Abstract
The portfolio of SARS-CoV-2 small molecule drugs is currently limited to a handful that are either approved (remdesivir), emergency approved (dexamethasone, baricitinib) or in advanced clinical trials. We have tested 45 FDA-approved kinase inhibitors in vitro against murine hepatitis virus (MHV) as a model of SARS-CoV-2 replication and identified 12 showing inhibition in the delayed brain tumor (DBT) cell line. Vandetanib, which targets the vascular endothelial growth factor receptor (VEGFR), the epidermal growth factor receptor (EGFR), and the RET-tyrosine kinase showed the most promising results on inhibition versus toxic effect on SARS-CoV-2-infected Caco-2 and A549-hACE2 cells (IC50 0.79 μM) while also showing a reduction of > 3 log TCID50/mL for HCoV-229E. The in vivo efficacy of vandetanib was assessed in a mouse model of SARS-CoV-2 infection and statistically significantly reduced the levels of IL-6, IL-10, TNF-α, and mitigated inflammatory cell infiltrates in the lungs of infected animals but did not reduce viral load.
Vandetanib rescued the decreased IFN-1β caused by SARS-CoV-2 infection in mice to levels similar to that in uninfected animals. Our results indicate that the FDA-approved vandetanib is a potential therapeutic candidate for COVID-19 positioned for follow up in clinical trials either alone or in combination with other drugs to address the cytokine storm associated with this viral infection.
Keywords: Antiviral, SARS-CoV-2, Spike protein, entrectinib, vandetanib
Introduction
Currently three vaccines are approved for SARS-CoV-2 in the USA1, 2, 3. In contrast, there are relatively few small-molecule drugs that are approved for use including remdesivir4, while molnupiravir is approved in the UK. An emergency use authorization allows the protein kinase inhibitor baricitinib to be combined with remdesivir in hospitalized adults and children 2 years and older who require respiratory support5. The NIH COVID-19 treatment guidelines recommend the use of dexamethasone in certain patients hospitalized with severe COVID-19 based on results from the RECOVERY trial6. As these limited treatment options and several drugs in clinical trials7 attest, there is a global need for more therapeutic options for COVID-19, such as small molecule antivirals that can be used outside of hospitals and treatments to address the many symptoms of COVID-19 that are termed long-COVID8, 9.
Drug repurposing is an essential strategy used to accelerate the discovery of a small molecule treatments for COVID-19 that can enable expedited clinical progression10, 11. Repurposing efforts have already identified molecules (such as remdesivir) originally developed for other viruses and approved outside the USA with potent in vitro activity against SARS-CoV-212. Small to medium scale assays and high-throughput screening (HTS) campaigns13 have been performed for testing FDA-approved drugs. The catalytic activity of the viral targets Mpro and PLpro are essential for viral replication, making inhibition of these enzymes a compelling strategy for antiviral therapy14. The discovery of PF-008352313 as a covalent active-site-directed inhibitor of SARS-CoV Mpro in 2003 allowed the translation of this agent into clinical trials for SARS-CoV-2 in 202015. Pfizer also developed the SARS-CoV-2 inhibitor PF-07321332 targeting Mpro, in combination with ritonavir (PAXLOVID™), and an interim analysis of the Phase 2/3 EPIC-HR study showed it reduced risk of death or hospitalization by 89 %. All of these direct acting antivirals target early-stage virus replication and have a short therapeutic window which would render them less effective if administered during the immunopathogenic phase of the disease.
When SARS-CoV-2 invades the body, it can cause an imbalance in the immune system that may result in a cytokine storm16. COVID-19 patients deteriorate over a short period, leading to acute respiratory distress syndrome (ARDS), coagulation disorders, and eventually multiple organ failure16, 17. COVID-19 displays an “inflammatory signature”, characterized by increased levels of soluble biomarkers (cytokine and chemokines) which are involved in the recruitment and activation of several immune cell types, like monocyte/macrophages, neutrophils, T-lymphocytes, and many others18. These immune-active biomarkers, measured either early upon patient admission or throughout hospitalization, may provide clinically relevant information in predicting a more or less severe course of the disease as well as in estimating the mortality risk of infected patients19. Targeting the cytokine storm to ameliorate the state of hyperinflammation has been proposed as a novel therapeutic approach in the treatment of COVID-1918, 20, 21, 22. It was previously demonstrated for many viruses that the modulation of host cell signaling is crucial for viral replication and it may exhibit strong therapeutic potential23, 24. To date, the FDA has approved over 60 small molecule protein kinase inhibitors and most of these are used in the treatment of cancers (e.g. leukemias, breast and lung cancers) whereas several are for non-malignancies25. Subsequently, protein kinase inhibitors have been proposed for treating SARS-CoV-2 and have demonstrated in vitro26, 27, 28, 29, 30, 31 and in vivo activity while several are also in clinical trials32, 33. In the current study, we have performed in vitro screening of 45 approved protein kinase inhibitors in delayed brain tumor (DBT) cells infected with murine hepatitis virus (MHV), followed by evaluation of entrectinib and vandetanib in A549-ACE2 cells infected by SARS-CoV-2. Finally, we have evaluated the in vivo efficacy of vandetanib in an acute infection model using K-18-hACE2 mice challenged with SARS-CoV-2.
Results
Vandetanib inhibits SARS-CoV-2 replication in vitro without cell toxicity
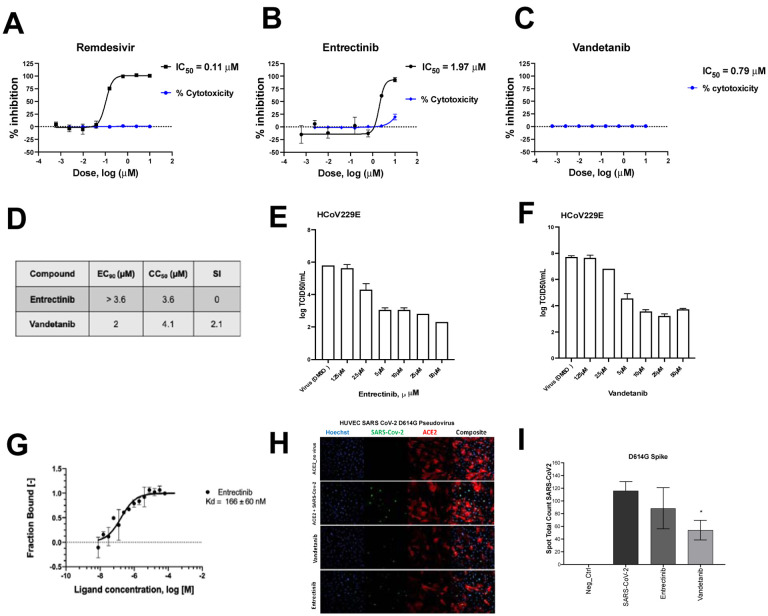
We used DBT cells infected with murine hepatitis virus (MHV), a model of SARS-CoV-2 replication to evaluate the antiviral activity of 45 kinase inhibitors (Table S1). Among them, 12 showed close to 100% inhibition, and 11 showed moderate activity >60% and <83% at 10 μM (Table S1). Two of the most active hits, entrectinib and vandetanib were also characterized in A549-ACE2 cells infected with SARS-CoV-2 using remdesivir as a positive control. Cells were pretreated with compounds 1 h prior to SARS-CoV-2 infection. Both entrectinib (IC50 1.97 μM) and vandetanib (IC50 0.79 μM) showed lower potency than remdesivir (IC50 of 0.11 μM) (Figure 1, A–C). Entrectinib was further tested in Caco-2 and in Calu-3 cell lines infected with SARS-CoV-2, however, this compound was toxic at the concentrations tested (Figure 1D, S1). Entrectinib (5 μM) was tested in Huh-7 cells infected with the human coronavirus 229E (HCoV-229E)34, 35, 36 demonstrating a decrease of 2.7 logTCID50/ml (Figure 1E). In contrast, vandetanib was active in Caco-2 cells (IC90 2 μM, Figure 1D) and showed a reduction of > 3 logTCID50/mL with HCoV-229E when tested at 5 μM (Figure 1 F). Further characterization showed that entrectinib binds to the SARS-CoV-2 Spike RBD with a KD of 166 ± 60 nM (Figure 1G) using microscale thermophoresis (MST)37, 38, an approximately 10 times weaker affinity when compared to ACE2 binding, which has a KD of 15 nM39, 40, which might not be able to protect against virus entry infection and the mechanism might be through inhibition of kinases, since it was reported that growth factor receptor signaling pathways have been reported to be highly activated upon SARS-CoV-2 infection41. SARS-CoV-2 Spike protein-mediated entry was measured in VSV-pseudotype SARS-CoV-2 assays demonstrating that vandetanib was active at 1 μM in the SARS-CoV-2 D614G strain, whereas entrectinib had no significant activity (Figure 1 H, I).
Figure 1.
Characterization of entrectinib and vandetanib. SARS-CoV-2 inhibition in A549-ACE2 cell lines and cytotoxicity of A) remdesivir B) entrectinib and C) vandetanib. D) EC90 and CC50 values for entrectinib and vandetanib (strain USA_WA1/2020) in Caco-2 cells. Only VYR data was reported. E) HCoV229E antiviral assay and in Huh-7 cell line with entrectinib and F) vandetanib. G) MicroScale Thermophoresis binding analysis for the interaction between SARS-CoV-2 Spike RBD and entrectinib. H) Pseudo SARS-CoV-2 D614G baculovirus (Montana Molecular #C1110G, #C1120G) assay in presence of vandetanib at 1 μM and its I) graphical analysis.
In vivo efficacy of vandetanib in mice
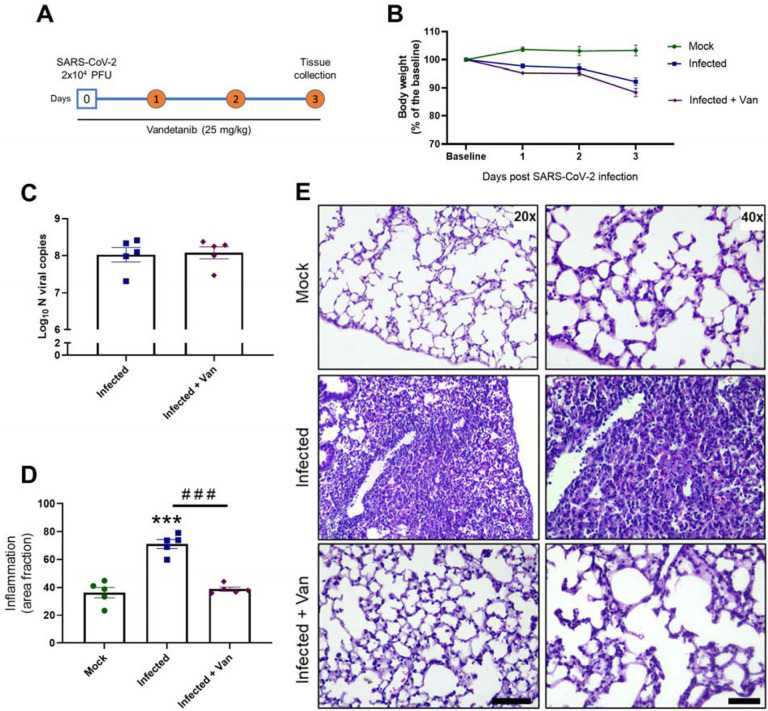
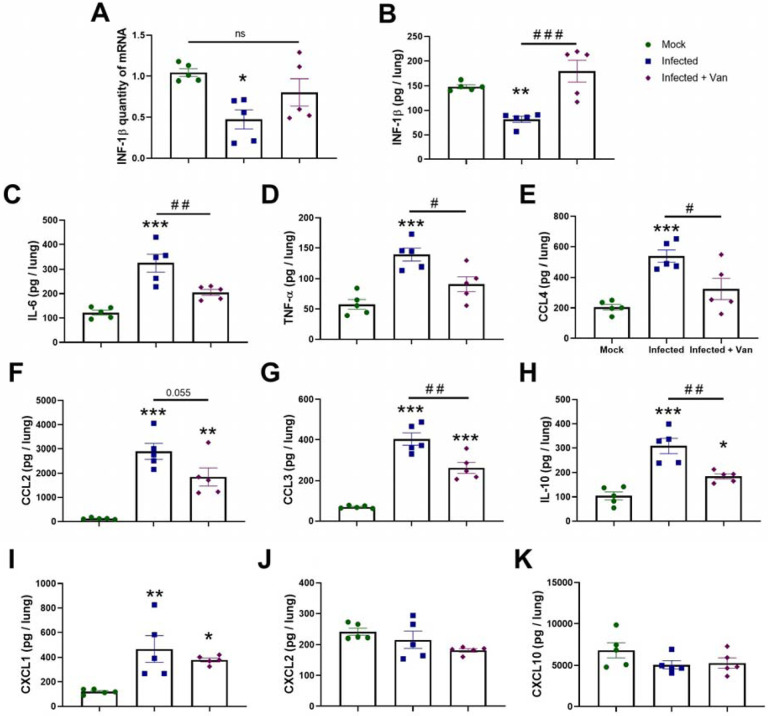
Vandetanib in vivo efficacy was assessed in the K-18-hACE2 mouse model of COVID-1942, 43, 44 (8-week-old females, challenged with SARS-Cov-2 2 ×104 PFU, intranasally). Vandetanib (25 mg/kg) was administered i.p. 1 h before infection and once daily up to day 3 post-infection (3 dpi) (Figure 2A). On 3 dpi, mice were euthanized, and viral load, cytokines and lung histopathology were evaluated. In all groups tested, mice lost weight compared to uninfected animals that received only vehicle formulation (Figure 2B). Lung viral load was evaluated by RT-PCR, and, although vandetanib reduced SARS-Cov-2 infection in A549-ACE2 cells, no significant reduction in viral RNA levels was observed in vivo when compared with the infected untreated mice (Figure 2C). While vandetanib did not decrease the viral load, it had a clear protective effect on lung pathology (Figure 2D and E). Infected untreated mice showed severe pathological changes with inflammatory cell infiltrates. In contrast, vandetanib treatment exhibited improved morphology and milder infiltration even in the absence of effect on viral load. These results might indicate an effect on the virus-induced inflammatory process. Further analyses also showed that vandetanib treatment restored the levels of IFN-1β (Figure 3A and B) and prevented the increase of the levels of the most widely evaluated inflammatory cytokines/chemokines observed in infected mice. Vandetanib reduced IL-6, TNF-α, and CCL4 (compared to infected untreated animals) to similar levels found in uninfected animals (Figure 3C, D and E). Vandetanib also significantly reduced the levels of CCL2, CCL3, and IL-10 compared to infected animals (Figure 3F, G and H). CXCL1 was not affected by the treatment (Figure 3I). CXCL2 and CXCL10 were not elevated in infected animals (Figure 3J and K). Altogether, these results indicate that the in vivo efficacy of vandetanib in the COVID-19 mouse model is more likely related to the reduction in the cytokine storm than reduction in SARS-CoV-2 replication.
Figure 2: In vivo efficacy of Vandetanib in a mouse model of COVID-19.
A) Experimental timeline: K18-hAce2 tg mice were infected with SARS-CoV-2 (2 × 104 PFU/40 μL saline, intranasal) or mock. One group of mice was treated with Vandetanib (25 mg/kg i.p.) 1 h before virus inoculation. B) Body weight was evaluated daily. C) At 3 DPI, mice were euthanized and the C) lung viral load, and D-E) Lung histopathology were evaluated. *** p<0.001 as compared with mock group after one-way ANOVA followed by Tukey post-hoc test. ### p<0.001 as compared with infected group after one-way ANOVA followed by Tukey post-hoc test. Bar scales = 20x – 125 μm; 40x – 50 μm.
Figure 3: Effects of Vandetanib on SARS-CoV-2-induced lung inflammation in a mouse model of COVID-19.
A) Expression of INF-1β quantified by qPCR. Levels of B) INF-1β, C) IL-6, D) TNF-α, E) CCL4, F) CCL2, G) CCL3, H) IL-10, I) CXCL1, J) CXCL2, and K) CXCL10 measured by ELISA. * p<0.05, ** p<0.01, and *** p<0.001 as compared with mock group after one-way ANOVA followed by Tukey post-hoc test. # p<0.05 and ## p<0.01 as compared with infected group after one-way ANOVA followed by Tukey post-hoc test.
Discussion
With a mounting infection and death toll to date, there has been a focus on ensuring the global population are vaccinated against SARS-CoV-2. Still, we are in a race against rapidly emerging viral variants that may hamper the vaccines effectiveness in future45, while many countries still have little or no access to the vaccines that are available in the USA and Europe46. There is also a critical need to develop treatments that do not require cold chain storage and can be used outside of hospitals. Repurposing approved small molecule drugs represents the fastest route to the clinic if they can demonstrate statistically significant efficacy in animal models.
Growth factor receptor signaling pathways have been reported to be highly activated upon SARS-CoV-2 infection, hence inhibition of these pathways prevents replication in cells47. After screening 45 kinase inhibitors against MHV, we narrowed them down to entrectinib and vandetanib. Vandetanib is an FDA approved drug used to treat thyroid gland tumors (targeting VEGFR, EGFR and RET-tyrosine kinase48) and was active in Caco-2 cell, A549-ACE2 infected by SARS-CoV-2 and against HCoV-229E. Based on this in vitro activity profile it was selected for testing in a COVID-19 mouse model.
One of the major causes of ARDS and multi-organ dysfunction syndrome (MODS) observed in severe SARS-CoV-2 infection is the cytokine storm17, 49. Studies have revealed higher levels of cytokine storm associated with more severe COVID development17. In these patients, accumulation and exudation of inflammatory substances from the cytokine storm destroys tissues, leading to multi-organ failure and ARDS49, an important cause of death in severe and critical cases of COVID-19. Capillary leakage is also major component of deteriorating lung function in COVID-19, resulting in ARDS due to inflammation driven by TNF, IL-1, IL-6, IL-8, and VEGF17. Abnormal coagulation can result in organ failure and death in patients with severe COVID-19, caused by the cytokine storm17. IL-1, IL-6, TNF, signal transducer and transcriptional activator 3 (STAT3), NF-kB, and lipopolysaccharides were all identified as regulators of thrombotic markers in COVID-19 patients with the severe-to-critical disease50. We are not aware of any current therapies that target the cytokine storm that have reversed disease.
Our evaluation of the in vivo efficacy of vandetanib in a murine model of infection demonstrated that vandetanib reduced IL-6, IL-10, and TNF-α compared to infected untreated animals to similar levels found in uninfected animals. In patients with severe COVID-19, the levels of several inflammatory cytokines and chemokines including PDGF, TNF, IL-6, and VEGF are significantly increased51. IL-6 has also been shown to correlate with respiratory failure and adverse clinical outcomes49, 52. Furthermore, a recent clinical study showed that the ARDS group had higher levels of IL-6, IL-8, and IL-10 than the non-ARDS group, and the levels of these cytokines correlated significantly with coagulation parameters and disseminated intravascular coagulation (DIC)49. The levels of IL-6 and TNF-α correlated with the levels of creatinine and urea nitrogen and were also higher in ARDS patients with acute kidney injury (AKI)49. Thus, reducing the levels of these mediators upon treatment with vandetanib could improve prognosis in COVID-19. The host innate immune response releases cytokines such as type I interferon (IFN α and β) during infection, thereby initiating antiviral activity. However, this particular interferon response is interrupted by factors such as SARS-CoV-2 non-structural proteins, aging, diabetes, and germ-line errors eventually making the host more susceptible to illness53, 54, 55, 56, 57. One interesting observation is that vandetanib rescued the decreased IFN-1β caused by SARS-CoV-2 infection in mice to levels similar to that in uninfected animals (Figure 3A). Vandetanib also decreased CCL2, CCL3, CCL4 and CXCL1 compared to infected animals (Figures 3F, 3G, 3E, 3I). These chemokines have been reported to be increased in patients with COVID-1919. CXCL1 is a known chemoattractant for neutrophils, and it was identified, among others, to be the one of the more abundantly expressed by macrophages involved in SARS-CoV2 infection, especially in more severe cases58 CCL2 is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages and CCL2 levels were also independently associated (together with other immune soluble biomarkers) with mortality in COVID-19 patients59. Finally, RNA isolated from nasopharyngeal swab samples from COVID-19 positive patients showed that CCL2 (and CCL3) expression was significantly higher in patients with unfavorable outcomes than cases with a favorable evolution or with negative controls60. Targeting chemokine/chemokine receptor binding might suppress hyper immune activation in critical COVID-19 patients58.
The lung histological examination for mice treated with vandetanib showed a significant treatment effect with mild infiltration, looking similar to uninfected mice. ARDS and dyspnea create hypoxia in lung tissues and other organs. Hypoxia induces VEGF expression. VEGF is a potent vascular permeability factor that induces vascular leakiness in COVID-19 infected lung tissues, resulting in plasma extravasation and pulmonary edema, which further increases tissue hypoxia61, 62 and VEGF participates in lung inflammation63. Blocking VEGF and the VEGF receptor (VEGFR)-mediated signaling would improve oxygen perfusion and anti-inflammatory response and alleviate clinical symptoms in patients with severe COVID-19. In agreement, bevacizumab, a humanized anti-VEGF monoclonal antibody was employed for treating patients with severe COVID-19 (NCT0427541464). Relative to comparable controls, bevacizumab showed clinical efficacy by improving oxygenation and shortening oxygen-support duration, and by day 28, 92% of patients show improvement in oxygen-support status, 65% patients were discharged, and none showed worsened oxygen-support status nor died64. Therefore modulating VEGF using drugs such as vandetanib48 may have clinical utility.
Many FDA-approved kinase inhibitors have been proposed as broad-spectrum antiviral therapies65 because they have multiple protein targets, including those in the host cell required for viral life cycle, replication, and infection of multiple virus types. Kinase inhibitors also have anti-inflammatory and cytokine inhibitory activity properties which may address lung damage from respiratory virus infections65. Host-target antivirals also offer the advantage that they can exploit the host protein and pathways needed for the replication of the virus and resistance may be less likely to develop against them.
Although we observed vandetanib reduced infection in i) DBT cells infected by MHV and in ii) A549-ACE2 infected by SARS-CoV-2, iii) showed a reduction of > 3 logTCID50/mL of HCoV-229E and iv) decreased viral entry in the pseudovirus assay, we did not observe a statistically significant reduction in the viral load in the murine SARS-CoV-2 infected model. We would likely need much longer in vivo studies to demonstrate a reduction in viral replication. We also did not observe a decrease in remdesivir viral load although it has been previously reported that remdesivir (25mg/kg) subcutaneous studies in mice should use Ces1c−/− which lack the serum esterase in order to mirror pharmacokinetics exposure that is seen in humans66. While our results may represent a suboptimal remdesivir dose it demonstrated a positive effect on the lung pathology in mice comparable to vandetanib. The positive effects shown in our study regarding modulation of the main inflammatory cytokines and chemokines as well as prevention of lung damage and fibrosis, demonstrated that vandetanib likely has the potential to address the cytokine storm associated with SARS-CoV-2 infection. Although the antiviral remdesivir was shown to reduce the length of hospital stay for those with COVID-1967, only anti-inflammatory approaches have improved survival in these patients, such as dexamethasone when given to those with an oxygen requirement68. Most recently, a randomized, placebo-controlled trial of Janus-kinase inhibition using tofacitinib has been reported to improve COVID-19 survival, in the presence of background glucocorticoid treatment (received by 89% of patients)69. The SAVE-MORE trial showed that blockade of the cytokine IL-1 via the recombinant human IL-1 receptor antagonist anakinra in patients with COVID-19, guided by suPAR levels in patients hospitalized with moderate and severe COVID-19 significantly reduced the risk of worse clinical outcome at day 2870.
Minimizing or preventing the cytokine storm is still a significant challenge, as it will require definition of the timing immunosuppressive or immunomodulatory agent administration. Knowing the cytokines to target in severe COVID-19 pneumonia and providing more-targeted therapeutic approaches may allow for the earlier introduction of anti-cytokine treatment. We now report that vandetanib can decrease levels of several such important cytokines that are significantly elevated in the cytokine storm, such as IL-6 and TNF-α.
In conclusion, there is continued interest in kinase inhibitors for treating COVID-19 and several such as masitinib71 and others are in clinical trials26. We now report that the FDA approved kinase inhibitor vandetanib could be a potential drug to target the cytokine storm and prevent patients from developing ARDS. Treatment with vandetanib in the mouse model also reduced key inflammatory cytokines. Vandetanib is well absorbed from the gut, reaching peak blood plasma concentrations 4 to 10 h after application, and has an average half-life of 19 days72, 73. The pharmacokinetic properties of vandetanib were linear over the dosage range of 50 to 1200 mg/d. The maximum plasma concentration of 857 ng/mL is usually reached after 6 h in patients with thyroid medullary cancer at a dose of 300 mg. Vandetanib is highly protein-bound (92–94%) and has a terminal excretion half-life of 20 d. It is metabolized by cytochrome P450 3A4 (CYP3A4) and is predominantly excreted via the feces and urine (44% and 25%, respectively48). In another study in healthy patients with dose escalation up to 1200 mg/d, vandetanib appeared to be well tolerated in the populations studied and at the dose of 800 mg/kg, vandetanib can reach a Cmax of 1 μM72, that is above the IC50 reported in our study infection of SARS-CoV-2 in A549-ACE2 cells. When combined these pharmacokinetic effects and positive impacts on the cytokine storm and preventing lung inflammation, may suggest it is worthy of clinical studies for COVID-19. While ee are aware of at least one report of a patient treated with vandetanib who had COVID-19 and recovered74, to date it has not been accessed further in a clinical trial which this current study may point towards.
Supplementary Material
Acknowledgments
We graciously thank Dr. Sara Cherry and Dr. David Schultz for the Calu-3 high-content SARS-CoV-2 studies performed by the University of Pennsylvania High-throughput Screening Core and the Cherry laboratory. Dr. Mindy Davis and colleagues are gratefully acknowledged for assistance with the NIAID virus screening capabilities. The authors gratefully acknowledge the technical assistance of Marcella Daruge Grando, Ieda Regina dos Santos, Juliana Trench Abumansur, and Felipe Souza. We kindly thank Dr. Anne. M. Quinn from Montana Molecular for her generous assistance with pseudovirus testing.
Grant information
We kindly acknowledge NIH funding: 1R43AT010585-01 from NIH/NCCAM to SE, AI142759 and AI108197 to RSB. This project was also supported by the North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill with funding from the North Carolina Coronavirus Relief Fund established and appropriated by the North Carolina General Assembly.
J.A. Levi and N.J. Johnson were supported by the Comparative Medicine Institute at North Carolina State University through its CAVE initiative.
Dr. Glaucius Oliva and colleagues acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - project 88887.516153/2020-00) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP project 2013/07600-3).
Collaborations Pharmaceuticals, Inc. has utilized the non-clinical and pre-clinical services program offered by the National Institute of Allergy and Infectious Diseases. TMC, JCAF and FQC received funding from the São Paulo Research Foundation (FAPESP) under grant agreement 2013/08216-2 (Center for Research in Inflammatory Disease) and 2020/04860-8 and from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (project 88887.507155/2020-00).
Collaborations Pharmaceuticals, Inc. has utilized the non-clinical and pre-clinical services program offered by the National Institute of Allergy and Infectious Diseases.
ABBREVIATIONS USED
- ACE2
Angiotensin converting enzyme 2
- BMP
Bis(monoacylglycero)phosphate
- COVID-19
Coronavirus disease
- MST
Microscale Thermophoresis
- MERS-CoV
Middle East Respiratory Syndrome coronavirus
- SARS-CoV-2
severe acute respiratory coronavirus 2
Footnotes
Competing interests:
SE is CEO of Collaborations Pharmaceuticals, Inc. ACP is an employee at Collaborations Pharmaceuticals, Inc. All other c-authors have no conflicts of interest.
References
- 1.Rehman M.F.U. et al. Novel coronavirus disease (COVID-19) pandemic: A recent mini review. Comput Struct Biotechnol J 19, 612–623 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyriakidis N.C., López-Cortés A., González E.V., Grimaldos A.B. & Prado E.O. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines 6, 28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H.Y. et al. Landscape and progress of global COVID-19 vaccine development. Hum Vaccin Immunother, 1–5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastman R.T. et al. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent Sci 6, 672–683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalil A.C. et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med 384, 795–807 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P. et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 384, 693–704 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apaydin C.B., Cinar G. & Cihan-Ustundag G. Small-molecule antiviral agents in ongoing clinical trials for COVID-19. Curr Drug Targets (2021). [DOI] [PubMed] [Google Scholar]
- 8.Nalbandian A. et al. Post-acute COVID-19 syndrome. Nat Med 27, 601–615 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva Andrade B. et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekins S. et al. Deja vu: Stimulating open drug discovery for SARS-CoV-2. Drug Discov Today 25, 928–941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muratov E.N. et al. A critical overview of computational approaches employed for COVID-19 drug discovery. Chem Soc Rev 50, 9121–9151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puhl A.C. et al. Repurposing the Ebola and Marburg Virus Inhibitors Tilorone, Quinacrine, and Pyronaridine: In Vitro Activity against SARS-CoV-2 and Potential Mechanisms. ACS Omega 6, 7454–7468 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riva L. et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586, 113–119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin S.A., Banerjee S., Ghosh K., Gayen S. & Jha T. Protease targeted COVID-19 drug discovery and its challenges: Insight into viral main protease (Mpro) and papain-like protease (PLpro) inhibitors. Bioorg Med Chem 29, 115860 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman R.L. et al. Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19. J Med Chem 63, 12725–12747 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C. & Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 54, 62–75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R. et al. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front Immunol 12, 589095 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roshanravan N., Seif F., Ostadrahimi A., Pouraghaei M. & Ghaffari S. Targeting Cytokine Storm to Manage Patients with COVID-19: A Mini-Review. Arch Med Res 51, 608–612 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coperchini F. et al. The cytokine storm in COVID-19: Further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev 58, 82–91 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felsenstein S., Herbert J.A., McNamara P.S. & Hedrich C.M. COVID-19: Immunology and treatment options. Clin Immunol 215, 108448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peter A.E., Sandeep B.V., Rao B.G. & Kalpana V.L. Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front Pharmacol 11, 583777 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moradian N. et al. Cytokine release syndrome: inhibition of pro-inflammatory cytokines as a solution for reducing COVID-19 mortality. Eur Cytokine Netw 31, 81–93 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beerli C. et al. Vaccinia virus hijacks EGFR signalling to enhance virus spread through rapid and directed infected cell motility. Nat Microbiol 4, 216–225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pleschka S. et al. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol 3, 301–305 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol Res 165, 105463 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Weisberg E. et al. Repurposing of Kinase Inhibitors for Treatment of COVID-19. Pharm Res 37, 167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baranov M.V., Bianchi F. & van den Bogaart G. The PIKfyve Inhibitor Apilimod: A Double-Edged Sword against COVID-19. Cells 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang T.N. et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 184, 460–475 e421 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q. et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov 6, 80 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riva L. et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586, 113–119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H., Mendenhall M. & Deininger M.W. Imatinib is not a potent anti-SARS-CoV-2 drug. Leukemia 34, 3085–3087 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghuvanshi R. & Bharate S.B. Recent Developments in the Use of Kinase Inhibitors for Management of Viral Infections. J Med Chem (2021). [DOI] [PubMed] [Google Scholar]
- 33.Drayman N. et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corman V.M. et al. Link of a ubiquitous human coronavirus to dromedary camels. Proc Natl Acad Sci U S A 113, 9864–9869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim Y.X., Ng Y.L., Tam J.P. & Liu D.X. Human Coronaviruses: A Review of Virus-Host Interactions. Diseases 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fehr A.R. & Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282, 1–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seidel S.A. et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 59, 301–315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willemsen M.J. et al. MicroScale Thermophoresis: Interaction analysis and beyond. J Mol Structure 1077, 101–113 (2014). [Google Scholar]
- 39.Wrapp D. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan K.K. et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 369, 1261–1265 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klann K. et al. Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication. Mol Cell 80, 164–174 e164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCray P.B. Jr. et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81, 813–821 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oladunni F.S. et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun 11, 6122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao L. et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583, 830–833 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Harvey W.T. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19, 409–424 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forni G., Mantovani A. & COVID-19 Commission of Accademia Nazionale dei Lincei, R.m. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ 28, 626–639 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klann K. et al. Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication. Mol Cell 80, 164–174.e164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frampton J.E. Vandetanib: in medullary thyroid cancer. Drugs 72, 1423–1436 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Wang J. et al. Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction. Virol J 18, 117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aid M. et al. Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell 183, 1354–1366 e1313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herold T. et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 146, 128–136.e124 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia H. et al. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep 33, 108234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuen C.K. et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg Microbes Infect 9, 1418–1428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molony R.D. et al. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci Signal 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu R., Xia C.Q., Butfiloski E. & Clare-Salzler M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: Implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection. Clin Immunol 195, 139–148 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chi Y. et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J Infect Dis 222, 746–754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abers M.S. et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sierra B. et al. Association of Early Nasopharyngeal Immune Markers With COVID-19 Clinical Outcome: Predictive Value of CCL2/MCP-1. Open Forum Infect Dis 7, ofaa407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaner R.J. et al. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 22, 657–664 (2000). [DOI] [PubMed] [Google Scholar]
- 62.Marti H.H. & Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci U S A 95, 15809–15814 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee C.G. et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 10, 1095–1103 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang J. et al. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nat Commun 12, 814 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weisberg E. et al. Repurposing of Kinase Inhibitors for Treatment of COVID-19. Pharm Res 37, 167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheahan T.P. et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beigel J.H. et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 383, 1813–1826 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Group R.C. et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 384, 693–704 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guimaraes P.O. et al. Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med 385, 406–415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kyriazopoulou E. et al. Author Correction: Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 27, 1850 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drayman N. et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science 373, 931–936 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin P. et al. Pharmacokinetics of vandetanib: three phase I studies in healthy subjects. Clin Ther 34, 221–237 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Jeong W., Doroshow J.H. & Kummar S. United States Food and Drug Administration approved oral kinase inhibitors for the treatment of malignancies. Curr Probl Cancer 37, 110–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prete A. et al. Thyroid cancer and COVID-19: experience at one single thyroid disease referral center. Endocrine 72, 332–339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.