Abstract
Background
Several studies have demonstrated that the preoperative Glasgow prognostic score (GPS) and modified GPS (mGPS) reflected the prognosis in patients undergoing curative surgery for colorectal cancer. However, there are no reports on long-term prognosis prediction using high-sensitivity mGPS (HS-GPS) in colorectal cancer. Therefore, this study aimed to calculate the prognostic value of preoperative HS-GPS in patients with colon cancer.
Methods
A cohort of 595 patients with advanced resectable colon cancer managed at our institution was analysed retrospectively. HS-GPS, GPS, and mGPS were evaluated for their ability to predict prognosis based on overall survival (OS) and recurrence-free survival (RFS).
Results
In the univariate analysis, HS-GPS was able to predict the prognosis with significant differences in OS but was not superior in assessing RFS. In the multivariate analysis of the HS-GPS model, age, pT, pN, and HS-GPS of 2 compared to HS-GPS of 0 (2 vs 0; hazard ratio [HR], 2.638; 95% confidence interval [CI], 1.046–6.650; P = 0.04) were identified as independent prognostic predictors of OS. In the multivariate analysis of the GPS model, GPS 2 vs 0 (HR, 1.444; 95% CI, 1.018–2.048; P = 0.04) and GPS 2 vs 1 (HR, 2.933; 95% CI, 1.209–7.144; P = 0.017), and in that of the mGPS model, mGPS 2 vs 0 (HR, 1.51; 95% CI, 1.066–2.140; P = 0.02) were independent prognostic predictors of OS. In each classification, GPS outperformed HS-GPS in predicting OS with a significant difference in the area under the receiver operating characteristic curve. In the multivariate analysis of the GPS model, GPS 2 vs 0 (HR, 1.537; 95% CI, 1.190–1.987; P = 0.002), and in that of the mGPS model, pN, CEA were independent prognostic predictors of RFS.
Conclusion
HS-GPS is useful for predicting the prognosis of resectable advanced colon cancer. However, GPS may be more useful than HS-GPS as a prognostic model for advanced colon cancer.
Keywords: Glasgow prognostic score, Modified Glasgow prognostic score, High-sensitivity modified Glasgow prognostic score, Colon cancer
Background
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer-related mortality worldwide [1]. CRC prognosis is based on the Union for International Cancer Control (UICC) tumour node metastasis (TNM) classification; however, differences in outcomes have been reported among patients presenting with the same disease stage [2, 3]. Concurrently, various inflammatory biomarkers have been suggested as relevant survival predictors in this patient group [4, 5].
Several studies have demonstrated that preoperative Glasgow prognostic score (GPS) and modified GPS (mGPS) reflected the prognosis in patients with CRC or colon cancer (CC) who were undergoing curative surgery [2–4]. It was reported that high-sensitivity mGPS (HS-GPS) was useful for changing the cut-off value of GPS and mGPS in other cancer types and identifying higher number of patients with poor prognosis. However, the HS-GPS developed for prediction of prognosis in CRC or CC patients has not been reported in detail [5]. Furthermore, there are no reports on the value of HS-mGPS in predicting long-term prognosis in patients with colorectal cancer. Therefore, this study aimed to calculate the prognostic value of preoperative HS-GPS in patients with advanced resectable CC and compare it with those of GPS and mGPS.
Methods
Patients
We retrospectively examined the data of 636 consecutive patients with advanced resectable CC who underwent surgery at the Tokyo Medical University Hospital between 2000 and 2015. Of these, 41 patients with missing test data, severe obstructive enteritis or gastrointestinal perforation requiring emergency surgery, or organ failure (including patients on dialysis and those having liver cirrhosis) were excluded. In our study, though 31 patients had obstructive enteritis, we inserted a transanal tube or stent and performed surgery after a waiting period of 10 to 22 days; consequently, these cases were included in the study. The remaining 595 patients were divided into groups based on HS-GPS values. The CC stage was classified according to the eighth edition of the UICC TNM classification system. Patient blood test results analysed in this study used those from the samples that were obtained shortly before the surgery, most of which were collected 1 or 2 days before surgery. All patients underwent curative surgery.
This study was approved by the institutional review board of Tokyo Medical University Hospital.
Postoperative follow-up
After surgery, patients were followed up every 3 or 6 months for 5 years or longer with detailed examination that included blood sampling, imaging, and endoscopy assessments. These examinations and postoperative adjuvant chemotherapy (ADJ) were performed at our institution according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines for each age [6]. Chemotherapy was administered to 10% of stage II patients and 64% of stage III patients. ADJ included the 5-fluorouracil-based regimen. If CRC recurred, most patients were treated according to the JSCCR guidelines [6]. Overall survival (OS) was calculated from the date of colectomy to the date of death or last follow-up. Recurrence-free survival (RFS) was calculated from the date of colectomy to the date of either recurrence or death or the last follow-up.
Criteria of each GPS related score
The HS-mGPS was calculated based on the cut-off values of 0.3 mg/dL for C-reactive protein (CRP) and 3.5 g/dL for albumin levels. Patients with an elevated CRP (> 0.3 mg/dL) and hypoalbuminemia (< 3.5 mg/dL) were assigned a score of 2; those with an elevated CRP alone were assigned a score of 1; and patients without an elevated CRP (≤ 0.3 mg/dL), regardless of albumin levels, were assigned a score of 0 [5]. The GPS was scored by allocating one point each for elevated CRP (> 1.0 mg/dL) and hypoalbuminemia (< 3.5 mg/dL). tPatients with both, either, or none of these laboratory parameters were assigned scores of 2, 1, or 0, respectively. For the mGPS, patients with an elevated CRP (> 1.0 mg/dL) and hypoalbuminemia (< 3.5 mg/dL) were assigned a score of 2; those with an elevated CRP alone, a score of 1; and those with a normal CRP regardless of the albumin levels, a score of 0 [7] .
Statistical analysis
Patient baseline characteristics in each HS-GPS group were compared using either the χ2 and Fisher’s exact tests. The association of GPS with OS and RFS was analysed using the Kaplan–Meier method and log-rank test. Multivariate Cox regression analysis was also performed. Statistical significance was set at P < 0.05. The Cox proportional hazards regression model was used to estimate the hazard ratio (HR) at 95% confidence intervals (CI). All statistical analyses were performed using the Statistical Package for Social Science software package (SPSS Inc., Tokyo, Japan). Receiver operating characteristic (ROC) curve analysis was performed using the EZR software package (EZR v1.51, Tokyo, Japan).
Ethics statement
This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Tokyo Medical University Hospital (T2019–0054). This study was approved by the institutional ethics board, and informed consent was obtained from all patients.
Results
Patient clinicopathological characteristics in association with GPS scores
The baseline characteristics of the 595 patients who underwent curative surgery for CC are presented in Tables 1, 2, and 3. The study sample included 362 men (65.7%) and 232 women (34.3%). The median age of the patients was 69.7 (range, 30–95) years. Table 1 shows patient characteristics according to the HS-GPS model. The HS-GPS score significantly correlated with age (P = 0.046), BMI (P = 0.039), tumour location (P = 0.008), surgical approach (P = 0.02), tumour size (P < 0.001), pT (P = 0.011), and pN (P = 0.016). Table 2 shows patient characteristics according to the GPS model. The GPS score significantly correlated with age (P = 0.046), BMI (P = 0.039), tumour location (P = 0.008), surgical approach (P = 0.02), tumour size (P < 0.001), pT (P = 0.011), and pN (P = 0.016). Table 3 shows patient characteristics in accordance with the mGPS model. The mGPS score significantly correlated with the surgical approach (P = 0.001), tumour size (P < 0.001), pT (P = 0.014), and pN (P = 0.17).
Table 1.
The relationship between HS-GPS scores and clinicopathological characteristics
| variable | HS-GPS 0 | HS-GPS 1 | HS-GPS 2 | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| (N = 437) | (N = 107) | (N = 52) | ||||||
| Age | (years) | 0.031 | ||||||
| < 75 | 313 | 52.7% | 67 | 11.3% | 29 | 4.9% | ||
| ≥75 | 124 | 20.9% | 38 | 6.4% | 23 | 3.9% | ||
| Sex | 0.996 | |||||||
| female | 171 | 28.8% | 41 | 6.9% | 20 | 3.4% | ||
| male | 266 | 44.8% | 64 | 10.8% | 32 | 5.4% | ||
| BMI | 0.016 | |||||||
| ≥18.5 | 363 | 64.7% | 85 | 15.2% | 42 | 7.5% | ||
| < 18.5 | 41 | 7.3% | 20 | 3.6% | 10 | 1.8% | ||
| tumor location | 0.084 | |||||||
| right side | 178 | 30.0% | 49 | 8.2% | 29 | 4.9% | ||
| left side | 259 | 43.6% | 56 | 9.4% | 233.9 | 4.9% | ||
| operation approach | 0.001 | |||||||
| open | 249 | 42.0% | 78 | 13.2% | 38 | 6.4% | ||
| lap | 187 | 31.5% | 27 | 4.6% | 14 | 2.4% | ||
|
Tumor size T |
(cm) | < 0.001 | ||||||
| < 5 | 291 | 50.5% | 45 | 7.8% | 11 | 1.9% | ||
| ≥5 | 134 | 23.3% | 55 | 9.5% | 40 | 5.9% | ||
| 0.174 | ||||||||
| T2/3 | 218 | 36.8% | 56 | 9.4% | 33 | 5.6% | ||
| T4 | 218 | 36.8% | 17 | 8.3% | 19 | 3.2% | ||
| N | 0.016 | |||||||
| N0 | 196 | 33.1% | 67 | 11.3% | 44 | 7.4% | ||
| N1/2/3 | 213 | 35.8% | 50 | 8.4% | 24 | 4.0% | ||
| CEA | (ng/ml) | 0.621 | ||||||
| < 5 | 276 | 51.4% | 66 | 12.3% | 29 | 5.4% | ||
| ≥5 | 122 | 22.7% | 27 | 5.0% | 17 | 3.2% | ||
| CA19–9 | (U/ml) | 0.204 | ||||||
| < 37 | 320 | 59.9% | 79 | 14.8% | 29 | 8.1% | ||
| ≥37 | 79 | 14.8% | 15 | 2.8% | 12 | 2.2% | ||
Abbreviation: HS-GPS high sensivity-Glasgow Prognostic Score, BMI body mass index, CEA carcinoembryonic antigen; CA19–9 = carbohydrate antigen 19–9
P-values < 0.05 were considered to indicate statistical significance
Table 2.
The relarionship between GPS score and clinicopathological characteristics
| variable | GPS 0 | GPS 1 | GPS 2 | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| (N = 409) | (N = 117) | (N = 68) | ||||||
| Age | (years) | 0.046 | ||||||
| < 75 | 290 | 48.8% | 81 | 13.6% | 38 | 6.4% | ||
| ≥75 | 119 | 20.0% | 36 | 19.7% | 30 | 11.4% | ||
| Sex | 0.075 | |||||||
| female | 168 | 28.3% | 35 | 5.9% | 29 | 4.9% | ||
| male | 241 | 40.6% | 117 | 19.7% | 68 | 11.4% | ||
| BMI | 0.039 | |||||||
| ≥18.5 | 328 | 58.5% | 108 | 19.3% | 54 | 9.6% | ||
| < 18.5 | 48 | 8.6% | 9 | 1.6% | 14 | 2.5% | ||
| tumor location | 0.008 | |||||||
| right side | 159 | 26.8% | 60 | 10.1% | 37 | 6.2% | ||
| left side | 250 | 42.1% | 57 | 9.6% | 31 | 5.2% | ||
| opearation approach | 0.02 | |||||||
| open | 236 | 39.8% | 80 | 13.5% | 49 | 8.3% | ||
| lap | 172 | 29.0% | 37 | 6.2% | 19 | 3.2% | ||
| Tumor size | (cm) | < 0.001 | ||||||
| < 5 | 279 | 48.4% | 52 | 9.0% | 16 | 2.8% | ||
| ≥5 | 118 | 20.5% | 60 | 10.4% | 51 | 8.9% | ||
| T | 0.011 | |||||||
| T2/3 | 342 | 57.6% | 99 | 16.7% | 47 | 7.9% | ||
| T4 | 67 | 11.3% | 18 | 3.0% | 21 | 3.5% | ||
| N | 0.016 | |||||||
| N0 | 196 | 33.1% | 67 | 11.3% | 44 | 7.4% | ||
| N1/2/3 | 213 | 35.8% | 50 | 8.4% | 24 | 4.0% | ||
| CEA | (ng/ml) | 0.361 | ||||||
| < 5 | 263 | 49.0% | 69 | 12.8% | 39 | 7.3% | ||
| ≥5 | 108 | 20.1% | 35 | 6.5% | 23 | 4.3% | ||
| CA19–9 | (U/ml) | 0.588 | ||||||
| < 37 | 302 | 56.5% | 83 | 15.5% | 43 | 8.1% | ||
| ≥37 | 74 | 13.9% | 18 | 3.4% | 14 | 2.6% | ||
Abbreviation: GPS Glasgow Prognostic Score, BMI body mass index, CEA carcinoembryonic antigen; CA19–9 carbohydrate antigen 19–9
P-values < 0.05 were considered to indicate statistical significance
Table 3.
The relarionship between mGPS score and clinicopathological characteristics
| variable | mGPS 0 | mGPS 1 | mGPS 2 | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| (N = 493) | (N = 49) | (N = 49) | ||||||
| Age | (years) | 0.101 | ||||||
| < 75 | 345 | 58.1% | 35 | 5.9% | 29 | 4.9% | ||
| ≥75 | 148 | 24.9% | 14 | 2.4% | 23 | 3.9% | ||
| Sex | 0.283 | |||||||
| female | 198 | 33.3% | 14 | 2.4% | 20 | 3.4% | ||
| male | 295 | 49.7% | 35 | 5.9% | 32 | 5.4% | ||
| BMI | 0.302 | |||||||
| ≥18.5 | 404 | 87.8% | 44 | 7.8% | 42 | 7.5% | ||
| < 18.5 | 56 | 10.0% | 5 | 0.9% | 10 | 1.8% | ||
| tumor location | 0.153 | |||||||
| right side | 159 | 26.8% | 60 | 10.1% | 37 | 6.2% | ||
| left | 250 | 42.1% | 57 | 9.6% | 31 | 5.2% | ||
| opearation approach | 0.001 | |||||||
| open | 287 | 48.4% | 40 | 6.7% | 38 | 6.4% | ||
| lap | 205 | 34.6% | 9 | 1.5% | 14 | 2.4% | ||
| Tumor size | (cm) | < 0.001 | ||||||
| < 5 | 317 | 55.0% | 19 | 3.3% | 11 | 1.9% | ||
| ≥5 | 163 | 28.3% | 26 | 4.5% | 40 | 6.9% | ||
| T | 0.014 | |||||||
| T2/3 | 412 | 69.4% | 41 | 6.9% | 35 | 5.9% | ||
| T4 | 81 | 13.6% | 8 | 1.3% | 17 | 2.9% | ||
| N | 0.17 | |||||||
| N0 | 247 | 41.7% | 27 | 4.6% | 33 | 5.6% | ||
| N1/2/3 | 245 | 41.3% | 22 | 3.7% | 19 | 3.2% | ||
| CEA | (ng/ml) | 0.629 | ||||||
| < 5 | 312 | 58.1% | 30 | 5.6% | 29 | 5.4% | ||
| ≥5 | 137 | 25.5% | 12 | 2.2% | 17 | 3.2% | ||
| CA19–9 | (U/ml) | 0.274 | ||||||
| < 37 | 365 | 68.4% | 34 | 6.4% | 29 | 5.4% | ||
| ≥37 | 87 | 16.3% | 7 | 1.3% | 12 | 2.2% | ||
Abbreviation: mGPS modified-Glasgow Prognostic Score, BMI body mass index, CEA carcinoembryonic antigen; CA19–9 carbohydrate antigen 19–9
P-values < 0.05 were considered to indicate statistical significance
Recurrence was classified as distant recurrence (DR) and local recurrence (LR). LR was defined as any clinical or histological evidence of tumour regrowth near the primary site. DR was defined as all recurrence types except those classified as LR. Among 88 cases (20.1%) of recurrence with an HS-GPS score of 0 or 1, the initial recurrence type was DR in 20 cases (liver, 37; lung, 23; lymph node, 7; peritoneal, 9; other type, 8) and LR in 18 cases. Among 14 cases (26.9%) of recurrence with an HS-GPS score of 2, the initial recurrence type was DR in 15 cases (liver, 6; lung, 5; lymph node, 1; peritoneal, 1; other type, 2) and LR in 2 cases. There were no significant differences between any of the groups. Treatment after recurrence was performed according to JSCCR guidelines [6].
Association of the various GPS scores and other clinicopathological factors with OS
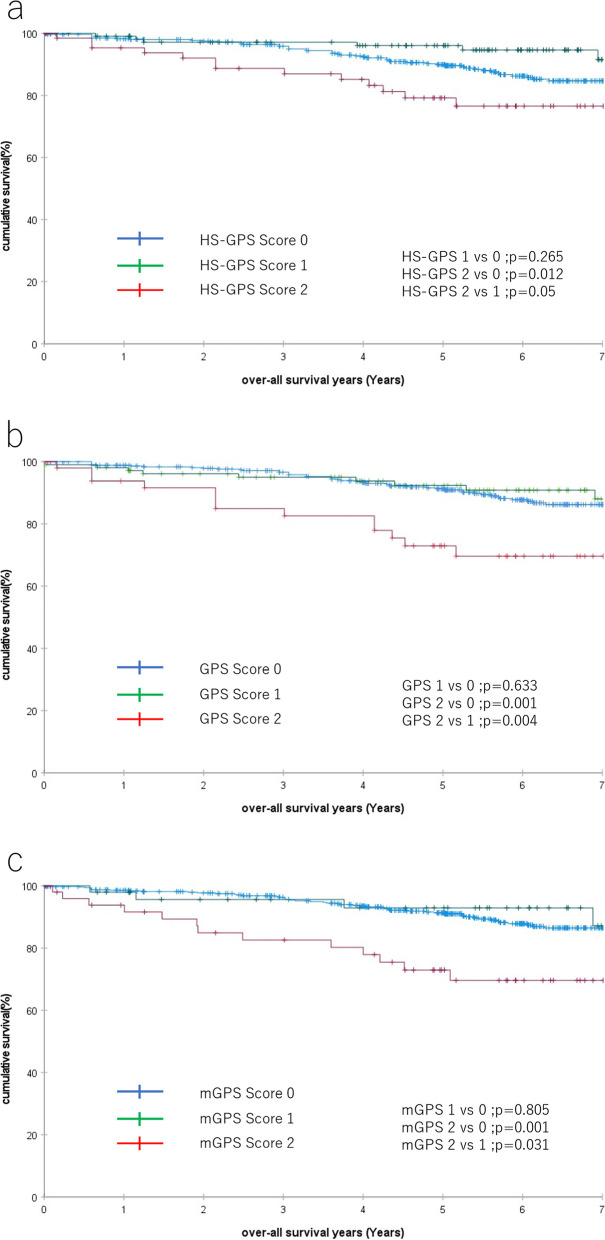
Kaplan–Meier survival analysis was performed to assess the differences between the HS-GPS, GPS, and mGPS in evaluating OS. The log-rank test showed that HS-GPS 2 was a poor prognostic factor compared to HS-GPS 0 (HS-GPS 2 vs. 0; P = 0.012) and HS-GPS 1 (HS-GPS 2 vs 1; P = 0.05) (Fig. 1a). For the GPS, the test showed a significant difference in OS between GPS 2 vs 0 (P = 0.001) and 2 vs 1 (P = 0.04) (Fig. 1b). Further analysis for the mGPS showed a significant difference in OS between mGPS 2 vs 1 (P = 0.001) and 2 vs 1 (P = 0.031) (Fig. 1c). Of the three inflammation-based prognostic scores, a score of 2 was shown to be a poor prognostic factor.
Fig. 1.
Overall survival in patients with colorectal cancer according to HS-GPS (a), GPS (b), and mGPS (c). GPS, Glasgow prognostic score; HS-GPS, high-sensitivity mGPS; mGPS, modified Glasgow prognostic score
The area under the curve (AUC) values of GPS, mGPS, and HS-mGPS for predicting OS were 0.540, 0.528, and 0.51, respectively. The AUC value of GPS was significantly higher than that of HS-GPS (p = 0.007). There was no significant difference in the AUC values between GPS and mGPS (p = 0.407), or mGPS and HS-GPS (p = 0.179).
Table 4 shows the results of the univariate and multivariate analyses of the predictive impacts of the three inflammation-based prognostic score models and other clinicopathological factors of OS. Univariate analysis showed that age, BMI, pT factor, pN factor, CEA, and CA19–9 were significantly different in OS prediction. Multivariate analysis was performed using the factors that showed significant differences (P < 0.05) in univariate analysis among inflammation-based prognostic scores and other clinicopathological factors. For the HS-GPS model, multivariate analysis confirmed that age (HR, 2.049; 95% CI, 1.195–3.413; P = 0.009), pT (HR, 2.254; 95% CI, 1.272–3.992; P = 0.005), pN (HR, 1.925; 95% CI, 1.081–3.429; P = 0.026), and HS-GPS 2 vs 0 (HR, 2.638; 95% CI, 1.046–6.650; P = 0.04) were independent prognostic predictors of OS. For the GPS model, pT (HR, 2.381; 95% CI, 1.315–4.311; P = 0.004), pN (HR, 2.014; 95% CI, 1.016–3.670; P = 0.022), GPS 2 vs 0 (HR, 1.444; 95% CI, 1.018–2.048; P = 0.04) were independent prognostic predictors. Further, for the mGPS model, age (HR, 1.98; 95% CI, 1.163–3.371; P = 0.02), pT (HR, 1.955; 95% CI, 1.090–3.508; P = 0.025), pN (HR, 2.04; 95% CI, 1.157–3.598; P = 0.014), mGPS 2 vs 0 (HR, 1.51; 95% CI, 1.066–2.140; P = 0.02) were independent prognostic predictors.
Table 4.
Results of univariate and multivariate analysis of clinicopathological factors affecting OS
| variable | univariate analysis | multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HS-GPS model | GPS model | mGPS model | |||||||||
| p value | HR | 95%CI | p value | HR | 95%CI | p value | HR | 95%CI | p value | ||
| Age | (years) | 0.001 | 2.049 | 1.195–3.413 | 0.009 | 2.052 | 1.180–3.568 | 0.11 | 1.98 | 1.163–3.371 | 0.02 |
| < 75 | |||||||||||
| ≥75 | |||||||||||
| Sex | 0.764 | ||||||||||
| female | |||||||||||
| male | |||||||||||
| BMI | 0.035 | 0.562 | 0.300–1.054 | 0.072 | 0.502 | 0.270–1.003 | 0.051 | 0.601 | 0.321–1.125 | 0.111 | |
| ≥18.5 | |||||||||||
| < 18.5 | |||||||||||
| tumor location | 0.725 | ||||||||||
| right side | |||||||||||
| left side | |||||||||||
| operation approach | 0.895 | ||||||||||
| open | |||||||||||
| lap | |||||||||||
| Tumor size | (cm) | 0.344 | |||||||||
| < 5 | |||||||||||
| ≥5 | |||||||||||
| T | < 0.001 | 2.254 | 1.272–3.992 | 0.005 | 2.381 | 1.315–4.311 | 0.004 | 1.955 | 1.090–3.508 | 0.025 | |
| T2/3 | |||||||||||
| T4 | |||||||||||
| N | 0.001 | 1.925 | 1.081–3.429 | 0.026 | 2.014 | 1.016–3.670 | 0.022 | 2.04 | 1.157–3.598 | 0.014 | |
| N0 | |||||||||||
| N1/2/3 | |||||||||||
| CEA | (ng/ml) | 0.019 | 1.547 | 0.894–2.674 | 0.119 | 1.583 | 0.913–2.746 | 1.102 | 1.601 | 0.941–2.721 | 0.082 |
| < 5 | |||||||||||
| ≥5 | |||||||||||
| CA19–9 | (U/ml) | 0.038 | 1.238 | 0.630–2.611 | 0.493 | 0.906 | 0.421–1.948 | 0.8 | 1.13 | 0.561–2.276 | 0.733 |
| < 37 | |||||||||||
| ≥37 | |||||||||||
| HS-GPS | 0 | reference | |||||||||
| 1 | 0.265 | ||||||||||
| 2 | 0.012 | 2.638 | 1.046–6.650 | 0.04 | |||||||
| GPS | 0 | reference | |||||||||
| 1 | 0.633 | ||||||||||
| 2 | 0.001 | 1.444 | 1.018–2.048 | 0.04 | |||||||
| mGPS | 0 | reference | |||||||||
| 1 | 0.805 | ||||||||||
| 2 | 0.001 | 1.51 | 1.066–2.140 | 0.02 | |||||||
Abbreviation: HS-GPS high sensivity modified-Glasgow Prognostic Score, GPS Glasgow Prognostic Score, m-GPS modified-GPS, BMI body mass index, CEA carcinoembryonic antigen, CA19–9 carbohydrate antigen 19–9
P-values < 0.05 were considered to indicate statistical significance
Association of the various GPS scores and other clinicopathological factors with RFS
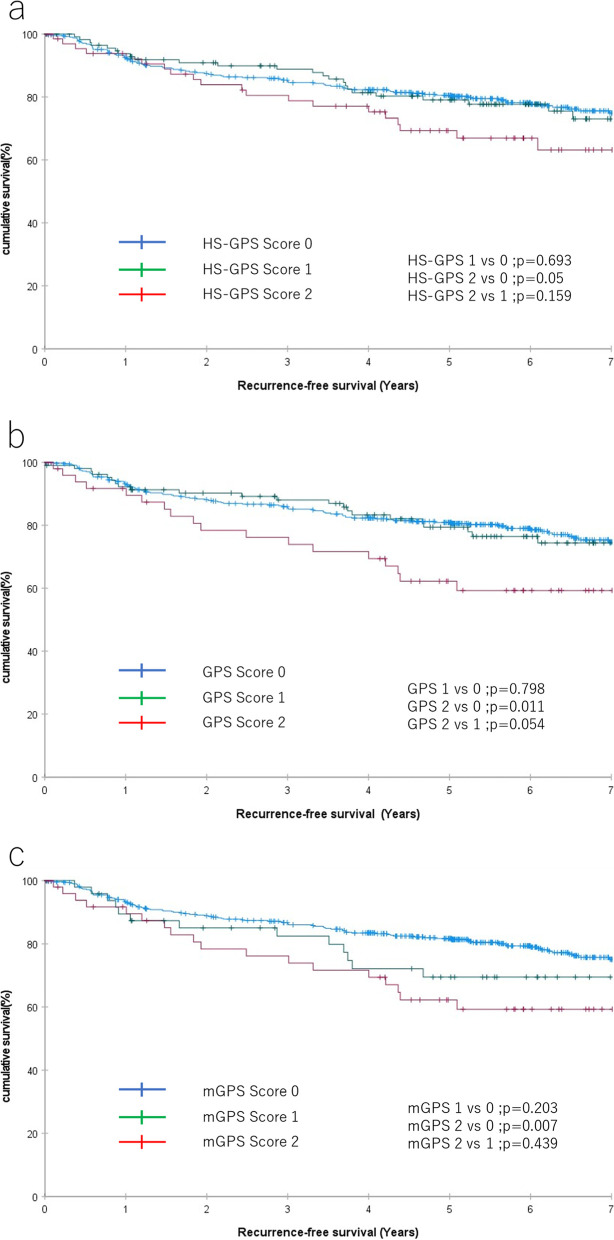
Kaplan–Meier survival analysis was performed to assess the differences between the HS-GPS, GPS, and mGPS in evaluating RFS. The log-rank test did not show a significant difference in RFS between HS-GPS 2 vs 0 (P = 0.05) (Fig. 2a). For the GPS, the test showed a significant difference in GPS 2 vs 0 (P = 0.011) (Fig. 2b). For the mGPS, the test showed a significant difference in mGPS 2 vs 0 (P = 0.007) (Fig. 2c).
Fig. 2.
Recurrence-free survival in patients with colorectal cancer according to HS-GPS (a), GPS (b), mGPS (c). GPS, Glasgow prognostic score; HS-GPS, high-sensitivity mGPS; mGPS, modified Glasgow prognostic score
Table 5 summarises the results of the univariate and multivariate analyses of the predictive ability of each inflammation-based prognostic score model and other clinicopathological factors for RFS. Univariate analysis showed that age, pT factor, pN factor, and CEA showed significant differences in RFS prediction. Multivariate analysis was performed for the factors that showed significant differences (P < 0.05) in the univariate analysis among inflammation-based prognostic scores and other clinicopathological factors. In the multivariate analysis of the HS-GPS model, pN (HR, 2.747; 95% CI, 1.797–4.199; P < 0.001) and CEA (HR, 2.093; 95% CI, 1.420–3.086; P < 0.001) were independent prognostic predictors of RFS. For the GPS model, multivariate analysis showed that pN (HR, 2.549; 95% CI, 1.648–3.857; P < 0.001), CEA (HR, 2.093; 95% CI, 1.420–3.086; P = < 0.001), and GPS 2 vs 0 (HR, 1.537; 95% CI, 1.190–1.987; P = 0.002) were independent prognostic predictors of RFS. In the analysis of the mGPS model, pN (HR, 2.55; 95% CI, 1.715–3.793; P < 0.001), CEA (HR, 1.998; 95% CI, 1.376–2.902; P < 0.001), and mGPS 2 vs 0 (HR, 1.555; 95% CI, 1.206–2.005; P = 0.004) were independent prognostic predictors.
Table 5.
Results of univariate and multivariate analysis of clinicopathological factors affecting RFS
| variable | univariate analysis | multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HS-GPS model | GPS model | mGPS model | |||||||||
| p value | HR | 95%CI | p value | HR | 95%CI | p value | HR | 95%CI | p value | ||
| Age | (years) | 0.027 | 1.349 | 0.897–2.028 | 0.151 | 1.281 | 0.848–1.934 | 0.24 | 1.232 | 0.830–1.830 | 0.3 |
| < 75 | |||||||||||
| ≥75 | |||||||||||
| Sex | 0.545 | ||||||||||
| female | |||||||||||
| male | |||||||||||
| BMI | 0.174 | ||||||||||
| ≥18.5 | |||||||||||
| < 18.5 | |||||||||||
| tumor location | 0.912 | ||||||||||
| right side | |||||||||||
| left side | |||||||||||
| operation approach | 0.078 | ||||||||||
| open | |||||||||||
| lap | |||||||||||
| Tumor size | (cm) | 0.086 | |||||||||
| < 5 | |||||||||||
| ≥5 | |||||||||||
| T | < 0.001 | 1.571 | 0.777–3.179 | 0.209 | 1.572 | 0.78–3.618 | 0.206 | 1.555 | 0.802–3.014 | 0.191 | |
| T2/3 | |||||||||||
| T4 | |||||||||||
| N | 0.001 | 2.747 | 1.797–4.199 | < 0.001 | 2.549 | 1684–3.857 | < 0.001 | 2.55 | 1.715–3.793 | < 0.001 | |
| N0 | |||||||||||
| N1/2/3 | |||||||||||
| CEA | (ng/ml) | < 0.001 | 2.093 | 1.420–3.086 | < 0.001 | 2.093 | 1.420–3.086 | < 0.001 | 1.998 | 1.376–2.902 | < 0.001 |
| < 5 | |||||||||||
| ≥5 | |||||||||||
| CA19–9 | (U/ml) | 0.064 | |||||||||
| < 37 | |||||||||||
| ≥37 | |||||||||||
| HS-GPS | 0 | reference | |||||||||
| 1 | 0.693 | ||||||||||
| 2 | 0.051 | ||||||||||
| GPS | 0 | reference | |||||||||
| 1 | 0.798 | ||||||||||
| 2 | 0.011 | 1.537 | 1.190–1.987 | 0.002 | |||||||
| mGPS | 0 | reference | |||||||||
| 1 | 0.203 | ||||||||||
| 2 | 0.007 | 1555 | 1.206–2.005 | 0.004 | |||||||
Abbreviation: HS-GPS high sensivity modified-Glasgow Prognostic Score, GPS Glasgow Prognostic Score, m-GPS modified-GPS, BMI body mass index, CEA carcinoembryonic antigen, CA19–9 carbohydrate antigen 19–9
P-values <0.05 were considered to indicate statistical significance
Discussion
CRC prognosis is based on the UICC TNM classification; however, differences in outcomes have been reported among patients presenting with the same disease stage [8, 9]. CRC has different treatment strategies and prognoses owing to various characteristics such as stage, localisation (right or left; colon or rectum), and genotype. Therefore, biomarkers using nutritional or immunological factors associated with cancer progression and carcinogenesis have been established for improving prediction accuracy; furthermore, it is more useful to perform classification that considers each stage and localisation [10–12]. In CRC, which has a variety of treatment strategies, surgery is the mainstay of almost all approaches to resectable advanced. We considered that this group of patients was the most suitable for evaluating the biological characteristics of CRC.
Forrest et al. reported on the GPS model as a cumulative score of CRP and albumin levels, showing a significant prognostic effect in patients with lung cancer [13]. Subsequently, McMillan et al. reported that the mGPS predicted CRC patient prognosis more accurately [14]. Prediction of prognosis for CRC or other cancers using these scores has already been reported, including meta-analysis studies [2, 15, 16].
The accuracy of the three inflammation-based prognostic scoring systems is a frequent topic of discussion. GPS scores are determined using both CRP and albumin values; a score of 0 in both the mGPS and HS-mGPS systems is determined using CRP alone, regardless of albumin levels. Therefore, though hypoalbuminemia is more likely to occur secondary to elevated CRP levels, a crucial difference between the GPS and mGPS or HS-GPS is the inclusion of patients with hypoalbuminemia in the absence of elevated CRP levels. Thus, both the inflammatory response and nutritional status must be considered to predict the prognoses of cancer patients more accurately [17]. Our study was consistent with past reports. Though HS-GPS was able to predict the prognosis by evaluating the OS, it was not an independent marker of prognosis prediction for cancer recurrence by evaluating RFS. A disadvantage of the mGPS scoring system is that patients with abnormal values are a minority, and studies may only select certain minority patients with an unfavourable prognosis, which may introduce a bias. To address these problems, Proctor et al. confirmed that the HS-mGPS could enhance the prognostic values of the GPS and mGPS in a large cohort of 12,119 patients with cancer [5]. However, the number of patients with CRC in this study was limited, and the patients examined had significantly different clinicopathological classification among different cancer types. There is an increasing evidence that the inflammatory indices of HS-GPS play important roles in predicting survival in many cancers. However, Hirahara et al. reported that GPS was more reliable in evaluating prognosis in gastric cancer than HS-GPS, and the true effectiveness of HS-GPS needed to be verified in each cancer [17]. There are currently no reports analysing the prognostic impact of HS-GPS in CRC alone. Our study is the first to investigate the effectiveness of HS-GPS in patients with CRC. In our study, it was possible to predict the long-term prognosis using HS-GPS in a limited way; however, and it was not useful in predicting long-term prognosis as that using GPS. This is similar to the results of a study by Hirahara et al. on gastric cancer, and the effectiveness of HS-GPS in predicting prognosis in CRC may be limited.
This study has several limitations. First, selection bias may have been introduced because this was a single-institution, retrospective study. Though our study did not demonstrate that HS-GPS was useful in assessing long-term prognosis using RFS, we did determine that the HS-GPS score 2 group tended to have a poor prognosis. This suggests that having a larger sample size may provide useful results. Second, we did not consider ADJ. Approximately 30% of patients receive ADJ, but it is difficult to analyse the specific regimen used and other associated parameters. Chemotherapy used for CRC varies by country and age, and the choice of the multitude of regimens available serves as a prognostic factor. In particular, elderly and undernourished patients may have difficulty tolerating anticancer drugs, and it is difficult to completely eliminate this effect. Third, the relationship with other markers of systemic inflammation and nutrition is unknown. Further improvement in the prognosis of CRC may not be possible with a single marker.
Conclusion
HS-GPS was useful for predicting the OS prognosis of resectable advanced CC. Long-term prediction of RFS only showed a dominant tendency. HS-GPS may be less effective as a prognostic score for CC than GPS.
Acknowledgements
Not applicable.
Authors’ contributions
KK contributed to the concept and design of the study, collection and interpretation of data, writing of the manuscript, and revision of the manuscript. ME and KK contributed to the concept and design of the study, interpretation of data, and revision of the manuscript. RU, TI, JM, TT, TY, YN, and AT contributed to the collection and interpretation of data and revision of the manuscript. All the authors approved the final version of the manuscript.
Funding
The authors declare that they have received no grants/funds from any institution/organization/person for the purpose of this study.
Availability of data and materials
The datasets during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Tokyo Medical University Hospital (T2019–0054). Informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest directly relevant to the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lu X, Guo W, Xu W, Zhang X, Shi Z, Zheng L, et al. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta-analysis of 9,839 patients. Cancer Manag Res. 2019;11:229–249. doi: 10.2147/CMAR.S185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 4.Son W, Shin SJ, Park SH, Lee SK, Park EJ, Baik SH, et al. Clinical impact of combined modified Glasgow prognostic score and C-reactive protein/albumin ratio in patients with colorectal cancer. Diagnostics (Basel) 2020;10:859. doi: 10.3390/diagnostics10110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow inflammation outcome study. Cancer. 2013;119:2325–2332. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 6.Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines:1–42. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines:1–42. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshmukh SK, Srivastava SK, Poosarla T, Dyess DL, Holliday NP, Singh AP, et al. Inflammation, immunosuppressive microenvironment and breast cancer: opportunities for cancer prevention and therapy. Ann Transl Med. 2019;7:593. doi: 10.21037/atm.2019.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson AB, 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370–398. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 9.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazaki J, Katsumata K, Kasahara K, Tago T, Wada T, Kuwabara H, et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20:922. doi: 10.1186/s12885-020-07429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265:539–546. doi: 10.1097/SLA.0000000000001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi S, Basso M, Strippoli A, Schinzari G, D'Argento E, Larocca M, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16:264–274. doi: 10.1016/j.clcc.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Color Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 15.Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7:16717. doi: 10.1038/s41598-017-16955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, He X, Pan J, Chen S, Wang L. Prognostic role of Glasgow prognostic score in patients with colorectal cancer: evidence from population studies. Sci Rep. 2017;7:6144. doi: 10.1038/s41598-017-06577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirahara N, Matsubara T, Kaji S, Kawabata Y, Hyakudomi R, Yamamoto T, et al. Glasgow prognostic score is a better predictor of the long-term survival in patients with gastric cancer, compared to the modified Glasgow prognostic score or high-sensitivity modified Glasgow prognostic score. Oncotarget. 2020;11:4169–4177. doi: 10.18632/oncotarget.27796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study are available from the corresponding author upon reasonable request.