Abstract
Purpose
To present a nomogram to predict overall survival in patients with FIGO-2018 II to III squamous cell cervical carcinoma undergoing radical radiotherapy.
Patients and Methods
Patients diagnosed with FIGO-2018 II to III squamous cell cervical cancer between December 2013 and December 2014 were analyzed retrospectively. The optimal cutoff point for tumor length and width were determined by R package. We identified prognostic factors by univariate and multivariate Cox proportional-hazard regression, then built a nomogram to visualize the prediction model. Our model was compared to the 2018 FIGO staging prediction model. Harrell’s concordance index, receiver operating characteristic curve, calibration plot were used to evaluate the discriminability and accuracy of the predictive models, and decision curve analysis (DCA) was used to show the net benefits.
Results
Data from 469 patients were included in the final analyses. The cutoff values of tumor length and width were 5.10 cm and 4.13 cm, respectively. Four independent prognostic variables—tumor length, tumor width, lower one-third vaginal involvement, and lymph node metastases—were used to establish the nomogram. The C-index of the nomogram was 0.71 (95%, CI = 0.66–0.77), which was better than that of the 2018 FIGO stage prediction model (C-index: 0.62, 95% CI = 0.58–0.66, p = 0.009). The calibration plot of the nomogram was a good fit for both 3-year and 5-year overall survival predictions. And DCA curves showed that net benefits for our model were higher than FIGO-2018 staging system.
Conclusion
A clinically useful nomogram for calculating overall survival probability in FIGO-2018 II to III squamous cell cervical cancer patients who had received radical radiotherapy was developed. Tumor length, tumor width, lower one-third vaginal involvement, and lymph node metastases were found to be independent prognostic factors. Our model performed better than the 2018 FIGO staging model. The findings could help clinicians in China to predict the survival of these patients in clinical care and research.
Keywords: cervical carcinoma, overall survival, radiotherapy, nomogram
Introduction
Cervical carcinoma, killing approximately 300,000 women and affecting nearly 600,000 yearly, particularly middle-aged women and those living in low-resource settings, is one of the most common malignant tumors of the female genital tract worldwide.1 It is estimated that, with the improvements of the human papilloma virus vaccination rate, the incidence will decrease in the next few decades. However, in today’s China, about 131,500 cases of cervical carcinoma are newly diagnosed each year, accounting for approximately one-fourth of the new cases worldwide, and its incidence is on the rise.2 Therefore, cervical carcinoma currently still is a serious issue, endangering the health of the Chinese female population.
The International Federation of Gynecology and Obstetrics’ (FIGO) staging system for cervical carcinoma is the main staging system used in clinical diagnoses and treatment. The 2009 FIGO staging system for cervical carcinoma, which is based on physical examinations, showed some deficiencies in clinical practice. The most significant problem was that lymph node status was not considered in the prognosis. However, many studies demonstrated that lymph node (LN) metastasis significantly affects cervical carcinoma prognosis.3–5 A new staging system, the 2018 FIGO staging system, which incorporates radiographic data, was launched in 2018.6 Multiple studies have shown that the 2018 FIGO staging system can distinguish the real risk factors influencing a patient’s prognosis, whereas the 2009 FIGO system could not.7,8
Nowadays, therapeutic strategies for cervical carcinoma mainly include surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy.9 Although the screening for cervical carcinoma is simple and easily applied, most cervical carcinoma patients in China are already at advanced stages when diagnosed, owing to different economic conditions. Radiotherapy continues to play a significant role in the treatment for locally advanced cervical carcinoma.
The 2018 FIGO staging system has been commonly used for the clinical evaluation of patients with cervical carcinoma.10,11 However, it is also used to assess the whole cervical carcinoma population prior to therapeutic treatment. Several studies established prognostic models for patients who had received radiotherapy, using nomograms.12–14 Annually, the diagnosed new cases of cervical carcinoma in China account for about 25% of total cases worldwide, with the most common pathological type being squamous cell carcinoma; however, there are few prognostic models for Chinese female population of cervical carcinoma.
A nomogram transforms the complex regression equation into a simple and visual graph, which makes the results of the prediction model more readable and of higher use value. This advantage enables nomograms to receive more attention and application in medical research and clinical practice. Some studies have used nomograms to predict the prognosis of patients with cervical cancer. However, some studies were based on the FIGO 2009 staging system.15,16 Others were focused on or enrolled in patients who had received surgery, most of whom were at early stages of cervical cancer.11,17 In the study conducted by Rose et al, they found that prognosis of Asian patients was better than other races.12
Thus, we aimed to establish a nomogram to predict the overall survival (OS) of FIGO-2018 II to III squamous cell cervical carcinoma in Chinese patients receiving radiotherapy in this study. Our purpose was to help clinicians accurately predict prognoses mainly through imaging data in clinical care and research for cervical carcinoma patients in China.
Patients and Methods
Study Population
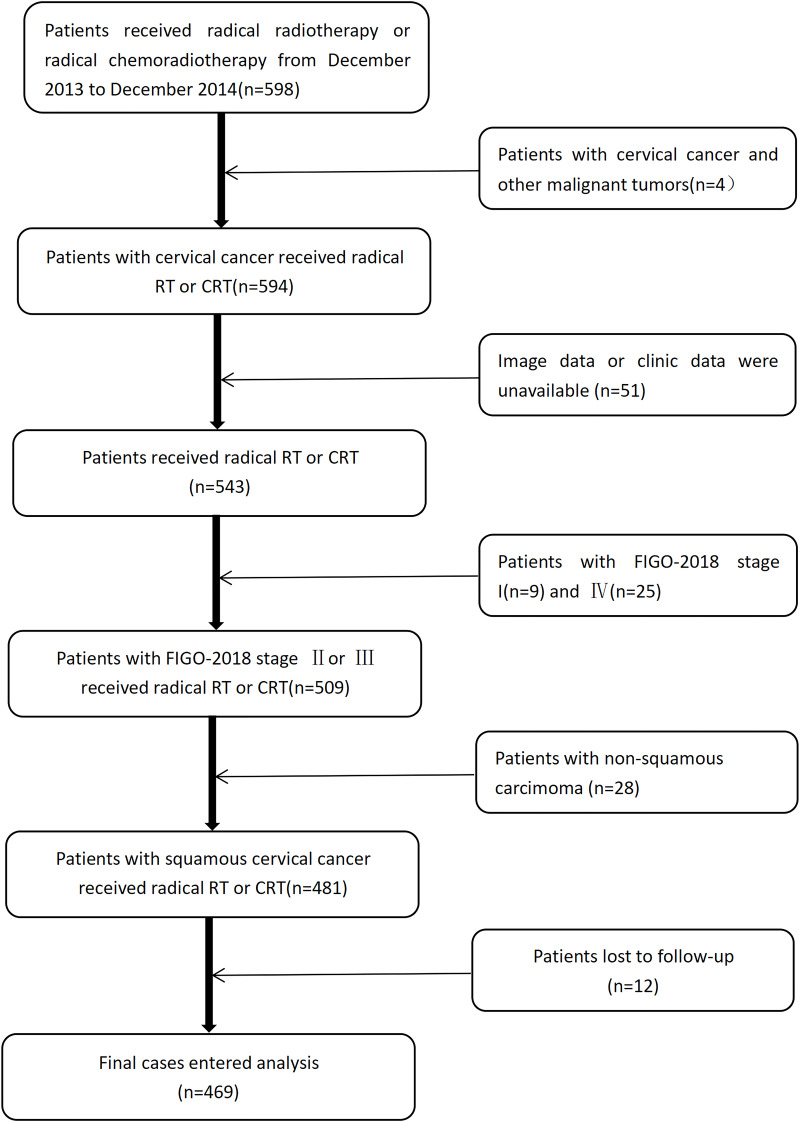
From December 2013 to December 2014, patients with squamous cervical carcinoma who had received radical radiotherapy, were recruited for this study. The 2018 FIGO staging system was used to re-stage all patients. We included patients (1) with histologically proven squamous cervical carcinoma; (2) who were at an advanced stage (2018 FIGO stage II to III); and (3) who had received radical radiotherapy. The exclusion criteria were (1) other malignant tumors; (2) incomplete clinical or imaging data; and (3) patients who could not be followed up. A flow chart showing the eventual study population is shown in Figure 1.
Figure 1.
Flow chart of the patients’ enrollment and exclusion.
Abbreviations: RT, radiotherapy; CRT, chemoradiotherapy; FIGO, International Federation of Gynecology and Obstetrics.
Ethical Considerations
This study was conducted under the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Fujian Cancer Hospital (k2021-087-1), which waved the need for individual informed consent because the patient medical and follow-up were extracted retrospectively. Patients’ records and information were anonymized before analysis. Only members directly involved in this study had access to the information.
Variable Definition and Stratification
Patient age was defined as the age at the time of cervical carcinoma diagnosis. Imaging data, blood routine and serum squamous cell carcinoma (SCC) antigen were performed within 2 weeks, before treatment commenced. Radiological images were evaluable for all the patients. Tumor diameters were measured according to corresponding imaging. For patients who underwent magnetic resonance imaging (MRI) or positron emission tomography/computed tomography (PET/CT) scanning before any treatment, the length and width of tumors were evaluated on axial sections that demonstrated maximum cervical diameter. As CT does not have sufficient soft tissue contrast resolution to allow direct tumor visualization, the cervical size was measured instead of the true tumor size.3 Metastatic lymph nodes were determined if the short diameter was greater than 1.0 cm on the MR or CT. Focal increased fluorodeoxyglucose uptake, which could be detected in pelvic or paraaortic lymph node sites, was considered to indicate malignancy. The lower one-third vaginal involvement (LTI) and parametrial extension were determined via pelvic examinations. All pelvic examinations and analyses of imaging data were performed by two dedicated gynecologic oncologists and two radiologists with more than 10 years’ experience in gynecological oncology.
All of the patients in this study received radical radiotherapy composed of external beam radiation therapy (EBRT) and brachytherapy (BT). EBRT was performed by means of two-dimensional radiotherapy or intensity modulated radiotherapy (IMRT) techniques according to patients’ selection and physicians’ recommendations. The dose of EBRT ranged from 45―52Gy, 1.80―2.0Gy/F. BT was administered after half of EBRT, once a week. All patients received intracavitary treatment 3–7 times, with a dose of A point 6―7Gy/F. 187 patients received vaginal brachytherapy 1―4 times depending on the vaginal invasion before treatment and tumor regressions during treatment, with a dose of reference point 6Gy/F. The reference point was defined as the submucous membrane 5 mm from the mucosal surface. 94 patients received para-aortic radiotherapy, with the dose ranging from 45―61.25Gy, 1.80―2.45Gy/F. Time of radiotherapy was defined as extending from the first day of EBRT to the last BT. In some patients, Glycididazole Sodium was administered intravenously as radiation sensitizer 30 minutes before EBRT at a dose of 800mg/m2. Some patients did not receive chemotherapy owing to contraindications or patients’ refusal. The chemotherapy regimens contained a single platinum agent or paclitaxel, and combination regimens were based on platinum and taxane.
Outcome Definition
The primary outcome for this study is the OS, which was defined as the time from diagnosis to death of any cause.
Statistical Analysis
Considering that all the continuous variables were redefined as categorical variables, all the variables in this study are presented as n (%) and compared using the chi-square test or Fisher’s test. The optimal cut-off point for continuous variables was determined by using the “surv_cutpoint” function of the R package’s “survminer” to establish the cutoff values of tumor length and width. A univariate analysis was used to screen for parameters associated with the prognoses. The factors with P < 0.05 were included in the multivariate Cox regression to identify the independent factors for OS.
A nomogram, which integrated all independent prognostic factors was established, on the basis of the results of the multivariate analysis. The predictive accuracy of the constructed nomogram was evaluated using the concordance index and the area under the time-dependent receiver operating characteristic curve (AUC). The accuracy of the nomogram was verified using a bootstrapped resample with 1000 iterations. Decision curves analysis (DCA) was used to show the net benefits.
Statistical tests were conducted using RStudio (version 1.3.1073), including xlsx, Table 1, survminer, survival, rms, time ROC and ggDCA packages. All statistical tests were two-tailed, and P < 0.05 was considered to be statistically significant.
Table 1.
Patients’ Characteristics
| Characteristics | Value | Characteristics | Value |
|---|---|---|---|
| Age (years) | CT | 58 (12.30%) | |
| ≤60 | 355 (75.69%) | MRI | 397 (84.60%) |
| >60 | 114 (24.31%) | PET/CT | 14 (2.90%) |
| Hemoglobin | Parametrial invasion | ||
| ≥120g/L | 345 (73.56%) | No | 40 (8.53%) |
| <120g/L | 124 (26.44%) | Parametrial extension | 235 (50.11%) |
| White blood cell counts | Pelvic wall involvement | 194 (41.36%) | |
| <10*10E91/L | 371 (79.10%) | FIGO | |
| ≥10*10E91/L | II | 167 (35.61%) | |
| SCC | 98 (20.90%) | III | 302 (64.39%) |
| ≤2ng/mL | 111 (23.67%) | Chemotherapy | |
| >2ng/mL | No | 78 (16.63%) | |
| Tumor length | 358 (76.33%) | Yes | 391 (83.37%) |
| ≤5.1cm | 283 (60.34%) | Radiotherapy | |
| >5.1cm | 186 (39.66%) | Conventional | 270 (57.57%) |
| Tumor width | IMRT | 199 (42.43%) | |
| ≤4.13cm | 362 (77.19%) | Time of Radiotherapy | |
| >4.13cm | 107 (22.81%) | ≤56days | 172 (36.67%) |
| LTI | >56days | 297 (63.33%) | |
| No | 330 (70.36%) | Glycididazole Sodium | |
| Yes | 139 (29.64%) | No | 411 (87.63%) |
| LN metastasis | Yes | 58 (12.37%) | |
| No | 277 (59.06%) | Thermotherapy | |
| Yes | 192 (40.94%) | No | 346 (73.77%) |
| Imaging | Yes | 123 (26.23%) |
Results
Patients’ Characteristics
A total of 469 patients were included in the final analysis. Patients’ clinical data are shown in Table 1. In this cohort, 114 (24.31%) patients were older than 60 years of age. A total of 139 cases (29.64%) had lower one-third vaginal involvement.
Forty (8.53%) patients had no parametrial invasion; however, the tumor extended from the cervix to the parametrium for 235 (50.11%) patients, and to the pelvic walls for 194 (41.36%). Furthermore, 167 patients (35.61%) were in stage II. A total of 78 (16.63%) patients had not received chemotherapy due to intolerance or treatment refusal. Additionally, 270 patients (57.57%) had received conventional radiotherapy, and 58 patients (12.37%) had received glycididazole sodium as a radiosensitizer three times a week. Lastly, 123 patients (26.23%) had received thermotherapy twice a week along with radiotherapy. The estimated survival rates in the patient cohort at years 3 and 5, after diagnosis, were 0.92 and 0.86, respectively.
Determination of the Cutoff Values of Tumor Length and Width
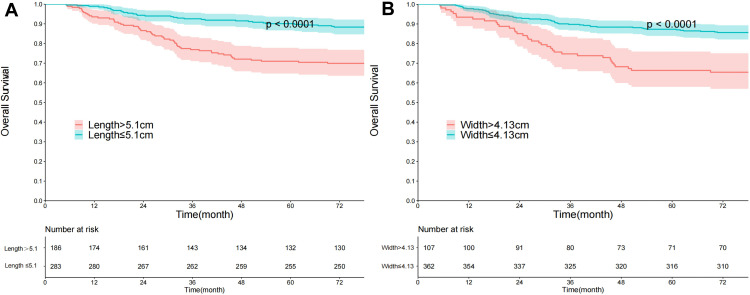
The cutoff values of tumor length and width were 5.10 cm and 4.13 cm, respectively (Figure 2). The tumor length of 283 patients (60.34%) was no longer than 5.10 cm, the hazard ratio (HR) was 2.88, the 95% confidence interval (CI) was 1.88 to 4.40, and the p-value was < 0.0001. The tumor width of 362 patients (77.19%) was not wider than 4.13 cm, the HR was 2.76, 95% CI ranged from 1.82 to 4.19, and the p-value was < 0.0001.
Figure 2.
The cut-off values of tumor length and width. (A) Tumor length; (B) tumor width.
Univariate and Multivariate Analysis of Factors for OS
Since the accuracy of pelvic examination is highly dependent on the physician experience,18 we did not include parametrial involvement in the analyses. Univariate analyses showed that hemoglobin (p = 0.037), white blood cell counts (p = 0.010), tumor length (p < 0.001), tumor width (p < 0.001), LTI (p = 0.005), and LN metastasis (p < 0.001), were associated with OS. The following factors were found to be independently associated with a significantly increased risk of shorter OS: tumor length > 5.1 cm (HR = 1.77, 95% CI = 1.06–2.94, p = 0.028), tumor width > 4.13 cm (HR = 1.68, 95% CI = 1.03–2.76, p = 0.039), LTI (HR =1.59, 95% CI = 1.05–2.43, p = 0.03), and LN metastasis (HR = 1.96, 95% CI = 1.27–3.02, p = 0.002). The results are presented in Table 2.
Table 2.
Results of Univariable and Multivariate Analysis
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| Age | 1.11 (0.70–1.77) | 0.664 | – | – |
| Hemoglobin | 1.58 (1.03–2.43) | 0.037 | 1.04 (0.66–1.64) | 0.850 |
| White blood cell counts | 1.80 (1.15–2.81) | 0.010 | 1.17 (0.73–1.87) | 0.508 |
| SCC | 1.61 (0.93–2.81) | 0.091 | – | – |
| Tumor Length | 2.88 (1.88–4.40) | <0.001 | 1.77 (1.06–2.94) | 0.028 |
| Tumor Width | 2.76 (1.82–4.19) | <0.001 | 1.68 (1.03–2.76) | 0.039 |
| LTI | 1.80 (1.19–2.74) | 0.005 | 1.59 (1.05–2.43) | 0.030 |
| LN metastasis | 2.43 (1.59–3.69) | <0.001 | 1.96 (1.27–3.02) | 0.002 |
| Chemotherapy | 0.64 (0.40–1.05) | 0.077 | – | – |
| Radiotherapy | 1.17 (0.78–1.77) | 0.451 | – | – |
| Time of Radiotherapy | 1.43 (0.91–2.25) | 0.118 | – | – |
| Glycididazole Sodium | 1.12 (0.61–2.05) | 0.722 | – | – |
| Thermotherapy | 1.45 (0.94–2.25) | 0.093 | – | – |
Abbreviations: SCC, squamous cell carcinoma antigen; LTI, lower one third vaginal involvement; LN, lymph node; IMRT, intensity modulated radiotherapy.
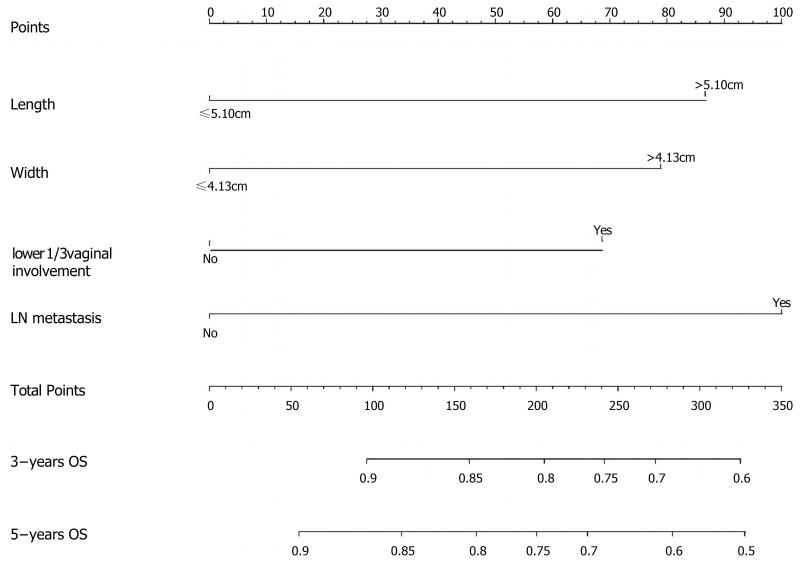
Establishment of Nomogram
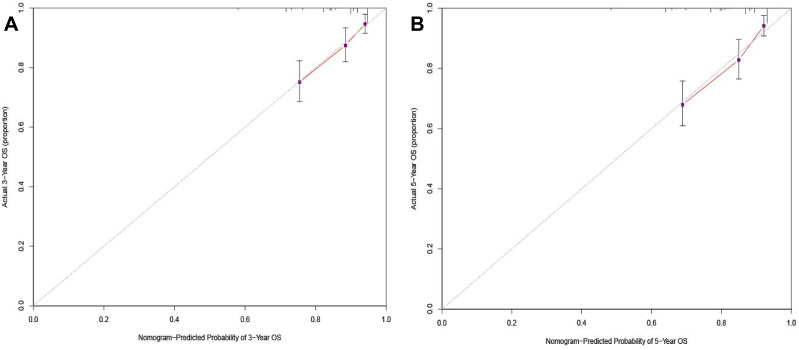
A nomogram was established to predict the 3- and 5-year OS probabilities, based on the multivariate analysis, as shown in Figure 3. The calibration plots indicate a strong consistency between the observed and the nomogram-predicted probabilities (Figure 4).
Figure 3.
Nomogram for predicting the overall survival probability in cervical cancer patients whose FIGO-2018 stage were II and III.
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Figure 4.
Internal calibration of nomogram of 3-year and 5-year survival. (A) Internal calibration of nomogram of 3-year overall survival; (B) internal calibration of nomogram of 5-year overall survival.
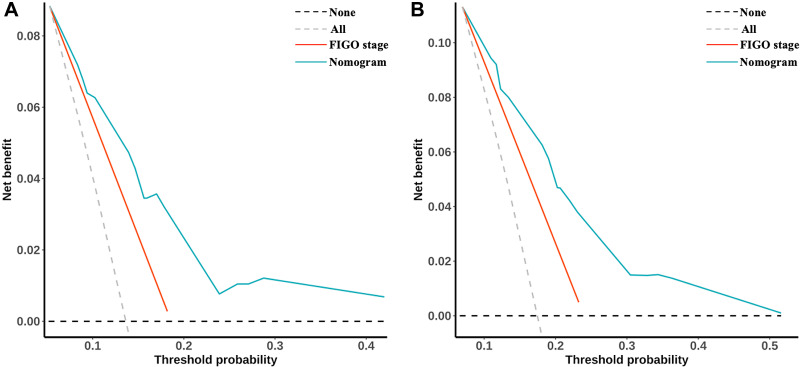
The C-index of the current nomogram was as high as 0.71 (0.66–0.77), which was significantly higher than that of the FIGO-staging system (C-index = 0.62, 95% CI = 0.58–0.66, p = 0.009). Similar advantages of the current model were also observed in terms of AUC at the 3- and 5-year marks (Table 3). DCA curves showed that net benefits for our nomogram were higher than for the FIGO-2018 staging system both for 3- and 5-year OS (Figure 5).
Table 3.
Comparisons of the Time-Dependent AUC and C-Index Between the Nomogram and FIGO-2018
| Variables | FIGO-2018 | Nomogram | P value |
|---|---|---|---|
| C-index (95% CI) | 0.62 (0.58–0.66) | 0.71 (0.66–0.77) | 0.009 |
| 3-year AUC (95% CI) | 0.63 (0.59–0.68) | 0.72 (0.66–0.79) | 0.039 |
| 5-year AUC (95% CI) | 0.64 (0.60–0.69) | 0.73 (0.67–0.79) | 0.020 |
Abbreviations: AUC, area under the curve; CI, confidence interval; FIGO, Federation of Gynecology and Obstetrics.
Figure 5.
Decision curve analysis (DCA) for 3- and 5-year OS. (A) DCA curve of nomogram and FIGO-2018 staging system of 3-year overall survival; (B) DCA curve of nomogram and FIGO-2018 staging system of 5-year overall survival.
Discussion
In this study, we established a predictive model for FIGO-2018 II to III squamous cervical carcinoma patients undergoing radical radiotherapy. This nomogram is an OS probability prediction tool, comprising four pretreatment prognostic factors selected through multivariate analysis. These factors, including tumor length, tumor width, lower one-third vaginal involvement and lymph node metastasis, can be accurately obtained through imaging data and are common clinical factors that may be useful for some patients.
The multivariate analysis in our study identified tumor length and width as independent factors of patient prognosis and indicates that tumor size influences the outcome. This is consistent with the results of previous studies (Table 4).3,17,19 Tumor size is an independent prognostic factor of locally advanced cervical carcinoma. A possible explication for this result may be as follows: When the diameter of the tumor is less than 1 mm, tumor cells can exchange oxygen, nutrients, and metabolic substances by simple diffusion, so they are considered to be under adequate oxygenation. However, tumor hypoxia is a common phenomenon for a tumor diameter of more than 1 mm.20 Larger tumors are usually associated with tumor hypoxia.21 Tumor hypoxia is a well-known and important determinant of the response to anti-tumor treatments, particularly radiotherapy. In our study, we employed both tumor length and tumor width as study variables, and the results show that both are independent prognostic factors for survival. As the tumor shape is irregular, two directions of tumor diameter would result in a better estimate of tumor size than a single diameter. Recent studies often evaluated the measurements of tumor size through physical examination or simply did not mention them; our study used more accurate imaging methods, and reduced the bias caused by clinical experience.
Table 4.
References Talking About the Prognostic Factors
| References | Published Year | Population | Year of Case Collection | Case Number | Stage Systm | Stage | Treatment | Prognostic Factor Discussed | Measurement Methods |
|---|---|---|---|---|---|---|---|---|---|
| Toita et al.3 | 1995 | Japan | 1985–1991 | 70 | FIGO | IIB-IIIB | RT | Cervical diameter > 6 cm | CT |
| Polterauer et al.17 | 2012 | Austria | 1996–2009 | 528 | FIGO | IA1-IVB | S, RT, CT | Tumor size>2cm | Not mentioned |
| Wang et al19 | 2015 | China | 2005–2008 | 284 | FIGO | IA-IIIB | S, RT | Tumor size>4cm | Not mentioned |
| Kobierski, J. et al.4 | 2002 | Poland | NA | 499 | FIGO | I-IIA | S | Lymph node metastasis | Surgical pathology |
| Bae, H.S. et al.22 | 2016 | Korea | 2001–2010 | 397 | FIGO | IB2-IVA | CRT | Lymph node metastasis | MRI |
| Kim et al.23 | 2020 | Korea | 2000–2014 | 897 | FIGO | IB-IIA | S+adjuvant CRT | Lymph node metastasis | Surgical pathology |
| Chen et al.24 | 2020 | America | 2010–2015 | 19,377 | TNM | NA | S | Lymph node metastasis | Surgical pathology |
| China | 2008–2018 | 14 | FIGO | IB1-IIA2 | S | ||||
| Kilic et al.25 | 2021 | Turkey | 1993–2019 | 197 | FIGO 2009 | IB1–IIIB | S, RT, CCRT | Lymph node metastasis | Surgical pathology/imaging |
| Grigsby et al.26 | 2020 | America | 1997–2019 | 1282 | FIGO 2009 | I-IV | NA | Stage | PET |
| Brodeur et al.8 | 2021 | Canada | 2010–2018 | 216 | FIGO 2009 | IA1-IVA | RT, CRT | Stage | MRI and CT/PET. |
| Gurram et al.27 | 2020 | India | 2010–2016 | 118 | FIGO 2009 | IIIA, IIIB | RT, CRT | LTI | NA |
| Katanyoo et al.28 | 2017 | Thailand | 1995–2012 | 216 | FIGO | IIIB | RT, CCRT | LTI | Pelvic examination |
| Fang et al.29 | 2021 | China | 2007–2014 | 622 | FIGO 2009 | IIIB | RT, CRT | LTI | NA |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; S, surgery; RT, radiotherapy; CT, chemotherapy; CT, computerized tomography; CRT, chemoradiation; MRI, magnetic resonance imaging; CCRT, concurrent chemoradiotherapy; NA, not available; PET, positron emission tomography; LTI, lower third of vaginal invasion.
We also found that lymphatic metastasis was a negative prognostic factor for squamous cell cervical carcinoma patients who had received radical radiotherapy. Several studies have shown that lymph node metastasis is an independent prognostic factor for overall survival time,4,22 and is associated with distant metastases in early-stage cervical cancer,23,24 especially the presence of paraaortic lymph node metastasis was significantly associated with distant recurrence.25 There have been many similar studies, and this has been the main reason why FIGO incorporates lymph node metastatic conditions into the staging system it published in 2018. Grigsby et al analyzed a database cohort of 1282 patients newly diagnosed with cervical carcinoma from 1997 to 2019, and found that FIGO 2018 improves survival discriminatory ability for stages I and IV patients.26 Brodeur et al performed a retrospective cohort study on 216 adult cervical carcinoma patients treated with definitive chemoradiotherapy between 2010 and 2018, and found that the 2018 FIGO staging reflects patient prognosis more accurately.8
To our knowledge, few studies have investigated the prognostic significance of LTI. Gurram et al confirmed that FIGO IIIA patients do better than IIIB patients with lower vaginal involvement.27 Katanyoo et al and Fang et al found that stage IIIB cervical carcinoma patients with LTI had poorer survival outcomes than those without LTI.28,29 Our study showed that LTI is associated with poor prognosis in patients with locally advanced squamous cell cervical carcinoma who had received radiotherapy. The details of the references regarding the prognostic factors are summarized in Table 4.
In this study, we established a nomogram to predict the overall survival of patients with squamous cell cervical carcinoma in FIGO-2018 stages II-III under radical radiotherapy. The C-index of our nomogram is significantly higher than that of the FIGO-2018 stage, which means its’ predictive capacity is better at predicting patients’ 3-year and 5-year survival. The four factors used to establish our nomogram were mainly extracted from imaging data and clinical factors that were not difficult to obtain. Some studies have shown that in operable cervical cancer patients, the accuracy of pelvic examination in evaluating parametrial invasion is about 50%-80%30–32 and MRI’s accuracy is higher than that of clinical evaluation.33 However, in patients with advanced stage cancer, it is not feasible to compare pelvic examination with surgical pathology because surgery is not applicable for them. Moreover, MRI is contraindicated for patients with metallic objects, such as intrauterine device and pacemakers. Instead, we included imaging factors and clinical factors to establish predictive models; then our nomogram showed that it can predict OS well.
Several studies about nomograms have predicted the OS of cervical cancer patients undergoing radiotherapy. Sturdza et al recently published their results which showed that clinical target volume at high risk at first BT significantly affects the prognoses of locally advanced cervical cancer patients treated by radio-chemotherapy including image guided brachytherapy.15 The staging system used in their study was FIGO 2009. The ethnic composition of the study’s population was not mentioned. They finally built a nomogram based on factors about modern image guided brachytherapy. It is not feasible to evaluate it in our study, because all patients received conventional BT. And we mainly focused on evaluating the value of imaging data before treatment, which is a more objective assessment measure. Two studies that enrolled more than 2000 patients, based on the Surveillance, Epidemiology, and End Results (SEER) database, showed that there were factors outside the FIGO-2009 stage influencing the survival of cervical cancer patients.14,16 We restaged all the patients through the FIGO-2018 staging system and established a predictive model with an imaging aspect. Our model can provide much more customized survival predictions to Chinese patients with FIGO-2018 II to III squamous cell cervical carcinoma under radical radiotherapy than those based on coarse groupings of large numbers of heterogeneous patients.
Our study had some limitations. First, it was conducted retrospectively and was inherently subjective to the specific biases of a retrospective study. Although the cases were collected in 13 months, the diagnosis and treatment strategies were similar. Second, this was a single-center retrospective study; hence, the inclusion of some factors may be overestimated and the conclusion needs to be further explored by incorporating multiple centers. Third, these findings are preliminary results and the nomogram needs to be further validated.
Conclusion
In this study, we developed a nomogram for FIGO-2018 II to III squamous cell cervical carcinoma in patients who had received radical radiotherapy in China, based on clinical and imaging information. Tumor length, tumor width, LTI, and LN metastasis were found to be independent prognostic factors. These factors are common clinical factors and can be extracted accurately from imaging data. The nomogram performed better than the FIGO stage prediction model and could help clinicians precisely predict prognosis in clinical care and research in China.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ping L. Evaluation of big data of clinical epidemiology of cervical cancer in 13 years in Chinese mainland (in Chinese).Chinese JPractical Gynecology and Obstetrics.. 2018;34(01):41–45. [Google Scholar]
- 3.Toita T, Nakano M, Higashi M, Sakumoto K, Kanazawa K. Prognostic value of cervical size and pelvic lymph node status assessed by computed tomography for pa tients with uterine cervical cancer treated by radical radiation therapy. Int J Radiat Oncol Biol Phys. 1995;33(4):843–849. doi: 10.1016/0360-3016(95)00204-5 [DOI] [PubMed] [Google Scholar]
- 4.Kobierski J, Emerich J, Kr¨®likowska B, Majdak E. [Lymph node metastasis as a prognostic factor in cervical carcinoma]. Ginekol Pol. 2002;73(11):925–929. Polish [PubMed] [Google Scholar]
- 5.Yan X, Li G, Shang H, Lin F, Yang X, Zheng F. Outcome and prognostic factors of laparoscopic radical hysterectomy and pelvic lymphadenectomy in 148 patients with stage IB1 cervical cancer. Int J Gynecol Cancer. 2012;22(2):286–290. doi: 10.1097/IGC.0b013e318233d549 [DOI] [PubMed] [Google Scholar]
- 6.Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. doi: 10.1002/ijgo.12611 [DOI] [PubMed] [Google Scholar]
- 7.Tomizawa K, Kaminuma T, Murata K, et al. FIGO 2018 staging for cervical cancer: influence on stage distribution and outcomes in the 3D-image-G uided brachytherapy era. Cancers. 2020;12(7):1770. doi: 10.3390/cancers12071770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodeur MN, Dejean R, Beauchemin MC, et al. Oncologic outcomes in the era of modern radiation therapy using FIGO 2018 staging system for cervical cancer. Gynecol Oncol. 2021;162(2):277–283. doi: 10.1016/j.ygyno.2021.05.023 [DOI] [PubMed] [Google Scholar]
- 9.Lan C, Shen J, Wang Y, et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): a multicenter, open-label, single-arm, phase II trial. J Clin Oncol. 2020;38(34):4095–4106. doi: 10.1200/JCO.20.01920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152(1):87–93. doi: 10.1016/j.ygyno.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang X, Guo C, Liu S, Guo J, Hua K, Qiu J. A novel prognostic nomogram utilizing the 2018 FIGO staging system for cervical cancer: a large multi center study. Int J Gynaecol Obstet. 2021;155(1):86–94. doi: 10.1002/ijgo.13644 [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, Java J, Whitney CW, et al. Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG oncology/Gynecologic oncology group randomized trials of chemoradiotherapy. J Clin Oncol. 2015;33(19):2136–2142. doi: 10.1200/JCO.2014.57.7122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti C, De Felice F, Di Pinto A, et al. Survival nomograms after curative neoadjuvant chemotherapy and radical surgery for stage IB2-IIIB cervical cancer. Cancer Res Treat. 2018;50(3):768–776. doi: 10.4143/crt.2017.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Tian G, Pan Z, et al. Nomograms for predicting the survival rate for cervical cancer patients who undergo radiation therapy: a SEER analysis. Fut Oncol. 2019;15(26):3033–3045. doi: 10.2217/fon-2019-0029 [DOI] [PubMed] [Google Scholar]
- 15.Sturdza AE, P?tter R, Kossmeier M, et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with r adiochemotherapy including image-guided brachytherapy: a retro-EMBRACE study. Int J Radiat Oncol Biol Phys. 2021;111(1):168–177. doi: 10.1016/j.ijrobp.2021.04.022 [DOI] [PubMed] [Google Scholar]
- 16.Xie G, Wang R, Shang L, et al. Calculating the overall survival probability in patients with cervical cancer: a nomogram and decisio n curve analysis-based study. BMC Cancer. 2020;20(1):833. doi: 10.1186/s12885-020-07349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polterauer S, Grimm C, Hofstetter G, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer. 2012;107(6):918–924. doi: 10.1038/bjc.2012.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padilla LA, Radosevich DM, Milad MP. Limitations of the pelvic examination for evaluation of the female pelvic organs. Int J Gynaecol Obstet. 2005;88(1):84–88. doi: 10.1016/j.ijgo.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wang T, Yang YY, Chai YL, Shi F, Liu ZI. Patient age, tumor appearance and tumor size are risk factors for early recurrence of cervical cancer. Mol Clin Oncol. 2015;3(2):363–366. doi: 10.3892/mco.2014.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley JA, Shipley WU, Steel GG. Influence of tumour size on hypoxic fraction and therapeutic sensitivity of Lewis lung tumour. Br J Cancer. 1977;36(1):105–113. doi: 10.1038/bjc.1977.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Klem J, Wyrick JB, et al. Detection of hypoxia in human brain tumor xenografts using a modified comet assay. Neoplasia. 2003;5(4):288–296. doi: 10.1016/S1476-5586(03)80022-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae HS, Kim YJ, Lim MC, et al. Predictors of radiation field failure after definitive chemoradiation in patients with locally advanc ed cervical cancer. Int J Gynecol Cancer. 2016;26(4):737–742. doi: 10.1097/IGC.0000000000000662 [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Cho WK, Kim YJ, Kim YS, Park W. Significance of the number of high-risk factors in patients with cervical cancer treated with radical hysterectomy and concurrent chemoradiotherapy. Gynecol Oncol. 2020;157(2):423–428. doi: 10.1016/j.ygyno.2020.02.031 [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Chen L, Zhu H, Tao J. Risk factors and prognostic predictors for cervical cancer patients with lung metastasis. J Cancer. 2020;11(20):5880–5889. doi: 10.7150/jca.46258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilic C, Kimyon Comert G, Cakir C, et al. Recurrence pattern and prognostic factors for survival in cervical cancer with lymph node metastasis. J Obstet Gynaecol Res. 2021;47(6):2175–2184. doi: 10.1111/jog.14762 [DOI] [PubMed] [Google Scholar]
- 26.Grigsby PW, Massad LS, Mutch DG, et al. FIGO 2018 staging criteria for cervical cancer: impact on stage migration and survival. Gynecol Oncol. 2020;157(3):639–643. doi: 10.1016/j.ygyno.2020.03.027 [DOI] [PubMed] [Google Scholar]
- 27.Gurram L, Patil R, Chopra S, et al. Evaluation of outcomes in patients of cervical cancer with lower one third vaginal involvement: a single institutional experience. Gynecol Oncol. 2020;159(2):359–364. doi: 10.1016/j.ygyno.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 28.Katanyoo K. Comparing treatment outcomes of stage IIIB cervical cancer patients between those with and without lo wer third of vaginal invasion. J Gynecol Oncol. 2017;28(6):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang C, Zhang P, Yu A, Yang Y, Zhang J. Different prognosis of stage IIIB cervical cancer patients with lower third of vaginal invasion and t hose without. Gynecol Oncol. 2021;162(1):50–55. doi: 10.1016/j.ygyno.2021.04.005 [DOI] [PubMed] [Google Scholar]
- 30.Innocenti P, Pulli F, Savino L, et al. Staging of cervical cancer: reliability of transrectal US. Radiology. 1992;185(1):201–205. doi: 10.1148/radiology.185.1.1523308 [DOI] [PubMed] [Google Scholar]
- 31.Yang WT, Walkden SB, Ho S, et al. Transrectal ultrasound in the evaluation of cervical carcinoma and comparison with spiral computed to mography and magnetic resonance imaging. Br J Radiol. 1996;69(823):610–616. [DOI] [PubMed] [Google Scholar]
- 32.Qin Y, Peng Z, Lou J, Liu H, Deng F, Zheng Y. Discrepancies between clinical staging and pathological findings of operable cervical carcinoma with stage IB-IIB: a retrospective analysis of 818 patients. Aust N Z J Obstet Gynaecol. 2009;49(5):542–544. doi: 10.1111/j.1479-828X.2009.01065.x [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Choi BI, Lee HP, et al. Uterine cervical carcinoma: comparison of CT and MR findings. Radiology. 1990;175(1):45–51. doi: 10.1148/radiology.175.1.2315503 [DOI] [PubMed] [Google Scholar]