Abstract
p27 is a key regulator of cell proliferation through inhibition of G1 cyclin-dependent kinase (CDK) activity. Translation of the p27 mRNA is an important control mechanism for determining cellular levels of the inhibitor. Nearly all eukaryotic mRNAs are translated through a mechanism involving recognition of the 5′ cap by eukaryotic initiation factor 4E (eIF4E). In quiescent cells eIF4E activity is repressed, leading to a global decline in translation rates. In contrast, p27 translation is highest during quiescence, suggesting that it escapes the general repression of translational initiation. We show that the 5′ untranslated region (5′-UTR) of the p27 mRNA mediates cap-independent translation. This activity is unaffected by conditions in which eIF4E is inhibited. In D6P2T cells, elevated cyclic AMP levels cause a rapid withdrawal from the cell cycle that is correlated with a striking increase in p27. Under these same conditions, cap-independent translation from the p27 5′-UTR is enhanced. These results indicate that regulation of internal initiation of translation is an important determinant of p27 protein levels.
In normal cells, the cyclin-dependent kinase (CDK) inhibitor p27 mediates cell cycle arrest during contact inhibition, during cellular differentiation, and in response to numerous other cellular modulators (3). p27 arrests cells in G1 primarily by inhibition of CDK2, which is required for the transition into S phase. In cancer cells, p27 is generally downregulated and there is a strong inverse correlation between tumor progression and the levels of p27 (3, 20). Loss of p27 expression in tumor cells is not due to mutations in the gene that encodes the inhibitor (4, 7, 14, 32) and so must reflect changes in the regulatory pathways that control p27 expression. In addition, changes in p27 protein levels in normal cells do not in general correlate with changes in transcription of the p27 gene. Rather, p27 is most commonly regulated by changes in the rate of proteasome-mediated degradation (30) or the rate at which its mRNA is translated (1, 10, 25).
Nearly all nucleus-encoded eukaryotic proteins are translated through a mechanism that involves recognition of the mRNA 5′ 7-methylguanosine cap by eukaryotic initiation factor 4E (eIF4E). Through its association with eIF4G, eIF4E binding to the cap results in recruitment of the 40S ribosomal subunit. eIF4E cap recognition has been shown to be the rate-limiting factor in translational initiation (6). eIF4E activity is modulated by several mechanisms. Its gene is transcriptionally regulated in response to mitogenic stimulation and may be a direct target for c-myc (13). The cap-binding activity of eIF4E is enhanced by phosphorylation. This is mediated by the protein kinase Mnk (40), which is downstream of the mitogen-activated protein kinase signaling pathway and therefore activated in response to numerous mitogenic signals. Finally, eIF4E is inhibited by interacting with 4E binding protein (4E-BP, also called PHAS). 4E-BP interacts with the same region of eIF4E that is bound by eIF4G (9, 22) and thus blocks assembly of the initiation complex at the 5′ end of mRNAs. Phosphorylation of 4E-BP through the phosphoinositol 3-kinase-dependent pathway blocks its ability to bind eIF4E (34).
The net result of these eIF4E regulatory mechanisms is that cap-dependent translation rates are lower in quiescent cells than in proliferating cells. However, even though global rates of protein synthesis are lower, the p27 mRNA is translated more efficiently in cells that are quiescent (1, 10, 25). We hypothesized, therefore, that the p27 mRNA escapes the effects of downregulation of cap-dependent translation by being translated through a cap-independent mechanism. In support of this hypothesis, the results reported here indicate that the 5′ untranslated region (5′-UTR) of the p27 mRNA functions as an internal ribosome entry site (IRES) and that translation through this element is enhanced under conditions in which p27 expression is elevated.
MATERIALS AND METHODS
Cell culture.
NIH 3T3 and D6P2T cells were grown in Dulbecco's modified Eagle medium (DMEM) containing 10% newborn calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Northern blotting.
Stably transfected NIH 3T3 cells were harvested in Dulbecco's phosphate-buffered saline and centrifuged to pellet the cells. A portion of the cells was removed for chloramphenicol acetyltransferase (CAT) and luciferase assays (see below). RNA was isolated from the remainder of the cells using TRI Reagent according to the manufacturer's directions. The RNA was separated on a 1% agarose gel containing 2.2 M formaldehyde. Following electrophoresis, the ribosomal bands were visualized by staining in SybrGold (Molecular Probes) and the RNA was transferred to a nylon membrane (Schleicher & Schuell). Hybridization was carried out in PerfectHyb buffer (Sigma) at 68°C. 32P-labeled probes, consisting of either the CAT coding sequence or the luciferase coding sequence, were labeled using the RediPrime labeling system (Amersham). Free 32P was removed by passage through a Sephadex G-50 spin column. Following hybridization, the nylon filter was washed and the blot was subjected to autoradiography.
Transfections and reporter assays.
Cells were transfected using GenePorter (Gene Therapy Systems) in medium without added serum or growth factors. Four hours after transfection, 2 volumes of medium containing 20% calf serum was added to the cultures. NIH 3T3 cells were harvested 1 day and D6P2T cells were harvested either 1 or 3 days after transfection. Cells in 35-mm-diameter culture dishes were harvested by addition of 200 μl of reporter lysis buffer (Promega) and scraping from the bottom of the dish.
Luciferase activity was determined using either the LucLite substrate (Packard) or the Steady-Glo substrate (Promega) according to the manufacturer's protocol, using 50 to 200 μl of cellular extract. CAT activity was determined as previously described (29).
Plasmid constructs.
The mouse p27 5′-UTR was amplified from the full-length cDNA (a gift of Tony Hunter) by PCR with primers CTGCGCCTCTCTTCCCCAGA and CTTCCTCCTCGGGCGGGTGT. The PCR product was ligated into the HindIII site of pGL2CAT/Luc (a gift of R. E. Rhoads) which had been end filled and dephosphorylated. Deletion constructs of the p27 5′-UTR were prepared by PCR amplification using primers CTGCGCCTCTCTTCCCCAGA and CGACTGCACACAGACAGTCA for region 1–90 and TGACTGTCTGTGTGCAGTCG and CTTCCTCCTCGGGCGGGTGT for region 71–217. Both PCR products were cloned into pGL2CAT/Luc as described above. To prepare constructs carrying inverted repeat sequences, the luciferase coding region, with or without the p27 5′-UTR at the 5′ end, was amplified by PCR and inserted into the end-filled BamHI site of pcDNA3.1hygro (Invitrogen). A double-stranded oligonucleotide containing an inverted repeat sequence (CTAGGGGCGCGTGGTGGCGGCTGCAGCCGCCACCACGCGCCC) was then ligated into the NheI site within the multiple cloning site of the vector.
p27 expression constructs were prepared by amplifying the mouse p27 cDNA using the appropriate primers. For pcDNA-p27-217, the primers used were CTGCGCCTCTCTTCCCCAGA and ATGTCTTCCTTGCTTCATAAAGCAG. For pcDNA-p27-3 the primers used were AAGATGTCAAACGTGAGAGTG and ATGTCTTCCTTGCTTCATAAAGCAG. The PCR products were phosphorylated and made blunt ended using a Perfectly Blunt cloning kit (Novagen). They were then ligated into the NheI site of pcDNA3.1hygro which had been end filled.
Western blotting.
For Western blotting, cultured cells were harvested directly in sodium dodecyl sulfate (SDS)-sample buffer. Nucleic acids were sheared by sonication. The samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to Immobilon P membranes using a semidry transfer apparatus. p27 was detected using a monoclonal antibody (Transduction Laboratories), a horseradish peroxidase-conjugated secondary antibody, and SuperSignal chemiluminescence reagents (Pierce Chemical Co.). 4E-BP was detected using a polyclonal antibody to PHAS (Zymed). V5 epitope-tagged p27 was detected using a monoclonal antibody to V5 (Invitrogen).
Polysome gradients.
Cycloheximide (90 μg/ml, final concentration) was added to D6P2T cells 30 min prior to harvesting in polysome solution (0.3 M NaCl, 10 mM Tris-Cl [pH 7.4], 10 mM MgCl2, 1.2% Triton X-100, 90 μg of cycloheximide per ml, 1 mg of heparin per ml). Cells were homogenized and centrifuged at 15,000 × g for 5 min, and the supernatant was layered onto 0.5 to 1.5 M sucrose gradients. The gradients were centrifuged for 90 min at 49,600 rpm in an SW65 rotor. Fractions were collected from the bottom. RNA was isolated from each fraction by using TRI Reagent as described above. The RNA pellet was dissolved in RNase-free water and analyzed by Northern blotting as described above. The probe used for detection of p27 mRNA from the polysome gradients was prepared as described above, using the p27 cDNA. mRNA levels in each fraction were quantitated with a Chemilmager (Alpha Innotech).
Pulse-labeling and immunoprecipitation of p27.
The cells were washed three times with DMEM lacking Met and Cys and then incubated in 3 ml of the same medium containing 200 μCi of 35S-labeled Met and Cys (Tran35S-label [ICN], 1,175 Ci/mmol) for 1 h. The cells were harvested by trypsinization and then resuspended in DMEM containing 10% calf serum to inactivate the trypsin. The cell number was determined with a hemacytometer; the cells were pelleted and then washed with Dulbecco's phosphate-buffered saline three times. The cells were suspended in a mixture containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris-Cl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 0.2 mM sodium orthovanadate, 1 mM NaF, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, and 10% glycerol at 1.4 × 104 cells per μl. The cell suspension was briefly sonicated and then centrifuged at full speed in a microcentrifuge at 4°C for 20 min. A portion of the supernatant representing 1.4 × 105 cells was precipitated with trichloroacetic acid to estimate the level of total protein synthesis. The remainder of the cell extract was incubated with 2 μg of rabbit anti-p27 polyclonal antibody (sc-776; Santa Cruz Biotechnology) for 7 h with gentle shaking at 4°C. Protein A-conjugated agarose beads (30 μl) were added, and the incubation continued for 1 h on a rotator. The mixture was centrifuged at 1,200 × g to pellet the beads. The beads were washed five times with lysis buffer. After the final wash, the supernatant was discarded and 60 μl of SDS-sample buffer was added. After heating at 50°C for 5 min, the sample was loaded on an SDS–10% polyacrylamide gel.
RESULTS
To test the hypothesis that the 5′-UTR of the p27 mRNA can mediate cap-independent translation, we used the bicistronic expression vector pGL2CAT/Luc (8). The mRNA expressed from this vector carries both the CAT and luciferase coding regions separated by a short spacer region. The CAT coding region is near the 5′ end and is translated in a cap-dependent manner. However, since the luciferase coding region is downstream of the CAT stop codon, it is expressed only if a sequence is inserted into the spacer region that can mediate cap-independent translation. For these experiments, we used the p27 5′-UTR from a cDNA cloned by Toyoshima and Hunter (39) from a mouse macrophage cell line. This fragment, which includes 217 bases upstream of the AUG start codon, was inserted between the CAT and luciferase genes in the bicistronic vector to construct pGL2CAT/Luc-p27UTR.
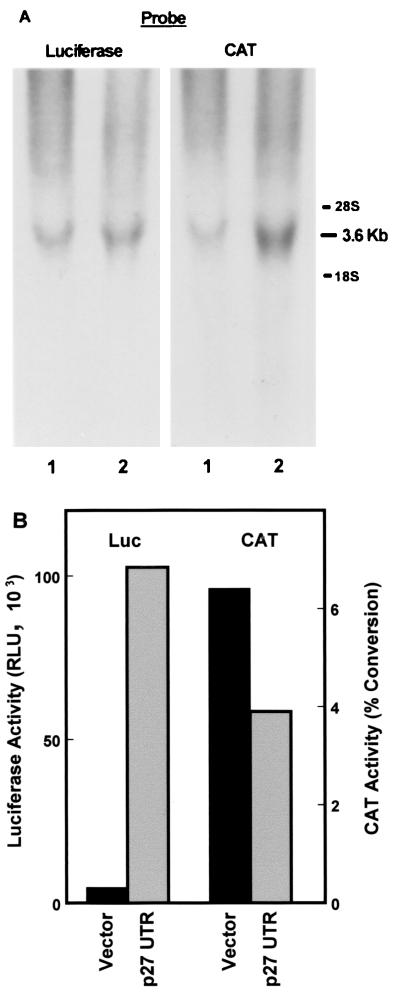
pGL2CAT/Luc-p27UTR and pGL2CAT/Luc were stably transfected into NIH 3T3 cells. These constructs should express a single mRNA that encodes for both the CAT and luciferase genes. This was confirmed by isolating RNA from the transfected cells and estimating the size of the mRNAs expressed by Northern blotting (Fig. 1A). The blots were first probed with a luciferase gene probe and then stripped and reprobed with a CAT gene probe. For each construct, both probes identify a single band that migrates in the range of 3.6 kb, which is near the predicted size of the expected mRNAs. No signal at all was detected in untransfected 3T3 cells (not shown). The resolution of the gel is not sufficient to distinguish the 217-nucleotide difference in size of the mRNAs encoded by the vector and the construct carrying the p27 5′-UTR. Thus, as expected, the CAT and luciferase coding regions are expressed on a single mRNA molecule.
FIG. 1.
Expression of bicistronic CAT/luciferase constructs in stably transfected NIH 3T3 cells. (A) RNA was isolated from NIH 3T3 cells stably transfected with either pGL2CAT/Luc vector (lane 1) or pGL2CAT/Luc-p27UTR (lane 2). The RNA was analyzed by Northern blotting using the probe indicated. The positions of 28S and 18S rRNAs are indicated at the right. (B) A portion of the same population of cells stably transfected with either pGL2CAT/Luc (vector) or pGL2CAT/Luc-p27UTR (p27 UTR) was harvested and assayed for both luciferase (Luc) and CAT activities.
A portion of the same cell populations used to perform the Northern blots described above was used for analysis of both CAT and luciferase activities (Fig. 1B). Both the vector and the construct carrying the p27 5′-UTR efficiently expressed the CAT gene. However, only the construct with the p27 5′-UTR insert expressed significant levels of luciferase activity, indicating that this sequence is able to mediate cap-independent translation through an internal ribosome entry mechanism.
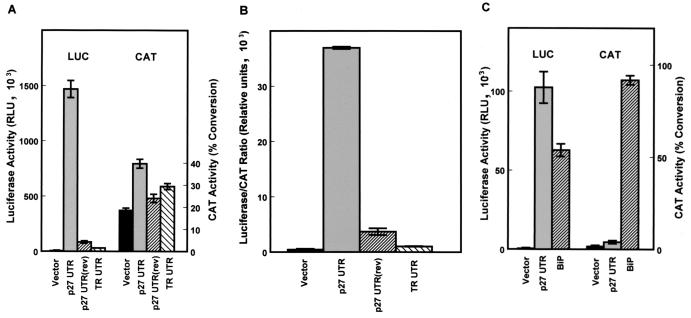
The ability of the p27 5′-UTR to mediate cap-independent translation was further compared to those of other sequences inserted into the spacer region between the CAT and luciferase coding regions. These included the p27 5′-UTR inserted in the reverse orientation and a 200-bp segment of the human transferrin receptor (TR) 5′-UTR. These constructs were transiently transfected into D6P2T cells and harvested for analysis of CAT and luciferase activities after 1 day. All of the constructs expressed similar levels of CAT activity, but only the construct carrying the p27 5′-UTR in the forward orientation expressed high levels of luciferase activity (Fig. 2A). Both the inverted p27 5′-UTR and the TR 5′-UTR constructs expressed slightly higher levels of luciferase than the pGL2CAT/Luc vector with no insert; however, the luciferase to CAT ratio was only a small fraction of that observed with the p27 5′-UTR in the correct orientation (Fig. 2B). The activity of the p27 5′-UTR was also compared to that of a well-characterized mammalian IRES, the BiP 5′-UTR (21). Bicistronic constructs carrying these inserts were transiently transfected into D6P2T cells. Both sequences were able to mediate cap-independent expression of luciferase, although higher levels were observed with the p27 5′-UTR. Also, the BiP construct, which utilizes the cytomegalovirus promoter rather than the simian virus 40 promoter, expresses a much higher level of CAT than the p27 5′-UTR construct, indicating a higher level of transcription from this construct. Therefore, the p27 5′-UTR is more efficient at mediating cap-independent translation than the BiP IRES.
FIG. 2.
Cap-independent translation mediated by the p27 5′-UTR. Bicistronic mRNAs encoding CAT upstream of luciferase were expressed in proliferating D6P2T cells. Cells were transfected with either pGL2CAT/Luc (vector), which has a short spacer region between the CAT and luciferase coding regions, pGL2CAT/Luc-p27UTR, which carries the region encoding the mouse p27 5′-UTR inserted within the spacer region, pGL2CAT/Luc-p27UTR(rev), which carries the region encoding the mouse p27 5′-UTR inserted in reverse orientation within the spacer region, or pGL2CAT/Luc-TR-UTR, which carries 200 bp of the TR 5′-UTR inserted within the spacer region. After cell lysis, CAT and luciferase (LUC) activities were determined for each extract (A), and the relative level of cap-independent translation was estimated from the luciferase/CAT ratio (B). The luciferase/CAT ratio was determined by dividing the relative light units (luciferase) by the percentage of substrate converted to the acetylated form (CAT). (C) D6P2T cells were transfected with either pGL2CAT/Luc (vector), pGL2CAT/Luc-p27UTR, or pcBiP, which carries the BIP 5′-UTR inserted between CAT and luciferase in the plasmid pcDNA1. The cells were harvested 1 day after transfection and assayed for CAT and luciferase activities. All data represent the mean of a minimum of three replicates plus or minus the standard error.
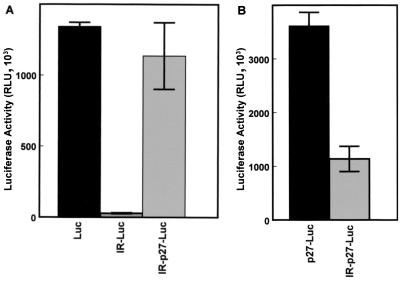
To further confirm these results, luciferase reporter constructs were prepared in which an inverted repeat was inserted near the 5′ end of the transcribed region. In the mRNAs transcribed from these constructs, the inverted repeat will result in the formation of a stable hairpin-loop (ΔG = −61 kcal/mol) just downstream of the 5′ cap (16). Formation of this hairpin-loop will effectively block cap-dependent initiation of translation but should have little effect on internal ribosome assembly (16, 31). In transiently transfected NIH 3T3 cells, the control construct, in which the luciferase coding region is under the control of the cytomegalovirus promoter (Fig. 3A, Luc), is efficiently expressed. Placing a hairpin-loop upstream of the luciferase coding region near the 5′ end of the transcript (IR-Luc) nearly eliminates luciferase expression (Fig. 3A). This indicates that, as expected, the hairpin-loop blocks cap-dependent translation. However, when the p27 5′-UTR is inserted between the hairpin-loop and the luciferase coding region (IR-p27-Luc), high-level expression of luciferase is restored. There is a 40-fold increase in expression when the p27 5′-UTR is placed downstream of the hairpin-loop, further demonstrating that the p27 5′-UTR is able to mediate cap-independent translation.
FIG. 3.
Cap-independent translation of p27 in the presence of a strong hairpin-loop. NIH 3T3 cells were transfected with the indicated expression constructs (see text for descriptions), harvested after 1 day, and assayed for luciferase activity. All data represent the mean of a minimum of three replicates plus or minus the standard error.
It is notable that there is about a threefold increase in expression when the p27 5′-UTR is placed upstream of the luciferase coding region (p27-Luc) compared to the same construct without the 5′-UTR (Luc) (Fig. 3). This suggests that the p27 5′-UTR is able to enhance translational initiation. In addition, although the construct encoding the hairpin-loop upstream of the p27 5′-UTR (IR-p27-Luc) is expressed at significant levels, its activity is only about 30% of that of the construct without the inverted repeat (p27-Luc) (Fig. 3B). This suggests that p27 can be translated in a cap-dependent manner as well as through an internal ribosome entry mechanism. However, it is also possible that the presence of the strong hairpin-loop affects the conformation of the 5′-UTR and therefore interferes with cap-independent initiation.
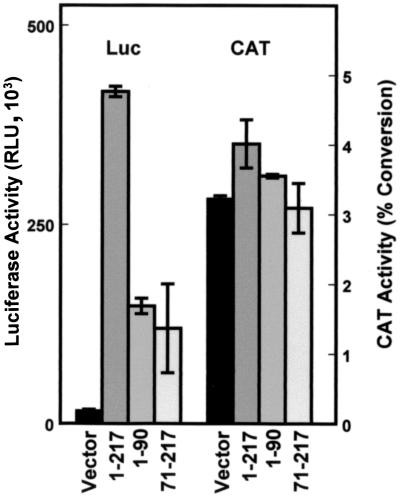
The p27 5′-UTR sequence used in the experiments described above includes the 217 nucleotides immediately upstream of the p27 start codon. This region contains some features commonly found in other IRESs, including a polypyrimidine tract upstream of the start codon and the potential to form extensive secondary structure (11). To determine the position of the sequences that are able to promote internal ribosome assembly, two additional bicistronic constructs were made. One construct encompasses the proximal portion of the 5′-UTR (nucleotides 71 to 217) and includes the polypyrimidine tract; the other construct encompasses the distal portion (nucleotides 1 to 90) of the p27 5′-UTR. In transfection assays in D6P2T cells, both the proximal and distal portions of the p27 5′-UTR were able to mediate luciferase expression (Fig. 4). However, neither was able to support the level of expression observed with the full-length 5′-UTR. CAT expression levels were nearly identical with all of the constructs, indicating that the differences in luciferase activity reflect differences in the efficiency of internal ribosome assembly. Apparently the IRES within the p27 5′-UTR consists of multiple sequence elements, which is consistent with reports on other IRESs (8, 36).
FIG. 4.
Cap-independent translation mediated by p27 5′-UTR deletion mutants. D6P2T cells were transfected with CAT/luciferase (Luc) constructs containing inserts within the spacer region as indicated. Region 1-217 encodes the entire 5′-UTR (pGL2CAT/Luc-p27UTR), region 71-217 encodes the region immediately proximal to the p27 AUG start codon, and region 1-90 encodes the distal region of the 5′-UTR. All data represent the mean of a minimum of three replicates plus or minus the standard error.
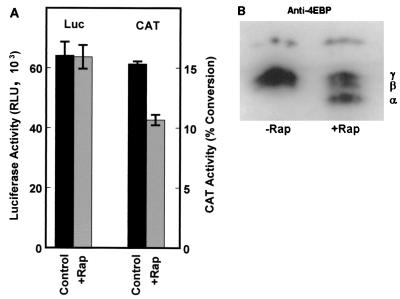
Internal, cap-independent initiation of translation through the p27 5′-UTR should not be significantly affected by conditions that lead to inhibition of eIF4E. eIF4E activity is blocked by hypophosphorylated 4E-BP, resulting in a general inhibition of cap-dependent translation. Phosphorylation of 4E-BP results in dissociation from eIF4E and enhanced cap-dependent translation. The drug rapamycin blocks 4E-BP phosphorylation and has previously been shown to inhibit cap-dependent translation but not IRES-dependent translation (2, 33). When D6P2T cells were transfected with the p27 5′-UTR bicistronic construct and then treated with rapamycin, there was no effect on luciferase expression whereas CAT expression was reduced by nearly one-third (Fig. 5A). The decrease in CAT activity corresponds to the appearance of the active hypophosphorylated form (α form) of 4E-BP, as shown by Western blotting (Fig. 5B). These results support the conclusion that the p27 5′-UTR mediates cap-independent translation that is resistant to rapamycin, similar to previous findings for other IRESs (2, 33).
FIG. 5.
Inhibition of cap-dependent translation does not affect p27 5′-UTR IRES activity. (A) D6P2T cells were transfected with the bicistronic construct carrying the full-length p27 5′-UTR, pGL2CAT/Luc-p27UTR. The cells were then treated with rapamycin (200 ng/ml) as indicated (+Rap). Each extract was analyzed for luciferase (Luc) and CAT activities. All data represent the mean of a minimum of three replicates plus or minus the standard error. (B) D6P2T cells were left untreated or treated with rapamycin as in panel A. Cells were lysed, and the extracts were subjected to Western blotting for 4E-BP. α represents the position of the hypophosphorylated form of 4E-BP; β and γ represent positions of the hyperphosphorylated forms.
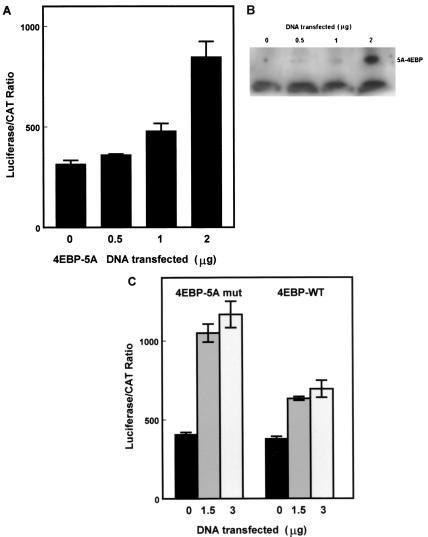
The results above suggest that p27 5′-UTR-mediated internal initiation allows translation to continue even when cap-dependent translation is inhibited. To verify this, the bicistronic p27 5′-UTR construct was cotransfected into D6P2T cells with an expression vector encoding a mutant 4E-BP1. This 4E-BP mutant is altered at five amino acid positions that are normally targets for phosphorylation (27). Mothe-Satney et al. (27) have shown that since the expressed protein cannot be phosphorylated at the appropriate sites, it is constitutively active in the inhibition of eIF4E and cap-dependent translation. They have also shown that the mutant protein does not interfere with IRES activity (27). Coexpression of the mutant 4E-BP with the p27 5′-UTR bicistronic reporter in D6P2T cells led to increased luciferase/CAT ratios (Fig. 6A) that paralleled increased levels of mutant 4E-BP (Fig. 6B). This is the expected result if cap-dependent translation is inhibited. In addition, the activity of the mutant 4E-BP was compared with the activity of the wild-type 4E-BP in cotransfection experiments with the p27 5′-UTR bicistronic construct in NIH 3T3 cells (Fig. 6C). Both the wild-type and mutant proteins were able to enhance the luciferase/CAT ratio. However, the activity of the mutant was approximately twofold greater than the wild-type level, which is consistent with the previous findings of Mothe-Satney et al. (27) for the ability of these two proteins to inhibit cap-dependent translation. These results further verify that the p27 5′-UTR is able to mediate cap-independent translation that is resistant to inhibition of eIF4E activity.
FIG. 6.
Coexpression of 4E-BP does not affect p27 5′-UTR IRES activity. (A) D6P2T cells were cotransfected with the bicistronic construct carrying the full-length p27 5′-UTR and increasing amounts of the same vector encoding a mutant form of 4E-BP-1 which is constitutively active in inhibiting elF4E. The amount of DNA transfected was held constant by including the appropriate level of empty expression vector. Each extract was analyzed for luciferase and CAT activity. The luciferase/CAT ratio was determined by dividing the relative light units (luciferase) by the percentage of substrate converted to the acetylated form (CAT). All data represent the mean of a minimum of three replicates plus or minus the standard error. (B) D6P2T cells transfected as in panel A were harvested for Western blot analysis of 4E-BP levels. Equal amounts of protein from each extract were loaded in each lane. The position of the constitutively active 4E-BP mutant is indicated (5A-4EBP). The lower band, which is a nonspecific band recognized by the antibody, indicates that equal levels of protein were loaded in each lane. (C) NIH 3T3 cells were cotransfected with the bicistronic construct carrying the full-length p27 5′-UTR and 0, 1.5, or 3 μg of expression constructs encoding either the wild-type 4E-BP-1 (4EBP-WT) or constitutively active mutant 4E-BP (4EBP-5A mut). Extracts from transfected cells were assayed for CAT and luciferase activities. All data represent the mean of a minimum of three replicates plus or minus the standard error.
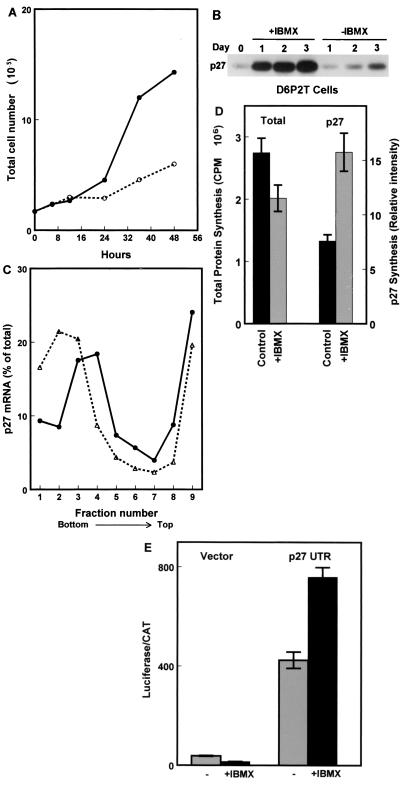
We have previously shown that when D6P2T cells are treated with reagents that elevate cyclic AMP levels, they rapidly exit the cell cycle (reference 8 and Fig. 7A). Western blotting indicates that cell cycle exit induced by the addition of isobutylmethylxanthine (IBMX) is accompanied by a striking increase in p27 protein levels (Fig. 7B). Coincident with the increase in p27 protein levels there is a shift of p27 mRNA molecules to denser polysomes, as determined by Northern blotting of fractions from polysome sucrose gradients (Fig. 7C). In addition, experiments using pulse-labeling with 35S-labeled amino acids followed by immunoprecipitation of p27 show about a twofold increase in p27 synthesis 24 h after treatment with IBMX (Fig. 7D). These experiments indicate that translational initiation of the p27 mRNA is enhanced when D6P2T cells exit the cell cycle. This occurs despite the fact that total protein synthesis is decreased by ∼27% (Fig. 7D).
FIG. 7.
Enhanced p27 5′-UTR-mediated cap-independent translation in quiescent cells. (A) D6P2T cells were induced to differentiate by the addition of IBMX to 500 μM. At various times after the addition of IBMX, the cells were harvested by trypsinization and counted. The graph shows total cell number over time in untreated (solid line) and IBMX-treated (dashed line) cultures. (B) D6P2T cells were induced to differentiate by the addition of IBMX to 500 μM. Cell extracts were prepared over a 3-day period and used to detect p27 by Western blotting. (C) D6P2T cells were left untreated or induced to differentiate by addition of IBMX to 500 μM. After 3 days, the cells were harvested for polysome gradient analysis. Northern blots of RNA isolated from each gradient fraction were used to estimate the level of p27 mRNA. The percentage of the total p27 mRNA from gradient fractions from untreated cells (●) or IBMX-treated cells (▵) was plotted. The data are from a representative experiment. (D) D6P2T cells were pulse-labeled for 1 h with 35S-amino acids in the presence or absence of IBMX. Following the pulse, p27 was immunoprecipitated, and the resulting precipitate was run on a polyacrylamide gel. The gel was then subjected to autoradiography to determine the level of newly synthesized p27. The level of total protein synthesis was estimated by trichloroacetic acid precipitation of an aliquot of the extract representing 1.4 × 105 cells prior to immunoprecipitation. (E) D6P2T cells were transfected with pGL2CAT/Luc (vector) or pGL2CAT/Luc-p27UTR and then treated with or without IBMX. The normalized luciferase activity (luciferase/CAT ratio) for the transfected cells is shown. All data represent the mean of three replicates plus or minus the standard error.
In D6P2T cells transfected with the bicistronic reporter constructs, addition of IBMX leads to enhanced cap-independent translation mediated by the p27 5′-UTR (Fig. 7E). This is indicated by a ∼2-fold increase in normalized luciferase activity (luciferase/CAT ratio) in IBMX-treated cells compared to untreated cells. The slight activity observed for the pGL2CAT/Luc vector is actually inhibited under similar conditions. Thus, the process of internal ribosome assembly within the p27 5′-UTR appears to be specifically activated as the cells stop proliferating.
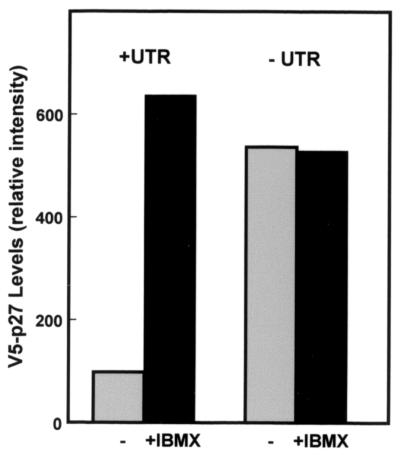
We further analyzed the activity of the 5′-UTR by expressing epitope-tagged p27 in D6P2T cells (Fig. 8). Two constructs were prepared; one retained the full 217-bp p27 5′-UTR described in the experiments above, and the other retained only 3 bp upstream of the AUG start codon. For both constructs, the 5′ end of encoded mRNAs includes a ∼115-bp sequence derived from the vector. Therefore, the only difference between the expressed sequence of the two clones is the presence or absence of 214 bp of the p27 5′-UTR in its normal position relative to the start codon. These two constructs were transfected into D6P2T cells that had been treated with or without IBMX. The construct lacking the p27 5′-UTR was expressed equally well and at high levels whether or not the cells were proliferating or had exited the cell cycle in response to IBMX. However, the construct which retained the p27 5′-UTR in its normal position relative to the start codon was expressed at significantly lower levels in proliferating cells than in IBMX-treated cells. Thus, the p27 5′-UTR in its normal context appears to limit expression in the proliferating cells but allow induction of high level expression in quiescent cells. This activity coincides with increased p27 5′-UTR-mediated cap-independent translation.
FIG. 8.
The p27 5′-UTR in its native context mediates inducible expression in D6P2T cells. Constructs encoding V5-epitope tagged p27 cDNA linked to either the first 3 nucleotides upstream of the AUG (−UTR) or the entire p27 5′-UTR (+UTR) were transfected into D6P2T cells. The cells were left untreated or treated with 500 μM IBMX. Two days after transfection, the cells were harvested and the level of V5-tagged p27 protein was determined by Western blotting with an anti-V5 antibody. The relative level of epitope-tagged p27 was determined by densitometric scanning. Data from a representative experiment of three repetitions are shown.
DISCUSSION
The results shown here demonstrate that the 5′-UTR of the p27 mRNA is capable of mediating cap-independent translation. This conclusion is supported by a number of different experimental approaches. The p27 5′-UTR supports translation of luciferase when its coding region is placed downstream of the CAT coding region in a bicistronic mRNA. This activity is not blocked by rapamycin, which specifically represses cap-dependent translation but not cap-independent translation (2, 33). Also, p27 5′-UTR-mediated cap-independent translation is not inhibited by coexpression with constitutively active 4E-BP, which binds to and inactivates eIF4E, the rate-limiting factor for cap-dependent translation (6). Finally, positioning a very stable hairpin-loop upstream of the start codon in a luciferase expression construct leads to a nearly complete inhibition of expression. This inhibition is reversed by inserting the p27 5′-UTR between the hairpin-loop and the luciferase start codon, strongly supporting the conclusion that the 5′-UTR mediates internal initiation of translation.
Using mouse T cells, Kwon et al. (17) found two major transcription start sites which mapped 200 or 252 nucleotides upstream of the AUG start codon. In contrast, using mouse kidney RNA, Zhang and Lin (41) found evidence for a major transcriptional start site ∼500 nucleotides upstream of the AUG start codon. The p27 5′-UTR used for our experiments was derived from a mouse macrophage cDNA cloned by Toyoshima and Hunter (39) and includes 217 nucleotides upstream of the AUG start codon. Therefore, the 5′ end of the sequence that we have analyzed lies between the two start sites of Kwon et al. (17). Deletion analysis has indicated that multiple elements within this 217-bp sequence contribute to IRES activity. Deletion of either 70 bases from the 5′ end or 127 bases from the 3′ end of the 5′-UTR reduces but does not eliminate IRES activity. Interestingly, the 3′ end deletion removes a polypyrimidine tract ∼40 nucleotides upstream of the start codon. A similar sequence in the human p27 mRNA has recently been shown to enhance translational initiation and to bind several proteins, including HuR, hnRNP C1, and hnRNP C2 (24). Zhao et al. (42) identified an endonuclease which is able to cleave within the HuR binding site of the human p27 5′-UTR and have shown that HuR inhibits cleavage by the endonuclease. This suggests that the polypyrimidine tract may function in message stability. It is not known whether it also affects cap-independent translation. However, our results (Fig. 4 and unpublished data) indicate that the polypyrimidine tract is not absolutely essential for IRES activity.
The importance of cap-independent translation of the p27 mRNA is that it provides a mechanism to escape the effects of global inhibition of cap-dependent translation that occur when eIF4E activity is downregulated. This occurs in response to contact inhibition, mitogen deprivation, amino acid starvation, and numerous other treatments. These are precisely the conditions in which p27 must be expressed at elevated levels in order to facilitate cell cycle arrest. By mediating internal initiation of translation, the p27 5′-UTR bypasses the need for eIF4E and the inhibitor can continue to be synthesized at significant levels.
It is possible that the p27 mRNA can be translated through both cap-dependent and cap-independent mechanisms. This is suggested by the fact that a luciferase construct containing a stable hairpin-loop upstream of the p27 5′-UTR is expressed at about 30% of the level of the same construct without the hairpin-loop. This may be because the stable hairpin-loop blocks scanning initiated from the 5′ cap, but it is also possible that the hairpin-loop influences internal initiation by affecting the structure of the 5′-UTR. If the p27 mRNA can be translated through both mechanisms, it is expected that cap-independent initiation will be the predominant mode under conditions where eIF4E activity is low.
It is very likely that modulation of p27 5′-UTR IRES activity is an important mechanism in determining the cellular levels of p27. We have shown in quiescent D6P2T cells, cap-independent translation through the 5′-UTR is specifically enhanced and may be an important factor in the observed increase in p27 protein levels. Presumably, internal ribosome entry is initiated by proteins that recognize specific sequences or structures within the p27 5′-UTR, as shown for other IRESs (12, 35, 37). Such factors would functionally replace eIF4E and eliminate the need for 5′ cap recognition. The activity of these factors may be modulated in response to differentiation signals and downregulated or inactivated in proliferating cells.
Finally, the inability to translate p27 by a cap-independent mechanism could contribute to the loss of p27 expression observed in many tumor cells. It is interesting that eIF4E is expressed at elevated levels in many tumors (5, 15, 19, 26, 28) and that overexpression of eIF4E leads to transformation of cultured cells (18, 23, 38). It is possible that high levels of eIF4E lead to sequestration of factors that are also needed for internal initiation of translation and that this contributes to inefficient synthesis of the p27 protein.
ACKNOWLEDGMENTS
We thank T. Hunter for the mouse p27 cDNA construct, R. E. Rhoads for pGL2CAT/Luc, L. Hudson for the BiP construct, and John Lawrence, Jr., for the mutant PHAS-1 construct. We thank Lew Bowman for protocols and advice for polysome gradient experiments.
This work was supported by NIH grants NS36164 (R.M.) and CA84325 (W.K.M.).
REFERENCES
- 1.Agrawal D, Hauser P, McPherson F, Dong F, Garcia A, Pledger W J. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol Cell Biol. 1996;16:4327–4336. doi: 10.1128/mcb.16.8.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beretta L, Gingras A C, Svitkin Y V, Hall M N, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 3.Clurman B E, Porter P. New insights into the tumor suppression function of P27(kip1) Proc Natl Acad Sci USA. 1998;95:15158–15160. doi: 10.1073/pnas.95.26.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahia P L, Aguiar R C, Honegger J, Fahlbush R, Jordan S, Lowe D G, Lu X, Clayton R N, Besser G M, Grossman A B. Mutation and expression analysis of the p27/kip1 gene in corticotrophin-secreting tumours. Oncogene. 1998;16:69–76. doi: 10.1038/sj.onc.1201516. [DOI] [PubMed] [Google Scholar]
- 5.De Benedetti A, Harris A L. eIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31:59–72. doi: 10.1016/s1357-2725(98)00132-0. [DOI] [PubMed] [Google Scholar]
- 6.Duncan R, Milburn S C, Hershey J W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987;262:380–388. [PubMed] [Google Scholar]
- 7.Ferrando A A, Balbin M, Pendas A M, Vizoso F, Velasco G, Lopez-Otin C. Mutational analysis of the human cyclin-dependent kinase inhibitor p27kip1 in primary breast carcinomas. Hum Genet. 1996;97:91–94. doi: 10.1007/BF00218840. [DOI] [PubMed] [Google Scholar]
- 8.Gan W, Celle M L, Rhoads R E. Functional characterization of the internal ribosome entry site of eIF4G mRNA. J Biol Chem. 1998;276:5006–5012. doi: 10.1074/jbc.273.9.5006. [DOI] [PubMed] [Google Scholar]
- 9.Haghighat A, Mader S, Pause A, Sonenberg N. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 1995;14:5701–5709. doi: 10.1002/j.1460-2075.1995.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hengst L, Reed S I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 11.Houdebine L M, Attal J. Internal ribosome entry sites (IRESs): reality and use. Transgenic Res. 1999;8:157–177. doi: 10.1023/a:1008909908180. [DOI] [PubMed] [Google Scholar]
- 12.Hunt S L, Hsuan J J, Totty N, Jackson R J. unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev. 1999;13:437–448. doi: 10.1101/gad.13.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones R M, Branda J, Johnston K A, Polymenis M, Gadd M, Rustgi A, Callanan L, Schmidt E V. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Mol Cell Biol. 1996;16:4754–4764. doi: 10.1128/mcb.16.9.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamata N, Morosetti R, Miller C W, Park D, Spirin K S, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995;55:2266–2269. [PubMed] [Google Scholar]
- 15.Kerekatte V, Smiley K, Hu B, Smith A, Gelder F, De Benedetti A. The proto-oncogene/translation factor eIF4E: a survey of its expression in breast carcinomas. Int J Cancer. 1995;64:27–31. doi: 10.1002/ijc.2910640107. [DOI] [PubMed] [Google Scholar]
- 16.Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989;9:5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon T K, Nagel J E, Buchholz M A, Nordin A A. Characterization of the murine cyclin-dependent kinase inhibitor gene p27Kip1. Gene. 1996;180:113–120. doi: 10.1016/s0378-1119(96)00416-7. [DOI] [PubMed] [Google Scholar]
- 18.Lazaris-Karatzas A, Sonenberg N. The mRNA 5′ cap-binding protein, eIF-4E, cooperates with v-Myc or E1A in the transformation of primary rodent fibroblasts. Mol Cell Biol. 1992;12:1234–1238. doi: 10.1128/mcb.12.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B D, Liu L, Dawson M, De Benedetti A. Overexpression of eukaryotic initiation factor 4E (eIF4E) in breast carcinoma. Cancer. 1997;79:2385–2390. [PubMed] [Google Scholar]
- 20.Lloyd R V, Erickson L A, Jin L, Kulig E, Qian X, Cheville J C, Scheithauer B W. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macejak D G, Sarnow P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature. 1991;353:90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- 22.Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKendrick L, Pain V M, Morley S J. Translation initiation factor 4E. Int J Biochem Cell Biol. 1999;31:31–35. doi: 10.1016/s1357-2725(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 24.Millard S S, Vidal A, Markus M, Koff A. A U-rich element in the 5′ untranslated region is necessary for the translation of p27 mRNA. Mol Cell Biol. 2000;20:5947–5959. doi: 10.1128/mcb.20.16.5947-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millard S S, Yan J S, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- 26.Miyagi Y, Sugiyama A, Asai A, Okazaki T, Kuchino Y, Kerr S J. Elevated levels of eukaryotic translation initiation factor eIF-4E, mRNA in a broad spectrum of transformed cell lines. Cancer Lett. 1995;91:247–252. [Google Scholar]
- 27.Mothe-Satney I, Yang D, Fadden P, Haystead T A, Lawrence J C., Jr Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol Cell Biol. 2000;20:3558–3567. doi: 10.1128/mcb.20.10.3558-3567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C A, Franklin S, Abreo F W, Nassar R, De Benedetti A, Williams J, Stucker F J. Expression of eIF4E during head and neck tumorigenesis: possible role in angiogenesis. Laryngoscope. 1999;109:1253–1258. doi: 10.1097/00005537-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang Q, Bommakanti M, Miskimins W K. A mitogen-responsive promoter region that is synergistically activated through multiple signalling pathways. Mol Cell Biol. 1993;13:1796–1804. doi: 10.1128/mcb.13.3.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 32.Ponce-Castaneda M V, Lee M H, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Sheinfeld J, Massague J. p27Kip1: chromosomal mapping to 12p12–12p13.1 and absence of mutations in human tumors. Cancer Res. 1995;55:1211–1214. [PubMed] [Google Scholar]
- 33.Pyronnet S, Pradayrol L, Sonenberg N. A cell cycle-dependent internal ribosome entry site. Mol Cell. 2000;5:607–616. doi: 10.1016/s1097-2765(00)80240-3. [DOI] [PubMed] [Google Scholar]
- 34.Rhoads R E. Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem. 1999;274:30337–30340. doi: 10.1074/jbc.274.43.30337. [DOI] [PubMed] [Google Scholar]
- 35.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 36.Sella O, Gerlitz G, Le S Y, Elroy-Stein O. Differentiation-induced internal translation of c-sis mRNA: analysis of the cis elements and their differentiation-linked binding to the hnRNP C protein. Mol Cell Biol. 1999;19:5429–5240. doi: 10.1128/mcb.19.8.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sizova D V, Kolupaeva V G, Pestova T V, Shatsky I N, Hellen C U. Specific interaction of eukaryotic translation initiation factor 3 with the 5′ nontranslated regions of hepatitis C virus and classical swine fever virus RNAs. J Virol. 1998;72:4775–4782. doi: 10.1128/jvi.72.6.4775-4782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith M R, Jaramillo M, Liu Y L, Dever T E, Merrick W C, Kung H F, Sonenberg N. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 1990;2:648–654. [PubMed] [Google Scholar]
- 39.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 40.Waskiewicz A J, Johnson J C, Penn B, Mahalingam M, Kimball S R, Cooper J A. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–1880. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Lin S C. Molecular characterization of the cyclin-dependent kinase inhibitor p27 promoter. Biochim Biophys Acta. 1997;1353:307–317. doi: 10.1016/s0167-4781(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Z, Chang F C, Furneaux H M. The identification of an endonuclease that cleaves within an HuR binding site in mRNA. Nucleic Acids Res. 2000;28:2695–2701. doi: 10.1093/nar/28.14.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]