Abstract
Purpose:
The study provides a comprehensive assessment of how determinants of health across demographic, psychological, mobility-related, health, environmental, and economic domains are associated with the diagnosis of osteoporosis and tests three hypotheses, including: 1) a diverse set of variables across domains will predict osteoporosis, 2) chronic inflammation as a result of stress (represented by high-sensitivity C-reactive protein) will not be associated with osteoporosis, and 3) the model developed will have high accuracy in predicting osteoporosis.
Methods:
Logistic regression and Cox proportional hazards models of osteoporosis diagnosis were estimated using data from 14,792 and 13,169 participants (depending on model) in the 2012–2016 waves of the Health and Retirement Study, including the Biomarker Study, the Contextual Data Resource, and validated measures of childhood socio-economic status. Predictive accuracy was assessed using k-Nearest Neighbors Discriminant Analysis.
Results:
Demographic, environmental, and health-related factors were associated with osteoporosis diagnosis, and predictive accuracy of the models was good. High-sensitivity C-reactive protein was not associated with osteoporosis diagnosis.
Conclusion:
Social determinants identified indicate access to health care, inequalities in the greater social environment (e.g., access to resources), and overall health (i.e., underlying medical conditions) are key components for developing osteoporosis and indicate underlying health inequities in this sample. There is a need to further address the interplay between primary health care and social determinants of health.
Keywords: social determinants of health, bone health, biomarkers, longitudinal analysis, aging
Mini Abstract
Chronic stress from social/environmental pressures has been proposed to affect bone health through increased inflammation. We demonstrate that inflammation from prolonged stress does not cause changes to bone health through inflammation but instead impacts access to health care, social inequalities, and overall health, which in turn impact bone health.
Importance of Understanding Osteoporosis Determinants
Osteoporosis leads to more than 2 million factures in the United States each year [1], and older women (50+) are particularly susceptible. These fractures lead to morbidity, mortality, and increased health care spending [1]. The annual cost related to fractures in the United States was estimated at 57 billion dollars for 2018 and is forecasted to rise to 95 billion dollars by the year 2040 [2]. These costs may be further increased by individuals impacted by accelerated aging. Therefore, it is important from economic and quality of life perspectives to identify and quantify the risk factors that affect osteoporosis in order to implement preventative measures and reduce the risk of developing disease.
Accelerated aging is associated with the development of advanced age-related diseases [3], such as cardiovascular disease (CVD) [4]. Aging and accelerated aging are linked to increased inflammation, which leads to the development of advanced age-related disease and/or mobility limitations in a process known as “inflammaging” [5]. More specifically, increased inflammation as measured through high sensitivity C-reactive protein (hs-CRP) has been linked to increased risk for CVD and osteoporosis [6], although findings have been mixed on the nature of this latter relationship, as others have reported increased hs-CRP as protective of bone mineral density [BMD; 7]. Our study investigates determinants across six domains (demographic, psychological, mobility-related, health, environmental, and economic) that are associated with increased inflammation and/or the development of osteoporosis, using a conceptual model derived from the social epidemiological literature (Fig. 1). Our approach is relatively unique, incorporating a wide range of contexts to examine bone health.
Fig. 1.
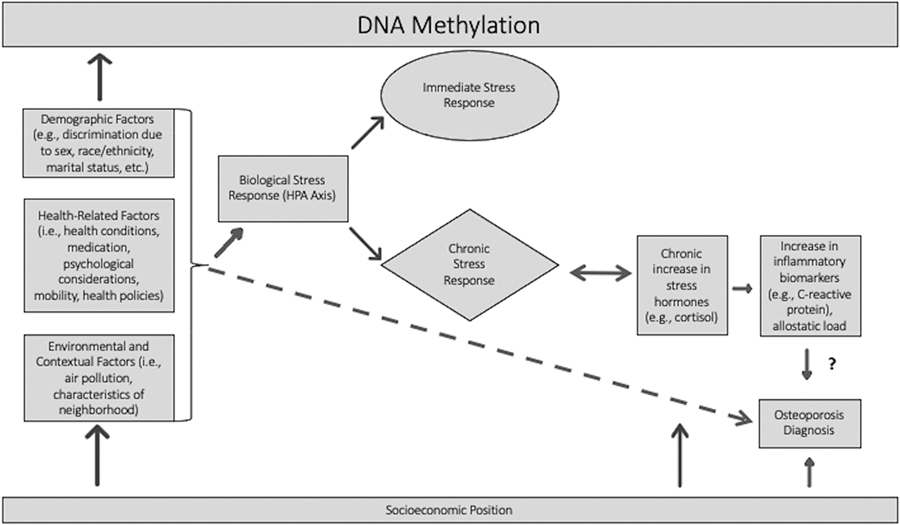
Conceptual Model derived from Barr [8], Gough and Godde [9], Kubzansky [4], and Riancho and Brennan-Olsen [10]
The underlying premise behind this conceptual model is that various social and environmental factors can lead to biological changes in the body that then have cascading consequences. For example, social position and social inequality are posited to promote chronic stress exposure [8]. Similarly, social adversity is hypothesized to influence health and disease through social and physical environments, behaviors, and psychosocial stress and cognitive processes [4]. These mechanisms may then provoke a biological response that eventually leads to health problems or disease [4]. Barr [8] describes how the experience of stress from a variety of sources causes a hypothalamic-pituitary-adrenal (HPA) axis response. This reaction can lead to both acute and chronic stress responses in the body, the combination of which is considered allostatic load. A rise in allostatic load then can lead to a chronic increase in stress hormones, which precipitate physiologic injury, such as an increase in inflammatory biomarkers [8].
The final step in the conceptual figure is the link to osteoporosis diagnosis. Riancho and Brennan-Olsen [10] conceptualize osteoporotic fracture risk as an outcome of lifestyle, risk factors, and the aforementioned inflammatory state that arises from the chronic stress response. Thus, theoretically we might expect to see direct effects of some exposure mechanisms on risk of osteoporosis, along with indirect effects operating through inflammatory processes, the consequences of which can be measured through inflammatory biomarkers. However, recent empirical research draws into question the existence of a true causal link between inflammatory biomarkers and osteoporosis, as opposed to a confounding relationship [11–13].
In the osteoporosis literature, there is a lack of research examining associations between mobility measures, long-term exposure to pollutants, childhood socioeconomic status, and allostatic load. Here, we endeavor to estimate the role of six domains (demographic, psychological, mobility-related, health, environmental, and economic) in the development of osteoporosis, along with more commonly investigated risk factors. We hypothesize that: 1. A wide variety of variables across all domains will be predictive of osteoporosis diagnosis; 2. Net of the factors tested in Hypothesis 1, and consistent with recent research, chronic inflammation (as a result of prolonged stress), as measured by hs-CRP, will not be associated with increased risk of osteoporosis diagnosis; and 3. After accounting for variables across all domains, the model will have a strong capacity to predict osteoporosis diagnosis.
Data and Methods
Data
The data come from the 2012–2016 waves of the Health and Retirement Study (HRS) (the years for which osteoporosis diagnosis is available). We combine data from the RAND Longitudinal File1 with additional variables from the Core Interviews (including the osteoporosis indicator) and data from the sensitive health Biomarker studies, the restricted Contextual Data Resource (CDR), and validated measures of childhood socio-economic status [14]. The RAND HRS Longitudinal file is data derived from all waves of the HRS. It contains cleaned and processed variables, that are largely harmonized across waves, from the Core and Exit Interviews of the HRS. RAND-developed variables cover demographics, health insurance, family structure, health, mobility, economic, and other factors [15, 16]. In each wave half of the sample has biomarker data collected for the Biomarker Study. For example, one half had biomarker data collected in 2012 and the other half in 2014; 2012–2016 Biomarker Study data are used. Finally, we include data from the CDR to provide contextual information. We use data from the Decennial Census and American Community Survey data files [17], the EPA-derived pollution files [18, 19], and the Uniform Crime Report files [20]. The sample includes all respondents between the ages of 50 and 90 for whom data are available (N = 14,792 [model without hs-CRP] and 13,169 [model with hs-CRP]). The research in this paper was determined to be exempt by the University of La Verne Institutional Review Board.
Variables
The dependent variable is osteoporosis diagnosis, which is a binary variable taking a value of 1 if the respondent reports being told by a doctor that they have osteoporosis and 0 otherwise. The independent variables were identified based on theory and prior research and then narrowed down using a combination of Spearman correlations and change-in estimate variable selection methods [21]. The change-in estimate method evaluates the change in the main predictor variable’s coefficient, and variables that impact it by 10% or more (which are confounders) are retained in the model. This approach has been shown to be excellent at identifying confounders [21], which were an important part of our modeling process. The variables selected from this routine are discussed, below.
Demographic and socioeconomic variables include age (continuous), sex (binary male/female), race and ethnicity (1-White, 2-Black, 3- Some other race/ethnicity, the latter of which could not be disaggregated due to sample size), household income (continuous), and mother’s education level (continuous; in years). These come from the RAND Longitudinal file, with the exception of race and ethnicity, which also incorporates the HRS Restricted Race data. In addition, the Cox proportional hazards model incorporates marital status (measured as: married/cohabiting, separated/divorced, widowed, never married), and education (measured as: less than high school, GED, high school graduate, some college, and college or more). The Cox model also contains two variables specific to the respondent’s childhood: the number of household adults [measured as lived with both parents (biological) = 0, lived with both parents (one biological) = .1, had both parents but did not live together = .2, did not have both parents = .3; , 14], and maternal investment [measured effort, teaching, and attention by mother; , 14], which represents the quality of the respondent’s relationship with their mother [14].
To understand which potential health-related factors could be predictors for osteoporosis, health behaviors and health condition variables were obtained from the RAND Longitudinal data and Biomarker study data. For mental health, we use the respondent’s score on the Center for Epidemiological Studies Depression Scale that measures depressive symptoms (continuous) and a question asking if the respondent has psychological problems (0- No, 1- Yes). For health behaviors, the respondent’s alcohol consumption was measured through the number of alcoholic drinks a respondent consumed on a given day. Respondents were also asked about current or previous health problems measured categorically: heart problems (0- No, 1- Yes) and arthritis (0-No, 1-Yes). Health characteristics include the respondent’s weight (recorded in kilograms) and several measures that collectively represent a measure of allostatic load. Allostatic load is measuring using an index created by the authors, and partially based on McCrory et al. [22], that ranges from 0–8. A value of 1 is assigned when the respondent has a value greater than the 75th percentile for each of the following variables: systolic blood pressure, diastolic blood pressure, pulse, total cholesterol, hs-CRP, A1c, Cystatin C; or has waist circumference greater than 35 inches for women or 40 inches for men. We then sum across each of these to get the index score. To measure access to health care, we include whether the respondent is covered by Medicare (0- No, 1- Yes). Finally, to evaluate overall health, a measure of the number of times respondents spent the night in the hospital over the prior two years is used. Cox models also incorporate a measure of self-reported health (measured continuously, ranging from excellent to poor) and a categorical measure of diabetes created by the authors (0= no diabetes, 1=diabetes with medication, 2=diabetes with no medication).
Mobility and measures of activity were obtained from the RAND Longitudinal dataset. Most of these variables are measured continuously as indices. The Activities of Daily Living Index summarizes the respondent’s difficulty with performing daily tasks such as bathing, eating, dressing, walking across a room, and getting in or out of bed. The Instrumental Activities of Daily Living Index summarizes the respondent’s difficulty with using a telephone, taking medication, and handling money. The Change in Gross Motor Skills Index is the change in the respondent’s difficulty with walking one block, walking across the room, climbing one flight of stairs, and bathing. The following mobility variables are measured categorically: the respondent’s difficulty with sitting for two hours (0- No, 1- Yes) and difficulty getting up from a chair (0- No, 1- Yes). Cox models also include the Mobility Index (index of difficulty walking one block, walking several blocks, walking across a room, climbing one flight of stairs, and climbing several flights of stairs) and the Large Muscle Index (index of difficulty sitting for two hours, getting up from a chair, stooping or kneeling and crouching, and pushing or pulling a large object).
Environmental factors span measures of demographic, air pollution, and crime data for the respondent’s area in which they lived at the time of data collection. The first measure is the percent of non-Hispanic Black/African American residents living in the respondent’s county; this comes from the US Decennial Census and American Community Survey component of the CDR. The percent of non-Hispanic White residents living in the respondent’s county is also included. The following measures come from HRS-CDR Pollution: Mean O3 (ozone) for April, Mean O3 for November, and Mean PM 2.5 for March. The Cox model also includes Mean O3 for the second (April-June) and third (July-September) quarters, Mean PM 2.5 for February, and the annual count of rapes by county, which comes from the HRS-CDR Uniform Crime Report data.
To look at inflammation’s relationship to osteoporosis, some models include measures of hs-CRP; hs-CRP is measured in two ways: high-low hs-CRP and high-medium-low hs-CRP. High hs-CRP is defined as hs-CRP greater than 3 mg/dl [e.g., 23]. In the tri-level version of hs-CRP, low hs-CRP is less than 1 mg/dl and medium hs-CRP is between 1 mg/dl and 3 mg/dl [24]. Values of hs-CRP greater than 10 mg/dl are dropped, as they indicate probable underlying medical conditions [25].
Analytic Strategy
Two statistical approaches were employed to examine the six variable domains’ associations with osteoporosis. Because osteoporosis diagnosis is a binary outcome variable, we estimate logistic regression models, which provide odds ratios for a positive osteoporosis diagnosis for each predictor variable. The logit was evaluated with McFadden’s Pseudo R2. To examine age-dependency of a positive osteoporosis diagnosis, we also estimate Cox proportional hazards models. Cox regression is a semi-parametric survival analysis that estimates the hazard of a positive osteoporosis diagnosis for each covariate. Age at survey, irrespective of age at which osteoporosis was diagnosed, is the time scale variable. As these are longitudinal panel data, we include a variable to denote wave. The Cox model calculates the cumulative hazard for receiving a positive osteoporosis diagnosis at a specific age (time t) as shown in Equation 1:
| (1) |
In this equation h(t) is the expected hazard at a certain age and h(t0) is the baseline at birth (when all covariates are zero). The proportionality assumption of the Cox model that assumes the covariates’ effect on survival (i.e., living without osteoporosis) is constant over time was evaluated using a test of the Schoenfeld residuals. Both the logit and Cox models were examined for multicollinearity via the variance inflation factor (VIF). Kaplan-Meier survival plots were also produced and log-rank tests examined sex differences within osteoporosis diagnosis status.
The HRS uses a complex survey sampling design, so we estimate our models using appropriate survey sampling methodology (sample stratum, sample PSU, and weights), instituting the svy commands in Stata 16.0 and the survey package [26] in R [27]. Missing data is addressed using listwise deletion for those variables with small amounts of missing data. Finally, we use k-Nearest Neighbors Discriminant Analysis (k-NNDA, k=3), which is a nonparametric machine learning algorithm that sorts observations into clusters utilizing neighboring data values and using Mahalanobis distances derived from the pooled covariance matrix. k-NNDA assessed the prediction accuracies of the independent variables selected in the logit and Cox models using cross-validation [28] in SAS 9.4.
Results
The original sample prior to application of statistical modeling, less missing strata and zero weights, was 18,900 (Supplementary Information Table 1). Survey-weighted descriptive statistics for the analytic sample are shown in Table 1. Approximately 8% of the sample reported an osteoporosis diagnosis. About 38% of the sample has moderate hs-CRP levels, while 26% has high hs-CRP levels. More than 80% of the sample identifies as White, with 10% identifying as Black/African American, and 6% identifying as some other race/ethnicity. The survey-weighted descriptive statistics for the Kaplan-Meier plots and log-rank tests can be found in Supplementary InformationTable 1.
TABLE 1.
Descriptive Statistics for Analytic Sample without and with hs-CRP
| Without hs-CRP |
With hs-CRP |
|||
|---|---|---|---|---|
| Variable | Mean (SE)/ Proportion | N | Mean (SD)/ Proportion | N |
| Osteoporosis- Yes | 0.078 | 14,792 | 0.078 | 13,169 |
| Osteoporosis- No | 0.922 | 14,792 | 0.922 | 13,169 |
| Sex- Female | 0.512 | 14,792 | 0.508 | 13,169 |
| Age | 65.673 (0.261) | 14,792 | 65.705 (0.272) | 13,169 |
| Race/Ethnicity | ||||
| Black/African American | 0.096 | 14,792 | 0.088 | 13,169 |
| Another Race/Ethnicity | 0.063 | 14.729 | 0.063 | 13,169 |
| Respondent’s Mother’s Education Level | 10.688 (0.091) | 14,792 | 10.715 (0.088) | 13,169 |
| Total Household Income | 92040.81 (2711.999) | 14,792 | 94702.72 (2881.841) | 13,169 |
| Center for Epidemiological Studies Depression Scale | 1.267 (0.025) | 14,792 | 1.213 (0.024) | 13,169 |
| Psychological Problems- Yes | 0.193 | 14,792 | 0.189 | 13,169 |
| # of Alcoholic Drinks | 0.954 (0.024) | 14,792 | 0.965 (0.024) | 13,169 |
| Heart Problems- Yes | 0.227 | 14,792 | 0.221 | 13,169 |
| Arthritis- Yes | 0.570 | 14.792 | 0.562 | 13,169 |
| Weight in Kilograms | 83.64 (0.238) | 14,792 | 82.806 (0.231) | 13,169 |
| Allostatic Load | 2.168 (0.021) | 14,792 | 2.122 (0.022) | 13,169 |
| Number of times respondent spent night in the hospital | 0.388 (0.012) | 14,792 | 0.359 (0.011) | 13,169 |
| Covered by Medicare- Yes | 0.507 | 14,792 | 0.502 | 13,169 |
| Activities of Daily Living Index | 0.236 (0.008) | 14,792 | 0.211 (0.008) | 13,169 |
| Instrumental Activities of Daily Living Index | 0.095 (0.005) | 14,792 | 0.088 (0.004) | 13,169 |
| Change in Gross Motor Skills Index | 0.075 (0.007) | 14,792 | 0.069 (0.008) | 13,169 |
| Difficulty Sitting for Two Hours- Yes | 0.183 | 14,792 | 0.174 | 13,169 |
| Difficulty Getting Up from a Chair- Yes | 0.353 | 14,792 | 0.339 | 13,169 |
| Mean Ozone for April | 46.086 (0.160) | 14,792 | 46.115 (0.162) | 13,169 |
| Mean Ozone for November | 31.518 (0.144) | 14,792 | 31.536 (0.145) | 13,169 |
| Mean PM 2.5 for March | 8.693 (0.070) | 14,792 | 8.690 (0.071) | 13,169 |
| Proportion Non-Hispanic Black | 0.119 | 14,729 | 0.118 | 13,169 |
| Proportion Non-Hispanic White | 0.683 | 14,729 | 0.687 | 13,169 |
| Wave 11 | 0.371 | 14,792 | 0.374 | 13,169 |
| Wave 12 | 0.371 | 14,792 | 0.320 | 13,169 |
| Wave 13 | 0.313 | 14,792 | 0.305 | 13,169 |
| High sensitivity C-reactive protein (mg/dl) | ||||
| Moderate: greater than 1–3 | 0.377 | 13,169 | ||
| High: greater than 3 | 0.257 | 13,169 | ||
Results for the logit models are shown in Table 2. Model 1 (without hs-CRP as a predictor) has a McFadden’s pseudo R2 value of 0.19, indicating a very good model fit [29]. Examining demographic and economic factors first, the results show that female respondents have a much higher odds ratio for reporting an osteoporosis diagnosis. By contrast, individuals identifying as Black/African American have a much lower odds ratio for reporting an osteoporosis diagnosis compared to those reporting a White race/ethnicity. All other demographic variables and the only economic variable (total household income) are confounders.
TABLE 2.
Logistic regression models of osteoporosis diagnosis
McFadden Pseudo R2: 0.19
| Model 1: No hs-CRP (N=14,792) |
Model 2: With binary hs-CRP (N=13,169) | Model 3: With tri-level hs-CRP (N=13,169) | ||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p |
| Sex- Female | 5.8885 (4.5990, 7.5395) | <0.001 | 6.3028 (4.888, 8.1272) | <0.001 | 6.2714 (4.8608, 8.0914) | <0.001 |
| Age | 1.0062 (0.9943, 1.0183) | 0.3158 | 1.0049 (0.9931, 1.0168) | 0.4270 | 1.0048 (0.993, 1.0167) | 0.4344 |
| Race/Ethnicity | ||||||
| Black/African American | 0.6151 (0.4598, 0.8230) | 0.0027 | 0.6272 (0.4546, 0.8653) | 0.0081 | 0.6279 (0.4548, 0.867) | 0.0086 |
| Another Race/Ethnicity | 0.8758 (0.6268, 1.2238) | 0.4432 | 0.9099 (0.6568, 1.2605) | 0.5743 | 0.9095 (0.6565, 1.26) | 0.5731 |
| Respondent’s Mother’s Education Level | 0.9992 (0.9802, 1.0186) | 0.9356 | 0.9993 (0.9785, 1.0206) | 0.9492 | 0.9995 (0.9788, 1.0207) | 0.9649 |
| Total Household Income | 0.9999 (0.9999, 1.0000) | 0.3672 | 0.9999 (0.9999, 1.0000) | 0.2713 | 0.9999 (0.9999, 1.0000) | 0.2780 |
| Center for Epidemiological Studies Depression Scale | 1.0485 (1.0124, 1.0859) | 0.0126 | 1.0518 (1.0101, 1.0953) | 0.0208 | 1.0519 (1.0103, 1.0952) | 0.0206 |
| Psychological Problems- Yes | 1.3159 (1.0894, 1.5894) | 0.0079 | 1.3508 (1.1056, 1.6504) | 0.0064 | 1.3517 (1.107, 1.6506) | 0.0062 |
| # of Alcoholic Drinks | 0.9614 (0.8938, 1.0342) | 0.2994 | 0.9242 (0.8574, 0.9961) | 0.0484 | 0.9245 (0.8575, 0.9967) | 0.0502 |
| Heart Problems- Yes | 1.062 (0.8708, 1.2953) | 0.5569 | 1.0576 (0.8611, 1.299) | 0.5973 | 1.0575 (0.8611, 1.2986) | 0.5979 |
| Arthritis- Yes | 2.1600 (1.7527, 2.6619) | <0.001 | 2.1054 (1.7047, 2.6002) | <0.001 | 2.1041 (1.7044, 2.5976) | <0.001 |
| Weight in Kilograms | 0.9821 (0.9772, 0.9869) | <0.001 | 0.9809 (0.9752, 0.9866) | <0.001 | 0.9806 (0.9749, 0.9865) | <0.001 |
| Allostatic Load | 0.8810 (0.8331, 0.9317) | <0.001 | 0.8901 (0.8372, 0.9464) | <0.001 | 0.8883 (0.835, 0.945) | <0.001 |
| Number of Times Respondent Spent Night in the Hospital | 1.0954 (1.0384, 1.1555) | 0.0022 | 1.1173 (1.0512, 1.1876) | <0.001 | 1.1173 (1.0513, 1.1874) | 0.0013 |
| Covered by Medicare- Yes | 1.4085 (1.0336, 1.9193) | 0.0381 | 1.4104 (1.0503, 1.894) | 0.0297 | 1.4128 (1.0527, 1.8961) | 0.0289 |
| Activities of Daily Living Index | 1.2080 (1.1092, 1.3157) | <0.001 | 1.2027 (1.0855, 1.3326) | <0.001 | 1.2036 (1.0868, 1.3329) | 0.0014 |
| Instrumental Activities of Daily Living Index | 0.7910 (0.6577, 0.9512) | 0.0185 | 0.7495 (0.6122, 0.9177) | 0.0092 | 0.7504 (0.6133, 0.9183) | 0.0094 |
| Change in Gross Motor Skills Index | 0.9988 (0.9094, 1.0969) | 0.9799 | 1.0118 (0.9065, 1.1294) | 0.8351 | 1.0115 (0.9061, 1.1292) | 0.8397 |
| Difficulty Sitting for Two Hours- Yes | 1.0690 (0.8860, 1.2897) | 0.4918 | 1.0729 (1.0499, 1.0963) | 0.5284 | 1.0707 (0.8628, 1.3285) | 0.5404 |
| Difficulty Getting Up from a Chair- Yes | 1.3320 (1.1168, 1.5887) | 0.0033 | 1.3640 (1.1219, 1.6583) | <0.001 | 1.3626 (1.1213, 1.6558) | 0.0043 |
| Mean 03 for April | 1.0312 (1.0086, 1.0543) | 0.0108 | 1.0355 (1.0121, 1.0594) | 0.0056 | 1.0354 (1.0121, 1.0594) | 0.0058 |
| Mean 03 for November | 1.0059 (0.9857, 1.0265) | 0.5718 | 1.0022 (0.9838, 1.021) | 0.8168 | 1.0023 (0.9839, 1.0211) | 0.8082 |
| Mean PM2.5 for March | 0.9858 (0.9515, 1.0212) | 0.4330 | 0.9828 (0.9466, 1.0204) | 0.3721 | 0.9834 (0.9473, 1.0208) | 0.3871 |
| Percent Non-Hispanic Black in County | 1.1124 (0.5620, 2.2016) | 0.7619 | 0.8575 (0.4434, 1.6584) | 0.6513 | 0.8550 (0.4421, 1.6536) | 0.6453 |
| Percent Non-Hispanic White in County | 1.0742 (0.8195, 1.4081) | 0.6081 | 1.0856 (0.8338, 1.4133) | 0.5467 | 1.0842 (0.8331, 1.4110) | 0.5521 |
| Wave | 0.5449 (0.4985, 0.5957) | <0.001 | 0.5531 (0.4997, 0.6122) | <0.001 | 0.5537 (0.5006, 0.6125) | <0.001 |
| High-Sensitivity C-reactive Protein (mg/dl) - Greater than 3 | 0.9056 (0.7161, 1.1453) | 0.4147 | ||||
| High-Sensitivity C-reactive Protein (mg/dl) | ||||||
| Greater than 1 – 3 | 1.0549 (0.8996, 1.2370) | 0.5157 | ||||
| Greater than 3 | 0.9357 (0.7239, 1.2097) | 0.6163 | ||||
Turning next to mental/physical health/health behaviors/medical coverage, having a higher score on the CESD (indicative of worse depressive symptoms) and reporting psychological problems are associated with higher odds of an osteoporosis diagnosis. Further, if the respondent reported having arthritis they also have significantly increased odds of reporting an osteoporosis diagnosis. Higher weight in kilograms and higher allostatic load are associated with a lower odds ratio for reporting an osteoporosis diagnosis; the significance of allostatic load is probably driven by the inclusion of waist circumference in the index. Conversely, a greater number of hospital stays in the prior two years is associated with a higher odds ratio for reporting an osteoporosis diagnosis, as is Medicare coverage compared to no Medicare coverage. All other health/health behavior variables are confounders.
Focusing on the mobility variables, a higher score on the ADL index (indicating greater difficulty with ADLs) is associated with a higher odds ratio for reporting an osteoporosis diagnosis, while a higher IADL score yields a lower odds ratio for reporting an osteoporosis diagnosis. Difficulty getting up from a chair on one’s own increases odds of reporting an osteoporosis diagnosis. The remaining mobility variables are confounders.
We also examine contextual variables related to where the respondent lived. A higher level of ozone exposure in April is associated with increased risk of reporting an osteoporosis diagnosis. Other contextual variables, including mean ozone for November, mean PM 2.5 for March, percent non-Hispanic Black in the respondent’s county, and percent non-Hispanic White in the respondent’s county are confounders. The k-NNDA accuracy of the same independent variables is 77.31%.
Models 2 and 3 present results with binary hs-CRP and tri-level hs-CRP as predictors. The hs-CRP variables are not significant predictors of osteoporosis in either model, contrary to some expectations in the literature, but in support of recent research and our second hypothesis. The k-NNDA accuracies are 77.06% and 76.93%, respectively.
Results for the Cox models are shown in Table 3. In Model 4, without CRP as a predictor, females have a higher hazard ratio than males, consistent with the logit models. Identifying as a member of a race/ethnicity other than White or Black/African American is associated with a higher hazard ratio of reporting osteoporosis diagnosis. For education, compared to having less than a high school education, those with more education have an increased hazard of reporting an osteoporosis diagnosis. In terms of family background variables, having a good quality relationship with one’s mother is associated with a reduced hazard ratio for an osteoporosis diagnosis. Compared to being married or cohabiting, widowed respondents have a lower hazard of reporting an osteoporosis diagnosis. Family structure during childhood (number of adults in the household) is a confounder of osteoporosis diagnosis.
TABLE 3.
Cox proportional hazards model results with osteoporosis diagnosis as the outcome variable and age-at-wave the time scale
| Model 1: No hs-CRP (N=11,206) |
Model 2: With binary hs-CRP (N=10,024) | Model 3: With tri-level hs-CRP (N=10,024) | ||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Sex- Female | 7.4335 (5.8046, 9.5196) | <0.0001 | 8.2340 (6.2223, 10.9121) | <0.0001 | 8.2400 (6.2174, 10.9206) | <0.0001 |
| Race/Ethnicity | ||||||
| Black/African American | 0.7781 (0.5479, 1.1051) | 0.1612 | 0.7975 (0.5519, 1.1523) | 0.2282 | 0.7969 (0.5517, 1.1511) | 0.2263 |
| Another Race/Ethnicity | 1.4309 (1.0529, 1.9446) | 0.0221 | 1.4351 (1.0609, 1.9411) | 0.0191 | 1.4343 (1.0606, 1.9397) | 0.0192 |
| Education Level | ||||||
| GED | 1.9150 (1.2773, 2.8709) | 0.0017 | 1.8493 (1.2136, 2.8179) | 0.0042 | 1.8452 (1.2107, 2.8123) | 0.0044 |
| High School Grad | 1.3288 (1.0429, 1.6931) | 0.0214 | 1.2389 (0.9604, 1.5981) | 0.0992 | 1.2375 (0.9575, 1.5995) | 0.1034 |
| Some College | 1.6164 (1.2679, 2.0607) | 0.0001 | 1.4960 (1.1716, 1.9102) | 0.0012 | 1.4947 (1.1687, 1.9115) | 0.0014 |
| College and Above | 1.6601 (1.2948, 2.1285) | <0.0001 | 1.5325 (1.1911, 1.9718) | 0.0009 | 1.5297 (1.1864, 1.9725) | 0.0010 |
| Marital status | ||||||
| Separated/Divorced | 0.9853 (0.8058, 1.2048) | 0.8850 | 0.9604 (0.7624, 1.2098) | 0.7318 | 0.9610 (0.7621, 1.2118) | 0.7365 |
| Widowed | 0.4929 (0.4297, 0.5654) | <0.0001 | 0.4710 (0.4054, 0.5471) | <0.0001 | 0.4710 (0.4056, 0.5470) | <0.0001 |
| Never Married | 0.7715 (0.5001, 1.1902) | 0.2409 | 0.7592 (0.4919, 1.1717) | 0.2135 | 0.7592 (0.4920, 1.1715) | 0.2132 |
| Family Structure: Number of Household Adults during Childhood | 1.7114 (0.9776, 2.9959) | 0.0600 | 1.6540 (0.9299, 2.9419) | 0.0868 | 1.6558 (0.9319, 2.9422) | <0.0001 |
| Maternal Investment | 0.6876 (0.5830, 0.8110) | <0.0001 | 0.7031 (0.5943, 0.8319) | <0.0001 | 0.7029 (0.5940, 0.8318) | <0.0001 |
| Self-Reported Health | 1.1055 (1.0237, 1.1938) | 0.0105 | 1.0916 (0.9996, 1.1920) | 0.0512 | 1.0917 (0.9997, 1.1921) | 0.0511 |
| Center for Epidemiological Studies Depression Scale | 1.0813 (1.0467, 1.1171) | <0.0001 | 1.0881 (1.0489, 1.1287) | <0.0001 | 1.0877 (1.0484, 1.1286) | <0.0001 |
| Diabetes | ||||||
| Yes and take medication | 0.6147 (0.4830, 0.7823) | <0.0001 | 0.5913 (0.4542, 0.7696) | <0.0001 | 0.5916 (0.4548, 0.7694) | <0.0001 |
| Yes and do not take medication | 1.0074 (0.7842, 1.2942) | 0.9541 | 1.0897 (0.8180, 1.4516) | 0.5571 | 1.0885 (0.8147, 1.4542) | 0.5664 |
| Arthritis: Yes | 1.3219 (1.0769, 1.6227) | 0.0076 | 1.3177 (1.0724, 1.6191) | 0.0086 | 1.3177 (1.0726, 1.6188) | 0.0086 |
| # of Alcoholic Drinks | 1.0340 (0.9389, 1.1386) | 0.4963 | 1.0290 (0.9306, 1.1379) | 0.5779 | 1.0291 (0.9309, 1.1377) | 0.5759 |
| Mobility Index | 0.9538 (0.8925, 1.0193) | 0.1634 | 0.9549 (0.8901, 1.0243) | 0.1969 | 0.9549 (0.8902, 1.0244) | 0.1973 |
| Large Muscle Index | 1.0635 (0.9930, 1.1391) | 0.0783 | 1.0672 (0.9923, 1.1476) | 0.0801 | 1.0676 (0.9927, 1.1481) | 0.0780 |
| Activities of Daily Living Index | 0.9993 (0.9099, 1.0974) | 0.9881 | 0.9614 (0.8604, 1.0742) | 0.4864 | 0.9612 (0.8604, 1.0737) | 0.4838 |
| Mean O3 for Second Quarter (April-June) | 1.0344 (1.0133, 1.0559) | 0.0013 | 1.0381 (1.0170, 1.0597) | 0.0004 | 1.0381 (1.0172, 1.0595) | 0.0003 |
| Mean O3 for Third Quarter (July - September) | 0.9890 (0.9736, 1.0046) | 0.1673 | 0.9868 (0.9712, 1.0026) | 0.1009 | 0.9869 (0.9715, 1.0025) | 0.1010 |
| Mean PM 2.5 February | 0.9731 (0.9477, 0.9992) | 0.0429 | 0.9716 (0.9475, 0.9963) | 0.0249 | 0.9715 (0.9473, 0.9964) | 0.0249 |
| Annual Count of Rapes by County | 1.0001 (0.9999, 1.0002) | 0.1365 | 1.0001 (0.9999, 1.0002) | 0.0689 | 1.0001 (0.9999, 1.0003) | 0.0686 |
| Wave | 0.5591 (0.0539, 0.6204) | <0.0001 | 0.5618 (0.5440, 0.5802) | <0.0001 | 0.5617 (0.4980, 0.6335) | <0.0001 |
| High-Sensitivity C-reactive Protein (mg/dl) - Greater than 1–3 | 0.8814 (0.7187, 1.0808) | 0.2130 | ||||
| High-Sensitivity C-reactive Protein (mg/dl) | ||||||
| Greater than 1 – 3 | 0.9818 (0.8409, 1.1462) | 0.8156 | ||||
| Greater than 3 | 0.8727 (0.6936, 1.0980) | 0.2451 | ||||
For health-related variables, worse self-reported health is associated with a greater hazard of reporting an osteoporosis diagnosis, as is a higher CESD score, the latter of which is consistent with the logit model results. Respondents who reported a diabetes diagnosis and taking medication for their diabetes have a lower hazard ratio for osteoporosis diagnosis than those without a diabetes diagnosis. Consistent with the logit models, arthritis displays an increased hazard of reporting an osteoporosis diagnosis. Other health- and mobility-related variables, including number of alcoholic drinks, the mobility index, the large muscle index, and ADLs are confounders of osteoporosis.
Finally, for the variables describing the context in which the respondent lived, similar to the logit model results, ozone exposure for the second quarter (April-June) generates a higher hazard ratio for reporting an osteoporosis diagnosis, while PM 2.5 for February is associated with a lower hazard. Other variables related to the context in which the respondent lived, including mean ozone for the third quarter (July-September), and the annual count of rapes at the county level are confounders. The prediction accuracy from k-NNDA is slightly lower than the logit models: 74.33%.
Models 5 and 6 present results with binary and tri-level hs-CRP as predictors. hs-CRP is not a significant predictor of reporting an osteoporosis diagnosis in either model. However, controlling for CRP does modestly attenuate the results for a few variables. The VIF for all models was less than 10. k-NNDA accuracy is 74.09% and 74.12%, respectively.
The Kaplan-Meier survivorship plots (Figs. 2 & 3) show that in the sample of respondents without osteoporosis, females live longer after about the age of 60 (Fig. 2). Alternatively, in the sample with osteoporosis, males live dramatically longer than females, starting at around age 55 (Fig. 3). In comparison to one another, the sample with osteoporosis displays increased survivorship over the sample without osteoporosis, starting at age 55 in both sexes (Figs. 2 & 3). Log rank tests indicate these trends between the sexes are significant (Fig. 2: χ= 80.6596, p-value = <0.0001; Fig. 3: χ2 = 305.9969, p-value = <0.0001).
Fig. 2.
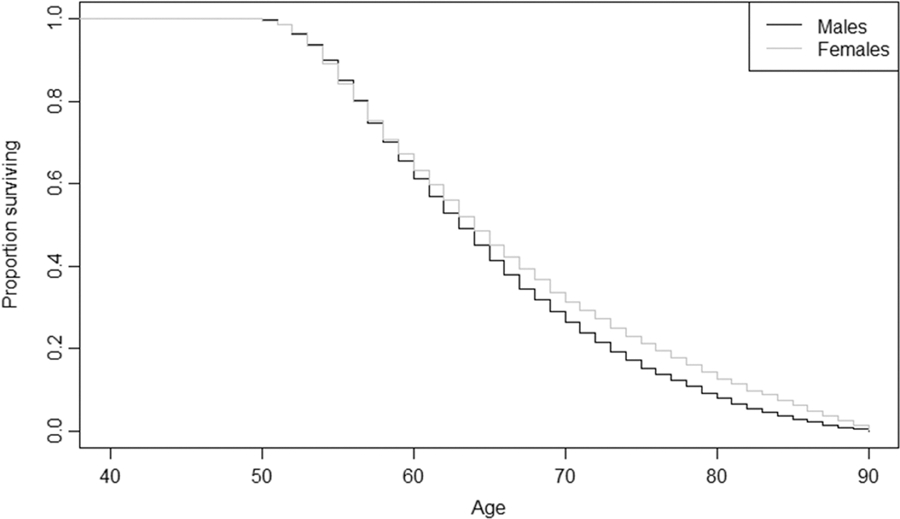
Kaplan-Meier Survivorship Plot for Sex Differences in Respondents without Osteoporosis
Fig. 3.
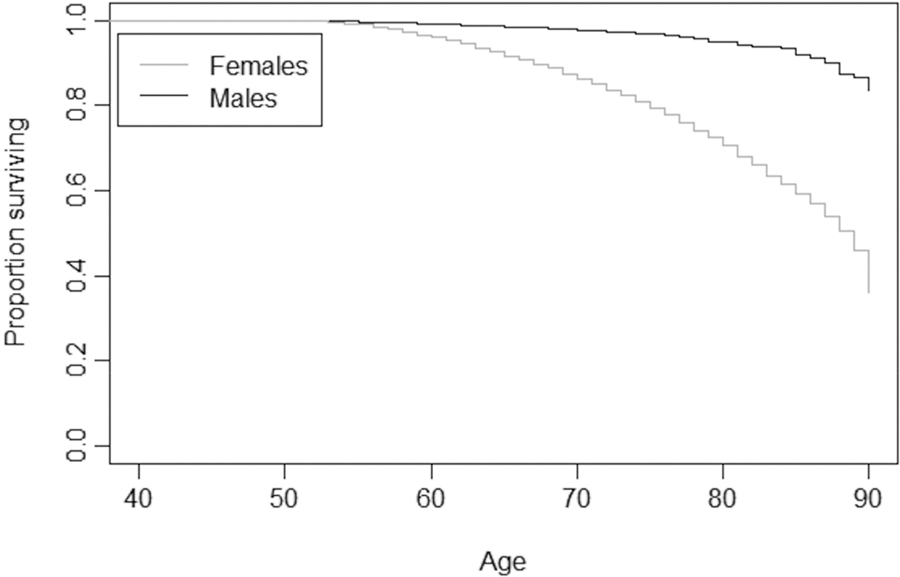
Kaplan-Meier Survivorship Plot for Sex Differences in Respondents with Osteoporosis
Discussion
Accelerated aging was examined for osteoporosis outcomes, using social determinants of health. We find support for all three hypotheses. We investigated the role of inflammation in influencing osteoporosis, showing that hs-CRP does not play a role in increasing the odds/hazards ratios of diagnosis with osteoporosis net of demographic, health-related, and other factors. Instead, logit models point to sex, psychological and physical health, Medicare coverage, and ozone exposure as potential contributors to being diagnosed with osteoporosis at any age. Cox models, which elicit age-dependent information, also point to race/ethnicity and respondent education as predictors of osteoporosis. Thus, determinants across several domains are important predictors of osteoporosis diagnosis. The predictive accuracy of the final models is good.
The prevalence of osteoporosis in our analytic sample from the HRS is 7.8%, which is low in comparison to other studies focused on the United States [e.g., 30, 31], irrespective of demographics. Limiting the HRS sample to people who identify as Non-Hispanic White, the most commonly reported sub-group, results in a prevalence of 9.64%, which aligns well with the nationally representative NHANES 2017–2018 (33: 12%) and NHANES 2005–2006 (34: 10%) samples, years that closely pre-date and post-date our sample. The HRS sample reported here is nationally representative not only for people identifying as Non-Hispanic White, but also for a broad range of racial and ethnic groups, which provides greater insight into social determinants influencing risk of osteoporosis diagnosis in the American population. It is further insightful for other populations by providing a framework of how various risk factor domains affect bone health.
Consistent with prior literature, sex is important to explaining osteoporosis diagnosis; women lose BMD at a higher rate and earlier age compared to men and have a four times higher risk of developing osteoporosis compared to men [32]. While our Kaplan-Meier plots show that males in our sample living with osteoporosis survive longer than females living with it (the opposite of the pattern for those without osteoporosis), the sample with osteoporosis is predominately female (88.33%), as younger males with fatal medical conditions or who reached the end of their lifespan likely already exited the sample due to death, leaving more robust males remaining. Likewise, females are older in the sample with osteoporosis as younger individuals were removed from the sample via death or dropout. This provides a unique opportunity to examine survivorship in males living to older ages and provides novel insight into what risk factors affect their probability of developing osteoporosis.
Aligned with our current findings, prior research indicates that osteoporosis is less common among Black/African Americans as compared to individuals who identify as White, Hispanic, or Asian American [33]; one study found the highest risk to be among Asian Americans [33]. Moreover, access to preventative measures and treatment of osteoporosis differs by race/ethnicity [34], which is likely reflected in the significant coefficients in both the logit and Cox models. Further, the literature indicates higher education levels are protective over bone density [35], but our results demonstrate the opposite pattern: those with at least a GED have a higher risk of osteoporosis diagnosis than those without a high school education. Not surprisingly, evidence for the relationship between BMD and education level is not strong. Other work has noted this trend and suggested understanding it requires a cohort study examining changes in SES (including education) over time [35]. Our study does this to some extent with household income and educational attainment, still yielding similar results.
In terms of childhood socioeconomic environment and the life course, one predictor (maternal investment) is significant in the Cox model, indicating an age-dependency component. Childhood socioeconomic advantage (defined as educational level of male and female heads of household, welfare status, and childhood financial status) displays varied results in the literature; it is positively associated with lumbar spine BMD, but not femoral [36]. When adult attainment was added to a model with childhood advantage, childhood advantage’s significance was reduced [36]. In this study, the Cox model has a highly significant p-value for the measure of maternal investment when evaluated with adult education level in the same model, which is a strong result in a longitudinal sample and demonstrates childhood and adulthood SES are important factors for osteoporosis risk. Specifically for childhood environment, increased maternal investment is protective.
Somewhat surprisingly, widowed respondents in our sample have a lower hazard of reporting an osteoporosis diagnosis in the Cox model after accounting for age and other important characteristics, although their unadjusted level of osteoporosis diagnosis is higher. However, a descriptive analysis of widowed respondents (Supplementary Information Table 2) indicates that despite them being much older on average and having slightly worse health, they are advantaged in ways that may offset their risk factors after accounting for age, including higher levels of positive social support, better self-rated health, and greater social engagement.
Turning to health, worse self-reported health is associated with a higher hazard of an osteoporosis diagnosis and taking diabetic medication as a diabetes patient is protective over bone health in the Cox model. In a previous study, researchers found that a high self-reported frailty score was associated with low calcaneal BMD among a community dwelling elder population [37]. For mental health, a higher CESD score and psychological problems are associated with higher risk of reporting an osteoporosis diagnosis in our sample, which is consistent with other research [38]. A greater number of hospital stays means higher odds of osteoporosis diagnosis in our sample, likely due to comorbidities with osteoporosis and stays due to osteoporotic fracture, which are costly both clinically and economically [1, 2].
In our sample, respondents with Medicare coverage have a higher risk for osteoporosis diagnosis compared to respondents not covered by Medicare. King and Fiorentino [39] found that cuts to Medicare Part B in 2007 impeded access to DXA scans. In 2011, the Patient Protection and Affordable Care Act (ACA) removed some barriers to getting DXA scans [1], making them more accessible. The change in Medicare likely introduced heterogeneity in the dataset, and subsequently our analysis, for respondents falling under the different provisions. Further complicating matters for osteoporosis patients with Medicare, among Medicare beneficiaries who suffered from frailty fractures, little attention has been given to osteoporosis after fractures take place, and treatment is even lower in select subpopulations with an overall low level of care [40].
For mobility, difficulty on the Activities of Daily Living Index and getting up from a chair are associated with an increased risk for osteoporosis, though difficulties on the Instrumental Activities of Daily Living Index are associated with reduced risk. Individuals with osteoporosis have difficulty with mobility [41], as it affects the bony microstructure and weakens the cancellous bone. It is therefore expected that we issues with mobility would be detected in our sample of patients living with osteoporosis, and that there may be heterogeneous relationships.
The impact by the physical environment, i.e., air pollution, is minimal with only mean ozone for April (and the second quarter) yielding an increased odds ratio for reporting an osteoporosis diagnosis. By contrast, in the Cox model, greater PM 2.5 exposure in February is associated with a lower hazard of reporting an osteoporosis diagnosis. Research indicates that ozone [42], among other types of air pollution, is detrimental to bone health, probably due to decreased vitamin D uptake, which may be the mechanism for increased risk of osteoporosis from pollution exposure. Air pollution may also limit the amount of time individuals spend outside, thus increasing risk of vitamin D deficiency in a second way [42]. The results of PM 2.5 are not surprising given the mixed nature of other studies’ findings [43]. Further, the temporal component is February, a month where few people are spending time outside to have exposure to PM 2.5. Therefore, this information, combined with the larger, but still significant p-value, suggests this finding may be spurious.
Neither the logit nor the Cox models show a significant predictive relationship between hs-CRP and osteoporosis diagnosis. While it was long established that hs-CRP was linked to bone remodeling via interleukin 6 (IL-6) [44], and associated with osteoclastogenesis, osteoclastic activity, and reduced osteoclastic apoptosis [45], recent research shows this relationship is flawed [12] and hs-CRP is likely a confounder and not a causal predictor [13]. hs-CRP inhibits osteoblast and osteoclast differentiation [12], causing effects that cancel one another out. More specifically, Mendelian randomization of genetic data finds no causal relationship between hs-CRP and BMD [11].
The main limitation of the study is the measurement of the outcome variable. Osteoporosis diagnosis is an important condition to explore, but diagnosis can only occur through interaction with the health care system. Thus, individuals without access to health care may not know they have osteoporosis. As mentioned earlier, screening and treatment rates for osteoporosis are low, even among those with a prior fracture. Thus, our outcome measure likely underestimates the true proportion of the HRS with osteoporosis. Studying objectively measured BMD may provide additional insights with regard to the role of variables across the six domains studied for osteoporosis risk, but these data are not available in most longitudinal surveys in the United States. Similarly, our self-reported health variable may not have captured undiagnosed medical conditions, which would bias our estimates of the relationship between self-reported health and osteoporosis and underestimate the impact of poor self-rated health on osteoporosis diagnoses. In other words, our estimate of the odds of poor self-rated health as a risk factor for developing osteoporosis is a minimum value. Further, our race/ethnicity variable lacked the resolution to divide it into more meaningful categories due to sample size. Finally, some of the predictor variables are measured in less precise ways than is ideal. For example, the arthritis variable does not distinguish between osteoarthritis and rheumatoid arthritis, and the measure of diabetes does not distinguish between Type 1 and Type 2. Nonetheless, this study offers one of the first comprehensive examinations of the role of a wide range of social determinants for osteoporosis diagnosis among older adults.
This study utilized a social epidemiological approach to examine the social determinants that potentially cause accelerated aging and may be associated with osteoporosis. A variety of predictors were identified that indicate access to health care, inequalities in the greater social environment (e.g., access to resources), and overall health (i.e., underlying medical conditions) are the most important factors for receiving an osteoporosis diagnosis and indicate underlying health inequities in this sample. Our analysis supports the need to address the interplay between primary health care and social determinants of health together.
Supplementary Material
Acknowledgements
Thank you to Valerie Pasillas, Angelyn Mendoza, Josephine Roberts, America Sanchez, and Haylie Wise who contributed as research assistants for this work. This analysis uses data from the Contextual Data Resource (CDR): US Decennial Census and American Community Survey Data, 1990–2018, Version 2.0 as of September 2020, developed by Jennifer Ailshire, Sarah Mawhorter, and Eun Young Choi at the USC/UCLA Center on Biodemography and Population Health, with funding from the National Institute on Aging (R21 AG045625, P30 AG017625); the Contextual Data Resource (CDR): United States Environmental Protection Agency Ozone FAQSD Files by Census Tract, 2002–2016, Version 2.0 as of September 2020, developed by Jennifer Ailshire and Hyewon Kang at the USC/UCLA Center on Biodemography and Population Health; the Contextual Data Resource (CDR): United States Environmental Protection Agency Particulate Matter 2.5 FAQSD Files by Census Tract, 2002–2016, Version 2.0 as of September 2020, developed by Jennifer Ailshire and Hyewon Kang at the USC/UCLA Center on Biodemography and Population Health; and the Contextual Data Resource (CDR): Uniform Crime Reports by County, 1994–2016, Version 2.0 as of February 2020, developed by the USC/UCLA Center on Biodemography and Population Health. The development of the CDR was funded by the National Institute on Aging (R21 AG045625, P30 AG017625). For more information, please refer to https://hrs.isr.umich.edu/data-products/restricted-data/available-products
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award number R15AG063330, as part of an award totaling $389,611. For this publication, 91% of the work was funded by NIA and 9% was funded by non-governmental sources, as noted below. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional non-governmental support was provided by a La Verne Academy grant from University of La Verne.
Footnotes
Declarations:
Conflicts of Interest
Margaret Gough Courtney, Yadira Quintero, and K. Godde declare that they have no conflict of interest.
Availability of Data and Material
Data for this study are available through the restricted-use application process of the Health and Retirement Study.
Code Availability
Model code can be made available upon request, subject to Michigan Center on the Demography of Aging Virtual Data Enclave export guidelines.
Ethics Approval
This study was determined to be exempt by the Institutional Review Board at the University of La Verne.
The RAND HRS Longitudinal File is an easy-to-use dataset based on the HRS core data. This file was developed at RAND with funding from the National Institute on Aging and the Social Security Administration.
Reference Page
- 1.Dane Hansen FSA, Bazell C, Pelizzari P, Bruce Pyenson FSA (2019) Medicare cost of osteoporotic fractures: The clinical and cost burden of an important consequence of osteoporosis. Miliman Resaerch Report 839:1–41 [Google Scholar]
- 2.Lewiecki EM, Ortendahl JD, Vanderpuye‐Orgle J, et al. (2019) Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the United States. JBMR Plus 3:1–7. 10.1002/jbm4.10192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson JB, Porges EC, Lamb DG, Porges SW (2015) Maladaptive autonomic regulation in PTSD accelerates physiological aging. Front Psychol 5:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubzansky L, Seeman TE, Glymour MM (2014) Biological pathways linking social conditions and health: plausible mechanisms and emerging puzzles. In: Berkman L, Kawachi I, Glymour MM (eds) Social Epidemiology: New Perspectives on Social Determinants of Global Population Health Oxford University Press, Oxford, pp 512–561 [Google Scholar]
- 5.Ferrucci L, Fabbri E (2018) Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15:505–522. 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM (2003) Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107:363–369. 10.1161/01.CIR.0000053730.47739.3C [DOI] [PubMed] [Google Scholar]
- 7.Berglundh S, Malmgren L, Luthman H, et al. (2015) C-reactive protein, bone loss, fracture, and mortality in elderly women: a longitudinal study in the OPRA cohort. Osteoporos Int 26:727–735. 10.1007/s00198-014-2951-7 [DOI] [PubMed] [Google Scholar]
- 8.Barr DA (2014) Health Disparities in the United States, 2nd edn. Johns Hopkins University Press, Baltimore, Md [Google Scholar]
- 9.Gough M, Godde K (2018) A multifaceted analysis of social stressors and chronic inflammation. SSM Popul Health 6:136–140. 10.1016/j.ssmph.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riancho JA, Brennan-Olsen SL (2017) The epigenome at the crossroad between social factors, inflammation, and osteoporosis risk. Clinic Rev Bone Miner Metab 15:59–68. 10.1007/s12018-017-9229-5 [DOI] [Google Scholar]
- 11.Huang JV, Schooling CM (2017) Inflammation and bone mineral density: a Mendelian randomization study. Sci Rep 7:1–7. 10.1038/s41598-017-09080-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho I-J, Choi KH, Oh CH, et al. (2016) Effects of c-reactive protein on bone cells. Life Sci 145:1–8. 10.1016/j.lfs.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 13.Oei L, Campos-Obando N, Dehghan A, et al. (2014) Dissecting the relationship between high-sensitivity serum C-reactive protein and increased fracture risk: the Rotterdam Study. Front Psychol 25:1247–1254. 10.1007/s00198-013-2578-0 [DOI] [PubMed] [Google Scholar]
- 14.Vable AM, Gilsanz P, Nguyen TT, et al. (2017) Validation of a theoretically motivated approach to measuring childhood socioeconomic circumstances in the Health and Retirement Study. PloS one 12:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health and Retirement Study (2020) RAND HRS Longitudinal File 2016 (V2) public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740) Ann Arbor, MI [Google Scholar]
- 16.RAND HRS Longitudinal File 2016 | Health and Retirement Study (2020) RAND HRS Longitudinal File 2016 (V2). Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration Santa Monica, CA. Accessed 4 Feb 2021 [Google Scholar]
- 17.Ailshire J, Mawhorter S, Choi EY (2020) Contextual Data Resource (CDR): US Decennial Census and American Community Survey Data, 1990–2018, Version 2.0 [Google Scholar]
- 18.Ailshire J, Kang H (2020) United States Environmental Protection Agency Ozone FAQSD Files by Census Tract, 2002–2016, Version 2.0 [Google Scholar]
- 19.Ailshire J, Kang H (2020) Contextual Data Resource (CDR): United States Environmental Protection Agency Particulate Matter 2.5 FAQSD Files by Census Tract, 2002–2016, Version 2.0 [Google Scholar]
- 20.Ailshire J, Mawhorter S, Kang H (2020) Contextual Data Resource (CDR): Uniform Crime Reports by County, 1994–2016. Version 2.0 [Google Scholar]
- 21.Maldonado G, Greenland S (1993) Simulation study of confounder-selection strategies. Am J Epidemiol 138:923–936 [DOI] [PubMed] [Google Scholar]
- 22.McCrory C, Fiorito G, Ni Cheallaigh C, et al. (2019) How does socio-economic position (SEP) get biologically embedded? A comparison of allostatic load and the epigenetic clock(s). Psychoneuroendocrinology 104:64–73. 10.1016/j.psyneuen.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 23.Farmer HR, Wray LA, Xian Y, et al. (2020) Racial Differences in Elevated C-Reactive Protein Among US Older Adults. J Am Geriatr Soc 68:362–369. 10.1111/jgs.16187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alley DE, Seeman TE, Kim JK, et al. (2006) Socioeconomic status and c-reactive protein levels in the US population: NHANES IV. Brain Behav Immun 20:498–504 [DOI] [PubMed] [Google Scholar]
- 25.Pearson TA, Mensah GA, Alexander RW, et al. (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511. 10.1161/01.cir.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- 26.Lumley T (2004) Analysis of complex survey samples. J Stat Softw 9:1–19 [Google Scholar]
- 27.R Core Team (2020) R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna [Google Scholar]
- 28.Parzen E (1962) On estimation of a probability density function and mode. Ann Math Stat 33:1065–1076 [Google Scholar]
- 29.McFadden D (1977) Quantitative methods for analysing travel behaviour of individuals Yale University, New Haven, CT [Google Scholar]
- 30.Sarafrazi N, Wambogo EA, Shepherd JA (2021) Osteoporosis or Low Bone Mass in Older Adults: United States, 2017–2018. NCHS Data Brief 1–8 [PubMed] [Google Scholar]
- 31.Looker AC, Melton LJ III, Harris TB, et al. (2010) Prevalence and trends in low femur bone density among older US adults: NHANES 2005–2006 compared with NHANES III. Journal of Bone and Mineral Research 25:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Center JR, Nguyen TV, Schneider D, et al. (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. The Lancet 353:878–882 [DOI] [PubMed] [Google Scholar]
- 33.Cheng H, Gary LC, Curtis JR, et al. (2009) Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int 20:1507–1515. 10.1007/s00198-009-0835-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cauley JA (2011) Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res 469:1891–1899. 10.1007/s11999-011-1863-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brennan SL, Pasco JA, Urquhart DM, et al. (2011) Association between socioeconomic status and bone mineral density in adults: a systematic review. Osteoporos Int 22:517–527. 10.1007/s00198-010-1261-y [DOI] [PubMed] [Google Scholar]
- 36.Crandall C, Merkin SS, Seeman TE, et al. (2012) Socioeconomic status over the life-course and adult bone mineral density: the Midlife in the U.S. Study. Bone 51:107–113. 10.1016/j.bone.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma S-L, Oyler J, Glavin S, et al. (2009) Self-reported frailty is associated with low calcaneal bone mineral density in a multiracial population of community-dwelling elderly. Osteoporos Int 20:1837–1846. 10.1007/s00198-009-0884-3 [DOI] [PubMed] [Google Scholar]
- 38.Robbins J, Hirsch C, Whitmer R, et al. (2001) The association of bone mineral density and depression in an older population. J Am Geriatr Soc 49:732–736. 10.1046/j.1532-5415.2001.49149.x [DOI] [PubMed] [Google Scholar]
- 39.King AB, Fiorentino DM (2011) Medicare payment cuts for osteoporosis testing reduced use despite tests’ benefit in reducing fractures. Health Aff (Millwood) 30:2362–2370. 10.1377/hlthaff.2011.0233 [DOI] [PubMed] [Google Scholar]
- 40.Liu SK, Munson JC, Bell J-E, et al. (2013) Quality of osteoporosis care of older Medicare recipients with fragility fractures: 2006 to 2010. J Am Geriatr Soc 61:1855–1862. 10.1111/jgs.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr C, Bottomley C, Shingler S, et al. (2017) The importance of physical function to people with osteoporosis. Osteoporos Int 28:1597–1607. 10.1007/s00198-017-3911-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cevei M, Stoicănescu D (2010) Air pollution and genetic influences on bone mineral density and osteoporosis. Fascicula Biologie TOM XVII:84–89 [Google Scholar]
- 43.Pang K-L, Ekeuku SO, Chin K-Y (2021) Particulate Air Pollution and Osteoporosis: A Systematic Review. Risk Manag Healthc Policy 14:2715–2732. 10.2147/RMHP.S316429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt C, Pedersen BK (2010) The Role of Exercise-Induced Myokines in Muscle Homeostasis and the Defense against Chronic Diseases. J Biomed Biotechnol 2010:1–6. 10.1155/2010/520258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofbauer LC, Khosla S, Dunstan CR, et al. (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


