Abstract
Hepatocyte growth factor (scatter factor) (HGF/SF) is a pleiotrophic mediator of epithelial cell motility, morphogenesis, angiogenesis, and tumorigenesis. HGF/SF protects cells against DNA damage by a pathway from its receptor c-Met to phosphatidylinositol 3-kinase (PI3K) to c-Akt, resulting in enhanced DNA repair and decreased apoptosis. We now show that protection against the DNA-damaging agent adriamycin (ADR; topoisomerase IIα inhibitor) requires the Grb2-binding site of c-Met, and overexpression of the Grb2-associated binder Gab1 (a multisubstrate adapter required for epithelial morphogenesis) inhibits the ability of HGF/SF to protect MDCK epithelial cells against ADR. In contrast to Gab1 and its homolog Gab2, overexpression of c-Cb1, another multisubstrate adapter that associates with c-Met, did not affect protection. Gab1 blocked the ability of HGF/SF to cause the sustained activation of c-Akt and c-Akt signaling (FKHR phosphorylation). The Gab1 inhibition of sustained c-Akt activation and of cell protection did not require the Gab1 pleckstrin homology or SHP2 phosphatase-binding domain but did require the PI3K-binding domain. HGF/SF protection of parental MDCK cells was blocked by wortmannin, expression of PTEN, and dominant negative mutants of p85 (regulatory subunit of PI3K), Akt, and Pak1; the protection of cells overexpressing Gab1 was restored by wild-type or activated mutants of p85, Akt, and Pak1. These findings suggest that the adapter Gab1 may redirect c-Met signaling through PI3K away from a c-Akt/Pak1 cell survival pathway.
Hepatocyte growth factor (scatter factor) (HGF/SF), a pleiotropic mediator of epithelial cell motility, proliferation, morphogenesis, angiogenesis, and tumorigenesis, has been implicated as a mediator of cell survival, via its ability to activate an antiapoptosis pathway(s) (6, 17). HGF/SF blocked apoptosis of Madin-Darby canine kidney (MDCK) epithelial cells induced by the loss of adherence to the substratum (termed anoikis) (17), suggesting that HGF/SF can compensate for the loss of an integrin-linked cellular survival signal. A constitutively active, oncogenic form of the c-Met tyrosine kinase, the HGF/SF receptor (8), blocked apoptosis and allowed immortalization of cultured hepatocytes (5).
In recent studies, HGF/SF, through the c-Met receptor, was found to protect epithelial, carcinoma, and glioma cell lines against cytotoxicity and apoptosis induced by DNA-damaging agents, such as ionizing radiation and various chemotherapy drugs (9, 12). Interestingly, HGF/SF not only blocked DNA damage-induced apoptosis but also enhanced the rate of repair of DNA strand breaks (11). The maximal protection of epithelial cells by HGF/SF required preincubation for 48 h before exposure to the DNA-damaging agent, suggesting a requirement for new protein synthesis; the protection was completely blocked by a highly specific c-Met receptor antagonist (NK1) (12). In MDA-MB-453 human breast cancer cells, the ability of HGF/SF to protect against the DNA-damaging agent adriamycin (ADR) correlated with its ability to prevent the ADR-induced down-regulation of the levels of the antiapoptotic protein Bcl-XL (11, 12). These findings raise the possibility that HGF/SF, which accumulates within breast cancers, gliomas, and other tumor types, could mediate radioresistance and chemoresistance in these settings.
The signaling pathway, for HGF/SF-mediated cell protection and DNA repair has not been fully elucidated. However, in MDA-MB-453 cells and in glioma cell lines, this protection pathway appears to involve signaling through phosphatidylinositol 3′-kinase (PI3K) and activation of the serine/threonine kinase c-Akt (protein kinase B) (9, 11). Thus, disruption of the enzymatic activities of PI3K or c-Akt significantly attenuated the ability of HGF/SF to protect breast cancer and glioma cell lines against DNA-damaging agents and to stimulate the repair of DNA strand breaks. c-Akt has previously been implicated as a mediator of other cell survival pathways, such as that activated by insulin-like growth factor 1 (16, 25).
Much of the c-Met receptor signaling for cell motility, morphogenesis, and transformation involves association of signaling intermediary proteins to a unique multifunctional docking site in the intracellular portion of the activated receptor, 1349YVHVXXX1356YVNV (32, 47). Mutation of either tyrosine of the multifunctional docking site, especially 1356Y, significantly reduces the biologic functions of the receptor. The Grb2-associated binder (Gab1) has recently been identified as a multisubstrate adapter protein of the insulin-responsive substrate 1 family that associates with the c-Met receptor and mediates epithelial morphogenesis (i.e., the formation of a three-dimensional network of branching tubules by MDCK cells cultured within a collagen gel) (21, 44). Thus, overexpression of Gab1 in MDCK cells restored the ligand-induced tubulogenesis mediated by a chimeric c-Met receptor defective in the multisubstrate docking site (28).
The ability of Gab1 to mediate the MDCK tubulogenic response required PI3K-dependent activation of Gab1 as well as the localization of Gab1 to sites of cell-cell contact, mediated by the amino-terminal pleckstrin homology (PH) domain of Gab1 (28). Within the PH domain of Gab1, a conserved inositol phospholipid-binding site was found to be essential for Gab1-mediated epithelial morphogenesis (29).
Although overexpression studies have implicated Gab1 as a positive regulator of c-Met-mediated epithelial morphogenesis, a positive or negative role for Gab1 in the regulation of other c-Met functional activities has not been established. In this study, we found that Gab1 is a potent inhibitor of HGF/SF–c-Met signaling pathways for cell survival and DNA repair downstream of PI3K. We also demonstrated that the structural requirement for Gab1 regulation of cell survival and DNA repair is qualitatively distinct from that for epithelial morphogenesis.
MATERIALS AND METHODS
Sources of reagents and antibodies.
Recombinant human HGF/SF was provided by Ralph Schwall, Department of Endocrine Research, Genentech, Inc. (South San Francisco, Calif.). ADR (doxorubicin hydrochloride) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye (thioazyl blue) were purchased from Sigma Chemical Co. (St. Louis, Mo.). The PI3K inhibitor wortmannin was obtained from Biomol. Cell-permeable caspase-3 inhibitor I (235423) and caspase-6 inhibitor II (218767) were obtained from Calbiochem-Novabiochem Corporation (La Jolla, Calif.). The final concentrations of inhibitors used in this study were as follows: wortmannin, 50 nM; caspase inhibitors, 10 μM each.
The primary antibodies used for Western blotting were specific to hemagglutinin (HA) (HA.11; 1:500 dilution; BAbCO, Richmond, Calif.), p85 (PI3K regulatory subunit) (B-9) (Catalog no. sc-1637, mouse monoclonal immunoglobulin G [IgG]; 1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, Calif.), p110 (PI3K catalytic subunit) (catalog no. I-19; 1:300 dilution; Santa Cruz), Gab1 (C-20; catalog no. sc-6292; 1:200 dilution; Santa Cruz), α-actin (I-19; 1:1,000 dilution; Santa Cruz), total c-Akt (antibody 9272; 1:500 dilution; New England Biolabs, Inc., Beverly, Mass.), phospho-Akt (Ser-473) (antibody 9271S; 1:500 dilution; New England Biolabs), total forkhead family transcription factor FKHR (antibody 9462; 1:500 dilution; New England Biolabs), and phospho-FKHR (Ser-256) (antibody 9461; 1:500 dilution; New England Biolabs).
Cell lines and culture.
MDCK cells were originally obtained from the American Type Culture Collection (Manassas, Va.). Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal calf serum, 5 mM glutamine, streptomycin (100 μg/ml), and penicillin (100 U/ml). Cell culture reagents were obtained from BioWhittaker (Walkersville, Md.).
MDCK cell lines stably expressing chimeric receptors consisting of the extracellular ligand-binding domain of the colony-stimulating factor 1 (CSF-1) receptor and the intracellular portion of the wild-type (wt) or mutant c-Met receptor have been described earlier (15, 48). MDCK cell clones expressing amino-terminal HA-tagged wt or mutant Gab1 and wt or mutant c-Cb1 have also been described previously (21, 22, 28, 43). See Results for further descriptions of these constructs and cell lines.
Expression vectors and cell transfections.
The HA-Gab2 expression vector (in the pcDNA3.1 vector) was provided by Gen-Sheng Feng (The Burnham Institute, La Jolla, Calif.) (46). The Akt expression vectors were provided by Michael Quon (National Heart Lung and Blood Institute, Bethesda, Md). These included vectors encoding wt Akt, a constitutively active myristolated Akt (Akt-myr), and a dominant negative (DN) kinase-inactive (K179A) Akt (DN Akt) (10). Expression vector pFLAG-CMV-PTEN was used to express PTEN (19). The PTEN vector was provided by M. M. Georgescu (Rockefeller University, New York, N.Y.). Wild-type and kinase-dead (K299R) DN mutant p21-associated kinase (Pak1) expression vectors were used to experimentally manipulate the intracellular Pak1 protein levels or activity (1). Expression vectors encoding wt p110 (catalytic subunit of PI3K) or constitutively membrane-localized forms of p110 (p110-myr and p110CAAX) have been described earlier (22). Plasmid pRSV-Ras-N17, encoding a DN Ras protein, was provided by Richard Pestell (Albert Einstein College of Medicine, Bronx, N.Y.) (2). The DN p85 expression vector [p85(DN)] encodes a protein containing a deletion within the Src homology 2 (SH2) domain of the regulatory subunit of PI3K.
For transient transfections, subconfluent proliferating cells were transfected overnight using Lipofectamine (Life Technologies) (10 μg of plasmid DNA per 100-mm-diameter dish) and then washed to remove the excess vector and Lipofectamine. As a control for transfection efficiency, cultures were cotransfected with 10 μg of a β-galactosidase (β-Gal) expression vector (pSV-β-gal; Promega, Madison, Wis.) under parallel conditions; β-Gal was detected using a 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining kit (Gene Therapy Systems, Inc., San Diego, Calif.).
[ADR] treatment.
Subconfluent proliferating cells in 100-mm-diameter plastic dishes or 96-well plates were preincubated with or without HGF/SF (100 ng/ml for 48 h) and then sham treated (control) or treated with the indicated concentration of ADR (2 h at 37°C) in complete culture medium (Dulbecco modified Eagle medium plus 10% fetal calf serum). Cultures were then washed twice to remove the ADR, postincubated in fresh drug-free complete culture medium at 37°C for 24 or 72 h, and then harvested for assays of DNA strand breakage (24 h), apoptosis (72 h), or cell viability (72 h).
MTT cell viability assay.
The assay is based on the ability of viable mitochondria to convert MTT, a soluble tetrazolium salt, into an insoluble formazan precipitate, which is dissolved in dimethyl sulfoxide and quantitated by spectrophotometry (3). Cells were seeded into 96-well dishes (2,000 cells per well) in standard growth medium, incubated for 24 to 48 h to allow attachment and entry into the cell cycle, preincubated with or without HGF/SF (100 ng/ml for 48 h), treated with ADR for 2 h, postincubated for 72 h, and tested for MTT dye conversion. Cell viability was calculated as the amount of MTT dye conversion relative to sham-treated control cells.
DNA filter elution assays.
Subconfluent proliferating cells were labeled with [3H]thymidine (0.02 μCi/ml for 32 h), chased for 2 h in isotope-free medium, exposed to ADR (see above), washed twice, incubated in fresh drug-free complete culture medium for 24 h, and counted by hemacytometer. Equal numbers of cells (2 × 106) were loaded onto nonproteinizing polycarbonate filters, lysed, and subjected to alkaline elution or neutral elution (7). Radioactivity in the DNA fractions was counted, and the fraction of DNA eluted was calculated as elution fraction/[filter + lysis + elution fraction]. Elution of DNA under alkaline conditions reflects the presence of single-strand breaks (SSBs); elution under neutral conditions reflects double-strand breaks (DSBs).
DNA fragmentation (apoptosis) assays.
DNA fragmentation was assessed by agarose gel electrophoresis (20). Cells were harvested by centrifugation, washed twice in phosphate-buffered saline (PBS), and then resuspended in 200 μl of lysis buffer (50 mM Tris-HCl [pH 7.5], 0.1 M EDTA, 1% NP-40). The supernatants were incubated with 1% sodium dodecyl sulfate (SDS) and 5 mg of RNase A per ml for 2 h at 37°C and treated overnight with 2.5 mg of proteinase K per ml at 56°C. DNA was precipitated with 1/2 volume of 10 M ammonium acetate and 2.5 volumes of 95% ethanol. Precipitated DNA was dissolved in gel loading buffer and analyzed by electrophoresis on a 1.5% agarose gel containing 0.1 mg of ethidium bromide per ml to visualize DNA ladders. The gels were photographed under UV light.
IP.
Subconfluent proliferating cells in 150-cm2 dishes were harvested, and whole-cell extracts were prepared, as described below. Each immunoprecipitation (IP) was carried out using 6 μg of antibody and 1,000 μg of total extract protein. Precipitated proteins were collected using protein G beads, washed, eluted in boiling Laemmli sample buffer, and subjected to Western blotting. The IP antibodies were anti-human Gab1 (catalog no. 06-579, rabbit polyclonal IgG; Upstate Biotechnology, Lake Placid, N.Y.), c-Met C-terminal [c-met (C-Term)] antibody SP260 (catalog no. sc-162; Santa Cruz), and anti-HA (HA.11, mouse monoclonal; BAbCO). The control IP antibody was an equivalent quantity (6 μg) of normal mouse IgG (Santa Cruz).
Western blotting.
Western blotting was performed essentially as described earlier (13). Cells were centrifuged, washed with PBS, and lysed at 0°C for 30 min in lysis buffer [1% NP-40 in PBS containing 2 mM 4-(2-aminoethylbenzenesulfonyl fluoride, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 mM NaF, 1 mM sodium orthovandate, 5 mM sodium pyrophosphate; 100 to 200 μl per 100-mm-diameter dish]. Protein content was determined by the Bio-Rad (Hercules, Calif.) dye-binding microassay and 100 μg of protein per lane was electrophoresed on SDS–12% polyacrylamide gels after boiling for 5 min in Laemmli sample buffer. Proteins were blotted onto Immobilon membranes (Millipore, Bedford, Mass.). Equal protein loading was confirmed by fast green staining of the membrane and by blotting for α-actin as a control for loading and transfer. Colored markers (Bio-Rad) were used as size standards.
After electroblotting, the membranes were blocked in PBS-Tween blocking buffer containing 5% nonfat dry milk, washed with PBS-Tween buffer, and incubated with the primary antibody (see above) diluted in blocking buffer for 1 h. Primary antibody dilutions were those recommended by the manufacturers and ranged from 1:300 to 1:1,000. Membranes were then washed, incubated with the appropriate second antibody (1:3,000) in blocking buffer for 1 h, and rewashed. Blotted proteins were detected using an enhanced chemiluminescence detection system (Amersham Life Sciences). Where indicated, protein bands were quantitated by densitometry and expressed relative to α-actin.
Statistical analyses.
Where appropriate, statistical comparisons were made using the two-tailed Student t test.
RESULTS
Structural requirements for c-Met receptor-mediated cell signaling for cytoprotection.
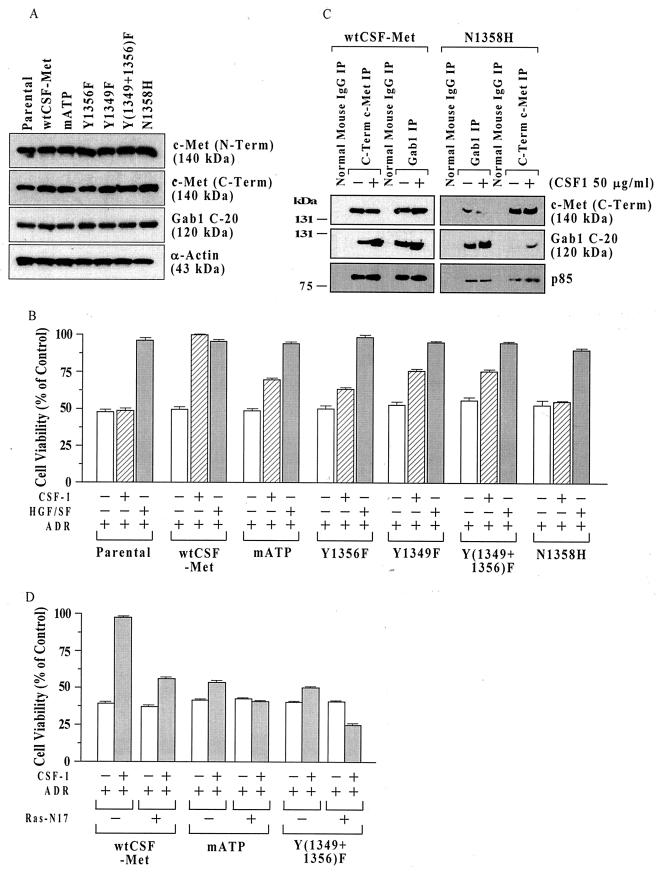
To identify sites within c-Met essential for signaling for survival, we used a series of MDCK cell lines expressing chimeric receptors composed of the extracellular ligand-binding domain of the CSF-1 receptor linked to wt or mutant forms of the intracellular portion of the c-Met receptor (CSF-Met) (15, 48). Figure 1A shows Western blot characterization of these cell lines using antibodies against the N terminus of c-Met (N-Term) (ligand-binding domain, β- chain), c-Met (C-Term) (intracellular region, β chain), and the multi substrate adapter protein Gab1. The c-Met (N-Term) antibody detects only the endogenous wild-type HGF/SF receptor, while the c-Met (C-Term) antibody should detect both the endogenous and chimeric Met receptors. There were no obvious differences in the levels of endogenous c-Met receptor or Gab1 in the different cell lines. However, compared to the other cell lines, the parental cells showed somewhat decreased levels of c-Met (C-Term), consistent with the absence of chimeric receptor in these cells.
FIG. 1.
Structural requirements for c-Met signaling for cell survival in MDCK cells. (A) c-Met protein levels in cell lines expressing different chimeric Met receptors. Subconfluent proliferating cells were harvested, and equal aliquots of total cell protein (50 μg per lane) were Western blotted, using antibodies directed against c-Met (N-Term), c-Met (C-Term), the multisubstrate adapter Gab1, and α-actin (control for loading and transfer). (B) MTT assays. Parental MDCK cells or clones expressing wt or mutant chimeric CSF-Met receptors were tested for protection against ADR by the chimeric receptor. Subconfluent proliferating cells in 96-well dishes were sham treated (negative control) or preincubated with recombinant human HGF/SF (100 ng/ml; positive control) or recombinant human CSF-1 (50 ng/ml) for 48 h, exposed to ADR (15 μM for 2 h), washed, postincubated in fresh drug-free culture medium for 72 h, and assayed for cell viability using the MTT assay. Cell viability values (relative to sham-treated controls) are means ± standard errors of 10 replicate wells. The survival of untransfected parental cells was not altered by CSF-1, while in wt CSF-Met cells, the protection by CSF-1 was similar to that of HGF/SF. For cells with mutant CSF-Met but not for wt CSF-Met cells, the viability of CSF-1–ADR-treated cells was less than that for HGF/SF-ADR-treated cells (P < 0.001, two-tailed t tests). (C) Decreased association of Gab1 and p85 with N1358H mutant Met receptor. Subconfluent proliferating cultures of MDCK cells expressing wt CSF-Met or N1358H mutant Met receptors were treated with or without CSF-1 (50 ng/ml for 48 h) and subjected to IP using antibodies against c-Met (C-Term), Gab1, or normal mouse IgG as a control. IPs were Western blotted for c-Met (C-Term), Gab1 (C-20), or p85 (the catalytic subunit of PI3 kinase). (D) Inhibition of protection by mATP and Y(1349+1356)F receptors by DN Ras. Subconfluent proliferating cells in 100-mm-diameter dishes were transfected overnight without or with 10 μg of a DN Ras expression vector (Ras-N17), washed, subcultured into 96-well dishes, preincubated with or without CSF-1 (50 ng/ml for 48 h), exposed to ADR (15 μM for 2 h), postincubated for 72 h, and assayed for MTT dye conversion. Ras-N17 caused a significant reduction in the survival of CSF-1-treated cells for all three cell types (P < 0.001) but did not affect the cell survival in the absence of CSF-1.
These cells lines, which contain little or no endogenous CSF-1 receptor, were preincubated with CSF-1 (50 ng/ml for 48 h), exposed to ADR (a DNA topoisomerase IIα inhibitor that causes SSBs and DSBs), postincubated in drug-free medium, and assayed for cell viability using the MTT dye conversion assay. As controls, preincubation with CSF-1 strongly protected wt CSF-Met cells but not untransfected parental MDCK cells, which express little or no endogenous CSF-1 receptor, against ADR-induced cytotoxicity (Fig. 1B). On the other hand, preincubation with HGF/SF (100 ng/ml for 48 h) protected wt CSF-Met, parental MDCK, and each mutant CSF-Met cell line against ADR, consistent with the presence of the endogenous wt c-Met receptor in all of these cell lines.
Chimeric receptors with mutations mapping to ATP binding site of the kinase catalytic domain (mATP) or the tyrosine residues of the multifunctional docking site [Y1349F, Y1356F, and Y(1349+1356)F] were all defective in the ability to mediate protection against ADR. In different experiments, these mutations reduced the degree of protection (i.e., the increment in survival induced by CSF-1) to 15 to 50% of that of the wt CSF-Met receptor. However, in multiple independent experiments, the double tyrosine mutant [Y(1349+1356)F] did show some residual degree of protection by CSF-1, indicating that the loss of these tyrosines does not fully abrogate the protection pathway. Interestingly, the N1358H mutation, which selectively abolishes Grb2 binding (15, 35), nearly fully abolished cytoprotection, suggesting the importance of this site for antiapoptotic signaling.
Since Gab1 binding to the Met receptor is thought to be mediated, in part, indirectly through interaction with Grb2 (31, 37), we compared the association of Gab1 with the wt CSF-Met versus the N1358H mutant receptor by IP-Western blotting. Although it was possible to immunoprecipitate roughly equal quantities of c-Met (C-Term) and Gab1 from these two cell types, the quantities of Gab1 in the c-Met (C-Term) IP and of Met in the Gab1 IP were considerably reduced for the N1358H mutant compared with the wt CSF-Met cell line (Fig. 1C). Furthermore, the amount of p85 (regulatory subunit of PI3 kinase) associated with the Met receptor was also decreased in the N1358H mutant cell line.
Although the multifunctional docking site (1349YVHVXXX1356YVNV) is known to mediate most c-Met receptor signaling activities, a recent study suggests that mutations of both tyrosine residues as well as other tyrosines of the Met intracellular domain does not abrogate Ras signaling or cell scattering (42). In the experiment shown in Fig. 1D, cells were transiently transfected with a DN Ras expression vector (Ras-N17) and then assayed for protection against ADR by CSF-1, through the chimeric CSF-Met receptor. The DN Ras significantly reduced but did not abrogate protection by the wt CSF-Met receptor. However, the residual receptor-mediated protection by the Y(1349+1356)F and mATP mutants was abrogated by the DN Ras. The potential significance of these findings is considered in Discussion.
MDCK cell lines overexpressing wt and mutant signaling adapters.
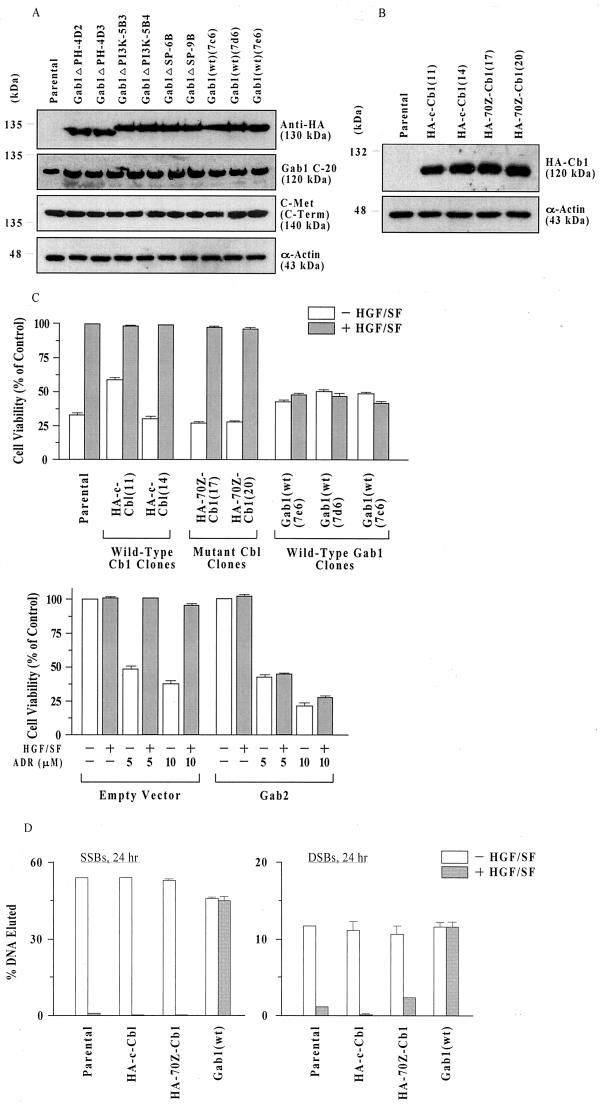
To investigate the role of multisubstrate adapter proteins in the regulation of the c-Met-mediated cell survival and cytoprotection response, we studied a series of MDCK cell lines overexpressing wt or mutant forms of Gab1 and c-Cb1, each expressed with an HA tag, to allow convenient immunodetection. The Gab1-expressing cell lines included three cell clones expressing wt Gab1 [Gab1(wt)] and two clones each expressing Gab1 mutants defective in the PH domain [Gab1ΔPH], SHP2 phosphatase-binding domain [Gab1ΔSP], and PI3K-binding domain [Gab1ΔPI3K]. The Cb1 cell lines included two clones each expressing wt Cb1 (HA-c-Cb1) and mutant Cb1 (HA-70Z-Cb1). Anti-HA Western blots verified the expression of all of the tagged proteins (Fig. 2A and B).
FIG. 2.
Overexpression of Gab1 but not c-Cb1 blocks HGF/SF protection of MDCK. (A) Anti-HA, Gab1 (C-20), and c-Met (C-Term) Western blots of MDCK cell lines stably expressing different forms of HA-Gab1. Subconfluent proliferating cells were harvested, and equal aliquots of total cell protein (50 μg per lane) were blotted as described in Materials and Methods. (B) Anti-HA Western blots of MDCK cell lines stably expressing HA-Cb1 cDNAs. Assays were performed as described for panel A. (C) Gab1 and its homolog Gab2 block protection against ADR-induced toxicity. (Top) Subconfluent proliferating cells in 96-well dishes were preincubated with or without HGF/SF (100 ng/ml for 48 h), exposed to ADR (15 μM for 2 h), washed, postincubated in fresh drug-free culture medium for 72 h, and assayed for MTT dye conversion. Cell viability values are means ± standard errors for cell lines expressing wt Cb1 (HA-c-Cb1), mutant Cb1 (HA-70Z-Cbl), Gab1 (wt) (Bottom) and parental cells. Values plotted were calculated from 10 replicate wells. (Bottom) Parental MDCK cells were transfected overnight with Gab2 or empty vector (10 μg of plasmid DNA per 100-mm-diameter dish) plus Lipofectamine, inoculated in 96-well dishes, and assayed as described above. For each experimental condition, two separate dishes were transfected; 10 replicate wells were tested per dish. Values are means ± ranges of the two dishes. For each ADR dose, cell survival based on pooled data for the two dishes was significantly increased by HGF/SF treatment of empty vector (P < 0.001) but not Gab2-transfected (P > 0.05) cells. (D) Gab1 blocks HGF/SF-stimulated repair of DNA strand breaks. Subconfluent proliferating cells in 100 mm-diameter dishes were preincubated with or without HGF/SF (100 ng/ml for 48 h), exposed to ADR (20 μM for 2 h), washed, and postincubated for 24 h in fresh drug-free culture medium. The number of SSBs or DSBs was determined by DNA filter elution assays and expressed as the fraction of DNA eluted minus that for sham-treated control cells. Results are shown for the same cell types as in panel C. Values are means ± ranges or standard errors for two or three clones of each type.
Note that all of the Gab1 cell lines contain endogenous (canine) Gab1, which migrates slightly more rapidly (120 kDa) than the murine HA-Gab1 (130 kDa) and is detected by an antibody raised against the C terminus of human Gab1 (C-20). However, the C-20 antibody does not detect the murine HA-Gab1 (Fig. 2A). Furthermore, these cell lines and the parental cells contained roughly equal quantities of c-Met receptor protein.
The Gab1 and c-Cb1 mutants have been described before (21, 22, 28). The Gab1ΔPI3K mutant contains three Y→F mutations in the carboxyl-terminal portion of the Gab1 protein (amino acids 447, 472, and 589) corresponding to putative binding motifs (YVPM) for the SH2 domains of the regulatory subunit (p85) of PI3K (22). The substitution of phenylalanines for tyrosines at these sites results in the loss of p85 binding to Gab1. The Gab1ΔPH mutant is missing the entire amino-terminal PH domain and is composed of amino acids 116 to 695 of the murine Gab1 cDNA; the Gab1ΔSP mutant has an inactivating point mutation at the SHP2-binding site (Y627F) (21). HA-70Z-Cb1 encodes a mutant Cb1 with an internal deletion of 17 amino acids within the amino-terminal C3HC4 ring finger motif. This mutation results in constitutive phosphorylation of Cb1, its association with growth factor receptors, and its dominant oncogenic activity (18, 43).
Overexpression of Gab1 but not c-Cb1 abrogates HGF/SF-mediated cytoprotection.
MDCK cell lines stably expressing cDNAs for wt c-Cb1, mutant Cb1, and Gab1 (wt) were tested for HGF/SF-mediated protection against ADR, using assays of (i) cell viability (MTT dye conversion assay) and (ii) repair of DNA strand breaks (DNA filter elution assay). Two clones each of HA-c-Cb1, HA-70Z-Cb1, and Gab1(wt) were tested. Compared with untransfected parental cells as a positive control, clones expressing wt or mutant Cb1 showed similarly strong degrees of HGF/SF-mediated protection against loss of cell viability (Fig. 2C, top). However, in contrast to cell clones overexpressing Cb1, clones overexpressing Gab1(wt) showed abrogation of or a greatly decreased degree of protection by HGF/SF in the MTT assay.
Recently, a structural and functional homolog of Gab1 designated Gab2 was identified and cloned (46). When an expression vector for Gab2 was introduced into the parental MDCK cells by transient transfection, we found that like Gab1, Gab2, but not the empty vector, abrogated the ability of HGF/SF to protect against ADR (Fig. 2C, bottom panel). Furthermore, the overexpression of Gab1(wt) but not of c-Cb1 (wt or mutant) also blocked the enhancement of DNA repair activity induced by treatment with HGF/SF (Fig. 2D). These findings suggest that members of the Gab1 gene family but not c-Cb1 can act to regulate HGF/SF-mediated cell protection and DNA repair.
Since PI3K signaling has been implicated in HGF/SF protection of other cell types, we performed MTT assays of ADR sensitivity of MDCK cells treated with HGF/SF with or without wortmannin (50 nM), a selective inhibitor of PI3K. Wortmannin partially, but significantly, inhibited HGF/SF-mediated protection in parental cells and in two clones each of wt and mutant Cb1 cells (data not shown). The degree of protection was reduced by about half in the presence of wortmannin. However, in the Gab1(wt) cell clones, there was little or no protection by HGF/SF in the absence or presence of wortmannin. Higher doses of wortmannin were toxic to MDCK cells, and so it is not clear if the 50% reduction of protection was the maximum achievable by PI3K inhibition.
The PI3K-binding domain of Gab1 is required for the regulation of HGF/SF–c-Met-mediated cytoprotection.
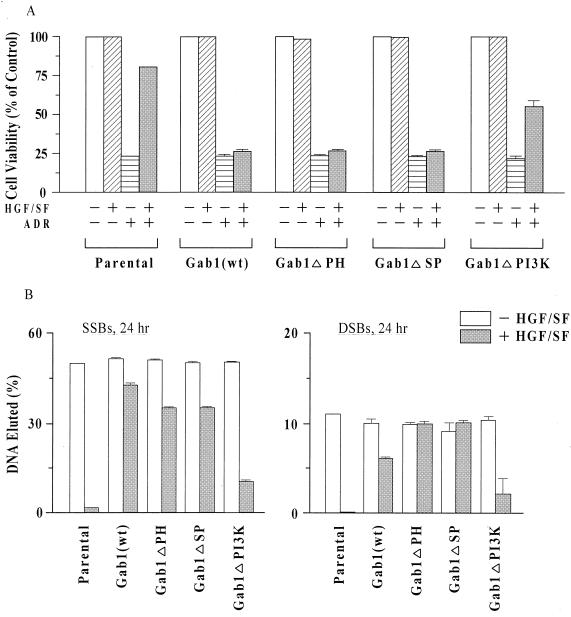
To assess the structural-functional requirement for Gab1 abrogation of HGF/SF-mediated cell protection and DNA repair, we studied a set of MDCK cell clones expressing the three different mutant forms of Gab1 described above (Fig. 2A). The responses to ADR were determined for two independent clones each of MDCK cells expressing the defective Gab1 proteins. These responses were compared to that of three clones of MDCK Gab1(wt) cells, as a control for the Gab1 abrogation of HGF/SF protection, and to parental MDCK cells, as a control to demonstrate the baseline extent of HGF/SF mediated protection and DNA repair.
Based on the MTT assays, Gab1(wt), Gab1ΔPH, and Gab1ΔSP cell lines all showed little or no evidence of protection: cell viability was less than 5% higher in cultures treated with HGF/SF plus ADR compared with ADR alone (Fig. 3A). In contrast, Gab1ΔPI3K cells showed a large degree of cytoprotection, although the survival of HGF/SF-ADR-treated Gab1ΔPI3K cells was usually lower than that of similarly treated parental cells. DNA filter elution assays revealed that HGF/SF-ADR-treated Gab1ΔPI3K cells repaired DNA SSBs and DSBs more efficiently than similarly treated Gab1(wt), Gab1ΔPH, and Gab1ΔSP cell lines; i.e., there were fewer residual DNA strand breaks at 24 h after ADR exposure (Fig. 3B). Thus, with respect to HGF/SF-mediated cell survival and DNA repair, the Gab1ΔPH and Gab1ΔSP cell types behaved similarly to the Gab1(wt) cells, while the Gab1ΔPI3K cells behaved more similarly to the untransfected parental cells. These findings suggest that the PI3K-binding domain, but not the PH or SHP2-binding domains, of Gab1 is essential for regulation of HGF/SF-mediated protection.
FIG. 3.
Ability of Gab1 to abrogate HGF/SF-mediated protection requires the PI3K-binding domain of Gab1. (A) MTT assays of cell viability. Assays were carried out as described for Fig. 2C. Cell viability values (relative to sham-treated control cells) are means ± standard errors for three separate Gab1(wt) cell clones and means ± ranges for two separate clones each of Gab1ΔPH, Gab1ΔSP, and Gab1ΔPI3K. For each cell line, 10 replicate wells were tested. Cell viability values were significantly higher in HGF/SF-ADR-treated parental or Gab1ΔPI3K cells than in the corresponding ADR-treated cells (P < 0.001). (B) DNA filter elution assays of DNA strand breaks. Assays were performed as described for Fig. 2D, using the same clonal types as in panel A. Values are means ± standard errors or ranges for two or three clones of each type.
Overexpression of Gab1 abrogates sustained HGF/SF-induced activation of c-Akt.
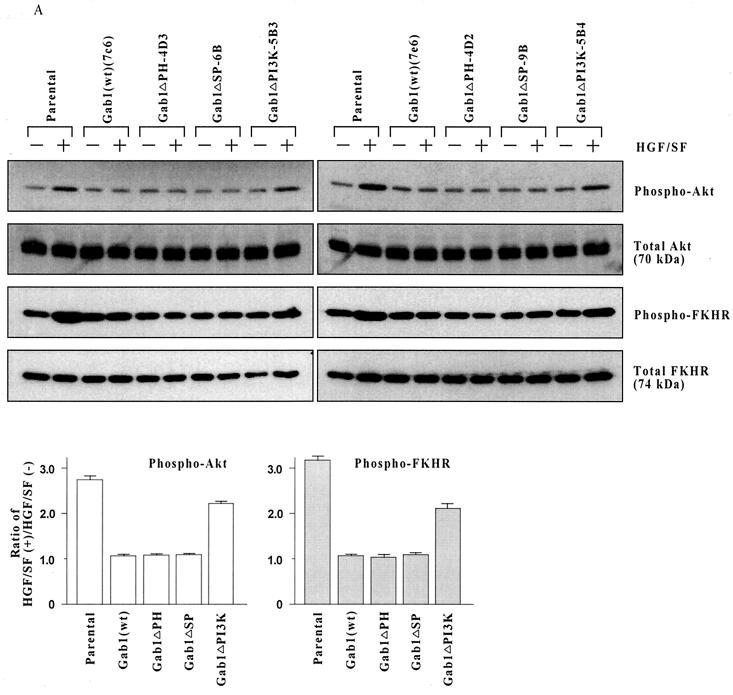
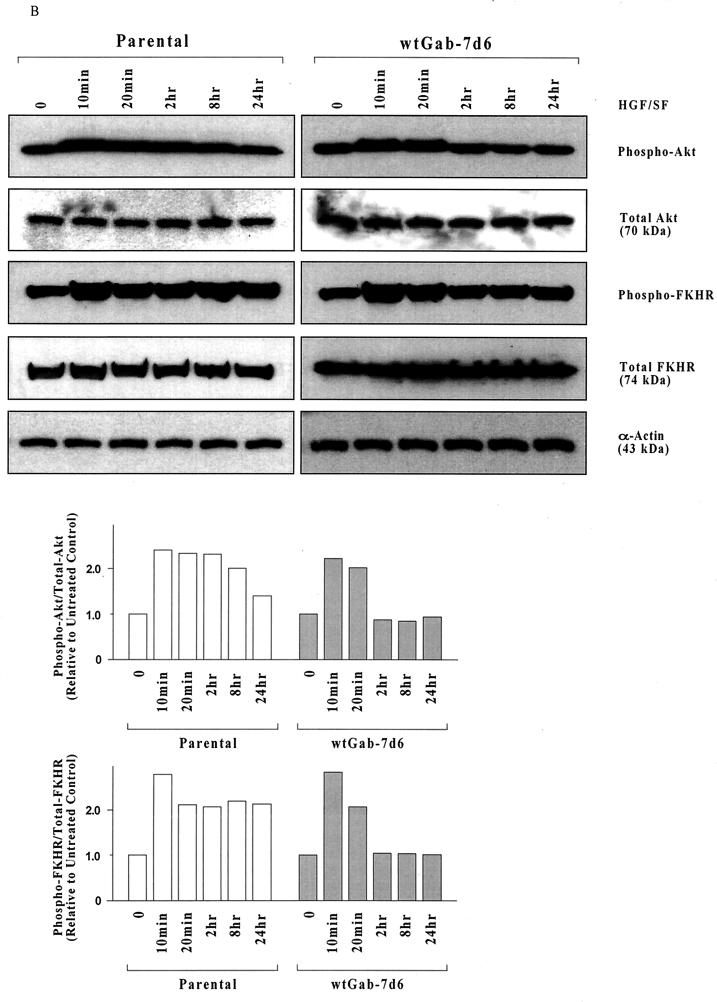
We had previously implicated signaling through c-Akt in HGF/SF-stimulated protection and DNA repair in human breast cancer cell line MDA-MB-453 (11). Thus, c-Akt is a logical target for the Gab1 regulation of HGF/SF cell survival signaling. To determine if Gab1 regulates the ability of HGF/SF to induce phosphorylation and activation of c-Akt, MDCK cells were incubated with or without HGF/SF (100 ng/ml for 48 h) and subjected to Western blotting using antibodies that detect all forms of Akt (total Akt) or only Akt phosphorylated on Ser-473 (phospho-Akt). Ser-473 phosphorylation of c-Akt usually correlates with Akt activation.
In contrast to the parental cells, Gab1(wt) cells failed to show an increase in the proportion of phospho-Akt to total Akt in response to HGF/SF (Fig. 4A). Similarly, the Gab1ΔPH and Gab1ΔSP cell clones failed to exhibit any induction of Akt phosphorylation in response to HGF/SF. However, Gab1ΔPI3K cells showed an easily detectable increase in Akt phosphorylation after treament with HGF/SF. Thus, the abrogation of signaling through c-Akt may contribute to the loss of cytoprotection in cells overexpressing Gab1.
FIG. 4.
Gab1 overexpression inhibits HGF/SF-induced sustained activation of c-Akt. (A) HGF/SF induces prolonged c-Akt activation and FKHR phosphorylation in parental but not Gab1(wt) cells. Subconfluent proliferating cultures of two clones of each type in 100-mm-diameter dishes were preincubated with or without HGF/SF (100 ng/ml for 48 h), harvested, and Western blotted to detect total Akt, Akt phosphorylated on Ser-473 (phospho-Akt), total FKHR, or FKHR phosphorylated on Ser-256 (phospho-FKHR). Each lane corresponds to an aliquot of 50 μg of total cell protein. The bar graphs show the ratios of phospho-Akt and of phospho-FKHR in cells treated with (+) HGF/SF relative to cells treated without (−) HGF/SF, as determined by densitometry. Values are means ± ranges for two clones of each clonal type. (B) HGF/SF causes only transient c-Akt activation and FKHR phosphorylation in Gab1(wt) cells. Assays were performed as for panel A except that shorter incubations with HGF/SF (10 min to 24 h) were used.
The forkhead family transcription factor FKHR, which activates proapoptotic genes such as Fas ligand, was identified as a substrate for Akt-mediated phosphorylation (41). Phosphorylation of FKHR blocks its nuclear translocation and therefore its transcriptional activity. We used an antibody which detects only FKHR that has been phosphorylated on one of three specific Akt phosphorylation sites, Ser-256 (within the forkhead domain), to assess Akt signaling. Similar to results for c-Akt, HGF/SF-induced FKHR phosphorylation was detected in parental and to a lesser extent, Gab1ΔPI3K cells but was not detected in the Gab1(wt), Gab1ΔPH, or Gab1ΔSP cell clones (Fig. 4A).
The assays in Fig. 4A were performed after 48 h of HGF/SF stimulation. When shorter time points were tested, we found that Gab1(wt) cells showed initial activation of c-Akt and phosphorylation of FKHR at 10 to 20 min, which then returned to baseline by 2 h or earlier (Fig. 4B). However, the parental cells showed Akt and FKHR phosphorylation that remained above baseline levels for up to 24 h (Fig. 4B) and 48 h (Fig. 4A). These findings suggest that overexpression of Gab1 blocks sustained signaling through the Akt pathway but does not block the early activation of Akt induced by HGF/SF.
Overexpression of the PI3K regulatory subunit (p85) but not the catalytic subunit (p110) blocks the Gab1 inhibition of HGF/SF protection.
To determine if overexpression of the catalytic subunit of PI3K (p110) could stimulate protection of MDCK cells, parental cells as well as a Gab1(wt) clone were transiently transfected with expression vectors for wt p110 or constitutively active membrane-localized forms of p110 (p110-myr and p110CAAX) and then assayed for HGF/SF-mediated protection against ADR. Transient transfection of each of the three p110 vectors resulted in a significant increase in the total p110 protein levels (as detected using an anti-p110 antibody), indicating that the p110 genes are each well expressed. However, in several independent experiments, none of the three forms of p110 significantly altered the survival of parental cells treated with ADR alone or HGF/SF plus ADR (data not shown). Furthermore, there was little or no increase in the survival of wt p110-, p110-myr-, or p110CAAX-transfected Gab1(wt) cells (data not shown). Thus, different forms of p110 failed to significantly enhance survival of either parental or Gab1-transfected MDCK cells.
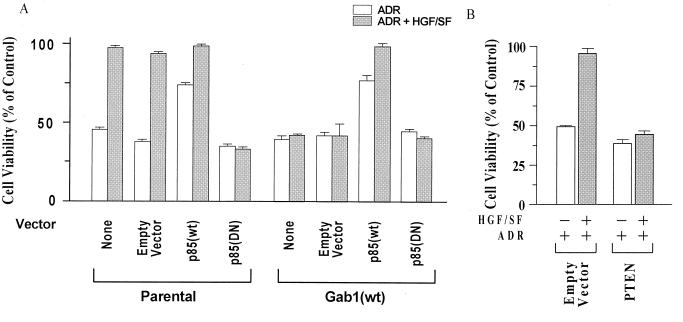
Although the ectopic expression of p110 failed to overcome the Gab1 inhibition of HGF/SF protection, this and prior studies document that pharmacologic inhibitors of PI3K (wortmannin and LY294002) inhibit the protection by HGF/SF of various cell types, including MDCK cells (9, 11). Since it is the regulatory subunit of PI3K (24) that is directly recruited to the activated c-Met receptor and Gab1, we next tested the effect of p85 expression constructs on HGF/SF-mediated cell protection. In contrast to p110, transient transfection of p85(wt) protected both parental and Gab1(wt) cells against ADR in the absence of HGF/SF and enhanced the degree of protection of Gab1(wt) cells in the presence of HGF/SF (Fig. 5A). Furthermore, p85(DN) abrogated the HGF/SF-mediated protection of parental cells against ADR.
FIG. 5.
Effects of wt and mutant p85 (PI3K) and of wt PTEN expression vectors on HGF/SF-mediated protection of MDCK cells. (A) The regulatory subunit of PI3K (p85) modulates MDCK survival. Subconfluent proliferating cells in 100-mm-diameter dishes were transiently transfected overnight, using Lipofectamine and 10 μg of each vector per dish: empty vector (control), p85(wt), or p85(DN). Cells were washed, subcultured into 96-well dishes, preincubated with or without HGF/SF (100 ng/ml for 48 h), exposed to ADR (15 μM for 2 h), washed three times to remove ADR, postincubated for 72 h in fresh drug-free medium, and assayed for MTT dye conversion. Values of cell viability are based on 10 replicate wells. p85(wt) protected parental and Gab1 (wt) cells against ADR in the absence of HGF/SF (P < 0.001); p85(DN) blocked the HGF/SF-mediated protection of parental cells against ADR (P < 0.001). (B) PTEN blocks HGF/SF-mediated protection of parental MDCK cells. Transient transfection assays were performed as for panel A. For the pooled data from the two dishes for each assay condition, cells treated with empty vector plus HGF/SF and ADR showed a significantly reduced cell viability compared with cells treated with (PTEN, HGF/SF, and ADR) (P < 0.001); there was little or no increase in survival of cells treated with PTEN, HGF/SF, and ADR as compared with PTEN plus ADR.
Consistent with these findings, transient transfection of the tumor suppressor PTEN, which blocks signaling downstream of PI3K (16, 19), blocked the ability of HGF/SF to protect parental MDCK cells against ADR (Fig. 5B). It was also noted that the survival (percent cell viability) of PTEN-transfected, ADR-treated cells was 10 to 15% lower than that of non-PTEN-transfected cells. This reduction in the survival of cells not pretreated with HGF/SF could be due to PTEN-mediated inhibition of a survival signal due to a small amount of HGF/SF present in the fetal calf serum or to other growth factors present in the serum. Taken together, these findings suggest that signaling pathways downstream of PI3K that are required for HGF/SF protection are inhibited by Gab1.
c-Akt modulates HGF/SF signaling for cell survival in MDCK cells.
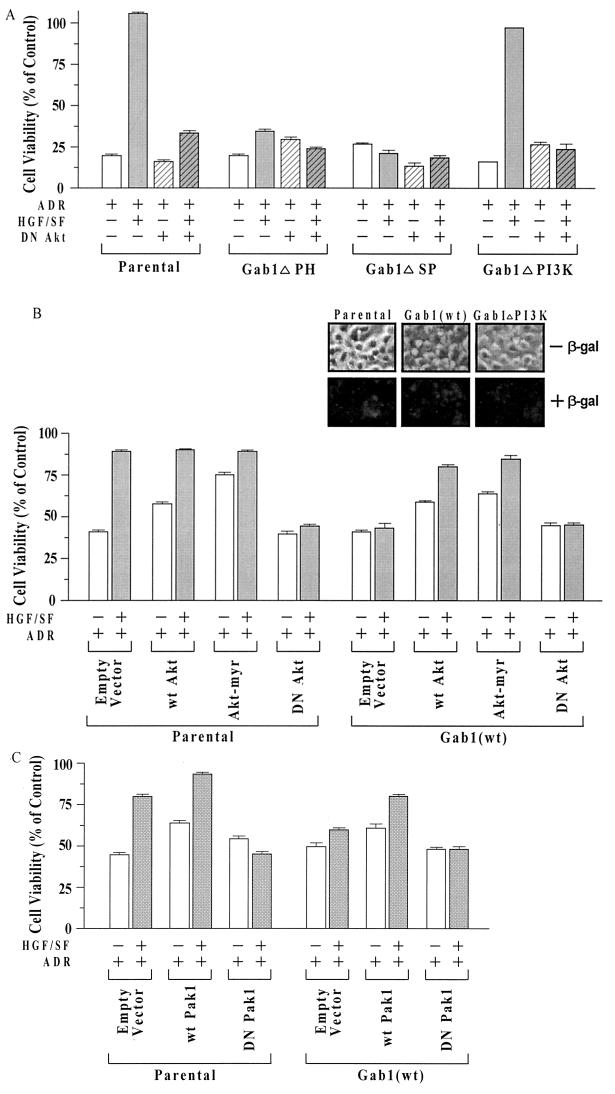
To further investigate the role of Akt in the cytoprotection pathway and its regulation by Gab1, cells were transiently transfected with expression vectors for different Akt mutants and assayed for HGF/SF-mediated protection against ADR. Parental cells and the different Gab1 mutant cell lines (ΔPH, ΔSP, and ΔPI3K) were tested for the effect of DN Akt (10) on cell survival, using the MTT assay (Fig. 6A). In the parental cells, DN Akt reduced the degree of HGF/SF-mediated protection by about 85%. In the Gab1ΔPH and Gab1ΔSP clones, which already showed little or no HGF/SF protection, there was hardly any effect of the DN Akt vector; in the Gab1ΔPI3K clones, the HGF/SF-mediated protection was completely lost. These findings suggest that a pathway(s) downstream of c-Akt is predominantly responsible for HGF/SF-mediated protection in MDCK parental and Gab1ΔPI3K cells.
FIG. 6.
Effect of mutant Akt constructs on HGF/SF-mediated cytoprotection in MDCK cells expressing wt or mutant forms of Gab1. (A) DN Akt strongly inhibits HGF/SF-mediated protection. Transient transfection assays were performed analogously to those shown in Fig. 5A. Briefly, cells were transiently transfected overnight, washed, subcultured into 96-well dishes, preincubated with or without HGF/SF, exposed to ADR (15 μM for 2 h), postincubated for 72 h, and assayed for MTT dye conversion. For the parental and Gab1ΔPI3K cell types, DN Akt caused a large and significant reduction in the survival of HGF/SF-ADR-treated cells (P < 0.001). (B) wt Akt and Akt-myr enhance cell survival without HGF/SF and overcome Gab1 inhibition of HGF/SF protection. Assays were performed as described for panel A. Compared with the empty vector controls, wt Akt and Akt-myr enhanced the survival of ADR-treated parental MDCK cells and of Gab1(wt) cells in the absence of HGF/SF. The survival of wt Akt and Akt-myr cells but not empty vector-transfected Gab1(wt) cells, was significantly increased in cells treated with HGF/SF plus ADR compared to ADR alone (P < 0.001). (Inset) Parental cells and one clone each of Gab1(wt) and Gab1ΔPI3K cells were cotransfected with wt Akt and with plasmid pSV-β-gal, under conditions similar to those described above, to assess transfection efficiency. At 48 h after transfection, cultures were stained using X-Gal to detect β-Gal staining. (C) Pak1 positively modulates HGF/SF protection. Transient transfection assays were performed as above, using wt and DN Pak1 expression vectors. wt Pak1 enhanced the survival of parental or Gab1(wt) cells in the absence or presence of HGF/SF (P < 0.001); DN Pak1 abolished the ability of HGF/SF to protect the parental cells.
It should be noted here that in different experiments, the baseline cell survival (viability) of MDCK parental or Gab1(wt) cells treated with ADR (15 μM for 2 h) and postincubated for 72 h varied from about 25 to 60%. This variability may be related to the degree of confluency of the cells at the time of drug exposure: the more confluent, the higher the survival level. However, multiple independent experiments revealed that the ability of HGF/SF to protect cells, the ability of Gab1 to block the protection, and the ability (or lack thereof) of exogenously expressed expressed factors, such as p85, p110 or Akt, to modulate the protection was not altered by the baseline survival levels.
Overexpression of Akt blocks Gab1 inhibition of HGF/SF-mediated cell protection.
In additional experiments, we tested the ability of expression vectors for wt Akt and Akt-myr (10) to restore HGF/SF-mediated protection of MDCK Gab1(wt) cells or to establish protection against ADR in the absence of HGF/SF. As illustrated in Fig. 6B, both wt Akt and Akt-myr enhanced the survival of ADR-treated parental MDCK cells in the absence of HGF/SF. In Gab1(wt) cell clones, wt Akt and Akt-myr not only enhanced the survival of ADR-treated cells in the absence of HGF/SF but also restored the ability of HGF/SF to further protect the cells against ADR. In the same set of experiments, there was little or no HGF/SF-mediated protection of either parental or Gab1(wt) cells against ADR in the presence of the DN Akt vector. These findings suggest that c-Akt acts downstream of Gab1 and is a major target for the Gab1-mediated regulation of HGF/SF cell survival signaling.
Transfection experiments using plasmid pSV-β-gal, followed by X-Gal staining, indicate that transfection efficiencies are very high and are about equal in parental, Gab1(wt), and Gab1ΔPI3K cells (Fig. 6B, inset). This experiment showed transfection efficiencies of over 95%. Note that some regions of the culture show less extensive β-Gal staining than others, even though over 95% of the cells show some staining under the microscope. Thus, although the degree of expression of transfected genes may not be uniform over the whole cell population, these genes are well expressed.
Role of Pak1 in HGF/SF-mediated cytoprotection.
Pak1 has recently been found to modulate extracellular matrix-mediated for cell survival (antiapoptosis) (4), but its role in HGF/SF-mediated cell survival signaling has not been established. To determine if Pak1 is involved in HGF/SF-mediated cell protection, MDCK parental and Gab1(wt) cells were transiently transfected with a wt or DN Pak1 expression vector, pretreated with or without HGF/SF, then treated with or without ADR, and assayed for cell viability using the MTT assay. For parental MDCK cells, wt Pak1 moderately enhanced the survival of ADR-treated cells in the absence or presence of HGF/SF, while the DN Pak1 vector completely abolished HGF/SF-mediated cell protection (Fig. 6C). For the Gab1(wt) cell clones, the survival levels were moderately but significantly higher in wt Pak1 cells treated in either the absence or presence of HGF/SF.
Notably, the survival of Gab1(wt) MDCK cell clones treated with-wt Pak1 and HGF/SF was similar to that of non-Pak1-transfected parental cells treated with HGF/SF (about 80%). The small amount of residual protection of control Gab1(wt) cells by addition of HGF/SF (10% in this experiment) was completely abolished by the DN Pak1 vector. These findings suggest that, like c-Akt, Pak1 acts downstream of Gab1 and is an essential component of HGF/SF signaling for cell survival.
Gab1ΔPI3K associates with c-Met but not with the PI3K regulatory subunit, p85.
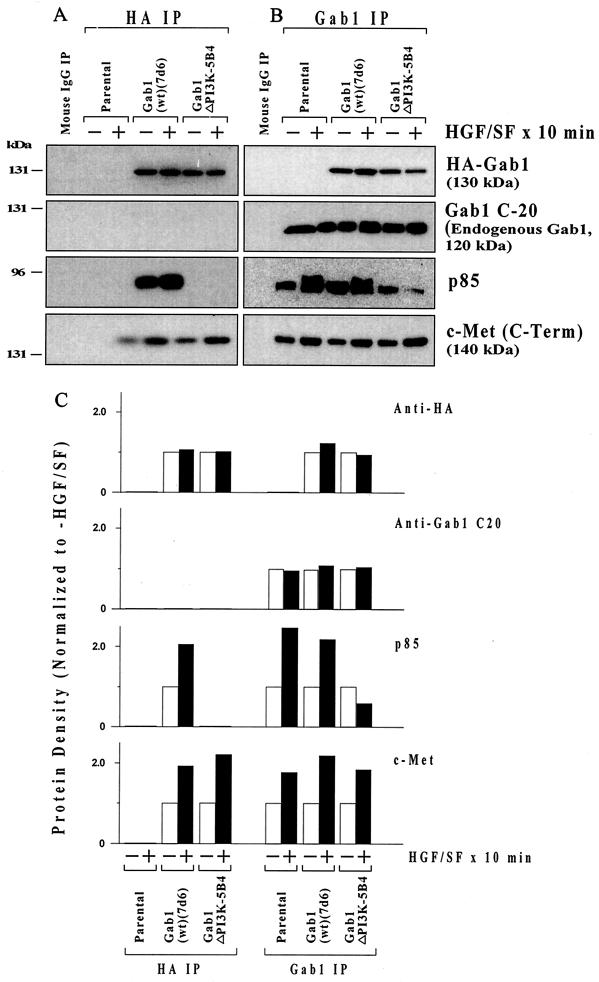
As demonstrated above, the Gab1ΔPI3K mutation, unlike Gab1(wt), Gab1ΔPH, or Gab1ΔSP, blocks the Gab1 inhibition of HGF/SF protection. We performed IP-Western blotting to confirm that Gab1ΔPI3K fails to associate with the p85 subunit of PI3K. MDCK parental, HA-Gab1(wt), or HA-Gab1ΔPI3K cell cultures were treated with or without HGF/SF for 10 min and then subjected to IP using an anti-HA antibody or an antibody against Gab1 (Fig. 7). The IP with anti-HA (with precipitates HA-Gab1 but not endogenous Gab1) shows that HGF/SF enhanced the association of p85 and of c-Met with HA-Gab1(wt) (Fig. 7A). However, there was no evidence of association of p85 with HA-Gab1ΔPI3K, even though roughly equal quantities of HA-Gab1(wt) and HA-Gab1ΔPI3K were immunoprecipitated with the anti-HA antibody. There were no bands in the IP of the parental cells, which lack any HA-tagged proteins.
FIG. 7.
Failure of Gab1ΔPI3K to associate with the PI3K regulatory subunit (p85). Subconfluent proliferating parental, HA-Gab1(wt), or HA-Gab1ΔPI3K cell cultures were treated without (−) or with (+) 100 ng of HGF/SF per ml for 10 min and then harvested for IP. Cells were subjected to IP using an anti-HA (A) or anti-human Gab1 (B) antibody. As a control, parental MDCK cells were subjected to IP using an equivalent quantity of normal mouse IgG. The precipitates were subjected to Western blotting using anti-HA antibody, anti-Gab1 antibody C-20 (different from the Gab1 IP antibody), and an anti-p85 antibody. Protein densities (+HGF/SF/−HGF/SF ratio) were determined by densitometry (C).
IP was also performed with an anti-human Gab1 antibody (Fig. 7B), which is capable of precipitating the endogenous (canine) Gab1 as well as the HA-Gab1 (which is of murine origin) but which does not work for Western blotting. Thus, the anti-HA Western of the Gab1 IP shows the exogenous HA-Gab1, which migrates at 130 kDa, while the Gab1 Western blotting antibody (C-20) detects the endogenous canine Gab1 (120 kDa) but not the exogenous murine HA-Gab1. Figure 7B shows HGF/SF-stimulated association of p85 with Gab1 for the parental cells and the Gab1(wt) clone but not for the Gab1ΔPI3K clone. The small degree of association of Gab1 and p85 for the Gab1ΔPI3K clone in this IP experiment probably reflects endogenous Gab1 rather than HA-Gab1ΔPI3K. Note that the Gab1ΔPI3K mutation did not affect the association of Gab1 to the receptor c-Met in either IP experiment.
Role of caspases in HGF/SF-mediated protection against ADR.
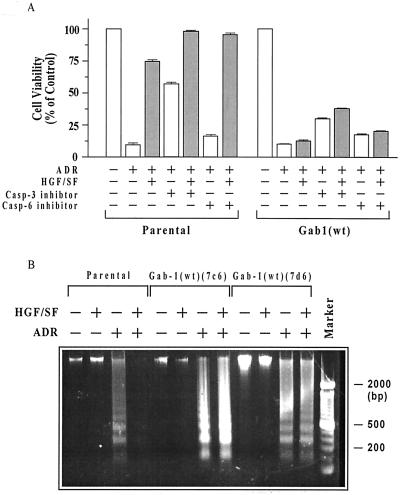
As illustrated in Fig. 8A, incubation with a cell-permeable caspase-3 inhibitor significantly (46%) enhanced the survival of ADR-treated MDCK parental cells in the absence of HGF/SF and improved survival to a smaller extent in the presence of HGF/SF. Improvement in cell survival was also observed in Gab1(wt) cells (20 to 25%) treated without or with HGF/SF. A cell-permeable caspase-6 inhibitor induced much smaller increments in cell survival in both cell types. Thus, a caspase-3 inhibitor partially restored cell survival in ADR-treated cells, simulating the effect of preincubation with HGF/SF.
FIG. 8.
Contribution of apoptosis to ADR cytotoxicity and Gab1 inhibition of protection. (A) Inhibition of caspase-3 partially reverses ADR toxicity in parental and Gab1(wt) cells. Subconfluent proliferating parental MDCK cells and two clones of Gab1 (wt) cells in 96-well dishes were preincubated with or without HGF/SF for 48 h, treated with ADR (15 μM for 2 h), washed, and postincubated for 72 h in the presence of caspase-3 inhibitor (10 μM), caspase-6 inhibitor (10 μM), or neither (see Materials and Methods). Cells were then assayed for MTT dye conversion. For each individual cell line, 10 replicate wells were assayed, and the mean cell viability values were calculated. For parental cells, values plotted are means ± standard errors of the 10 replicate wells. For the Gab1(wt) cells, the values are means ± ranges of the two separate clones. For both parental and Gab1(wt) cells treated with HGF/SF plus ADR or with ADR alone, the survival was significantly greater for cells treated with caspase-3 inhibitor compared with no inhibitor (P < 0.001 for all comparisons). (B) Gab1 blocks HGF/SF-mediated protection against ADR-induced apoptosis. Parental cells and two Gab1(wt) cell clones were treated with or without HGF/SF (100 ng/ml) and/or ADR (15 μM for 2 h), postincubated for 72 h in drug-free medium, and harvested for assays of DNA laddering as described in Materials and Methods. Pretreatment with HGF/SF blocked the formation of DNA ladders in the parental cells but not in the Gab1(wt) cell clones.
Finally, we examined the role of apoptosis in HGF/SF-mediated protection (or lack thereof) in our MDCK cell lines. Parental cells and two Gab1(wt) cell clones were treated with or without HGF/SF and/or ADR as before, and the cells were harvested for assessment of DNA fragmentation (i.e., DNA laddering), which is indicative of apoptosis. Pretreatment with HGF/SF blocked the formation of DNA ladders in the parental cells but not in either of the Gab1(wt) cell clones. These findings suggest that at least part of the ADR-induced cytotoxicity is due to apoptosis.
DISCUSSION
c-Cb1 and Gab1 are multisubstrate signaling adapters that link activated growth factor and cytokine receptors to specific downstream signaling pathways (14, 18, 21, 26, 27, 33, 44). Both proteins have been implicated in HGF/SF→c-Met receptor signaling pathways (14, 28–30, 44). In particular, several studies have implicated Gab1 as an essential mediator of HGF/SF-induced morphogenesis (e.g., branching tubulogenesis of MDCK epithelial cells) (28, 29, 44). Thus, overexpression of Gab1 could restore tubulogenesis mediated by a chimeric c-Met receptor defective in the ability to associate with Gab1 (28). The Met→Gab1 tubulogenesis pathway required localization of Gab1 at sites of cell-cell contact, which, in turn, required the Gab1 PH domain and functional PI3K but did not require the PI3K-binding domain of Gab1. In the present study, overexpression of Gab1 but not of wt or mutant forms of Cb1 blocked HGF/SF protection against ADR-induced cytotoxicity and DNA damage. Interestingly, even a constitutively active mutant form of Cb1 (70Z) with transforming activity (43) failed to modulate the ability of HGF/SF to protect MDCK epithelial cells. However, the Gab1 homolog Gab2 strongly inhibited HGF/SF protection of MDCK cells.
These findings implicate Gab1 as a regulator of an HGF/SF-mediated cell survival pathway(s). The overexpression of Gab1 did not induce cytotoxicity by itself, nor did it have a significant effect on sensitivity to ADR in the absence of HGF/SF, suggesting that effect of Gab1 was mainly restricted to the increased survival due to c-Met signaling. Studies of MDCK cell lines expressing mutant Gab1 proteins revealed that neither the PH nor the SHP2-binding domain was required for regulation of cell survival or DNA repair, but the PI3K-binding domain of Gab1 was essential for these activities. Further studies revealed that in parental MDCK cells, HGF/SF induced the sustained activation of c-Akt, which persisted at levels significantly greater than the unstimulated control after 24 and 48 h. In contrast, in cell lines overexpressing Gab1(wt) or the PH and SHP2 domain mutants, HGF/SF induced only transient activation (phosphorylation) of c-Akt, which returned to baseline by 2 h or less. However, cells expressing the PI3K-binding domain mutant Gab1 exhibited significant HGF/SF activation of c-Akt at the 48 h time point.
The forkhead family transcription factor FKHR, which induces the transcription of proapoptotic genes, is phosphorylated and functionally inactivated by Akt (41). Utilizing a phosphospecific FKHR antibody, we found that the phosphorylation of FKHR in the different Gab1-expressing cell lines and the time course for HGF/SF-induced phosphorylation of FKHR correlated strongly with the HGF/SF-induced Akt activation. Coupled with the observation that inhibition of PI3K signaling by wortmannin reduced the degree of HGF/SF protection in untransfected MDCK cells, these findings suggest that Gab1 functions to regulate the PI3K→c-Akt cell survival pathway.
Consistent with this idea, the ectopic expression of p85(wt) overcame the inhibition of protection in Gab1-overexpressing cell lines and protected cells even in the absence of HGF/SF, while p85(DN) blocked the HGF/SF-mediated protection of parental MDCK cells. Somewhat surprisingly, the overexpression of wt or constitutive membrane-localized forms of p110, the catalytic subunit of PI3K, failed to overcome the Gab1 inhibition of HGF/SF protection or to significantly enhance the baseline MDCK cell survival, as did overexpression of p85, c-Akt, or Pak1. It should be recognized that c-Met, Gab1, and other signaling intermediaries such as c-Cb1 interact with the regulatory subunit of PI3K (p85) rather than the catalytic subunit (p110). Our findings suggest, although they do not formally prove, that for protection to occur, PI3K must be targeted to the c-Met receptor and that membrane targeting of the catalytic subunit of PI3K is not sufficient for protection.
The ability of HGF/SF to protect MDCK cells was abrogated by the overexpression of PTEN, a lipid 3′-phosphatase that converts phosphatidylinositol-(3,4,5)-triphosphate [PI(3,4,5)P3] to PI(4,5)P2. This finding suggests that the major lipid product of PI3K enzymatic activity [PI(3,4,5)P3] is essential for the HGF/SF survival signal, although it is noted PI3K has protein kinase activity, which might also play a role in HGF/SF-mediated protection. Prior studies have suggested that PTEN blocks c-Akt-mediated signaling pathways for cell survival (39). The Gab1 regulation of cell survival and DNA repair required the PI3K-binding but not the PH domain of Gab1. Thus, localization of Gab1 to cell-cell contacts, which is mediated by the PH domain (28), is not required for cell survival. However, the failure of Gab1ΔPI3K to inhibit cell protection suggests that a physical interaction between Gab1 and PI3K is required to regulate cell survival. These findings are consistent with a model in which the recruitment of PI3K by Gab1 to a specifically constituted signaling complex determines which signaling pathway(s) downstream of PI3K is activated by HGF/SF.
Expression of DN mutants of either c-Akt or Pak1 strongly inhibited the ability of HGF/SF to protect parental MDCK cells, while the expression of wt or constitutively active mutant forms of c-Akt and Pak1 partially restored the HGF/SF-mediated protection in Gab1-overexpressing cell lines. These findings implicate c-Akt and Pak1 as signaling intermediaries for cell survival that function downstream of Gab1. In some cellular contexts, Pak1 may act downstream of c-Akt to cause the phosphorylation and inactivation of Bad, a cell death agonist that binds to and inhibits the cell survival mediator Bcl-XL (38). Although Pak1 activity is required for HGF/SF protection, and two Rho family GTPases that can function as activators of Pak1 (cdc42 and Rac) (45) are activated by HGF/SF in MDCK cells (36), preliminary studies indicate that expression of DN inhibitors of these GTPases (cdc42-N17 and Rac-N17) do not block HGF/SF protection of MDCK or MtLn3 rat mammary carcinoma cells (unpublished findings). Thus, the HGF/SF-mediated c-Akt/Pak1 survival pathway may be independent of cdc42 and Rac.9
We previously reported that HGF/SF blocks apoptosis induced by DNA-damaging agents such as ADR in various epithelial cell types, including MDCK cells (12). HGF/SF inhibition of apoptosis was demonstrated by a significant reduction in the extent of DNA fragmentation caused by ADR. Consistent with this finding, a caspase-3 inhibitor and, to a much less extent, a caspase-6 inhibitor enhanced cell survival in ADR-treated MDCK cells in the absence of HGF/SF. Although the increase in survival attributable to the caspase-3 inhibitor in Gab1(wt) cell clones was relatively modest (20 to 25%), this increase was observed in the absence or presence of HGF/SF, suggesting that Gab1 functions to inhibit an antiapoptosis pathway that ultimately prevents the activation of caspase-3. The inability of the caspase-3 inhibitor to fully restore cell viability, especially in the Gab1(wt)-transfected cell lines, could have several explanations: (i) the inhibitor only partially blocked caspase-3 activity; (ii) additional caspases can independently mediate ADR-induced cell death; and (iii) ADR also induces a component of nonapoptotic cell death.
Several studies suggest that Gab1 associates with the c-Met receptor by at least two distinct mechanisms: one involving recruitment to the canonical Grb2-binding site (1356YVNV) of c-Met by interaction with Grb2, and the second governed by a non-Grb2-dependent mechanism (27, 37). The overexpression of Gab1 restored c-Met-mediated tubulogenesis even in cells with chimeric receptors mutated at the Grb2-binding site (i.e., 1356Y→F and 1358N→H) (28), suggesting that a Grb2-independent pathway may contribute to tubulogenesis. Our studies using the chimeric CSF-Met-transfected MDCK cell lines suggest that the Grb2-binding site is particularly important for c-Met-mediated cell survival signaling, although other are also involved. Thus, Gab1 may compete with or sequester other substrates that associate with c-Met through the Grb2 site, possibly including the p85 regulatory subunit of PI3K. This hypothesis is consistent with the finding that the N1358H mutant receptor showed significantly reduced association with Gab1 and with p85 compared with the wt CSF-Met receptor.
Previous studies have established that PI3K can be activated through an interaction with Ras (34) and that Ras can be recruited to tyrosine kinase receptors through interaction with Sos (Son of sevenless), a protein that functions as a guanine nucleotide exchange factor for Ras and interacts with tyrosine kinase receptors (23, 30). Sos, which interacts with the multifunctional docking site of the Met receptor (1349YVHVXXX1356YVNV) (32), also binds to the Grb2 adapter. Interestingly, a recent study suggests that the multifunctional docking site of Met is not required for receptor-mediated Ras signaling or cell scattering (42). Thus, residual Ras signaling could explain why there is some protection by mutant receptors lacking either or both tyrosines (1349Y and 1356Y). Thus, expression of a DN Ras (Ras-N17) abrogated the protection mediated by the Y(1349+1356)F mutant receptor and partially but significantly inhibited protection by the wt chimeric CSF-Met receptor. Alternatively, the small degree of protection observed by some of the mutant chimeric Met receptors, but not observed for the N1358H mutant, could be due to clonal variability.
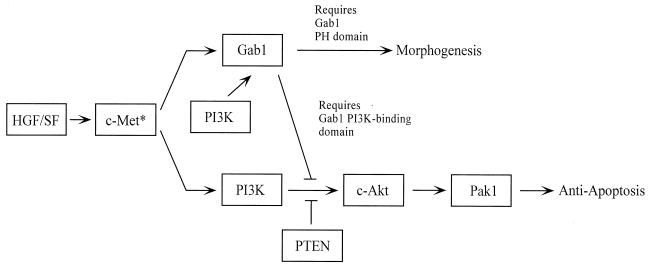
Figure 9 shows one possible model for Gab1-dependent signaling pathways in MDCK cells. In this scheme, PI3K is an effector for both the morphogenesis and antiapoptosis pathways, and Gab1, through its PI3K-binding domain, directs the signal away from the antiapoptosis pathway. Several features of this model are noteworthy: (i) PI3K functions both to activate Gab1 morphogenesis signaling (28) and, in a different context, to activate the c-Akt/Pak1 survival pathway; and (ii) Gab1 acts upstream of c-Akt and Pak1 to regulate cell survival signaling.
FIG. 9.
Model for Gab1 signaling pathways in MDCK cells. See text for discussion.
This model may also have implications for understanding the role of HGF/SF in embryonic morphogenesis. Since regulated apoptosis is essential during embryonic development, Gab1 may function to render cells undergoing morphogenesis more susceptible to cell death signals required for the next embryonic transition. In our studies, overexpression of Gab1 had a much greater effect on the HGF/SF-stimulated cell survival than on the baseline survival in the absence of exogenous HGF/SF. Although the hypothesis is speculative, our findings support a significant role for Gab1 in the regulation of cell survival pathways in epithelial cells.
ACKNOWLEDGMENTS
We are grateful to Morag Park (Department of Molecular Oncology, Royal Victoria Hospital and McGill University, Quebec, Quebec, Canada) for providing the cell lines used in these studies (chimeric CSF-Met receptor MDCK cell lines, wt and mutant HA-Gab1 cell clones, and wt and mutant Cb1 clones) and for advice and helpful discussions.
This study was supported in part by USPHS research grants R01-ES09169, R01-CA82599, and R01-CA80000 (E.M.R.).
REFERENCES
- 1.Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem. 2000;275:12041–12950. doi: 10.1074/jbc.275.16.12041. [DOI] [PubMed] [Google Scholar]
- 2.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 3.Alley M C, Scudieco D A, Monks A, Hursey M L, Czerwinski M J, Fine D L, Abbott B J, Mayo J G, Shoemaker R H, Boyd M R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 4.Almeida E A, Ilic D, Han Q, Hauck C R, Jin F, Kawakatsu H, Schlaepfer D D, Damsky C H. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amicone L, Spagnoli F M, Spath G, Giordano S, Tommasini C, Bernardini S, De Luca V, Della Roca C, Weiss M C, Comoglio P M, Tripoldi M. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO J. 1997;16:495–503. doi: 10.1093/emboj/16.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardelli A, Longatti P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio P M. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996;15:6205–6212. [PMC free article] [PubMed] [Google Scholar]
- 7.Bertrand R, Pommier Y. Assessment of DNA damage in mammalian cells by DNA filter elution methodology. In: Studzinski G P, editor. apoptosis. New York, N.Y: Oxford University Press; 1995. pp. 97–117. [Google Scholar]
- 8.Bottaro D P, Rubin J S, Faletto D L, Chan A M-L, Kmiecik T E, Vande Woude G F, Aaronson S A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 9.Bowers D C, Fan S, Walter K, Abounder R, Williams J A, Rosen E M, Laterra J. Scatter factor/hepatocyte growth factor activates AKT and protects against cytotoxic death in human glioblastoma cells via PI3-kinase-dependent pathways. Cancer Res. 2000;60:4277–4283. [PubMed] [Google Scholar]
- 10.Cong L N, Chen H, Zhou L, McGibbon M A, Taylor S C, Quon M J. Physiologic role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 11.Fan S, Ma Y X, Wang J-A, Yuan R-Q, Meng Q, Laterra J J, Goldberg I D, Rosen E M. The cytokine scatter factor inhibits apoptosis and enhances DNA repair by a common mechanism involving signaling through phosphatidylinositol 3′ kinase. Oncogene. 2000;19:2212–2223. doi: 10.1038/sj.onc.1203566. [DOI] [PubMed] [Google Scholar]
- 12.Fan S, Wang J-A, Yuan R-Q, Rockwell S, Andres J, Zlatapolskiy A, Goldberg I D, Rosen E M. Scatter factor protects epithelial and carcinoma cells against apoptosis induced by DNA-damaging agents. Oncogene. 1998;17:131–141. doi: 10.1038/sj.onc.1201943. [DOI] [PubMed] [Google Scholar]
- 13.Fan S, Wang J-A, Yuan R-Q, Ma Y X, Meng Q, Goldberg I D, Rosen E M. BRCA1 as a human prostate tumor suppressor: modulation of proliferation, damage responses, and expression of regulatory proteins. Oncogene. 1998;16:3069–3083. doi: 10.1038/sj.onc.1202116. [DOI] [PubMed] [Google Scholar]
- 14.Fixman E D, Holgado-Madruga M, Nguyen L, Kamikura D M, Fournier T M, Wong A J, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cb1 and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- 15.Fournier T M, Kamikura D, Teng K, Park M. Branching tubulogenesis but not scatter of Madin-Darby canine kidney cells requires a functional Grb2 binding site in the Met receptor tyrosine kinase. J Biol Chem. 1996;271:22211–22217. doi: 10.1074/jbc.271.36.22211. [DOI] [PubMed] [Google Scholar]
- 16.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. . (Review). [DOI] [PubMed] [Google Scholar]
- 17.Frisch S M, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galisteo M L, Dikie I, Batzer A G, Langdon W V, Schlessinger J. Tyrosine phosphorylation of the c-cb1 proto-oncogene protein product and association with the epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem. 1995;270:20242–20245. doi: 10.1074/jbc.270.35.20242. [DOI] [PubMed] [Google Scholar]
- 19.Georgescu M M, Kirsch K H, Akagi T, Shishido T, Hanafusa H. The tumor suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad USA. 1999;96:10182–10186. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann M, Lorenz H-M, Voll R, Grunke M, Woith W, Kalden J R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 22.Holgado-Madruga M, Moscatello D K, Emley D R, Dieterich R, Wong A J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12319–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlovich C A, Bonfini L, McCollam L, Rogge R D, Daga A, Czech M P, Banerjee U. In vivo functional analysis of the Ras exchange factor son of sevenless. Science. 1995;268:576–579. doi: 10.1126/science.7725106. [DOI] [PubMed] [Google Scholar]
- 24.Klippel A, Escobedo J A, Hirano M, Williams L T. The interaction of small domains between the subunits of phosphatidylinositol-3-kinase determines enzyme activity. Mol Cell Biol. 1991;14:2675–2685. doi: 10.1128/mcb.14.4.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolik G, Klippel A, Weber J J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korhonen J M, Said F A, Wong A J, Kaplan D R. Gab1 mediates neurite outgrowth, DNA synthesis, and survival in PC12 cells. J Biol Chem. 1999;274:37307–37314. doi: 10.1074/jbc.274.52.37307. [DOI] [PubMed] [Google Scholar]
- 27.Lock L S, Royal I, Naujokas M A, Park M. Identification of an atypical Grb2 carboxy-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem. 2000;275:31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 28.Maroun C R, Holgado-Madruga M A, Royal I, Naujokas M A, Fournier T M, Wong A J, Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the Met receptor kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maroun C R, Moscatello D K, Naujokas M A, Holgado-Madruga M A, Wong A J, Park M. A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis. J Biol Chem. 1999;274:31719–31726. doi: 10.1074/jbc.274.44.31719. [DOI] [PubMed] [Google Scholar]
- 30.McCollam L, Bonfini L, Karlovich C A, Conway B R, Kozma L M, Banerjee U, Czech M P. Functional roles for the pleckstrin and Db1 homology regions in the Ras exchange factor Son-of-sevenless. J Biol Chem. 1995;270:15954–15957. doi: 10.1074/jbc.270.27.15954. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen L, Holgado-Madruga M, Maroun C, Fixman E D, Kamikura D, Fournier T, Charest A, Tremblay M L, Wong A J, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 32.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla-Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio P M. A multifunctional docking site mediates signalling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 33.Rocchi S, Tartare-Deckert S, Murdaca J, Holgado-Madruga M, Wong A J, Van Obberghen E. Determination of Gab1 (Grb2-associated binder-1) interaction with insulin receptor signaling molecules. Mol Endocrinol. 1998;12:914–923. doi: 10.1210/mend.12.7.0141. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Viciana P, Warne P H, Vanhaesebroeck B, Waterfield M D, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 35.Royal I, Fournier T M, Park M. Differential requirement of Grb2 and PI3-kinase in HGF/SF-induced cell motility and tubulogenesis. J Cell Physiol. 1997;173:196–201. doi: 10.1002/(SICI)1097-4652(199711)173:2<196::AID-JCP20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 36.Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaeper U, Gehring N H, Fuchs K P, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schurmann A, Mooney A F, Sanders L C, Sells M A, Wang H G, Reed J C, Bokoch G M. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Tak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 40.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:238–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 41.Tang E D, Nunez G, Barr F G, Guan K L. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 42.Tulasne D, Paumelle R, Weidner K M, Vandenbunder B, Fafeur V. The multisubstrate docking site of the MET receptor is dispensible for MET-mediated RAS signaling and cell scattering. Mol Biol Cell. 1999;19:551–565. doi: 10.1091/mbc.10.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Leeuwen J E, Paik P K, Samelson L E. The oncogenic 70Z Cb1 mutation blocks the phosphotyrosine binding domain-dependent negative regulation of ZAP-70 by c-Cb1 in Jurkat T cells. Mol Cell Biol. 1999;19:6652–6664. doi: 10.1128/mcb.19.10.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 45.Zhang S, Han J, Sells M A, Chernoff J, Knaus U G, Ulevich R J, Bokoch G M. Rho family GTPases regulate p38 mitogen-activated protein kinase activity through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 46.Zhao C, Yu D H, Shen R, Feng G S. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, Fixman E D, Naujokas M A, Torissian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for transduction of signals for motility and morphogenesis. J Biol Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]
- 48.Zhu H, Naujokas M A, Park M. Receptor chimeras indicate that the met tyrosine kinase mediates motility and morphogenic responses of hepatocyte growth factor/scatter factor. Cell Growth Differ. 1994;5:359–366. [PubMed] [Google Scholar]