Abstract
Imprinted maternal-allele-specific expression of the mouse insulin-like growth-factor type 2 receptor (Igf2r) gene depends on a 3.7-kb element named region 2, located in the second intron of the gene. Region 2 carries a maternal-allele-specific methylation imprint and contains an imprinted CpG island promoter (Air) that expresses a noncoding antisense RNA from the paternal inherited allele only. Here, we use transgenes to test the minimal requirements for imprinting of Air and to test if the action of region 2 is restricted to Igf2r. Transgenes up to 9 kb with Air as a single promoter are expressed but not imprinted. When coupled to the Igf2r CpG island promoter on a 44-kb transgene, Air was imprinted in one of three lines. However, Air on a 4.6-kb fragment is also imprinted in 2 of 14 lines when inserted in an intron of an adenine phosphoribosyltransferase (Aprt) transgene, and in one line, the imprinted methylation and expression of Air have been transferred onto the Aprt CpG island promoter. These data suggest that a dual CpG island promoter setting may facilitate Air imprinting as a short transgene and also show that Air can transfer imprinting onto other genes. However, for reliable Air imprinting, elements are necessary that are located outside a 44-kb region spanning the Air-Igf2r promoters.
Within the mammalian genome, more than 40 imprinted genes have been identified (for an up-to-date list, see www.mgu.har.mrc.ac.uk/imprinting/). Imprinted genes show parental-allele-specific expression such that only one of the two parental copies present in a diploid cell is expressed. The hallmark of an imprinted gene is the presence of an imprint that ultimately allows the cellular transcription machinery to discriminate between the two parental alleles. Because imprinting can occur in inbred mice with genetically identical parental alleles, imprints cannot be acquired in diploid cells but instead must be set when the parental alleles are in physically separate compartments, during germ cell development or before nuclear fusion in the zygote. In addition, the imprint must be epigenetic, stably inherited through mitosis in embryonic development, and reset upon germ line formation (for a review, see references 7, 28, and 35). Several possibilities exist for an imprint mark, such as chromatin composition, organization, and histone acetylation or methylation state (21, 24). However, DNA methylation is by far the best candidate as it fulfils all the desired criteria; in particular, de novo methylation patterns that are differently acquired by the male and female gamete could function as the imprinting mark. Support for a role of DNA methylation comes from the demonstration that mice lacking a functional maintenance methyltransferase gene (Dnmt1) show a loss of imprinted expression, observed as either biallelic silencing or biallelic expression (13, 16, 25, 26). All tested imprinted genes have been associated with allelic methylation differences in somatic tissue, and some also show gamete-specific methylation (26, 31, 36). Finally, for three imprinted genes, deletion of the sequences carrying the methylation imprint resulted in loss of imprinting, thereby identifying these methylated sequences as imprint control elements (8, 29, 34, 39, 40). Methylation in the mammalian genome is present on CpG dinucleotides, and DNA elements that carry a methylation imprint can be defined as CpG islands, since they contain clustered CpGs and are associated with active transcription. However, methylated CpG island promoters are unusual, as the majority of CpG island promoters are unmethylated regardless of expression state (3).
The reason why imprinted CpG islands attract a methylation imprint during gametogenesis has not yet been resolved, but two models have been proposed. First, specialized sequences are proposed to exist within the imprinted CpG island that either prevent or attract de novo methylation activity in one gamete. An allele discrimination signal (ADS) has been proposed to exist in the insulin-like growth factor type 2 receptor gene (Igf2r) imprinted intron 2 CpG island that attracts gamete-specific shielding factors, thereby protecting a nearby de novo methylation signal (DNS) from methylation. In this model, the primary allele discrimination is not de novo methylation in the oocyte, but the shielding that prevents methylation in spermatozoa. De novo methylation can subsequently occur later in gametogenesis or even after fertilization (9). Second, an alternative sequence-independent model proposed that the presence of direct repeats that have also been found in several imprinted CpG islands attracts methylation in the gamete as a response to unusual secondary structure formation. The ability of methylation to target these repeats in imprinted CpG islands was suggested to have evolved from a host defense response to suppress genomic parasitic DNA elements (5, 20, 38).
In order to identify genes involved in the imprint methylation pathway, the DNA sequences necessary and sufficient to attract and maintain the methylation imprint need to be characterized. Targeted deletions from the endogenous locus can be used to identify essential elements necessary for methylation, but only transgenes can identify the minimal configuration sufficient for the imprint. Such a transgenic approach has been successful in the identification of the minimally imprinted configuration for the maternally expressed H19 and paternally expressed insulin-like growth factor type 2 (Igf2) neighboring loci. Results have shown that large 130-kb transgenes that contain both the H19 and Igf2 genes as well as 140-kb transgenes that contain only the H19 gene are reliably imprinted (1, 15). H19 transgenes as small as 10 kb can be imprinted, but mostly as multicopy transgenes and with less than 100% frequency (12, 14, 23). These results indicate that an imprinted H19 transgene demands multiple elements in addition to the sequences that carry the methylation imprint, the H19 promoter, and the downstream situated enhancers. The G-rich repetitive element located upstream of the H19 promoter is, however, dispensable (12, 14, 23, 30). A transgenic approach has also been applied to the paternally expressed Snrpn gene, and results show that 75- to 85-kb multicopy transgenes containing the entire Snrpn gene and flanking DNA can be imprinted (10, 27). A minimal imprinted configuration has also been delineated on multicopy transgenes as a dual element of 1.2 kb composed of a 200-bp minimal Snrpn promoter coupled to a 1.0-kb human imprint regulatory element located 35 kb upstream of the human SNRPN promoter (27).
The maternally expressed Igf2r gene differs from the above imprinted genes, since it contains, in intron 2, a promoter generating a paternally expressed imprinted antisense RNA (Air) (17) that overlaps the Igf2r promoter (see Fig. 1 for an overview of the imprinted Air-Igf2r locus). This Air RNA is an unusual genomic transcript that is noncoding with no or few introns. An imprinted configuration for Igf2r has been identified on 300-kb transgenes that contain the whole 87-kb Igf2r gene and the 108-kb Air RNA plus additional flanking genes. Igf2r contains a methylation imprint inherited from the oocyte on a 3.7-kb fragment known as region 2 that is located within intron 2 (31). Region 2 contains direct repeats (20), an ADS-DNS motif (9), and the CpG island Air promoter (17). Region 2 is necessary for Igf2r imprinting in a transgene situation, as deletion results in Igf2r expression from both parental alleles (39), and is also necessary for imprinting the endogenous locus (40). In contrast, region 2 is not sufficient for Air imprinting, because short multicopy Air transgenes that contain all or part of region 2 are not imprinted by methylation (39). Imprinting of small Air transgenes might therefore demand multiple elements, similar to H19 and Snrpn transgenes.
FIG. 1.
The maternal and paternal endogenous (wt) Air-Igf2r loci are represented by the first two lines. The Air and Igf2r promoters are CpG island promoters (ellipses), and the arrows indicate the transcription starts. The methylated CpG islands are shown as stippled ellipses. Region 2 is presented as a line with the ADS-DNS motif depicted with an asterisk. The Igf2r exons are shown as numbered black boxes. Transgenic constructs REP, LAI, SAI, RAA, and FAA are derived from the endogenous Air-Igf2r locus. Construct REP contains a 9-kb Air fragment (including Igf2r exons 3 through 5) coupled to an EGFP reporter (stippled box), SV40 intron, and polyadenylation cassette (black triangle). The LAI construct spans 44 kb, from the Igf2r promoter up to Igf2r exon 7, and contains an EGFP-tagged (stippled box) Igf2r exon 1. 5′ SV40 polyadenylation cassette and 3′ rabbit β-globin polyadenylation cassettes are shown as black triangles. The small Air-Igf2r SAI construct is similar to LAI, but lacks Igf2r intron 1 and is shorter at the 3′ end, lacking Igf2r exons 6 and 7. Constructs RAA and FAA contain a complete mouse Aprt gene with a rabbit β-globin polyadenylation cassette inserted at the 5′ end (black triangles), the endogenous Aprt polyadenylation signal is used 3′. The Aprt exons are shown as open boxes. In construct RAA, the Air promoter is inserted in an antisense orientation with respect to the Aprt promoter, whereas in construct FAA the Air promoter is inserted in a sense orientation. The probes used for methylation analyses (LA, MC, MS, SB, and SE) and for expression analyses (SVA, GFPAIR, GFPIGF2R, MlMs1, PS, and DE) are shown. For details of the constructs and the probes, see Materials and Methods.
Here, we have tested whether stable Air expression and/or the presence of the Igf2r promoter and Igf2r expression are sufficient for imprinting of small Air transgenes. Our results show that in contrast to multicopy H19 transgenes, multicopy Air-expressing transgenes are not imprinted. However, the Air promoter can, albeit at a low frequency, recapitulate imprinting on a fragment as small as 4.6 kb when embedded in an adenine phosphoribosyltransferase (Aprt) transgene and can, in one instance, transfer imprinting onto the Aprt gene. Interestingly, including the Igf2r CpG island promoter and all flanking sequences up to the Air promoter does not increase the imprinting frequency for Air above that seen in combination with the Aprt CpG island promoter. Thus, this study indicates that a dual-promoter setting may facilitate Air imprinting and that elements for reliable Air imprinting are contained outside a 44-kb region spanning the Air-Igf2r promoters but inside the previously defined 300-kb region.
MATERIALS AND METHODS
DNA constructs. (i) REP.
Construct REP (for reporter) consists of a 9.1-kb SpeI fragment (nucleotide bp 120364 to 132177, GenBank accession number AJ249895) containing region 2 isolated from cosmid 940PS (31) ligated into the BglII site of reporter plasmid pEGFP-1 (Clontech). The pEGFP-1 reporter has two modifications. An internal ribosome entry site sequence was introduced into the SmaI site, and a simian virus 40 (SV40) intron obtained as a 179-bp XhoI/NotI fragment from plasmid pCMVβ (Clontech) was inserted into the NotI site of pEGFP-1. The 10.8-kb construct was excised from the vector by SpeI/SspI digestion.
(ii) LAI.
Construct LAI (for large Air-Igf2r construct) was built in a modified pBeloBAC-II vector (Research Genetics) that allowed excision of the final 44-kb LAI construct with RsrII/BsiWI digestion. LAI consists of a 40-kb NotI fragment containing region 2 (bp 98069 to 138490; AJ249895) from cosmid OT1 (17) ligated to a 4.0-kb EcoRI/NotI fragment (bp 94101 to 98069; AJ249895) containing the Igf2r promoter isolated from cosmid 3L (31). A 59-bp AgeI/NotI fragment (bp 98010 to 98069; AJ249895) from Igf2r exon 1 has been replaced in frame by a 735-bp AgeI/NotI fragment of reporter pEGFP-1 (Clontech). In a BglII site (bp 94139; AJ249895) upstream to the Igf2r promoter, a 198-bp BamHI fragment from pCMVβ (Clontech) containing the SV40 polyadenylation signal was introduced in an antisense orientation to terminate the Air transcript. The 3′ end of the construct contains a 1-kb HincII fragment from the rabbit β-globin gene, which includes part of intron 2, and complete exon 3 with its polyadenylation signal to terminate the Igf2r transcript.
(iii) SAI.
Construct SAI (for small Air-Igf2r construct) was built in the modified pBeloBAC-II vector and excised as a 20-kb RsrII/BsiWI fragment. It consists of the H/X-14 construct (39), which is a HindII/XhoI fragment containing region 2 (bp 133005 to 118712; AJ249895), and is ligated to the same enhanced green fluorescent protein (EGFP)-tagged EcoRI/NotI Igf2r promoter fragment that was used in construct LAI. The 5′ and 3′ polyadenylation cassettes were identical to that in the LAI construct.
(iv) RAA and FAA.
Constructs RAA (for reverse Air-Aprt) and FAA (for forward Air-Aprt) contain a 5-kb NcoI fragment with the entire mouse Aprt gene (a gift from Harry Vrieling, Leiden University). A 1.2-kb EcoRI fragment from rabbit β-globin, including part of exon 2, complete intron 2, and exon 3 containing a polyadenylation signal, was inserted in an antisense orientation into the EcoRV site (bp 108; M11310) upstream of the Aprt promoter. A 4.6-kb SpeI/BglII fragment (bp 120364 to 127575; AJ249895) from cosmid 940PS containing region 2 was inserted into the HindIII site (bp 1694; M11310) of Aprt intron 2 in an antisense (in construct RAA) or sense (in construct FAA) orientation with respect to the Aprt promoter. Both constructs were released from the pBluescript II vector (Stratagene) by BssHII/NotI digestion.
Transgenic mice.
Transgenic mice were generated by injecting DNA into FVB/N-fertilized oocytes by approved procedures (2). Approximately 300 oocytes injected with the REP construct generated 11 founder transgenes, 550 oocytes injected with the LAI construct generated 3 founder transgenes, 450 oocytes injected with the SAI construct generated 2 founder transgenes, 300 oocytes injected with the RAA construct generated 5 founder transgenes, and 200 oocytes injected with the FAA construct generated 9 founder transgenes. Transgenes were bred hemizygously onto an FVB/N background and identified by Southern blotting or PCR on genomic tail DNA.
DNA and methylation analyses.
Genomic DNA was isolated by the sodium dodecyl sulfate-proteinase K procedure (2). The following oligonucleotides were used: for typing construct REP, GFPF (5′-CTGGTGAACCGCATCGAGCTGAA-3′) and GFPAR (5′-ACCTCTACAAATGTGGTATGGCTG-3′); for constructs LAI and SAI, EFP1F (5′-GTAAACGGCCACAAGTTCAGC-3′) and EFP2R (5′-GGTGTTCTGCTGGTAGTGGTC-3′); for construct FAA, APRTEX2F (5′-TATCTCGCCCCTCTTGAAAGACC-3′) and M404F (5′-GTGACTCACTTTTGAGAAC-3′); for construct RAA, APRTEX3R (5′-CCATACTCCAGAGAATAGGAGGC-3′) and M404F. Methylation analysis probes (see also Fig. 1) were as follows: for SB, a 723-bp HindIII/BglI fragment (bp 971 to 1694; M11310) from the Aprt gene; for SE, a 700-bp EcoRV/SmaI fragment (bp 108 to 808; M11310) upstream from the Aprt promoter; for LA, a 984-bp SacI/AgeI fragment (bp 97026 to 98010; AJ249895) from the Igf2r promoter; for MS, SfuI/MluI (bp 124992 to126086; AJ249895); for MC, MluI/BglII (bp 126086 to 127575; AJ249895). The digestion of methylation-sensitive enzymes was regularly monitored by hybridization to mitochondrial DNA as previously described (38). Methylation was quantified with a PhosphorImager (Fujix) and expressed as a percentage in multiples of 10. Transgene copy numbers were determined by comparing the transgene to the endogenous signal in a Southern blot with a PhosphorImager (Fujix). Single-copy lines were confirmed by hybridizing with an end fragment from the transgene.
RNA analyses.
Total RNA was isolated with Tri Reagent (Molecular Research Center) according to the manufacturer's protocol. For the RNase protection assay (RPA) the RPAIII kit (Ambion) was used, and for the Northern blot assay the formaldehyde method was used (2). Expression analysis probes were as follows: SVA, a 350-bp HpaI fragment from construct REP, protects a 240-bp fragment from the SV40 polyadenylation signal; A3, a 252-bp XhoI/XbaI fragment (bp 2165 to 2417; M11310) protects Aprt exon 3 (134 bp); GFPIGF2R and GFPAIR, templates made by PCR with T3IGGFP (5′-AATTAACCCTCACTAAAGGGAAAACTTGTGGCCGTTTAC-3′) and T7IGEX1 (5′-TAATACGACTCACTATAGGGAGTCACGGAGCGCCTCCTC-3′) on construct LAI (281 bp); GFPAIR detects endogenous Air (185 bp) and transgenic Air (275 bp). GFPIGF2R detects endogenous (188, 163, 143, and 132 bp) and transgenic (278, 253, 233, and 222 bp) Igf2r RNA; MlMs1 is a MluI/MseI fragment (bp 126086 to 126293; AJ249895) (17) that detects unspliced (207, 171, and 148 bp) or spliced (112, 76, and 53 bp) Air RNA; Gapdh, templates generated by PCR with GAPDHF (5′-CGGGGTACCACAGCCGCATCTTCTTGTGCAG-3′) and GAPDHR (5′-CGTCTAGATGGGTGGTCCAGGGTTTCTTAC-3′); PS, a 1.5-kb EcoRI/HindIII fragment (bp 1 to 1694; M11310) containing Aprt exons 1 and 2 (19); DE, a 1.3-kb HindIII fragment (bp 1694 to 3066; M11310) containing Aprt exons 3 to 5 (19).
RESULTS
Single-promoter Air transgenes are expressed but not imprinted.
Previously, it has been shown that short transgenes from 1 to 14 kb containing the intronic CpG island known as region 2 (see Fig. 1) do not carry a methylation imprint (39). These transgenes were not characterized for expression and may have lacked sequences needed for Air promoter activity and termination of the Air transcript. To test whether imprinting of Air requires stable Air transcription, we generated the REP construct that contains 9 kb, including region 2, coupled to an EGFP reporter, as described in Materials and Methods (Fig. 1). Five REP transgenic lines were generated and analyzed for Air imprinting upon hemizygous transmission through the paternal and maternal germ lines.
Methylation of the Air promoter was analyzed using the methylation-sensitive restriction enzymes on genomic DNA from adult tail and spleen and embryo 13.5 days postconception (dpc). These enzymes can only digest unmethylated DNA and are consequently informative for the methylation status of the recognition site. For all transgenes, we used the methylation-sensitive enzymes MluI and SfuI that cut within the core of the Air CpG island. The methylation status of these restriction sites is representative for the whole CpG island and identical for tail, spleen, and embryo DNA (references 31 and 39 and data not shown). Imprinted methylation was absent in all REP lines (Table 1). Four lines had no methylation on the Air promoter following transmission from either parent; one line, REP-30, showed 50% methylation that was unchanged following maternal and paternal transmission (Fig. 2A; Table 1).
TABLE 1.
Imprinting status of Air transgenesa
| Construct | Line | Copy no. |
Air
|
Aprt-Igf2r
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methylation
|
Expression
|
Methylation
|
Expression
|
|||||||
| p | m | p | m | p | m | p | m | |||
| REP | 26 | 2 | 0 | 0 | + | + | ||||
| 27 | 2 | 0 | 0 | + | + | |||||
| 30 | 10–15 | 50 | 50 | + | + | |||||
| 65 | 4 | 0 | 0 | + | + | |||||
| 90 | 2 | 0 | 0 | + | + | |||||
| 13 | 2 | 30 | ND | ND | ND | |||||
| 24 | 3 | ND | 0 | ND | ND | |||||
| 36 | 10–15 | 40 | ND | ND | ND | |||||
| 77 | 3 | ND | 40 | ND | ND | |||||
| 78 | 3 | 20 | ND | ND | ND | |||||
| 87 | 5 | 30 | ND | ND | ND | |||||
| LAI | 46 | 1 | 0 | 100 | + | − | 100 | 70 | − | + |
| 48 | 2 | 0 | 0 | + | + | 80 | 80 | + | + | |
| 313 | >30 | 0 | 0 | + | + | 80 | 80 | + | + | |
| SAI | 112 | 2 | 0 | 0 | + | + | 100 | 100 | − | − |
| 119 | 2 | 0 | 0 | + | + | 50 | 50 | + | + | |
| RAA | 14 | 1 | 0 | 100 | + | − | 0 | 0 | + | + |
| 21 | 2 | 0 | 0 | + | + | 0 | 0 | + | + | |
| 27 | 10–15 | 40 | 40 | + | + | ND | ND | ND | ND | |
| 30 | 2 | 0 | 0 | + | + | ND | ND | ND | ND | |
| 58 | 10–15 | 50 | 50 | + | + | ND | ND | ND | ND | |
| FAA | 3 | 3 | 0 | 0 | + | + | ND | ND | ND | ND |
| 4 | 1 | 0 | 100 | + | − | 0 | 100 | + | − | |
| 8 | 2 | 0 | 0 | + | + | ND | ND | ND | ND | |
| 21 | 1 | 0 | 0 | + | + | ND | ND | ND | ND | |
| 26 | 3 | 10 | 10 | + | + | ND | ND | ND | ND | |
| 30 | 4 | 20 | 20 | + | + | ND | ND | ND | ND | |
| 37 | 1 | 0 | 0 | + | + | 0 | 0 | ND | ND | |
| 57 | 2 | 50 | 50 | + | + | ND | ND | ND | ND | |
| 67 | 2 | 0 | 0 | + | + | ND | ND | ND | ND | |
The methylation and expression status of the Air promoter on all transgenes is shown. Methylation and expression of either the Igf2r promoter (in LAI and SAI constructs) or the Aprt promoter (in RAA and FAA constructs) were determined only for transgenes linked to an imprinted Air promoter and for some representative transgenes linked to a nonimprinted Air promoter. Methylation is given as a percentage. The data for imprinted transgenes generated in this study are shown in boldface. Construct, abbreviated transgene name, as described in Materials and Methods; line, the number assigned to the independent integration sites of each transgene; p and m, paternal and maternal expression of the transgene, respectively; ND, not determined.
FIG. 2.
Imprinting analyses of transgenic lines REP-65 and REP-30. (A) Methylation analyses of the Air promoter. A Southern blot of tail genomic DNA digested with SacI (− lanes) or SacI and MluI (+ lanes) and hybridized with probe MS detecting endogenous (wt) and transgenic (tg) bands (sizes in kilobases are indicated) is shown. (B) Expression analyses of the transgenic Air promoter. An RPA was carried out of adult cardiac tissue total RNA with the transgene-specific probe (SVA) from the polyadenylation cassette in combination with an Aprt exon 3 probe (A3) as loading control. Lanes: i, input probes; c, tRNA hybridization to the probes; p and m, paternal and maternal transmission of the transgene, respectively; wt, a nontransgenic sample.
Transgenic Air expression was then analyzed in cardiac and 13.5-dpc embryo tissue, as these samples have a high level of endogenous Air expression (17, 39). All the REP transgenes expressed high levels of Air (data not shown), thus confirming that sequences needed for Air promoter activity are contained on the 9-kb fragment. When analyzing the REP lines for Air expression, we found that in contrast to the endogenous locus, the transgenic Air RNA is spliced. A reverse transcription (RT)-PCR product was obtained using primers present in the polyadenylation cassette of the EGFP reporter and the mapped Air transcription start. The sequence of the RT-PCR product identified the splice donor GAACTGAG-GTAAGC located 53 bp downstream of the major transcription start for the Air RNA (17). The acceptor (GTCCCGGA) is part of an SV40 intron, located between the EGFP reporter and the polyadenylation cassette. An RPA with a transgene-specific probe (SVA) from the polyadenylation cassette showed an absence of imprinted expression for the transgenic Air RNA, as all five REP lines have equal expression levels for both parental transmissions (Fig. 2B; Table 1). An additional six REP lines were generated and analyzed for methylation differences by comparing the founder to its progeny with the MluI and SfuI sites in the Air CpG island promoter. Air methylation was unchanged in offspring, following germ line transmission from four male founders and two female founders, similar to all other REP lines and nonimprinted lines (Table 1 and data not shown). Based on the absence of a methylation change upon germ line transmission, these lines were excluded from detailed expression analyses. Thus, a total of 11 REP lines lack imprinted characteristics for Air.
Low-frequency imprinting of Air-Igf2r transgenes.
Previously, it has been shown that large, 300-kb transgenes that contain both the Air and Igf2r promoters were successfully imprinted in three of three sites when integrated into autosomes (39). During the same experiment, a related transgene with no Igf2r expression was inadvertently derived that failed to be imprinted. This result, combined with the failure of imprinting of small Air transgenes, suggested the possibility that normal Igf2r expression is a requirement for Air imprinting. To test if Igf2r expression is sufficient for imprinting of Air on short transgenes, we generated two differently sized constructs that contain both the Air and Igf2r promoters. The LAI construct is 44 kb and spans from 3.8 kb upstream of the Igf2r promoter up to Igf2r exon 7 and includes an EGFP-tagged Igf2r exon 1 (Fig. 1). The 20-kb SAI construct is similar to LAI but lacks the 20-kb Igf2r intron 1 and only extends up to Igf2r exon 5 (Fig. 1). Both constructs are flanked 5′ and 3′ by polyadenylation signals to terminate the transcripts from the Air and Igf2r promoters. Three LAI and two SAI transgenic lines were generated.
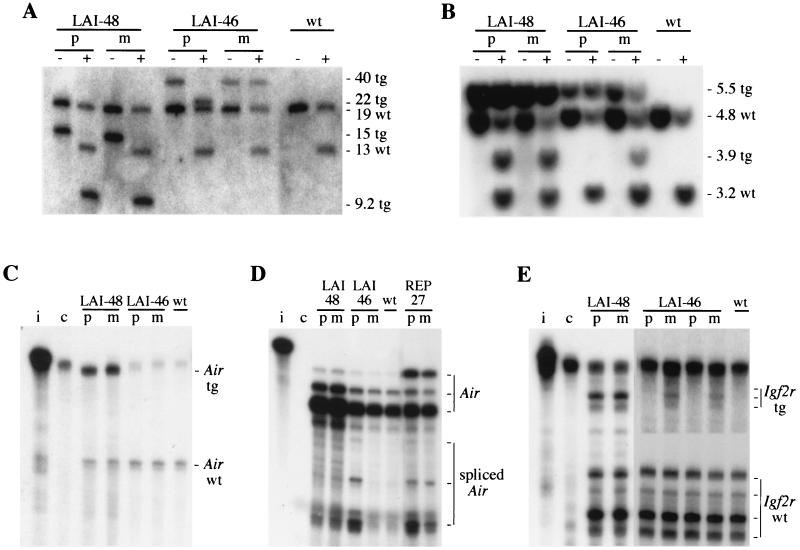
Methylation of the Air promoter was investigated using the methylation-sensitive restriction enzymes MluI and SfuI. Of five lines, only line LAI-46 revealed a difference in methylation of Air on the 40-kb transgene-specific fragment (Fig. 3A; Table 1). The maternal transmission of LAI-46 has a 100% methylated Air promoter whereas the paternal transmission has no methylation. LAI-48, LAI-31, and the two SAI lines have an unmethylated or hypomethylated Air promoter that was unchanged on maternal and paternal transmission (Fig. 3A; Table 1).
FIG. 3.
Imprinting analyses of transgenic lines LAI-48 and LAI-46. (A) Methylation analyses of the Air promoter by Southern blotting of adult spleen genomic DNA cut with HindIII (− lanes) or HindIII and SfuI (+ lanes) hybridized with probe MC detecting both endogenous (wt) and transgenic (tg) fragments (sizes in kilobases are indicated). Note that HindIII cuts inside the LAI construct and that LAI-46 shows an aberrant fragment due to a deletion of the 3′ polyadenylation cassette. (B) Methylation analyses of the Igf2r promoter. A Southern blot of adult tail genomic DNA cut with SspI (− lanes) or with SspI and SalI (+ lanes) hybridized with probe LA detecting endogenous (wt) and transgenic fragments (tg) is shown (sizes in kilobases are indicated). (C ) Expression analyses of endogenous (wt) and transgenic (tg) Air RNA in cardiac tissue by RPA with probe GFPAIR that covers part of the EGFP-tagged Igf2r exon 1. (D) Expression of Air RNA in cardiac tissue with RPA probe MlMs1 that covers multiple transcription starts at the Air promoter, yielding multiple protected bands for both spliced and unspliced Air RNA. The transgenic line REP-27 is used as a control for spliced Air RNA. (E) Expression of Igf2r RNA detected by RPA with probe GFPIGF2R on cardiac (LAI-48) or embryonic 12.5-dpc (LAI-46) total RNA. Note that this probe covers multiple transcription starts of the Igf2r promoter and part of the EGFP tag, resulting in multiple protected fragments for both the endogenous (wt) and transgenic (tg) Igf2r RNA. The part of the LAI-46 line showing transgenic Igf2r RNA (top) has been overexposed compared to the the bottom panel, showing endogenous Igf2r RNA. Lanes: i, input probe; c, tRNA hybridization to the probe; p and m, paternal and maternal transmission of the transgene, respectively; wt, nontransgenic sample.
The LAI and SAI lines were then investigated for Air expression by RPA using a probe (GFPAIR) that spans part of the EGFP-tagged Igf2r exon 1. The endogenous Air RNA runs through the Igf2r promoter, and this probe can detect endogenous and transgenic Air RNA as differently sized fragments. Both parental transmissions of the LAI-313 and LAI-48 lines that lack a methylation imprint express equal levels of transgenic Air RNA (Fig. 3C; Table 1). However, for the LAI-46 line and the two SAI lines no Air RNA could be detected with the GFPAIR probe (Fig. 3C and data not shown). Based on the splicing of Air RNA that occurs in the REP lines, we investigated whether the Air RNA in the LAI and SAI lines is also spliced by using RPA with probe MlMs1 that covers the splice donor and the Air transcription starts (17). The two SAI lines have equal spliced Air expression upon both parental transmissions (Table 1). Line LAI-46 produces spliced Air RNA upon paternal transmission but no Air RNA upon maternal transmission (Fig. 3D). Thus, LAI-46 has imprinted Air expression. Splicing of the Air RNA is similar (i.e., with the same splice donor) to the REP-27 line that was used as a splicing control (Fig. 3D) and explains why no Air RNA could be detected through the Igf2r promoter by probe GFPAIR (Fig. 3C).
The relationship between the methylation-expression status of the Air promoter and that of the linked Igf2r promoter in LAI and SAI transgenes was then investigated (Table 1). Methylation of the Igf2r promoter was analyzed for a SalI (for LAI) or NotI (for SAI) and two SmaI (for LAI and SAI) sites that are located within the Igf2r promoter CpG island. For all lines, the NotI and SalI sites have a degree of methylation identical to that of the SmaI sites (data not shown). The Igf2r promoter in line LAI-46 has 70 and 100% methylation after maternal and paternal transmission, respectively (Fig. 3B). In lines LAI-48 and LAI-313, the Igf2r promoter has 80% methylation for both parental transmissions; in the SAI-112 and SAI-119 lines, the Igf2r promoter has 100 and 50% methylation, respectively, for both transmissions (Fig. 3B and Table 1 and data not shown).
Expression of the Igf2r promoter in the LAI and SAI transgenic lines in cardiac tissue was analyzed by RPA with probe GFPIGF2R. This probe covers multiple Igf2r transcription start sites (31, 33) and part of the EGFP tag, thereby detecting both endogenous and transgenic Igf2r RNA as differently sized fragments. Transgenes LAI-48, LAI-313, and SAI-119 do not have imprinted Igf2r expression and express similar levels of Igf2r RNA upon both parental transmissions (Fig. 3E and Table 1 and data not shown). SAI-112 has no detectable Igf2r expression on any transmission (Table 1 and data not shown). Neither of the two parental transmissions for LAI-46 had any detectable Igf2r expression in cardiac tissue, despite the fact that maternal transmission showed 30% reduction in Igf2r methylation (Fig. 3B and data not shown). We therefore analyzed Igf2r expression of line LAI-46 in 12.5-dpc embryos, as these embryos have higher levels of Igf2r RNA and reduced methylation on the Igf2r promoter (31). The Igf2r promoter of LAI-46 12.5-dpc embryos is 80% methylated on paternal transmission and 50% methylated on maternal transmission (data not shown) and shows weak but maternal-allele-specific Igf2r expression as analyzed by RPA (Fig. 3E; Table 1). So in summary, of five lines that contain both the Air and Igf2r promoters, only line LAI-46 is imprinted for Air, and this correlates with Igf2r imprinting.
Low-frequency imprinting of Air-Aprt transgenes.
The observation that Air can be imprinted when combined with the Igf2r promoter prompted us to test if nonimprinted CpG island promoters could also facilitate Air imprinting. Two constructs were generated that positioned the Air promoter in opposite orientations into a mouse Aprt transgene. Construct RAA contains the complete Aprt gene, polyadenylation signal, and promoter, plus a 4.6-kb fragment containing region 2 inserted into intron 2 of the Aprt gene so that the Air promoter faces the Aprt promoter and potentially generates an antisense RNA. Upstream of the Aprt promoter, a polyadenylation cassette was introduced to terminate Air RNA (Fig. 1). Five transgenic RAA lines were generated and investigated for imprinting of Air and Aprt.
Methylation analyses of the Air promoter showed no methylation for low-copy-number lines RAA-21 and RAA-30 and a low level of methylation (40 to 50%) for high-copy-number lines RAA-27 and RAA-58 (Table 1). The methylation status was unchanged on parental transmission with the exception of one line, RAA-14. This line showed imprinted methylation for Air, gaining 100% methylation upon maternal transmission and losing all methylation upon paternal transmission (Fig. 4A). Methylation of the maternally derived RAA-14 transgene was, however, inconsistent between individuals from one litter. For example, of 15 offspring with a maternally inherited transgene, 12 had a methylated Air promoter but 3 were unmethylated. Female offspring with a methylated or unmethylated transgenic Air promoter subsequently produced offspring with the same variability of methylated transgenes (data not shown). Paternal transmission showed no variability and always resulted in an unmethylated Air promoter (Fig. 4A).
FIG. 4.
Imprinting analyses of transgenes RAA-21 and RAA-14. (A) Methylation analyses of the Air promoter. A Southern blot is shown of genomic spleen DNA digested with EcoRV (− lanes) or EcoRV and MluI (+ lanes) and hybridized with probe MS detecting both endogenous (wt) and transgenic (tg) Air fragments (sizes in kilobases are indicated). (B) Methylation analyses of the Aprt promoter. A Southern blot is shown of spleen DNA cut with NcoI (− lanes) or NcoI and SmaI (+ lanes) and hybridized with probe SE, which detects both endogenous (wt) and transgenic (tg) Aprt fragments (sizes in kilobases are indicated). (C) Expression of the Aprt and Air promoters by Northern blot analyses of adult cardiac tissue total RNA. Probe PS consists of Aprt exon 1 and 2 (top) and detects both endogenous and transgenic Aprt RNA and transgenic Air RNA due to the reverse orientation of the Air promoter (see Fig. 1). The signal from transgenic Aprt is not visible at this exposure with this short probe. Probe DE consists of Aprt exon 3 to 5 (middle) and detects endogenous and transgenic Aprt RNA as indistinguishable bands. Transgenic Aprt expression from line RAA-14 was analyzed in an Aprt−/− background. Gapdh was used as a loading control (bottom). Lanes: p and m, paternal and maternal transmission of the transgene, respectively; wt, nontransgenic sample.
The expression of the RAA transgenes was investigated by Northern blot analyses. Two probes, PS (covering Aprt exons 1 and 2) and DE (covering Aprt exons 3 to 5), were used to discriminate between the Air and Aprt RNA, respectively. Note that the Air RNA in the RAA lines was spliced, using the same donor identified in the REP lines, but spliced to a novel acceptor (GTGAGTGG) located in antisense orientation within Aprt exon 2. The Air RNA had equal levels of expression upon both transmissions in all lines, except in RAA-14 (Fig. 4C and Table 1). Line RAA-14 showed Air expression upon paternal transmission, which corresponds to the unmethylated status of the Air promoter. Maternally transmitted RAA-14 transgenes with a methylated Air promoter (80% of offspring) completely lacked Air expression, whereas the 20% of offspring with an unmethylated Air promoter expressed spliced Air (Fig. 4C).
To address whether the imprinted Air promoter of RAA-14 is able to imprint the Aprt promoter, methylation and expression analyses of Aprt were performed. Methylation of the Aprt promoter for lines RAA-21 and RAA-14 was analyzed for two SmaI sites located within the Aprt CpG island promoter. For both transgenic lines, both sites are unmethylated on maternal and paternal transmission, as is the endogenous Aprt promoter (Fig. 4B; Table 1). Aprt expression was analyzed by RT-PCR. Both RAA-21 and RAA-14 lines produce transgenic Aprt RNA upon both transmissions, as detected by RT-PCR (Table 1). This transgenic Aprt RNA includes the 125-bp Igf2r exon 3, due to the orientation of region 2 (data not shown). However, this RNA overlaps with endogenous Aprt on a Northern blot, so we analyzed Aprt expression of line RAA-14 in an Aprt−/− background (Fig. 4C). The paternal and maternal transmissions express equal levels of the transgenic Aprt RNA when compared to Gapdh, indicating that the Aprt promoter has no imprinted expression (Fig. 4C; Table 1).
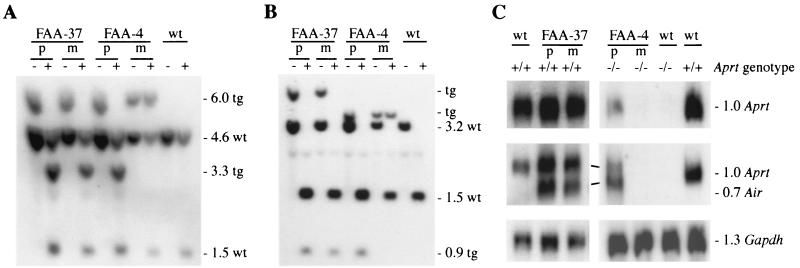
Construct FAA is the same basic construct as RAA but has the 4.6-kb region 2-containing fragment inserted in a forward orientation, so that both the Aprt and Air promoters have the same direction (Fig. 1). Nine FAA lines were generated and analyzed for Air imprinting. One line, FAA-4, had imprinted methylation of the Air promoter, being unmethylated on paternal transmission and methylated on maternal transmission (Fig. 5A; Table 1). The remaining eight lines had no or low-level Air methylation, which was identical upon both parental transmissions (Fig. 5A; Table 1). Expression, analyzed by Northern blotting in an Aprt−/− background, shows that line FAA-4 has paternal-allele-specific Air expression that inversely correlates with methylation status (Fig. 5C). The transgenic Air RNA was again spliced, using the same donor as the REP and RAA lines, but spliced to the normal Aprt exon 3 acceptor (GTCTAGAC). Remarkably, Northern blotting also showed that the Aprt promoter has paternal-allele-specific expression similar to that of the Air promoter on the same transgene (Fig. 5C; Table 1).
FIG. 5.
Imprinting analyses of transgenes FAA-37 and FAA-4. (A) Methylation of the Air promoter was analyzed by Southern blotting with enzymes HincII (− lanes) or HincII and MluI (+ lanes) and hybridized with probe MC, which detects both endogenous (wt) and transgenic (tg) Air fragments (sizes are indicated in kilobases). (B) Methylation analyses of the transgenic (tg) and endogenous (wt) Aprt promoters. A Southern blot of spleen DNA cut with PstI (− lanes) or PstI and SmaI (+ lanes) and hybridized with probe SB is shown. Because PstI cuts outside the transgene at the 5′ end, it generates differently sized fragments for each transgenic line and confirms the single-copy integration site. (C) Expression analyses of the Aprt and Air promoters by Northern blotting of total embryo (14.5 dpc) RNA. Note that due to the orientation of the Air promoter, probe PS consisting of Aprt exons 1 and 2 (top) only detects endogenous and transgenic Aprt RNA, whereas probe DE consisting of Aprt exons 3 to 5 (middle) now detects both transgenic and endogenous Aprt RNA and transgenic Air RNA. Line FAA-4 was analyzed in an Aprt−/− background. Gapdh was used as a loading control (bottom). Lanes: p and m, paternal and maternal transmission of the transgene, respectively; wt, nontransgenic sample.
Methylation analyses for the Aprt promoter performed as for the RAA lines indicated that the Aprt promoter of FAA-4 acquired a maternal-allele-specific methylation imprint, as did the Air promoter on this transgene (Fig. 5B; Table 1). In contrast to the RAA lines where the Igf2r exon 3 is spliced into the transgenic Aprt RNA, the Aprt RNA produced from FAA-4 is functional due to the orientation of the region 2 fragment. This allowed us to rescue the phenotype from Aprt −/− mice (37) in an imprinted manner by crossing in the FAA-4 line. Aprt−/− mice (six of six) and Aprt−/− mice with a maternally transmitted FAA-4 transgene (four out of four) have up to 50% less body weight than Aprt+/− (eight of eight) littermates and died within 4 months (37). However, upon paternal transmission of FAA-4, Aprt−/− mice (nine of nine) were indistinguishable from Aprt+/− mice and had normal body weight, indicating functional Aprt expression from the transgene. In summary, 2 of 14 Air-Aprt transgenes have an imprinted Air promoter which is maternally methylated and silent and paternally unmethylated and expressed. In one of these lines, the Aprt promoter has the same imprinted and paternal specific expression as the Air promoter and rescued the Aprt−/− phenotype upon paternal transmission.
DISCUSSION
Air behaves as a nonimprinted CpG island promoter by default.
Exploiting transgenes, we have investigated the minimal setting sufficient to imprint the Air promoter. Although all transgenes carried the ADS, the DNS, and the direct repeat array that have all been postulated to be involved in attracting the methylation imprint (9, 20), in 27 of 30 transgenic lines Air is not imprinted and has no or low-level methylation. The results presented here that examined transgene methylation either in mid-gestation embryos or in adult tissues do not discriminate between a failure to gain a methylation imprint and a failure to maintain the imprint. Despite this, these results may indicate that the default state for the Air CpG island is absence of methylation. If so, this contrasts with other experiments (9) that examined transgenic founders as pre- and postimplantation embryos and showed that a multimerized 113-bp fragment from the core of the Air promoter attracts and maintains a maternal-allele-specific methylation imprint up to blastocyst stages. Results from these early-embryo founder transgenes indicated that methylation was the default state of the maternal allele and that the paternal allele was protected from methylation. Our results that examined adult founders, F1-F2 embryos, and adults containing low- to high-copy-number transgenes offer support for the opposite proposal—that absence of methylation is the default state and that the maternal allele actively acquires its methylation imprint.
Air is not imprinted in a single-promoter setting.
We have tested whether imprinting of Air requires stable Air expression with the REP reporter construct that contains the Air promoter on a 9-kb fragment in a single-promoter setting. This 9 kb is sufficient for Air to function as a promoter in an integration-independent manner but not enough for Air imprinting, as none of the 11 lines are imprinted. Considering seven additional transgenic lines ranging from 3 to 14 kb that contain the Air promoter but do not recapitulate the methylation imprint (39), we conclude that, as distinct from H19 and Snrpn (10, 12, 27), Air as a single expressed promoter in low- to high-copy-number transgenes is not sufficient to be imprinted.
Air can be imprinted in a dual-promoter setting.
The hypothesis that Air imprinting might need an additional CpG island promoter arose from an observation that 300-kb Air-Igf2r transgenes that were inadvertently derived with an inactive Igf2r promoter also lacked imprinting for both Air and Igf2r (39). Considering these transgenes, we tested whether Igf2r promoter activity would be sufficient for Air imprinting with the LAI and SAI lines that contain both the Air and Igf2r CpG island promoters. In addition, we tested whether any nonimprinted CpG island promoter could establish an imprinted configuration for Air with the RAA and FAA lines that contain the Air promoter coupled to the Aprt promoter. We chose the mouse Aprt gene, as the Aprt promoter is a CpG island with a broad expression profile and thus similar to the Igf2r promoter. In addition, transgenic studies have shown that Aprt is unmethylated as a transgene but can be methylated and silent under some circumstances (11, 18, 19). In a dual-promoter setting, Air imprinting was established in 3 of 19 lines (16%), indicating that a second CpG island promoter may facilitate Air imprinting. The frequency of Air imprinting when coupled to the Igf2r promoter (1 in 5 lines) is comparable to the situation when the Air promoter is coupled to the Aprt promoter (2 in 14 lines). This shows that Air on a 4.6-b fragment can be imprinted in the absence of the Igf2r promoter and also indicates that the Aprt promoter is as good at facilitating Air imprinting as an Igf2r promoter. Moreover, the orientation of the Air promoter with respect to the Aprt promoter does not influence the result, since one in five lines in which both promoters face each other (the RAA lines) and one in nine lines with both promoters in the same orientation (the FAA lines) are imprinted for Air. Imprinting of the RAA-14 line is stable with paternal transmission but less robust with maternal transmission, adding further support to the proposal that the maternal methylation is the limiting step in imprinting and that the paternal absence of methylation is the default state. Comparable imprinted behavior has also been observed for a 300-kb Igf2r-Air transgene that integrated on the X chromosome (39) and for some H19 transgenes (12, 14).
An additional point is that the three imprinted dual promoter lines generated in this study are all single copies (as shown by end fragment analysis ) (Table 1). No multicopy-imprinted Air transgenes were identified. The numbers of transgenes generated here are small but may indicate that two heterologous promoters in a single-copy setting may facilitate imprinting of short transgenes. This study and a previous study (39) did not generate any single-copy transgenes with Air as a single promoter (e.g., all REP transgenes were multicopy transgenes). It therefore remains a possibility that single copies of the Air promoter are permissive for imprinting in the absence of a dual-promoter setting. Based on a comparative analysis of 1 Mbp spanning the homologous human- and mouse-imprinted domains containing Igf2-H19 and many other imprinted genes, Onyango et al. (22) postulated the “two CpG island rule” for imprinted genes. They observed that imprinted genes are associated with more than one CpG island, whereas nonimprinted genes harbor one or no CpG island. The results here support this hypothesis.
A low level of background imprinting has been reported for randomly integrated transgenes (reviewed in Surani et al. [32]). Despite the low frequency of Air imprinting, even in a dual-promoter setting (16%), we consider this to recapitulate imprinting of the endogenous locus, since neither the 11 short single-promoter transgenes described here nor any of the 12 short transgenes containing all or part of the Air promoter previously described (39) are imprinted. However, compared to H19 transgenes from 10 to 18 kb which are imprinted with a frequency between 43 to 80%, depending on size and type of construct (12, 14, 23, 30), and Snrpn (exon 1 plus upstream sequences) transgenes of 1.2 kb, which are imprinted with an 85% frequency (27), the imprinted frequency for Air transgenes (16%) is relatively low. The basis of this difference is not clear. It may result from a difference in the mechanism that imprints Igf2r compared to that imprinting H19 and Snrpn. Alternatively, more-consistent imprinting of Air transgenes may demand additional elements besides the two CpG island promoters. These elements may include enhancers for either Igf2r or Air expression. Epigenetic regulation of access to shared enhancers has been shown to play an essential role in imprinting the Igf2-H19 gene pair (reviewed in reference 6). We argue, however, that the high level of Air expression in comparison to that of the endogenous Air locus shown by all 30 transgenes described in this study indicates that Air enhancers are not absent. Elements that influence the gain or maintenance of the maternal methylation imprint may also be missing. The LAI transgene that spans 44 kb of the endogenous Igf2r locus is imprinted in only one of three cases, whereas 300-kb transgenes are imprinted in four of four cases on three autosomal locations and one X-linked location (39). Thus, the missing sequences for reliable imprinting reside outside the 44 kb but within 300 kb. However, it also remains possible that the difference in transgene size is sufficient to shield Air from position effects that influence the gain or maintenance of the methylated state.
Silencing of linked promoters by region 2.
Imprinting of the Igf2r promoter depends on the presence of region 2 as deletion of the paternal region 2, including the unmethylated and expressed Air promoter, derepresses the paternal Igf2r promoter on transgenes (39) and in the endogenous locus (40). Because region 2 is present in all our transgenes, we investigated whether it is capable of silencing the linked Igf2r and Aprt promoters in the LAI-SAI and RAA-FAA lines. With line LAI-46, we have shown that imprinted expression of Air correlates with reciprocal expression of the linked Igf2r gene. Note that in contrast to the endogenous locus, the Air RNA in line LAI-46 is spliced. The functional significance of the absence of splicing at the endogenous locus is unclear, since at least for line LAI-46 splicing of Air is compatible with silencing of the linked Igf2r promoter. However, it remains unclear why in the LAI-313, LAI-48, and the SAI-119 lines an expressed unmethylated Air promoter does not silence the cis-linked Igf2r promoter as predicted by the expression-competition models proposed for sense-antisense pairs of imprinted genes (4). In the two cases of Air imprinting in an Aprt transgene, neither showed reciprocal effects on the cis-linked Aprt promoter. Although these numbers are too small for conclusions, they may indicate that not all CpG island promoters can be imprinted by region 2. In one Aprt transgene (line FAA-4), the linked Air and Aprt promoters have the same imprinted methylation and expression, in contrast to the endogenous locus, where Air and Igf2r are reciprocally expressed and methylated. Although the Air promoter in FAA-4 has imprinted Aprt, we argue that it has arisen from a mechanism different from that seen at the Air-Igf2r locus. A possible explanation is that the methylation imprint of the Air promoter has spread onto the Aprt promoter because the two CpG island promoters are closely located within a 2-kb fragment, due to the forward orientation of the region 2 insert in these FAA transgenes. Despite the fact that this transgene may not have recapitulated the mechanism that imprints Igf2r, it has generated an imprinted and paternally expressed Aprt transgene that rescues an Aprt-deficient phenotype in a paternal-allele-specific manner. This result indicates a possibility of imprinting other nonimprinted genes by proximity to the Air CpG island with potential application in creating parent-specific phenotypes.
ACKNOWLEDGMENTS
We thank Rene Bobeldijk, Karin van Veen, Karin van het Wout, and Paul Krimpenfort for generating the transgenic mice; Loes Rijswijk, Fina van der Ahé, Tania Maidment, and Nel Bosnie for care of the mice; Harry Vrieling, Leiden University, for Aprt-deficient mice and an Aprt genomic clone; Mitchell Turker, University of Oregon, for an Aprt genomic clone; Anton Berns and all members of H5 for discussion; and Hein te Riele and Piet Borst for reading the manuscript.
This research was supported by the Dutch Cancer Society (KWF).
REFERENCES
- 1.Ainscough J F, Koide T, Tada M, Barton S, Surani M A. Imprinting of Igf2 and H19 from a 130 kb YAC transgene. Development. 1997;124:3621–3632. doi: 10.1242/dev.124.18.3621. [DOI] [PubMed] [Google Scholar]
- 2.Akagi K, Sandig V, Vooijs M, Van der Valk M, Giovannini M, Strauss M, Berns A. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25:1766–1773. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antequera F, Bird A. CpG islands as genomic footprints of promoters that are associated with replication origins. Curr Biol. 1999;9:R661–R667. doi: 10.1016/s0960-9822(99)80418-7. [DOI] [PubMed] [Google Scholar]
- 4.Barlow D P. Competition—a common motif for the imprinting mechanism. EMBO J. 1997;16:6899–6905. doi: 10.1093/emboj/16.23.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow D P. Methylation and imprinting: from host defense to gene regulation. Science. 1993;260:309–310. doi: 10.1126/science.8469984. [DOI] [PubMed] [Google Scholar]
- 6.Bell A C, West A G, Felsenfeld G. Insulators and boundaries: versatile regulatory elements in the eukaryotic genome. Science. 2001;291:447–450. doi: 10.1126/science.291.5503.447. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Porath I, Cedar H. Imprinting: focusing on the center. Curr Opin Genet Dev. 2000;10:550–554. doi: 10.1016/s0959-437x(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 8.Bielinska B, Blaydes S M, Buiting K, Yang T, Krajewska-Walasek M, Horsthemke B, Brannan C I. De novo deletions of SNRPN exon 1 in early human and mouse embryos result in a paternal to maternal imprint switch. Nat Genet. 2000;25:74–78. doi: 10.1038/75629. [DOI] [PubMed] [Google Scholar]
- 9.Birger Y, Shemer R, Perk J, Razin A. The imprinting box of the mouse Igf2r gene. Nature. 1999;397:84–88. doi: 10.1038/16291. [DOI] [PubMed] [Google Scholar]
- 10.Blaydes S M, Elmore M, Yang T, Brannan C I. Analysis of murine Snrpn and human SNRPN gene imprinting in transgenic mice. Mamm Genome. 1999;10:549–555. doi: 10.1007/s003359901042. [DOI] [PubMed] [Google Scholar]
- 11.Brandeis M, Frank D, Keshet I, Siegfried Z, Mendelsohn M, Nemes A, Temper V, Razin A, Cedar H. Sp1 elements protect a CpG island from de novo methylation. Nature. 1994;371:435–438. doi: 10.1038/371435a0. [DOI] [PubMed] [Google Scholar]
- 12.Brenton J D, Drewell R A, Viville S, Hilton K J, Barton S C, Ainscough J F, Surani M A. A silencer element identified in Drosophila is required for imprinting of H19 reporter transgenes in mice. Proc Natl Acad Sci USA. 1999;96:9242–9247. doi: 10.1073/pnas.96.16.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspary T, Cleary M A, Baker C C, Guan X J, Tilghman S M. Multiple mechanisms regulate imprinting of the mouse distal chromosome 7 gene cluster. Mol Cell Biol. 1998;18:3466–3474. doi: 10.1128/mcb.18.6.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elson D A, Bartolomei M S. A 5′ differentially methylated sequence and the 3′-flanking region are necessary for H19 transgene imprinting. Mol Cell Biol. 1997;17:309–317. doi: 10.1128/mcb.17.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaffer C R, Srivastava M, Park K, Ives E, Hsieh S, Batlle J, Grinberg A, Huang S, Pfeifer K. A transcriptional insulator at the imprinted H19/Igf2 locus. Genes Dev. 2000;14:1908–1919. [PMC free article] [PubMed] [Google Scholar]
- 16.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 17.Lyle R, Watanabe D, te Vruchte D, Lerchner W, Smrzka O W, Wutz A, Schageman J, Hahner L, Davies C, Barlow D P. The imprinted antisense RNA at the Igf2r locus overlaps but does not imprint Mas1. Nat Genet. 2000;25:19–21. doi: 10.1038/75546. [DOI] [PubMed] [Google Scholar]
- 18.Macleod D, Charlton J, Mullins J, Bird A P. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 1994;8:2282–2292. doi: 10.1101/gad.8.19.2282. [DOI] [PubMed] [Google Scholar]
- 19.Mummaneni P, Walker K A, Bishop P L, Turker M S. Epigenetic gene inactivation induced by a cis-acting methylation center. J Biol Chem. 1995;270:788–792. doi: 10.1074/jbc.270.2.788. [DOI] [PubMed] [Google Scholar]
- 20.Neumann B, Kubicka P, Barlow D P. Characteristics of imprinted genes. Nat Genet. 1995;9:12–13. doi: 10.1038/ng0195-12. [DOI] [PubMed] [Google Scholar]
- 21.Ng H H, Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev. 1999;9:158–163. doi: 10.1016/s0959-437x(99)80024-0. [DOI] [PubMed] [Google Scholar]
- 22.Onyango P, Miller W, Lehoczky J, Leung C T, Birren B, Wheelan S, Dewar K, Feinberg A P. Sequence and comparative analysis of the mouse 1-megabase region orthologous to the human 11p15 imprinted domain. Genome Res. 2000;10:1697–1710. doi: 10.1101/gr.161800. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer K, Leighton P A, Tilghman S M. The structural H19 gene is required for transgene imprinting. Proc Natl Acad Sci USA. 1996;93:13876–13883. doi: 10.1073/pnas.93.24.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rea S, Eisenhaber F, O'Carroll D, Strahl B D, Sun Z W, Schmid M, Opravil S, Mechtler K, Ponting C P, Allis C D, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt J V, Levorse J M, Tilghman S M. Enhancer competition between H19 and Igf2 does not mediate their imprinting. Proc Natl Acad Sci USA. 1999;96:9733–9738. doi: 10.1073/pnas.96.17.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shemer R, Birger Y, Riggs A D, Razin A. Structure of the imprinted mouse Snrpn gene and establishment of its parental-specific methylation pattern. Proc Natl Acad Sci USA. 1997;94:10267–10272. doi: 10.1073/pnas.94.19.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shemer R, Hershko A Y, Perk J, Mostoslavsky R, Tsuberi B, Cedar H, Buiting K, Razin A. The imprinting box of the Prader-Willi/Angelman syndrome domain. Nat Genet. 2000;26:440–443. doi: 10.1038/82571. [DOI] [PubMed] [Google Scholar]
- 28.Sleutels F, Barlow D P, Lyle R. The uniqueness of the imprinting mechanism. Curr Opin Genet Dev. 2000;10:229–233. doi: 10.1016/s0959-437x(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava M, Hsieh S, Grinberg A, Williams-Simons L, Huang S P, Pfeifer K. H19 and Igf2 monoallelic expression is regulated in two distinct ways by a shared cis acting regulatory region upstream of H19. Genes Dev. 2000;14:1186–1195. [PMC free article] [PubMed] [Google Scholar]
- 30.Stadnick M P, Pieracci F M, Cranston M J, Taksel E, Thorvaldsen J L, Bartolomei M S. Role of a 461-bp G-rich repetitive element in H19 transgene imprinting. Dev Genes Evol. 1999;209:239–248. doi: 10.1007/s004270050248. [DOI] [PubMed] [Google Scholar]
- 31.Stöger R, Kubicka P, Liu C G, Kafri T, Razin A, Cedar H, Barlow D P. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993;73:61–71. doi: 10.1016/0092-8674(93)90160-r. [DOI] [PubMed] [Google Scholar]
- 32.Surani M A, Reik W, Allen N D. Transgenes as molecular probes for genomic imprinting. Trends Genet. 1988;4:59–62. doi: 10.1016/0168-9525(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 33.Szebenyi G, Rotwein P. The mouse insulin-like growth factor II/cation-independent mannose 6-phosphate (IGF-II/MPR) receptor gene: molecular cloning and genomic organization. Genomics. 1994;19:120–129. doi: 10.1006/geno.1994.1021. [DOI] [PubMed] [Google Scholar]
- 34.Thorvaldsen J L, Duran K L, Bartolomei M S. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 1998;12:3693–3702. doi: 10.1101/gad.12.23.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilghman S M. The sins of the fathers and mothers: genomic imprinting in mammalian development. Cell. 1999;96:185–193. doi: 10.1016/s0092-8674(00)80559-0. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay K D, Saam J R, Ingram R S, Tilghman S M, Bartolomei M S. A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nat Genet. 1995;9:407–413. doi: 10.1038/ng0495-407. [DOI] [PubMed] [Google Scholar]
- 37.Van Sloun P P, Wijnhoven S W, Kool H J, Slater R, Weeda G, van Zeeland A A, Lohman P H, Vrieling H. Determination of spontaneous loss of heterozygosity mutations in Aprt heterozygous mice. Nucleic Acids Res. 1998;26:4888–4894. doi: 10.1093/nar/26.21.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh C P, Bestor T H. Cytosine methylation and mammalian development. Genes Dev. 1999;13:26–34. doi: 10.1101/gad.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wutz A, Smrzka O W, Schweifer N, Schellander K, Wagner E F, Barlow D P. Imprinted expression of the Igf2r gene depends on an intronic CpG island. Nature. 1997;389:745–749. doi: 10.1038/39631. [DOI] [PubMed] [Google Scholar]
- 40.Wutz A, Theussl H C, Dausman J, Jaenisch R, Barlow D P, Wagner E F. Non-imprinted Igf2r expression decreases growth and rescues the Tme mutation in mice. Development. 2001;128:1881–1887. doi: 10.1242/dev.128.10.1881. [DOI] [PubMed] [Google Scholar]