolfe et al. (42) had an inadequate measure of mixed venous blood, and were thus not able to truly measure either lactate or pyruvate kinetics. Sampling as they did, the investigators experimentally excised the pulmonary bed from their estimations of lactate flux. Moreover, even if Wolfe et al. (42) had simultaneously sampled pulmonary arterial and arterial blood they would have missed contributions of the lung parenchyma to lactate flux. The pulmonary mesenchyme richly expresses LDH and monocarboxylate transport proteins (MCTs) that are involved in the conversion of mixed-venous pyruvate to lactate and subsequent release into the pulmonary vein and arterial blood via a Transpulmonary Lactate Shuttle (52, 53). Again, such results involving lung and heart parenchyma were unanticipated (41, 42).
As already described, based on the results of tracer studies on heparinized dog blood under stasis conditions and anaesthetized dogs improperly infused and sampled it was concluded that lactate tracers yielded results to pyruvate, as opposed to lactate metabolism (41, 42). Those data and interpretations were in contrast to those previously obtained on small (34), medium sized (33), or large mammals (36) as well as humans (37, 39, 59, 64).
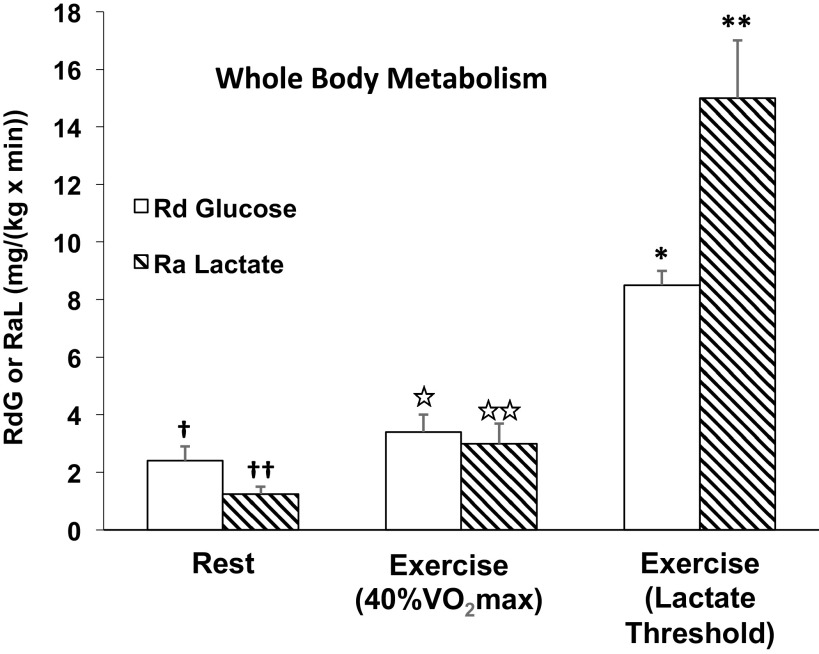
And finally, it was disappointing that Wolfe et al. neglected to make easy checks on their conclusion that measures of lactate kinetics using carbon-labeled tracers reflected whole body pyruvate kinetics. For instance, the rate of glucose disposal (Rd) is the best measure of whole body glycolysis, hence pyruvate production. However, as illustrated in Fig. 3, in resting men tracer-measured lactate Ra was approximately half of glucose disposal rate (Rd). During exercise at 40% V̇o2max, glucose Rd and lactate rate of appearance (Ra) were approximately equal, but during exercise at 65% of V̇o2max (≈ the lactate threshold), due to the major contribution of glycogen to glycolytic flux lactate Ra was twice the glucose Rd (8, 38, 59, 65). Thus, depending on the metabolic rate, as determined by exercise intensity or other factors such as epinephrine stimulation (52, 66, 67), lactate flux can be less, the same, or greater than that of glucose. Hence, estimating glycolytic flux rates by making precursor-product comparisons using tracers does not support the thesis that lactate tracers estimate pyruvate flux.
Figure 3.
Illustration of the relationships between whole body glucose rate of disappearance (Rd) and lactate appearance (Ra) in 10 men during rest, exercise at 40% V̇o2max (59), and lactate threshold (≈65% V̇o2max; 6 men) (38). Symbols: for rest glucose † and lactate †† fluxes significantly different from each other; for 40% V̇o2max exercise, glucose ⋆ and lactate ⋆⋆ fluxes not significantly different from each other, but increased from corresponding values at rest; for exercise at the LT glucose flux * significantly greater than that at rest and 40% V̇o2max, and lactate flux ** significantly greater than corresponding values at rest, 40% V̇o2max and the corresponding glucose flux. Lactate flux in excess of glucose flux is attributable to the contribution of glycogenolysis to glycolytic carbon flux (18).
Another way Romijn et al. could have checked on veracity of their claim that the use of carbon-labeled lactate tracers reflects whole body pyruvate kinetics would have been to determine pyruvate and lactate oxidation rates and compare results against those obtained by indirect calorimetry. Since Zuntz and Schumburg (68) and Lusk (69) countless others, including us (70), have used indirect calorimetry to assess metabolic rate and energy substrate partitioning. At no time in resting or exercising subjects, when lactate disposal via oxidation approximates 100%, has the whole body carbohydrate oxidation estimated from pulmonary gas exchange been shown to equal the tracer-measured lactate, or pyruvate oxidation rates (6, 37–39).
Tracer Validation Using Exogenous Infusion
Yet another way to validate the use of carbon-labeled tracers to measure body lactate turnover is to mimic the euglycemic, hyperinsulinemic procedure in which endogenous glucose production is determined by inhibiting endogenous production and infusing a quantity of exogenous glucose so that euglycemia is achieved (i.e., the “glucose clamp”). By analogy, blood lactate kinetics can be assessed by simultaneously infusing 13C-lactate tracers along with exogenous, unlabeled lactate raising arterial lactate concentration to an intended value (e.g., 4 mM), thus performing a “lactate clamp” (LC). Such LC procedures have been employed by at least two sets of investigators, including Miller et al. (71) and Searle et al. (72). Results of those experiments not only validate the use of carbon-labeled tracers to study lactate metabolism, but they are also informative of glucose-lactate and lactate-lactate source interactions in humans. Simply, with regard to the latter effects of lactate, endogenous lactate can substitute for glucose, thus reducing glucose flux, whereas exogenous lactate can reduce both glucose disposal and endogenous lactate appearance. Competitive inhibition among energy substrates has long been recognized in the field of cardiac metabolism (8, 73, 74).
In the study by Miller et al., the investigators studied endurance-trained humans at rest and during moderate intensity exercise while blood lactate concentration was clamped at 4 mM using an exogenous intravenous infusion of sodium lactate. Using a [3-13C]lactate tracer, they demonstrated that lactate Ra was increased at rest and during exercise to an extent equal to the exogenous infusion rate; rephrased, the tracer-measured lactate flux equaled the endogenous lactate production plus exogenous lactate infusion rates. In addition, by simultaneously infusing [6,6-2H2]glucose (i.e., D2-glucose) tracer, Miller et al. (75) observed that raising the arterial [lactate] by exogenous lactate infusion decreased glucose disposal. Furthermore, as described earlier regarding competitive substrate inhibition, Miller et al. showed that LC increased lactate oxidation rate (Rox) and decreased glucose Rox.
In their studies, Searle et al. (72) showed that by infusing large quantities of lactate, i.e., “excess lactate,” the infused material could suppress endogenous lactate production. In this way, the two sets of investigators demonstrated for whole body metabolism what has previously been shown of cardiac muscle, which is competitive inhibition of one substrate by another (8).
And finally on the matter of lactate as a preferred fuel over glucose, the phenomenon is seen in brain slices of rodent models (76, 77) and intact healthy as well as brain injured humans (9). In fact, in patients with post-traumatic brain injury (TBI), gluconeogenesis from lactate provides substrate for most (2/3) of body glucose production. Thus, either directly via net lactate uptake or indirectly via gluconeogenesis, lactate provides most fuel for the injured brain (10). Implications for the use of exogenous lactate for providing nutritive support to the injured brain have been discussed (78).
[LIE]:[PIE] Measurements from Mass Spectrometry, Numbers Matter
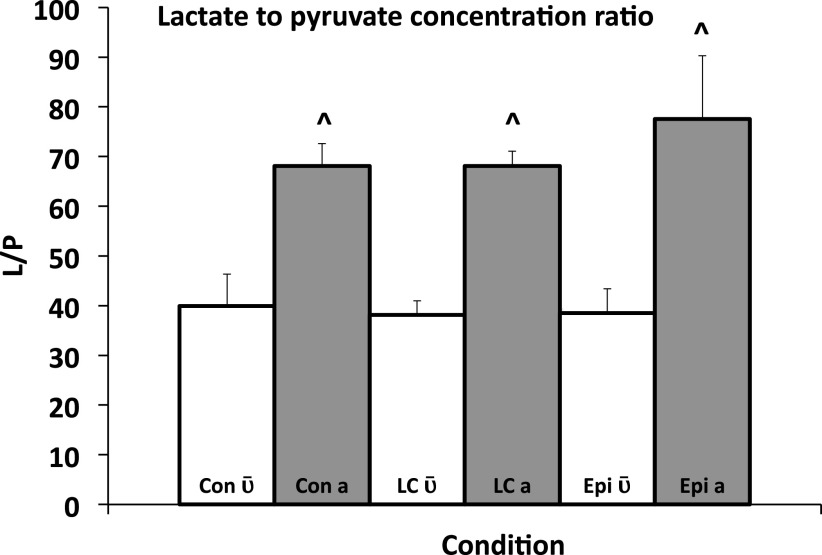
Readers need to appreciate that this discussion around the use of carbon-labeled isotope tracers to study lactate metabolism is a subject of discussion among colleagues who have successfully worked together in the past (57, 64, 79), but who disagree on matters of tracer methodology and data interpretation. Specifically, on the matter of isotopic equilibration between lactate and pyruvate in the blood of study subjects, the work of Johnson et al. (52) on documenting the presence of the Transpulmonary Lactate Shuttle in rats is relevant. In that effort, blood lactate concentration was raised by exogenous infusion of unlabeled lactate (lactate clamp procedure) and epinephrine infusion; other anesthetized animals served as controls. In their experiments, Johnson et al. placed a leg (saphenous) vein catheter for exogenous lactate and epinephrine infusions. In addition, right atrial and arterial catheters were placed to measure L and P concentrations and isotopic enrichments and thus determine metabolite exchange across the body including the lungs. As well, [U-13C]lactate was infused to determine metabolite exchange as well as assess the extent of lactate-pyruvate equilibration in mixed venous and arterial blood samples. Briefly, under control and lactate clamp conditions, the lungs took up lactate whereas epinephrine stimulation caused the lungs to release lactate on a net basis. The lungs also took up pyruvate and converted it to lactate such that the L/P rose significantly across the lungs (Fig. 4). More importantly and relevant to the issue at hand, although lactate and pyruvate isotopic enrichments following [U-13C]lactate infusion were loosely correlated at both mixed venous and arterial blood sites, the values were higher on the arterial site and not equivalent at either site (Fig. 5).
Figure 4.
Lactate-to-pyruvate concentration ratio (L/P) in central mixed venous () and arterial blood (a), of anesthetized rats studied under control (Con), exogenous lactate infusion (lactate clamp, LC) and epinephrine infusion (Epi). Values are expressed as means ± SD. Metabolic activity of the lung parenchyma in converting pyruvate to lactate is apparent under all conditions studied. ^Significantly different than the corresponding trial , P < 0.05. From Ref. 52. Mixed venous and arterial lactate/pyruvate ratios are different clearly indicating roles of heart and lung parenchyma.
Figure 5.
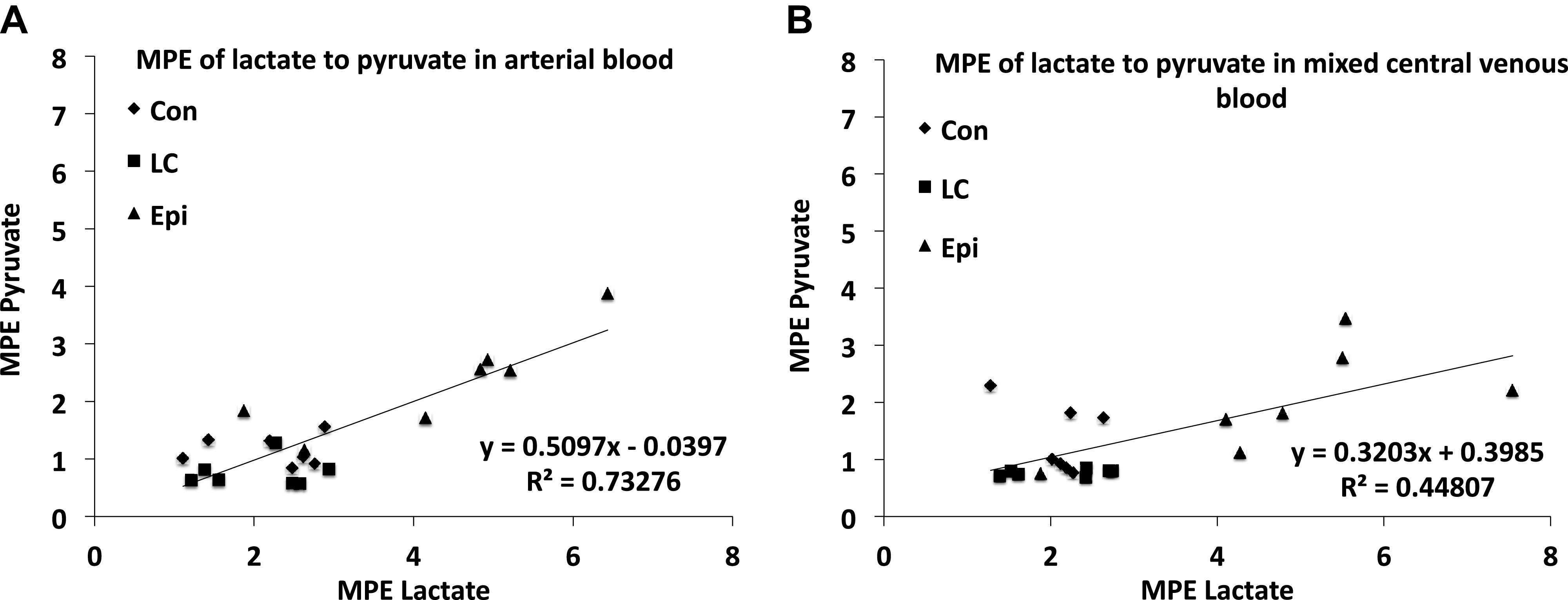
A: relationship between mixed central venous lactate and pyruvate isotopic enrichment expressed as molar percent excess (MPE) in rat blood during control (Con), exogenous unlabeled lactate infusion, lactate clamp (LC), and epinephrine infusion (Epi) conditions, respectively. B: relationship between arterial lactate and pyruvate MPE in rat blood during Con, LC, and Epi conditions, respectively. Lactate and pyruvate enrichments following [U-13C]lactate infusion are correlated, but not equivalent, n = 7 each condition. From Ref. 52.
Recent Use of Mass Spectrometry That Corroborate Efficacious Use of Lactate Tracers
In addition to the earlier-cited work showing that carbon-labeled tracers accurately measure lactate metabolism as assessed by mass balance and indirect calorimetry in humans in vivo (6), more recently using a mouse model Hui et al. (3) have independently reproduced, and reaffirmed typically used tracer calculations to measure lactate flux (6, 33, 35, 39, 40, 64). Unfortunately in their studies, Hui et al. were limited in the ability to determine tissue blood flows in general, and specifically across the heart (8) and lungs (52).
Other Ways to Look at Issues Related to Metabolic Regulation in Vivo
Mass spectrometry as applied to calculations of metabolite flux rates following administration of isotope tracers is a powerful tool for studying metabolite flux rates; however, mass spectrometric analyses of isotopic enrichment of blood metabolites following tracer administration is not the only available tool, as there are a host of related tools and techniques. These include magnetic resonance spectroscopy, cell fractionation, and organelle analysis that have provided valuable insights into metabolic regulation.
[LIE]:[PIE] Measurements from Magnetic Resonance Spectroscopy
Even when tracers are used in conjunction with determinations on arterial-venous concentration and isotopic enrichment differences, the results cannot distinguish between contributions of red versus white muscle fibers, recruited or quiescent muscle fibers, or indeed adipose, connective, or osseous tissues. In an attempt to obtain data of greater tissue specificity, investigators have used magnetic resonance spectroscopy (MRS) technology. Using rat hindquarter preparations with MRS and 13C-lactate and -acetate tracers, Bertocci et al. (80) evaluated the role of lactate in muscle energy transduction. By tracking the conversion of 13C-lactate into glutamate (in equilibrium with TCA cycle intermediate α-ketoglutarate) in a rat muscle preparation, it was confirmed that lactate directly enters the TCA cycle and is readily oxidized by skeletal muscle during rest and contraction (81). More recently, Park et al. (30) used hyperpolarized 13C-NMR technology to study lactate kinetics on intact rat skeletal muscle. On separate occasions, investigators used hyperpolarized [1-13C]lactate and [2-13C]pyruvate as well as dichloroacetate (DCA, an inhibitor of pyruvate dehydrogenase kinase, and thus increase overall PDH activity) to measure the rapid lactate kinetics in muscle. From 13C-NMR determinations of intracellular H13CO3−, results showed rapid lactate oxidation apparently without equilibration with cytosolic pyruvate or alanine. Thus, even considering limitations in MRS technology for distinguishing between metabolism in distinct cell compartments, the hypothesis of a dynamic role of lactate in maintaining cellular energy homeostasis developed on the bases of tissue metabolite concentration and isotope tracer flux studies was supported. Hence, like many others before (82–84), Park et al. asserted that simple measures of blood or muscle lactate concentration underestimate the system’s capacity to respond to changes in nutritive state, power output, duration of activity, and arterial lactate concentration (6, 57). Park et al. (30) concluded: “The dynamic nature of lactate metabolism provokes a reconsideration of many key concepts in biology.”
13C-Lactate and Mass Spectrometry to Detect Gene Expression via Lactylation
A recent contribution showing the importance of L over P in the regulation of metabolism using 13C-lactate tracers and mass spectrometry comes from Zhang et al. (23) who showed that cellular lactate regulates gene expression through chemical labeling of histone proteins. Lactylation of histones was discovered by means of using mass spectrometry to identify the mass shift associated with exogenous 13C-lactate labeling of lysine amino acid residues of histone tails. In addition, the authors used 13C-labeled glucose to produce 13C-labeled lactate through glycolysis that was also identified by the same mass shift of the lactylated lysine amino acid residue. Thus, histone lactylation, not “pyruvation” affects gene expression in a manner consistent with the large physiological dynamic range of lactate concentrations that respond rapidly based on diet, exercise, endocrine signaling, injury, or disease state (85).
The Mitochondrial l-Lactate Dehydrogenase Affair
Staid concepts of the relationships between glycolytic and oxidative metabolism as expressed in decades of textbooks impede understanding and progress and lead to unnecessary distractions such as the L/P isotope equilibration issue (46). Regrettably, such issues are recurrent. Consider for instance “The Mitochondrial l-lactate Dehydrogenase Affair” eloquently articulated by Passarella et al. (86). Recognition that there are two LDH pools, cytoplasmic and mitochondrial should have turned cerebral lights on concerning the linkage between glycolytic and oxidative (so called “anaerobic” and “aerobic” metabolism) (18). Unfortunately, biological significance of the discovery of mLDH (12), and its annotation in the MitoCarta have yet to impress, and deniers of the linkage between glycolytic and oxidative metabolism dependent on lactate flux persist.
The oxidative apparatus in heart and skeletal muscle, and likely most other tissues, exists as a tubular network that extends from the plasma membrane deep within cells tissues (87–89). Hence, the globular appearance of mitochondria apparent in electron micrographic thin sections has been misinterpreted. Rather than mitochondria being portrayed and thought of as powerhouses of the cell, e.g., see Ref. 46, in fact the cellular mitochondrial reticulum represents a vast, interconnected cellular energy highway (89, 90). Although the word “mitochondrial” is used in this paper, it is to be appreciated that isolated mitochondria are remnants of destructive processes that render the mitochondrial reticulum into discrete “mitochondria,” that while robust in their capabilities, are damaged fragmentation residues. Hopefully, contemporary textbooks will get the morphology right and describe the cellular respiratory apparatus as a network, the mitochondrial reticulum (90–92).
Despite evidence from MRS on direct mitochondrial lactate oxidation (vide supra), the discovery that mammalian mitochondrial preparations oxidize lactate has been controversial; some investigators produced such preparations that could (11, 12, 86, 93–98), whereas others (99–101) produced preparations that could not oxidize lactate. With clear demonstration of lactate oxidation in mitochondrial preparations from human skeletal muscle (102) along with supportive data from MRS (1, 30, 81), one might think that the issue of mitochondrial lactate oxidation has been resolved; still dissenters persist. Difficulties in obtaining vesicular mitochondrial preparations from mammalian tissues likely have to do with disruption of the mitochondrial reticulum (89) during isolation, and labiality and fragility of the l-lactate dehydrogenase during isolation of mitochondrial vesicles. Loss of a mitochondrial preparation’s (103) capacity to respire lactate is not unique reflecting a similar problem with oxidation of long-chain fatty acids attributable to the use of the proteolytic enzyme Nagarse in mitochondrial isolation (104). Respectfully, part of the problem has also been a loss of physiological perspective by some (99–101, 105). If tissues such as the heart and red skeletal muscle take up and oxidize fatty acids in vivo, but mitochondria isolated from those tissues fail to recapitulate what is known to happen in vivo (104), then the obvious conclusion should have been that the mitochondrial preparations were defective and not representative of the tissues and organs from which they came. Similarly, the heart, red skeletal muscle, and brain take up and oxidize lactate in vivo, but if mitochondrial preparations from those tissues fail to oxidize lactate, the appropriate conclusion should have been that the preparations were defective and not representative of the tissues and organs from which they came!
Discovery of the Intracellular Lactate Shuttle and a Mitochondrial Lactate Oxidation Complex (mLOC) (31) further obviates discussions of blood lactate and pyruvate isotopic equilibration. As described elsewhere (14, 18, 20, 106), the mLOC was developed on the basis of a series of tracer (33, 34, 107), muscle compartment and fiber-type analyses (28, 108, 109), cell fractionation and mitochondrial isolation (13, 76, 77, 110), mitochondrial respiration (11, 12, 94, 97, 98, 111), and immuno-coprecipitation and immuno-imaging studies (31, 32, 112).
And finally, on the importance of mLDH in metabolic regulation, it is helpful in terms of explaining extant data. For instance, in 1995 Sumegi et al. (113) reported on studies of systemic and cardiac metabolism using infusions of [3-13C]pyruvate and [3-13C]propionate in rats. They observed that the infused [3-13C]pyruvate was quickly converted to [3-13C]lactate in the blood and that the [3-13C]lactate from labeled pyruvate infusion was well metabolized in normoxic and ischemic hearts. They went on to show that “the extracellular lactate is preferentially taken up by a portion of cytoplasm which converts the lactate to pyruvate and transfers it to the mitochondrial reticular network.” A retrospective interpretation is that the action of mLDH explained this aspect of their results on rat hearts in vivo and illustrates the Intracellular Lactate Shuttle. LDH in their figure 4 is mLDH. Cardiac lactate metabolism and its relationship to whole body lactate metabolism has recently been reviewed (8).
CONCLUSIONS
The assertion that plasma lactate isotopic enrichment determined by mass spectrometry after carbon tracer administration yields results about body pyruvate production is not supported. Technically, the results of lactate⇔pyruvate isotopic equilibration are not reproducible, ignore basic anatomy and physiology, and miss contributions of the lungs and heart to pyruvate and lactate dynamics. Furthermore, the assertion of lactate⇔pyruvate equilibration is unconfirmed by others using similar or different methods, e.g., MRS. Moreover, the assertion was made without consideration of direct mitochondrial lactate oxidation as part of the Intracellular Lactate Shuttle or redox control of cellular metabolism (44, 45). Regrettably, as noted by us and others (3, 54), the litany of errors in interpreting L⇔P interactions and consequent inability to distinguish between results of metabolite flux and concentration data persists; for example Ref. 55. Nonetheless, because of its position as a fulcrum in metabolic regulation (18, 20), we maintain that carbon-labeled lactate tracers can be used to quantitate tissue (6, 9) and whole body lactate fluxes (6, 10). Finally, whether from standpoints of energy flux or metabolite signaling we maintain that knowing lactate concentrations and flux rates are of as great, or greater significance than knowing those of pyruvate.
GRANTS
This study was supported by NIH 1 R01 AG059715-01, Pac-12 Conference Grant No. 3-02-Brooks-17, and the UCB Center for Research and Education on Aging (CREA) (to G. A. Brooks).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.A.B. conceived and designed research; G.A.B. and M.A.H. performed experiments; G.A.B. and M.A.H. analyzed data; G.A.B., A.D.O., R.G.L., C.C.C., J.A.A., and J.D. interpreted results of experiments; G.A.B. prepared figures; G.A.B. drafted manuscript; G.A.B., A.D.O., R.G.L., C.C.C., J.A.A., J.D., and M.A.H. edited and revised manuscript; G.A.B., A.D.O., R.G.L., C.C.C., J.A.A., and J.D. approved final version of manuscript.
REFERENCES
- 1.Chen YJ, Mahieu NG, Huang X, Singh M, Crawford PA, Johnson SL, Gross RW, Schaefer J, Patti GJ. Lactate metabolism is associated with mammalian mitochondria. Nat Chem Biol 12: 937–943, 2016. doi: 10.1038/nchembio.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 118: 691–728, 2018. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 3.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J, White E, Rabinowitz JD. Glucose feeds the TCA cycle via circulating lactate. Nature 551: 115–118, 2017. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinowitz JD, Enerbäck S. Lactate: the ugly duckling of energy metabolism. Nat Metab 2: 566–571, 2020. doi: 10.1038/s42255-020-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Pasquale N, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee MY, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P, Kalliokoski KK, Nielsen S, Pedersen BK, Kahn CR, Tseng YH, Goodyear LJ. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 1: 291–303, 2019. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol (1985) 87: 1684–1696, 1999. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- 7.Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J Physiol 587: 2087–2099, 2009. doi: 10.1113/jphysiol.2008.168286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks GA. Role of the heart in lactate shuttling. Front Nutr 8: 663560, 2021. doi: 10.3389/fnut.2021.663560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda D, Vespa PM, Brooks GA. Lactate: brain fuel in human traumatic brain injury: a comparison to normal healthy control subjects. J Neurotrauma 32: 820–832, 2015. doi: 10.1089/neu.2014.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenn TC, Martin NA, McArthur DL, Hovda DA, Vespa P, Johnson ML, Horning MA, Brooks GA. Endogenous nutritive support after traumatic brain injury: peripheral lactate production for glucose supply via gluconeogenesis. J Neurotrauma 32: 811–819, 2015. doi: 10.1089/neu.2014.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt RB, Laux JE, Spainhour SE, Kline ES. Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys 259: 412–422, 1987. doi: 10.1016/0003-9861(87)90507-8. [DOI] [PubMed] [Google Scholar]
- 12.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA 96: 1129–1134, 1999. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passarella S, de Bari L, Valenti D, Pizzuto R, Paventi G, Atlante A. Mitochondria and l-lactate metabolism. FEBS Lett 582: 3569–3576, 2008. doi: 10.1016/j.febslet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 14.Brooks GA, Arevalo JA, Osmond AD, Leija RG, Curl CC, Tovar AP. Lactate in contemporary biology: a phoenix risen. J Physiol. In press. doi: 10.1113/JP280955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster DW. Banting lecture 1984. From glycogen to ketones—and back. Diabetes 33: 1188–1199, 1984. doi: 10.2337/diab.33.12.1188. [DOI] [PubMed] [Google Scholar]
- 16.Woerle HJ, Meyer C, Dostou JM, Gosmanov NR, Islam N, Popa E, Wittlin SD, Welle SL, Gerich JE. Pathways for glucose disposal after meal ingestion in humans. Am J Physiol Endocrinol Physiol 284: E716–E725, 2003. doi: 10.1152/ajpendo.00365.2002. [DOI] [PubMed] [Google Scholar]
- 17.Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Physiol 278: E244–E251, 2000. doi: 10.1152/ajpendo.2000.278.2.E244. [DOI] [PubMed] [Google Scholar]
- 18.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab 27: 757–785, 2018. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24: 382–391, 2001. doi: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 20.Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol 35: 101454, 2020. doi: 10.1016/j.redox.2020.101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab 11: 311–319, 2010. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab 35: 176–185, 2015. doi: 10.1038/jcbfm.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, Becker L, Zhao Y. Metabolic regulation of gene expression by histone lactylation. Nature 574: 575–580, 2019. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc 17: 22–34, 1985. [PubMed] [Google Scholar]
- 25.Brooks GA. Glycolytic end product and oxidative substrate during sustained exercise in mammals—the “lactate shuttle”. In: Comparative Physiology and Biochemistry—Current Topics and Trends Vol A, Respiration—Metabolism—Circulation, edited by Gilles R. Berlin: Springer-Verlag, 1985, p. 208–218. [Google Scholar]
- 26.Brooks GA. Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc 45: 2924–2929, 1986. [PubMed] [Google Scholar]
- 27.Baldwin KM, Campbell PJ, Cooke DA. Glycogen, lactate, and alanine changes in muscle fiber types during graded exercise. J Appl Physiol Respir Environ Exerc Physiol 43: 288–291, 1977. doi: 10.1152/jappl.1977.43.2.288. [DOI] [PubMed] [Google Scholar]
- 28.Hooker AM, Baldwin KM. Substrate oxidation specificity in different types of mammalian muscle. Am J Physiol Cell Physiol 236: C66–C69, 1979. doi: 10.1152/ajpcell.1979.236.1.C66. [DOI] [PubMed] [Google Scholar]
- 29.Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol (1985) 85: 627–634, 1998. doi: 10.1152/jappl.1998.85.2.627. [DOI] [PubMed] [Google Scholar]
- 30.Park JM, Josan S, Mayer D, Hurd RE, Chung Y, Bendahan D, Spielman DM, Jue T. Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J Exp Biol 218: 3308–3318, 2015. doi: 10.1242/jeb.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One 3: e2915, 2008. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto T, Masuda S, Taguchi S, Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol 567: 121–129, 2005. doi: 10.1113/jphysiol.2005.087411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depocas F, Minaire Y, Chatonnet J. Rates of formation and oxidation of lactic acid in dogs at rest and during moderate exercise. Can J Physiol Pharmacol 47: 603–610, 1969. doi: 10.1139/y69-106. [DOI] [PubMed] [Google Scholar]
- 34.Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol Endocrinol Physiol 244: E83–E92, 1983. doi: 10.1152/ajpendo.1983.244.1.E83. [DOI] [PubMed] [Google Scholar]
- 35.Freminet A, Bursaux E, Poyart CF. Effect of elevated lactataemia on the rates of lactate turnover and oxidation in rats. Pflugers Arch 346: 75–86, 1974. doi: 10.1007/BF00592652. [DOI] [PubMed] [Google Scholar]
- 36.Weber JM, Parkhouse WS, Dobson GP, Harman JC, Snow DH, Hochachka PW. Lactate kinetics in exercising thoroughbred horses: regulation of turnover rate in plasma. Am J Physiol Regul Integr Comp Physiol 253: R896–R903, 1987. doi: 10.1152/ajpregu.1987.253.6.R896. [DOI] [PubMed] [Google Scholar]
- 37.Mazzeo RS, Brooks GA, Schoeller DA, Budinger TF. Disposal of blood [1-13C]lactate in humans during rest and exercise. J Appl Physiol (1985) 60: 232–241, 1986. doi: 10.1152/jappl.1986.60.1.232. [DOI] [PubMed] [Google Scholar]
- 38.Messonnier LA, Emhoff C-AW, Fattor JA, Horning MA, Carlson TJ, Brooks GA. Kinetics at the lactate threshold in trained and untrained men. J Appl Physiol 114: 1593–1602, 2013. doi: 10.1152/japplphysiol.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanley WC, Gertz EW, Wisneski JA, Morris DL, Neese RA, Brooks GA. Systemic lactate kinetics during graded exercise in man. Am J Physiol Endocrinol Physiol 249: E595–E602, 1985. doi: 10.1152/ajpendo.1985.249.6.E595. [DOI] [PubMed] [Google Scholar]
- 40.Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL, Brooks GA. Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol (1985) 60: 1116–1120, 1986. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- 41.Romijn JA, Chinkes DL, Schwarz JM, Wolfe RR. Lactate-pyruvate interconversion in blood: implications for in vivo tracer studies. Am J Physiol Endocrinol Physiol 266: E334–E340, 1994. doi: 10.1152/ajpendo.1994.266.3.E334. [DOI] [PubMed] [Google Scholar]
- 42.Wolfe RR, Jahoor F, Miyoshi H. Evaluation of the isotopic equilibration between lactate and pyruvate. Am J Physiol Endocrinol Physiol 254: E532–E535, 1988. doi: 10.1152/ajpendo.1988.254.4.E532. [DOI] [PubMed] [Google Scholar]
- 43.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol (1985) 76: 2253–2261, 1994. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- 44.Krebs HA, Veech RL. Equilibrium relations between pyridine nucleotides and adenine nucleotides and their roles in the regulation of metabolic processes. Adv Enzyme Regul 7: 397–413, 1969. doi: 10.1016/0065-2571(69)90030-2. [DOI] [PubMed] [Google Scholar]
- 45.Veech RL, Lawson JW, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. J Biol Chem 254: 6538–6547, 1979. [PubMed] [Google Scholar]
- 46.Lehninger AL. Biochemistry: The Molecular Basis of Cell Structure and Function. New York, NY: Worth Publishers, 1970. [Google Scholar]
- 47.Henderson GC, Horning MA, Wallis GA, Brooks GA. Pyruvate metabolism in working human skeletal muscle. Am J Physiol Endocrinol Physiol 292: E366, 2007. doi: 10.1152/ajpendo.00363.2006. [DOI] [PubMed] [Google Scholar]
- 48.Huckabee WE. Relationships of pyruvate and lactate during anaerobic metabolism. II. Exercise and formation of O-debt. J Clin Invest 37: 255–263, 1958. doi: 10.1172/JCI103604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huckabee WE. Relationships of pyruvate and lactate during anaerobic metabolism. III. Effect of breathing low-oxygen gases. J Clin Invest 37: 264–271, 1958. doi: 10.1172/JCI103605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marik P, Bellomo R. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Critical Care 1: 3–5, 2013. doi: 10.13172/2052-9309-1-1-431. [DOI] [Google Scholar]
- 51.Stein NR, McArthur DL, Etchepare M, Vespa PM. Early cerebral metabolic crisis after TBI influences outcome despite adequate hemodynamic resuscitation. Neurocrit Care 17: 49–57, 2012. doi: 10.1007/s12028-012-9708-y. [DOI] [PubMed] [Google Scholar]
- 52.Johnson ML, Emhoff CA, Horning MA, Brooks GA. Transpulmonary lactate shuttle. Am J Physiol Regul Integr Comp Physiol 302: R143–R149, 2012. doi: 10.1152/ajpregu.00402.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson ML, Hussien R, Horning MA, Brooks GA. Transpulmonary pyruvate kinetics. Am J Physiol Regul Integr Comp Physiol 301: R769–R774, 2011. doi: 10.1152/ajpregu.00206.2011. [DOI] [PubMed] [Google Scholar]
- 54.Bartman CR, TeSlaa T, Rabinowitz JD. Quantitative flux analysis in mammals. Nat Metab 3: 896–908, 2021. doi: 10.1038/s42255-021-00419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stellingwerff T, Leblanc PJ, Hollidge MG, Heigenhauser GJ, Spriet LL. Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. Am J Physiol Endocrinol Physiol 290: E1180–E1190, 2006. doi: 10.1152/ajpendo.00499.2005. [DOI] [PubMed] [Google Scholar]
- 56.Brooks GA, Wolfel EE, Butterfield GE, Cymerman A, Roberts AC, Mazzeo RS, Reeves JT. Poor relationship between arterial [lactate] and leg net release during exercise at 4,300 m altitude. Am J Physiol Endocrinol Physiol 275: R1192–R1201, 1998. doi: 10.1152/ajpregu.1998.275.4.R1192. [DOI] [PubMed] [Google Scholar]
- 57.Brooks GA, Wolfel EE, Groves BM, Bender PR, Butterfield GE, Cymerman A, Mazzeo RS, Sutton JR, Wolfe RR, Reeves JT. Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. J Appl Physiol (1985) 72: 2435–2445, 1992. doi: 10.1152/jappl.1992.72.6.2435. [DOI] [PubMed] [Google Scholar]
- 58.Mazzeo RS, Brooks GA, Budinger TF, Schoeller DA. Pulse injection, 13C tracer studies of lactate metabolism in humans during rest and two levels of exercise. Biomed Mass Spectrom 9: 310–314, 1982. doi: 10.1002/bms.1200090708. [DOI] [PubMed] [Google Scholar]
- 59.Stanley WC, Wisneski JA, Gertz EW, Neese RA, Brooks GA. Glucose and lactate interrelations during moderate-intensity exercise in humans. Metabolism 37: 850–858, 1988. doi: 10.1016/0026-0495(88)90119-9. [DOI] [PubMed] [Google Scholar]
- 60.Halestrap AP. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J 156: 193–207, 1976. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skelton MS, Kremer DE, Smith EW, Gladden LB. Lactate influx into red blood cells of athletic and nonathletic species. Am J Physiol Regul Integr Comp Physiol 268: R1121–R1128, 1995. doi: 10.1152/ajpregu.1995.268.5.R1121. [DOI] [PubMed] [Google Scholar]
- 62.Katz J. Importance of sites of tracer administration and sampling in turnover studies. Fed Proc 41: 123–128, 1982. [PubMed] [Google Scholar]
- 63.Edwards JD, Mayall RM. Importance of the sampling site for measurement of mixed venous oxygen saturation in shock. Crit Care Med 26: 1356–1360, 1998. doi: 10.1097/00003246-199808000-00020. [DOI] [PubMed] [Google Scholar]
- 64.Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT. Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol (1985) 71: 333–341, 1991. doi: 10.1152/jappl.1991.71.1.333. [DOI] [PubMed] [Google Scholar]
- 65.Brooks GA. The precious few grams of glucose during exercise. Int J Mol Sci 21: 5733, 2020. doi: 10.3390/ijms21165733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamann JJ, Kelley KM, Gladden LB. Effect of epinephrine on net lactate uptake by contracting skeletal muscle. J Appl Physiol (1985) 91: 2635–2641, 2001. doi: 10.1152/jappl.2001.91.6.2635. [DOI] [PubMed] [Google Scholar]
- 67.Kjaer M, Farrell PA, Christensen NJ, Galbo H. Increased epinephrine response and inaccurate glucoregulation in exercising athletes. J Appl Physiol (1985) 61: 1693–1700, 1986. doi: 10.1152/jappl.1986.61.5.1693. [DOI] [PubMed] [Google Scholar]
- 68.Zuntz N, Schumburg W. Studien zu einer Physiologie des Marsches. Berlin: Hirschwald, 1901. [Google Scholar]
- 69.Lusk G. Animal calorimetry twenty-fourth paper. Analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem 59: 41–42, 1924. [Google Scholar]
- 70.Bergman BC, Brooks GA. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J Appl Physiol (1985) 86: 479–487, 1999. doi: 10.1152/jappl.1999.86.2.479. [DOI] [PubMed] [Google Scholar]
- 71.Miller BF, Fattor JA, Jacobs KA, Horning MA, Suh SH, Navazio F, Brooks GA. Metabolic and cardiorespiratory responses to “the lactate clamp”. Am J Physiol Endocrinol Physiol 283: E889–E898, 2002. doi: 10.1152/ajpendo.00266.2002. [DOI] [PubMed] [Google Scholar]
- 72.Searle GL, Feingold KR, Hsu FS, Clark OH, Gertz EW, Stanley WC. Inhibition of endogenous lactate turnover with lactate infusion in humans. Metabolism 38: 1120–1123, 1989. doi: 10.1016/0026-0495(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 73.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 16: 504–515, 1954. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- 74.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest 82: 2017–2025, 1988. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI, Brooks GA. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol 544: 963–975, 2002. doi: 10.1113/jphysiol.2002.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schurr A. Lactate: a major and crucial player in normal function of both muscle and brain. J Physiol 586: 2665–2666, 2008. doi: 10.1113/jphysiol.2008.155416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schurr A, Payne RS. Lactate, not pyruvate, is neuronal aerobic glycolysis end product: an in vitro electrophysiological study. Neuroscience 147: 613–619, 2007. doi: 10.1016/j.neuroscience.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Brooks GA, Martin NA. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front Neurosci 8: 408, 2014. doi: 10.3389/fnins.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT. Increased dependence on blood glucose after acclimatization to 4,300 m. J Appl Physiol (1985) 70: 919–927, 1991. doi: 10.1152/jappl.1991.70.2.919. [DOI] [PubMed] [Google Scholar]
- 80.Bertocci LA, Haller RG, Lewis SF. Muscle metabolism during lactate infusion in human phosphofructokinase deficiency. J Appl Physiol (1985) 74: 1342–1347, 1993. doi: 10.1152/jappl.1993.74.3.1342. [DOI] [PubMed] [Google Scholar]
- 81.Bertocci LA, Lujan BF. Incorporation and utilization of [3-13C]lactate and [1,2-13C]acetate by rat skeletal muscle. J Appl Physiol (1985) 86: 2077–2089, 1999. doi: 10.1152/jappl.1999.86.6.2077. [DOI] [PubMed] [Google Scholar]
- 82.Alpert NR. Lactate production and removal and the regulation of metabolism. Ann N Y Acad Sci 119: 995–1012, 1965. doi: 10.1111/j.1749-6632.1965.tb47457.x. [DOI] [PubMed] [Google Scholar]
- 83.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans 30: 258–264, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Eldridge FL. Relationship between turnover rate and blood concentration of lactate in exercising dogs. J Appl Physiol 39: 231–234, 1975. doi: 10.1152/jappl.1975.39.2.231. [DOI] [PubMed] [Google Scholar]
- 85.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21: 2602–2612, 2007. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 86.Passarella S, Paventi G, Pizzuto R. The mitochondrial l-lactate dehydrogenase affair. Front Neurosci 8: 407, 2014. doi: 10.3389/fnins.2014.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 501: 349–369, 1978. doi: 10.1016/0005-2728(78)90104-4. [DOI] [PubMed] [Google Scholar]
- 88.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523: 617–620, 2015. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirkwood SP, Munn EA, Brooks GA. Mitochondrial reticulum in limb skeletal muscle. Am J Physiol Cell Physiol 251: C395–C402, 1986. doi: 10.1152/ajpcell.1986.251.3.C395. [DOI] [PubMed] [Google Scholar]
- 90.Brooks GA, Fahey TD. Exercise Physiology: Human Bioenergetics and Its Applications. New York: Wiley, 1984. [Google Scholar]
- 91.Brooks GA, Fahey TD, Baldwin KM. Exercise Physiology: Human Bioenergetics and Its Applications. Lexington, KY: Kindle Direct Publishing, 2019. [Google Scholar]
- 92.Urry LA, Cain ML, Wasserman SL, Minorsky PV, Orr O. Campbell biology. In: Campbell Biology (12 ed.). Pearson, 2020, p. 164–170. [Google Scholar]
- 93.Baba N, Sharma HM. Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J Cell Biol 51: 621–635, 1971. doi: 10.1083/jcb.51.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brooks GA, Brown MA, Butz CE, Sicurello JP, Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol (1985) 87: 1713–1718, 1999. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- 95.De Bari L, Atlante A, Valenti D, Passarella S. Partial reconstruction of in vitro gluconeogenesis arising from mitochondrial l-lactate uptake/metabolism and oxaloacetate export via novel l-lactate translocators. Biochem J 380: 231–242, 2004. doi: 10.1042/BJ20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dianzani MU. [Distribution of lactic acid oxidase in liver and kidney cells of normal rats and rats with fatty degeneration of the liver]. Arch Fisiol 50: 181–186, 1951. [PubMed] [Google Scholar]
- 97.Kline ES, Brandt RB, Laux JE, Spainhour SE, Higgins ES, Rogers KS, Tinsley SB, Waters MG. Localization of l-lactate dehydrogenase in mitochondria. Arch Biochem Biophys 246: 673–680, 1986. doi: 10.1016/0003-9861(86)90323-1. [DOI] [PubMed] [Google Scholar]
- 98.Young A, Oldford C, Mailloux RJ. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol 28: 101339, 2020. doi: 10.1016/j.redox.2019.101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rasmussen HN, van Hall G, Rasmussen UF. Lactate dehydrogenase is not a mitochondrial enzyme in human and mouse vastus lateralis muscle. J Physiol 541: 575–580, 2002. doi: 10.1113/jphysiol.2002.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sahlin K, Fernström M, Svensson M, Tonkonogi M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J Physiol 541: 569–574, 2002. doi: 10.1113/jphysiol.2002.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshida Y, Holloway GP, Ljubicic V, Hatta H, Spriet LL, Hood DA, Bonen A. Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. J Physiol 582: 1317–1335, 2007. doi: 10.1113/jphysiol.2007.135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacobs RA, Meinild AK, Nordsborg NB, Lundby C. Lactate oxidation in human skeletal muscle mitochondria. Am J Physiol Endocrinol Physiol 304: E686–E694, 2013. doi: 10.1152/ajpendo.00476.2012. [DOI] [PubMed] [Google Scholar]
- 103.Boussouar F, Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab 15: 345–350, 2004. doi: 10.1016/j.tem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 104.Pande SV, Blanchaer MC. Preferential loss of ATP-dependent long-chain fatty acid activating enzyme in mitochondria prepared using Nagarse. Biochim Biophys Acta 202: 43–48, 1970. doi: 10.1016/0005-2760(70)90216-x. [DOI] [PubMed] [Google Scholar]
- 105.Ponsot E, Zoll J, N'Guessan B, Ribera F, Lampert E, Richard R, Veksler V, Ventura-Clapier R, Mettauer B. Mitochondrial tissue specificity of substrates utilization in rat cardiac and skeletal muscles. J Cell Physiol 203: 479–486, 2005. doi: 10.1002/jcp.20245. [DOI] [PubMed] [Google Scholar]
- 106.Brooks GA. Lactate shuttling and the mitochondrial lactate oxidation complex. In: Mitochondrial Signaling in Health and Disease (Oxidative Stress and Disease), edited by Orrenius S, Packer L, Cardenas E.. Boca Raton, FL: CRC Press, 2012, p. 530. [Google Scholar]
- 107.Brooks GA, Donovan CM. Effect of endurance training on glucose kinetics during exercise. Am J Physiol Endocrinol Physiol 244: E505–E512, 1983. doi: 10.1152/ajpendo.1983.244.5.E505. [DOI] [PubMed] [Google Scholar]
- 108.Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA, Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol 222: 373–378, 1972. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- 109.Molé PA, Baldwin KM, Terjung RL, Holloszy JO. Enzymatic pathways of pyruvate metabolism in skeletal muscle: adaptations to exercise. Am J Physiol 224: 50–54, 1973. doi: 10.1152/ajplegacy.1973.224.1.50. [DOI] [PubMed] [Google Scholar]
- 110.Atlante A, de Bari L, Bobba A, Marra E, Passarella S. Transport and metabolism of L-lactate occur in mitochondria from cerebellar granule cells and are modified in cells undergoing low potassium dependent apoptosis. Biochim Biophys Acta 1767: 1285–1299, 2007. doi: 10.1016/j.bbabio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Brandt RB, Laux JE, Spainhour SE, Bear HD, Kline ES. Cytosolic-mitochondrial interactions (mitochondrial control of glycolysis). Prog Clin Biol Res 292: 497–506, 1989. [PubMed] [Google Scholar]
- 112.Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Physiol 290: E1237–E1244, 2006. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- 113.Sumegi B, Podanyi B, Forgo P, Kover KE. Metabolism of [3-13C]pyruvate and [3-13C]propionate in normal and ischaemic rat heart in vivo: 1H- and 13C-NMR studies. Biochem J 312: 75–81, 1995. doi: 10.1042/bj3120075. [DOI] [PMC free article] [PubMed] [Google Scholar]