This study examines COVID-19 infections among 4791 vaccinated and unvaccinated patients receiving dialysis and describes the longitudinal antibody-based response up to 5 to 6 months after full vaccination and its relationship with breakthrough infections in 2563 patients.
Visual Abstract. SARS-CoV-2 Vaccine Response and Breakthrough Infection in Patients Receiving Dialysis.
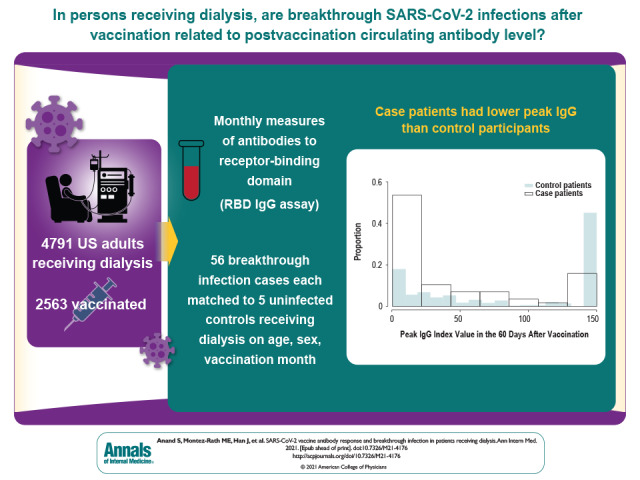
This study examines COVID-19 infections among 4791 vaccinated and unvaccinated patients receiving dialysis and describes the longitudinal antibody-based response up to 5 to 6 months after full vaccination and its relationship with breakthrough infections in 2563 patients.
Abstract
Background:
Whether breakthrough SARS-CoV-2 infections after vaccination are related to the level of postvaccine circulating antibody is unclear.
Objective:
To determine longitudinal antibody-based response and risk for breakthrough infection after SARS-CoV-2 vaccination.
Design:
Prospective study.
Setting:
Nationwide sample from dialysis facilities.
Patients:
4791 patients receiving dialysis.
Measurements:
Remainder plasma from a laboratory processing routine monthly tests was used to measure qualitative and semiquantitative antibodies to the receptor-binding domain (RBD) of SARS-CoV-2. To evaluate whether peak or prebreakthrough RBD values were associated with breakthrough infection, a nested case–control analysis matched each breakthrough case patient to 5 control patients by age, sex, and vaccination month and adjusted for diabetes status and region of residence.
Results:
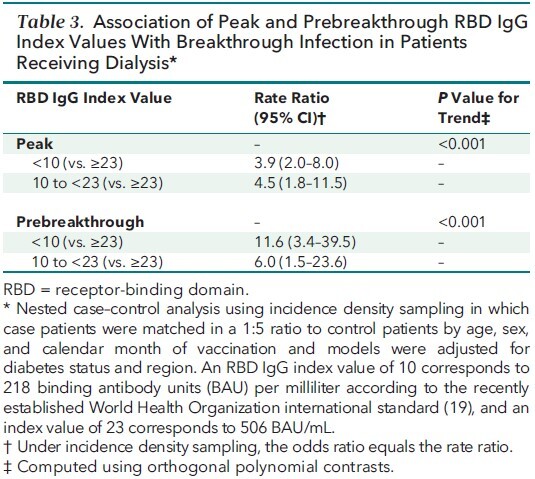
Of the 4791 patients followed with monthly RBD assays, 2563 were vaccinated as of 14 September 2021. Among the vaccinated patients, the estimated proportion with an undetectable RBD response increased from 6.6% (95% CI, 5.5% to 7.8%) 14 to 30 days after vaccination to 20.2% (CI, 17.0% to 23.3%) 5 to 6 months after vaccination. Estimated median index values decreased from 91.9 (CI, 78.6 to 105.2) 14 to 30 days after vaccination to 8.4 (CI, 7.6 to 9.3) 5 to 6 months after vaccination. Breakthrough infections occurred in 56 patients, with samples collected a median of 21 days before breakthrough infection. Compared with prebreakthrough index RBD values of 23 or higher (equivalent to ≥506 binding antibody units per milliliter), prebreakthrough RBD values less than 10 and values from 10 to less than 23 were associated with higher odds for breakthrough infection (rate ratios, 11.6 [CI, 3.4 to 39.5] and 6.0 [CI, 1.5 to 23.6], respectively).
Limitations:
Single measure of vaccine response; ascertainment of COVID-19 diagnosis from electronic health records.
Conclusion:
The antibody response to SARS-CoV-2 vaccination wanes rapidly in persons receiving dialysis. In this population, the circulating antibody response is associated with risk for breakthrough infection.
Primary Funding Source:
Ascend Clinical Laboratory.
Vaccinations are typically administered on a routine schedule, with no postvaccine measurement of immune response. Data linking circulating antibody titers to risk for reinfection are sparse, and the Advisory Committee on Immunization Practices recommends against checking antibody titers after vaccination in healthy persons (1, 2). However, postvaccination circulating antibody titers have been used as correlates of protection in various clinical scenarios (3, 4).
Among patients receiving dialysis, there is a precedent for testing response to vaccination in order to inform vaccination schedules (5, 6). Ample data indicate lower rates of seroconversion after hepatitis B and influenza vaccination (7–9); moreover, the response is shorter than in healthy controls (7). Thus, patients receiving dialysis with hepatitis B surface antibody titers below 10 IU/mL 2 months after the primary vaccination series are revaccinated or receive a booster if titers (measured annually) fall below 10 IU/mL (6).
Although a majority of patients receiving dialysis experience seroconversion after SARS-CoV-2 vaccination, we have previously found that the early response was diminished in up to 15% and differed by vaccine type (10). The duration of circulating antibody levels after vaccination is unknown. Moreover, evidence from randomized controlled trials of mRNA-1273 (11) and ChAdOx1 (12) vaccination indicates a higher risk for postvaccination (“breakthrough”) infection among persons with lower neutralizing, spike, or receptor-binding domain (RBD) titers in the early postvaccination period. Real-world data from Israeli health care workers who received the BNT162b2 vaccine also showed an association between lower peri-infection antibody titers and breakthrough infection (13). Knowing the strength and duration of antibody response to SARS-CoV-2 vaccination in high-risk groups could help to optimize their immunization schedules and strategies for preexposure or postexposure prophylaxis. In this study, we sought to delineate the duration of antibody response to SARS-CoV-2 vaccination among patients receiving dialysis and to determine whether antibody titers to SARS-CoV-2 could identify patients receiving dialysis who are at risk for breakthrough infection.
Methods
Starting in January 2021, we tested monthly remainder plasma samples from a cohort of persons receiving dialysis at U.S. Renal Care, a dialysis network with more than 350 facilities nationwide. In partnership with Ascend Clinical, a central laboratory processing routine monthly tests from persons receiving dialysis at several dialysis networks, including U.S. Renal Care, we tested these samples for RBD antibody and ascertained patient characteristics, vaccination status, and COVID-19 diagnoses using electronic health records. The study received ethics approval from Stanford University. Stanford University investigators received anonymized data, and the Institutional Review Board waived the requirement for consent.
Sampling
In the first 2 weeks of January 2021, before widespread rollout of COVID-19 vaccines, we tested the SARS-CoV-2 antibody status of 21 570 patients receiving dialysis, of whom 17 390 were seronegative (no evidence of prior SARS-CoV-2 infection) (14). On the basis of prior data on nonresponse to hepatitis B vaccination (15), we initially selected a sufficient sample size to estimate age-stratified proportions of nonresponse to COVID-19 vaccination with 2% precision. To randomly select these patients from all patients without prior evidence of SARS-CoV-2 infection, we used systematic sampling with fraction intervals; we sorted the data by ZIP code, age, sex, and race/ethnicity and then selected every fourth patient in the sampling frame. We also followed a cohort of patients with evidence of prior SARS-CoV-2 infection by RBD antibody testing in whom we knew the date of seroconversion, yielding a final sample of 4884 patients selected for monthly antibody testing (see the Appendix for more details).
Patient Demographic Characteristics and COVID-19 Diagnosis
We extracted routinely collected data on age, sex, self-reported race/ethnicity, and diabetes, along with data collected by U.S. Renal Care on date and type of SARS-CoV-2 vaccination. U.S. Renal Care instituted a questionnaire-based health screening for all patients. If a patient reported relevant symptoms, dialysis staff requested that the patient be tested, and if the patient had SARS-CoV-2 infection, staff recorded the date of the diagnosis. In addition, if the patient was hospitalized with COVID-19 before completing the questionnaire, U.S. Renal Care recorded the date of diagnosis. We also extracted data on hospitalizations in the 7 days before or the 14 days after the COVID-19 diagnosis date.
Laboratory Testing for RBD Antibodies
We tested remainder samples using the Siemens total RBD Ig assay. The assay is reported by the manufacturer to have 100% sensitivity and 99.8% specificity if performed 14 or more days after a positive result on a reverse transcriptase polymerase chain reaction (RT-PCR) test (16), and it has been independently validated, with similar performance characteristics (17, 18). On a monthly basis starting in February 2021, we used this assay to test remainder samples from patients in whom antibodies had not been detected in the prior month. After a positive total RBD Ig result, the positive sample and all subsequent monthly samples were tested using a semiquantitative Siemens RBD IgG assay (Atellica IM sCOVG assay) (16). This assay is a 2-step sandwich indirect chemiluminescent assay with manufacturer-reported sensitivity of 95.6% (95% CI, 92.2% to 97.8%) and specificity of 99.9% (CI, 99.6% to 99.9%) for tests performed 21 or more days after a positive RT-PCR result. An index value of 1.0 corresponds to 21.8 binding antibody units (BAU) per milliliter according to the recently established World Health Organization (WHO) international standard (19). An index value of 1.0 or greater (≥21.8 BAU/mL) is considered reactive on this assay, and an index value of 150 (3270 BAU/mL) is the upper limit of quantification.
Statistical Analysis
Two of the authors (M.E.M. and J.H.) led the statistical analyses. For all patients followed with monthly laboratory tests, we described demographic data and laboratory values by vaccination status using proportions, means and SDs, or medians and interquartile ranges, as applicable. We categorized patients as fully vaccinated 14 days after they completed vaccination, whether they received 2 doses of an mRNA vaccine according to the recommended schedule or a single dose of the attenuated adenovirus vaccine. In this cohort, we first used robust Poisson regression models (20) to assess the proportion of patients without a detectable IgG response during follow-up, overall and then stratified by RBD IgG antibody status before vaccination and vaccine type. We assumed that the presence of RBD IgG before vaccination indicated prior SARS-CoV-2 infection. Because patients receiving dialysis are tested monthly on or around the same date each month, we reported data using discrete postvaccination 30-day time windows, except for the first period, which spanned 14 to 30 days after vaccination.
Next, among patients with a total RBD response, we estimated medians and 95% CIs for RBD IgG index values using quantile regression with robust SEs to account for multiple observations per patient (21), as implemented in the Stata qreg and margins commands. In this longitudinal data analysis, model parameters have a population-average interpretation (22). We used quantile regression and, in particular, the median to describe the data because it is invariant to data truncation and estimable in all of the analyses presented. We ascertained effect modification by separately adding to the quantile regression model the interaction between time window and RBD antibody status before vaccination, vaccine type, age, and diabetes status. We adjusted all models for age, sex, diabetes status, RBD IgG antibody status before vaccination, and vaccine type, as applicable.
Finally, to evaluate the association between the circulating RBD IgG titer and risk for breakthrough infection, we used a nested case–control design with incidence density sampling (Stata command sttocc). The cohort comprised fully vaccinated patients. We set the time at which vaccination was completed to be time 0. We then defined case patients as all patients in the cohort who had been diagnosed with COVID-19 before 14 September 2021. We matched each case patient to 5 control patients (patients who were still alive, were not infected, and were receiving dialysis at a U.S. Renal Care facility) by age (5-year categories), sex, and calendar month of vaccination. Given the incidence density sampling design, we performed conditional logistic regression with adjustment for diabetes status and region of residence to estimate the rate ratio of a COVID-19 case for the discrete index values less than 10 and from 10 to less than 23 compared with 23 or higher (the reference group) (23, 24). We similarly evaluated risk using the peak RBD IgG attained within the first 60 days after vaccination. We performed trend tests using orthogonal polynomial contrasts. We selected the first cut point based on data showing that index values of 10 or greater corresponded with pseudovirus neutralization titers (25) above 1:500 and a positive predictive value of 100% for a PRNT50 titer above 1:80 in Siemens studies (26, 27). The cut point of 23 corresponds to 506 BAU/mL according to the WHO international standard (19) and has been shown to correspond to 80% vaccine efficacy against symptomatic infection (12). We assumed statistical significance at an α level of 0.05. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute), or Stata/MP 17 (StataCorp).
Role of the Funding Source
Ascend Clinical Laboratory funded the remainder plasma testing for this study. Coauthors from Ascend Clinical Laboratory participated in data collection.
Results
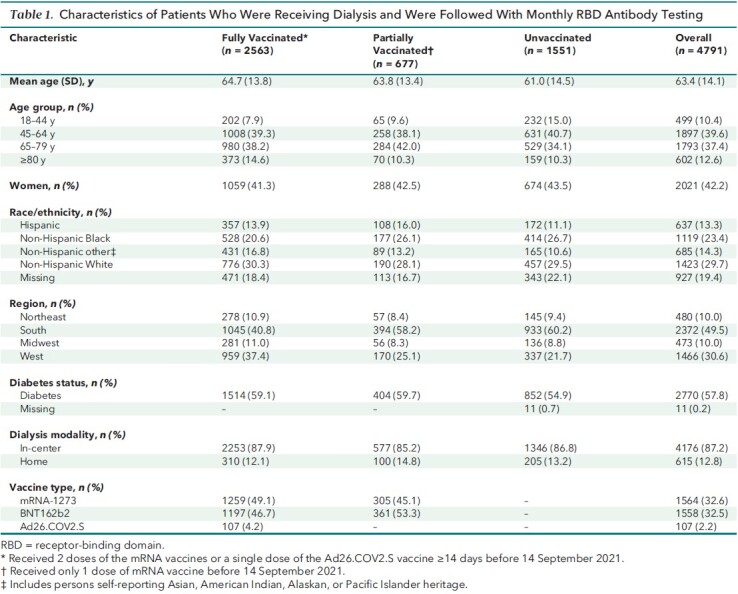
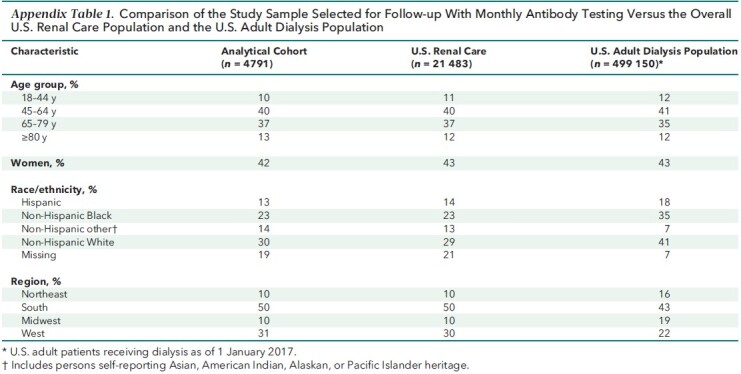
Of the 4884 patients we selected to follow as of January 2021, 4791 (98%) were able to be followed (Appendix Figure 1). Among these 4791, 2563 (54%) completed vaccination (Table 1). The sample was representative of the U.S. Renal Care patient population receiving dialysis. Compared with the U.S. adult population receiving dialysis, age and sex distributions were similar, but the study sample had fewer patients from the Northeast and Midwest (Appendix Table 1).
Appendix Figure 1. Flowchart of study participants.
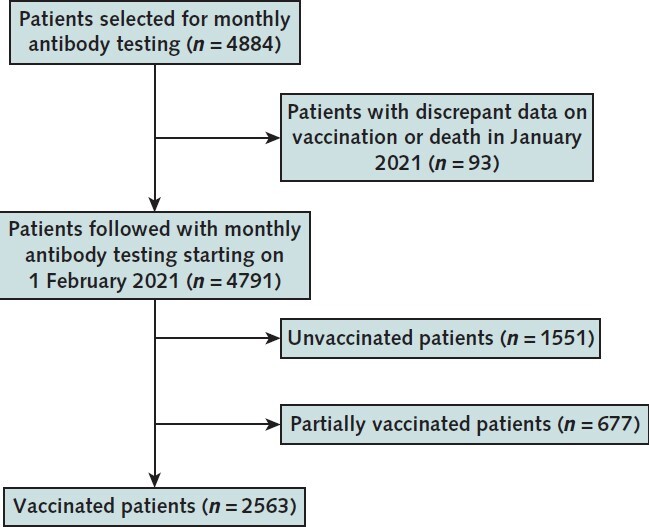
Table 1.
Characteristics of Patients Who Were Receiving Dialysis and Were Followed With Monthly RBD Antibody Testing
Appendix Table 1.
Comparison of the Study Sample Selected for Follow-up With Monthly Antibody Testing Versus the Overall U.S. Renal Care Population and the U.S. Adult Dialysis Population
During follow-up, among 1551 unvaccinated patients (median follow-up, 256 days), 173 (11%) had clinically documented COVID-19. Among the 677 partially vaccinated patients (median follow-up, 158 days), 20 (3%) had clinically documented COVID-19 after a single dose of one of the mRNA vaccines. Among 2563 fully vaccinated patients (median follow-up, 152 days), 56 (2%) had clinically documented COVID-19 after being vaccinated.
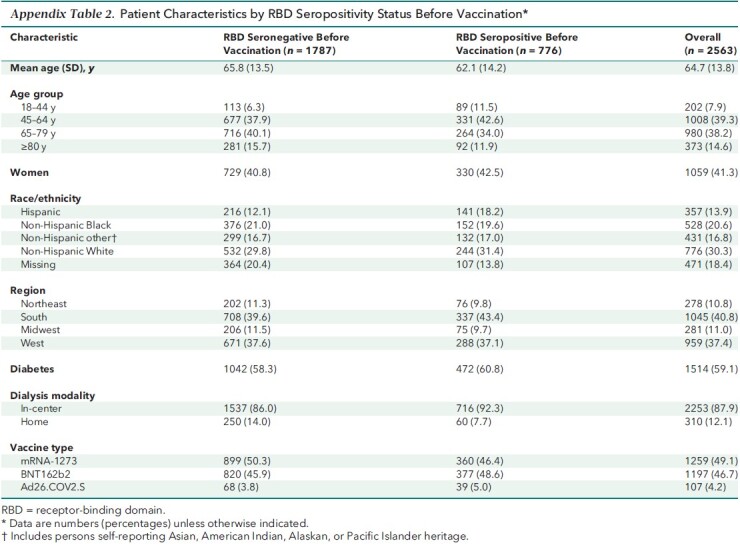
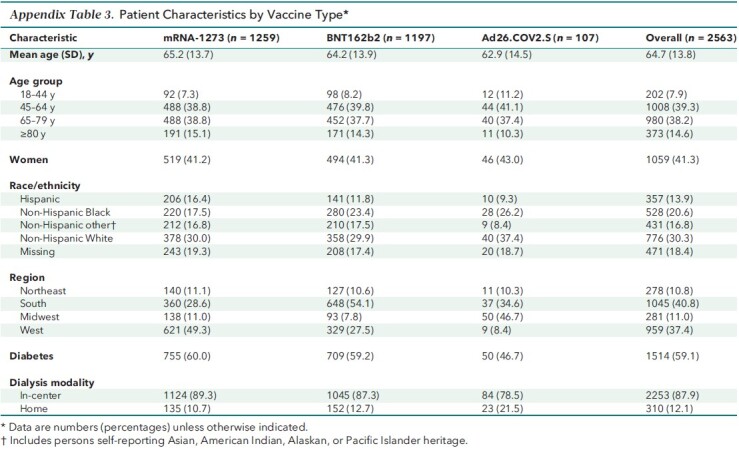
The remainder of the analyses evaluated antibody responses among this fully vaccinated cohort. Patients with presence of RBD before vaccination (that is, evidence of prior SARS-CoV-2 infection) were younger (Appendix Table 2). Compared with patients vaccinated with mRNA-1273, those vaccinated with BNT162b2 were more likely to reside in the South (Appendix Table 3).
Appendix Table 2.
Patient Characteristics by RBD Seropositivity Status Before Vaccination*
Appendix Table 3.
Patient Characteristics by Vaccine Type*
Proportion of Patients With Detectable Postvaccination RBD IgG
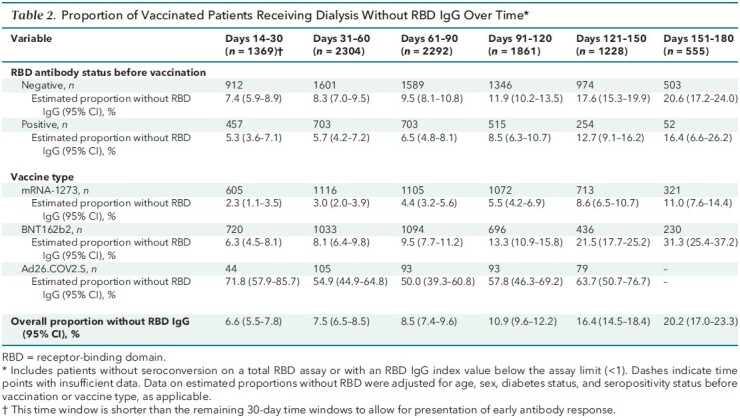
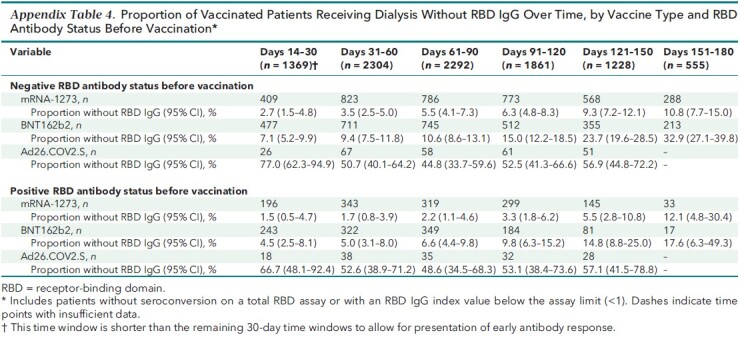
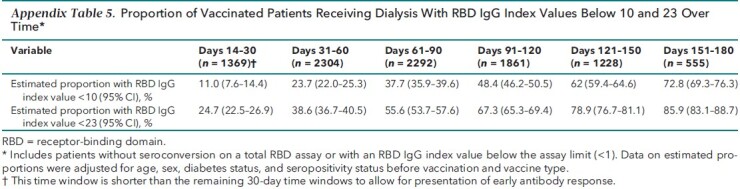
Table 2 shows the proportion of patients who lost detectable RBD IgG response over time up to the 5- to 6-month period after vaccination, accounting for age, sex, diabetes status, vaccine type, and RBD serostatus before vaccination, as applicable. Over time, a greater proportion of patients lost antibody response after vaccination; those who were vaccinated with mRNA-1273 had the smallest proportion without RBD IgG response at each follow-up time point. This pattern was observed regardless of RBD serostatus before vaccination and across vaccine types (Table 2; Appendix Table 4). A majority of patients (72.8% [CI, 69.3% to 76.3%]) had estimated RBD IgG index values below 10 in the 5- to 6-month period after vaccination (Appendix Table 5).
Table 2.
Proportion of Vaccinated Patients Receiving Dialysis Without RBD IgG Over Time*
Appendix Table 4.
Proportion of Vaccinated Patients Receiving Dialysis Without RBD IgG Over Time, by Vaccine Type and RBD Antibody Status Before Vaccination*
Appendix Table 5.
Proportion of Vaccinated Patients Receiving Dialysis With RBD IgG Index Values Below 10 and 23 Over Time*
Semiquantitative Postvaccination RBD IgG Values
Index values of RBD IgG decreased over time among patients with a detectable total RBD antibody response. By 5 to 6 months after vaccination, the median index values for the overall cohort were 8.4 (CI, 7.6 to 9.3) compared with a median peak index value of 91.9 (CI, 78.6 to 105.2) in the 14 to 30 days after vaccination completion (Figure 1, A). The overall trajectory of the response differed by SARS-CoV-2 infection status before vaccination, vaccine type, and age group but not by diabetes status (Figure 1, A to D; P for interaction <0.001 for the first 3 subgroups). The peak response was higher among patients with evidence of prior SARS-CoV-2 infection, but by the end of follow-up, the difference was attenuated (Figure 1, A; P = 0.077 for the difference in median index values at the end of follow-up).
Figure 1. RBD IgG index values over time among patients receiving dialysis, in the overall cohort and by prior SARS-CoV-2 infection (A), by vaccine type (B), by age group (C), and by diabetes status (D).
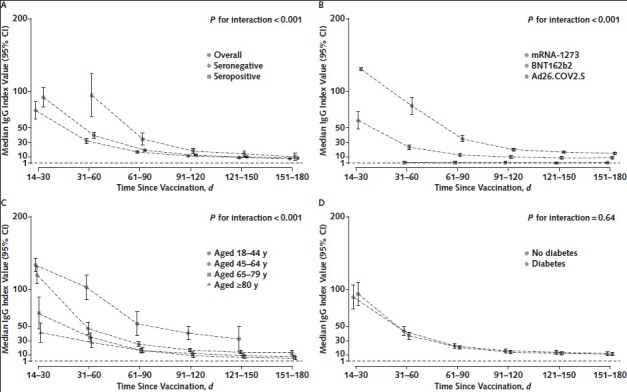
Median RBD IgG index values among patients who had seroconversion on the total RBD Ig assay are graphed by time since vaccination, with error bars representing 95% CIs for the median values. Index values account for age, sex, diabetes status, SARS-CoV-2 RBD serostatus before vaccination, and vaccine type, as applicable. A missing time point indicates insufficient data for the subgroup at that time point. An index value of 1 corresponds to 21.8 BAU/mL according to the World Health Organization standard. Index values <1 indicate a “negative” result on the assay. P values tested for interaction by subgroup, and a significant P value indicates that the trajectory of the response differed by the subgroup depicted. BAU = binding antibody units; RBD = receptor-binding domain.
Association of Postvaccination RBD IgG Values With Breakthrough Infection
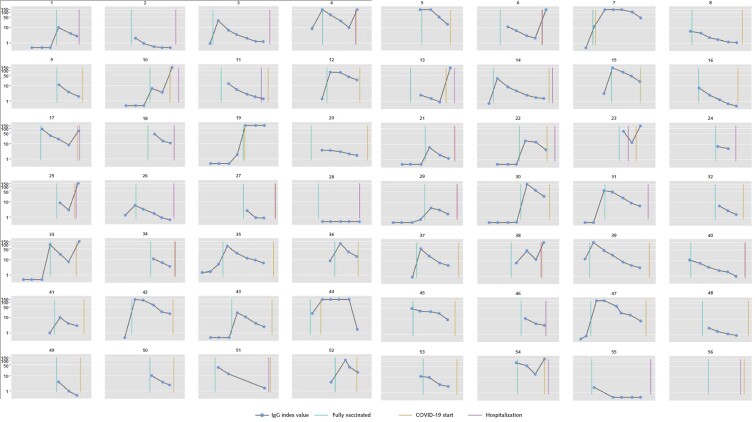
As of 14 September 2021, there were 56 breakthrough cases of COVID-19 (median time from vaccination to infection, 110 days [interquartile range, 85 to 143 days]). Appendix Figure 2 graphs the serial IgG index values of each case patient with markers for vaccination, COVID-19 diagnosis, and hospitalization. Among these 56 case patients, 25 (45%) were hospitalized with admission or discharge diagnoses indicating COVID-19, and an additional 15 (27%) had documented symptoms, with fever, cough, and chills the most common. The matching procedure achieved balance in the distribution of age, sex, and calendar month of vaccination among case patients and control patients (Appendix Table 6). Case patients had lower peak and prebreakthrough RBD IgG index values than control patients (Figure 2). Low prebreakthrough index values were associated with breakthrough infection among case patients (Table 3).
Appendix Figure 2. Serial IgG index values among patients with breakthrough infection.
Some panels are missing the gold lines indicating the start of COVID-19 because this date overlapped with the start of hospitalization.
Appendix Table 6.
Distributions of Age, Sex, and Diabetes Status in Case Patients and Control Patients
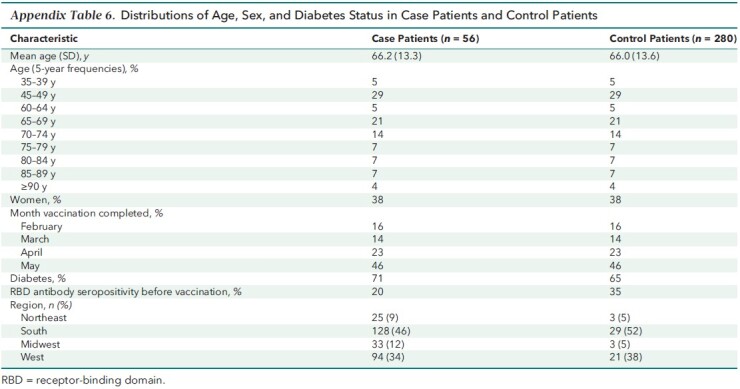
Figure 2. RBD IgG index values among case patients versus control patients.
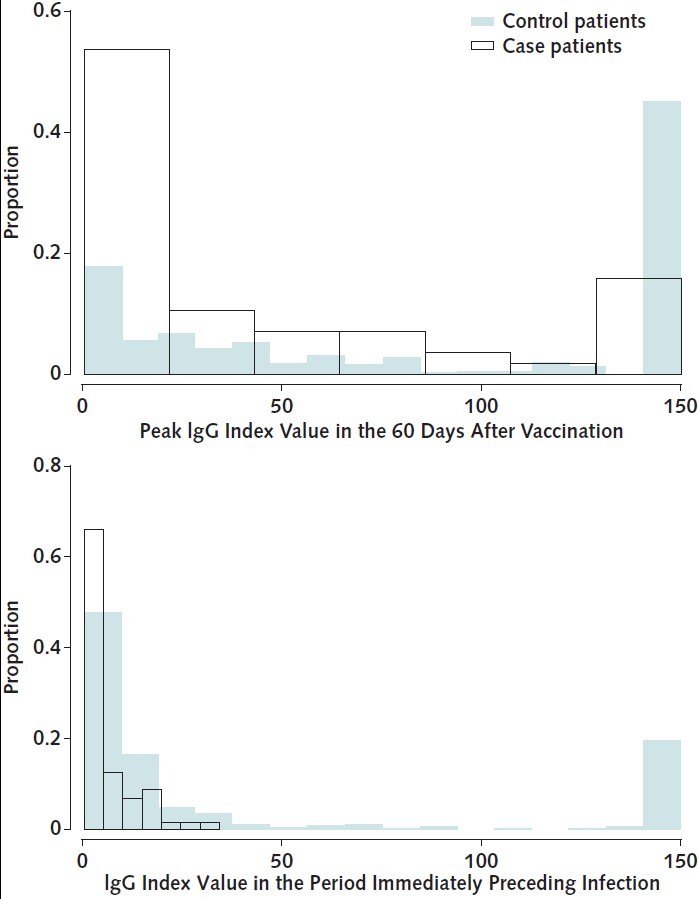
Overlapping histograms of peak RBD IgG values obtained within 60 days after vaccination (top) and IgG values obtained in the period immediately preceding infection (bottom) are graphed by case patient versus control patient status. The median time between prebreakthrough IgG values and COVID-19 diagnosis was 21 days (interquartile range, 14 to 28 days); the corresponding time for control patients was 21 days (interquartile range, 12 to 27 days). Median peak RBD IgG values were 98.0 (95% CI, 22.3 to 150) versus 15.1 (CI, 6.3 to 71.5) and prebreakthrough values were 11.0 (CI, 2.3 to 47.8) versus 2.8 (CI, 1.2 to 8.6) for control patients versus case patients, respectively. RBD = receptor-binding domain.
Table 3.
Association of Peak and Prebreakthrough RBD IgG Index Values With Breakthrough Infection in Patients Receiving Dialysis*
Discussion
In our diverse national cohort of patients receiving dialysis, 1 in 5 had lost a detectable RBD antibody response within 6 months after vaccination. Low levels of circulating RBD antibody were associated with risk for breakthrough infection. Although peak median antibody values were higher among patients with SARS-CoV-2 infection before vaccination, the proportion without a detectable response and median antibody values were largely similar among the patients with and without prior SARS-CoV-2 infection by the end of follow-up. Vaccine type was associated with the strength of the RBD antibody response throughout follow-up, with mRNA-1273 conferring the highest median RBD index values and the smallest proportion of patients without a detectable response.
Large national studies (28, 29) demonstrating waning vaccine effectiveness in parallel with a decline in postvaccination antibody response (30) provide indirect evidence that circulating antibody response could be a useful marker of vaccine effectiveness (31). Furthermore, participants from vaccine trials who had breakthrough infections were found to have lower antibody responses soon after vaccination than those without breakthrough infections (11, 12). However, current data, which emphasize neutralizing antibodies (12, 32, 33) or draw from clinical trials (11, 12) or health care worker cohorts (13), do not have broad applicability. Furthermore, most studies have evaluated peak or early (<60-day) vaccine response (11, 12).
In our cohort, we were able to implement an unbiased monthly serologic testing strategy to study postvaccination response in a geographically diverse population with sizeable proportions of racial and ethnic minority groups and patients with chronic illnesses (such as heart failure or diabetes). Using these real-world data from a time when the Delta variant of SARS-CoV-2 was also circulating widely in the United States, we found a clinically meaningful indication that antibody values measured using an accessible assay and at time points remote from vaccination are strongly associated with risk for breakthrough infection. This brings us closer to defining a “persisting antibody” threshold (34) for immunity. The relative importance of such a threshold may be greater for high-risk or immunocompromised groups compared with otherwise healthy persons because many components of their immune response may be impaired (35). This is evident in our data, as 40% of patients with breakthrough infection were hospitalized.
Our analysis suggests that a majority of vaccinated patients have circulating antibody levels 5 months after vaccination that render them vulnerable to a breakthrough infection. Although the overall number of breakthrough infections was low, even in the group with low antibody levels, exposure to SARS-CoV-2 is not uniform and patients are likely to have used other mitigation strategies, such as masking and social distancing. Nationwide data indicate that up to one quarter of patients receiving dialysis who have been hospitalized with COVID-19 have died (36), and similar or higher mortality rates are reported among immunocompromised groups with COVID-19. Thus, it is critical to identify high-risk persons who need heightened protection. That antibody thresholds may lack sensitivity is counterbalanced by the relatively low risk associated with enhanced mitigation for those classified as “at risk.” Research could also assess whether serologic testing improves uptake of the recent recommendations to add doses to the initial vaccination schedule. Uptake of these boosters, which may eventually be integrated into the recommended vaccine schedule, was below 20% in the United States as of November 2021 (37). Among high-risk groups, serologic testing of postvaccination antibody levels could inform decision making about the requirement for additional vaccine doses or other protective measures.
Several studies have reported a stronger initial antibody response with mRNA-1273 than with BNT162b2, putatively due to the higher mRNA dose in the mRNA-1273 formulation (35, 38). Our longitudinal analyses confirmed that persons vaccinated with mRNA-1273 maintained slightly higher index values than those receiving BNT162b2 throughout the 6-month follow-up. A single dose of Ad26.COV2.S did not yield a detectable antibody response in more than half of patients. The manufacturers of Ad26.COV2.S recently submitted data showing improved efficacy of this vaccine with 2 doses given 2 months apart (39). Finally, although healthy persons with SARS-CoV-2 infection before vaccination seem to mount peak antibody responses that are more than 2-fold higher than among those who were not infected (40), we found that antibodies among patients receiving dialysis wane over time regardless of prior SARS-CoV-2 infection and are equivalently low 6 months after vaccination. Patients with prior SARS-CoV-2 infection also experienced breakthrough infection in our cohort.
The limitations of this study include the reliance on RBD as the single measure of antibody response, without measuring the entire breadth of humoral response and any innate immune responses. Memory T-cell and B-cell responses likely play key roles (34) in protection against COVID-19 but are harder to measure (31, 34) and correlate with measured antibody responses even among immunosuppressed persons (35). We also used electronic health records to detect cases. Although this strategy is pragmatic and likely detects clinically meaningful events, it misses asymptomatic cases and those with minor symptoms. Vaccine types were not randomly allocated, and the number of patients who received Ad26.COV2.S was low; therefore, comparisons of response by vaccine type should be interpreted with caution. The number of breakthrough infections was relatively small but was proportionately higher than in healthy cohorts, and the absolute numbers were equivalent to or higher than those reported in clinical trials (11, 12). Furthermore, the observed associations despite the modest sample size and number of events highlight the relevance of our findings. We have limited data on comorbid conditions and reasons for loss to follow-up. In our prior longitudinal analysis, sensitivity analyses using multiple imputation to account for missingness yielded similar results (27). Finally, recommended immunization schedules are changing, and our study did not evaluate response to the now universal recommendation for third doses for adults.
In summary, among patients receiving dialysis, the humoral response to SARS-CoV-2 vaccination wanes and is associated with risk for breakthrough infection. Serologic testing using commercially available high-throughput assays could inform vaccination and enhanced mitigation strategies and potentially improve uptake of additional vaccination doses in immunocompromised and other high-risk populations. Even as new vaccine platforms and immunization schedules are used, further research investigating the associations between antibody response and risk for infection may guide management for immunocompromised hosts.
Appendix: Sample Size of Cohort Selected for Monthly Antibody Testing
Our study was conducted in partnership with the dialysis network U.S. Renal Care and Ascend Clinical Laboratory. Ascend Clinical tested remainder plasma from patients for SARS-CoV-2 antibody and anonymized all patient demographic, comorbidity, and laboratory data before transfer to Stanford University. We selected 4348 patients to follow from the 17 390 patients receiving dialysis without prior evidence of SARS-CoV-2 infection as of January 2021 in the U.S. Renal Care network. To estimate sample size, we used data on hepatitis B vaccination nonresponse by age strata that were previously published by Bruguera and colleagues (15), who evaluated immune response in 270 patients. In that study, the rates of nonresponse among persons aged 20 to 40 years, aged 40 to 60 years, and older than 60 years were 7%, 13%, and 35%, respectively. Our estimates of nonresponse among persons aged 18 to 44 years, 45 to 64 years, and 65 years or older were 5%, 15%, and 30%, respectively. Estimating these proportions of nonresponse with an absolute precision of 2% and oversampling by 15% resulted in a sample size estimate of 4222 (see Appendix Table 7).
Appendix Table 7.
Population and Subpopulation Sizes by Age and Number of Patients Required to Obtain a Prevalence Estimate With the Specified Absolute Precision and the Specified Proportion of Nonresponse to the Vaccine
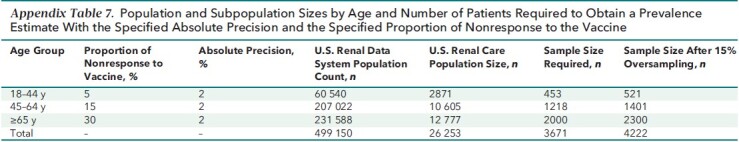
We used systematic sampling with fractional intervals. In systematic sampling, the patients are selected from the list using a fixed selection interval, calculated by dividing the total number of patients in the list by the desired number (17 390 / 4222 = 4.1). We thus randomly selected one number between 1 and 4 and then selected every fourth patient in the sampling frame sorted by ZIP code, age, sex, and race/ethnicity. This resulted in a sample size of 4344 patients receiving dialysis. In addition, since August 2020, we have followed 6551 patients among whom a subset had seroconversion before vaccination (that is, developed evidence of natural infection). All 540 U.S. Renal Care patients who were seropositive as of January 2021 were also selected to be followed. This resulted in a final sample of 4884 patients in whom we initiated monthly antibody testing; 4791 were able to be followed starting in February 2021, and 2563 were vaccinated and had at least 14 days of available data as of 14 September 2021 (see Appendix Figure 1 for the final vaccinated cohort).
Footnotes
This article was published at Annals.org on 14 December 2021.
* Drs. Block, Chertow, and Parsonnet contributed equally to this work.
References
- 1. Centers for Disease Control and Prevention. Table 1. Recommended Adult Immunization Schedule for ages 19 years or older, United States, 2020. Accessed at http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html on 14 September 2021.
- 2. Kroger A, Bahta L, Hunter P. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Accessed at www.cdc.gov/vaccines/hcp/acip-recs/general-recs/intro.html on 4 October 2021.
- 3. Plotkin SA . Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401-9. [PMID: ] doi: 10.1086/589862 [DOI] [PubMed] [Google Scholar]
- 4. Marin M , Marlow M , Moore KL , et al. Recommendation of the Advisory Committee on Immunization Practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Morb Mortal Wkly Rep. 2018;67:33-8. [PMID: ] doi: 10.15585/mmwr.mm6701a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mast EE , Weinbaum CM , Fiore AE , et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1-33. [PMID: ] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Guidelines for Vaccinating Kidney Dialysis Patients and Patients with Chronic Kidney Disease. December 2012. Accessed at www.cdc.gov/dialysis/PDFs/Vaccinating_Dialysis_Patients_and_Patients_dec2012.pdf on 10 June 2021.
- 7. Buti M , Viladomiu L , Jardi R , et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in hemodialysis patients. Am J Nephrol. 1992;12:144-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 8. Broeders NE , Hombrouck A , Lemy A , et al. Influenza A/H1N1 vaccine in patients treated by kidney transplant or dialysis: a cohort study. Clin J Am Soc Nephrol. 2011;6:2573-8. [PMID: ] doi: 10.2215/CJN.04670511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peces R , de la Torre M , Alcázar R , et al. Prospective analysis of the factors influencing the antibody response to hepatitis B vaccine in hemodialysis patients. Am J Kidney Dis. 1997;29:239-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 10. Anand S , Montez-Rath ME , Han J , et al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol. 2021;32:2435-8. [PMID: ] doi: 10.1681/ASN.2021050611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbert PB , Montefiori DC , McDermott AB , et al; Immune Assays Team. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2021:eab3435. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng S , Phillips DJ , White T , et al; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032-40. [PMID: ] doi: 10.1038/s41591-021-01540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergwerk M , Gonen T , Lustig Y , et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474-84. [PMID: ] doi: 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anand S , Montez-Rath M , Han J , et al. Estimated SARS-CoV-2 seroprevalence in US patients receiving dialysis 1 year after the beginning of the COVID-19 pandemic. JAMA Netw Open. 2021;4:e2116572. [PMID: ] doi: 10.1001/jamanetworkopen.2021.16572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruguera M , Rodicio JL , Alcazar JM , et al. Effects of different dose levels and vaccination schedules on immune response to a recombinant DNA hepatitis B vaccine in haemodialysis patients. Vaccine. 1990;8 Suppl:S47-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 16. U.S. Food and Drug Administration. EUA Authorized Serology Test Performance. Accessed at www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance on 4 December 2021.
- 17. Schnurra C , Reiners N , Biemann R , et al. Comparison of the diagnostic sensitivity of SARS-CoV-2 nucleoprotein and glycoprotein-based antibody tests. J Clin Virol. 2020;129:104544. [PMID: ] doi: 10.1016/j.jcv.2020.104544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Public Health England; University of Oxford; Oxford University Hospitals NHS Foundation Trust. Evaluation of sensitivity and specificity of four commercially available SARS-CoV-2 antibody immunoassays. July 2020. Accessed at www.gov.uk/government/publications/covid-19-head-to-head-laboratory-evaluation-of-4-commercial-serological-assays on 8 July 2020.
- 19. Kristiansen PA , Page M , Bernasconi V , et al. WHO international standard for anti-SARS-CoV-2 immunoglobulin [Letter]. Lancet. 2021;397:1347-8. [PMID: ] doi: 10.1016/S0140-6736(21)00527-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou G . A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 21. Hao L, Naiman DQ. Quantile Regression. Sage; 2007.
- 22. Marino MF, Farcomeni A. Linear quantile regression models for longitudinal experiments: an overview. METRON. 2015;73:229-47. doi:10.1007/s40300-015-0072-5
- 23. Knol MJ , Vandenbroucke JP , Scott P , et al. What do case–control studies estimate? Survey of methods and assumptions in published case–control research. Am J Epidemiol. 2008;168:1073-81. [PMID: ] doi: 10.1093/aje/kwn217 [DOI] [PubMed] [Google Scholar]
- 24. Labrecque JA , Hunink MMG , Ikram MA , et al. Do case–control studies always estimate odds ratios. Am J Epidemiol. 2021;190:318-21. [PMID: ] doi: 10.1093/aje/kwaa167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legros V , Denolly S , Vogrig M , et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318-27. [PMID: ] doi: 10.1038/s41423-020-00588-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Addetia A , Crawford KHD , Dingens A , et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58. [PMID: ] doi: 10.1128/JCM.02107-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anand S , Montez-Rath ME , Han J , et al. Serial SARS-CoV-2 receptor-binding domain antibody responses in patients receiving dialysis. Ann Intern Med. 2021;174:1073-80. [PMID: ] doi: 10.7326/M21-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chemaitelly H , Tang P , Hasan MR , et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021. [PMID: ] doi: 10.1056/NEJMoa2114114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg Y , Mandel M , Bar-On YM , et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021. [PMID: ] doi: 10.1056/NEJMoa2114228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levin EG , Lustig Y , Cohen C , et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021. [PMID: ] doi: 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krammer F . A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27:1147-8. [PMID: ] doi: 10.1038/s41591-021-01432-4 [DOI] [PubMed] [Google Scholar]
- 32. Earle KA , Ambrosino DM , Fiore-Gartland A , et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423-8. [PMID: ] doi: 10.1016/j.vaccine.2021.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khoury DS , Cromer D , Reynaldi A , et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205-11. [PMID: ] doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 34. Amanna IJ , Slifka MK . Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011;411:206-15. [PMID: ] doi: 10.1016/j.virol.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stumpf J , Siepmann T , Lindner T , et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [PMID: ] doi: 10.1016/j.lanepe.2021.100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu CM , Weiner DE , Aweh G , et al. COVID-19 among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77:748-756.e1. [PMID: ] doi: 10.1053/j.ajkd.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention. COVID-19 Vaccinations in the United States. Accessed at https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total on 23 November 2021.
- 38. Steensels D , Pierlet N , Penders J , et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533-5. [PMID: ] doi: 10.1001/jama.2021.15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson & Johnson. Johnson & Johnson Announces Real-World Evidence and Phase 3 Data Confirming Strong and Long-Lasting Protection of Single-Shot COVID-19 Vaccine in the U.S. 21 September 2021. Accessed at www.jnj.com/johnson-johnson-announces-real-world-evidence-and-phase-3-data-confirming-strong-and-long-lasting-protection-of-single-shot-covid-19-vaccine-in-the-u-s on 6 October 2021.
- 40. Anderson M , Stec M , Rewane A , et al. SARS-CoV-2 antibody responses in infection-naive or previously infected individuals after 1 and 2 doses of the BNT162b2 vaccine. JAMA Netw Open. 2021;4:e2119741. [PMID: ] doi: 10.1001/jamanetworkopen.2021.19741 [DOI] [PMC free article] [PubMed] [Google Scholar]