Abstract
Background:
Chronotype characterizes individual differences in sleep/wake rhythm timing, which can also impact light exposure patterns. The present study investigated whether early and late chronotypes respond differently to controlled advancing and delaying light exposure patterns while on a fixed, advanced sleep/wake schedule.
Methods:
In a mixed design, 23 participants (11 late chronotypes and 12 early chronotypes) completed a 2-week, advanced sleep/wake protocol twice, once with an advancing light exposure pattern and once with a delaying light exposure pattern. In the advancing light exposure pattern, the participants received short-wavelength light in the morning and short-wavelength-restricting orange-tinted glasses in the evening. In the delaying light exposure pattern, participants received short-wavelength-restricting orange-tinted glasses in the morning and short-wavelength light in the evening. Light/dark exposures were measured with the Daysimeter. Salivary dim light melatonin onset (DLMO) was also measured.
Results:
Compared to the baseline week, DLMO was significantly delayed after the delaying light intervention and significantly advanced after the advancing light intervention in both groups. There was no significant difference in how the two chronotype groups responded to the light intervention.
Conclusions:
The present results demonstrate that circadian phase changes resulting from light interventions are consistent with those predicted by previously published phase response curves (PRCs) for both early and late chronotypes.
INTRODUCTION
The circadian system regulates daily variations in performance, behavior, endocrine functions, and the timing of sleep. Chronotype is used to describe individual differences in the timing of the sleep/wake rhythm. Early chronotypes have earlier sleep times, an earlier peak in alertness, and an earlier minimum core body temperature than late types. Because of later bedtimes and fixed wake times due to social and work obligations, late chronotypes tend to accumulate more sleep debt over the course of the working week compared to early chronotypes [1]. In fact, it has been suggested that late chronotypes suffer from a chronic form of jet lag [2] because their sleep/wake schedules are not well aligned with their social schedules. This chronic jet lag, also known as “social jet lag,” has been linked to an increased risk of obesity [3], depression [4], and cardiovascular disease [5].
Sleep is governed by the interaction between the homeostatic and the circadian systems. The homeostatic system increases sleep pressure as a nonlinear function of time awake. An increase in adenosine over the course of the day has been associated with an increase in sleep pressure [6]. The circadian system sends an alerting signal to the brain during daytime hours and a sleep signal during nighttime hours. In entrained people, the circadian and homeostatic systems work together to assure wakefulness during daytime and consolidated sleep at night. Studies have shown that adolescents and late chronotypes are slower to build up sleep pressure, even though they seem to dissipate sleep homeostasis similarly [7–9].
Light exposure on the retina determines the phase of the circadian system. Phase response curves (PRCs) can be used to characterize the magnitude and direction of light-induced phase adjustments of the master pacemaker. Light exposure in the early evening and first half of the night will delay the circadian phase, whereas light in the latest part of the night and in the morning hours will advance the timing of the pacemaker [10]. It has been hypothesized that similar light exposures in the phase advance and phase delay portions of the PRC might be differentially effective for early chronotypes and for late chronotypes, and their respective sleep/wake schedules may indirectly reflect this difference.
Sharkey et al. [11] studied two groups of young, late types, both of which were placed on a 1.5-h earlier sleep/wake schedule than their normal schedule; one group received 1 h of short-wavelength (blue) light within 15 min of waking while the other group was not exposed to the blue light in the morning. Personal light exposures were continuously monitored for all subjects throughout the study. Subjects in both groups exhibited similar circadian phase advances after 1 week on the advanced sleep/wake schedule. Because the total measured daily light exposures for both groups were not statistically different, the authors concluded that the daily environmental light exposures associated with the prescribed (earlier) sleep/wake schedule were sufficient to advance the circadian phase in young adults who would otherwise exhibit a delayed pattern, with or without a morning blue light intervention.
Appleman et al. [12] placed participants on a 1.5-h advanced sleep/wake schedule, with half receiving a light intervention designed to advance the circadian phase (short-wavelength light exposure from blue light-emitting diodes or LEDs in the morning and short-wavelength-restricting orange-tinted glasses in the evening) congruent with their advanced sleep schedule, while the other half received a delaying light intervention (short-wavelength-restricting orange-tinted glasses in the morning and short-wavelength light exposure from blue LEDs in the evening) incongruent with their advanced sleep schedule. Subjects who received the advancing light intervention advanced the circadian phase, while those who received the delaying light treatment delayed their circadian phase irrespective of their earlier sleep/wake schedule.
The present study was designed to extend from those by Sharkey et al. [11] and Appleman et al. [12] by investigating whether those with earlier sleep schedules (early chronotypes) and those with later sleep schedules (late chronotypes) respond differently to controlled advancing and delaying light exposure patterns while on a fixed, advanced sleep/wake schedule. Using a mixed experimental design, 23 participants (11 late chronotypes and 12 early chronotypes) completed a 2-week, advanced sleep/wake schedule protocol twice, once with an advancing light exposure pattern and once with a delaying light exposure pattern. For both sessions, following a baseline week, both groups were placed on a 1.5-h advanced sleep/wake schedule during the second, intervention week. We speculated that if the circadian phase, as measured by dim light melatonin onset (DLMO), were similar, but bedtimes were different between the early and late chronotypes, the controlled light schedules would fall at different parts of their PRCs, which, in turn, would lead to differential effects for the controlled advancing and delaying light patterns of the two groups [7–9,13,14]. Therefore, we hypothesized that for the controlled delaying light exposure pattern (light in the evening) the late chronotypes would receive the delaying light at a later circadian phase than the early types, resulting in greater phase delays for the late chronotypes than for the early chronotypes. For the controlled advancing light exposure pattern (light in the morning), the early chronotypes would receive the advancing light at an earlier circadian time than the late chronotypes, resulting in greater phase advances for the early chronotypes than for the late chronotypes.
MATERIALS AND METHODS
Participants
Twenty-four participants were recruited through website advertisement, word of mouth, and e-mail announcements. One subject dropped out of the experiment at the start of the intervention week because he could not comply with the advanced sleep/wake schedule. The results for the remaining 23 subjects who completed the entire experimental protocol are reported here. They were selected based on their self-reported chronotype, as described in the Munich Chronotype Questionnaire (MCTQ) [1]. In brief, the subjects were asked to rate themselves as extremely early type (0), moderate early type (1), slight early type (2), normal type (3), slight late type (4), moderate late type (5), and extreme late type (6). Those who rated themselves as extreme, moderate, and slight early chronotypes (MCTQ = 0–2; n = 12; Early Group) or moderate and extreme late chronotypes (MCTQ = 5–6; n = 11; Late Group) and also reported regular sleep patterns (i.e., no diagnosed sleep disturbances) were accepted into the study. The mean ± standard deviation (SD) in chronotype score was 1.0 ± 0.6 in the Early Group and 5.4 ± 0.5 in the Late Group. Using the calculation procedure published in Roenneberg et al. [1], we also calculated chronotype scores using the corrected mid-sleep on free days with adjustments for sleep debt accumulated during the work week (MSFsc) from baseline actigraph data. The average ± SD MSFsc for the Early Group was 2.5 (0230) ± 0.3 and 5.5 (0530) ± 1.0 for the Late Group.
All participants reported that they had no major health concerns and that they did not take pharmaceuticals, except for women taking birth control pills. Participants (17 females) ranged in age from 18 to 51 years old (mean age ± SD, 31.1 ± 11.1). The participants’ mean ± SD age was 40 ± 7.4 in the Early Group (nine females) and 21.5 ± 2.3 in the Late Group (eight females). Each participant selected for the study had to demonstrate an ability to use instant messaging and to respond quickly with his or her own personal mobile device to electronic prompts from the researcher. All participants were provided written informed consent approved by Rensselaer’s Institutional Review Board and were paid for their participation in the study.
Study overview
Two 13-day sessions were employed in the present study. The protocol was the same as that employed by Appleman et al. [12], except that every subject in the present study experienced both an advancing and a delaying light intervention, as described below. In brief, every participant was asked to continuously wear a Daysimeter-D [15–17] on the wrist at all times, except when showering and swimming. During both baseline weeks (6 days each), participants wore the device while keeping their regular schedule. At the end of each baseline week, participants reported to the laboratory for collection of evening saliva samples used to assess DLMO. For the intervention weeks (7 days each) immediately following each baseline week, all participants were placed on an advanced sleep/wake schedule that was 1.5 h earlier than their regular sleep/wake schedule. Immediately following saliva collection after the first baseline week, subjects were randomly assigned to receive either the advancing or the delaying light intervention during the subsequent intervention week. At the end of this first intervention week, participants again reported to the laboratory for evening saliva sample collection to assess DLMO. After a 3-week washout period, subjects completed the second 13-day session; those who received the advancing light intervention first received the delaying light intervention second, and vice versa.
Home monitoring
Personal light/dark and activity/rest patterns were continuously monitored for each participant with a Daysimeter-D. The device was worn on the nondominant wrist at all times, except for swimming and showering, over the course of both 13-day sessions. Participants were asked to avoid covering the device with clothing.
Figueiro et al. [16,17] previously documented the physical and calibration characteristics of the Daysimeter-D. Briefly, light sensing by the Daysimeter-D is performed with an integrated circuit (IC) sensor array that includes optical filters for four measurement channels: red (R), green (G), blue (B), and infrared (IR). The R, G, B, and IR photoelements have peak spectral responses at 615, 530, 460, and 855 nm, respectively. The Daysimeter-D is calibrated in terms of photopic illuminance (lux) and of circadian illuminance (CLA). CLA calibration represents the spectral sensitivity of the human circadian system, which is based on nocturnal melatonin suppression. Values of CLA are scaled so that 1000 lux of CIE Illuminant A (incandescent source at 2856 K) is equivalent to 1000 units of CLA. Circadian stimulus (CS) is calculated from the recorded CLA values. CS represents the input–output operating characteristics of the human circadian system from threshold to saturation. CS values are proportional to the levels of nocturnal melatonin suppression, from 0% at threshold to 70% at saturation, during the midpoint of melatonin production after 1 h of light exposure for a 2.3-mm-diameter pupil [18]. The Daysimeter-D also has three orthogonally oriented, solid-state accelerometers that are calibrated in terms of gravitational forces on the device, which allows for continuous measures of rest/activity patterns.
Baseline sleep schedule
During the 6-day baseline week in both sessions, participants were required to keep their normal sleep schedule. Bedtimes and wake times were not fixed, and light/dark exposures were not controlled, only monitored by the wrist-worn Daysimeter-D.
Light intervention
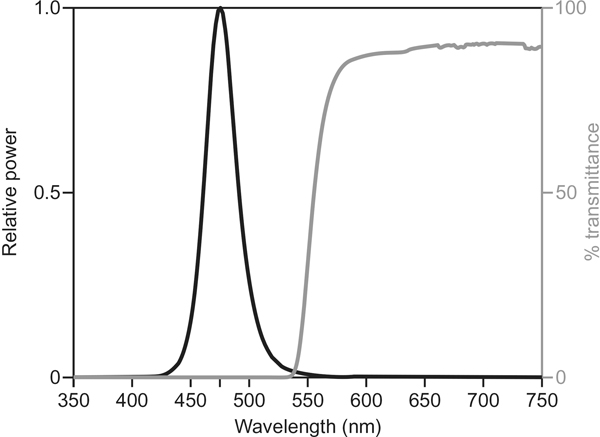
Two types of glasses were used in the study for the advancing and the delaying light interventions: (1) short-wavelength-restricting orange-tinted glasses (Orange Glasses #I005–017, UV Process Supply, Chicago, IL, USA) that filter nearly all optical radiation below 525 nm and (2) blue-light glasses consisting of four LEDs (λmax = 476 ± 1 nm, full-width half-maximum ~20 nm), two LEDs mounted on each lens of clear safety glasses. A translucent polycarbonate filter (Roscolux #116, Rosco, Stamford, CT, USA) was used to diffuse the light emitted by the LEDs, which minimizes glare and the risk of blue light hazard [19]. Prior to the study, each set of blue-light glasses was calibrated in the laboratory using a spectrometer (Oriel Instaspec IV spectrometer, Oriel Instruments, Stratford, CT, USA) with an ultraviolet–visible (UV–VIS) optical fiber ending in a Lambertian diffuser. The current from a remote 9-V battery was adjusted with a control circuit until the mean corneal illuminance at the left and right lenses reached 40 lux (40 μW/cm2). The spectral transmittance of the orange-tinted glasses was also measured. Figure 1 shows the spectral transmittance of the orange-tinted glasses and the relative spectral power distribution of the LEDs used in the blue-light glasses. A Daysimeter-D was added to both types of glasses to monitor participant compliance with the protocol. At the end of each intervention week, the researcher downloaded the activity data to verify compliance by subjects.
Fig. 1.
Spectral transmittance of the orange-tinted glasses and the relative spectral power distribution of the LEDs used in the blue-light glasses.
Sleep schedule intervention
During the intervention week, participants were required to be in bed, with lights off and trying to fall asleep at prescribed bedtimes. Compliance with the sleep schedule was confirmed through instant or text messaging to the researcher at bedtimes and later confirmed by inspecting the activity patterns from the wrist-worn Daysimeter-D.
Circadian phase assessment
Circadian phase assessments were based on melatonin concentrations from evening saliva samples obtained using the Salivette system (SciMart, Saint Louis, MO, USA). To prevent contamination, participants were not allowed to eat or drink between saliva sampling times, except for sipping water soon after a saliva sample was collected. Saliva samples were centrifuged and frozen at −20 °C until assayed for melatonin levels by radioimmunoassay using a commercially available kit from Labor Diagnostika Nord (Nordhorn, Germany). The limit of detection was 1.4 pg/mL and the intra-assay and inter-assay coefficients of variability were determined to be 11.4% and 14.6%, respectively. All saliva samples from one evening were assayed in the same batch. Saliva collection began 2 h prior to the estimated time of DLMO and continued every 20 min until 2 h after the predicted time of DLMO. Predicted DLMO was determined using the algorithm by Martin and Eastman [20] and was based on subjects’ self-reported sleep times (sleep logs).
Subjective sleepiness assessment
During both 13-day sessions, participants completed upon waking, at bedtime and at four prescribed times, 4 h apart, throughout the day (0800, 1200, 1600, and 2000), the Karolinska Sleepiness Scale (KSS) survey sent by the researcher using an instant or text messaging system. If the researcher did not hear from a participant at a designated time, messages were sent until the participant returned a response. Because the majority of the participants in the Late Group were not awake at 0800, KSS values collected at this time were not included in the statistical analyses.
Procedures
Upon acceptance into the study, participants were asked to come to the laboratory to sign the consent form and pick up a wrist-worn Daysimeter-D to start their baseline week data collection. At the end of the first baseline week, participants came to the laboratory at an appointed time to provide saliva samples for circadian phase assessment. Prior to leaving the laboratory, participants were given orange-tinted glasses, blue-light glasses, fresh 9-V batteries (for the blue-light glasses), and a blank sleep log. Participants also received written instructions for their new bedtimes and wake times and the light intervention schedule, detailing the times when they should wear each type of glasses during the following week. Participants were reminded that both the orange-tinted and the blue-light glasses had the Daysimeter-D attached to them to verify compliance with the protocol. Participants assigned to the advancing light intervention were asked to wear the blue-light glasses for 2 h upon waking and the orange-tinted glasses for 3 h prior to their designated bedtimes. As short-wavelength light was provided in the morning and restricted during the evening, this light intervention was designed to advance their circadian phase in concert with the advanced sleep/wake schedule. Participants assigned to the delaying light intervention were asked to wear the orange-tinted glasses for 2 h upon waking and the blue-light glasses for 3 h prior to their designated bedtimes. This light intervention was incongruent with the advanced sleep/wake schedule.
At the end of the first intervention week, participants returned to the laboratory at their designated times to provide saliva samples for a second circadian phase assessment. At the end of the night, participants were allowed to go home and maintain their regular schedules for the following 4 weeks. At the end of the third week of the washout period, participants came to the laboratory to pick up the Daysimeter-D and sleep logs. They repeated the same 13-day protocol (6 days of baseline data collection and 7 days of light exposure and sleep schedule interventions), except those who experienced the advancing light intervention in the first session were assigned the delaying light intervention in the second session, and vice versa. All participants continued to maintain a 1.5-h advanced sleep/wake schedule and returned to the laboratory for saliva sample collection at the end of the intervention week. At the end of the two sessions, all subjects experienced both the advancing and the delaying light interventions. All participants complied with the experimental protocol.
Data analyses
Using two techniques published in the literature, DLMO thresholds for each subject’s melatonin profile were calculated by taking the average of the three lowest points plus twice the standard deviation of these points (“3L”) [21] and by taking the average of the five continuous lowest points plus 15% of the five continuous highest points (“5H/5L”) [22]. The two different DLMO threshold calculations were employed to account for the different melatonin profiles in the subjects, as shown by visual inspection of the data [23]. Subjects whose DLMO thresholds were calculated using the 3L method had steeper melatonin profiles, while subjects whose DLMO thresholds were calculated using the 5H/5L method exhibited less steep melatonin profiles at the start of data collection until later in the evening, when melatonin levels went up. Therefore, the 5H/5L method, which included the higher levels at the end of the night in the calculation, better characterized DLMO thresholds for these subjects, because the first three lowest points were very often the same value. The DLMO time for each melatonin profile was the time (as determined by linear interpolation) that the fitted curve reached and remained above the calculated DLMO threshold [12]. If the fitted curve had not reached and remained above the calculated DLMO threshold by the end of the data collection period, DLMO time was taken at the last data collection time.
For each session, phase shifts were determined by subtracting DLMO values obtained after the baseline week from those obtained at the end of the intervention week. A negative difference would mean the participant exhibited a circadian phase delay after the intervention relative to the baseline week, whereas a positive difference would mean that the participant exhibited a circadian phase advance after the intervention week relative to the baseline week.
Average KSS scores were calculated five times during the day: upon waking, at bedtime, and at 1200, 1600, and 2000, throughout the baseline and intervention weeks for both sessions.
The photopic and circadian illuminance values were determined for the first 2 h after waking (phase advance portion of the PRC) and for the last 3 h before bedtime (phase delay portion of the PRC). Logarithmic transforms of the raw illuminance values were performed due to their highly skewed distributions. Three measures were used in the analyses: CS, log CLA, and log lux.
For DLMO times, a one-between (chronotype group (Early vs. Late)) and two-within (light intervention (advancing vs. delaying) and weeks (baseline vs. intervention)) mixed analysis of variance (ANOVA) was performed. In addition, a one-between (chronotype group (Early vs. Late)) and one-within (light intervention (advancing vs. delaying)) mixed ANOVA was performed for the DLMO phase shift (difference between DLMO at baseline and DLMO after the intervention week).
For the average KSS scores, a one-between (chronotype group (Early vs. Late)) and three-within (light intervention (advancing vs. delaying), weeks (baseline vs. intervention), and time of day (upon waking, at bedtime, 1200, 1600, and 2000)) mixed ANOVA was performed.
For each of the three light exposure measures, a one-between (chronotype group (Early vs. Late)) and three-within (sampling intervals (morning vs. evening), light intervention (advancing vs. delaying), and weeks (baseline vs. intervention)) mixed ANOVA was conducted.
Two-tailed paired (when comparing within subjects) and unpaired (when comparing between chronotype groups) Student’s t-tests were used to further compare the main effects and interactions. All statistical analyses were performed using PASW Statistics 18.0 software (SPSS, Chicago, IL, USA).
RESULTS
Circadian phase (DLMO)
DLMO times following baseline and intervention periods, DLMO phase shifts, baseline-reported bedtimes, wake times, and sleep times for both chronotype groups and for the two light intervention periods are listed in Table 1.
Table 1.
DLMO times following baseline and intervention weeks, DLMO phase shifts, reported bedtimes, wake times, and sleep times for both chronotype groups and for both light interventions.
| Participant characteristics | Early group (n = 12) | Late group (n = 11) | ||
|---|---|---|---|---|
|
|
|
|||
| Mean [SD] | Range | Mean [SD] | Range | |
| Age (years) | 40 [7.9] | 25–48 | 21.5 [2.3] | 18–25 |
| MCTQ weekday wake times (h, min) | 0632 [34] | 0530–0730 | 0851 [33] | 0800–1000 |
| MCTQ weekday bedtimes (h, min) | 2242 [27] | 2200–2300 | 0024 [32] | 2300–0200 |
| MCTQ weekend wake times (h, min) | 0725 [38] | 0600–0800 | 1005 [32] | 0900–1100 |
| MCTQ weekend bedtimes (h, min) | 2307 [22] | 2230–0000 | 0151 [54] | 0030–0300 |
| MCTQ midpoint of sleep, free days (h, min) | 0315 [25] | 0215–0345 | 0600 [23] | 0530–0630 |
|
| ||||
| Early group (n = 12) | Advancing light | Delaying light | ||
|
|
|
|||
| Mean [SD] | Range | Mean [SD] | Range | |
|
| ||||
| Reported bedtimes | ||||
| Baseline (h, min) | 2306 [42] | 2200–0030 | 2312 [35] | 2230–0030 |
| Intervention (h, min) | 2136 [42] | 2030–2300 | 2142 [35] | 2100–2300 |
| Reported wake times | ||||
| Baseline (h, min) | 0636 [31] | 0545–0730 | 0638 [37] | 0530–0730 |
| Intervention (h, min) | 0506 [31] | 0415–0600 | 0508 [37] | 0400–0600 |
| Baseline total sleep time (min) | 450 [36] | 390–510 | 446 [37] | 390–495 |
| Intervention sleep time (min) | 450 [36] | 390–510 | 446 [37] | 390–495 |
| DLMO | ||||
| Baseline DLMO time (h, min) | 2048 [28] | 2003–2130 | 2007–2219 | |
| Intervention DLMO time (h, min) | 1845 [30] | 1804–1940 | 2136 [47] | 2019–2319 |
| DLMO shift (min) | 122 [34] | 58–165 | −37 [38] | 20 to −116 |
| Phase angle between DLMO and bedtime | ||||
| Night 1, baseline (min) | 138 [46] | 67–220 | 0212 [51] | 30–230 |
| Night 2, intervention (min) | 171 [57] | 80–290 | 0006 [31] | −109 to 90 |
|
| ||||
| Late group (n = 11) | Advancing light | Delaying light | ||
|
|
|
|||
| Mean [SD] | Range | Mean [SD] | Range | |
|
| ||||
| Reported bedtimes | ||||
| Baseline (h, min) | 0100 [70] | 2330–0400 | 0049 [59] | 0000–0300 |
| Intervention (h, min) | 2332 [70] | 2200–0230 | 2319 [59] | 2230–0130 |
| Reported wake times | ||||
| Baseline (h, min) | 0909 [68] | 0800–1130 | 0913 [57] | 0800–1100 |
| Intervention (h, min) | 0739 [68] | 0630–1000 | 0743 [57] | 0630–0930 |
| Baseline total sleep time (min) | 487 [55] | 420–600 | 499 [41] | 420–570 |
| Intervention sleep time (min) | 487 [55] | 420–600 | 499 [41] | 420–570 |
| DLMO | ||||
| Baseline DLMO time (h, min) | 2131 [33] | 2053–2226 | 2137 [28] | 2100–2240 |
| Intervention DLMO time (h, min) | 1943 [46] | 1840–2113 | 2236 [45] | 2152–2349 |
| DLMO shift (min) | 108 [28] | 73–160 | −59 [28] | −33 to −116 |
| Phase angle between DLMO and bedtime | ||||
| Night 1, baseline (min) | 208 [75] | 129–400 | 0312 [77] | 79–350 |
| Night 2, intervention (min) | 228 [92] | 133–469 | 0043 [76] | −19 to 217 |
The ANOVA using DLMO times revealed significant main effects of the chronotype group (F1,21 = 29.6; p < 0.0001), of weeks (F1,21 = 41.9; p < 0.0001), and of light intervention (F1,21 = 66.1; p < 0.0001). There was also a significant weeks × light intervention interaction (F1,21 = 343.2; p < 0.0001). The interaction between weeks and chronotype group did not reach significance (F1,21 = 3.0; p = 0.098). The t-tests revealed that DLMO time at baseline was statistically different (p < 0.01) between the two groups, but this difference was not statistically significant after the advancing and the delaying light interventions.
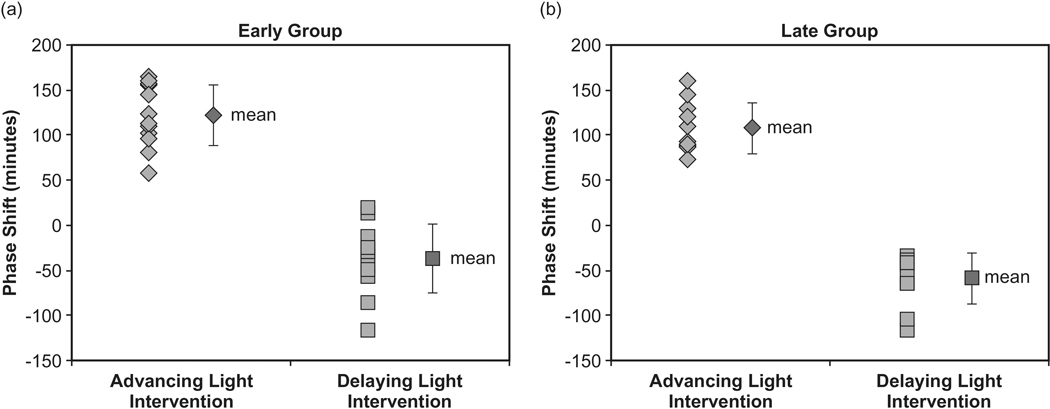
The ANOVA using DLMO phase shift as the dependent measure revealed a significant main effect of light intervention (F1,21 = 440.7; p < 0.0001). There was no significant difference between the chronotype groups (F1,21 = 2.6; p = 0.1), nor was there an interaction between light intervention and the chronotype group (F1,21 = 0.19; p = 0.67). In the Early Group, the mean ± SD phase advance was 122 ± 34 min when participants were in the advancing light intervention, and the mean ± SD phase delay was −37 ± 38 min when participants experienced the delaying light intervention. In the Late Group, the mean ± SD phase advance was 108 ± 28 min and the mean ± SD phase delay was −59 ± 28 min when participants experienced the advancing and the delaying light interventions, respectively. Figure 2a illustrates the phase shifts for the 12 participants in the Early Group. Figure 2b illustrates phase shifts for the 11 participants in the Late Group. Given the small difference in baseline DLMO between the two groups, a post hoc analysis was performed where participants were re-classified as Late or Early Types based on the baseline DLMO collected at the start of the study. Participants who had DLMO occurring before 2100 were classified as Early Types (n = 11) and those with DLMO after 2100 were classified as Late Types (n = 12). The mean ± SD baseline DLMO times were 2038 h ± 17 min and 2136 h ± 26 min in the Early and Late Types, respectively. The calculated phase shifts showed that the Early Types exhibited a mean ± SD phase advance of 112 ± 32 min when participants were in the advancing light intervention, and the mean ± SD phase delay was −43 ± 36 min when participants experienced the delaying light intervention. In the Late Types, the mean ± SD phase advance was 118 ± 33 min, and the mean ± SD phase delay was −52 ± 34 min when participants experienced the advancing and the delaying light interventions, respectively.
Fig. 2.
(a) Phase shifts for the 12 participants in the Early Group. The mean ± SD phase advance was 122 ± 34 min when participants were in the advancing light intervention and the mean ± SD phase delay was −37 ± 38 min when participants experienced the delaying light intervention. (b) Phase shifts for the 11 participants in the Late Group. The mean ± SD phase advance was 108 ± 28 min and the mean ± SD phase delay was −59 ± 28 min when participants experienced the advancing and the delaying light interventions, respectively.
Subjective sleepiness (KSS)
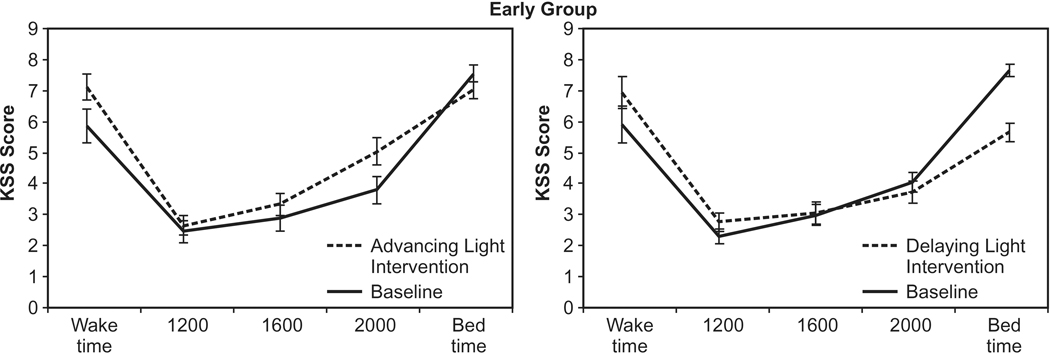
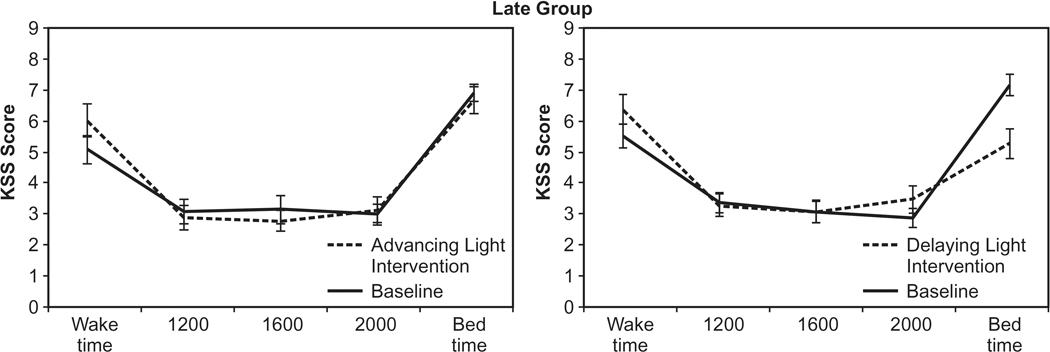
The ANOVA revealed a significant main effect of time of day (F4,84 = 94.6; p < 0.0001). The chronotype group was not significantly different (F4,84 = 0.865; p > 0.05). Participants in both the Early Group and the Late Group felt significantly sleepier (p < 0.05) at bedtimes than at any other time of the day. Participants also reported feeling sleepier (p < 0.05) at waking than at 1200, 1600, and 2000. The following two-way interactions were statistically significant: light intervention by chronotype group (F1,21 = 4.5; p = 0.047), chronotype group by time of day (F4,84 = 2.9; p = 0.27), weeks by light intervention (F1,1 = 19.5; p < 0.0001), weeks by time of day (F4,84 = 21.9; p < 0.0001), and light intervention by time of day (F4,84 = 5.9; p < 0.0001). Two three-way interactions, weeks by light intervention by chronotype group (F1,21 = 8.1; p = 0.01) and weeks by light intervention by time of day (F4,84 = 7.6; p < 0.0001), were significant as was the four-way interaction, weeks by light intervention by time of day and by chronotype group (F4,84 = 3.2; p = 0.016). Compared to the baseline week, the t-tests showed that the Early Group reported feeling significantly sleepier during the advancing light intervention at waking (p = 0.002), at 2000 (p = 0.0007), and at bedtime (p < 0.0001), and the Late Group reported feeling significantly sleepier at waking (p = 0.007). Compared to the baseline week, the Early Group reported feeling significantly less sleepy at bedtimes (p < 0.0001) and the Late Group reported feeling sleepier at 2000 (p = 0.03) and significantly less sleepy at bedtimes (p = 0.0008). When comparing the light interventions (advancing vs. delaying), the Early Group reported feeling significantly less sleepy after the delaying light intervention at 0800 (p = 0.0001) and at bedtimes (p < 0.0001), while the Late Group reported feeling significantly less sleepy only at bedtimes (p = 0.0008). Figures 3 and 4 show the KSS scores for both groups after experiencing both light interventions.
Fig. 3.
KSS scores for the Early Group after experiencing both light interventions. Compared to the baseline week, the Early Group reported feeling significantly sleepier during the advancing light intervention at waking (p = 0.002), at 2000 (p = 0.0007), and at bedtime (p < 0.0001) and significantly less sleepy after the delaying light intervention at bedtimes (p < 0.0001).
Fig. 4.
KSS scores for the Late Group after experiencing both light interventions. Compared to the baseline week, the Late Group reported feeling significantly sleepier during the advancing light intervention at waking (p = 0.007). After the delaying light intervention, the Late Group reported feeling significantly sleepier at 2000 (p = 0.03) and significantly less sleepy at bedtimes (p = 0.0008).
Light exposures
Table 2 lists the CS, log CLA, and log lux for both chronotype groups (early and late) and both light interventions (advancing and delaying). One Daysimeter-D failed to record data for one subject during the baseline week; this subject was removed from the statistical analyses for all three light exposure measures. There was a significant main effect of weeks for CS (F1,20 = 847; p < 0.0001), log CLA (F1,20 = 22.4; p < 0.0001), and log lux (F1,20 = 6.1; p = 0.023). As expected, as a result of the light intervention imposed by the study protocol, light exposures experienced by participants during the baseline weeks were significantly less (p < 0.05) than those during the intervention weeks. There was also a significant main effect of time of day for log CLA (F1,20 = 35.8; p < 0.0001) and log lux (F1,20 = 48.6; p < 0.0001). The following two-way interactions were statistically significant for all three light exposure measures: time of day by chronotype group (F1,20 = 8.1, p = 0.01; F1,20 = 14.1, p = 0.001; and F1,20 = 15.1, p = 0.001 for CS, log CLA, and log lux, respectively), weeks by time of day (F1,20 = 8.4, p = 0.009; F1,20 = 39, p < 0.0001; and F1,20 = 55.2, p < 0.0001 for CS, log CLA, and log lux, respectively), and light intervention by time of day (F1,20 = 2403, p < 0.0001; F1,20 = 1223, p < 0.0001; and F1,20 = 951, p < 0.0001 for CS, log CLA, and log lux, respectively). The following three-way interactions were statistically significant: weeks by time of day by chronotype group (F1,20 = 7.6, p = 0.12; F1,20 = 12.3, p = 0.002; and F1,20 = 12.5, p = 0.002 for CS, log CLA, and log lux, respectively) and weeks by light intervention by time of day (F1,20 = 2000, p < 0.0001; F1,20 = 1204, p < 0.0001; and F1,20 = 856, p < 0.0001 for CS, log CLA, and log lux, respectively).
Table 2.
CS, log CLA, and log lux for both chronotype groups (Early and Late) and both light interventions (advancing and delaying).
| Baseline | Intervention | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| CS (SD) | log CLA (SD) | log lux (SD) | CS (SD) | log CLA (SD) | log lux (SD) | |
| First 2 h after wake | ||||||
| Early Group, advancing light | 0.04 (0.03) | −2.27 (1.34) | −2.24 (1.29) | 0.47 (0.01) | 6.52 (0.09) | 4.13 (0.16) |
| Early Group, delaying light | 0.03 (0.03) | −2.64 (1.41) | −2.65 (1.35) | 0.00 (0.00) | −4.61 (0.00) | −4.61 (0.00) |
| Late Group, advancing light | 0.08 (0.05) | −0.39 (1.92) | −0.33 (1.76) | 0.49 (0.04) | 6.74 (0.46) | 4.56 (0.65) |
| Late Group, delaying light | 0.10 (0.07) | 0.35 (2.23) | 0.47 (2.04) | 0.00 (0.00) | −4.61 (0.00) | −4.61 (0.00) |
| Last 3 h before bed | ||||||
| Early Group, advancing light | 0.11 (0.06) | 1.58 (1.57) | 1.91 (1.43) | 0.00 (0.00) | −4.61 (0.00) | −4.61 (0.00) |
| Early Group, delaying light | 0.09 (0.05) | 1.56 (0.92) | 1.91 (0.83) | 0.46 (0.01) | 6.49 (0.03) | 4.12 (0.22) |
| Late Group, advancing light | 0.08 (0.05) | 0.88 (1.53) | 1.36 (1.39) | 0.00 (0.00) | −4.61 (0.00) | −4.61 (0.00) |
| Late Group, delaying light | 0.09 (0.05) | 1.14 (1.09) | 1.57 (1.06) | 0.47 (0.01) | 6.51 (0.08) | 4.24 (0.29) |
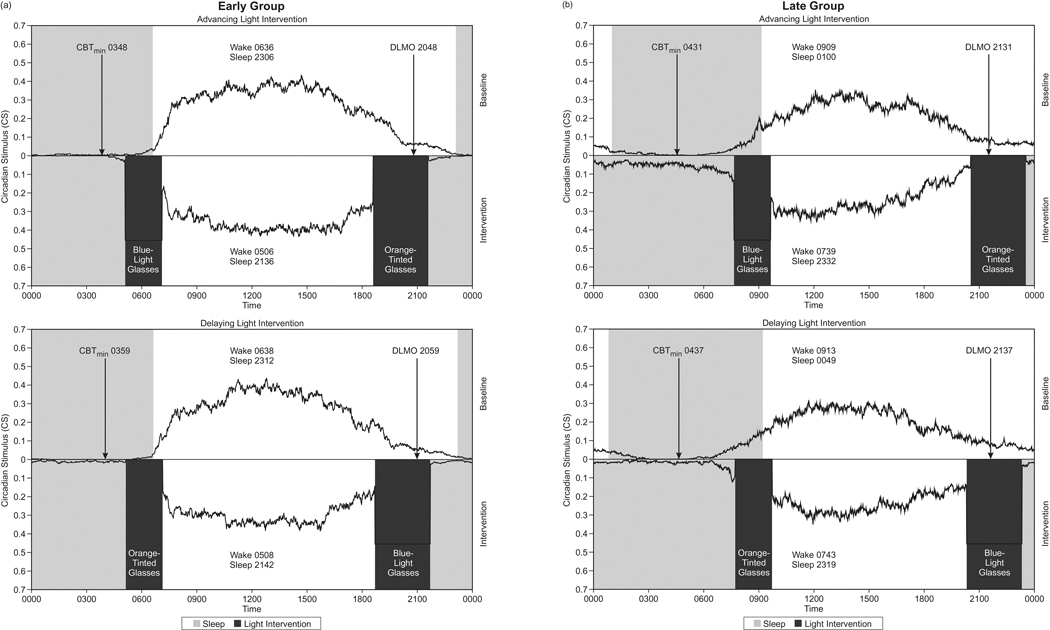
The ANOVA also revealed a significant main effect of the chronotype group for all three light exposure measures (F1,20 = 4.4, p = 0.048; F1,20 = 5.0, p = 0.036; and F1,20 = 7.9, p = 0.01 for CS, log CLA, and log lux, respectively). On average, the Late Group received more light than the Early Group; this difference was greater in the first 2 h after waking than in the last 3 h prior to bedtimes. As both the intervention blue-light glasses and the ambient lighting contribute to the measured light exposures, and because the treatment (blue-light glasses) was the same for both groups, the Late Group subjects likely received more ambient light (such as daylight) than the Early Group subjects in the morning. Moreover, the timing of the start of the blue-light treatment also differed between groups. On average, the Early Group started receiving the advancing light intervention 8.3 h after DLMO and the delaying light intervention 2.3 h before DLMO, while the Late Group started receiving the advancing light intervention 10.1 h after DLMO and the delaying light intervention 1.3 h before DLMO. Figures 5a and 5b illustrate the CS at baseline and intervention weeks for the Early and Late Groups, respectively. Also shown in these two figures are the timing of the advancing and delaying light interventions with respect to the baseline DLMO and the predicted minimum core body temperature (CBTmin), calculated by adding 7 h to baseline DLMO.
Fig. 5.
(a) Average 7-day circadian stimulus experienced by the Early Group, measured over the course of the 24-h day, during the baseline week (top) and the intervention week (bottom). The dark rectangles represent the morning and evening light intervention times. The lighter rectangles represent the sleep periods. Predicted minimum core body temperature (CBTmin) was calculated by adding 7 h to the average baseline DLMO. On average, the Early Group started receiving the advancing light intervention 8.3 h after DLMO and the delaying light intervention 2.3 h before DLMO. The midpoint of the blue-light glasses intervention was 0606 in the advancing light intervention and 2012 in the delaying light intervention. The midpoint of the orange-tinted glasses intervention was 2006 in the advancing light intervention and 0608 in the delaying light intervention. (b) Average 7-day circadian stimulus experienced by the Late Group, measured over the course of the 24-h day, during the baseline week (top) and the intervention week (bottom). The dark rectangles represent the morning and evening light intervention times. The lighter rectangles represent the sleep periods. Predicted minimum core body temperature (CBTmin) was calculated by adding 7 h to the average baseline DLMO. On average, the Late Group started receiving the advancing light intervention 10.1 h after DLMO and the delaying light intervention 1.3 h before DLMO. The midpoint of the blue-light glasses intervention was 0839 in the advancing light intervention and 2149 in the delaying light intervention. The midpoint of the orange-tinted glasses intervention was 2202 in the advancing light intervention and 0843 in the delaying light intervention.
DISCUSSION
The present results extend findings by Appleman et al. [12] showing that circadian phase changes resulting from a light intervention are consistent with those predicted by previously published PRCs [10] and are similar in people who have early and late sleep schedules, but similar circadian phases. Although the self-reported bedtimes in the Late Group were about 2 h later than in the Early Group and the mid-sleep in free days calculated using the baseline actigraphy data was 3 h later, baseline DLMO in the Early Group was only about 40 min earlier than the Late Group. We hypothesized that, due to the advanced sleep schedule and the resulting timing of the light interventions with respect to their PRCs, the Early Group would exhibit a greater phase advance than the Late Group when exposed to the same advancing light intervention, and that the Late Group would exhibit a greater phase delay than the Early Group when exposed to a delaying light intervention.
Although the Late Group delayed more than the Early Group for the delaying light intervention and advanced less for the advancing light intervention, as hypothesized, the t-tests revealed that this difference was not statistically significant. These results are consistent with those reported by Mongrain et al. [14], who showed that when morning types and evening types with overlapping circadian phase are compared, bedtimes and wake times are significantly different, despite the similar circadian phase. As a result of this overlap in circadian phases between the two groups even though they had significantly different bedtimes, the controlled light exposure patterns experienced by the two groups during the intervention week at a fixed clock time were likely not given at the same circadian time. For the secondary analysis, where participants were re-classified as Late or Early Types based on their baseline DLMOs, the magnitudes of the phase advances and the phase delays were very similar for both classifications and, again, consistent with PRC predictions.
We also examined if there was a relationship between MSFsc and circadian phase shift and although the correlation was greatest when the Late Types experienced the delaying light intervention, none of the correlations were statistically significant (p > 0.05). Moreover, no correlations were observed between the phase shifts and baseline DLMO for either group.
One explanation for our results lies not with a differential sensitivity to light, but perhaps with regard to differences in sleep pressure buildup. Both Jenni et al. and Taylor et al. [8,9] reported that more mature adolescents accumulated sleep pressure slower when sleep deprived. In addition, sleep latency scores were significantly lower in prepubertal adolescents than in postpubertal adolescents. Our study was not designed to evaluate whether the two chronotype groups build up sleep pressure differently. The fact that the circadian phase and circadian phase shifts resulting from the light interventions did not differ between groups while the bedtimes were significantly different during baseline may suggest, however, that chronotypes differ in their sleep pressure buildup. Consistently, Taillard et al. [7] showed that the buildup of subjective sleepiness is slower in the evening types than in the morning types; their measure of minimum core body temperature, used as a marker of the circadian phase, occurred approximately 1.4 h earlier in the morning types than in the evening types.
The present findings, and those earlier by Sharkey et al. and Appleman et al. [11,12], indicate that controlling the entire light–dark exposure pattern is primarily important in determining the circadian phase for all chronotypes when subjects are placed on a controlled sleep/wake schedule. It is not known, however, whether the larger phase advances observed here and earlier by Appleman et al. [12] would persist after an extensive period of time. Saxvig et al. [24] showed that a gradual advancement of rise time resulted in a phase advance of DLMO during a 2-week intervention with and without bright light treatment, but that the observed advanced phase relapsed after that time period if the light treatment was removed. It does appear that those on the delaying light intervention would quickly return to their baseline sleep/wake schedule. The KSS scores clearly show that participants in both chronotype groups were less sleepy at the prescribed, advanced bedtime when placed on the delaying light intervention. Presumably, without the rigid sleep/wake schedule, participants on the delaying light intervention would not have gone to bed so early, would probably have stayed in bed later, and consequently would have returned to the later sleep/wake schedule observed during the baseline week.
It is also not known whether circadian phase delays resulting from a delaying light intervention would be greater with a delayed sleep/wake schedule or whether phase advances resulting from an advancing light intervention would be smaller with a delayed sleep/wake schedule, and, more importantly, whether this would differ between chronotype groups. Although a very interesting question, an experimental design using a delayed sleep/wake schedule is more difficult to achieve in a field study like that employed here. Most subjects have social or work obligations in the morning, so it would be impractical to conduct a field study where a delayed sleep/wake schedule was a central part of the protocol. A controlled laboratory study probably would be better for answering this particular question.
Finally, it is not known whether a light intervention by itself can shift the sleep/wake schedule, as a control condition without the imposed sleep schedule was not performed. Sleep/wake schedules are set largely by social and work requirements. Our participants did not have a free sleep/wake schedule during the intervention weeks, so we do not know if they would have intuitively responded to the shift in their circadian phase by shifting their sleep/wake schedule. If social and work requirements set behavior rather than endogenous time, it is certainly conceivable that their daily behavior might expose them to light during uncontrolled light exposure periods that might counteract the prescribed light intervention. Thus, it may be necessary to completely control the light/dark pattern as well as the sleep/wake schedule to achieve persistent circadian phase changes. It is also possible that sleep itself affects the timing of the circadian clock, but this is less likely given that sleep opportunity times during the two intervention weeks (advancing and delaying light interventions) were exactly the same.
One limitation of our study is the age difference between the groups. The Early Group was older than the Late Group. Studies have shown that middle-aged adults in their forties and fifties exhibit earlier sleep timing and earlier minimum core body temperature [25], which is consistent with our findings. In general, increasing age is associated with more morningness [26]. Our results are consistent with previous reports showing that older adults have earlier circadian phases, earlier bedtimes, and earlier wake times than younger adults (in their twenties). Another limitation is the sex differences. We had a larger number of females than males in our study, even though we had the same number of males and females in each chronotype group. It has been demonstrated that the DLMO phase was earlier in women than in men, and the phase angle between DLMO and bedtime was wider in women than in men [27]. As both groups experienced both light interventions, we do not believe that age and sex differences could have introduced a bias in our results.
Conflict between sleep/wake schedules and social schedules, along with the associated decrements in sleep, performance, and well-being, has been identified as a significant problem [1]. It appears, based upon the present and previous results [11,12], that the light/dark exposure pattern is a central consideration because it drives the circadian phase, but the homeostatic system also plays a role in affecting behavior, which may consequently affect sleep/wake schedules and light/dark exposures patterns. Thus, future studies should investigate the relationships between chronotype, social schedules, sleep/wake schedules, and personal light/dark exposure patterns, with the goal of improving sleep, performance, and well-being.
ACKNOWLEDGMENTS
The authors would like to acknowledge Brittany Wood, Levent Sahin, Sharon Lesage, Andrew Bierman, Geoff Jones, Petteri Teikari, Greg Ward, Dennis Guyon, Rebekah Mullaney, and Sarah Hulse of the Lighting Research Center for their technical and editorial assistance. The authors would also like to thank the anonymous reviewers who helped us improve the manuscript with their insightful comments.
FUNDING SOURCES
This study was funded by the Office of Naval Research (grant # N000141110572). The Daysimeter-D development was funded by the National Institute on Aging (grant # R01AG34157).
Footnotes
CONflICT OF INTEREST
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2014.07.009.
REFERENCES
- 1.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18:80–90. [DOI] [PubMed] [Google Scholar]
- 2.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int 2006;23:497–509. [DOI] [PubMed] [Google Scholar]
- 3.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol 2012;22:939–43. [DOI] [PubMed] [Google Scholar]
- 4.Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int 2011;28:771–8. [DOI] [PubMed] [Google Scholar]
- 5.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009;106:4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol 2004;73:379–96. [DOI] [PubMed] [Google Scholar]
- 7.Taillard J, Philip P, Claustrat B, Capelli A, Coste O, Chaumet G, et al. Time course of neurobehavioral alertness during extended wakefulness in morning and evening-type healthy sleepers. Chronobiol Int 2011;28:520–7. [DOI] [PubMed] [Google Scholar]
- 8.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep 2005;28:1446–54. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res 2005;14:239–44. [DOI] [PubMed] [Google Scholar]
- 10.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol 2003;549:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med 2011;12:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appleman K, Figueiro MG, Rea MS. Controlling light-dark exposure patterns rather than sleep schedules determines circadian phase. Sleep Med 2013;14:456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley SJ, Acebo C, Carskadon M. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med 2007;8:602–12. [DOI] [PubMed] [Google Scholar]
- 14.Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in Morningness-Eveningness. J Biol Rhythms 2004;19:248–57. [DOI] [PubMed] [Google Scholar]
- 15.Bierman A, Klein TR, Rea MS. The Daysimeter: a device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol 2005;16:2292–9. [Google Scholar]
- 16.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol 2013;45:421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field Measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis 2012;31:711–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms 2010;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bullough JD. The blue-light hazard: a review. J Illum Eng Soc 2000;29:6–14. [Google Scholar]
- 20.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int 2002;19:695–707. [DOI] [PubMed] [Google Scholar]
- 21.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 1997;12:457–66. [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med 2009;10:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int 2011;28:714–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxvig IW, Wilhelmsen-Langeland A, Pallesen S, Vedaa Ø, Nordhus IH, Bjorvatn B. A randomized controlled trial with bright light and melatonin for delayed sleep phase disorder: effects on subjective and objective sleep. Chronobiol Int 2014;31:72–86. [DOI] [PubMed] [Google Scholar]
- 25.Carrier J, Paquet J, Morettini J, Touchette E. Phase advance of sleep and temperature circadian rhythms in the middle years of life in humans. Neurosci Lett 2002;320:1–4. [DOI] [PubMed] [Google Scholar]
- 26.Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol 2007;72:293–9. [DOI] [PubMed] [Google Scholar]
- 27.Van Reen E, Sharkey KM, Roane BM, Barker D, Seifer R, Raffray T, et al. Sex of college students moderates associations among bedtime, time in bed, and circadian phase angle. J Biol Rhythms 2013;28:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]