Abstract
Objective:
Uterine serous carcinoma (USC) is an aggressive histologic variant of endometrial cancer which portends a poor prognosis. DHES0815A is a novel antibody-drug-conjugate (ADC) which binds specifically to HER2 overexpressing tumors at a distinct epitope from that bound by trastuzumab and pertuzumab after which it delivers the toxic payload, PBD-MA, a DNA mono-alkylating agent. The objective of this study was to evaluate the preclinical activity of DHES0815A against primary USC cell lines and xenografts.
Methods:
Twelve primary USC cell lines were assessed by immunohistochemistry (IHC) for HER2 protein expression and for C-erbB2 gene amplification using fluorescent in situ hybridization (FISH) analysis. Cell viability and bystander killing in USC cell lines after exposure to DHES0815A, the non-targeted ADC, and the unconjugated antibody (i.e. MHES0488A) were evaluated using flow cytometry-based-assays. In vivo activity of DHES0815A was tested against HER2/neu overexpressing USC xenografts.
Results:
High HER2/neu protein expression was seen in 25% (3/12) of the primary USC cell lines. USC cell lines overexpressing HER2/neu were significantly more sensitive to DHES0815A when compared to the non-targeted control ADC (p<0.001). DHES0815A did not induce significant bystander killing of HER2/neu negative tumors when admixed with HER2/neu positive tumors. DHES0815A caused growth-inhibition and increased survival in USC HER2/neu overexpressing xenografts when compared to controls (p<0.01).
Conclusions:
DHES0815A is both highly selective and toxic to USC tumors overexpressing HER2/neu both in vitro and in vivo. HER2-directed ADCs, alone or in combination with other HER2/neu targeted agents may represent a novel treatment option for patients with tumors harboring HER2/neu overexpression refractory to trastuzumab and traditional chemotherapy.
Keywords: DHES0815A, uterine serous carcinoma, antibody drug conjugate (ADC), uterine cancer
INTRODUCTION
In developing countries, endometrial cancer is the most prevalent of all gynecologic malignancies. In the USA an estimated 65,620 cases will be diagnosed in 2020 and this disease will account for an estimated 2,590 deaths [1]. If current trends continue the incidence of endometrial cancer will double by 2030 [2]. There are two broad histologic categories of endometrial carcinomas designated as type I and type II tumors that have different patterns of molecular alterations, which underlie their pathogenesis and clinical outcomes [3,4]. Approximately 80% of endometrial carcinomas are type I tumors, which are those with grade 1 or 2 endometrioid histology and have a favorable prognosis. Type II tumors comprise only about 20% of endometrial carcinomas and include grade 3 endometrioid tumors as well as other nonendometrioid histology’s such as serous and clear cell; however, these tumors have a more aggressive clinical course. In fact, approximately 70% of patient with uterine serous carcinoma (USC) present with stage III or IV disease [5,6]. Although USC accounts for only 3 to 10% of all endometrial carcinomas it is responsible for a disproportionate 39% of all uterine cancer related deaths with an overall 5-year survival rate of 45%, compared to 91% for those with endometrioid adenocarcinoma [7–9]. Despite surgery and multimodality adjuvant therapy the prognosis remains dismal for patients with USC. Therefore, the identification of novel treatment modalities for patients diagnosed with USC remains an unmet medical need. The human epidermal growth factor type 2 receptor (HER2 or HER2/neu) gene (i.e. c-erbB2) encodes the HER2/neu tyrosine kinase receptor which plays an important role in the coordination of the complex erbB signaling network that is responsible for the regulation of cell growth, survival and proliferation [10]. HER2 overexpression (i.e. HER2/neu 3+ expression by IHC or c-erbB2 gene amplification by FISH) is present in approximately 30% of USC [11–14]. HER2 overexpression and/or gene amplification appears to be a poor prognostic factor in both early as well as advanced USC patients [11–13]. The substantial unwanted toxic side effects of conventional chemotherapy treatments used in the fight against cancer highlights the need for newer agents that specifically target tumor cells while sparing the surrounding normal tissue. Trastuzumab (Herceptin, Genentech/Roche), a humanized antibody targeting HER2/neu has recently demonstrated significant clinical activity in patients with HER2-amplified advanced/recurrent USC overexpressing HER2/neu. Indeed, a recent multicenter randomized phase II trial demonstrated that trastuzumab in combination with carboplatin/paclitaxel resulted in a significant improvement in progression-free survival and overall survival in patients with both advanced and recurrent HER2/neu-positive USC [11–12]. Importantly, as a result of this trial, the National Comprehensive Cancer Network Uterine Neoplasm Guidelines have been revised and now endorse the addition of trastuzumab to standard cytotoxic chemotherapy as the preferred regimen for the treatment of Her2/Neu-positive, advanced or recurrent USC [14]. Combination treatments may prove to be a highly effective therapeutic strategy to overcome inborn and/or acquired resistance to HER2/neu-targeted therapies for patients harboring HER2-amplified USC tumors.
Antibody-drug conjugates (ADCs), such as T-DM1, have recently strengthened the armamentarium in the era of molecularly targeted therapy. ADCs are sophisticated in that they link a surface receptor-targeting antibody to a cytotoxic molecule [15]. This permits the selective delivery and internalization of a toxic payload to tumor cells expressing high levels of the antigen targeted by the ADC while sparing healthy tissue that expresses minimal levels of this targeted antigen [16]. T-DM1 is composed of trastuzumab covalently linked with a non-cleavable linker to the antimitotic agent emtansine (DM1). Once internalized into tumor cells DM1 it is cleaved from the antibody and binds to tubulin inhibiting microtubule assembly and resulting in apoptosis in dividing tumor cells [17–20]. However, there is a significant need for new HER2-targeted agents with novel mechanisms of action as well as acceptable toxicity profiles that may be combined with current established treatments to improve outcomes for patients with HER2-overexpressing/amplified USC tumors. In fact, results from studies of HER2-positive advanced breast cancer patients suggest that engagement of HER2 at multiple epitopes may confer an advantageous clinical benefit and that tumor-directed cytotoxic agents may yield a more optimal safety profile over systemic chemotherapy [21–23]. DHES0815A is a THIOMAB™ antibody-drug conjugate (TDC) that is comprised of an engineered humanized immunoglobulin G1 (IgG1) monoclonal antibody, MHES0488A, which binds specifically to HER2, conjugated to a novel DNA mono-alkylating agent, pyrrolo[2,1-c][1,4]benzodiazepine monoamide (PBD-MA), via a stable disulfide-based linker. DHES0815A was designed using THIOMAB™ technology enabling the conjugation of two PBD-MA linker drug molecules per antibody [24]. The released payload covalently binds to DNA, leading to DNA alkylation but not cross-linking. Importantly, only one of the two PBD moieties contains an amide functionality that is not involved in DNA alkylation, making it less potent than the corresponding bis-alkylator to potentially improve tolerability. In contrast to the ADC Trastuzumab emtansine (T-DM1), where the toxic payload DM1 is active only in dividing cells, PBD-MA has cytotoxic activity in dividing and non-dividing cells. Interestingly, DHES0815A binds to domain I of the HER2 extracellular domain and recognizes a distinct epitope from that bound by trastuzumab, pertuzumab and T-DM1 [25]. Taken together, DHES0815A will not compete with previously studied HER2 targeted agents when binding to the HER2 receptor and as such it can potentially be used in combination treatments while providing a more favorable toxicity profile for the patient. Therefore, we sought to evaluate the preclinical antitumor activity of DHES0815A in USC for the first time through in vitro and in vivo experiments using several primary USC cell line models expressing different levels of HER2/neu and xenograft models.
MATERIALS AND METHODS
Establishment of USC cell lines
The Institutional Review Board (IRB) at Yale University approved this study and patient consent was obtained prior to tissue collection according to institutional guidelines. Twelve primary USC cell lines were established from fresh tumor biopsy samples obtained from chemotherapy naïve patients at the time of primary surgical staging. These fresh tumor biopsy samples were processed in a sterile fashion as previously described by our laboratory [26, 27]. The primary cell lines were then authenticated via whole exome sequencing (WES) at the Yale Center for Genome Analysis and subsequently cryopreserved [27]. All revived cell lines were used within 20 passages and cultured for less than 6 months before these experiments. Tumors were staged according to the International Federation of Gynecology and Obstetrics staging system. The corresponding cell blocks were analyzed for HER2 surface expression by IHC and c-erbB-2 gene amplification by fluorescent in situ hybridization (FISH) as described below. Patient and cell line characteristics as well as their respective HER2/neu expression are presented in Table 1. As this study involves work performed with human samples, all the study methodologies conformed to the standards set by the Declaration of Helsinki (World Medical Association, 2001).
Table 1.
Patient and cell line characteristics and Her2/neu expression in tumors
| Patient | Age | Race | FIGO Stagea | Histology | FISH | IHC cell block |
|---|---|---|---|---|---|---|
|
| ||||||
| ARK1 b | 62 | AA | IVA | Pure | Amplified | 3+ |
| ARK2 | 63 | AA | IVB | Pure | Amplified | 3+ |
| ARK4 | 73 | C | IVB | Pure | Not amplified | 0/1+ |
| ARK6 | 62 | C | IB | Mixed | Not amplified | 1+ |
| ARK7 | 75 | C | IIC | Pure | Not amplified | 2+ |
| ARK11 | 80 | AA | IIIC | Mixed | Not amplified | 1+ |
| ARK19 | 65 | C | IA | Pure | Not amplified | 2+ |
| ARK20 | 42 | C | II | Mixed | Amplified | 3+ |
| ARK22 | 60 | C | IVB | Pure | Not amplified | 0 |
| ARK24 | 54 | AA | IIIC | Pure | Not amplified | 1+ |
| ARK25 | 77 | C | IIIA | Mixed | Not amplified | 0 |
| ARK26 | 68 | C | IVB | Pure | Not amplified | 1+ |
Abbreviations: AA, African American; C, Caucasian
FIGO, International Federation of Gynecology and Obstetrics stage 1988.
Primary cell lines used in vitro validation experiments are listed in bold.
Drugs
T-DM1 (batch N0001B02; Roche, Basel, Switzerland) was provided by Synthon Biopharmaceuticals BV, Nijmegen, the Netherlands. DHES0815A, the unconjugated antibody or unconjugated anti-HER2 THIOMAB™ (MHES0488A), and the non-targeting control ADC (CNJ3605) were obtained from Genentech, Inc. (South San Francisco, CA). DHES0815A and T-DM1 were dissolved in sterile phosphate-buffered saline (PBS) as 20 μg/μL stock solutions for the in vitro experiments. MHES0488A and the non-targeted control ADC were dissolved in PBS as 15.4 μg/μL and 13.71 μg/μL respectively. The drug-to-antibody ratio (DAR) of DHES0815A and the control ADC were both 2.0. DHES0815A is a THIOMAB™ antibody-drug conjugate (TDC) that is comprised of an engineered humanized immunoglobulin G1 (IgG1) monoclonal antibody, MHES0488A, which binds specifically to HER2, conjugated to a novel DNA mono-alkylating agent, pyrrolo[2,1-c][1,4]benzodiazepine monoamide (PBD-MA), via a stable disulfide-based linker. DHES0815A was designed using THIOMAB™ technology enabling the conjugation of two PBD-MA linker drug molecules per antibody [24]. To achieve high stability in circulation and fast payload release at target cells, the PBD-MA payload was conjugated directly via a methyl-disulfide linker to the free thiols of the THIOMAB™ antibody light chain (LC-K149C); the site empirically identified for optimal stability. The non-targeted control ADC is comprised of a non-HER2 antibody conjugated with the same PBD-MA as DHES0815A. Of note, due to the intrinsic toxicity of pyrrolo[2,1-c][1,4]benzodiazepine monoamide (PBD-MA) when used as a free drug (unconjugated to an antibody), in vivo experiment with PBD-MA alone were not performed.
Evaluation of HER2 expression via immunostaining of primary USC cell blocks
Cell blocks were obtained from all twelve USC cell lines and reviewed by a gynecologic surgical pathologist to confirm the presence of tumor cells. The level of HER2 expression was evaluated as previously described [26]. Briefly, HER2 immunohistochemical staining was performed on paraffin-embedded 5 μm sections of the cell blocks after deparaffinization and rehydration, using the c-erbB-2 antibody (Thermo Fisher Scientific) at a 1:800 dilution. HER2 protein expression was scored per the recent clinical trial scoring criteria [28].
Evaluation of HER2 expression via FISH analysis of primary USC cell blocks
FISH analysis was performed using the PathVysion HER2 DNA FISH Kit (Abbott Molecular Inc.) according to the manufacturer’s instructions. Cell block sections of 5 μm were deparaffinized and rehydrated, followed by acid pretreatment and proteinase K digestion. A probe mix containing an orange probe directed against the HER2 gene (Vysis, Inc., LSI HER2) and a green probe directed against the pericentromeric region of chromosome 17 (Vysis CEP 17) were added and specimens were denatured for 5 minutes at 73 °C. Slides were then incubated overnight in a humidified chamber at 37 °C and washed the day after when a fluorescence mounting medium, containing 4,6-diamidino-2-phenylindole (DAPI), was applied. Fluorescent signals in at least 30 nonoverlapping interphase nuclei with intact morphology were scored using a Zeiss Axioplan 2 microscope (Carl Zeiss Meditec, Inc.) with a 100x planar objective, using a triple band-pass filter that permits simultaneous blue, green, and red colors. Tumor cells were evaluated for the number of orange (HER2) and green (CEP 17) signals. A case was considered amplified when the ratio of the number of fluorescent signals of HER2 to CEP 17 was ≥2.0.
Flow-cytometry based cell viability assays
USC cell lines were plated in six-well tissue culture plates at a density of 25,000–100,000 cells/well in RPMI 1640 media supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% amphotericin. Cells were incubated at 37°C, 5% CO2 for 24 hours. After this brief incubation, cells were treated with DHES0815A, the non-targeted ADC, and MHES0488A at concentrations of 0, 0.01, 0.07, 0.1, 0.5, 5.0 μg/mL. The six-well plates were then incubated for 72 hours after which the cells were harvested and stained with propidium iodide (2μl of 500 μg/ml stock solution in PBS). The viable cells were then quantified using flow-cytometry as mean ± SEM relative to untreated cells as 100% viable controls. A minimum of 3 independent experiments per cell line were performed to determine the half maximal inhibitory concentration (IC50) of DHES0815A, the non-targeted ADC, and MHES0488A.
Bystander Effect
Briefly, a 1:1 ratio of 3+ HER2/neu USC cells (i.e. ARK2) and HER2/neu 0/1+ USC cells (i.e. ARK4) stably transfected with a Green Fluorescence Protein (GFP) plasmid (pCDH-CMV-MCSEF1-copGFP, a gift from Dr. Simona Colla, MDACC, Houston, TX), were mixed (40,000 cells/well of each cell type) and plated in 6-well plates (2 mL/well). After an overnight incubation, DHES0815A or non-targeted control ADC at a concentration of 1 μg/mL or vehicle were added. Cells were collected, centrifuged and stained with propidium iodide (2μL of 500 μg/mL stock solution in PBS) after 72 hours of incubation to identify percentages of live versus dead cells in each well. Flow cytometry-based assay was performed to quantify live cells as a mean ± SEM relative to untreated cells. Bystander effect was then assessed by comparing the percentage of live HER2/neu negative cells (i.e. GFP-ARK4) when they are co-cultured with HER2/neu positive cells (i.e. ARK2) and treated with the non-targeted control ADC versus they are co-cultured and treated with DHES0815A.
In vivo treatment
The in vivo antitumor activity of DHES0815A was tested in a xenograft model with 3+ HER2/neu expression. Briefly, 5- to 8-week-old severe combined immunodeficiency (SCID) mice (ENVIGO, Indianapolis, IN) were given a single subcutaneous injection of 7×106 USC ARK-2 cells (HER2/neu 3+) in approximately 300 μL of a 1:1 solution of sterile PBS containing cells and Matrigel (BD Biosciences). Once the tumor volume was approximately 0.2 cm3, the mice were randomized into treatment groups (n= 6); those treated with DHES0815A (10 mg/kg), T-DM1 (10 mg/kg), non-targeting control ADC (10 mg/kg), MHES0488A the unconjugated anti-HER2 THIOMAB™ (10 mg/kg), and the vehicle being sterile saline. Drug dosages were chosen and given as a single intravenous injection according to previously conducted studies on different xenograft models [36–38]. Mice were observed for overall survival as the primary outcome measure. Tumor volume was determined using the formula (A2 x B)/2, where A and B represent the smallest perpendicular tumor diameter and largest tumor diameter respectively. The mice were euthanized when the tumor volume reached 1.0 cm3 or at the end of the study. All animal care and euthanasia were carried out according to the rules and regulations as set forth by the Institutional Animal Care and Use Committee (IACUC) which are in accordance with National Institutes of Health guide (NIH) for the care and use of Laboratory animals.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8 (GraphPad Software, San Diego, CA). The differences in the inhibition of proliferation in the USC cell lines after exposure to treatments were evaluated by two-tailed unpaired student t-test. Tumor volume differences at specific time points were compared using an unpaired student t-test. Overall survival data were analyzed using the Kaplan-Meier method. Survival curves were compared using the log-rank test. A two-sided p-value < 0.05 was considered to be significant.
RESULTS
HER2/neu expression in primary USC cell lines
We evaluated HER2/neu protein expression by IHC and C-erbB2 gene amplification by FISH in twelve primary USC culture cell lines (Table 1). Gene amplification and high levels (3+ staining) of HER2 protein expression by IHC were detected in 25% of the USC cell lines (i.e. 3/12). Seven cell lines had low (1+) or negligible (0/1+) HER2 expression on IHC, while the remaining two had 2+ expression (Table 1).
In vitro viability assays with DHES0815A, non-targeted control ADC, and MHES0488A in primary USC cell lines
Three primary HER2/neu 3+ USC cell lines (i.e. ARK-2, ARK-20 and ARK-1) and one primary HER2/neu 0/1+ USC cell line (ARK-4) were treated with scalar concentrations of DHES0815A, the non-targeted control ADC, and the unconjugated antibody MHES0488A for a total of 72 hours as described in the materials and methods section. The IC50 of DHES0815A, the non-targeted control ADC, and the unconjugated antibody MHES0488A were determined as described in the materials and methods section. As shown in Figure 1, DHES0815A was significantly more potent (i.e. 53-fold, 19-fold, and a 11-fold increase in cell cytotoxicity against ARK-2, ARK-20, and ARK-1 respectively) when compared to the non-targeted control ADC (p<0.05). Unconjugated antibody MHES0488A (ie, naked antibody) demonstrated no significant toxicity against the primary USC cell lines (data not shown). When the HER2/neu negative cell line (i.e. ARK-4) was challenged with DHES0815A, there was no increase in cell cytotoxicity when compared to the control ADC (p=0.1) (Figure 1).
Figure 1:
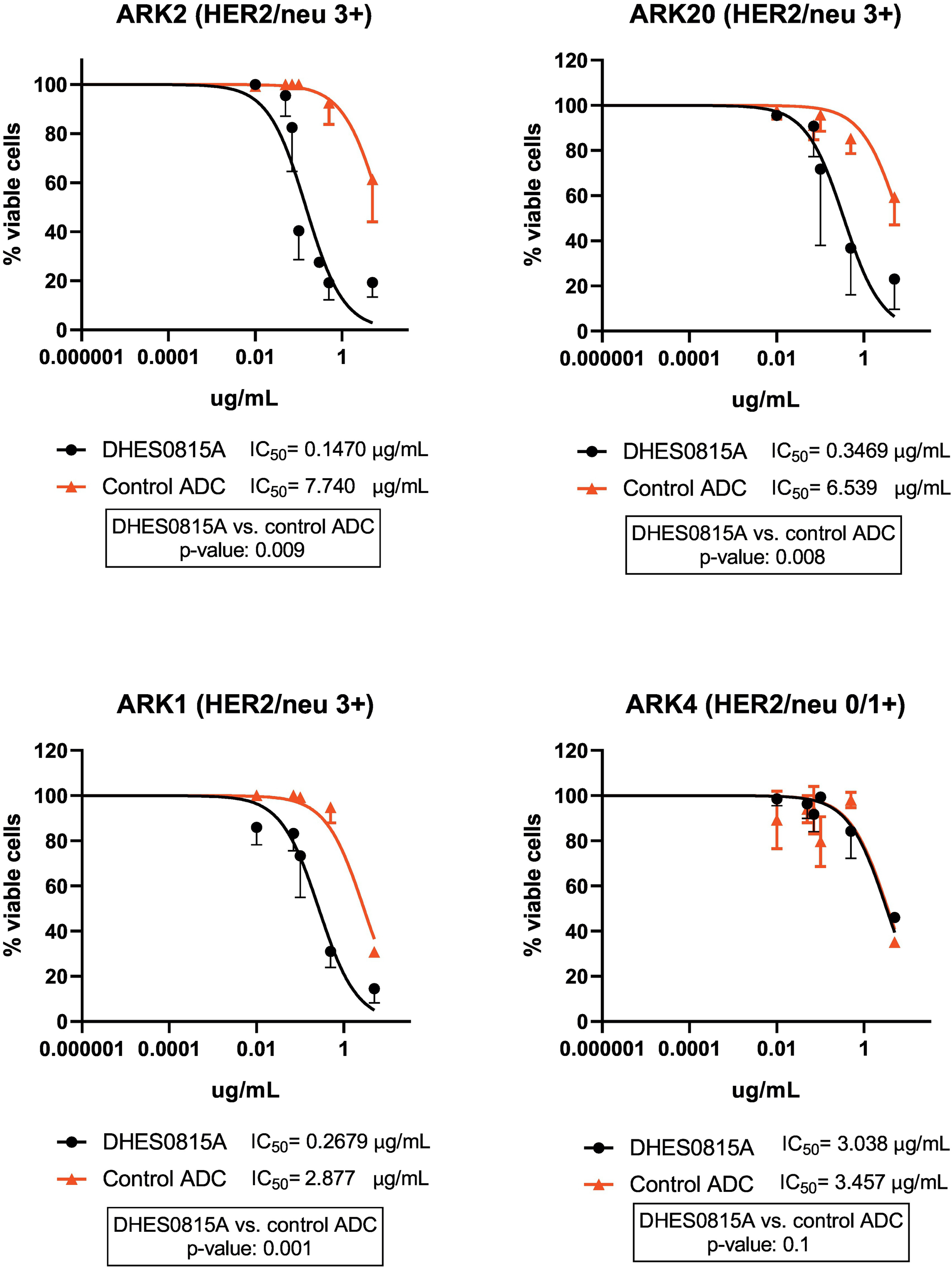
IC50 of DHES0815A compared to control (non-targeted control in USC cell lines with high level of HER2/neu expression versus negative HER2/neu expression. High HER2/neu (3+) expressing USC cell lines (ARK2, ARK20, ARK1) demonstrated significantly lower IC50 values when compared to the non-targeted control ADC (53-fold, 19-fold, and a 11-fold decrease, p<0.05). USC cell lines with negative HER2/neu (0/1+) expression (ARK4) showed no difference in IC50 values of DHES0815A when compared to the non-targeted control ADC.
DHES0815A does not demonstrate bystander effects in vitro
The ability of DHES0815A to induce a bystander killing effect against USC with heterogeneous HER2/neu expression was tested by admixing ARK-2 USC cells (i.e. high HER2/neu expression) in vitro with low/negligible HER2/neu expressing cells (i.e. GFP-ARK4 cells) for 72 hours as described in the methods and materials section. As shown in Figure 2 and consistent with the non-cleavable nature of the methyl-disulfide linker, there was no increase in bystander cytotoxicity of GFP-ARK4 cells seen when these and ARK-2 were treated as co-cultures with 1 μg/ml of DHES0815A when compared to DHES0815A -treated ARK-4 monocultures (p>0.05).
Figure 2: Bystander assay-.
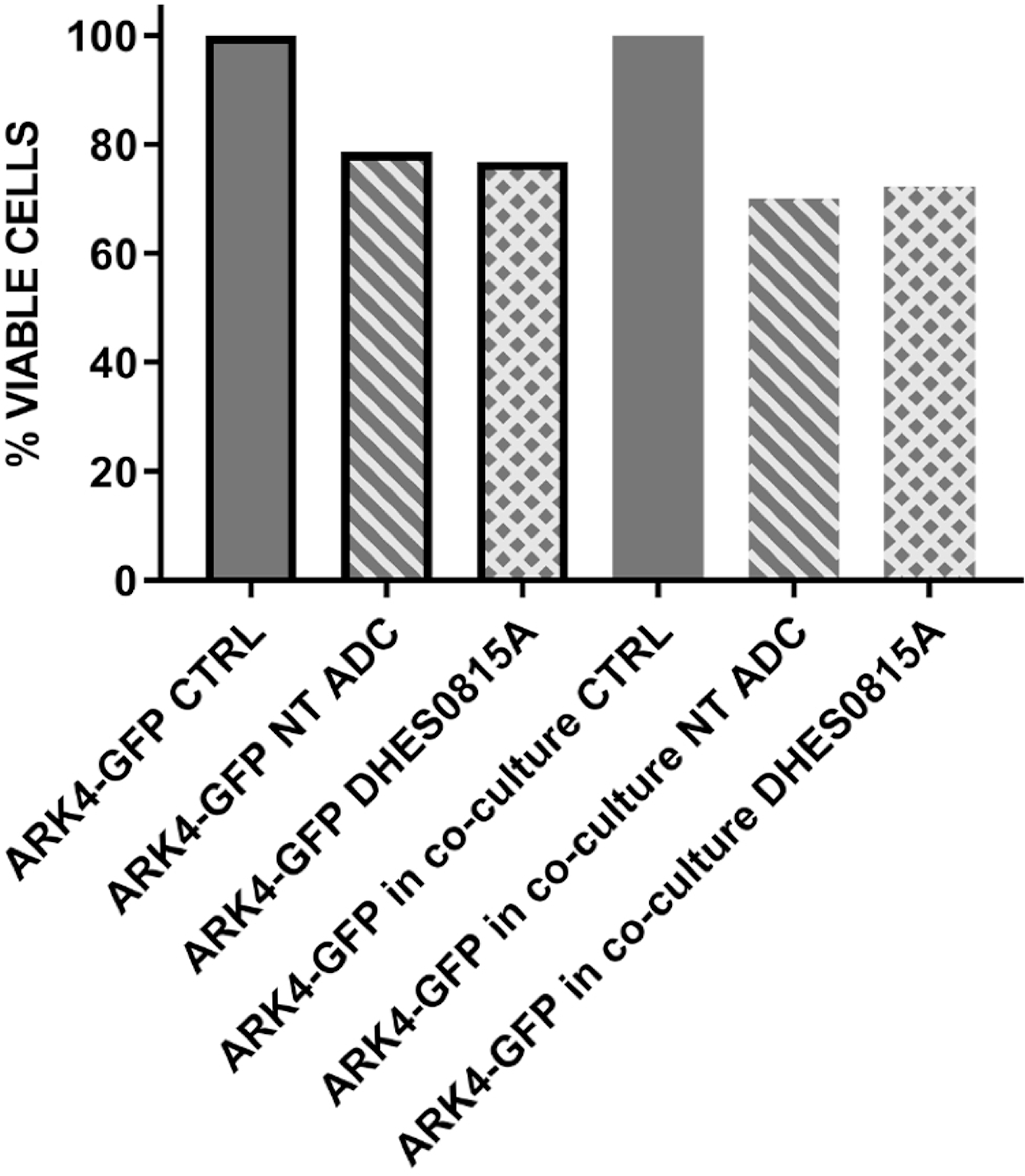
Solid gray bar no-border: Low/negative HER2/neu expressing ARK4 cell (ARK4-GFP cells), co-cultured without any treatment (control, CTRL). Stripes bar with no-border: Low/negative HER2/neu expressing ARK4 cell (ARK4-GFP cells), co-cultured with high HER2/neu expressing cells (ARK2) and treated with control ADC at 1 μg/mL. Squares bar no-border: Low/negative HER2/neu expressing ARK4 cell (ARK4-GFP cells), co-cultured with high HER2/neu expressing cells (ARK2) and treated with DHES0815A at 1 μg/mL concentration.
In vivo antitumor activity
In vivo experiments comparing the antitumor activity of DHES0815A, the non-targeting control ADC, MHES0488A, T-DM1, and saline (control) were conducted in xenograft models with 3+ HER2/neu expression (i.e. ARK-2). Drug dosages were given as a single intravenous injection. The treatment with DHES0815A showed a statistically significant difference in tumor growth inhibition compared to the saline (control) (p=0.0001) and the non-targeting control ADC (p=0.006) (Figure 3A) that began at days 16 and 19 of the treatment respectively. The significant antitumor effects were evidenced throughout the study up to the time the control animals were euthanized due to disease progression (i.e. tumor volume >1.0 cm3). The mice treated with DHES0815A and T-DM1 had no tumor growth while on treatment, whereas mice treated with saline solution, the non-targeted control ADC or the unconjugated control MHES0488A antibody all demonstrated rapid disease progression (Figure 3A). DHES0815A caused growth-inhibition and increased survival in HER2/neu overexpressing xenografts when compared to control (p<0.01). The in vivo activity of DHES0815A in the USC ARK2 xenograft model was similar to the in vivo activity of T-DM1 (Figure 3B). Animals tolerated DHES0815A treatment well with no significant change in their body weight (Figure 3C).
Figure 3A: In vivo efficacy of DHES0815A.
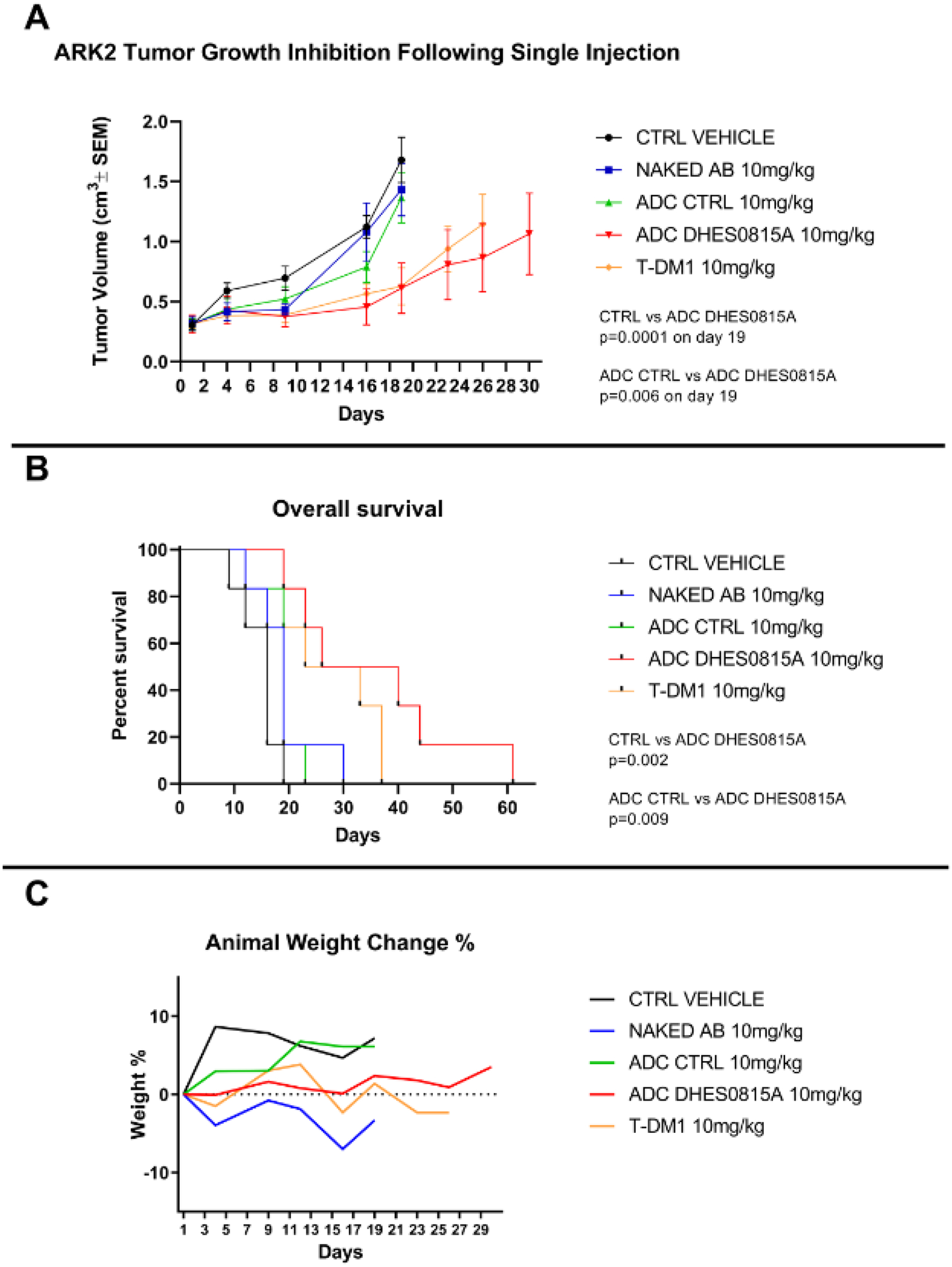
Antitumor activity of DHES0815A was compared to controls including the non-targeted ADC, the unconjugated antibody (MHES0488A), and saline (control) in xenograft models with 3+ HER2/neu expression. (i.e. ARK-2). Mice were treated with a single intravenous injection as described in the methods section. The treatment with DHES0815A, showed a statistically significant difference in tumor growth inhibition compared to the control (p=0.0001) and the non-targeted control ADC (p=0.006) that began at days 16 and 19 of the treatment respectively. Figure 3B: Overall survival. DHES0815A caused growth-inhibition and increased survival in HER2/neu overexpressing xenografts when compared to control (p<0.01). Figure 3C: Animal weight change. Animals tolerated DHES0815A well treatments with no significant change in their body weight.
DISCUSSION
Uterine serous carcinoma is a highly aggressive endometrial malignancy with limited effective therapeutic options and as such the prognosis for patients unfortunately remains dismal. As HER2 overexpression and amplification appears to be a poor prognostic factor and is found in approximately 30% of USC tumors, it represents an attractive molecular target for novel therapeutic agents [11–14]. There are currently four Food and Drug Administration (FDA) approved monoclonal HER2-directed antibody drugs to treat patients with tumors overexpressing HER2 which are Trastuzumab (T), Pertuzumab (P), Trastuzumab emtansine (T-DM1, Kadcyla, Genentech/Roche) and Fam-trastuzumab deruxtecan-nxki, formerly known as DS-8201 [29–36]. The HER2-directed antibody antitumor activity of these drugs may result from 1) the inhibition of dimerization and consequent decreased intracellular signaling via the phosphatidylinositol 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) pathways, 2) antibody-dependent and complement-dependent cytotoxicity (ie, ADCC and CDC) [37,38], and 3) the internalization of the cytotoxic payload linked to the antibody. While T-DM1 permits the selective delivery and internalization of its toxic payload, DM1, a maytansinoid, to tumor cells expressing only HER2/neu while sparing surrounding healthy tissue, other HER2/neu targeting ADC, (ie, SYD985, Synthon Biopharmaceuticals BV, Nijmegen, the Netherlands), which is composed of trastuzumab linked to the toxic payload valine-citrulline-seco DUocarmycin hydroxyBenzamide Azaindole (vc-seco-DUBA), via a cleavable linker, allows significant bystander killing of surrounding tumor cells that do not possess HER2/neu overexpression [29–31,39]. The toxic payload in SYD985 (i.e. DUBA), binds to the minor groove of DNA and subsequently causes irreversible DNA alkylation leading to DNA damage and ultimately cell death in both dividing as well as non-dividing cells [29–31,39]. SYD985 has demonstrated impressive preclinical antitumor activity in USC followed by clinical activity in a Phase II clinical trial (NCT02277717) [29–31,39].
In this present study, we investigated for the first time the in vitro and in vivo activity of DHES0815A, a novel ADC that couples a humanized HER2 monoclonal and PBD-MA via a stable linker, against primary USC cell lines and xenograft models. We found that primary tumor cell lines overexpressing HER2/neu were significantly more sensitive to DHES0815A with an 11-fold to 53-fold increase in cytotoxicity when compared to the non-targeted control ADC (p<0.05). In contrast, USC primary cell lines with low to negligible HER2/neu expression were not sensitive to the ADC. Additionally, a single DHES0815A infusion caused significant growth-inhibition and increased survival in HER2/neu overexpressing xenografts when compared to controls (p<0.0001). Taken together, these results suggest that the in vitro and in vivo cytotoxic effect of DHES0815A is the result of its high selectively to HER2/neu overexpressing tumor cells and mediated by the delivery of its toxic chemotherapy payload after ADC internalization.
One of the major challenges found with the use of targeted therapeutics in the treatment of gynecologic malignancies stems from the heterogeneity in the expression of target antigens. For instance, whole exome sequencing (WES) and confirmatory immunohistochemistry (IHC) and FISH studies demonstrated that over 50% of USC tumors overexpressing HER2/neu also displayed high heterogeneity (i.e. at least a two-degree difference in staining intensity involving tumor cells) [27, 40–41]. One approach to overcome such an issue which may result in resistance of a tumor to targeted treatment is the use of a cleavable linker whereby the toxic payload may be released in tumors both intracellularly as well as in the tumor microenvironment. While this allows the targeted killing of HER2/neu positive tumor cells by intracellular uptake and bystander killing of HER2/neu negative tumor cells by release of the toxic payload in the microenvironment, this strategy may also result in increased toxicity from such a drug given the instability of the linker. In this regard, and not surprisingly, DHES0815A did not induce bystander killing of HER2/neu negative tumors when admixed with HER2/neu positive tumors due to the fact that its toxic payload is linked to a HER2 targeted monoclonal antibody via a stable disulfide-based linker [25].
One of the unique features of DHES0815A is the ability of this ADC to target a distinct epitope on the HER2 receptor when compared to other FDA approved antibody treatments targeting HER2/neu. This feature may allow in future studies the evaluation of potential treatment combination with traditional chemotherapeutic agents or newer agents such as ADCs (i.e. T-DM1 or SYD985) with additive and/or synergistic cytotoxic activity against cells that are HER2/neu positive. Another relevant feature of DHES0815A is that the toxic payload, PBD-MA, is a mono-alkylating agent which makes it less potent that corresponding bis-alkylators to potentially improve tolerability. Nevertheless, DHES0815A, unlike T-DM1, also demonstrates cytotoxic activity against both dividing and non-dividing cells.
In conclusion, our work demonstrated for the first time remarkable preclinical activity of DHES0815A, a novel HER2 targeting ADC, against HER2/neu overexpressing USC primary cell lines and xenografts both in vitro and in vivo. Therefore, DHES0815A alone or in combination with other HER2/neu targeted agents may represent a novel and less toxic treatment option for patients with tumors harboring HER2/neu overexpression refractory to trastuzumab and traditional chemotherapy. Taken together, this preclinical data strongly supports the design of clinical trials of DHES0815A as either a single agent or in combination treatment for patients harboring USC overexpressing HER2/neu.
Highlights.
HER2/neu overexpressing USC cell lines are highly sensitive to DHES0815A in vitro.
DHES0815A targets HER2/neu positive cells without collateral damage to surrounding cells as seen by the bystander effect.
DHES0815A causes growth-inhibition and increased survival in HER2/neu overexpressing xenografts.
Financial support:
This work was supported in part by Gilead Sciences Inc., Foster City, CA, Puma Biotechnology, Inc, Los Angeles, CA, U01 CA176067-01A1 grants from NIH, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to ADS. This investigation was also supported by NIH Research Grant CA-16359 from the NCI and by Stand-up-to cancer (SU2C) convergence grant 2.0 to A.D. Santin.
Conflicts of Interest statement: All authors fulfill the conditions required for authorship. Dr. Santin reports grants from PUMA, grants from IMMUNOMEDICS, grants from GILEAD, grants from SYNTHON, grants from MERCK, grants from BOEHINGER-INGELHEIM, grants from GENENTECH, grants from TESARO, during the conduct of the study. A.M. declares that she receives research-funding support from Spanish Medical Oncology Society. D.A.S reports Intuitive Surgical, Medtronic, Olympus, and Zai Lab consultant/speaker fees.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020 CA Cancer J Clin. 2020. 2020; 70:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34. [DOI] [PubMed] [Google Scholar]
- [3].Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983; 15:10. [DOI] [PubMed] [Google Scholar]
- [4].Felix AS, Weissfeld JL, Stone RA, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 2010; 21:1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Slomovitz BM, Burke TW, Eifel PJ, et al. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol 2003; 91:463. [DOI] [PubMed] [Google Scholar]
- [6].Podratz KC, Mariani A. Uterine papillary serous carcinomas: the exigency for clinical trials. Gynecol Oncol 2003; 91:461. [DOI] [PubMed] [Google Scholar]
- [7].Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006; 95 Suppl 1:S105. [DOI] [PubMed] [Google Scholar]
- [8].Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer 2006; 94:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kosary C Cancer of the Corpus Uterus. Uterus SEER Surviv Monogr Cancer Surviv Adults US SEER Program 1988–2001 1988–2001 NCI SEER Program Natl Cancer Inst Bethesda MD. 2007. [Google Scholar]
- [10].Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501 [DOI] [PubMed] [Google Scholar]
- [11].Fader AN, Roque DM, Siegel E, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(20):2044–2051. doi: 10.1200/JCO.2017.76.5966 [DOI] [PubMed] [Google Scholar]
- [12].Fader AN, Roque DM, Siegel E, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplaitn-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2-/Neu (NCT01367002): Updated Overall Survival Analysis. Clin Cancer Res. 2020. August 1; 26 (15): 3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Erickson BK, Najjar Om, Damast S, et al. Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: A multi-institutional cohort study. Gynecol Oncol. 2020. October; 159(1): 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology-Uterine Neoplasms. Version 4.2019. 2019. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed October 6, 2019.
- [15].Nagayama A, Ellisen LW, Chabner B, Bardia A. Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments. Target. Oncol 2017;12:719–739. doi: 10.1007/s11523-017-0535-0. [DOI] [PubMed] [Google Scholar]
- [16].Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br. J. Cancer. 2017;117:1736–42. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barok M, Tanner M, Koninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171–9. [DOI] [PubMed] [Google Scholar]
- [18].Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–704. [DOI] [PubMed] [Google Scholar]
- [19].Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–63. [DOI] [PubMed] [Google Scholar]
- [20].Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baselga J, Javier Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krop IE, Kim S-B, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:6899. [DOI] [PubMed] [Google Scholar]
- [24].Junutula JR, Raab H, Clark S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 2008;26:925–32. [DOI] [PubMed] [Google Scholar]
- [25].hang D, Pillow TH, Ma Y et al. Linker Immolation Determines Cell Killing Activity of Disulfide-Linked Pyrrolobenzodiazepine Antibody-Drug Conjugates. ACS Med Chem Lett. 2016;7:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, et al. 2010. In vitro actvity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br. J. Cancer 102:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110:2916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buza N HER2 Testing in Endometrial Serous Carcinoma. Arch Pathol Lab Med 2020; doi: 10.5858/arpa.2020-0207-RA [DOI] [PubMed] [Google Scholar]
- [29].van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, et al. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol Cancer Ther. 2015;14:692–703. [DOI] [PubMed] [Google Scholar]
- [30].Dokter W, Ubink R, van der Lee M, van der Vleuten M, van Achterberg T, Jacobs D, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13:2618–29. [DOI] [PubMed] [Google Scholar]
- [31].Black J, Menderes G, Bellone S, Schwab CL, Bonazzoli E, Ferrari F, et al. SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine Serous Carcinoma with HER2/Neu Expression. Mol Cancer Ther. 2016;15:1900–9. [DOI] [PubMed] [Google Scholar]
- [32].Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- [33].Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. [DOI] [PubMed] [Google Scholar]
- [34].Amiri-Kordestani L, Blumenthal GM, Xu QC, Zhang L, Tang SW, Ha L, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014;20:4436–41. [DOI] [PubMed] [Google Scholar]
- [35].FDA hematology/oncology (cancer) approvals and safety notification. FDA website. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer. Published 2019. Accessed January 29, 2020. [Google Scholar]
- [36].Modi S, Saura C, Yamashita T, et al. ; DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer [published online December 11, 2019]. N Engl J Med. doi: 10.1056/NEJMoa1914510. [DOI] [Google Scholar]
- [37].Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sukawa Y, Yamamoto H, Nosho K, Ito M, Igarashi H, Naito T, et al. HER2 expression and PI3K-Akt pathway alterations in gastric cancer. Digestion. 2014;89:12–7. [DOI] [PubMed] [Google Scholar]
- [39].Elgersma RC, Coumans RG, Huijbregts T, Menge WM, Joosten JA, Spijker HJ, et al. Design, Synthesis, and Evaluation of Linker-Duocarmycin Payloads: Toward Selection of HER2-Targeting Antibody-Drug Conjugate SYD985. Mol Pharm. 2015;12:1813–35. [DOI] [PubMed] [Google Scholar]
- [40].Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod Pathol. 2013. December; 26(12): 1605–12. doi: 10.1038/modpathol.2013.113. Epub 2013 Jun 14 [DOI] [PubMed] [Google Scholar]
- [41].Buza N, Hui P. Marked heterogeneity of HER2/NEU gene amplification in endometrial serous carcinoma. Genes Chromosomes Cancer. 2013. December: 52(12): 1178–86. [DOI] [PubMed] [Google Scholar]


