Abstract
Liver transplantation (LT) recipients with hepatocellular carcinoma (HCC) receive a higher proportion of livers from donation after circulatory death (DCD) donors compared with non-HCC etiologies. Nevertheless, data on outcomes in patients with HCC receiving DCD grafts are limited. We evaluated the influence of DCD livers on post-LT outcome among HCC patients. We identified 7563 patients in the United Network for Organ Sharing (UNOS) database who underwent LT with Model for End-Stage Liver Disease score exceptions from 2012 to 2016, including 567 (7.5%) who received a DCD donor organ and 6996 (92.5%) who received a donation after brain death (DBD) donor organ. Kaplan-Meier probabilities of post-LT HCC recurrence at 3 years were 7.6% for DCD and 6.4% for DBD recipients (P = 0.67) and post-LT survival at 3 years was 81.1% versus 85.5%, respectively (P = 0.008). On multivariate analysis, DCD donor (hazard ratio, 1.38; P = 0.005) was an independent predictor of post-LT mortality. However, a survival difference after LT was only observed in subgroups at higher risk for HCC recurrence including Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score ≥4 (DCD 57.0% versus DBD 72.6%; P = 0.02), alpha-fetoprotein (AFP) ≥100 (60.1% versus 76.9%; P = 0.049), and multiple viable tumors on last imaging before LT (69.9% versus 83.1%; P = 0.002). In this analysis of HCC patients receiving DCD versus DBD livers in the UNOS database, we found that patients with a low-to-moderate risk of HCC recurrence (80%−90% of the DCD cohort) had equivalent survival regardless of donor type. It appears that DCD donation can best be used to increase the donor pool for HCC patients with decompensated cirrhosis or partial response/stable disease after locoregional therapy with AFP at LT <100 ng/mL.
Hepatocellular carcinoma (HCC) is the third highest cause of cancer mortality worldwide,(1,2) and liver transplantation (LT) remains the treatment with the highest probability of cure.(3,4) Because of organ shortages, LT candidates within conventional transplant criteria often have long wait times and risk wait-list dropout(5,6) while waiting for a donation after brain death (DBD) donor. To shorten wait times for LT, livers from donation after circulatory death (DCD) donors are occasionally used.(7,8)
There has been some concern regarding expanding the use of livers from DCD donors given that recipients of these grafts experience inferior graft survival that is mainly related to higher rates of biliary complications, notably ischemic cholangiopathy (IC).(7,9) However, when lower risk DCD livers with warm ischemia time <30 minutes and cold ischemia time (CIT) <10 hours were transplanted in robust patients (<60 years, creating [Cr] <2 mg/dL, admitted for LT from home), these patients achieved graft survival rates (67% at 3 years) not significantly different from recipients of DBD allografts.(9)
Patients with HCC have been shown to receive a higher proportion of DCD livers when compared with patients without HCC.(10) This is likely due to the tendency to use extended criteria organs in HCC patients who generally have more preserved liver synthetic function and the perception that these recipients are better able to tolerate these organs. Nevertheless, data on post-LT outcomes in HCC patients receiving DCD grafts are limited. A few recent studies showed no significant difference in recurrence-free survival between DBD and DCD groups. However, these studies were small and did not stratify patients by HCC recurrence risk categories.(11–13) Notably, these studies showed increased risk of biliary complications with DCD donors. Therefore, in this study, we aimed to use the large national United Network for Organ Sharing (UNOS) database to study the impact of DCD versus DBD donor on post-LT patient and graft survival and HCC recurrence, including stratification by HCC recurrence risk categories.
Patients and Methods
STUDY DESIGN AND PATIENT SELECTION
In this retrospective study using the UNOS database, we identified 7563 adult HCC patients who underwent LT with Model for End-Stage Liver Disease (MELD) score exception between April 1, 2012, and December 31, 2016. The study start date was chosen to coincide with UNOS/Organ Procurement and Transplantation Network initiating the explant pathology form. Exclusion criteria were patients with re-transplants (n = 9), patients with no evidence of HCC on explant despite not receiving locoregional therapy (LRT; n = 44), patients with “infiltrative” for number of tumors on explant (n = 46), and patients with cholangiocarcinoma because it is potentially a misdiagnosis of HCC (n = 39). This study received expedited study approval by the UCSF Committee for Human Research with minimal study risk assignment.
BASELINE VARIABLES
Study variables collected from the UNOS database at the time of listing included age, sex, race/ethnicity, and etiology of liver disease. Alpha-fetoprotein (AFP) levels and the size and number of HCC lesions at inclusion were determined at the time of initial MELD exception application. The percent of patients who underwent LRT while on the waiting list, time from initial exception to LT, and the time from MELD exception to LT were also collected as was the MELD score at LT. AFP and the radiographic tumor burden closest to the date of LT (within 90 days of LT) were obtained. Donor characteristics collected included donor age, donor risk index (DRI), steatosis, and CIT.
LT-RELATED VARIABLES
Presence of vascular invasion and the number and size of viable lesions were obtained from the explant data and used to determine both tumor stage and the individual Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score. The 3 components of the RETREAT score are AFP at LT, microvascular invasion, and the sum of the largest viable tumor plus the number of viable tumors on explant. The methods used to create the RETREAT score have previously been described.(14) Explant pathology data provided histological grade based on the modified Edmondson criteria.
HCC RECURRENCE
To identify patients with post-LT HCC recurrence, liver malignancy follow-up data and cause of death variables underwent physician review (N.M.). Records indicating posttransplant recurrence of pretransplant malignancy or a cause of death indicating HCC or metastatic malignancy were classified as having HCC recurrence.
OUTCOMES AND STATISTICAL ANALYSIS
The primary study outcome was post-LT patient survival, and the secondary outcomes were post-LT HCC recurrence and graft survival (defined as patient death or retransplantation). Outcomes were assessed for the overall cohort and stratified by type of donor (DCD versus DBD). Clinical and tumor characteristics were summarized using medians and interquartile ranges (IQRs) for continuous variables and proportions for categorical variables. Characteristics, including the RETREAT score, were stratified by tumor burden category and compared with Kruskal-Wallis and Pearson’s chi-square tests, as appropriate.
Observed post-LT patient and graft survival and HCC recurrence probabilities and 95% confidence intervals (CIs) were estimated at 1 and 3 years using the Kaplan-Meier method and compared by the type of donor using the log-rank test. For post-LT survival, follow-up was measured from the date of LT to retransplant, death, or last follow-up (whichever occurred first) with survival censored at retransplant (patient survival only) or last follow-up. For post-LT HCC recurrence, patient follow-up was measured from the date of LT to HCC recurrence or HCC-related death with patients censored at the date of non-HCC death or last follow-up. The association of post-LT outcomes with explanatory variables was explored using univariate and multivariate hazard ratios (HRs) and 95% CIs estimated by Cox proportional hazards regression for post-LT survival and HCC recurrence. Variables with a univariate P value <0.10 were included in the multivariate analysis with the final models selected by backward elimination (P value for removal >0.05).
Results
PATIENT CHARACTERISTICS AND EXPLANT DATA
There were 7563 total patients with HCC included in the cohort, out of which 6996 (92.5%) received DBD donor organs and 567 (7.5%) received DCD donor organs. Baseline, wait-list, and donor characteristics are described in Table 1. There was no difference in the baseline patient or tumor characteristics between groups including age, race, etiology of liver disease, and initial tumor size and number. The most common etiology of liver disease was hepatitis C virus (HCV; 63.0% of the overall cohort), most were male (77.4%), median age was 61 years (IQR, 57–65 years), and most patients initially had 1 lesion ≤3 cm (54.1% of the overall cohort). There was no difference in tumor burden on last imaging prior to LT between the DBD and DCD groups (P = 0.59). Nearly half of the cohort (48.4%) had no residual enhancing lesions on last imaging, whereas 13.8% had multiple enhancing lesions. Median AFP at LT was 8 ng/mL (IQR, 4–21 ng/mL) and was similar between groups. Median time from first exception to LT was 6.2 months (IQR, 3.1–10.1 months) for the DCD group compared with 6.9 months (3.4–12.4 months) for the DBD group (P < 0.001). Median donor age was 33 years (23–44) versus 45 years (29–56 years; P < 0.001), CIT was 5.4 hours (4.3–6.6 hours) versus 6.0 hours (4.7–7.6 hours; P < 0.001), and DRI was 1.73 (1.52–2.00) versus 1.36 (1.12–1.65) for the DCD versus DBD groups, respectively.
TABLE 1.
Baseline, Wait-list, and Donor Characteristics of the Study Cohort Stratified by Donor Type
| Overall (n = 7563) | DBD (n = 6996) | DCD (n = 567) | P Value | |
|---|---|---|---|---|
| Age at listing, years | 61 (57–65) | 61 (57–65) | 61 (57–65) | 0.89 |
| Sex, male | 5855 (77.4) | 5404 (77.2) | 451 (79.5) | 0.21 |
| Race/ethnicity | 0.43 | |||
| Caucasian | 5063 (66.9) | 4668 (66.7) | 395 (69.7) | |
| Hispanic | 1078 (14.3) | 1001 (14.3) | 77 (13.6) | |
| African American | 756 (10.0) | 708 (10.1) | 48 (8.5) | |
| Asian | 564 (7.5) | 527 (7.5) | 37 (6.5) | |
| Etiology of liver disease | 0.41 | |||
| HCV | 4764 (63.0) | 4409 (63.0) | 355 (62.6) | |
| NAFLD | 713 (9.4) | 668 (9.5) | 45 (7.9) | |
| Alcohol | 610 (8.1) | 554 (7.9) | 56 (9.9) | |
| HBV | 428 (5.7) | 395 (5.6) | 33 (5.8) | |
| Autoimmune* | 162 (2.1) | 151 (2.2) | 11 (1.9) | |
| Listing tumor burden | 0.35 | |||
| 1 lesion ≤3 cm | 3905 (54.1) | 3623 (54.3) | 282 (51.4) | |
| 1 lesion 3.1–5 cm | 1393 (19.3) | 1290 (19.3) | 103 (18.8) | |
| 2 lesions | 1491 (20.7) | 1364 (20.5) | 127 (23.1) | |
| 3 lesions | 429 (5.9) | 392 (5.9) | 37 (6.7) | |
| Initial AFP, ng/mL | 9 (5–28) | 9 (5–28) | 10 (5–27) | 0.62 |
| AFP at LT, ng/mL | 8 (4–21) | 8 (4–21) | 8 (4–20) | 0.62 |
| <20 ng/mL | 5395 (73.6) | 4982 (73.5) | 413 (74.4) | 0.92 |
| 20 to <100 ng/mL | 1321 (18.0) | 1222 (18.0) | 99 (17.8) | |
| 100 to ≤1000 ng/mL | 559 (7.6) | 519 (7.7) | 40 (7.2) | |
| >1000 ng/mL | 54 (0.7) | 51 (0.8) | 3 (0.5) | |
| MELD at LT | 12 (8–17) | 12 (8–17) | 12 (8–17) | 0.86 |
| Last total tumor diameter prior to LT, cm | 1.50 (0.00–2.80) | 1.50 (0.00–2.80) | 1.50 (0.00–2.80) | 0.86 |
| Tumor burden prior to LT | 0.59 | |||
| 0 lesions | 3618 (48.4) | 3361 (48.6) | 257 (45.8) | |
| 1 lesion ≤3 cm | 2247 (30.0) | 2077 (30.0) | 170 (30.3) | |
| 1 lesion 3.1–5 cm | 580 (7.8) | 535 (7.7) | 45 (8.0) | |
| 2 lesions | 793 (10.6) | 724 (10.5) | 69 (12.3) | |
| 3 lesions | 239 (3.2) | 219 (3.2) | 20 (3.6) | |
| Received LRT | ||||
| Any LRT | 7126 (94.3) | 6588 (94.2) | 538 (94.9) | 0.50 |
| Ablation at any time | 6422 (84.9) | 5945 (85.0) | 477 (84.1) | 0.59 |
| TACE at any time | 5122 (67.7) | 4759 (68.0) | 363 (64.0) | 0.05 |
| Y90 at any time | 372 (4.9) | 324 (4.6) | 48 (8.5) | <0.001 |
| Time from initial exception to LT, months | 6.8 (3.4–12.2) | 6.9 (3.4–12.4) | 6.2 (3.1–10.1) | <0.001 |
| Donor age, years | 44 (28–56) | 45 (29–56) | 33 (23–44) | <0.001 |
| DRI | 1.39 (1.14–1.69) | 1.36 (1.12–1.65) | 1.73 (1.52–2.00) | <0.001 |
| CIT | 6.0 (4.7–7.5) | 6.0 (4.7–7.6) | 5.4 (4.3–6.6) | <0.001 |
NOTE: Data are given as n (%) or median (IQR).
Includes autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis.
Explant characteristics are described in Table 2. No significant differences were observed between the 2 groups. The median RETREAT score was 1 (IQR, 1–2) for both groups, with 11.6% of DCD recipients and 11.8% of DBD recipients having a RETREAT score ≥4. The total tumor diameter in the explant was 2.4 cm (0.0–4.2 cm) for DCD recipients and 2.1 cm (IQR, 0.0–4.0) for DBD recipients. Tumors were most commonly moderately differentiated (45.7% for DCD and 46.3% for DBD), with only 6.2% and 6.8% poorly differentiated in DCD and DBD groups, respectively. Rates of microvascular invasion in DCD and DBD groups were 10.6% versus 12.9%, and explant stage beyond Milan were also similar (19.0% versus 17.3%).
TABLE 2.
Explant Characteristics of the Study Cohort Stratified by Donor Type (DCD Versus DBD)
| Overall (n = 7563) | DBD (n = 6996) | DCD (n = 567) | P Value | |
|---|---|---|---|---|
| Pathologic tumor stage | 0.29 | |||
| Within Milan | 6245 (82.6) | 5786 (82.7) | 459 (81.0) | |
| Beyond Milan | 1318 (17.4) | 1210 (17.3) | 108 (19.0) | |
| Total tumor diameter, cm | 2.1 (0.0–4.0) | 2.1 (0.0–4.0) | 2.4 (0.0–4.2) | 0.86 |
| Sum of the number of viable lesions and largest lesion, cm | 3.2 (0.0–4.9) | 3.2 (0.0–4.9) | 3.5 (0.0–5.0) | 0.06 |
| Histologic grade | 0.64 | |||
| Complete necrosis | 1457 (19.3) | 1353 (19.3) | 104 (18.3) | |
| Well differentiated | 1705 (22.6) | 1563 (22.4) | 142 (25.0) | |
| Moderately differentiated | 3494 (46.2) | 3235 (46.3) | 259 (45.7) | |
| Poorly differentiated | 514 (6.8) | 479 (6.8) | 35 (6.2) | |
| Microvascular invasion | 962 (12.7) | 902 (12.9) | 60 (10.6) | 0.11 |
| RETREAT scored(14) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.65 |
| 0 | 1738 (23.7) | 1619 (23.9) | 119 (21.4) | 0.66 |
| 1 | 2439 (33.3) | 2239 (33.1) | 200 (36.0) | |
| 2 | 1453 (19.8) | 1346 (19.9) | 107 (19.3) | |
| 3 | 832 (11.4) | 767 (11.3) | 65 (11.7) | |
| 4 | 533 (7.3) | 496 (7.3) | 37 (6.7) | |
| ≥5 | 334 (4.6) | 307 (4.5) | 27 (4.9) |
NOTE: Data are given as n (%) or median (IQR).
POST-LT SURVIVAL AND RECURRENCE BY DONOR TYPE
The median post-LT follow-up was 2.1 years (IQR, 1.1–3.8 years). The overall 1- and 3-year post-LT patient survival was 93.3% (95% CI, 92.7%−93.9%) and 85.2% (84.3%−86.1%), respectively. Stratified by donor type, post-LT patient survival at 1 and 3 years was 91.2% (88.5%−93.3%) and 81.1% (76.9%−84.6%), respectively, for DCD recipients versus 93.5% (92.9%−94.1%) and 85.5% (84.6%−86.5%), respectively, for DBD recipients (P = 0.008; Fig. 1). Overall graft survival was 91.4% (90.8%−92.1%) at 1 year and 82.9% (82.0%−83.9%) at 3 years. Stratified by donor type, post-LT 1- and 3-year graft survival was 88.2% (85.2%−90.6%) and 76.3% (71.9%−80.1%), respectively, for DCD recipients versus 91.7% (91.0%−92.3%) and 83.5% (82.5%−84.4%), respectively, for DBD recipients (P < 0.001; Fig. 2).
FIG. 1.
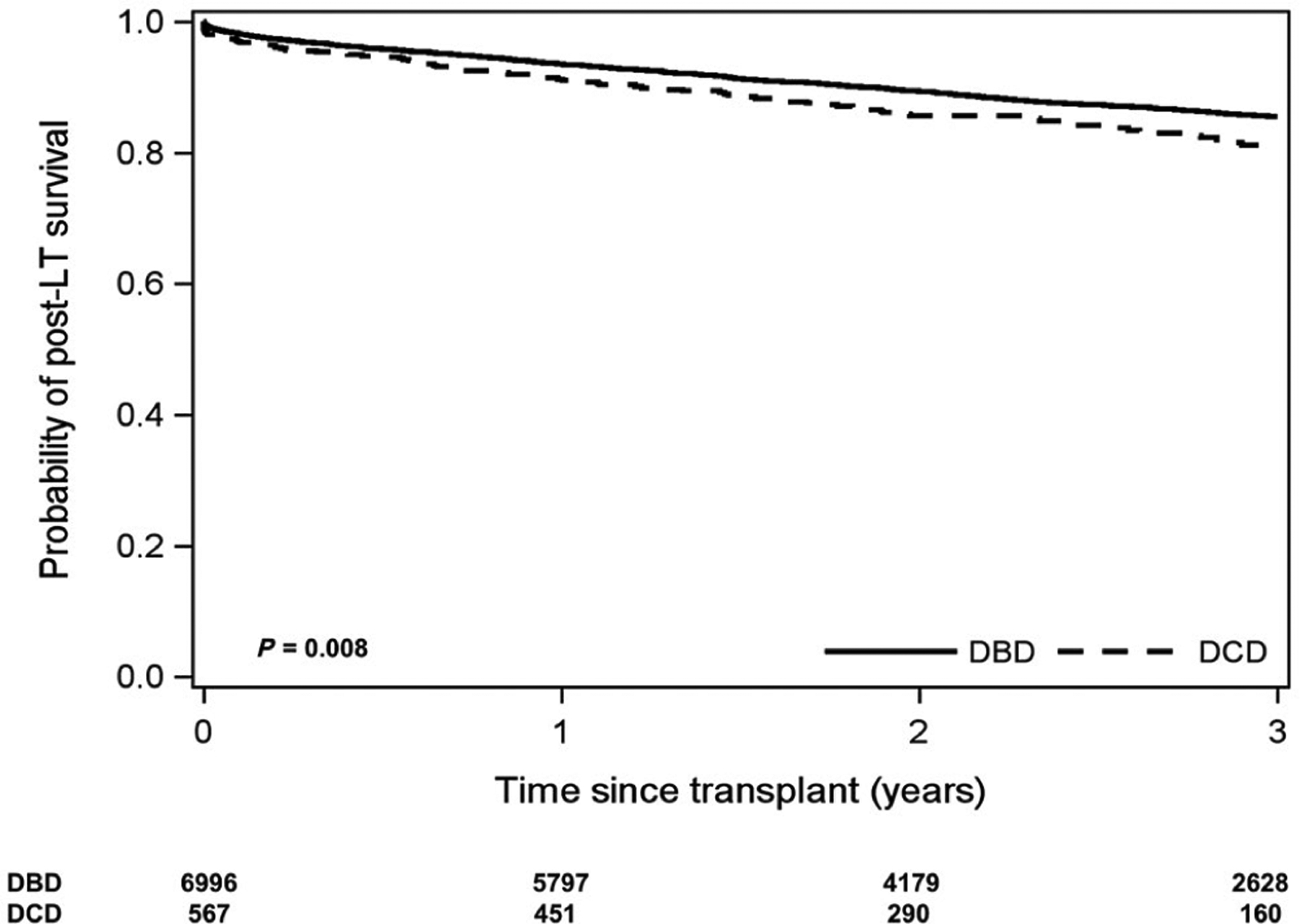
Kaplan-Meier curve of post-LT patient survival stratified by donor type for DCD versus DBD donors.
FIG. 2.

Kaplan-Meier curve of post-LT graft survival stratified by donor type for DCD versus DBD donors.
The overall 1- and 3-year post-LT HCC recurrence was 2.8% (95% CI, 2.4%−3.2%) and 6.5% (5.9%−7.2%). Stratified by donor type, post-LT HCC recurrence at 1 and 3 years was 2.0% (1.0%−3.7%) and 7.6% (5.3%−10.9%), respectively, for DCD recipients versus 2.8% (2.5%−3.3%) and 6.4% (5.8%−7.1%), respectively, for DBD recipients (P = 0.67; Fig. 3).
FIG. 3.

Kaplan-Meier curve of post-LT HCC recurrence rate stratified by donor type for DCD versus DBD donors.
POST-LT OUTCOMES BY DONOR TYPE AND RETREAT SCORE
When stratifying by RETREAT score and donor type, patients with RETREAT score <4 had no difference in overall patient survival (Fig. 4A,B). However, among those with RETREAT score ≥4 (11.9% of the overall cohort; n = 64 DCD and n = 803 DBD), the 3-year post-LT patient survival was significantly worse for DCD than DBD (57.0% versus 72.6%; P = 0.02; Fig. 4C). Graft survival at 3 years after LT was significantly worse for DCD recipients at all RETREAT scores, including 83.7% versus 77.3% (P = 0.003) for RETREAT score 1–3 and 70.4% versus 53.8% (P = 0.02) for RETREAT score ≥4. HCC recurrence rates did not differ when stratified by RETREAT score, including in patients with RETREAT score ≥4 (23.7% versus 26.8% at 3 years; P = 0.96).
FIG. 4.

Kaplan-Meier curve of post-LT patient survival stratified by donor type for DCD versus DBD donors. (A) RETREAT score 0. (B) RETREAT score 1–3. (C) RETREAT score ≥4.
POST-LT OUTCOMES BY DONOR TYPE AND AFP OR LAST RADIOGRAPHIC TUMOR BURDEN
For patients with AFP at LT <100 ng/mL (91.9% of the overall cohort), there was no difference in overall patient survival by donor type. However, for patients with AFP at LT ≥100 ng/mL (n = 43 DCD and n = 570 DBD), the 3-year overall patient survival was worse for DCD LT recipients (60.1% versus 76.9% for DBD; P = 0.049; Fig. 5A). There was no significant difference in 3-year recurrence risk by donor type stratified by AFP at LT, including for patients with AFP at LT ≥100 ng/mL (18.3% DCD versus 28.9% DBD; P = 0.23).
FIG. 5.
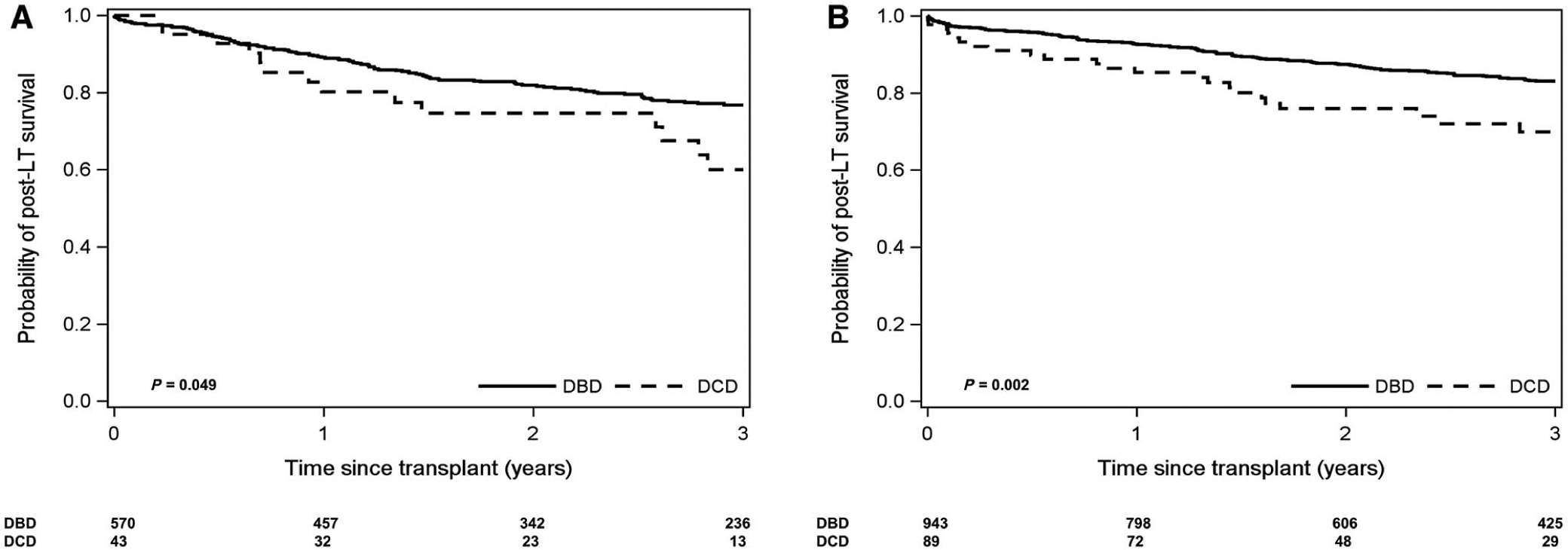
Kaplan-Meier curve of post-LT patient survival stratified by donor type for DCD versus DBD donors. (A) AFP at LT ≥100 ng/mL. (B) Multiple viable tumors on last imaging prior to LT.
In terms of last radiographic tumor size and number prior to LT, for patients with no viable tumors or a single tumor ≤5 cm (86.2% of the overall cohort), there was no difference in overall patient survival by donor type. However, for patients with multiple enhancing tumors on last imaging (n = 89 for DCD and n = 943 for DBD), the 3-year overall patient survival was worse for DCD LT recipients (69.9% versus 83.1% for DBD; P = 0.002; Fig. 5B).
PREDICTORS OF POST-LT SURVIVAL AND RECURRENCE
Predictors of post-LT patient survival from Cox proportional hazards univariate and multivariate analyses are summarized in Table 3. Receiving a DCD donor was a significant predictor of post-LT death in the univariate analysis as was increasing recipient and donor age, hepatitis B virus (HBV), increasing MELD score, DRI, AFP, radiographic tumor burden prior to LT, and RETREAT score. CIT, recipient sex, and receipt of ablation, transarterial chemoembolization (TACE), or yttrium-90 microspheres (Y90) prior to LT were not associated with post-LT survival in the univariate analysis. In the multivariate analysis, receipt of DCD donor (HR, 1.38; 95% CI, 1.10–1.72; P = 0.005) remained associated with worse post-LT patient survival as did increasing recipient age (HR, 1.02 per year; 95% CI, 1.01–1.03; P < 0.001), donor age (HR, 1.01; 95% CI, 1.00–1.01; P < 0.001), and RETREAT score (Table 3). When split by RETREAT group category, in the univariate analysis DCD donation compared with DBD was significantly associated with worse post-LT survival for RETREAT score ≥4 patients (HR, 1.70; 95% CI, 1.11–2.59; P = 0.01), whereas no significant association was seen for RETREAT score 1–3 (HR, 1.27; 95% CI, 0.95–1.70; P = 0.14) and RETREAT score 0 patients (HR, 1.31; 95% CI, 0.70–2.47; P = 0.38). When only factors known prior to LT were included (ie, exclusion of explant pathology), receipt of a DCD donor organ remained associated with worse post-LT survival as was increasing recipient and donor age, MELD score ≥15, and increasing AFP at LT. Patients with 3 viable lesions on last imaging prior to LT also had increased risk of post-LT death (HR, 1.39; 95% CI, 1.03–1.86; P = 0.03 compared with patients with no enhancing lesions) with a trend toward increased post-LT death for patients with a single 3.1–5 cm lesion or 2 lesions (Table 3).
TABLE 3.
Univariate and Multivariate Analyses of Predictors of Post-LT Death Using Cox Proportional Hazards Regression Among Patients With HCC (n = 7563)
| Predictor | HR (95% CI) | P Value |
|---|---|---|
| Univariate analysis | ||
| Age at listing (per year) | 1.02 (1.01–1.03) | <0.001 |
| Sex, female | 0.97 (0.83–1.13) | 0.68 |
| Etiology of liver disease (reference: HCV) | ||
| NAFLD | 0.95 (0.76–1.19) | 0.64 |
| Alcohol | 1.04 (0.83–1.31) | 0.74 |
| HBV | 0.67 (0.49–0.93) | 0.02 |
| Autoimmune* | 0.95 (0.61–1.49) | 0.83 |
| Received LRT prior to transplant | ||
| Any LRT | 1.00 (0.78–1.29) | 0.99 |
| Ablation at any time | 0.91 (0.77–1.07) | 0.26 |
| TACE or Y90 at any time | 1.12 (0.98–1.29) | 0.09 |
| Last imaging prior to LT (reference: 0 lesions) | ||
| 1 lesion ≤3 cm | 1.08 (0.93–1.25) | 0.32 |
| 1 lesion 3.1–5 cm | 1.34 (1.08–1.67) | 0.009 |
| 2 lesions | 1.28 (1.06–1.56) | 0.01 |
| 3 lesions | 1.57 (1.17–2.10) | 0.002 |
| Total tumor diameter (per cm) | 1.08 (1.04–1.11) | <0.001 |
| Number of lesions plus largest lesion diameter | 1.06 (1.03–1.10) | <0.001 |
| AFP at LT (reference: <20 ng/mL) | ||
| 20 to <100 ng/mL | 1.50 (1.29–1.74) | <0.001 |
| 100 to ≤1000 ng/mL | 1.95 (1.61–2.36) | <0.001 |
| ≥1000 ng/mL | 4.04 (2.59–6.31) | <0.001 |
| RETREAT score (reference: 0) | ||
| 1 | 1.24 (1.01–1.52) | 0.04 |
| 2 | 1.83 (1.48–2.26) | <0.001 |
| 3 | 2.26 (1.80–2.85) | <0.001 |
| 4 | 2.96 (2.33–3.77) (2.326–3.77) | <0.001 |
| ≥5 | 4.50 (3.51–5.78) | <0.001 |
| MELD score (per point) | 1.01 (1.00–1.02) | 0.03 |
| Donor factors | ||
| DCD versus DBD donor | 1.32 (1.06–1.64) | 0.01 |
| Donor age (per year) | 1.01 (1.00–1.01) | 0.001 |
| DRI | 1.41 (1.21–1.65) | <0.001 |
| CIT (hours) | 0.99 (0.96–1.01) | 0.28 |
| Multivariate analysis† | ||
| Age at listing (per year) | 1.02 (1.01–1.03) | <0.001 |
| Donor age (per year) | 1.01 (1.00–1.01) | <0.001 |
| DCD versus DBD donor (versus DBD) | 1.38 (1.10–1.72) | 0.005 |
| RETREAT score (reference: 0) | ||
| 1 | 1.25 (1.02–1.53) | 0.04 |
| 2 | 1.81 (1.47–2.24) | <0.001 |
| 3 | 2.26 (1.79–2.83) | <0.001 |
| 4 | 2.92 (2.30–3.72) | <0.001 |
| ≥5 | 4.41 (3.44–5.67) | <0.001 |
| Multivariate analysis with exclusion of explant features | ||
| Age at listing (per year) | 1.02 (1.01–1.03) | <0.001 |
| Last imaging prior to LT (reference: 0 lesions) | ||
| 1 lesion ≤3 cm | 1.05 (0.91–1.22) | 0.51 |
| 1 lesion 3.1–5 cm | 1.24 (0.99–1.54) | 0.06 |
| 2 lesions | 1.19 (0.97–1.45) | 0.09 |
| 3 lesions | 1.39 (1.03–1.86) | 0.03 |
| AFP at LT (reference: <20 ng/mL) | ||
| 20 to <100 ng/mL | 1.51 (1.29–1.75) | <0.001 |
| 100 to ≤1000 ng/mL | 1.93 (1.60–2.35) | <0.001 |
| ≥1000 ng/mL | 3.99 (2.55–6.25) | <0.001 |
| MELD score ≥15 | 1.16 (1.02–1.32) | 0.02 |
| Donor age (per year) | 1.01 (1.00–1.01) | <0.001 |
| DCD versus DBD donor | 1.42 (1.13–1.77) | 0.002 |
Includes autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis.
MELD (P = 0.12) and etiology of liver disease (P = 0.49) were tested and not significantly associated with survival.
Significant predictors of post-LT HCC recurrence in the univariate analysis were recipient sex, radiographic tumor burden prior to transplant, and RETREAT score. Receiving a DCD donor was not significantly associated with post-LT HCC recurrence (HR, 1.05; 95% CI, 0.71–1.53; P = 0.82) nor was DRI, donor age, donor steatosis, or CIT.
Discussion
Over the past decade, there has been an increasing number of patients awaiting LT.(15,16) As a result, more extended criteria donors are being considered, including DCD donors, in an attempt to increase the overall donor pool.(15,16) Livers from DCD donors have the potential to expand the available pool of livers by 1000 grafts/year.(17) However, DCD livers are still underused. In the most recent Scientific Registry of Transplant Recipients annual data report, there was only a moderate increase in the use of DCD livers from 5.1% of all LTs in 2007 to 6.9% in 2017, which is far lower in comparison to the United Kingdom where DCD livers account for approximately 17.8% of all LTs,(18) which is possibly due to shorter preservation times, lower recipient MELD scores, and a greater degree of clinical access to normothermic machine perfusion of the liver (NMP-L) and normothermic regional perfusion (NRP). In the United States, approximately 27% of DCD livers recovered for transplant are discarded (compared with <10% in DBD livers),(15) reflecting hesitation by many centers to use such grafts.(19) This is most likely due to the fact that patients who receive DCD livers have been shown to have worse overall patient survival and more biliary complications compared with those who receive DBD livers.(20–22) Most notably IC affects approximately 12% of recipients of DCD allografts.(23)
HCC patients receive a higher proportion of DCD donors than other LT patients.(10,24,25) However, the literature on outcomes for HCC patients receiving DCD donors is limited to studies with a very small sample of patients. Such studies have shown no overall differences in post-LT outcomes.(11–13,26) To the best of our knowledge, the current study is the first to compare post-LT survival and recurrence outcomes for HCC recipients of DCD versus DBD donors in a national database. The results of this study found inferior post-LT survival for HCC patients receiving a DCD donor organ compared with those receiving a DBD donor organ, with a nearly 40% increased risk of death for patients receiving a DCD organ. This discrepancy compared with prior studies is likely due to their small sample sizes with resulting low power to detect differences.(11–13,26) For example, 1 recently published study included only 18 HCC patients receiving a DCD donor and found no significant difference in 1-year survival, but the numerical differences (88% for DBD versus 75% for DCD) appear to be quite large.(26) In the present study, the observed survival difference was most notable in patients with high-risk features at transplant, including those with an AFP at LT ≥100 ng/mL, multiple enhancing lesions on last imaging prior to LT, and RETREAT score ≥4. Additionally, graft survival was worse for HCC patients who received DCD donors, regardless of the individual RETREAT score.
Within the first year after transplant, there are several common causes of death for patients with HCC. However, after the first year, recurrence of the primary liver disease is the main contributor.(27) The present study found no difference in HCC recurrence rates between the DCD and DBD groups, consistent with recent literature on the topic.(11,28) The fact that survival was worse in HCC patients at higher risk for recurrence who received a DCD liver but recurrence rates were similar between groups is meaningful and points toward the DCD graft itself as a contributor to the difference in survival. These findings support a “two-hit” hypothesis where patients who, first, are at high risk for recurrence and, then, receive a DCD liver have particularly poor post-LT patient and graft survival.
Incorporating these present data can better inform clinicians on which HCC patients should be considered candidates for DCD LT. Historically, HCC patients have received a higher proportion of DCD donors than non-HCC patients, which can be explained, in part, by an attempt to decrease wait-list dropout for these patients, especially after the Share 35 rule was enacted.(29) However, in areas with organ shortages, wait-list dropout rates for HCC patients are nearly 30%.(30) Expanding the pool of livers by using DCD donors has the potential to decrease this dropout rate, but properly selecting appropriate HCC candidates for DCD livers has been challenging.
Many models have attempted to predict increased wait-list dropout for HCC patients, and they typically consist of increasing MELD, Child-Pugh score, AFP, and tumor burden.(31–36) Patients with well-compensated disease and a single, small, well-treated tumor (approximately 20% of listed HCC patients(36)) most likely derive less benefit from receiving a DCD donor graft because that population can either wait for a standard criteria donor or perhaps avoid LT altogether (until tumor recurrence).(35) At the other extreme, in patients with high risk of HCC recurrence (eg, AFP at LT >100 ng/mL, progressive disease after LRT, or a RETREAT score >4),(37,38) the present findings suggest DCD livers worsen patient and graft survival. On one hand, these patients actually have a large mortality benefit from accepting an early offer DCD liver rather than waiting for a superior DBD liver because these patients are at high risk of wait-list dropout and/or death.(39) On the other hand, this must be weighed against the obligation to achieve a minimum survival threshold. Using a Markov model, Volk et al. has shown that the adverse effects of expanding LT criteria (eg, HCC patients beyond Milan) would out-weigh its benefits if the expected 5-year overall survival was <61%.(40) Therefore, caution should be made when deciding to give a DCD donor to this high-risk group. We believe that all other listed HCC patients such as those with decompensated liver disease, single viable lesion ≤5 cm, and/or partial response/stable disease after LRT likely benefit from accepting a DCD donor to avoid wait-list dropout(19,41) given that there does not appear to be a difference in post-LT survival for DCD versus DBD in such patients.
Although there does appear to be worse post-LT outcomes for HCC patients at high risk of recurrence who receive a DCD liver, DCD organs do have a promising future. Ischemia/reperfusion injury is greater in recipients of DCD grafts compared with DBD grafts, and this is the presumed mechanism of the increased risk of HCC recurrence some have described, and the increased risk of graft loss from IC.(21) Research is currently ongoing to identify the optimal method of preservation of DCD livers prior to transplant. To reduce the amount of hypoxia, NRP using extracorporeal membrane oxygenation during the time between the cardiac arrest and the beginning of organ preservation is being studied. Clinical and experimental data support its use in donors to aid the recovery of DCD organs from ischemic injury, showing additional benefits over traditional cold storage.(42) The logistics of NRP are challenging for donor procurement teams, but a recent study looking at 11 DCD LTs using NRP found no incidences of IC and only 1 primary graft nonfunction, rates similar to the control LT group who received DBD livers.(43) More clinical experience with NMP-L in humans has been generated in the last few years. A multicenter randomized control trial of 272 LT patients (78 DCD and 194 DBD patients) found promising findings for NMP-L when compared with conventional static cold storage. This study found that the NMP-L group had a 50% reduction in graft injury, measured by hepatocellular enzyme release (aspartate aminotransferase) with less early allograft dysfunction (10.1% versus 29.9%; P < 0.001) and much greater organ utilization, even though the trial was not designed to address organ discard.(44) Hypothermic oxygenated perfusion is another preservation strategy that is quickly gaining momentum,(45,46) as it has been shown to reduce the biliary injury of preservation, possibly even more effectively than NMP-L. Clinical trial data in humans are still mounting, but perhaps these preservation techniques will soon reduce the risk associated with DCD liver grafts, expanding the indication for transplant in patients with HCC.
Interestingly, in the univariate analysis, we found that HBV was a significant predictor of improved post-LT survival compared with HCV. Previous studies have shown that the probability of tumor progression was lower among patients with HBV compared with HCV, which may relate to the use of nucleos(t)ide analogues in patients with HBV cirrhosis. Treatment with nucleos(t)ide analogues in HBV cirrhosis has been shown to reduce the risk of HCC development(47,48) as well as HCC recurrence.(49) It is expected that most HBV patients in the current study had virological suppression with appropriate antiviral therapy. With the increased use of HCV treatment, presumably in the future we will see similarly improved post-LT prognosis for HCV patients. Therefore, the etiology of liver disease with respect to virological suppression, when applicable, could influence which HCC patients will be allocated a DCD graft.
There are several limitations of the present study. Most importantly, although this is the largest reported sample of HCC patients who received DCD livers, there was a smaller number of DCD (n = 567) patients compared with DBD (n = 6996). To this end, comparisons of post-LT survival and HCC recurrence by donor type, RETREAT score, and AFP included relatively small numbers in the highest-risk categories limiting our ability to detect statistically significant differences. Also, no mandate requires transplant centers to report HCC recurrence, and our need to capture explant data (available only since April 2012) resulted in a relatively short post-LT follow-up. These factors could result in underestimating post-LT recurrence, and thus, we focused on posttransplant patient survival as our primary outcome in this study. Finally, we captured graft survival, but the UNOS database does not report on the specific reason for graft failure, such as IC, and thus, we were unable to further study this secondary outcome.
In conclusion, in this large analysis examining post-LT outcomes of patients with HCC receiving DCD versus DBD livers, we found that patients with a low-to-moderate risk of HCC recurrence had equivalent survival (80%−90% of the DCD cohort). However, patients with an AFP at LT >100 ng/mL, multiple enhancing lesions on last imaging before LT, or RETREAT score ≥4 had worse post-LT survival with a DCD donor. Especially in areas with organ shortages and high wait-list dropout, it appears that DCD donation for patients with HCC can best be used to increase the donor pool for those with decompensated cirrhosis and/or partial response/stable disease after LRT with AFP at LT <100 ng/mL. Ongoing improvements in organ preservation of DCD grafts may further expand their utility in patients with HCC.
Acknowledgments
This work was supported by the Clinical and Translational Core of the University of California, San Francisco Liver Center (P30-DK-026473).
Abbreviations:
- AFP
alpha-fetoprotein
- CI
confidence interval
- CIT
cold ischemia time
- Cr
creatinine
- DBD
donation after brain death
- DCD
donation after circulatory death
- DRI
donor risk index
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IC
ischemic cholangiopathy
- IQR
interquartile range
- LRT
locoregional therapy
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- NMP-L
normothermic machine perfusion of the liver
- NRP
normothermic regional perfusion
- RETREAT
Risk Estimation of Tumor Recurrence After Transplant
- TACE
transarterial chemoembolization
- UNOS
United Network for Organ Sharing
- Y90
yttrium-90 microspheres
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1).Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986–994. [DOI] [PubMed] [Google Scholar]
- 2).Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 3).de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol 2012;56(suppl 1):S75–S87. [DOI] [PubMed] [Google Scholar]
- 4).Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–1255. [DOI] [PubMed] [Google Scholar]
- 5).Maddala YK, Stadheim L, Andrews JC, Burgart LJ, Rosen CB, Kremers WK, Gores G. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl 2004;10:449–455. [DOI] [PubMed] [Google Scholar]
- 6).Yao FY, Bass NM, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: lessons from the first year under the Model of End-Stage Liver Disease (MELD) organ allocation policy. Liver Transpl 2004;10:621–630. [DOI] [PubMed] [Google Scholar]
- 7).Monbaliu D, Pirenne J, Talbot D. Liver transplantation using donation after cardiac death donors. J Hepatol 2012;56:474–485. [DOI] [PubMed] [Google Scholar]
- 8).Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017;14:203–217. [DOI] [PubMed] [Google Scholar]
- 9).Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant 2006;6:791–796. [DOI] [PubMed] [Google Scholar]
- 10).Goh MA, Saeb-Parsy K, Pettigrew G. Liver transplantation from donation after circulatory death donors in patients with hepatocellular carcinoma results in good outcomes. Int J Surg 2013;11:706–707. [Google Scholar]
- 11).Croome KP, Lee DD, Burns JM, Musto K, Paz D, Nguyen JH, et al. The use of donation after cardiac death allografts does not increase recurrence of hepatocellular carcinoma. Am J Transplant 2015;15:2704–2711. [DOI] [PubMed] [Google Scholar]
- 12).Goldkamp W, Vanatta J, Nair S, Wong E, Dbouk N. Outcomes of patients with hepatocellular carcinoma receiving a donation after cardiac death liver graft. Am J Transplant 2015;15(suppl 3):D175. [Google Scholar]
- 13).El-Gazzaz G, Hashimoto K, Quintini C, Kelly D, Winans C, Eghtesad B, et al. Is liver transplantation outcome worse for HCC patients using organ donation after cardiac death? Am J Transplant 2013;13(suppl 5):A709. [Google Scholar]
- 14).Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, et al. OPTN/SRTR 2017 annual data report: liver. Am J Transplant 2019;19(suppl 2):184–283. [DOI] [PubMed] [Google Scholar]
- 16).Fayek SA, Quintini C, Chavin KD, Marsh CL. The current state of liver transplantation in the United States: perspective from American Society of Transplant Surgeons (ASTS) scientific studies committee and endorsed by ASTS Council. Am J Transplant 2016;16:3093–3104. [DOI] [PubMed] [Google Scholar]
- 17).Renz JF. Is DCD for liver transplantation DNR? Am J Transplant 2008;8:485–488. [DOI] [PubMed] [Google Scholar]
- 18).Johnson RJ, Bradbury LL, Martin K, Neuberger J; for UK Transplant Registry. Organ donation and transplantation in the UK-the last decade: a report from the UK national transplant registry. Transplantation 2014;97(suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]
- 19).Vining CC, Ecker BL, Abt PL, Olthoff KM. Donation after cardiac death in the hepatocellular carcinoma patient: same indication? Liver Transpl 2017;23(suppl 1):S27–S33. [DOI] [PubMed] [Google Scholar]
- 20).Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg 2005;242:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg 2011;253:259–264. [DOI] [PubMed] [Google Scholar]
- 22).Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, DʼAlessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg 2011;253:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Goldberg DS, Karp SJ, McCauley ME, Markmann JF, Croome KP, Taner CB, et al. Interpreting outcomes in DCDD liver transplantation: first report of the multicenter IDOL consortium. Transplantation 2017;101:1067–1073. [DOI] [PubMed] [Google Scholar]
- 24).McLean KA, Camilleri-Brennan J, Knight SR, Drake TM, Ots R, Shaw CA, et al. Decision modeling in donation after circulatory death liver transplantation. Liver Transpl 2017;23:594–603. [DOI] [PubMed] [Google Scholar]
- 25).Jay C, Ladner D, Wang E, Lyuksemburg V, Kang R, Chang Y, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant—an analysis of the national registry. J Hepatol 2011;55:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Martinez-Insfran LA, Ramirez P, Cascales P, Alconchel F, Ferreras D, Febrero B, et al. Early outcomes of liver transplantation using donors after circulatory death in patients with hepatocellular carcinoma: a comparative study. Transplant Proc 2019;51:359–364. [DOI] [PubMed] [Google Scholar]
- 27).Sposito C, Cucchetti A, Mazzaferro V. Assessing competing risks for death following liver transplantation for hepatocellular carcinoma. Dig Dis Sci 2019;64:1001–1007. [DOI] [PubMed] [Google Scholar]
- 28).Wallace D, Walker K, Charman S, Suddle A, Gimson A, Rowe I, et al. Assessing the impact of suboptimal donor characteristics on mortality after liver transplantation: a time-dependent analysis comparing HCC with non-HCC patients. Transplantation 2019;103:e89–e98. [DOI] [PubMed] [Google Scholar]
- 29).Croome KP, Lee DD, Harnois D, Taner CB. Effects of the Share 35 rule on waitlist and liver transplantation outcomes for patients with hepatocellular carcinoma. PLoS One 2017;12:e0170673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Increasing liver transplantation wait-list dropout for hepatocellular carcinoma with widening geographical disparities: implications for organ allocation. Liver Transpl 2018;24:1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Mehta N, Dodge J, Yao F. A novel dropout risk score in hepatocellular carcinoma (HCC) patients listed for liver transplant (LT) – identifying a threshold that predicts worse post-LT survival in short wait regions. Am J Transplant 2017;17(suppl 3):353.27401926 [Google Scholar]
- 32).Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant 2006;6:1416–1421. [DOI] [PubMed] [Google Scholar]
- 33).Toso C, Dupuis-Lozeron E, Majno P, Berney T, Kneteman NM, Perneger T, et al. A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list. Hepatology 2012;56:149–156. [DOI] [PubMed] [Google Scholar]
- 34).Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant 2010;10:1643–1648. [DOI] [PubMed] [Google Scholar]
- 35).Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Predictors of low risk for dropout from the liver transplant waiting list for hepatocellular carcinoma in long wait time regions: implications for organ allocation. Am J Transplant 2019;19:2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).De Giorgio M, Vezzoli S, Cohen E, Armellini E, Lucà MG, Verga G, et al. Prediction of progression-free survival in patients presenting with hepatocellular carcinoma within the Milan criteria. Liver Transpl 2010;16:503–512. [DOI] [PubMed] [Google Scholar]
- 38).Kim DJ, Clark PJ, Heimbach J, Rosen C, Sanchez W, Watt K, Charlton MR. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant 2014;14:1383–1390. [DOI] [PubMed] [Google Scholar]
- 39).Taylor R, Allen E, Richards JA, Goh MA, Neuberger J, Collett D, Pettigrew GJ; for Liver Advisory Group to NHS Blood and Transplant. Survival advantage for patients accepting the offer of a circulatory death liver transplant. J Hepatol 2019;70:855–865. [DOI] [PubMed] [Google Scholar]
- 40).Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant 2008;8:839–846. [DOI] [PubMed] [Google Scholar]
- 41).Lai Q, Vitale A, Iesari S, Finkenstedt A, Mennini G, Spoletini G, et al. ; for European Hepatocellular Cancer Liver Transplant Study Group. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017;66:1910–1919. [DOI] [PubMed] [Google Scholar]
- 42).Shapey IM, Muiesan P. Regional perfusion by extracorporeal membrane oxygenation of abdominal organs from donors after circulatory death: a systematic review. Liver Transpl 2013;19: 1292–1303. [DOI] [PubMed] [Google Scholar]
- 43).Rodríguez-Sanjuán JC, Ruiz N, Miñambres E, Toledo E, González-Noriega M, Fernández-Santiago R, Castillo F. Liver transplant from controlled cardiac death donors using normothermic regional perfusion: comparison with liver transplants from brain dead donors. Transplant Proc 2019;51:12–19. [DOI] [PubMed] [Google Scholar]
- 44).Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al. ; for Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature 2018;557:50–56. [DOI] [PubMed] [Google Scholar]
- 45).Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg 2015;262:764–770. [DOI] [PubMed] [Google Scholar]
- 46).Schlegel A, Muller X, Kalisvaart M, Muellhaupt B, Perera MTPR, Isaac JR, et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol 2019;70:50–57. [DOI] [PubMed] [Google Scholar]
- 47).Wong GLH, Chan HLY, Mak CWH, Lee SKY, Ip ZMY, Lam AT-H, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatol 2013;58:1537–1547. [DOI] [PubMed] [Google Scholar]
- 48).Liaw YF, Sung JJY, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–1531. [DOI] [PubMed] [Google Scholar]
- 49).Tan ZM, Sun BC. Effects of antiviral therapy on preventing liver tumorigenesis and hepatocellular carcinoma recurrence. World J Gastroenterol 2013;19:8895–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]


