Abstract
Recent estimates suggest that one in two sexually active individuals will acquire a sexually transmitted infection by age 25, an alarming statistic that amounts to over 1 million new infections per day worldwide. Vaccination against STIs is highly desirable for alleviating this global burden of disease. Vaginal immunization is a promising strategy to combat transmission via the vaginal mucosa. The vagina is typically considered a poor inductive site for common correlates of adaptive immunity. However, emerging evidence suggests that immune tolerance may be overcome by precisely engineered vaccination schemes that orchestrate cell-mediated immunity and establish tissue resident memory immune cells. In this review, we will discuss the unique immunological milieu of the vaginal mucosa and our current understanding of correlates of pathogenesis and protection for several common STIs. We then present a summary of recent vaginal vaccine studies and explore the role that mucosal adjuvants and delivery systems play in enhancing protection according to requisite features of immunity. Finally, we offer perspectives on the challenges and future directions of vaginal vaccine delivery, discussing remaining physiological barriers and innovative vaccine formulations that may overcome them.
Keywords: Vaginal vaccines, drug delivery, mucosal immunology, sexually transmitted infections, mucosal adjuvants
1. Introduction
The World Health Organization estimates that over 1 million sexually transmitted infections (STIs) are acquired worldwide each day. This immense burden of disease disproportionately effects women in low income countries and marginalized populations including sex workers and men who have sex with men. Complex socioeconomic challenges related to health services for STIs, including limited screening and prevention measures, lack of access to treatment, and social stigmatization, exacerbate health disparities among these vulnerable populations. Symptoms of inflammatory and ulcerative STIs can also increase the risk of coinfection with other mucosally transmitted pathogens, the consequence of which can result in serious reproductive complications or even death. As such, attention to these endemic infectious diseases, which claim millions of lives annually, must be consistently prioritized. Effective biomedical interventions are available for numerous STIs. Vaccines for human papillomavirus (HPV) and hepatitis B have been incorporated into routine vaccine programs worldwide and have prevented thousands of deaths from cervical cancer and chronic liver disease annually. Bacterial STIs – chlamydia, gonorrhoea, and syphilis – as well as one parasitic STI – trichomoniasis – are curable with existing antibiotic regimens. Nevertheless, asymptomatic infections with chlamydia and gonorrhoea are common and can result in serious complications such as infertility and adverse birth outcomes upon mother-to-child transmission. Due to recent increases in antibiotic resistance, preventive approaches are increasingly preferable to inhibit acquisition of bacterial infections. Antiviral drugs are also available to mediate, but not cure, disease progression of herpes simplex virus (HSV) and human immunodeficiency virus (HIV). However, current HIV antiretroviral (ARV) and pre-exposure prophylactic (PrEP) protocols require burdensome daily adherence regimens and are accompanied by adverse side effects. Vaccination strategies for STIs that act prophylactically or therapeutically are highly desirable and have potential to ameliorate morbidity and mortality associated with these viral infections worldwide.
Genital tissue is the predominant portal of entry for sexually transmitted pathogens, making mucosal immunity an important first level of defense. Urogenital, rectal, oral, and nasal vaccination are promising strategies to elicit protective immunity at genital sites of infection, and vice-versa, via the common mucosal immune system [1]. Unlike systemic vaccination, local immunogenic exposure can engender both proximal and distal mucosal tissue with innate signals that facilitate homing of adaptive immune cells primed by antigen in draining lymph nodes. Preferential leukocyte migration and residency in the exposed mucosa can more effectively clear infectious agents. Early comparison of mucosal immunization routes revealed that vaginal vaccination is capable of significantly increasing antigen-specific antibody secretion in the female genital tract, effectively neutralizing infectious organisms in the vagina [2]. Vaginal vaccination has since been studied for immunization against a number of STIs and has indeed proven effective at instilling protective populations of effector lymphocytes in cervicovaginal tissue. These findings are contrary to the traditionally held notion that the vagina is a poor inductive site for adaptive immunity, a discrepancy that is indicative of our overall lack of knowledge on basic mucosal immunology in the lower female reproductive tract (FRT). Indeed, further elucidation of the mechanisms by which immune tolerance is overcome in the genital mucosa to defend against incipient infections is needed in order to rationally design safe and effective vaginal vaccines. In addition to immunological advantages, vaginal mucosal vaccination has practical benefits over injectable methods. Needle-free vaccines offer superior patient comfort compared to intramuscular or subcutaneous delivery. Consequently, solid dosage modalities potentiate flexible formulation strategies that may enhance the functional capacity of administered active agents.
This review will describe the known features of innate and adaptive immunity in the vaginal mucosa and discuss the natural immune response to several relevant STIs, which have been the focus of numerous vaginal vaccine studies. We will highlight the correlates, or lack thereof, of mucosal immunity to establish design requirements for effective vaginal immunization. Informed by known or presumed correlates of immunity, we will evaluate recent vaginal vaccine studies in accordance with requisite criteria for protection, taking into consideration the use of mucosal adjuvants and advances in drug delivery systems designed to overcome physical barriers and the tolerogenic immunological milieu. This review will exclude discussion of STIs for which no vaginal vaccine studies have been attempted; however, this does not disqualify them as potential targets for vaginal vaccination. Prevention of STIs such as syphilis, trichomoniasis, bacterial vaginosis, and others may in fact benefit immensely from genital immunization and more consideration is warranted to determine the scientific premise of such investigation. Ultimately, innovations in vaginal delivery of vaccines may resolve gaps in our knowledge of vaginal immunity and address the enormous global burden of sexually transmitted infection.
2. Immunological milieu of the vaginal mucosa
The human vagina is a fibromuscular tube situated beneath the uterus that extends posterosuperiorly from the caudal end of the vulva to the cervix. Functionally, the vagina is a site of bidirectional transport, providing a channel for childbirth and menstruation as well as reception of seminal fluid. Due to its proximity to the exterior genitalia and contact with the external environment, the vaginal mucosa is a common site of infection and thus plays a dual role in defense against ascending pathogens and tolerance to both commensal bacteria and sperm. The vagina is lined by a non-keratinized stratified squamous epithelium that is covered apically by a layer of cornified cells that are permeable to microbes as well as cellular and molecular mediators of immunity [3]. This apical layer consists of flattened, mitotically-inactive cells containing large stores of cytoplasmic glycogen; it is void of both tight junctions and an impermeable intercellular lipid envelope, thus it permits transport of high molecular weight immunoglobulins [4]. The vaginal stratus corneum is frequently exfoliated along with luminal mucosal contents, clearing associated pathogenic infiltrate. This layer of the vaginal epithelium is host to a a proliferative population of commensal microbes that are important in defending against disease and maintaining healthy vaginal function. Beneath the cornified stratum are basal and suprabasal layers of epithelial cells which contain more extensive adhesions and exclusionary junctions extending into the underlying connective tissue. This type II mucosa within the lower genitourinary tract is rich in innate and adaptive immune cells, but lacks organized mucosa-associated lymphoid tissue (MALT) found in the type I mucosa of the upper FRT. As such, antigen presentation requires migration of mononuclear phagocytes through the epithelium and lamina propria to the draining lymph nodes (DLN), wherein naïve adaptive lymphocytes reside [5]. The upper two-thirds of the vaginal tract are drained by the Iliac lymph node, while the distal third is drained by the inguinal lymph node. The organizational and immunological differences between the upper and lower FRT are illustrated in Figure 1. Notably, the lack of MALT in the vaginal epithelium poses a barrier to initiating effective and lasting local immunity upon vaccination. Despite its unique immunological milieu, recent evidence confirms that the vagina is in fact an inductive site of cell-mediated immunity and inherent immunoregulatory barriers may be overcome to induce effective protection against infection.
Figure 1. Differences in the immunological milieu of the upper and lower female reproductive tract.
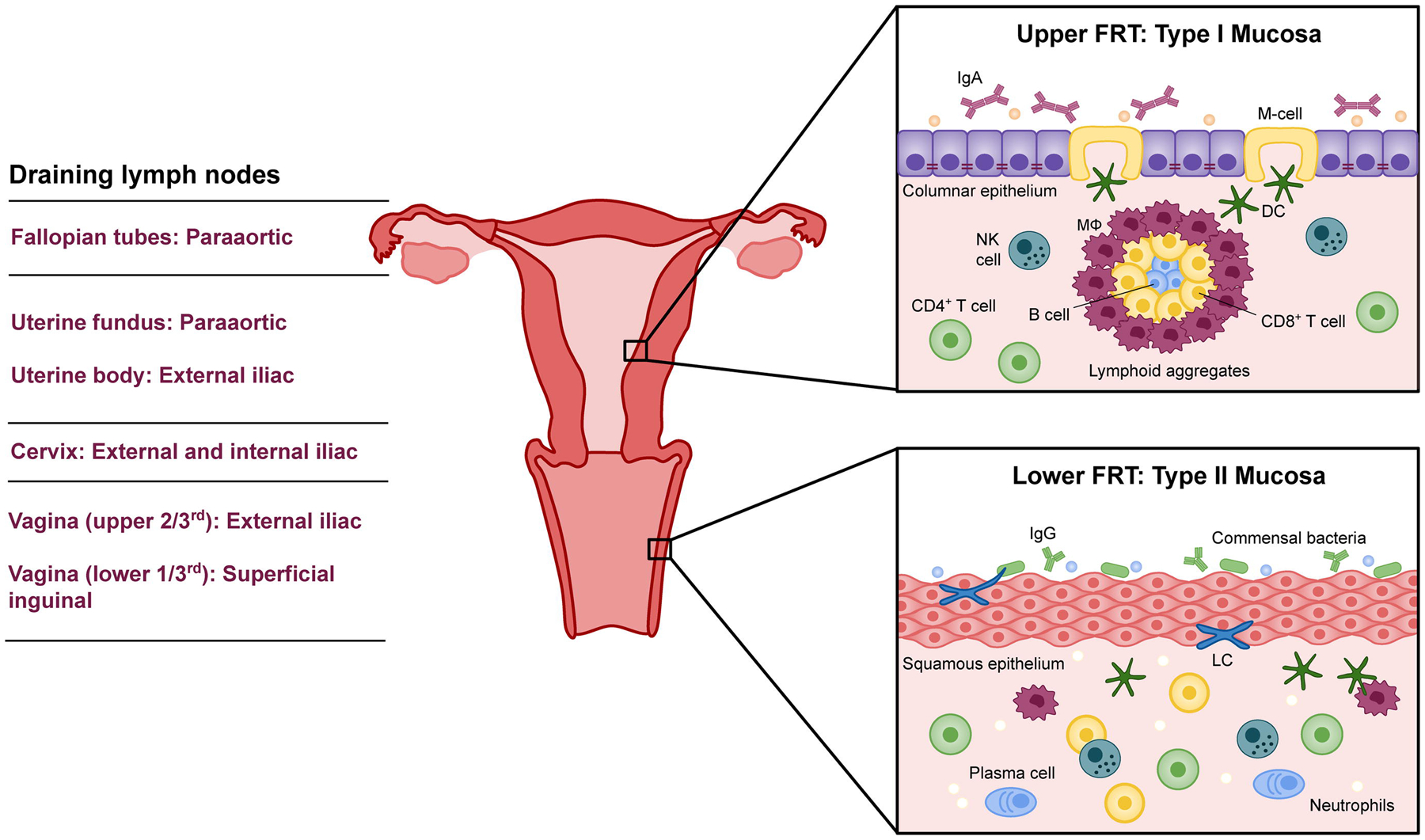
The upper FRT, comprised of the ovaries, fallopian tubes, uterus, and endocervix, is a sterile environment with type I mucosa. As such, it contains organized mucosa-associated lymphoid tissue (MALT) and exerts constitutive immune surveillance via intraepithelial M cells. IgA is the primary immunoglobulin secreted by the upper FRT, though IgG and IgM are also supplied. Antibodies and antimicrobial factors wash into the lower FRT, which is made up of type II mucosa. The ectocervix and vagina do not have MALT or M cells, but possess intraepithelial Langerhans cells which periodically patrol the vaginal lumen. A sequence of draining lymph nodes receive APCs from and prime adaptive immune cells for the FRT.
2.1. Innate immunity
Innate immunity within the vaginal mucosa is responsible for early and rapid clearance of pathogens by coordinating multiple nonspecific mechanisms of defense simultaneously to achieve protection. Elements of the innate immune system play a central role in orchestrating specific adaptive immune responses to prevent and combat recurrent infection with cognate antigen. The internal surface of the vagina is covered by a layer of mucus which is produced by secretory vaginal epithelial cells (VECs) and made up of high molecular weight glycoproteins called mucins. Vaginal mucus traps both harmless antigens and infectious microorganisms, serving as a physical barrier of protection for the underlying epithelium. Antimicrobial proteins produced by VECs, molecular immunological agents supplied by luminal secretions from the upper FRT, and the lectin and alternative compliment system are all innate immune components of the vaginal mucosa [6–9].
The innate immune system relies on genomically-encoded receptors, called pattern recognition receptors (PRRs), to recognize conserved pathogen-associated molecular patterns in a manner that is typically independent of prior or repeated exposure. Toll-like receptors (TLRs) are a group of PRRs expressed by epithelial cells, macrophages, dendritic cells (DCs), neutrophils, and natural killer (NK) cells. TLRs present in the vagina recognize and initiate signaling in response to a variety of pathogenic stimuli. The role of specific TLRs in detecting and responding to pathogens and their patterns of producing soluble immune factors has been reviewed in depth elsewhere [10,11]. Pro-inflammatory chemokines and cytokines secreted by TLRs are capable of mediating trans-epithelial leukocyte migration and are potent attractors of neutrophils [12]. Upon encountering incipient infection, neutrophils initiate swift bacterial clearance via phagocytosis, production of toxic oxidative compounds, release of microbicides by intracellular granules, and catalysis of leukotriene production to attract more neutrophils [8]. To counter this inflammatory milieu and support exogenous and commensal tolerance, VECs produce large amounts of immunosuppressive cytokines including TGFβ and IL-10 [13,14]. A number of regulatory leukocytes are also involved in dampening immunogenic responses in the lower FRT. The dynamics of soluble immune factors as they relate to coordinating inflammatory versus tolerogenic responses in the vaginal mucosa is illustrated in Figure 2. The homeostatic vagina is skewed towards immunosuppression, therefore the vaginal mucosa is typically considered an immune privileged site. Directing immunity away from this suppressive milieu is dependent on activating inflammatory PRR pathways present in cells of the vaginal mucosa, which are predominated by TLR2, 3, 5, and 6 [15]. This paradigm has significant consequences for vaginal vaccination, which will be discussed further throughout this review.
Figure 2. Soluble factors modulate immunogenic and tolerogenic responses in the vaginal mucosa.
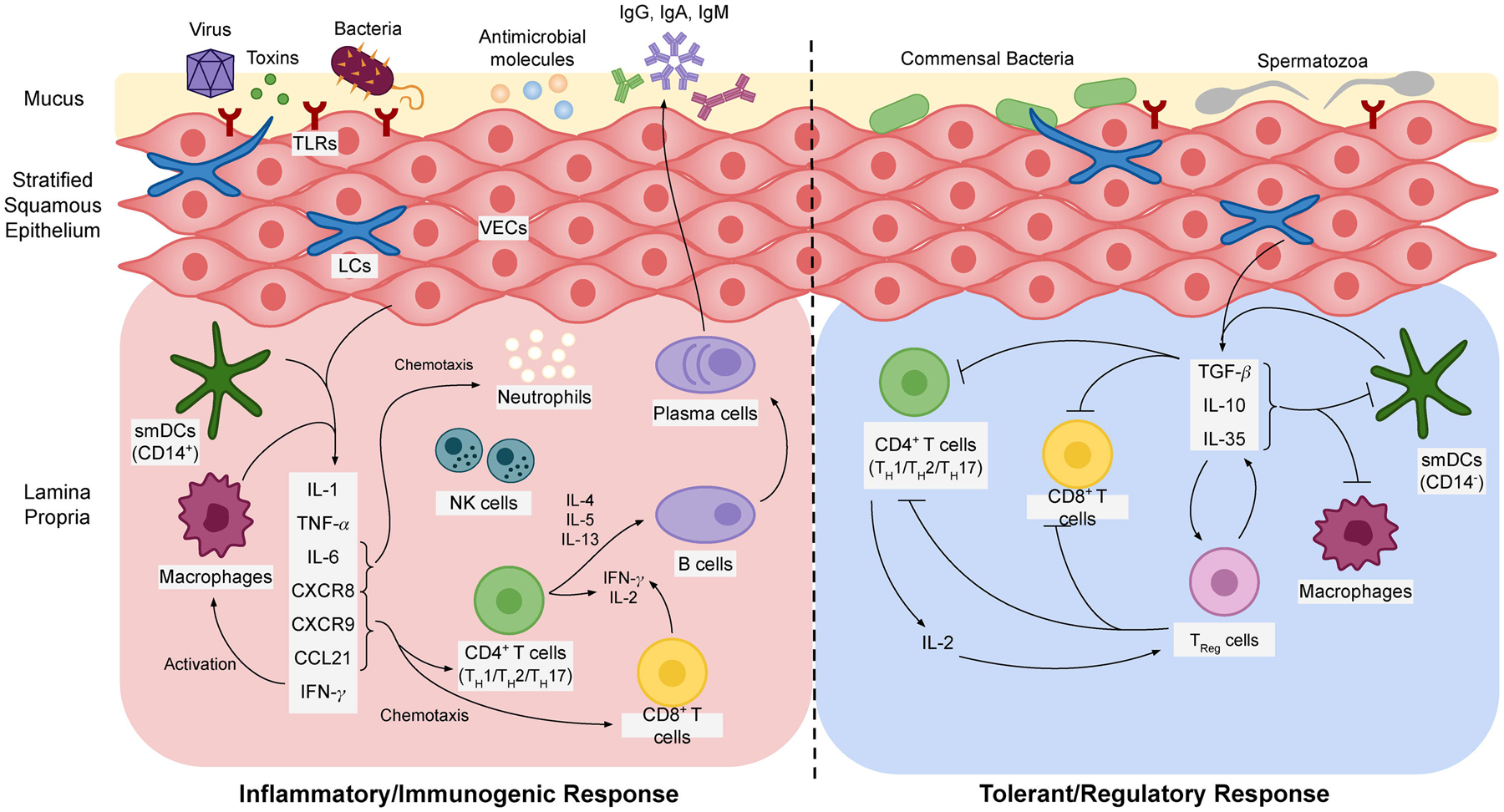
Inflammation or tolerance is largely determined by PRR signalling from specific APC subtypes. TLRs and C-type lectin receptors (CTR) on CD14+ smDCs and macrophages preferentially initiate release of several proinflammatory cytokines and chemokines with pleiotropic effects on immune cell recruitment and activation. On the other hand, VECs and cvLCs commonly patrol commensal bacteria and non-pathogenic infiltrate in the vaginal lumen and thus initiate tolerogenic signalling, primarily via release of TGF-β. Immunosuppressive cytokines inhibit antigen presentation and induce regulatory T-cell activation. TReg cells detect and suppress TH1, TH2, and TH17-type responses, inhibit CTL activity, and secrete additional cytokines to maintain a suppressive milieu.
The vaginal mucosa possesses a number of innate leukocytes that play a critical role in pathogen detection and generation of an adaptive immune response. Vaginal tissue resident antigen presenting cells (APCs) have been described thoroughly by Iijima et al. [16]. Of note, cervicovaginal Langerhans cells (cvLCs), which patrol the vaginal epithelium, have been shown to mediate HIV-1 uptake and degradation and inhibit viral T-cell infection [17]. As such, LCs are thought to provide a barrier to HIV-1 infection in the vaginal mucosa [18]. The vagina contains four distinct myeloid-derived APC subsets that differentially polarize CD4+ and CD8+ T-cell responses. Indeed, cvLCs and CD14− Lamina propria-dendritic cells (LP-DCs) preferentially induce T-helper type 2 (TH2) and TH22 responses, reflecting humoral and regulatory properties. CD14+ LP-DCs and Mϕs generate a TH1 response reflective of proinflammatory activity and T-cell mediated defense [19]. Additional innate lymphocytes such as NK cells and other granulocytes are widely distributed throughout the lamina propria, where they respond to infection by producing pro-inflammatory cytokines, promoting macrophage activation, and coordinating a cytotoxic T-cell response [20,21]. The effector functions of the innate immune system in the vaginal mucosa pose an formidable barrier to infections and coincidentally often impede vaginal vaccination attempts by generating a highly regulated immune environment. These barriers must be overcome by immunogens and complementary delivery systems in order to establish durable immunity.
2.2. Adaptive immunity
2.2.1. Induction of an adaptive immune response
Adaptive immunity in the cervicovaginal mucosa is responsible for antigen-specific responses to pathogens and encompasses activation of lymphocyte effector functions such as cytokine production, cytotoxicity, and antibody production. Protection is conferred by plasma cells, constituting humoral immunity, and T cells, which directly or indirectly control infection via cell-mediated immunity. Induction of an appropriate adaptive immune response begins with antigen-presenting cells in the vaginal mucosa processing antigen and travelling to the iliac and inguinal DLNs via afferent lymphatics. There, they stimulate expansion of naïve T and B cells, which in turn migrate back to the vaginal lamina propria via extravasation from adjacent blood vessels [22]. Certain DCs preferentially polarize specific T cell responses accordingly, as discussed previously (Section 2.1). CD3+ T cells are the predominant lymphocyte population in the vaginal lamina propria, of which the majority are CD8+ T cells of the effector phenotype [23]. The mucosa is populated to a lesser extent by CD4+ T cells and B cells, which represent the smallest proportion of CD45+ immune cells. Recruitment of these cell types is driven by VEC secretion of chemokines C-X-C motif ligand 9 (CXCL9) and CXCL10, and by cellular adhesion and selectin molecule upregulation [21,24]. Plasma cells also reside in the subepithelial tissue and, once induced by memory B cells, secrete immunoglobulins IgG, IgA, and IgM.
In addition to recruited circulating central memory (TCM) and effector memory (TEM) cells that infiltrate the lamina propria upon infection, tissue-resident memory CD4+ and CD8+ T (TRM) cells are instilled in mucosal tissue after primary infection and are able to respond rapidly to genital pathogens. A critical finding in defense of vaginal vaccination is that systemic vaccination is often insufficient at facilitating homing of effector lymphocytes into the vaginal mucosa in a timely manner to effectively combat incipient infection. Reynolds et al. showed that SIV-specific CD8+ lymphocytes generated upon vaginal inoculation were almost exclusively localized to vaginal tissue compared to other mucosal departments, and the inability to control natural infection is due to delayed lymphocyte infiltration that occurred after the time of peak viremia [25]. Vaginal inoculation caused significant systemic and local CD8+ T cell activation, suggesting that the vagina is not a poor inductive site incapable of generating immunity of sufficient amplitude, but naturally fails to do so in a timely manner. Therefore, establishing a population of CD8+ TRM cells that display potent viricidal activity is key to generating protective immunity in vaginal tissue [26]. Upon recognition of cognate antigens, vaginal CD8+ TRM cells commit swift cytotoxic activity and release IFN-γ to induce tissue inflammation and chemokine expression, thus initiating innate immune responses and recruiting circulating CD4+ T cells, which in turn facilitate entry of more effector lymphocytes [27]. It was previously thought that the residency of CD8+ T cells in the genital mucosa was established after primary T-cell differentiation of naïve lymphocytes in structured lymphoid organs. However, Wang et al. recently provided a new hypothesis in which the type-II vaginal mucosa itself acts as an inductive site for naïve CD8+ T-cell priming, characterized by antigen-specific activation and expansion following intravaginal vaccination in DLN-deficient mice [28]. In order to prove this theory, they first established that a naïve CD8+ T cell population is maintained in the vaginal lamina propria via thymic egress and replenished by lymphocyte circulation. They then adoptively transferred two aliquots of naïve CD8+ T cells with unique dyes both intravaginally (IVAG) and intravenously and used a lymphocyte circulation inhibitor (FTY720) to prevent infiltration of non-adoptively transferred naïve T cells into the vaginal mucosa. They found that only vaginally transferred cells were primed and activated in the vaginal mucosa after IVAG immunization, and that local priming occurred four hours faster than CD8+ T cell activation in the DLN. Finally, upon vaginal immunization with an HIV glycoprotein-specific adenoviral vector, they observed vaginal CD8+ T cell expansion in lymphocyte-ingress-restricted mice that was equivalent to FTY720-free mice. This study proves the existence of an entirely local T-cell priming and expansion mechanism, mediated by what is termed “inducible vaginal lymphoid tissue” (IVALT), that functions independently of efferent or afferent cell migration between the genital mucosa and the DLNs. These findings are substantiated by a study in lymph node-deficient and splenectomized mice that were vaccinated vaginally and demonstrated sterilizing protection against HSV [29]. This data provides a strong justification for vaginal immunization, wherein locally primed and activated CD8+ T cells, called cytotoxic lymphocytes (CTLs), may offer more swift and effective immune protection that does not rely on mucosal homing from systemic circulation or distal lymphatic sites. It is evident that establishing a population of local memory T cells in the vaginal mucosa is vital to protection against pathogens and thus a critical feature of effective vaccination efforts.
2.2.2. Functional activity of adaptive immunity
The adaptive immune system commits effector functions via two primary mechanisms: cellular-mediated immunity and humoral immunity. In the vaginal epithelium, T cell-mediated immunity (TH1) is critical for full protection against infections, particularly from viral pathogens such as HIV and HSV. Local effector T-cell function is orchestrated by activated CD4+ T cells, which coordinate CD8+ T cell mobility into restricted mucosal sites and cytotoxicity required for viral clearance [22]. Indeed, once granted entry or locally primed, effector CTLs eliminate cells infected with pathogen-presenting MHC class I molecules by targeted delivery of toxic effector molecules and recruit circulating T and B cells. Recently, Tan et al. showed that intravaginal delivery of recombinant influenza-HIV vectors resulted in durable instillation of CD8+ TRM cells in the vagina and caused a shift in T cell localization from the submucosa into the stratified epithelium [30]. These apically seeded TRM cells were able to recognize cognate antigen and respond rapidly, promoting inflammation and vascular cell adhesion molecule expression, which recruited peripheral CD8+ T cells, B cells, NK cells, and CD4+ T cells. This robust and durable response represents a strong premise for local vaccination as it facilitates rapid entry of a high volume of effector cell types. The genital mucosa also depends on local humoral (TH2) immunity to combat pathogens. Large-molecular-weight antibodies are permitted to infiltrate through the mucosa and into the vaginal lumen due to the lack of tight junctions in the apical layer of the stratified vaginal epithelium [4]. IgG is the primary immunoglobulin constituent of the vaginal type II mucosa, where it is produced by circulating plasma cells and trafficked across the genital tract epithelium by neonatal Fc receptor, FcRn, present on vaginal epithelial cells [31]. IgA and IgM are also present in the vaginal mucosa, though to a lesser extent than IgG. IgG has a functional role in coordinating opsonophagocytosis, neutralization, complement fixation, inhibition of epithelial transcytosis or cell-cell transmission, and antibody-dependent cellular cytotoxicity. As such, the induction of an antibody response is a key mechanism by which the adaptive immune system prevents viral or bacterial replication at mucosal surfaces. To that end, immunoglobulin activity in the vaginal mucosa is a central correlate of effective protection by vaccination.
2.3. Hormonal responsiveness
Nearly every aspect of mucosal immunity within the FRT is under strict hormonal regulation. In particular, estradiol (E2) and progesterone mediate immunity such that immunogenic and tolerogenic responses are complementary to reproductive function. Immunoregulation is modulated according to requisite physiological tasks, such as reception of sperm during the ovulatory phase and maintenance of an allogenic fetus during pregnancy. Hormonal dynamics throughout the menstrual cycle and their influence on vaginal physiology and immunity is depicted in Figure 3. Much of the focus regarding menstrual regulation of FRT immunology has been on the uterus. As such, hormonal responsiveness in the vaginal mucosa is largely understudied. However, a general consensus in the literature supports the hypothesis that estradiol performs a regulatory role in vaginal immunity, while progesterone, generally, results in an elevation of immunogenicity. Kaushic et al. reported an increase in macrophages, granulocytes, and dendritic cells in the subepithelial layers of rats at diestrus, when progesterone levels are high, compared to estrus [32]. Indeed, Wira and Rossoll found that antigen presentation in the vagina of ovariectomized rats was elevated when exogenous progesterone levels in the blood were high, an effect that could be reversed by administering estrogen [33]. These results were substantiated in vitro, where estradiol-treated primary VECs demonstrated attenuated production of antimicrobials HBD2 and Elafin as well as IL-8 in response to LPS [8]. Numerous innate immune mediators, including lactoferrin, SLPI, lysozyme, and secreted cytokines, have been shown to dramatically decrease at mid-cycle/ovulation (estradiol dominant) and increase during the secretory and menstrual phases (progesterone dominant) [34]. Humoral immunity also seems to be influenced by menstrual cyclicity. In a study of the effect of distal immunization on FRT antibody secretion in rats, antigen-specific vaginal IgG and IgA levels were markedly inhibited in animals treated with estradiol [35]. The reproductive significance of decreased immunogenicity during the ovulatory and secretory phases of the menstrual cycle, when E2 levels are high, may be explained by the expectation of spermatozoic reception during this stage of fertility.
Figure 3: Dominant menstrual hormones affect immunity in the vaginal mucosa.
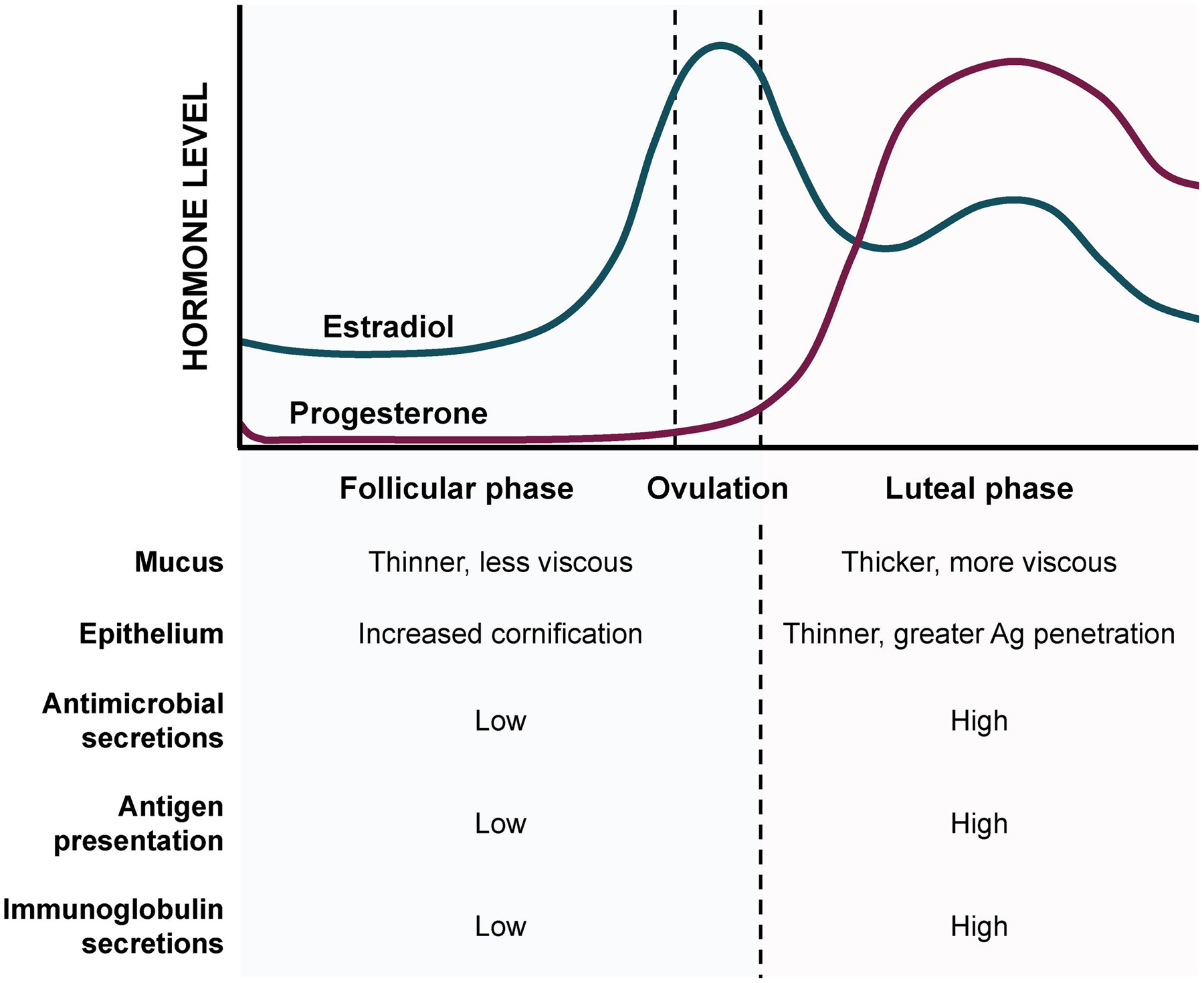
The follicular and preovulatory phases are dominated by estradiol, resulting in a general increase in immune tolerance. The luteal phase is dominated by progesterone, which causes significant thinning of the epithelium compensated by an increased immune secretory profile.
Conflicting reports comment on the influence progesterone and estradiol have on immune protection. Insofar as innate defense against pathogens, progesterone is a well-known mediator of vaginal epithelium thinning which is correlated with increased risk of HIV transmission in women [36,37]. This inducible susceptibility to immunogens is utilized intentionally in vaginal vaccine studies, wherein progesterone is usually administered to promote antigen penetration into the epithelium. In several studies, estradiol has been shown to improve immune protection against HIV, which is likely due to its role in enhancing epithelial integrity. This theory was confirmed in estrogen receptor Esr1-deficient mice, in which the vaginal epithelium lost its protective cornified layer and extensive epithelial lesions lead to a drastic influx of neutrophils and macrophages [38]. Estradiol has also been shown to preferentially generate a protective anti-viral TH17 CD4+ T-cell response to better combat HSV [39]. However, Gillgrass et al. showed that mice immunized intravaginally with HSV-2 antigen following estradiol treatment were not protected from vaginal challenge, whereas mice exposed to progesterone exhibited complete protection [40]. Estradiol appeared to prevent the formation of IVALT structures in vaginal tissue, aggregates which offered protection in effectively immunized mice. This effect may be correlated to E2-mediated inhibition of antigen presentation reported by Wira and Rossoll. The underlying mechanisms by which these menstrual hormones influence immunity in the vagina appear situationally-dependent and remain to be fully understood. It is evident that immunization attempts in the vaginal mucosa must take into account the stage of the menstrual cycle and influence of exogenous hormones upon antigen deposition in order to elicit an appropriate response that provides adequate protection.
3. Immunology and vaginal vaccination against viral sexually transmitted infections
Human immunodeficiency virus, genital herpes-simplex virus, and human papilloma virus are the three most common viral pathogens that infect the cervicovaginal mucosa, each contributing to a significant worldwide burden of disease. HIV-1 attacks the immune system, causing chronic lymphocyte depletion which leads to acquired immunodeficiency disease (AIDS), a disease which has claimed the lives of more than 32 million people since the epidemic began in the 1980s [41]. The prevalence of HSV-2 infection, which can cause incurable genital herpes, is currently rising in the US at an alarming rate and it is estimated that over 13% of the world population is currently living with HSV-2 [42]. HPV infection, while often asymptomatic, causes 90% of all cases of cervical cancer, which is the second leading cause of death among women worldwide [43]. The enormous epidemiological burden of these infections is met with significant efforts to develop prophylactic and therapeutic vaccines to prevent their transmission. Here, we discuss our current understanding of the invasion mechanisms and natural immune responses to these STIs in order to highlight requisite features of effective mucosal vaccination. While systemic vaccination typically relies on neutralizing antibodies and anatomically nonspecific localization of B cells to combat infection, this section will underscore the significance of generating resident memory T cells in vaginal tissue in order to better protect the genital mucosa.
3.1. Human papillomavirus
There are over 200 varieties of human papillomaviruses that infect humans via skin-to-skin or skin-to-mucosa contact, more than 40 of which are passed through sexual contact. HPV is the most common sexually transmitted infection resulting in a wide range of diseases, from benign lesions such as genital warts, to anogenital cancers, most commonly cervical cancer [44]. The incidence of cervical cancer in low- and middle-income countries remains alarmingly high, thus therapeutic vaccination is a highly desirable development goal. A comprehensive understanding of the mechanism of vaginal HPV infection and subsequent host immune response is critical to improving our understanding of how prophylactic vaccines currently in use prevent infection and for designing the next generation of therapeutic HPV vaccines.
3.1.1. Pathogenic infection and natural immune response
HPV is a small, non-enveloped DNA virus that can only productively infect terminally differentiated stratified squamous epithelial cells found near the basement membrane (BM) of the vaginal epithelium. Upon exposure to a chemically or mechanically traumatized epithelial surface, HPV accesses the BM and capsid proteins L1 and L2 bind to evolutionarily conserved heparan sulfate proteoglycan receptors [45]. DCs, LCs, macrophages, and B cells can bind to HPV and HPV virus-like particles (VLPs) via IgG receptor FcγRIII. Though these immunocytes are capable of internalizing virions, such interactions do not result in productive infection [46]. After binding to surface proteoglycans, the L2 site on the virion capsid is cleaved, exposing a region of the L1 biding site capable of binding to receptors on nearby epithelial cells, which internalize virus via an endocytic pathway 2–4 hours after cell surface attachment. This delayed period between virion adsorption and cellular entry is a notably large window in which the virus is highly susceptible to neutralization by antibodies, a unique characteristic that has been exploited by vaccination strategies [47]. During productive infection, expression of viral proteins E6 and E7 can alter epigenetic control over tumor suppressor genes in the host cell genome and promote cellular immortalization causing genital carcinomas [48]. Despite this oncogenic potential, the majority of HPV infections remain subclinical or asymptomatic as the virus remains dormant in non-replicating cells. The natural immune response clears HPV in an average of 8 months, benefiting from virucidal secretions from VECs, antibody production by plasma cells, and CD8+, MCH class I-restricted CTL-mediated killing of virus-infected host cells [48,49]. HPV is generally well-adapted to evade the mucosal immune system as its life cycle involves low levels of viral protein expression, low viremia, no inflammation, and suppression of interferon responses by E6 and E7 corresponding to malignant transformation [50]. As such, the primary correlates of natural immunity to HPV are specific T-cell proliferative responses and IL-2 release causing CTL activation [51].
3.1.2. Next generation design and delivery of HPV vaccines
HPV vaccines have been highly effective at decreasing HPV infection and related diseases worldwide. Three vaccines currently exist that together protect against nine types of HPV, including those that cause cervical cancer and condylomas. Gardasil, a quadrivalent HPV vaccine (Merck & Co., Kenilworth, NJ, USA), Cervarix, a bivalent HPV vaccine (GSK, Brentford, UK), and Gardasil 9 (Merck & Co., Kenilworth, NJ, USA) collectively offer protection against HPV6, 11, 16, 18, 31, 33, 45, 53, and 58. They cover 90% of HPV types that cause genital warts and 90% that cause cervical cancers [44]. These vaccines contain L1 virus-like particles (VLPs) that effectively generate a neutralizing antibody response in infected mucosa upon intramuscular administration. Because natural infection exhibits low viremia and poor access to the DLN, VLPs are weak immunogen candidates for vaginal administration. Intramuscular delivery grants direct antigenic access to the vasculature and lymphatics and is effective because humoral immunity is sufficient to protect against HPV infection. This is explained by the unique viral entry mechanisms described above in which HPV is not internalized by keratinocytes for at least 12–14 hours after exposure, providing Ig-rich serous exudate enough time to infiltrate the mucosa, bind to L1, and effectively neutralize the virus. If not for this distinct delay in internalization and viral integration, antibody-mediated defense alone may not be sufficient to prevent HPV infection. Though serum neutralizing antibodies are used to measure immune protection against HPV and proposed to play a functional role in viral inhibition, their mechanistic role as an immune correlate has not been verified [52]. Such identification would aid in generating next-generation HPV vaccines which will address remaining gaps, including targeting the L2 minor capsid protein that could provide broad cross-neutralizing antibody responses to HPV genotypes not covered by L1-VLPs [44]. Moreover, all current HPV vaccines are prophylactic and cannot treat existing genital lesions caused by latent HPV infection. This is of particular concern in the vagina, where, as discussed elsewhere, epithelial inflammation and trauma can increase susceptibility to other genitourinary infections. As such, there is increasing focus on therapeutic vaccines which are designed to leverage cell-mediated immunity to eliminate latently-infected cells. So, while systemic antibody-dependent prophylactic vaccines are highly effective in preventing HPV, vaginal vaccination may emerge as a strategy for therapeutic vaccination due to its ability to engender mucosal tissue with cytotoxic lymphocytes. However, VLP delivery strategies must account for the poor viremia and lymphatic access associated with HPV entry at genitourinary surfaces. Adjuvants that may stimulate LC activation and migration may enhance immunogenicity of vaginal HPV vaccines such that inherent mucosal immune tolerance is overcome.
Two studies in the past two decades have investigated vaginal HPV vaccination – both have advanced our understanding of vaginal vaccine delivery. Schreckenberger et al. injected plasmid DNA expressing HPV 6bL1 protein vaginally in rabbits with cholera toxin (CT), comparing intramuscular and rectal routes as well [53]. This study reported long lasting IgA in vaginal secretions with neutralizing activity against HPV-VLPs after vaginal immunization, but not rectal or intramuscular. This strategy may effectively prevent genital HPV-6 transmission, however, such immunity is currently provided by Gardasil and thus offers little clinical impact. However, of note is the fact that this is one of the first studies to test vaginal DNA vaccination in rabbits, which do not have an estrous cycle. Compared to vaginal DNA vaccination in cycling mice, the rabbit model used here showed stronger and more consistent immunity and represents an effective experimental method to control for confounding effects of endocrine influences on the vaginal immune system. Correcting for menstrual cyclicity may be useful for proof-of-concept studies on vaccine efficacy, but requires consideration for translation to humans where hormones might not be easily controlled. Subsequently, Park et al. studied vaginal immunization with HPV 16 L1 VLPs administered to mice with cholera toxin in a thermosensitive poloxamer (Pol) [54]. Intravaginal instillation of the Pol-CT vaccine resulted in high serum IgG and vaginal and salivary IgA secretions. Once again, this study has less clinical significance as it does investigational impact. Park et al. demonstrated significantly enhanced antibody responses using the in situ gelling poloxomer, which they optimized using variable polyethylene oxide content to achieve a syringeable, mucoadhesive, and appropriately thermosensitive formulation containing both antigen and adjuvant. The improved immunogenicity is likely the result of prolonged residency and penetration of active agents in the mucosa, a feature of many vaginal vaccine drug delivery systems highlighted in Table 1.
Table 1:
Delivery systems studied for vaginal vaccination
| Dosage form | Description | Vaccine type/Antigen | Adjuvanting activity | Features | Source |
|---|---|---|---|---|---|
| Vaginal ring | Poly(ethylene-co-vinyl acetate) (EVAc) | Protein Ferritin | No | Controlled release, retention time, antigen stability | [79] |
| Silicone elastomer + ag-loaded hydropropylmethylcellulose inserts | Protein BSA | No | Controlled release, retention time, formulation versatility, antigen stability | [80] | |
| Silicone elastomer + ag-loaded hydroxymethylcellulose inserts | Protein HIV-1 CN54 gp140 | No | [74] | ||
| Vaginal disk | EVAc | Plasmid DNA Sperm enzyme | No | Controlled release, retention time, antigen stability | [81] |
| EVAc | Protein Ovalbumin (OVA) | No | [82] | ||
| Nanoparticles/nanocomplexes | Anionic PEG amino nanocomplexes | Adenoviral vector HIV gag p24 | Yes: TAT peptide coating | Vaginal retention, controlled release, antigen stability, mucus penetration, cellular targeting, formulation versatility | [84] |
| Calcium phosphate (CAP) nanoparticles | Protein HSV-2 | Yes: CAP | [109] | ||
| Polystyrene nanospheres | Inactivated virus HIV-1 | Yes: Concavalin-A lecton | [83] | ||
| Gel | Poly-acrylic acid (Carbopol) gel | Protein HIV-1 CN54 gp140 | No | Vaginal retention, antigen stability, formulation versatility | [73,76,85] |
| Hydroxyethylcellulose (HEC) gel | Protein HIV-1 CN54 gp140 | No | Vaginal retention, mucoadhesion, antigen stability, formulation versatility | [87] | |
| Thermosensitive poloxamer | Protein HIV-1 CN54 gp140 | No | [54,88] | ||
| Liposome-loaded HEC gelling rods | Protein HIV-1 CN54 gp140 | No | [86] | ||
| Mucojet | Needle-free jet injector | Plasmid DNA OVA | No | Vaginal coating and retention, mucus penetration, antigen stability, formulation versatility | [89] |
| Needle-free jet injector + stearoyl (STR) peptide carrier | Plasmid DNA OVA | Yes: STR carrier | Vaginal coating and retention, mucus penetration, formulation versatility, antigen stability, cellular targeting | [90] | |
| Microneedle Array | Liposome-loaded MA | Protein HSV-2 gD | Yes: mannosylated/ stealth lipid A-coating + ammonium bicarbonate | Vaginal retention, mucus penetration, formulation versatility, antigen stability, cellular targeting, immune response modulation | [110] |
3.2. Human immunodeficiency virus
Despite advances in testing, transmission prevention, and treatment to achieve viral suppression, annual HIV incidence remains several times larger than global development goals. Developing an HIV vaccine is a highly sought after research aim that is considered the most likely mechanism by which to end the HIV pandemic. Preventing vaginal HIV transmission would have enormous implications for women and children, particularly in low-and-middle income regions and countries experiencing troubling surges in drug-resistant viral strains.
3.2.1. Pathogenic infection and natural immune response
HIV-1 is a lentivirus composed of two copies of single-stranded RNA enclosed by a capsid containing viral protein p24 and glycoproteins gp120 and gp41. HIV transmission occurs primarily at mucosal surfaces throughout the body, with vaginal transmission being the most common route of infection for adult women. Primary HIV infection was previously thought to occur only in the endocervix, which is lined by a vulnerable single layer of cuboidal epithelial cells. However, hysterectomized women and macaques lacking a cervix can be infected with HIV and simian immunodeficiency virus (SIV), respectively; thus it is evident that HIV can and does penetrate the stratified squamous epithelium of the vagina, though it does so relatively inefficiently [36]. Estimates suggest that heterosexual intercourse results in transmission in only 0.1% of exposures, and transmission is highly correlated with viral load in seminal fluid [55]. Much of this inefficiency is thought to be the result of innate physical barriers such as the presence of multiple cell layers in the vaginal epithelium which form a formidable barrier to accessing the lamina propria, wherein cells targeted for infection reside. This effect is enhanced during the follicular and ovulatory stages of the menstrual cycle, when a decrease in progesterone drives epithelial thickening [56,57]. Additionally, cervicovaginal mucus has been shown to impair the mobility of HIV molecules [58].
Despite these innate mechanisms of defense, there are numerous probable routes of transmission across an intact vaginal epithelium. Langerhans cells (LCs), which patrol the cervicovaginal mucosa, have dendritic cytoplasmic processes that extend into the vaginal lumen and sample antigens. Though they do not express CD4 or CCR5, LCs express surface HLA-DR, CD1a, and mannose-dependent C-type lectin receptors that may be involved in DC-mediated HIV transmission [59,60]. Upon capture, immature DCs in the epithelium migrate and present virus to CD4+CCR5+ T cells abundant in the lamina propria, thus permitting viral amplification. The precise dynamics of this antigen-presentation cascade are unclear, though it is thought to occur rapidly and results in transport of virus to underlying cells in a matter of minutes. Emerging evidence also points to direct primary infection of CD4+CCR5+ T cells as a mechanism of HIV transmission, wherein viral particles can access T cells typically residing in deeper layers of tissue via tears, inflammation, and hormone-induced thinning of the stratified epithelium. Coinfection with other STD’s can also increase susceptibility to vaginal HIV infection through epithelial weakening and immunogenic T cell recruitment [36]. Upon entry into the lamina propria via LC-mediated transport or direct contact, HIV enters CD4+CCR5+ target cells and generates a “nidus” of infection, infecting more and more recruited cells which eventually travel to secondary lymphatic tissue and systemic circulation. Thus, the vaginal mucosa serves as an effective portal of entry for systemic HIV infection, a process which can be exacerbated by adverse coincidental conditions of the mucosal microenvironment.
Depletion of CD4+ T cells is a primary hallmark of early HIV infection, as these cells express HIV-receptor CCR5 and are transcriptionally active. CD8+ T cells play a vital role in controlling HIV/SIV replication at mucosal surfaces early in infection, even more so than antibodies, which are produced later. This is evidenced by a study of vaginal HIV challenge where HIV-specific CTLs were identified in cervical cytobrush samples of women who remained seronegative after repeated exposure to HIV [61]. Memory CD8+ T cells play a central role in long term immunity owing to their unique ability to rapidly expand and re-express cytotoxic proteins upon restimulation by a cognate antigen [62]. Despite the potentially protective role of CTLs, a protective CTL phenotype has yet to be described. An impaired and ultimately ineffective natural CD8+ T cell response is likely due to the depletion of CD4+ T cell populations as infection becomes chronic. In the absence of programming from TH1-type CD4+ T cells and the IL-2 they generate, virus-specific CD8+ T cells enter a transcriptional state of exhaustion and exhibit a marked loss of function and cytotoxicity [62]. CD8+ T cells also require signaling from privileged CD4+ T cell subtypes in order to access restricted vaginal tissue during infection [63]. Indeed, Çuburu et al. showed that immunization of CD4+ T cell-deplete mice abrogated primary CD8+ T cell response in cervicovaginal tissue and only central memory CTLs were detected, resulting in increased viral susceptibility [64]. As such, despite CD4+ T cell depletion, this emerging evidence suggests that directing a precise CD4+ T cell response which can facilitate CD8+ T cell functionality in vaginal tissue, termed a “prime-pull” approach, may be a necessary component of controlling viremia [65]. It would be ideal to limit the population of activated CD4+ T cells to helper cells that are required to permit establishment of a local memory CD8+ T cell population, so as to not exacerbate the target cell population beyond a requisite level.
A strong mucosal humoral response is an important complement to cell-mediated protection against HIV. Primary HIV/SIV infection is marked by extreme follicular hyperplasia as B cells in secondary lymphoid tissues become activated and expand; however, as infection becomes chronic, this initial activity is replaced by lymphoid depletion caused by follicular fibrosis and involution, culminating in destruction of germinal centers [66]. B cell and T follicular helper (Tfh) cell impairment is consistent with a weak or ineffective neutralizing antibody response to HIV/SIV infection. This suggests another role for CD4+ T cells – cytolytic CD4+ T cells with effector functions that defend critical populations of Tfh cells can promote B cell expansion and facilitate a productive antibody response [67]. Vaccination strategies that ultimately generate binding, but not neutralizing, antibodies show protective promise in multiple human and macaque trials [68–71]. These antibodies function via antibody-dependent cellular cytotoxicity (ADCC), a process in which the IgG Fab fragment forms a complex with a viral cell surface protein, and the Ag Fc fragment binds to Fc receptors on effector cells, which include NK cells, macrophages, dendritic cells, γδ T cells, and neutrophils [36]. Owing to the abundance of locally-generated and transported IgG in the vaginal mucosa, eliciting an ADCC-mediated response via vaginal vaccination may be an effective correlate of local immunity to HIV. Ultimately, absolute correlates of mucosal immunity to HIV are undefined, which remains a major barrier to evaluating vaginal vaccines.
3.2.2. Vaginal vaccines for HIV
Despite the enormous global burden of HIV, no vaccines are currently available to prevent against its infection or associated disease. Significant research effort has gone into investigating an effective prophylactic vaccine that elicits a durable and protective immune response both systemically and in target mucosa. Intravaginal vaccination is a promising administration route to prevent HIV transmission. Protective mucosal immunity likely correlates with induction of a CD8+ T cell response by TRM cell populations coupled with antibody-dependent cellular cytotoxicity mediated by IgG and IgA, as discussed in section 3.2.1. Several studies in animals over the past 20 years have investigated vaginal HIV vaccination (Table 2). Comparison of various mucosal vaccination routes, including vaginal, for HIV immunization was reviewed in full by Yu and Vajdy [72]. Therefore, this review will focus on the outcomes of vaginal vaccination with limited comparison to alternate routes. Subunit vaccines have achieved vaginal inoculation of HIV-1 CN54 gp140 instilled in gel, with results ranging from modest systemic and mucosal antibody responses in rabbits to a large population of antigen-specific B cells in the DLN correlated with high IgG and IgA titers in the vaginal mucosa of sheep [73,74].
Table 2:
Summary of studies of vaginal HIV vaccination in animals
| Vaccine type | Animal model | Delivery modality | Prime/boost (routes) | Adjuvant | Systemic Response | Mucosal Response(a) | Remarks on efficacy | Source |
|---|---|---|---|---|---|---|---|---|
| Multicomponent peptide: HIV PND, HGP-30, CD4 binding site | Mice | Wax cylinder | Vaginal | IL-12, IL-4, CM-CSF, Cholera Toxin | High splenic CTL with CT; high serum IgG (IgG1) with IL-4 and GM-CSF | High sIgA with inhibitory activity against HIV gp’s; high IL-4 and IFN-γ producing intestinal and Peyer’s patch LC’s | Neutralizing effects suggest Ab titers are high enough to inhibit HIV infection. CT enhances cell-mediated immunity. | [130] |
| Viral vector: Sindbis virus replicon particles - HIV-1 gag | Mice | Solution | Vaginal | n/a | Lower CD8+ T cells in ILN compared to systemic routes | Lower CD8+ T cells and IFN-γ compared to systemic routes | Ivag vacc. prevented vaccina replication in ovaries | [146] |
| Inactivated virus: HIV-1 IIIB | Mice | Polystyrene nanosphere suspension | Vaginal | Concanavalin A | Not reported | No IgG, high IgA | Con-A NS enhanced immunogenicity of HIV-1 ag and produced secretory Abs with neutralizing activity against homologous HIV strains | [83] |
| Plasmid DNA: HIV env and rev | Mice | Wax cylinders | Vaginal | CM-CSF or IL-12 | High serum IgG1 with GM-CSF | High sIgA | CM-CSF enhanced Ab titers more than IL-12 and favors a TH2 response associated with ADCC | [131] |
| Viral vectors: Sindbis/Venezuelan equine encephalitis replicon particle - HIV-1 gag p55 | Mice | Solution | Vaginal | n/a | Low splenic and ILN CD8+ T cells; high HIV-gag-expressing cells in ILN | High IFN-γ homing receptor-expressing cells | Mucosal vacc. resulted in higher cellular responses and protective immunity after ivag challenge | [147] |
| Bacterial and adenoviral vectors: L. monocytogenes - HIV gag and rAd5 - HIV gag | Mice | Solution | Vaginal (L.mono)/ vaginal or i.m. (rAd5) | n/a | High splenic gag-specific CD8+ T cells after systemic boost; high IFN-γ expression | Gag-specific CD8+ T cells in lamina propria after ivag boost ; high cytolytic activity; TRM and protective TEM pop. | Mucosal boost with adenovirus significantly enhanced strength and duration of vaginal response | [148] |
| Protein: HIV-1 CN54 gp140 | Rabbits | Hydroxyethylcellulose rheologically structured vehicle (RSV) gel | Vaginal (9×) | n/a | Modest IgG, no IgA | IgG and IgA detected | Gel enhanced exposure of antigen to mucosa but resulted in modest antibody response | [87] |
| Protein: HIV-1 clade C gp140 | Rabbits | Poly-acrylic acid (Carbopol) gel | Vaginal (9×) | n/a | Modest IgG (enhanced with boosters), no IgA | High IgG (enhanced with boosters), no IgA | Serum and mucosal Abs neutralized heterologous gp-expressing pseudovirus | [73] |
| Protein: HIV-1 CN54 clade C gp140 | Macaques | Carbopol gel | Vaginal (9×) (some with single i.m. prime or boost) | n/a | IgG and IgA detected | IgG and IgA detected | Greater Ab response and neutralizing activity with i.m. boost or prime | [85] |
| Viral vector: recombinant HPV-SIV gag | Macaques | Solution (carboxymethyl cellulose) | Vaginal | Noxinol-9 | High gag-specific CD4+ TCM cells; high CD8+ TEM cells; high serum IgG; low serum IgA; gag-specific IFN-γ | Modest IgA; gag-specific CD4+ and CD8+ T cells at vaccination and 2 wks post infection | HPV PsVs infection enhanced by epithelial disruption (N9 or cytobrush) | [149] |
| Adenoviral vector: Helper-dependent Ad (HD-Ad) - HIV-1 gp140CF | Macaques | Solution or cervical injection | Intranasal/vaginal or i.m | n/a | Weaker CD8+ T cell response in PBMC than i.m.; high IL-10 and IL-12 | Stronger local CD8+ T cell response than i.m.; stronger CD4+ TCM response in colon | Ivag vacc. controlled viral replication and plasma viremia in rectal SHIV challenge better than i.m. | [150] |
| Viral vector: Recombinant influenza - HIV gp160 and gag192–208 | Mice | Solution | Vaginal | n/a | High splenic gp160-specific CD8+ T cells, modest serum IgG, poor gag-specific T cells response | High gp160-specific CD8+ T cells in ILN, poor gag-specific T cell response | Vaccine prevented ovarian infection after viral challenge. Pre-existing vector immunity restricted antigenicity and limited immune response | [151] |
| Viral vector: Recombinant influenza - HIV gag p24 | Mice | Solution | Intranasal/vaginal | n.a | Gag-specific CD8+ T cells | High CD8+ T cells in vagina and lung; CD8+ TRM pop. in vaginal epithelium > lamina propria | Ivag boost resulted in gag-specific TRM residence 30 days after vacc. CD8+ T cells reactivated by cognate peptide caused T cell, B cell, and NK cell infiltration | [30] |
| Protein: recombinant HIV-1 CN54 gp140 | Sheep | Vaginal ring: silicone elastomer with ag-loaded HPMC inserts | I.m. or vaginal/vaginal | R848 (TLR8/7 stimulant) | High serum IgG; serum IgA in ivag prime only; low splenic/peripheral ag-specific LC’s | High IgG and IgA (30× greater than systemic); high ag-specific B cells in DLN | R848 significantly enhanced systemic and mucosal humoral and cellular responses | [74] |
| Adenoviral vector: rAd5-HIV gag p24 | Mice | Anionic PEG amino (APS) nanocomplexes | Vaginal | TAT-peptide coating | Effective splenic CTL function; elevated IFN-γ and IL-4 in serum | Highest IgG and IgA with ivag nanocomplexes | PEG-TAT-coated rAd was more immunogenic than uni- or uncomplexed rAd | [84] |
Unless otherwise stated, mucosal antibody or cellular response refers to that detected in vaginal secretions or tissue
The immunogenicity of vaginal administration of HIV subunit vaccines in humans was subject to multiple clinical trials in the past two decades. In 2008, a phase 1 clinical trial tested vaginal and nasal immunization with recombinant HIV-1 gp160MN/LAI protein with or without DC-Cholesterol as an adjuvant [75]. After three doses, neither vaccination routes resulted in significant antibody response or anti-gp160 activity, suggesting insufficient immunogenicity of this antigen-adjuvant combination. Another phase 1 randomized controlled trial was conducted in 2011 in which women were given nine doses over three weeks (one menstrual cycle) of an HIV-1 CN54 gp140 subunit vaccine administered in Carbopol gel [76]. Serum and vaginal secretions showed no sustained IgG, IgA, or T cell responses. Though no adverse side effects were reported, this study is once again reflective of the weak immunogenicity of this vaccine type without adjuvant when administered intravaginally. This trial was followed up in 2014 with the same vaccine plus the addition of a 70 KDa heat shock protein adjuvant [77]. The primary objective of the study was to evaluate safety of this adjuvant after three immunizations. Generally, the vaccine was well tolerated with only mild-to-moderate and rapidly resolved adverse side effects reported. Promising secondary outcomes evaluating efficacy lend significance to this trial. Vaccination resulted in peripheral blood mononuclear cell (PBMC) upregulation of CC-class chemokines that induced downregulation of CCR5 on CD4+ T cells, indicating functional protection from CCR5-mediated HIV infection. Significant upregulation of the protective innate antiviral restriction factor, APOBEC3G, was also observed on CD4+ T cells in addition to overall PBMC, CD4+, and CD8+ T cell proliferation. An increase in serum IL-2 was measured in the majority of blood samples, indicating a TH1 CD4+ T cell response which may contribute to protection against HIV. Indeed, post-immunized PBMCs cultured ex vivo displayed significant inhibition of HIV-1 replication in CD4+ T cells. This trial shows immense promise for this vaccination protocol to protect women against HIV infection. The inverse correlation between CCR5 expression and PBMC and splenic CD4+ and CD8+ T cell proliferation indicates a protective response, in which adaptive cellular immunity proceeded while target cell availability was effectively suppressed. Though an important finding, this result would be more significant if it were seen in the vaginal lamina propria, where sterilizing immunity to HIV may be the effect of cell-mediated viral clearance in the absence of infectible CCR5+CD4+ T cells. The study reports low humoral responses in cervicovaginal secretions and does not report a mucosal cellular response. In order to induce protective mucosal immunity against HIV, cellular responses and ADCC must be directed to vaginal tissue, the site of infection. More extensive characterization of local immune responses in vaginal tissue is necessary to predict immunity as evaluating efficacy based on intravaginal HIV challenge is not feasible in human participants. The most recent clinical trial testing vaginal HIV vaccination compared intravaginal, intramuscular, and intranasal administration routes using the same HIV-1 CN54 gp140 vaccine without adjuvant [78]. This study showed very low systemic and mucosal IgG in one participant and no systemic or mucosal IgA or T cell responses after two vaginal vaccine doses. The primary difference between this trial and the successful 2014 trial is the incorporation of heat-shock protein in the latter, indicating the significance of administering a mucosal adjuvant to overcome protective barriers in the vaginal mucosa and stimulate immunity.
These clinical trials are limited for several reasons. Anatomical differences between species may explain the difficulty in translating promising results from animal studies (Table 2) to humans. Indeed, many small animals have a columnar single-cell layer epithelium throughout the cervico-vaginal tract, which is a less imposing barrier to antigen penetration than the human stratified squamous epithelium. Additionally, these studies enrolled small sample sizes ranging from 9 to 34 women, thus variability in volume and composition of biological samples may have significantly impacted the results of immunological assays. Lewis et al. reported low T cell numbers in cytobrush samples, complicating phenotype analysis and perhaps conflating reports of limited cellular activity with collection challenges. The added complication of menstrual cyclicity and/or exogenous hormones modulating vaginal immunity is difficult to control in human trials unlike animal studies. This challenge will be discussed in further detail in section 7. In sum, vaginal HIV vaccination shows promise, and investigating improved delivery or adjuvant strategies may yield successful local immunity.
3.2.3. Delivery systems for vaginal HIV vaccines
Several drug delivery systems for vaginal HIV vaccines have been attempted, primarily focused on addressing the challenge of leakage of free-flowing liquid vaccines from the vaginal cavity. Solid dosage modalities allow for extended residency of antigen in the vaginal mucosa. Vaginal rings and disks offer a convenient method of controlled protein and DNA vaccine release and have demonstrated efficacy in enhancing mucosal antibody responses to protein immunogens [74,79–82]. McKay et al. described a silicone elastomer vaginal ring containing three freeze-dried, rod-shaped hydroxypropylmethylcellulose (HPMC) inserts [74]. Each HPMC rod contained recombinant HIV-1 CN54gp140 protein with R848 as an adjuvant. Sheep immunized by three vaginal ring instillations, each one week in duration, exhibited mucosal antigen-specific IgG and IgA that were significantly greater than systemic levels and a high concentration of antigen-specific B cells in the DLN. This work demonstrated innovative biomaterial fabrication by incorporating bioactive molecules into modular components of a vaginal ring, which is typically limited in its ability to deliver biologics due to their poor stability, solubility, and diffusion through polymeric materials. The potential for codelivery of a topical antiretroviral presents a significant advantage of this strategy, as microbicides can provide protection during the vaccination window in which adjuvants may cause inflammation, possibly leaving the mucosa vulnerable to infection. Similar to vaginal rings, vaginal disks offer an alternative controlled delivery matrix that has been proven to effectively delivery protein vaccines and plasmid DNA to the vaginal mucosa [81,82]. These studies used poly(ethylene-co-vinyl acetate) loaded with model antigens to provide sustained release of DNA or protein into the vaginal mucosa for up to 60 days, resulting in high antigen-specific antibodies in vaginal exudate. The female genital tract inactivates DNA very easily and APCs patrol the vaginal mucosa at a frequency dependent on menstrual cyclicity. Thus, it is advantageous to control and sustain the release of plasmid DNA to increase the likelihood of cellular uptake. The tunability and versatility of solid dosage modalities potentiates their use for vaginal vaccination against any candidate STI, including HIV.
Nanoparticles (NPs) and nanocomplexes have also been investigated in several studies, and have the advantages of enhancing vaginal retention, mucus penetration, cellular targeting, and permitting versatile formulation approaches which may endow delivery vehicles with self-adjuvanting properties. Kawamura et al. conjugated concanavalin-A to polystyrene nanospheres for delivery of inactivated HIV-1, showing high anti-HIV-1 IgA in vaginal secretions after intravaginal vaccination with NPs compared to unformulated virus [83]. This study demonstrates efficient uptake of antigen and the ability to safely conjugate adjuvant to the polymer carrier. In order to investigate the role of NPs in generating cell-mediated immunity, Ji et al. developed anionic polyethylene glycol (PEG) amino nanocomplexes with a TAT-peptide coating to deliver HIV-gag protein expressing adenoviral vectors (rAd) to the vaginal mucosa (rAD-TAT-APS) [84]. The hydrophilic PEG derivative (APS) was shown to minimize hydrophobic entrapment in mucus, thereby enhancing penetration of encapsulated antigen into the epithelium. Moreover, the TAT-peptide coating facilitated transduction of epithelial cells with immunogenic cargo, offering efficient internalization by VECs and DCs and accessing intracellular antigen presentation pathways to a TH1-type response. Indeed, rAD-TAT-APS complexes resulted in significantly increased splenic populations IFN-γ-producing CD8+ T cells and IL-4-producing CD4+ T cells compared to unformulated antigen in intravaginally immunized mice. Further studies are warranted to investigate the establishment of resident T cell populations in vaginal tissue, however, this represents a promising method of enhancing delivery of genetic material for vaginal vaccination.
Numerous studies on vaginal vaccination have focused on gel-based approaches to solving the challenge of rapid clearance of liquid vaccines. Gels such as poly-acrylic-acid (Carbopol), hydroxyethylcellulose (HEC), thermosensitive poloxamer, and liposome-loaded HEC gelling rods have been studied for delivery of HIV subunit vaccines to enhance the duration of antigen exposure in the vagina [73,76,85–87]. Oh et al. developed a mucoadhesive in situ-gelling drug delivery system for vaginal codelivery of a subunit vaccine and a RANTES-expressing plasmid (pRANTES) adjuvant [88]. The temperature-sensitive poloxamer-based gel was designed to transition from a liquid at room temperature to a mucoadhesive gel at body temperature, offering prolonged mucosal exposure to antigen for over an hour after vaginal instillation. Administration of the hepatitis B surface antigen and pRANTES gel enhanced mucosal and systemic immune responses compared to unformulated vaccine/adjuvant and resulted in a durable antibody response 42 days after immunization. The use of a vaginal gel is highly amenable to encapsulation of a variety of antigens and adjuvants and is a promising advancement that parallels traditional suppository delivery systems in preparation and administration. For greater penetration than diffusion-based gel administration, Kanazawa et al. demonstrated the use of a jet injector for DNA vaccination in the vaginal mucosa [89,90]. Jet injectors function by delivering a liquid vaccine through a nozzle orifice at a high speed, effectively penetrating the stratus corneum and upper epithelial layers to increase antigenic uptake. Vaginal vaccination with the model plasmid ovalbumin antigen using the jet injector significantly enhanced vector expression and resulted in increased vaginal IgA secretion and IFN-γ expression compared to needle-syringe injection. A follow up study showed this effect could be enhanced by incorporating OVA into stearoyl peptide carrier and delivering with CpG-ODN; the addition of adjuvants increased serum and vaginal IgG compared to non-adjuvanted immunization. Improved immunity using the jet injector may be explained by its ability to better coat the vaginal mucosa with antigen; indeed, a wider distribution of pDNA solution within local tissue resulted in heightened plasmid uptake and expression. These approaches are also extensible to vaginal vaccination beyond HIV, and will likely significantly improve immunogenicity and multifunctionality of vaccine protocols.
3.3. Herpes simplex virus
Herpes simplex virus type 2 is a common sexually transmitted infection that primarily infects genital mucosal surfaces resulting in incurable genital herpes. This disease is particularly severe in neonates and immunocompromised individuals. In women, it causes ulcerative lesions at mucosal surfaces and can spread to the nervous system where it persists in ganglia and causes recurrent symptoms [91]. The importance of preventing and/or managing HSV-2 infection is two-fold: genital herpes contributes to significant worldwide morbidity and mortality, and the presence of resulting genital ulceration increases the risk of coincident STI transmission as it weakens epithelial barriers to viral entry [92,93].
3.3.1. Pathogenic infection and natural immune response
HSV-2 is a double stranded DNA virus encapsulated by a viral envelope containing 12 distinct glycoproteins which play a role in viral entry into vulvar epithelial cells. Infection begins with attachment of glycoproteins B and C (gB and gC) to heparan sulfate proteoglycans on host cells [94]. Cell entry occurs by direct fusion with the plasma membrane or by endocytosis [95]. After entering the cell, HSV-2 migrates to the cell nucleus and expresses structural viral proteins via a thymidine kinase (TK)-dependent pathway [94]. The virus then reassembles and replicated virions instigate acute primary infection. After the initial lytic infection and viral amplification in VECs, the virus rapidly migrates to sensory ganglia and enters a latent phase, evading host detection until reactivation by external stimuli to the neuron. Upon reactivation, the virus must egress the host neurons and travel in an anterograde fashion towards mucosal surfaces, where viral shedding and possible symptomatic disease progression reinitiates.
Over the course of HSV-2 infection, the virus encounters the host immune system at several locations that warrant understanding in order to guide development of therapeutic and prophylactic vaginal vaccines. It is impossible to study immune dynamics of neuronal interactions with HSV in humans and no immune correlate of protection has been established for any HSV induced disease in human or animal models. Here, we will focus on the dynamics of viral encounter with the vaginal epithelium, as this is most relevant to designing a vaginal vaccine and the most well characterized in humans. Like HIV, HSV-2 more efficiently infects the epithelium when cracks or abrasions exist in the protective stratus corneum. The first immune cells that interact with HSV are LCs in the vaginal mucosa that, along with VECs, express HSV receptors TLR-2 and TLR-9 [96]. Initial LC or VEC detection results in cytokine secretion, including IFN-α, IFN-β, IL-12, IL-6, and β-chemokines, which cause local inflammation and recruit effector monocytes [97]. β-chemokines also recruit T cells to the site of infection, where IL-12 entrains CD4+ T cells to a TH1 pattern that activates a strong cytotoxic response necessary for clearing virus. After antigen uptake in the vaginal epithelium, CD8+ DCs migrate to the DLN and present HSV antigens to CD8+ T cells, whereas CD11+ submucosal DCs stimulate CD4+ T cell activity [98]. Several studies show that the most effective adaptive immune response to HSV-2 is mediated by TH1 CD4+ T cells, which generate potent virus-specific IFN-γ capable of preventing lethal HSV-2 reinfection after vaccination in mice [99]. Cytotoxic T cells are another promising target for eliciting mucosal immunity to HSV; however, their functionality and residency must be considered. HSV suppresses MHC class I expression by infected cells, an effect that is countered by CD4+ T cell-released INF-γ [100]. Therefore, CD8+ T cells depend on activity from a TH1 response in order to recognize infected epithelial cells. Additionally, circulating effector memory CD8+ T cells have been shown to be ineffective at protecting against HSV challenge at epidermal and mucosal locations, likely due to the delay in peripheral immunosurveillance and recruitment of circulating lymphocytes [101]. Establishing local memory CD8+ T cells (TRM) that exhibit permanent lodgment in the vaginal epithelium is therefore crucial to viremic control. Similar to that described for HIV, HSV vaccination strategies would benefit from a “prime-pull” approach which engenders mucosal tissue with appropriate TH1-type cells or their effector chemokines necessary to recruit effector memory CD8+ T cells [102]. Finally, the role of B cells in mediating HSV-2 infection is not well understood in humans. In a vaccine trial including 8323 women vaccinated intramuscularly with a gD-2 HSV subunit vaccine, anti-gD-2 antibodies correlated to protection against HSV-1, which is transmitted by oral-to-oral contact, but not HSV-2 [103]. However, studies in mice show that IgG effectively neutralizes wild-type HSV-2 at mucosal surfaces to a greater extent than secretory IgA [91]. Thus, generation of antibody-mediated immunity by vaccination, particularly in the vagina which contains a high concentration of local IgG, may hold promise. Further study is warranted to define cellular and humoral correlates of genital immunity to HSV-2.
3.3.2. Vaginal vaccines for HSV
There exists a relatively large body of work focused on developing a vaginal HSV vaccine, much of which began in the 1990’s with Parr and Parr demonstrating sterilizing immunity after vaginal administration of wild-type HSV-2, which instilled long lasting IgG-secreting plasma cells in vaginal tissue [104]. Parr and Parr showed similar mucosal immunity marked by high vaginal Ab titers and local memory T cell populations in response to intravaginal delivery of attenuated HSV-2 antigen [105]. Using live-attenuated virus to access an MHC class I antigen presentation pathway is an effective approach to elicit a desirable CTL response. However, such vaccines are not translatable to human use due to safety concerns as live virus can establish latent infection in regional ganglia [106]. Thus, more recent attempts at achieving vaginal HSV immunity have been focused on enhancing the immunogenicity of non-live vectors, predominately subunit vaccines, in the genital mucosa (Table 3). Most studies effectively demonstrated protection against vaginal HSV challenge after vaginal vaccination and more recent reports demonstrate durable CTL responses in vaginal tissue lasting two to eight months after initial vaccination. These successful strategies involve the use of lipopeptide subunit vaccines that contain at least one CD8+ T cell-targeting epitope, an important finding in the quest for vaccine strategies that sufficiently engender mucosal tissue with protective T cells. Lipopeptides are a relatively low-cost method of generating immunogens, and reports suggest that conjugating adjuvant moieties directly to lipopeptides ensures a safer method of targeting TLRs for antigenic uptake. An intravaginal vaccination study in mice showed that extending an HSV-2 peptide targeting CD8+ T cells with a palmitic acid moiety, which is known to specifically target TLR-2, resulted in memory CD8+ T cell seeding in the vaginal mucosa [107]. Moreover, lipopeptide administration in TLR2−/− mice resulted in a significantly reduced lymphocyte response, faster disease progression, higher virulence and earlier death upon HSV-2 challenge. This reflects the significance of TLR targeting in overcoming immune tolerance and provides a strong premise for direct lipopeptide conjugation in order to functionalize subunit vaccines. In another attempt to generate safer HSV vaccines without compromising potency, Çuburu et al. used HPV pseudoviruses (PsVs) to deliver multiple HSV genes, also demonstrating improved outcomes after vaginal HSV challenge. Local antigen generation resulted in an amplified intraepithelial CD8+ T cell response, a feature which confers therapeutic potential to this vaccine [108]. The PsV delivery system is theoretically much safer than traditional attenuated viruses, thus this is a promising strategy that should be explored in higher order animals and potentially clinical trials.
Table 3:
Summary of studies of vaginal HSV vaccination in animals
| Vaccine type | Animal model | Delivery modality | Prime/boost (routes) | Adjuvant | Systemic Response | Mucosal Response | Remarks on efficacy | Source |
|---|---|---|---|---|---|---|---|---|
| Protein: HSV-2 gp | Mice | Nanoparticle suspension | Vaginal | Calcium phosphate (CAP) NPs | Enhanced IgG with CAP | Modest IgG and high IgA | CAP induced long-lived neutralizing Abs in serum and mucosa that protected against live HSV-2 infection | [109] |
| Protein: HSV recombinant gB (rgB) | Mice | Solution | Vaginal | CpG oligodeoxynucleotides (ODN) | Serum IgG and IgA | High vaginal IgG and IgA; high IgG at distal mucosal sites with CpG ODN | CpG ODN significantly enhanced mucosal response and survival against HSV challenge | [134] |
| Protein: HSV-2 gp | Mice | Solution | Vaginal/vaginal | CpG ODN | Strong TH1-like response in DLNs and spleen; splenic IgG | Rapid CC chemokine (MIP-1α, MIP-1β, RANTES) and CXC chemokine (MIP-2 and IP-10) response; high IgG | CpG ODN skewed a TH1 response that conferred protection against vaginal HSV challenge | [135] |
| Protein: Lipopeptide - HSV-2 gB498–505 - PAN DR (CD4+ and CD8+ T cell epitopes) | Mice | Solution | Vaginal/vaginal | Conjugated palmitic acid | Splenic gB-specific CTLs; high IFN-γ, IL-2 (TH1) and IL-12-producting CD8+ T cells in ILN | Induction of IFN-γ-producing CD8+ T cells; memory CD8+ T cell pop. 60 days after vacc. | Dual-epitope lipopeptide induced CD8+ T cell-mediated protection against HSV | [107] |
| Protein + viral vector: lipopeptide and adenovirus (rAdv5) - HSV-2 gB489–505 (CD8+ T cell epitope) | Mice | Solution | Vaginal (lipo)/vaginal (rAdv5) | n/a | Not reported | Induction of IFN-γ-producing CD8+ T cells; CTL responses 8 months after vacc. with lipo/rAdv5 | Heterologous prime/boost induced potent and durable CD8+ T cell response that protected against HSV | [152] |
| Viral vector: HPV pseudovirus (PsV) - HSV-2 gB or gD | Mice | Solution | Vaginal (+ i.m.) | n/a | Splenic memory CD8+ T cells; serum HSV-neutralizing Abs | IFN-γ and TNF-α-secreting CD8+ T cells; CD8+ TRM cells 4 wks after vacc. | Ivag and ivag + i.m. vacc. with combined gB and gD improved survival and reduced genital lesions and viral shedding | [108] |
In an impactful study, Shin and Iwasaki demonstrated an unconventional “prime-pull” vaccination strategy using subcutaneous vaccination with attenuated HSV-2 followed by topical vaginal application of CXCL9 and CXCL10 chemokines [102]. These chemokines recruit CXCL3+ T cells of the TH1 type as well as cytotoxic CD8+ T cells. Thus, this strategy was designed to prime lymphocytes systemically with HSV-2 antigen and pull activated effector cells to the vaginal mucosa. Results showed high concentrations of CD4+ and CD8+ T cells in the vaginal mucosa after the chemokine pull and tissue resident memory CD8+ T cells persisted four weeks after treatment. This approach prevented lethal HSV infection in mice and demonstrated that TH1-effector molecules alone are capable of coordinating a protective CD8+ T cell response. This study emphasizes the significance of orchestrating cellular-mediated immunity in vaginal tissue by local delivery of immunogenic agents and it may be extensible to other infections and pathogenic states that occur in restricted microenvironments. A drawback of this method is that it does not induce local amplification of recruited T cells, which may limit its efficacy. This limitation may be addressed by intravaginal boosting with antigen in addition to CXC chemokine application. Interestingly, this may be a promising approach for HIV vaccination as well, wherein induction of local CD4+ T cell expansion may increase risk of infection and viral amplification, but their effector chemokines are necessary for CD8+ T cell mobility to the vaginal mucosa.
3.3.3. Delivery systems for vaginal HSV vaccines
A few innovative studies have focused on advanced drug delivery systems for vaginal HSV vaccination. In an early study, He et al. used calcium phosphate (CAP), an adjuvant alternative to aluminum that is less irritating when applied topically, as a polymeric nanoparticle delivery vehicle for an HSV-2 protein [109]. This delivery system resulted in a near 5-fold increase in vaginal IgA and IgG lasting more than a month after vaccination, as well as a 5-fold decrease in clinical severity of disease upon vaginal challenge. The nanoparticles allowed for improved antigen retention and penetration in the vaginal mucosa, and self-adjuvanting properties of the polymer matrix offered a safe and effective means of enhancing immunogenicity. This study was limited in that it did not address cell-mediated immunity, though its authors posited that the size of CAP NPs is ideal for APC uptake and generation of TH1-type immunity. More research is warranted to prove this feature of CAP NP delivery. To more directly facilitate desirable MHC class I antigen presentation, Wang et al. recently developed microneedle arrays for vaginal delivery of protein-loaded liposomes [110]. This vaginal adjuvant-dual delivery system was administered by vaginal patching and resulted in enhanced penetration through the upper layers of the cornified epithelium. Released cargo exhibited a unique APC simulation profile in which mannosylated lipid-A liposomes (MLLs) were taken up by mucosal DCs and PEG-ylated stealth lipid A liposomes (SLLs) trafficked to DLN-resident APCs. SLLs were loaded with ammonium bicarbonate (NH4HCO3) in addition to antigenic cargo. Upon endosomal entrapment and liposome breakdown, the acidic endosomal environment catalyzed NH4HCO3 breakdown into NH4+ and CO2, the pressure of which caused endosomal rupture and release of antigen into the APC cytoplasm for MHC class I presentation. The resulting presentation of antigen by MHC class I and MHC class II molecules allowed for a mixed TH1/TH2 response and potent stimulation of CD8+ cytotoxic T cells. This approach also elicited a strong humoral response and generated memory B cells as well as TCM, and TEM CD4+ and CD8+ T cells upon antigen restimulation. The use of a microneedle array in combination with strategically engineered liposomes to direct antigen presentation illustrates a well engineered system that not only elicits effective immunity but may enhance patient comfort with a needleless delivery system.
Advances in delivery systems may allow us to realize not only prophylactic vaginal vaccination, but therapeutic vaccines as well. Agelidis et al. described a novel microbicidal and vaccine-like platform (termed microbivac) to combat primary and secondary female genital herpes infection [111]. The therapeutic vaccine-like technology uses zinc oxide tetrapod nanoparticles (ZOTEN) that entrap HSV-2 virions, prevent cell entry in the vaginal epithelium, and present antigen to APCs to generate an appropriate immune response. They found that vaginal treatment with ZOTEN-HSV-2 resulted in virus neutralization and a significant decrease in viral shedding marked by diminished vaginal lesions. However, they reported a decrease in intravaginal CD11+ DCs and CD45+ T cells in response to this treatment, reflecting a decline in immune surveillance and immune memory. This finding is in contrast to a previous study by this research group which showed that ZOTEN facilitated antigen uptake by ex vivo cultured DCs, and vaginal instillation caused proliferation of CD4+ and CD8+ T cells. Such data would reflect adjuvant properties and the potential of ZOTEN to transform live virus into a vaccine [112]. Authors reasoned that new reports of reduced immune cell activation is the result of decreased inflammation and viremic control, a product of the therapeutic activity of this microbivac. It is unclear how, then, ZOTEN may enhance adaptive immunity to HSV. Further elucidation of the nature of antigen sequestration and presentation is warranted to better understand the immune cascade responsible for bolstering adaptive immunity to HSV, thereby making this approach a true therapeutic vaccine, rather than prophylactic microbicide. This technology holds immense potential given further study, as the use of viral-sequestering oxidative material to neutralize live virus and transform it into an immune-bolstering vaccine may have significant implications for patients facing secondary infection with HSV-2.
4. Immunology and vaginal vaccination against bacterial sexually transmitted infections
Bacterial infections of the genitourinary tract contribute largely to the STI burden among women and individuals with FRT anatomy. Infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae can have detrimental effects on the female reproductive tract; both pathogens can cause pelvic inflammatory disease, increased risk of ectopic pregnancy, and result in tubal-factor infertility. Though these bacterial infections manifest most severely if they ascend into the upper reproductive tract, lower FRT infection is common and may be preventable by vaginal vaccination strategies. To date, very little investigation has gone into intravaginal vaccine delivery to combat these two pathogens, which is likely due to unique difficulties in eliciting protective immunity against reinfection and the prominence of effective antibiotic treatment options. Here, we will discuss the mechanisms by which C. trachomatis and N. gonorrhoeae fail to induce adaptive immune responses to provide background for the modest body of research focused on overcoming this challenge to develop vaginal mucosal vaccines.
4.1. Chlamydia trachomatis
4.1.1. Pathogenic infection and natural immune response
Chlamydia trachomatis is a gram-negative, intracellular parasitic bacteria that, much like other STIs discussed here, initially targets epithelial cells in the FRT via extracellular elementary body binding to the cell surface. Upon entry into a target epithelial cell, the bacteria becomes metabolically active and divides by binary fission, eventually occupying the majority of the host cell cytoplasm and ultimately causing cell lysis. Epithelial cells warn of infection after detection by TLRs via upregulation of proinflammatory cytokine expression, including IL-8, GROα, GM-CSF, IL-6, and IL-1α [113]. Unlike other mucosal infections, this inflammatory signaling is not transient, rather it is self-perpetuating and causes chronic inflammation marked by high-density neutrophil infiltration, tissue damage, and pathological scarring that is responsible for associated sequalae [114]. Studies indicate that adaptive T cells, specifically MHC class-II restricted CD4+ T cells, are required for protective immunity against C. trachomatis infection [115,116]. However, adaptive immunity responsible for natural clearance of C. trachomatis is not durable. Studies of vaginal inoculation in guinea pigs show that despite abbreviated cellular and humoral response time upon secondary infection, immunity waned when secondary exposure was delayed, resulting in repeated primary inflammation [117]. In natural infection, CD8+ T cell and antibody responses do not correlate with protective immunity, and the adaptive CD4+ T-cell response that does occur is of the TH17-type, which is associated with IL-17 production and neutrophil recruitment [114]. The lack of a productive immune memory upon C. trachomatis reinfection results in compounding tissue damage caused by recurrent inflammation and scarring. In order to combat the immunopathology of C. trachomatis infection, vaccination must lead to the induction of tissue-resident TH1 and TH2 responses which can dampen pro-inflammatory TH17 cells through INFγ and IL-12 secretion and resolve infection in a less destructive fashion. Based on data in animal models and available studies of human infection, recruitment of chlamydia-specific CD4+ lymphocytes to the vaginal mucosa and the presence of local neutralizing IgG antibodies are likely central correlates of protective immunity [118]. Considering deficiencies in human immune memory to Chlamydia, protective immunity is analogous to therapeutic vaccination with a goal of minimizing the duration of infection, enhancing immune response to reinfection, and reducing viral shedding to improve epidemiological outcomes.
4.1.2. Vaginal vaccine delivery for Chlamydia trachomatis
Vaginal vaccination against Chlamydia trachomatis must exhibit sufficient immunogenicity to elicit an adaptive immune response while avoiding immune mediation that results in TH17-type inflammatory signaling, which may worsen disease outcomes upon infectious challenge. Two studies have used subunit vaccines as a means of striking this immunogenic balance and directing a productive TH1 response. Singh et al. used a fusion protein consisting of C. trachomatis major outer membrane protein (MOMP) MoPn NiggII linked to cholera toxin [119]. They saw very modest mucosal and systemic IgG, specifically IgG2a, with no IgA secretion after vaginal vaccination. Alternatively, intranasal immunization increased mucosal antibody responses and resulted in a lower viral IFU after vaginal challenge. Similarly, Carmichael et al. attempted mucosal vaccination with a C. muridarum MOMP with CpG and montanide adjuvants in mice using a combined administration approach [120]. Primary mucosal immunization in the vagina and colon was followed by an identical mucosal booster or a systemic booster comprised of intramuscular and subcutaneous injections. They showed that systemic and mucosal antibodies, which favored IgG over IgA, as well as T-cell responses, were only elicited after the systemic booster. Further, systemic boosting was the only approach that provided protection against infertility after chlamydial challenge. These studies show that vaginal delivery of protein-based vaccines has been ineffective at eliciting even modest humoral responses and has not been protective against Chlamydia in mice models. Genetic immunization with viral vectors and plasmid DNA has proven more successful. Ekong et al. tested vaginal immunization in mice with a Vibrio cholerae ghost vector expressing C. trachomatis MOMP and CTA2B, a nontoxic CT adjuvant [121]. Vaccination elicited high systemic IgG2a titers and modest IgA with significant serum IFN-γ concentration indicative of a TH1 response; a similar response was seen in the vaginal mucosa with a preference for IgA over IgG2a. Mice immunized with antigen and adjuvant effectively cleared C. muridarium infection, a significant finding as animals were vaccinated with a human serovar-D derived MOMP. Thus, this vaccine offered cross-protection against a heterologous chlamydial serovar, indicating potentially broad protection by conserved neutralizing B cell epitopes. Slightly less success was observed in Schautteet et al.’s study investigating vaginal prime and boost in pigs with plasmid DNA expressing C. trachomatis E MOMP [122]. Vaccination with CM-CSF, LTA/B, and CpG induced high serum IgM, IgG, and IgA titers as well as PBMC proliferation after vaginal challenge. However, mean production of IFN-γ in lymphocyte cultures from the spleen, pelvic lymph node, and cervical lymph node were equivalent to unvaccinated animals or even reduced, indicating the lack of a productive TH1 response. Indeed, systemic IL-10, an anti-IFN-γ cytokine, expression increased modestly over a 72 hour time course after vaccination. Upon vaginal challenge with C. trachomatis, vaginally immunized pigs experienced significantly less macroscopic lesions, vaginal excretion and viral replication; however, all animals failed to completely clear infection. This study illustrates the importance of eliciting a local TH1 response to combat chlamydial infection as a strong humoral response was insufficient. Ultimately it seems genetic immunization holds the most promise for effective vaccination against Chlamydia, and the choice of genetic delivery vector may play a role in eliciting sufficient cell-mediated immunity. Incorporating adjuvants that stimulate TLRs which preferentially initiate a TH1-type response should also be further investigated.
4.2. Neisseria Gonorrhoeae
4.2.1. Pathogenic infection and natural immune response
Neisseria gonorrhoeae infection exhibits many similarities to that of Chlamydia. The gram-negative diplococci bacteria infects non-cornified epithelium in the lower genitourinary tract, primarily the cervix. Upon gonorrheal exposure, TLR4 on epithelial and local immune cells recognize the lipid A moiety of N. gonorrhoeae lipooligosaccharide [123]. Recognition results in upregulation of local proinflammatory signaling, causing a drastic influx of neutrophils and recruitment of a TH17 T cell response characterized by TGF-β and IL-10, much like Chlamydia. Despite its propensity for immunostimulation, N. gonorrhoeae is adept at evading host antibody-mediated immunity due to antigenic variation and dampening the TH1 and TH2 pathways, thereby manipulating cellular responses towards a nonprotective neutrophilic response. Not only does neutrophil infiltration result in tissue damage via protease and histone-mediated extracellular matrix degradation, any productive role it holds in combatting N. gonorrhoeae infection is thwarted by unique features of the bacteria which limit granule fusion between phagosomes and the cellular membrane [124]. Because of its ability to evade and diminish a natural immune response, gonorrheal infection requires antibiotic treatment to prevent ascension into the upper FRT, where it may cause serious complications in the fallopian tubes including tubal factor infertility. Vaccination against N. gonorrhoeae has become increasingly desirable due to the escalation of antibiotic resistance, however, there are several unique challenges to developing a systemic or mucosal vaccine. Like C. trachomatis, one exposure to N. gonorrhoeae does not confer protective immunity upon reinfection in humans. Additionally, determinants and correlates of immunity are unknown, and N. gonorrhoeae displays highly variable antigenicity, adding complexity to assessing specific immune responses to pathogenic variants of the Neisseria species [125]. It is likely that, in accordance with chlamydial vaccination strategies, polarizing a local TH1 response and suppressing the proinflammatory TH17 is necessary for protective immunity. A strategy that addresses gonorrheal tropism with multivalent antigens, for example, may be necessary to resolve challenges with antigenic variation. Further investigation is needed to inform precise correlates of mucosal immunity for the bacterial STIs described here.
4.2.2. Vaginal vaccine delivery for Neisseria Gonorrhoeae
There exists only a modest body of work focused on prophylactic vaginal vaccines to prevent N. gonorrhoeae infection. Liu et al. demonstrated the use of locally administered chemokines to induce a protective immune response to gonorrhea [126]. IL-12, a potent stimulator of TH1-associated cellular activity, was formulated in polylactic acid microparticles and delivered intravaginally. Polymeric encapsulation allowed for topical delivery of IL-12 that was nontoxic to the genital mucosa and resulted in dramatically enhanced local TH1 and antibody activity. The sustained release formulation allowed for a durable response that effectively defended against gonococcal challenge. The group also showed that microencapsulated anti-TGF-β and anti-IL-10 antibodies can effectively augment a productive immune response to N. gonorrhoeae, likely due to inhibition of suppressor functions. Vaginal instillation of encapsulated agents was significantly more effective in preventing infection at lower doses than parenteral injection and induced resistance to secondary infection. This study potentiates the use of microencapsulated cytokines as mucosal adjuvants capable of converting gonococcal infection into a live vaccine. In a later publication, this group combined their IL-12 microencapsulation strategy with a protein vaccine consisting of gonococcal outer membrane vesicle. Vaginal delivery of these agents resulted in a TH1-driven response with high mucosal and systemic IgA and IgG titers, specifically IgG2a, corresponding to TH1-mediated immunity. This immunity persisted for several months and conferred resistance to multiple heterologous strains of N. gonorrhoeae. Liu et al.’s work confirms the importance of augmenting a TH1 immune response and suppressing TH17 cell mediated immunity to prevent gonococcal infection, an approach which may be extensible to combatting Chlamydia as well. Significantly, they also elucidated a primary correlate of immunity to N. gonorrhoeae, which was shown to be centrally dependent on IFN-γ secretion. Thus, vaccine strategies that elicit local memory CD4+ T cell production of IFN-γ should be prioritized for prevention of bacterial STIs. Further, polymeric encapsulation of immunomodulatory agents is a promising strategy for vaginal delivery to elicit local recruitment of effector lymphocytes.
4.3. Vaginal vaccine delivery for other genitourinary infections
Several other common genital infections may be prevented or remediated by vaginal mucosal vaccination, though efforts to develop such vaccines have been less frequent and robust than the STIs discussed here. Of particular significance is a vaginal vaccine against urinary tract infection which was investigated by the Uehling group and culminated in multiple Phase 2 clinical trials [127,128]. This group modified the delivery of a UTI multivalent vaccine called Urovac, which was developed in Europe for parenteral administration and consists of a mixture of heat killed bacteria from 10 human uropathogenic E. coli strains. In their latest study, UTI-susceptible women were treated with Urovac administered in a PEG-based vaginal suppository. Patients given six vaccinations over the four month trial period showed delayed time until reinfection and 54% of participants in this group experienced no UTI’s by the final booster. Though this was a significant improvement over the untreated group and resulted in minimal side-effects, this study was not followed by a Phase III trial likely due to the modest efficacy at protecting against non E. coli infections and the need for a high number of treatments. These results indicate the relatively low immunogenicity of this vaccine when delivered vaginally and implies the need for further investigation of appropriate delivery systems and mucosal adjuvants to enhance immunity. Due to the increase in antibiotic resistance, the use of a vaccine to prevent recurrent UTIs is highly desirable and will likely see continued interest in the future.
5. Mucosal adjuvants for vaginal vaccination
Mucosal adjuvants play a critical role in successful vaccination. Effective adjuvants contain immune stimulatory motifs that bind host cell PRRs which upregulate pro-inflammatory signals and co-stimulatory molecules on APCs to activate naïve lymphocytes. At mucosal surfaces enriched with TGF cytokines, such as TGFβ in the vaginal mucosa, pro-inflammatory signaling typically induces a TH17-type response [129]. As previously discussed, this is a harmful consequence of natural infection with sexually transmitted pathogens. Thus, mucosal adjuvants for vaginal vaccination must achieve sufficient potency to stimulate long-lasting immune memory while not exacerbating harmful inflammation or damaging vulnerable mucosa. Mucosal adjuvants fall under several categories, including TLR agonists, cytokines, attenuated bacterial enterotoxins, and bioadhesives. The mechanisms by which these agents enhance mucosal immunity have been reviewed in full elsewhere [129]. Common adjuvants such as cholera toxin and granulocyte macrophage colony stimulating factor (GM-CSF) are often administered intravaginally with weakly immunogenic peptide and subunit antigens; both have been shown to enhance cell-mediated immunity and an ADCC response in the vagina [130–132]. CpG oligodeoxynucleotide (ODN), a TLR9 stimulant, has also been used as a mucosal adjuvant in several vaginal vaccination studies where it is associated with a protective TH1 response in vaginal tissue, increased NK cell activity, and enhanced Ab secretion [133–135]. Additionally, co-administration of polymeric penetration enhancers polyethyleneimine and chitosan has been shown to enhance trans-mucosal bioavailability of intravaginally administered vaccines, effectively augmenting Ag-specific IgG titers [136]. Adjuvants that specifically recruit memory T cells to mucosal tissue may be the most advantageous in preventing viral sexually transmitted infections. Tan et al. explored the use of all-trans retinoic acid (ATRA) as an adjuvant to enhance recruitment of effector and memory CD8+ T cells to mucosal tissues [137]. ATRA exerts pleiotropic effects on numerous cell types by binding to widely expressed nuclear retinoic acid receptors. It is known to induce T cell expansion and upregulate expression of CCR9 and the integrin α4β7 on lymphocytes, both of which mediate T cell migration to mucosal surfaces. Indeed, upon administration with a systemic vaccine, ATRA caused increased CD8+ T cell expression of CCR9 and α4β7 in the spleen, ILN, and vaginal mucosa after intravaginal vaccina virus challenge. This approach also facilitated establishment of a resident population of memory CD8+ T cells at mucosal sites, which contributed to better protection against vaginal challenge. This significant influence on T cell recruitment potentiates the use of retinoic acid as an adjuvant for vaginal vaccines against viral STIs, protection against which is correlated with local CTL-mediated immunity. Beyond traditional vaccination, the use of adjuvants alone has been shown to stimulate protective immunity in the vaginal mucosa. MacKay et al. demonstrated that intraepithelial CD8+ TRM cells can be generated in the absence of in situ antigen presentation. Indeed, inflammation caused by sensitizing agent 2,4-dinitrofluorobenzene and mechanical agitation enhanced TRM lodgment and these cells offered superior protection against mucosal infection than circulating memory T cells [101]. These findings agree with the idea that TRM cells are crucial for mucosal protection and substantiate studies that demonstrate the utility of inflammatory mediators as adjuvants for vaginal vaccines.
The choice of live or attenuated viral vectors can lend self-adjuvanting properties to a vaccine; indeed, the use of more potent viral vectors for genetic immunization has been shown to meaningfully enhance mucosal immunity. Çuburu et al. used an HPV PsV as a delivery vector for genetic immunization and showed that vaginal prime/boost with heterotypic HPV PsV’s overcame type-specific antibody neutralization, allowing for robust systemic and intraepithelial CD8+ T cell responses [64]. Their results demonstrated that the potency of HPV PsV’s was responsible for inducing maturation and expansion of tissue resident CD8+ T cells in the vaginal epithelium. Thus, HPV and other more immunogenic viral vectors can be said to have an adjuvant-like effect in directing durable immune response and memory. Self-adjuvating subunit vaccines have also been described. Zhang et al. showed that TLR2-stimulating lipopeptides extended with a palmitic acid moiety and delivering HSV glycoproteins elicited protective CD8+ T cell responses in the vaginal mucosa that effectively defended against HSV challenge [107]. The ability to conjugate adjuvant moieties directly to lipopeptides offers a safer and perhaps more effective means of stimulating immune mediators upon vaccination.
6. Challenges and future directions
There are numerous challenges associated with vaginal vaccination that have been highlighted here, including innate physical barriers and immune privilege of the FRT that prevent antigen penetration and suppress immunogenicity. The vaginal mucosa is generally considered a poor inductive site for humoral immunity, however promising evidence suggests that adaptive cellular responses may be locally orchestrated, potentiating vaccine strategies that leverage T-cell mediated immunity to prevent infection [28]. This represents a general paradigm shift in defining correlates of immunity and assessing vaccine efficacy. Typically, serum or secreted antibody titers are the primary indicators of immune protection generated by vaccines. It is clear that studies must assay local induction of protective CD4+ and CD8+ T cell responses as well as persistence of resident memory cell populations to fully characterize immunity in vaginal tissue. Study designs must also consider immune regulation mediated by the menstrual cycle, which is perhaps the most formidable barrier to effective vaginal vaccination. Administrating estrogen has been shown to significantly reduce immune protection afforded by vaginal vaccines and even induce tolerance, whereas progestin results in increased plasma cell localization in the vagina and enhanced antibody-mediated protection [105,138]. It is unclear the precise mechanisms by which estrogen commits immunosuppressive functions, but its effects are well documented – estrogen limits antigen presentation by DCs to naïve T cells and increases TGF-β production in vaginal tissue, which in turn regulates APC, NK cell, and T cell activity.
Due to significant interference imposed by reproductive hormones on immune regulation, controlled vaginal vaccine studies rely on exogenous hormone administration, typically Depo-Provera, a long acting progestin, to control estrous cyclicity. Depo-provera is effective at maintaining a cycling animal in diestrous, which correlates with an increase in mucosal CD8+ T cell localization, formation of IVALT, accumulation of CD11c+ cells, and thinning of the vaginal epithelium to enhance antigen penetration [28]. There is, however, a limit to the efficacy of progesterone in enhancing vaccine immunogenicity. Studies show that prolonged exposure to progesterone prior to intravaginal vaccination can attenuate immunity to viral pathogens. Gillgrass et al. demonstrated that exposure to Depo-Provera for 15 days prior to vaccination resulted in significantly decreased T cell proliferation and ag-specific IgG and IgA in vaginal secretions after HSV-2 challenge compared to exposure for 5 days [139]. It is expected that native susceptibility to viral challenge would increase after Depo treatment due to the thinner epithelium, but that this would be compensated by greater vaccine-induced immune protection due to improved antigen penetration. This was the case for a shorter interval of progesterone exposure, but longer exposure was shown to attenuate early IFN-γ secretion in the innate phase of antigen recognition. IFN-γ plays a central role in coordinating NK-cell mediated immunity and antigen presentation requisite for a an adaptive TH1 type response; thus, sustained progesterone exposure can suppress early signals necessary to develop a robust humoral and cell-mediated defense, thereby dampening immunity. The choice of animal model plays a role in methodology to control estrous cyclicity. Mice and pigs are polyestrous, thus have an estrous cycle throughout the year; sheep are seasonally polyestrous and therefore do not always require hormonal regulation. Conveniently, rabbits do not have an estrous cycle and only ovulate after coitus, so vaginal vaccination can be performed irrespective of treatment or annual/cyclic timing. However, the ability to control cyclicity in animal models does not necessarily address the challenge of translating vaginal vaccines to human use. Previous clinical trials have tried different approaches to either control or account for variations in menstrual cyclicity by immunizing at the same time or throughout the menstrual cycle, respectively [75,76]. It is difficult to extrapolate how menstrual timing affected vaccine success as neither intervention elicited any immunity. Further complicating this challenge is the fact that many women take exogenous hormones for contraception, which confounds epidemiological studies aimed at gaining a fundamental understanding of the influence of sex hormones on vaginal immunity. It is critical to better understand and address hormonal factors affecting vaginal vaccination success in humans such that promising vaccine candidates may move towards ameliorating the burden of STIs. Additionally, the influence of the vaginal microbiome on adaptive immunity is largely unknown and remains to be studied; as such, the interplay between the microbiome and vaginal vaccination has not yet been reported.
Intravaginal vaccination is a complex challenge, therefore several studies have explored other mucosal vaccination sites in pursuit of generating vaginal immunity. Reports demonstrate that intranasal (i.n.) vaccination is effective at eliciting antigen-specific antibodies in vaginal secretions [140,141]. One proposed benefit of i.n. vaccination is its superior ability to elicit IgA responses in the vagina, which is not typically considered an inductive site for IgA and favors a secretory IgG antibody profile. Conflicting reports support or contradict this hypothesis and it seems that vaccine type likely plays a role in dictating i.n. immunogenicity in the vagina [105,142]. Additionally, researchers may leverage novel drug delivery strategies for vaginal vaccination to achieve a variety of goals, including enhanced antigen uptake and the ability to engineer multifunctional immunogenic components to direct host response. These features may be attained by considering mucoadhesion, mucus penetration, inflammatory response to biomaterials, controlled release for automated prime/boost schemes, and so on. Solid dosage forms capable of encapsulating physiochemically diverse agents, such as electrospun nanofibers, microneedle arrays, thermosensitive polymers, and so on, are cost effective methods of enhancing antigenic exposure and conferring multifunctionality to vaccine delivery schemes. Eventual clinical translation of vaginal vaccine administration will likely necessitate administration methods that are amenable to clinician use and acceptable to patients. Strategies beyond traditional vaccination hold immense potential to enhance adaptive immunity in the vaginal mucosa. Cellular immunotherapy, such as adoptive transfer of immunogenic DCs, is an effective method of overcoming challenges imposed by classical mucosal vaccine delivery in solution such as the relatively low frequency of APC sampling. Adoptive transfer of autologous, antigen-loaded DCs has been successful in eliciting immune responses upon sublingual and intranasal administration [143,144]. Recently, Park et al. demonstrated that ex vivo programming of BMDCs with GM-CSF or human fms-related tyrosine kinase 3 ligand (Flt3-L) can differentially drive phenotypic and migratory behavior of adoptive DCs. Adoptive transfer of Flt3-L-programmed DCs in the genital mucosal using chitosan enhanced trans-epithelial migration and induced significant antigen-specific CD4+ and CD8+ T cell proliferation in the DLN [145]. Cell-based vaccination in the vaginal mucosa may be an effective means of overcoming physical and immunosuppressive barriers in the FRT to elicit enhanced local immunity. Future investigation is warranted to elucidate mechanisms by which we may control and enhance adoptively transferred DC activity.
7. Conclusion
Vaginal vaccination is a promising approach to alleviate the enormous burden of STIs on individuals with female reproductive anatomy. Advances in drug delivery will undoubtedly improve the efficacy and tolerability of immunization strategies, as will strategic codelivery of effective adjuvants to overcome innate immune barriers. Induction of local resident T cells is a necessary component of vaginal immunity and as such, future studies in animals and humans must be designed to evaluate meaningful correlates of adaptive mucosal immunity beyond humoral responses alone. Defining clear and reliable metrics of immunity will facilitate translation of prominent vaccine candidates, particularly for HIV and HSV, which seem the closest to realizing clinical use, to human trials. Studies may also use vaginal vaccines as a tool to gain fundamental knowledge about poorly understood immune mechanisms in the vaginal mucosa, such as induction of immune memory to combat bacterial STIs and the influence of menstrual cyclicity on vaccination. Advances in vaginal vaccination will continue to expand our understanding of genital mucosal immunology, prompt developments in drug delivery technology, and eventually challenge our ability to facilitate equitable and economic deployment of vaccines globally.
Acknowledgment
We thank funding supported by NIH grant R01AI14548, R01AI150325, and R56DE028539 to KAW; and the Fulbright U.S. Scholar Program to HMV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Holmgren J, Czerkinsky C, Mucosal immunity and vaccines, Nat. Med 11 (2005) S45. 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [2].Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP, Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women, Infect. Immun 65 (1997) 1387–1394. 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anderson DJ, Marathe J, Pudney J, The Structure of the Human Vaginal Stratum Corneum and its Role in Immune Defense, Am. J. Reprod. Immunol 71 (2014) 618–623. 10.1111/aji.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blaskewicz CD, Pudney J, Anderson DJ, Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia, Biol. Reprod 85 (2011) 97–104. 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iwasaki A, Mucosal dendritic cells, Annu. Rev. Immunol 25 (2007) 381–418. 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- [6].Han JH, Kim MS, Lee MY, Kim TH, Lee MK, Kim HR, Myung SC, Modulation of human β-defensin-2 expression by 17β-estradiol and progesterone in vaginal epithelial cells, Cytokine. 49 (2010) 209–214. 10.1016/j.cyto.2009.09.005. [DOI] [PubMed] [Google Scholar]
- [7].Spear GT, Kendrick SR, Chen HY, Thomas TT, Bahk M, Balderas R, Ghosh S, Weinberg A, Landay AL, Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ß and lactoferrin, PLoS One. 6 (2011) 1–7. 10.1371/journal.pone.0019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L, Innate and adaptive immunity in female genital tract: Cellular responses and interactions, Immunol. Rev 206 (2005) 306–335. 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- [9].Pellis V, De Seta F, Crovella S, Bossi F, Bulla R, Guaschino S, Radillo O, Garred P, Tedesco F, Mannose binding lectin and C3 act as recognition molecules for infectious agents in the vagina, Clin. Exp. Immunol 139 (2005) 120–126. 10.1111/j.1365-2249.2005.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L, Innate and adaptive immunity in female genital tract: Cellular responses and interactions, Immunol. Rev 206 (2005) 306–335. 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- [11].Hilbert DW, Vaginal Mucosal Immunity, Lab. 4 (2011) 1–6. [Google Scholar]
- [12].Godaly G, Bergsten G, Hang L, Fischer H, Frendéus B, Lundstedt AC, Samuelsson M, Samuelsson P, Svanborg C, Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection, J. Leukoc. Biol 69 (2001) 899–906. 10.1189/jlb.69.6.899. [DOI] [PubMed] [Google Scholar]
- [13].Fichorova RN, Anderson DJ, Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells, Biol. Reprod 60 (1999) 508–514. 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- [14].Wahl SM, Wen J, Moutsopoulos N, TGF-β: A mobile purveyor of immune privilege, Immunol. Rev 213 (2006) 213–227. 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- [15].Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, Chesson R, Spagnuolo RA, Pyles RB, Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia, Am. J. Reprod. Immunol 59 (2008) 212–224. 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- [16].Iijima N, Thompson JM, Iwasaki A, Dendritic cells and macrophages in the genitourinary tract, Mucosal Immunol. 1 (2008) 451–459. 10.1038/mi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pena-Cruz V, Agosto LM, Akiyama H, Olson A, Moreau Y, Larrieux JR, Henderson A, Gummuluru S, Sagar M, HIV-1 replicates and persists in vaginal epithelial dendritic cells, J. Clin. Invest 128 (2018) 3439–3444. 10.1172/JCI98943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Witte L, Nabatov A, Pion M, Fluitsma D, De Jong MAWP, De Gruijl T, Piguet V, Van Kooyk Y, Geijtenbeek TBH, Langerin is a natural barrier to HIV-1 transmission by Langerhans cells, Nat. Med 13 (2007) 367–371. 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- [19].Duluc D, Gannevat J, Anguiano E, Zurawski S, Carley M, Boreham M, Stecher J, Dullaers M, Banchereau J, Oh S, Functional diversity of human vaginal APC subsets in directing T-cell responses, Mucosal Immunol. 6 (2013) 626–638. 10.1038/mi.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brotman RM, Ravel J, Bavoil PM, Gravitt PE, Ghanem KG, Microbiome, sex hormones, and immune responses in the reproductive tract: Challenges for vaccine development against sexually transmitted infections, Vaccine. 32 (2014) 1543–1552. 10.1016/j.vaccine.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou JZ, Way SS, Chen K, Immunology of the Uterine and Vaginal Mucosae, Trends Immunol. 39 (2018) 302–314. 10.1016/j.it.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumamoto Y, Iwasaki A, Unique features of antiviral immune system of the vaginal mucosa, Curr. Opin. Immunol 24 (2012) 411–416. 10.1016/j.coi.2012.05.006.Unique. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee SK, Kim CJ, Kim D, Kang J, Immune Cells in the Female Reproductive Tract, 15 (2015) 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kelly KA, Rank RG, Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis, Infect. Immun 65 (1997) 5198–5208. 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma Z-M, Compton L, Napoé G, Wilson N, Miller CJ, Haase A, Watkins DI, CD8+ T-Lymphocyte Response to Major Immunodominant Epitopes after Vaginal Exposure to Simian Immunodeficiency Virus: Too Late and Too Little, J. Virol 79 (2005) 9228–9235. 10.1128/jvi.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D, Resident memory CD8 T cells trigger protective innate and adaptive immune responses, Science (80-.). 346 (2014) 98–101. 10.1128/isbn978-1-55581-603-2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schenkel Jason M., Fraser KA, Vezys V, Masopust D, Sensing and alarm function of resident memory CD8+ T cells, Nat. Rev. Immunol 14 (2013) 509–513. 10.1038/ni.2568.Sensing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Y, Sui Y, Kato S, Hogg AE, Steel JC, Morris JC, Berzofsky JA, Vaginal type-II mucosa is an inductive site for primary CD8+ T-cell mucosal immunity, Nat. Commun 6 (2015). 10.1038/ncomms7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Roth KL, Bhavanam S, Jiang H, Gillgrass A, Ho K, Ferreira VH, Kaushic C, Delayed but effective induction of mucosal memory immune responses against genital HSV-2 in the absence of secondary lymphoid organs, Mucosal Immunol. 6 (2013) 56–68. 10.1038/mi.2012.48. [DOI] [PubMed] [Google Scholar]
- [30].Tan HX, Wheatley AK, Esterbauer R, Jegaskanda S, Glass JJ, Masopust D, De Rose R, Kent SJ, Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization, Mucosal Immunol. 11 (2018) 994–1007. 10.1038/mi.2017.89. [DOI] [PubMed] [Google Scholar]
- [31].Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X, Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 4388–4393. 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR, Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract, Am. J. Reprod. Immunol 39 (1998) 209–216. 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- [33].Wira CR, Rossoll RM, Kaushic C, Antigen-presenting cells in the female reproductive tract: Influence of estradiol on antigen presentation by vaginal cells, Endocrinology. 141 (2000) 2877–2885. 10.1210/endo.141.8.7594. [DOI] [PubMed] [Google Scholar]
- [34].Wira CR, Patel MV, Ghosh M, Mukara L, Fahey JV, Innate Immunity in the Human Female Reproductive Tract: Endocrine Regulation of Endogenous Antimicrobial Protection Against HIV and Other Sexually Transmitted Infections, Am. J. Reprod. Immunol 65 (2011) 1–7. 10.1111/j.1600-0897.2011.00970.x.Innate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wira CR, Sandoe CP, Specific IgA and IgG antibodies in the secretions of the female reproductive tract: effects of immunization and estradiol on expression of this response in vivo., J. Immunol 138 (1987) 4159–64. http://www.ncbi.nlm.nih.gov/pubmed/3584972. [PubMed] [Google Scholar]
- [36].Xu H, Wang X, Veazey RS, Mucosal immunology of HIV infection, Immunol. Rev 254 (2013) 10–33. 10.1111/imr.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Heffron R, Celum C, Mugo N, Were E, De G, Donnell D, Hormonal contraceptive use and risk of HIV-1 transmission: a prospective cohort analysis, Lancet Infect. Dis 12 (2012) 19–26. 10.1016/S1473-3099(11)70247-X.Hormonal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li S, Herrera GG, Tam KK, Lizarraga JS, Beedle MT, Winuthayanon W, Estrogen Action in the Epithelial Cells of the Mouse Vagina Regulates Neutrophil Infiltration and Vaginal Tissue Integrity, Sci. Rep 8 (2018) 1–13. 10.1038/s41598-018-29423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Anipindi VC, Bagri P, Roth K, Dizzell SE, Nguyen PV, Shaler CR, Chu DK, Jiménez-Saiz R, Liang H, Swift S, Nazli A, Kafka JK, Bramson J, Xing Z, Jordana M, Wan Y, Snider DP, Stampfli MR, Kaushic C, Estradiol Enhances CD4+ T-Cell Anti-Viral Immunity by Priming Vaginal DCs to Induce Th17 Responses via an IL-1-Dependent Pathway, PLoS Pathog. 12 (2016) 1–27. 10.1371/journal.ppat.1005589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gillgrass AE, Tang VA, Towarnicki KM, Rosenthal KL, Kaushic C, Protection against Genital Herpes Infection in Mice Immunized under Different Hormonal Conditions Correlates with Induction of Vagina-Associated Lymphoid Tissue, J. Virol 79 (2005) 3117–3126. 10.1128/jvi.79.5.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].UNAIDS, Global HIV Statistics, End. AIDS Epidemic (2020) 1–3. [Google Scholar]
- [42].James C, Harfouche M, Welton NJ, Turner KME, Abu-Raddad LJ, Gottlieb SL, Looker KJ, Herpes simplex virus: Global infection prevalence and incidence estimates, 2016, Bull. World Health Organ 98 (2020) 315–329. 10.2471/BLT.19.237149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].de Martel C, Plummer M, Vignat J, Franceschi S, Worldwide burden of cancer attributable to HPV by site, country and HPV type, Int. J. Cancer 141 (2017) 664–670. 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cheng L, Wang Y, Du J, Human papillomavirus vaccines: An updated review, Vaccines. 8 (2020) 1–15. 10.3390/vaccines8030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schiller JT, Day PM, Kines RC, Current understanding of the mechanism of HPV infection, Gynecol. Oncol 118 (2010) S12–S17. 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Da Silva DM, Velders MP, Nieland JD, Schiller JT, Nickoloff BJ, Kast WM, Physical interaction of human papillomavirus virus-like particles with immune cells, Int. Immunol 13 (2001) 633–641. 10.1093/intimm/13.5.633. [DOI] [PubMed] [Google Scholar]
- [47].Culp TD, Christensen ND, Kinetics of in vitro adsorption and entry of papillomavirus virions, Virology. 319 (2004) 152–161. 10.1016/j.virol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [48].Brianti P, De Flammineis E, Mercuri SR, Review of HPV-related diseases and cancers, New Microbiol. 40 (2017) 80–85. [PubMed] [Google Scholar]
- [49].Scott M, Nakagawa M, Moscicki AB, Cell-mediated immune response to human papillomavirus infection, Clin. Diagn. Lab. Immunol 8 (2001) 209–220. 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stanley M, HPV - Immune response to infection and vaccination, Infect. Agent. Cancer 5 (2010) 19. 10.1186/1750-9378-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tsukui T, Hildesheim A, Schiffman MH, Lucci J, Contois D, Lawler P, Rush BB, Lorincz AT, Corrigan A, Burk RD, Qu W, Marshall MA, Mann D, Carrington M, Clerici M, Shearer GM, Carbone DP, Scott DR, Houghten RA, Berzofsky JA, Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: Correlation with cervical pathology, Cancer Res. 56 (1996) 3967–3974. [PubMed] [Google Scholar]
- [52].Turner TB, Huh WK, HPV vaccines: Translating immunogenicity into efficacy, Hum. Vaccines Immunother 12 (2016) 1403–1405. 10.1080/21645515.2015.1103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schreckenberger C, Sethupathi P, Kanjanahaluethai A, Müller M, Zhou J, Gissmann L, Qiao L, Induction of an HPV 6bL1-specific mucosal IgA response by DNA immunization, Vaccine. 19 (2000) 227–233. 10.1016/S0264-410X(00)00173-0. [DOI] [PubMed] [Google Scholar]
- [54].Park JS, Oh YK, Kang MJ, Kim CK, Enhanced mucosal and systemic immune responses following intravaginal immunization with human papillomavirus 16 L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems, J. Med. Virol 70 (2003) 633–641. 10.1002/jmv.10442. [DOI] [PubMed] [Google Scholar]
- [55].Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, De Bruyn G, Kiarie J, Inambao M, Kilembe W, Farquhar C, Celum C, Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples, J. Infect. Dis 205 (2012) 358–365. 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan Z, Morrison CS, Chen-Mok M, Archer DF, Gabelnick HL, The effect of one injection of Depo-Provera® on the human vaginal epithelium and cervical ectopy, Contraception. 60 (1999) 15–24. 10.1016/S0010-7824(99)00058-X. [DOI] [PubMed] [Google Scholar]
- [57].Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS, Cyclic changes in the vaginal epithelium of normal rhesus macaques, J. Endocrinol 190 (2006) 829–835. 10.1677/joe.1.06873. [DOI] [PubMed] [Google Scholar]
- [58].Anderson MR, Baig SM, Gioia CJ, Spongberg EJ, Sarah M, Human cervicovaginal mucus contains an activity that hinders HIV-1 movement, 6 (2013) 427–434. 10.1038/mi.2012.87.Human. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hu J, Pope M, Brown C, O’Doherty U, Miller CJ, Immunophenotypic characterization of simian immunodeficiency virus-infected dendritic cells in cervix, vagina, and draining lymph nodes of rhesus monkeys, Lab. Investig 78 (1998) 435–451. [PubMed] [Google Scholar]
- [60].Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stõssel H, Romani N, Piatak M, Lifson JD, Pope M, Cunningham AL, Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells, Blood. 103 (2004) 2170–2179. 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- [61].Kaul R, Thottingal P, Kimani J, Kiama P, Waigwa CW, Bwayo JJ, Plummer FA, Rowland-Jones SL, Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women, Aids. 17 (2003) 1139–1144. 10.1097/00002030-200305230-00004. [DOI] [PubMed] [Google Scholar]
- [62].Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R, Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection, Immunity. 27 (2007) 670–684. 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [63].Iwasaki A, Antiviral immune responses in the genital tract: clues for vaccines, Nat. Rev. Immunol 10 (2010) 699–711. 10.1038/nri2836.Antiviral. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Çuburu N, Graham BS, Buck CB, Kines RC, Pang YYS, Day PM, Lowy DR, Schiller JT, Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses, J. Clin. Invest 122 (2012) 4606–4620. 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nakanishi Y, Lu B, Gerard C, Iwasaki A, CD8 + T lymphocyte mobilization to virus-infected tissue requires CD4 + T-cell help, Nature. 462 (2009) 510–513. 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT, Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections, J. Clin. Invest 121 (2011) 998–1008. 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, Ranasinghe S, Lindqvist M, Davis I, Lane K, Rychert J, Rosenberg ES, Piechocka-Trocha A, Brass AL, Brenchley JM, Walker BD, Streeck H, HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome, Sci. Transl. Med 4 (2012) 1–18. 10.1161/CIRCULATIONAHA.110.956839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M, Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT, ADCC Develops Over Time during Persistent Infection with Live-Attenuated SIV and Is Associated with Complete Protection against SIVmac251 Challenge, PLoS Pathog. 8 (2012). 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Banks ND, Kinsey N, Clements J, Hildreth JEK, Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS, AIDS Res. Hum. Retroviruses 18 (2002) 1197–1205. 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- [70].Gómez-Román VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M, Vaccine-Elicited Antibodies Mediate Antibody-Dependent Cellular Cytotoxicity Correlated with Significantly Reduced Acute Viremia in Rhesus Macaques Challenged with SIV mac251, J. Immunol 174 (2005) 2185–2189. 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- [71].Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M, Contribution of Nonneutralizing Vaccine-Elicited Antibody Activities to Improved Protective Efficacy in Rhesus Macaques Immunized with Tat/Env Compared with Multigenic Vaccines, J. Immunol 182 (2009) 3718–3727. 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yu M, Vajdy M, Mucosal HIV transmission and vaccination strategies through oral compared to vaginal and rectal routes, Expert Opin. Biol. Ther 10 (2010) 1181–1195. 10.1517/14712598.2010.496776.Mucosal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cranage MP, Fraser CA, Stevens Z, Huting J, Chang M, Jeffs SA, Seaman MS, Cope A, Cole T, Shattock RJ, Repeated vaginal administration of trimeric HIV-1 clade C gp140 induces serum and mucosal antibody responses, Mucosal Immunol. 3 (2010) 57–68. 10.1038/mi.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].McKay PF, Mann JFS, Pattani A, Kett V, Aldon Y, King D, Malcolm RK, Shattock RJ, Intravaginal immunisation using a novel antigen-releasing ring device elicits robust vaccine antigen-specific systemic and mucosal humoral immune responses, J. Control. Release 249 (2017) 74–83. 10.1016/j.jconrel.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pialoux G, Hocini H, Pérusat S, Silberman B, Salmon-Ceron D, Slama L, Journot V, Mathieu E, Gaillard C, Petitprez K, Launay O, Chêne G, Phase I study of a candidate vaccine based on recombinant HIV-1 gp160 (MN/LAI) administered by the mucosal route to HIV-seronegative volunteers: The ANRS VAC14 study, Vaccine. 26 (2008) 2657–2666. 10.1016/j.vaccine.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [76].Lewis DJ, Fraser CA, Mahmoud AN, Wiggins RC, Woodrow M, Cope A, Cai C, Giemza R, Jeffs SA, Manoussaka M, Cole T, Cranage MP, Shattock RJ, Lacey CJ, Phase I randomised clinical trial of an HIV-1 CN54, clade C, trimeric envelope vaccine candidate delivered vaginally, PLoS One. 6 (2011) 1–11. 10.1371/journal.pone.0025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lewis DJM, Wang Y, Huo Z, Giemza R, Babaahmady K, Rahman D, Shattock RJ, Singh M, Lehner T, Effect of Vaginal Immunization with HIVgp140 and HSP70 on HIV-1 Replication and Innate and T Cell Adaptive Immunity in Women, J. Virol 88 (2014) 11648–11657. 10.1128/jvi.01621-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cosgrove CA, Lacey CJ, Cope AV, Bartolf A, Morris G, Yan C, Baden S, Cole T, Carter D, Brodnicki E, Shen X, Joseph S, DeRosa SC, Peng L, Yu X, Ferrari G, Seaman M, Montefiori DC, Frahm N, Tomaras GD, Stöhr W, McCormack S, Shattock RJ, Comparative immunogenicity of HIV-1 gp140 vaccine delivered by parenteral, and mucosal routes in female volunteers; MUCOVAC2, a randomized two centre study, PLoS One. 11 (2016) 1–18. 10.1371/journal.pone.0152038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wyatt TL, Whaley KJ, Cone RA, Saltzman WM, Antigen-releasing polymer rings and microspheres stimulate mucosal immunity in the vagina, J. Control. Release 50 (1998) 93–102. 10.1016/S0168-3659(97)00114-4. [DOI] [PubMed] [Google Scholar]
- [80].Morrow RJ, Woolfson AD, Donnelly L, Curran R, Sustained release of proteins from a modified vaginal ring device, 77 (2013) 3–10. 10.1016/j.ejpb.2010.10.010.Sustained. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shen H, Goldberg E, Saltzman WM, Gene expression and mucosal immune responses after vaginal DNA immunization in mice using a controlled delivery matrix, J. Control. Release 86 (2003) 339–348. 10.1016/S0168-3659(02)00354-1. [DOI] [PubMed] [Google Scholar]
- [82].Kuo-Haller P, Cu Y, Blum J, Appleton JA, Saltzman WM, Vaccine delivery by polymeric vehicles in the mouse reproductive tract induces sustained local and systemic immunity, Mol. Pharm 7 (2010) 1585–1595. 10.1021/mp100009e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kawamura M, Naito T, Ueno M, Akagi T, Hiraishi K, Takai I, Makino M, Serizawa T, Sugimura K, Akashi M, Baba M, Induction of mucosal IgA following intravaginal administration of inactivated HIV-1-capturing nanospheres in mice, J. Med. Virol 66 (2002) 291–298. 10.1002/jmv.2144. [DOI] [PubMed] [Google Scholar]
- [84].Ji Z, Xie Z, Zhang Z, Gong T, Sun X, Engineering intravaginal vaccines to overcome mucosal and epithelial barriers, Biomaterials. 128 (2017) 8–18. 10.1016/j.biomaterials.2017.03.007. [DOI] [PubMed] [Google Scholar]
- [85].Cranage MP, Fraser CA, Cope A, McKay PF, Seaman MS, Cole T, Mahmoud AN, Hall J, Giles E, Voss G, Page M, Almond N, Shattock RJ, Antibody responses after intravaginal immunisation with trimeric HIV-1CN54 clade C gp140 in Carbopol gel are augmented by systemic priming or boosting with an adjuvanted formulation, Vaccine. 29 (2011) 1421–1430. 10.1016/j.vaccine.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gupta PN, Pattani A, Curran RM, Kett VL, Andrews GP, Morrow RJ, Woolfson AD, Malcolm RK, Development of liposome gel based formulations for intravaginal delivery of the recombinant HIV-1 envelope protein CN54gp140, Eur. J. Pharm. Sci 46 (2012) 315–322. 10.1016/j.ejps.2012.02.003. [DOI] [PubMed] [Google Scholar]
- [87].Curran RM, Donnelly L, Morrow RJ, Fraser C, Andrews G, Cranage M, Malcolm RK, Shattock RJ, Woolfson AD, Vaginal delivery of the recombinant HIV-1 clade-C trimeric gp140 envelope protein CN54gp140 within novel rheologically structured vehicles elicits specific immune responses, Vaccine. 27 (2009) 6791–6798. 10.1016/j.vaccine.2009.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].OH Y, Park J, Yoon H, Kim C, Enhanced mucosal and systemic immune responses to a vaginal vaccine coadministered with RANTES-expressing plasmid DNA using in situ-gelling mucoadhesive delivery system, Vaccine. 21 (2003) 1980–1988. 10.1016/s0264-410x(02)00779-x. [DOI] [PubMed] [Google Scholar]
- [89].Kanazawa T, Takashima Y, Tamura T, Tsuchiya M, Shibata Y, Udagawa H, Okada H, Local gene expression and immune responses of vaginal DNA vaccination using a needle-free injector, Int. J. Pharm 396 (2010) 11–16. 10.1016/j.ijpharm.2010.05.040. [DOI] [PubMed] [Google Scholar]
- [90].Kanazawa T, Tamura T, Yamazaki M, Takashima Y, Okada H, Needle-free intravaginal DNA vaccination using a stearoyl oligopeptide carrier promotes local gene expression and immune responses, Int. J. Pharm 447 (2013) 70–74. 10.1016/j.ijpharm.2013.02.018. [DOI] [PubMed] [Google Scholar]
- [91].Parr MB, Parr EL, Vaginal immunity in the HSV-2 mouse model, Int. Rev. Immunol 22 (2003) 43–63. 10.1080/08830180305228. [DOI] [PubMed] [Google Scholar]
- [92].Greenblatt Ru.M., Lukehard SA, Plummer FA, Quinn TC, Critchlow CW, Ahsley RL, D’Costa LJ, Ndinya-Achola JO, Corey L, Ronald AR, Holmes KK, Genital ulceration as a risk factor for human immunodeficiency virus infection, AIDS. 2 (1988) 47–50. [DOI] [PubMed] [Google Scholar]
- [93].Galvin SR, Cohen MS, The role of sexually transmitted diseases in HIV transmission, Nat. Rev. Microbiol 2 (2004) 33–42. 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- [94].Jaishankar D, Shukla D, Genital herpes: Insights into sexually transmitted infectious disease, Microb. Cell 3 (2016) 438–450. 10.15698/mic2016.09.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Spear PG, Eisenberg RJ, Cohen GH, Three classes of cell surface receptors for alphaherpesvirus entry, Virology. 275 (2000) 1–8. 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- [96].Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, Douglas MW, The cycle of human herpes simplex virus infection: Virus transport and immune control, J. Infect. Dis 194 (2006) 11–18. 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- [97].Mikloska Z, Danis VA, Adams S, Lloyd AR, Adrian DL, Cunningham AL, In vivo production of cytokines and β (C-C) chemokines in human recurrent herpes simplex lesions - Do herpes simplex virus - Infected keratinocytes contribute to their production?, J. Infect. Dis 177 (1998) 827–838. 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- [98].Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A, Vaginal submucosal dendritic cells, but not langerhans cells, induce protective Th1 responses to herpes simplex virus-2, J. Exp. Med 197 (2003) 153–162. 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Harandi AM, Svennerholm B, Holmgren J, Eriksson K, Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice, J. Gen. Virol 82 (2001) 845–853. 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- [100].Mikloska Z, Kesson AM, Penfold MET, Cunningham AL, Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-γ, J. Infect. Dis 173 (1996) 7–17. 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- [101].Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T, Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 7037–7042. 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shin H, Iwasaki A, A vaccine strategy that protects against genital herpes by establishing local memory T cells, Nature. 491 (2012) 463–467. 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, Van Der Most R, Deal CD, Correlate of immune protection against HSV-1 genital disease in vaccinated women, J. Infect. Dis 209 (2014) 828–836. 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Parr EL, Parr MB, Immunoglobulin G, Plasma Cells, and Lymphocytes in the Murine Vagina after Vaginal or Parenteral Immunization with Attenuated Herpes Simplex Virus Type 2, J. Virol 72 (1998) 5137–5145. 10.1128/jvi.72.6.5137-5145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Parr EL, Parr MB, Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2, Immunology. 98 (1999) 639–645. 10.1046/j.1365-2567.1999.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Coen DM, Kosz-Vnenchak M, Jacobson JG, Leib DA, Bogard CL, Schaffer PA, Tyler KL, Knipe DM, Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate, Proc. Natl. Acad. Sci. U. S. A 86 (1989) 4736–4740. 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang X, Chentoufi A, Dasgupta G, Nesburn A, Wu M, Zhu X, Carpenter D, Wechsler S, You S, BenMohamed L, A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge, Mucosal Immunol. 2 (2009) 129–143. 10.1038/mi.2008.81.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Çuburu N, Wang K, Goodman KN, Pang YY, Thompson CD, Lowy DR, Cohen JI, Schiller JT, Topical Herpes Simplex Virus 2 (HSV-2) Vaccination with Human Papillomavirus Vectors Expressing gB/gD Ectodomains Induces Genital-Tissue-Resident Memory CD8 + T Cells and Reduces Genital Disease and Viral Shedding after HSV-2 Challenge, J. Virol 89 (2015) 83–96. 10.1128/jvi.02380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].He Q, Mitchell A, Morcol T, Bell SJD, Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2, Clin. Diagn. Lab. Immunol 9 (2002) 1021–1024. 10.1128/CDLI.9.5.1021-1024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wang N, Zhen Y, Jin Y, Wang X, Li N, Jiang S, Wang T, Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery system (VADDS), J. Control. Release 246 (2017) 12–29. 10.1016/j.jconrel.2016.12.009. [DOI] [PubMed] [Google Scholar]
- [111].Agelidis A, Koujah L, Suryawanshi R, Yadavalli T, Mishra YK, Adelung R, Shukla D, An intra-vaginal zinc oxide tetrapod nanoparticles (zoten) and genital herpesvirus cocktail can provide a novel platform for live virus vaccine, Front. Immunol 10 (2019) 1–9. 10.3389/fimmu.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Antoine TE, Hadigal SR, Yakoub AM, Mishra YK, Bhattacharya P, Haddad C, Valyi-Nagy T, Adelung R, Prabhakar BS, Shukla D, Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents against Genital Herpes, J. Immunol 196 (2016) 4566–4575. 10.4049/jimmunol.1502373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, Fierer J, Stephens RS, Kagnoff MF, Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis, J. Clin. Invest 99 (1997) 77–87. 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Darville T, Hiltke TJ, Pathogenesis of Genital Tract Disease due to Chlamydia trachomatis, J. Infect. Dis 201 (2010) S114–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li LX, McSorley SJ, A re-evaluation of the role of B cells in protective immunity to Chlamydia infection, Immunol. Lett 164 (2015) 88–93. 10.1016/j.imlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rank RG, Bowlin AK, Kelly KA, Characterization of lymphocyte response in the female genital tract during ascending chlamydial genital infection in the guinea pig model, Infect. Immun 68 (2000) 5293–5298. 10.1128/IAI.68.9.5293-5298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rank RG, Batteiger BE, Soderberg LSF, Susceptibility to reinfection after a primary chlamydial genital infection, Infect. Immun 56 (1988) 2243–2249. 10.1128/iai.56.9.2243-2249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Batteiger BE, Xu F, Johnson RE, Rekart ML, Protective Immunity to Chlamydia trachomatis Genital Infection: Evidence from Human Studies, J. Infect. Dis 201 (2010) S1. 10.7312/boas90468-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Singh SR, Hulett K, Pillai SR, Dennis VA, Oh MK, Scissum-Gunn K, Mucosal immunization with recombinant MOMP genetically linked with modified cholera toxin confers protection against Chlamydia trachomatis infection, Vaccine. 24 (2006) 1213–1224. 10.1016/j.vaccine.2005.08.097. [DOI] [PubMed] [Google Scholar]
- [120].Carmichael JR, Pal S, Tifrea D, De la Maza LM, Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine, Vaccine. 29 (2011) 5276–5283. 10.1016/j.vaccine.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ekong EE, Okenu DN, Mania-pramanik J, He Q, Joseph U, Ananaba GA, Lyn D, Black C, Eko FO, A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin, (2011) 1–19. 10.1111/j.1574-695X.2008.00493.x.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schautteet K, Stuyven E, Beeckman DSA, Van Acker S, Carlon M, Chiers K, Cox E, Vanrompay D, Protection of pigs against Chlamydia trachomatis challenge by administration of a MOMP-based DNA vaccine in the vaginal mucosa, Vaccine. 29 (2011) 1399–1407. 10.1016/j.vaccine.2010.12.042. [DOI] [PubMed] [Google Scholar]
- [123].Stevens JS, Criss AK, Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: Neutrophilic host response, sustained infection, and clinical sequelae, Curr. Opin. Hematol 25 (2018) 13–21. 10.1097/MOH.0000000000000394.Pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Handling JW, Criss AK, The Lipooligosaccharide Modifying Enzyme LptA Enhances Gonococcal Defense Against Human Neutrophils, Cell Microbiol. 17 (2015) 910–921. 10.1111/cmi.12411.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Russell MW, Jerse AE, Gray-Owen SD, Progress Toward a Gonococcal Vaccine: The Way Forward, Front. Immunol 10 (2019) 1–18. 10.3389/fimmu.2019.02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Liu Y, Egilmez NK, Russell MW, Enhancement of adaptive immunity to neisseria gonorrhoeae by local intravaginal administration of microencapsulated interleukin 12, J. Infect. Dis 208 (2013) 1821–1829. 10.1093/infdis/jit354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Uehling DT, Hopkins WJ, Elkahwaji JE, Schmidt DM, Leverson GE, Phase 2 clinical trial of a vaginal mucosal vaccine for urinary tract infections, J. Urol 170 (2003) 867–869. 10.1097/01.ju.0000075094.54767.6e. [DOI] [PubMed] [Google Scholar]
- [128].Hopkins WJ, Elkahwaji J, Beierle LM, Leverson GE, Uehling DT, Vaginal Mucosal Vaccine for Recurrent Urinary Tract Infections in Women: Results of a Phase 2 Clinical Trial, J. Urol 177 (2007) 1349–1353. 10.1016/j.juro.2006.11.093. [DOI] [PubMed] [Google Scholar]
- [129].Kozlowski PA, Aldovini A, Mucosal Vaccine Approaches for Prevention fo HIV and SIV Transmission, Curr. Immunol. Rev 15 (2019) 102–122. 10.2174/1573395514666180605092054.Mucosal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kato H, Bukawa H, Hagiwara E, Xin KQ, Hamajima K, Kawamoto S, Sugiyama M, Sugiyama M, Noda E, Nishizaki M, Okuda K, Rectal and vaginal immunization with a macromolecular multicomponent peptide vaccine candidate for HIV-1 infection induces HIV-specific protective immune responses, Vaccine. 18 (2000) 1151–1160. 10.1016/S0264-410X(99)00385-0. [DOI] [PubMed] [Google Scholar]
- [131].Hamajima K, Hoshino Y, Xin KQ, Hayashi F, Tadokoro K, Okuda K, Systemic and mucosal immune responses in mice after rectal and vaginal immunization with HIV-DNA vaccine, Clin. Immunol 102 (2002) 12–18. 10.1006/clim.2001.5141. [DOI] [PubMed] [Google Scholar]
- [132].Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, Anjuère F, Dendritic Cell-Mediated Induction of Mucosal Cytotoxic Responses following Intravaginal Immunization with the Nontoxic B Subunit of Cholera Toxin, J. Immunol 176 (2006) 2749–2757. 10.4049/jimmunol.176.5.2749. [DOI] [PubMed] [Google Scholar]
- [133].Harandi AM, Eriksson K, Holmgren J, A Protective Role of Locally Administered Immunostimulatory CpG Oligodeoxynucleotide in a Mouse Model of Genital Herpes Infection, J. Virol 77 (2003) 953–962. 10.1128/jvi.77.2.953-962.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kwant A, Rosenthal KL, Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV-2, Vaccine. 22 (2004) 3098–3104. 10.1016/j.vaccine.2004.01.059. [DOI] [PubMed] [Google Scholar]
- [135].Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM, Mucosal Administration of CpG Oligodeoxynucleotide Elicits Strong CC and CXC Chemokine Responses in the Vagina and Serves as a Potent Th1-Tilting Adjuvant for Recombinant gD2 Protein Vaccination against Genital Herpes, J. Virol 80 (2006) 5283–5291. 10.1128/jvi.02013-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Klein K, Mann JFS, Rogers P, Shattock RJ, Polymeric penetration enhancers promote humoral immune responses to mucosal vaccines, J. Control. Release 183 (2014) 43–50. 10.1016/j.jconrel.2014.03.018. [DOI] [PubMed] [Google Scholar]
- [137].Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD, Retinoic Acid as a Vaccine Adjuvant Enhances CD8+ T Cell Response and Mucosal Protection from Viral Challenge, J. Virol 85 (2011) 8316–8327. 10.1128/jvi.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Black CA, Rohan LC, Cost M, Watkins SC, Draviam R, Alber S, Edwards RP, Vaginal Mucosa Serves as an Inductive Site for Tolerance, J. Immunol 165 (2000) 5077–5083. 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- [139].Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C, Prolonged Exposure to Progesterone Prevents Induction of Protective Mucosal Responses following Intravaginal Immunization with Attenuated Herpes Simplex Virus Type 2, J. Virol 77 (2003) 9845–9851. 10.1128/jvi.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, Teresa De Magistris M, Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant, Infect. Immun 64 (1996) 974–979. 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bergquist C, Johansson EL, Lagergård T, Holmgren J, Rudin A, Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina, Infect. Immun 65 (1997) 2676–2684. 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Scott Gallichan W, Rosenthal KL, Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus, Vaccine. 13 (1995) 1589–1595. 10.1016/0264-410X(95)00100-F. [DOI] [PubMed] [Google Scholar]
- [143].Hervouet C, Luci C, Bekri S, Juhel T, Bihl F, Braud VM, Czerkinsky C, Anjuère F, Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells, Mucosal Immunol. 7 (2014) 280–291. 10.1038/mi.2013.45. [DOI] [PubMed] [Google Scholar]
- [144].Gao X, Wang S, Fan Y, Bai H, Yang J, Yang X, CD8+ DC, but not CD8-DC, isolated from BCG-infected mice reduces pathological reactions induced by mycobacterial challenge infection, PLoS One. 5 (2010). 10.1371/journal.pone.0009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Park J, Frizzell H, Zhang H, Cao S, Hughes SM, Hladik F, Koelle DM, Woodrow KA, Flt3-L enhances trans-epithelial migration and antigen presentation of dendritic cells adoptively transferred to genital mucosa, J. Control. Release (2020) 1–12. 10.1016/j.jconrel.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Vajdy M, Gardner J, Neidleman J, Cuadra L, Greer C, Perri S, O’hagan D, Polo JM, Human immunodeficiency virus type 1 gag-specific vaginal immunity and protection after local immunizations with sindbis virus-based replicon particles, J. Infect. Dis 184 (2001) 1613–1616. 10.1086/324581. [DOI] [PubMed] [Google Scholar]
- [147].Gupta S, Janani R, Bin Q, Luciw P, Greer C, Perri S, Legg H, Donnelly J, Barnett S, O’Hagan D, Polo JM, Vajdy M, Characterization of Human Immunodeficiency Virus Gag-Specific Gamma Interferon-Expressing Cells following Protective Mucosal Immunization with Alphavirus Replicon Particles, J. Virol 79 (2005) 7135–7145. 10.1128/jvi.79.11.7135-7145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR, Novel Vaccination Protocol with Two Live Mucosal Vectors Elicits Strong Cell-Mediated Immunity in the Vagina and Protects against Vaginal Virus Challenge, J. Immunol 180 (2008) 2504–2513. 10.4049/jimmunol.180.4.2504. [DOI] [PubMed] [Google Scholar]
- [149].Gordon SN, Kines RC, Kutsyna G, Ma Z-M, Hryniewicz A, Roberts JN, Fenizia C, Hidajat R, Brocca-Cofano E, Cuburu N, Buck CB, Bernardo ML, Robert-Guroff M, Miller CJ, Graham BS, Lowy DR, Schiller JT, Franchini G, Targeting the Vaginal Mucosa with Human Papillomavirus Pseudovirion Vaccines Delivering Simian Immunodeficiency Virus DNA, J. Immunol 188 (2012) 714–723. 10.4049/jimmunol.1101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Weaver EA, Nehete PN, Nehete BP, Yang G, Buchl SJ, Hanley PW, Palmer D, Montefiori DC, Ferrari G, Ng P, Sastry KJ, Barry MA, Comparison of Systemic and Mucosal Immunization with Helper-Dependent Adenoviruses for Vaccination against Mucosal Challenge with SHIV, PLoS One. 8 (2013). 10.1371/journal.pone.0067574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Garulli B, Di Mario G, Stillitano MG, Kawaoka Y, Castrucci MR, Exploring mucosal immunization with a recombinant influenza virus carrying an HIV-polyepitope in mice with pre-existing immunity to influenza, Vaccine. 32 (2014) 2501–2506. 10.1016/j.vaccine.2014.02.077. [DOI] [PubMed] [Google Scholar]
- [152].Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, BenMohamed L, Targeting the Genital Tract Mucosa with a Lipopeptide/Recombinant Adenovirus Prime/Boost Vaccine Induces Potent and Long-Lasting CD8 + T Cell Immunity against Herpes: Importance of MyD88, J. Immunol 189 (2012) 4496–4509. 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]


