Most patients with COVID-19 are asked to self-isolate and monitor their symptoms at home. However, their conditions may decline rapidly and unpredictably. The authors studied the use of a remote patient monitoring service via text messaging with clinical support to facilitate emergency department and hospital care for patients who require it.
Visual Abstract. Outcomes of Automated Home Monitoring for COVID-19.
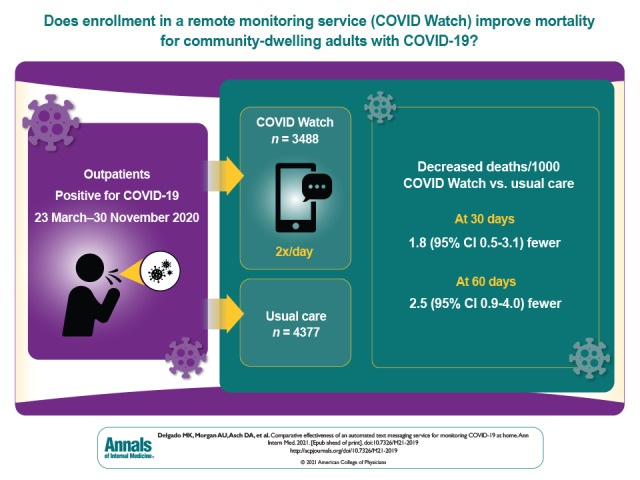
Most patients with COVID-19 are asked to self-isolate and monitor their symptoms at home. However, their conditions may decline rapidly and unpredictably. The authors studied the use of a remote patient monitoring service via text messaging with clinical support to facilitate emergency department and hospital care for patients who require it.
Abstract
Background:
Although most patients with SARS-CoV-2 infection can be safely managed at home, the need for hospitalization can arise suddenly.
Objective:
To determine whether enrollment in an automated remote monitoring service for community-dwelling adults with COVID-19 at home (“COVID Watch”) was associated with improved mortality.
Design:
Retrospective cohort analysis.
Setting:
Mid-Atlantic academic health system in the United States.
Participants:
Outpatients who tested positive for SARS-CoV-2 between 23 March and 30 November 2020.
Intervention:
The COVID Watch service consists of twice-daily, automated text message check-ins with an option to report worsening symptoms at any time. All escalations were managed 24 hours a day, 7 days a week by dedicated telemedicine clinicians.
Measurements:
Thirty- and 60-day outcomes of patients enrolled in COVID Watch were compared with those of patients who were eligible to enroll but received usual care. The primary outcome was death at 30 days. Secondary outcomes included emergency department (ED) visits and hospitalizations. Treatment effects were estimated with propensity score–weighted risk adjustment models.
Results:
A total of 3488 patients enrolled in COVID Watch and 4377 usual care control participants were compared with propensity score weighted models. At 30 days, COVID Watch patients had an odds ratio for death of 0.32 (95% CI, 0.12 to 0.72), with 1.8 fewer deaths per 1000 patients (CI, 0.5 to 3.1) (P = 0.005); at 60 days, the difference was 2.5 fewer deaths per 1000 patients (CI, 0.9 to 4.0) (P = 0.002). Patients in COVID Watch had more telemedicine encounters, ED visits, and hospitalizations and presented to the ED sooner (mean, 1.9 days sooner [CI, 0.9 to 2.9 days]; all P < 0.001).
Limitation:
Observational study with the potential for unobserved confounding.
Conclusion:
Enrollment of outpatients with COVID-19 in an automated remote monitoring service was associated with reduced mortality, potentially explained by more frequent telemedicine encounters and more frequent and earlier presentation to the ED.
Primary Funding Source:
Patient-Centered Outcomes Research Institute.
As of 24 August 2021, the United States is experiencing its fourth surge of SARS-CoV-2 (COVID-19) cases, averaging more than 153 000 cases per day, with several regions experiencing severe hospital-capacity strain (1). Nearly 90% of patients with COVID-19 are asked to self-isolate and monitor their symptoms at home (2–5). Remote outpatient monitoring of patients with COVID-19 has been needed because patients with SARS-CoV-2 infection can decline rapidly and unpredictably, and because of their own limited capacity to manage acute symptoms and concerns about staff safety, office-based outpatient practices often redirect patients with confirmed or suspected COVID-19 to hospitals. As a result, emergency departments (EDs) and hospitals have been overwhelmed during surge periods of high community incidence rates and prevalence (6, 7). However, patients with COVID-19 whose symptoms are worsening at home may delay or face delays in seeking care (8–10). Remote monitoring has the potential to facilitate ED- and hospital-level care for patients who require it while supporting access to care for patients who can safely remain at home (11–13).
Toward those goals, the University of Pennsylvania Health System (Penn Medicine) developed COVID Watch, an automated text message–based, remote monitoring program with 24/7 clinical support (12). This study compares outcomes for patients enrolled in COVID Watch with those of patients who were eligible to enroll but received usual care, with the hypothesis that enrollment in COVID Watch was associated with reduced mortality.
Methods
This is a retrospective cohort study that follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline for reporting observational studies. It was approved by the Institutional Review Board at the University of Pennsylvania.
Setting
Penn Medicine is an academic health system serving large portions of southeast Pennsylvania, New Jersey, and Delaware, with 6 hospitals and hundreds of outpatient practices.
Community–dwelling adults (aged >18 years) with presumed or confirmed COVID-19 could be enrolled in COVID Watch through an electronic health record order. The health care worker entering the electronic order did not have to be a physician and could include nurses, social workers, and care coordinators involved in the patient's care in the health system. Enrollment often occurred during or after a provider had a conversation with a patient. Patients were not required to have established care with a Penn Medicine provider, having only received COVID-19 testing at Penn Medicine, for example. The design of COVID Watch is detailed elsewhere (12) and in Supplement Figures 1 and 2. The first patient was enrolled on 23 March 2020.
The enrollment order results in an instantaneous text message sent to the patient introducing them to the program. Once enrolled and the patient texts back confirming they want to engage with the program, they begin receiving twice-daily automated text message check-ins asking the following question: “How are you feeling compared to 12 hours ago: better, same, or worse?” Patients replying “worse” receive a follow-up question: “Is it harder than usual for you to breath: yes or no?” For patients who respond “yes,” an alert is generated for a telemedicine clinician to contact the patient by telephone within 1 hour. Outside of the twice-daily check-ins, patients were instructed to text “worse” to trigger a clinician call back. During the daytime, nurses were the primary responders, with support from nurse practitioners and physicians. At night, escalations were directly relayed to on-call nurse practitioners and physicians.
The clinical team for COVID Watch received regular training and guidelines for managing patients with COVID-19. After an escalation, clinicians could provide reassurance, advise how best to manage symptoms at home, prescribe medications, or redirect patients to the ED. The program continued for 14 days from enrollment, at which point patients were offered the option to remain enrolled for 7 more days. A Spanish-language version of COVID Watch was made available on 18 May 2020.
Data Sources and Study Sample
We included all community-dwelling adults who tested positive for SARS-CoV-2 at Penn Medicine as outpatients between 23 March (the start of COVID Watch) and 30 November 2020 and determined if they were enrolled in COVID Watch versus usual care. We excluded patients who did not meet eligibility criteria for COVID Watch: those who were younger than 18 years, were actively enrolled in home health services or hospice, or were currently in long-term care (for example, skilled-nursing facility, long-term acute care, or acute rehabilitation). To further reduce the potential for bias, we excluded those who were tested in a location where COVID Watch enrollment had not yet begun, were previously in long-term care or hospice, or had a documented do not resuscitate or do not intubate code status before COVID-19 test collection. If patients had multiple positive test results for COVID-19, the first test with a positive result was chosen as the index test. We excluded patients enrolled in COVID Watch more than 7 days before or after the date of their index COVID-19 test collection to avoid attribution of outcomes to COVID Watch enrollment for other episodes of care (for example, repeated COVID-19 testing).
We derived patient-level sociodemographic and clinical characteristics from Penn Medicine's electronic health record. We derived patient death data, clinical encounter details (ED visits, hospitalizations, outpatient office visits, and telemedicine), and documented details (for example, encounter dates and in-hospital vs. out-of-hospital mortality) from Penn Medicine's electronic record and a regional health information exchange containing data from 53 surrounding hospitals in Pennsylvania, New Jersey, and Delaware (14).
Outcomes
Outcomes were ascertained within 30 days of the date of COVID-19 test collection. We used an intention-to-treat approach in which any patient enrolled in COVID Watch was included in the COVID Watch group even if they did not respond to any text messages. The primary outcome was any-site mortality. We separately analyzed deaths that occurred in hospital or out of hospital. Secondary outcomes included rates of total ED encounters (including discharges and hospitalizations), hospitalizations (inpatient admissions and observation), and outpatient encounters (in-person office visits and telemedicine, including video or telephone visits). We tabulated a composite outcome of days alive and out of the hospital, which factors in death and the ability to remain out of the hospital by accounting for ED visits and, if hospitalized, the length of stay (15–18). As a secondary analysis, we extended the follow-up window to 60 days for mortality and health care use measures.
Among patients who required acute care, we tabulated the time from the collection of the positive result to ED presentation, the ED vital signs, the length of stay if hospitalized, and whether the patient needed intubation and mechanical ventilation. Finally, we tabulated process outcomes among those enrolled in COVID Watch, including how many text message check-ins they responded to, the proportion who triggered an escalation to the on-call nurse, and the triage recommendations of the on-call nurse.
Covariates
We collected patients' COVID-19 test data, age, sex, race/ethnicity, primary insurance, county of residence, and household income derived from ZIP code median values. We included comorbidities known to be associated with severe COVID-19 illness or treatment adherence (19–24). We captured whether patients had a listed primary care provider and baseline health care use, including the frequency of encounters with Penn Medicine in the prior year—ED visits, hospitalizations, and outpatient encounters.
Statistical Analysis
All extracted variables were checked for outliers and missingness. Only 21 patients of 7865 who met inclusion criteria (0.27%) were missing any covariate data and excluded from the primary analysis. To account for imbalances on covariates between comparison groups in the outpatient cohort, we estimated propensity scores (the probability of enrollment in COVID Watch) using logistic regression. After using inverse probability of treatment weighting, we found that all covariates achieved balance between patients enrolled in COVID Watch and usual care with mean standardized differences of less than 0.1 (25). Outcome models weighted by inverse probability of treatment weights were either logistic for binary outcomes or linear for continuous outcomes. We did a priori subgroup analyses of outcomes by race/ethnicity.
To assess the robustness of our findings, we assessed the sensitivity of our mortality results to potential unmeasured confounding by using the Rosenbaum γ approach. We also tabulated any deaths that occurred in patients with missing covariates (n = 21). We also did a per protocol analysis, excluding any patients enrolled in COVID Watch who did not respond to any text messages, including the enrollment invitation text. Finally, we tabulated baseline characteristics and diagnoses of patients who died. All analyses were done using R, version 3.6.0 (R Project for Statistical Computing). All analyses used 2-sided statistical tests, and a P value less than 0.05 was considered statistically significant. Additional methodological details about the development of propensity scores can be found in the Supplement.
Role of the Funding Source
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Results
Baseline Characteristics
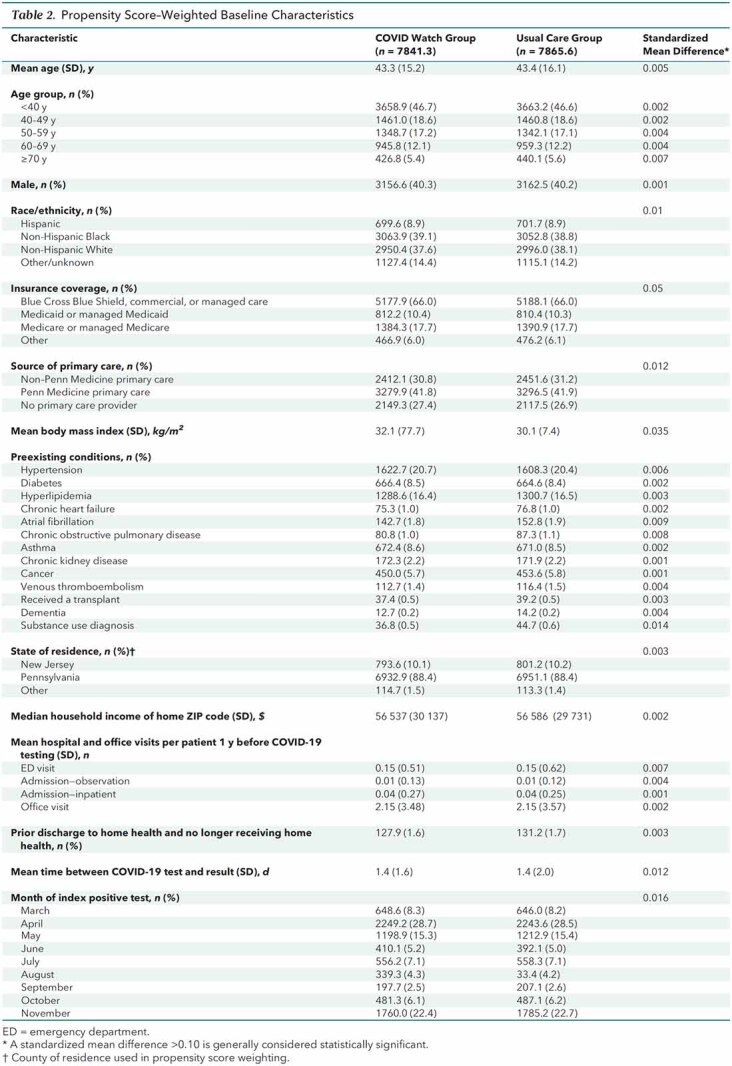
After ineligible patients (Appendix Figure) were excluded, 7865 outpatients were available for analysis: 3488 patients enrolled in COVID Watch and 4377 in usual care. Patients in COVID Watch were enrolled a mean of 1.8 days (SD, 2.3) after the date of COVID-19 test collection. Before propensity score weighting, patients enrolled in COVID Watch were similar to those who received usual care in age but were less likely to be male (37.1% vs. 42.5%) and were more likely to identify as non-Hispanic Black (47.9% vs. 32.1%), have public insurance (29.3% vs. 26.6%), not have a primary care provider (28.3% vs. 24.5%), have a higher mean body mass index (33.6 vs. 29.7 kg/m2), have certain comorbidities (hypertension, diabetes, and asthma), have a lower median household income ($51 491 vs. $60 638), have higher rates of ED and office visits in the past year, and be enrolled in the earlier months of the pandemic (Table 1; Supplement Figure 3). Covariates were well balanced after propensity score weighting (Table 2; Supplement Figure 4).
Appendix Figure. Study sample flowchart and patients excluded.
Table 1.
Unadjusted Baseline Characteristics
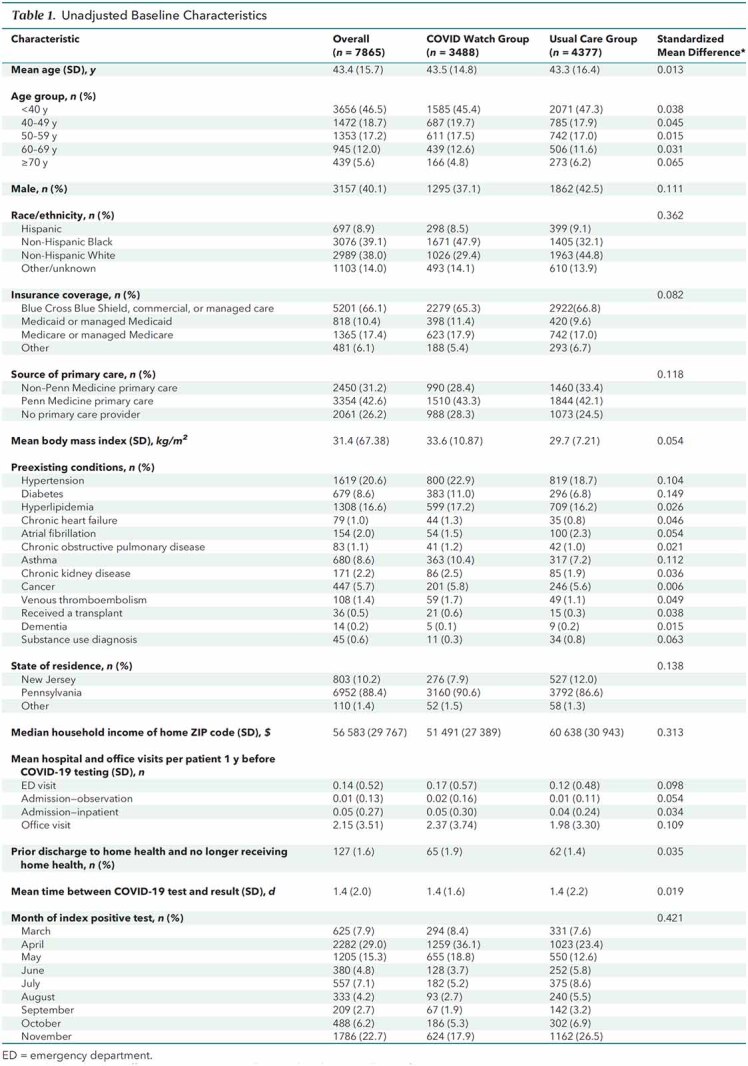
Table 2.
Propensity Score–Weighted Baseline Characteristics
Outcomes
Of the 3488 patients enrolled in COVID Watch, 3028 (86.8%) engaged by responding to at least 1 text (mean, 23 check-in responses). Of patients who engaged, 434 (14.3%) triggered an escalation to a registered nurse, with mean response time of 24 minutes (Supplement Table 1).
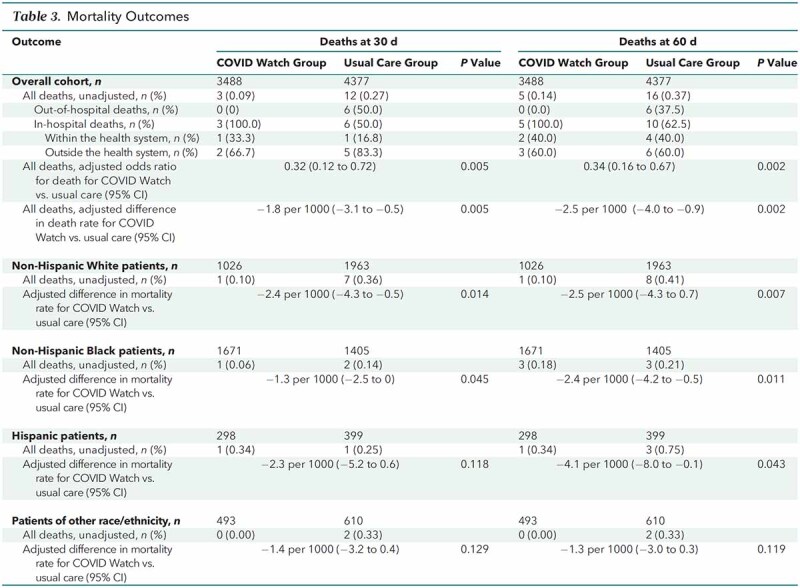
At 30 days, 3 of 3488 (0.09%) patients enrolled in COVID Watch died versus 12 of 4377 (0.27%) who received usual care (Table 3). Of the deaths, 0 in COVID Watch occurred outside the hospital versus 6 of those who received usual care. Among in-hospital deaths, 2 of the 3 in COVID Watch occurred in hospitals outside of Penn Medicine compared with 5 of 6 in the usual care group. At 60 days, there were 2 additional in-hospital deaths among those enrolled in COVID Watch and 4 among those who received usual care. In total, 37.5% of the deaths at 60 days in the usual care group occurred outside the hospital. After propensity score weighting and modeling, at 30 days, those enrolled in COVID Watch had an odds ratio for overall mortality of 0.32 (95% CI, 0.12 to 0.72), with a difference of −1.8 deaths per 1000 patients (CI, −3.1 to −0.5) (P = 0.005). At 60 days, COVID Watch outcomes remained consistently better, with a difference of −2.5 deaths per 1000 patients (CI, −4.0 to −0.9) (P = 0.002). Furthermore, we found that COVID Watch was associated with equitable treatment benefits, with White, Black, and Hispanic subgroups all having reduced mortality by 60 days (Table 3).
Table 3.
Mortality Outcomes
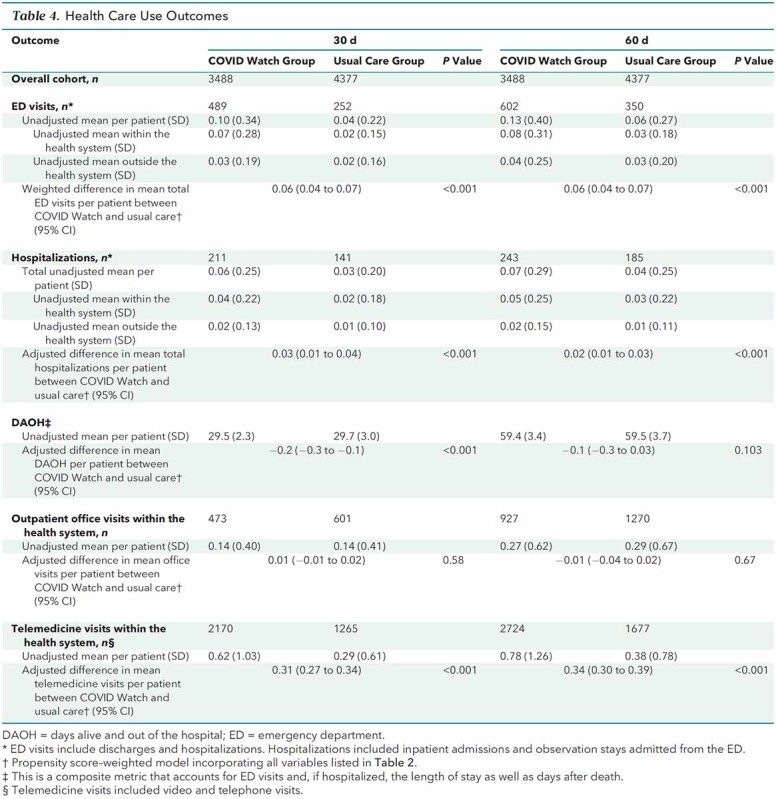
Within 30 days, COVID Watch patients had a total of 489 ED encounters (121 per 1000), with 384 (78.5%) occurring within Penn Medicine and 105 (21.5%) outside the health system. Of the 489 ED encounters by COVID Watch patients, only 62 (12.7%) were by those who never engaged with COVID Watch. Patients receiving usual care had a total of 252 ED encounters (50 per 1000), with 161 (63.9%) occurring within Penn Medicine and 91 (36.1%) outside the health system. After propensity score weighting, we found that COVID Watch patients had a higher mean number of ED encounters per patient (adjusted difference, 0.06 [CI, 0.04 to 0.07]; P < 0.001) and mean number of hospitalizations per patient (adjusted difference, 0.03 [CI, 0.01 to 0.04]; P < 0.001). Patients in COVID Watch had a similar number of office visits but a greater mean number of telemedicine encounters per patient (adjusted difference, 0.31 [CI, 0.27 to 0.34]; P < 0.001) than usual care patients within 30 days after their date of COVID-19 test collection.
When ED and hospital use was factored in with mortality, the mean number of days alive and out of the hospital within 30 days was marginally lower among COVID Watch patients (adjusted absolute difference, −0.2 [CI, −0.3 to −0.1]; P < 0.001) but was similar at 60 days (adjusted absolute difference, −0.1 [CI, −0.3 to 0.03]; P = 0.103) (Table 4) as deaths accrued and health care use diminished.
Table 4.
Health Care Use Outcomes
Among patients who presented to any hospital (within Penn Medicine or outside) for the first time in the 30 days after their date of COVID-19 test collection (Table 4), COVID Watch patients presented to the ED sooner (6.6 vs. 8.9 days) (propensity score–weighted difference, −1.9 days [CI, −2.9 to −0.9 days]; P < 0.001).
Among the subset of patients who presented to the ED within Penn Medicine (Table 5), COVID Watch enrollees compared with usual care patients presented to the ED even sooner (6.1 vs. 9.0 days) (propensity score–weighted difference, −2.9 days [CI, −4.1 to −1.7 days]; P < 0.001). During the ED evaluation, there were no statistically significant differences in vital signs or need for intubation and ventilation. However, compared with the 10.8% of usual care patients who received dexamethasone in Penn Medicine hospitals, the 11.3% of COVID Watch patients who were treated with dexamethasone received it sooner (propensity score–weighted difference, −3.0 days [CI, −5.6 to −0.4 days]; P = 0.026).
Table 5.
Timing and Severity of Initial Presentations to Health System EDs Within 30 Days*

Sensitivity Analyses
The Rosenbaum bounds analysis showed there would need to be 1.8 times greater odds of differential assignment to COVID Watch attributable to unobserved factors, a substantial amount of unmeasured confounding needed to reverse the statistically significant findings (Supplement Table 2). There were also no deaths among the 21 patients excluded from the outpatient cohort because of missing covariate data (Supplement Table 3). We also found that 2 of the 5 deaths in COVID Watch occurred among the 13.2% of the patients who never engaged with the system. The per protocol analysis of patients who engaged with COVID Watch indicated even stronger treatment effects: odds ratio for death of 0.25 (CI, 0.10 to 0.55), with a difference of −2.8 deaths per 1000 patients (CI, −4.3 to −1.3 deaths) (P = 0.001) (Supplement Table 4). Finally, baseline characteristics of patients who died varied but were not statistically significant from each other across treatment groups (Supplement Table 5), and coded diagnoses of in-hospital deaths were consistent with COVID-19 being the primary cause of death (Supplement Table 6).
Discussion
This study has 4 main findings. First, the mortality rate for community-dwelling adults with COVID-19 was significantly lower among those in COVID Watch compared with usual care, even after adjustment for differences in patients' clinical and sociodemographic characteristics. Second, more than one third of the deaths in the usual care group occurred outside the hospital versus none among those in COVID Watch. Third, patients in COVID Watch were more likely to present to the hospital, and they presented earlier. Fourth, all major racial and ethnic subgroups had reduced mortality rates when enrolled in COVID Watch.
These findings imply that COVID Watch is associated with a 64% relative reduction in the risk for death and that 1 life was saved for every 400 patients enrolled—or about 1 every 4 days during peak enrollment weeks. Although remote patient monitoring programs used to manage patients with COVID-19 outside of hospital settings have been described (26, 27), including 1 study from Kaiser Permanente that reported unadjusted morality rates of 2.3% in usual care versus 1.3% with remote monitoring (28), we believe ours to be the first risk-adjusted study to show improved survival.
Public health messaging strongly promoted staying home to promote social distancing and decrease hospital strain during the pandemic (29, 30). However, those messages were accompanied by decreases in emergent conditions presenting to the ED and increased out-of-hospital deaths (8, 9, 31–39). In this study, 37.5% of deaths among patients who received usual care occurred outside the hospital versus none among patients in COVID Watch, which is consistent with the interpretation that COVID Watch exerts its effect by increasing vigilance over those at home and efficiently sorting them into those who will benefit from the ED and those who will not (8, 32).
Further evidence supports this mechanistic hypothesis. Patients in COVID Watch were more likely to present to the hospital and presented earlier, likely improving their ability to benefit from the care they receive. For example, dexamethasone reduces mortality and length of stay for patients with COVID-19 (40), and the benefit may be larger if the drug is administered earlier in the disease course (41–45). We found that among those who received dexamethasone in Penn Medicine hospitals, COVID Watch patients received the medication 3 days earlier on average. We also found that the treatment effects associated with COVID Watch were stronger among those who engaged with the remote monitoring service; 2 of the 5 deaths in the COVID Watch group were among the 13% of enrolled patients who never engaged the system. The constellation of these findings is consistent with the view that COVID Watch operates as an early warning and referral system for community-dwelling patients (13).
The combination of technology-based, automated remote monitoring (46) backed by clinician support may be necessary ingredients for the observed clinical effect. Because COVID Watch was automated, only 2 to 4 staff members were required to oversee more than 1000 patients at a time, far fewer than personnel-intensive calling systems (26, 27). Because it relied on symptom self-report, COVID Watch did not require dedicated temperature sensors or pulse oximetry (12, 27, 47–49). The use of additional equipment in the home varies substantially across remote patient monitoring programs, and its incremental value is unknown (50). Future research is needed to determine whether this type of monitoring service could be adapted to other acute conditions (for example, pneumonia or cellulitis) and chronic conditions (for example, asthma or diabetes) in which automated text check-ins and low barrier access to rapid clinical assessment and ED triage could improve outcomes.
In our study, non-Hispanic Black patients were more likely to be enrolled in COVID Watch than usual care, and Hispanic patients were about as likely to be in either group. White, Black, and Hispanic populations also had significantly reduced mortality when enrolled in COVID Watch, and the overall mortality rates were lower relative to reports nationally (28, 39, 51, 52). These findings suggest no substantial racial or ethnic barriers to program enrollment or its effectiveness and that implementing this type of remote monitoring service has the potential to reduce racial disparities in regions in which Black and Hispanic patients have decreased access to care and higher mortality rates.
This study has limitations. First, we could observe deaths in our hospitals and hospitals outside our health system via a health information exchange linkage, but we may have incomplete ascertainment of out-of-hospital deaths. Death certificate linkage via the National Death Index was not available at the time of manuscript submission because of lag times in these databases. However, COVID Watch patients were highly engaged with Penn Medicine, replying to a mean of 23 text message check-ins through the program, which suggests that their deaths would more likely be ascertained.
Second, we cannot capture hospital use outside of the geography of health information exchange. Fortunately, 99.4% of our study sample had residential ZIP codes within the geographic region covered by the health information exchange, decreasing the potential for incomplete capture of hospital use.
Third, we cannot capture the reasons patients were enrolled in COVID Watch or whether patients were verbally offered COVID Watch and declined it. We also cannot capture social needs, the nature and timing of symptoms before the testing date, and other unobserved confounders that may affect outcomes. However, higher rates of characteristics associated with worse outcomes from COVID-19 were seen in the COVID Watch group, including the lack of a primary care physician, Black race, residing in a lower-income ZIP code, greater body mass index, higher rates of high-risk comorbidities, and higher proportion treated early in the pandemic (6, 24, 53–55), all suggesting higher expected mortality among the COVID Watch group. Furthermore, our sensitivity analyses indicated there would need to be a 1.8 times greater odds of differential assignment to COVID Watch versus the control group that was attributable to unobserved factors. Given the large number of important covariates we have accounted for in our analysis, it is unlikely that such an impactful covariate was not included.
Fourth, outcomes measured reflect care received at a single health system, a select set of hospitals in a specific region of the United States, which may limit generalizability of our findings. However, this study included populations with a diverse set of comorbidities and sociodemographic characteristics. Relatedly, we were unable to measure clinical status and treatments provided during hospital encounters outside our health system. Given the differential use of hospitals outside our health system, clinical treatment rates seen within our health system should not be extrapolated to the subset treated in hospitals outside our health system.
This study also has strengths. It reflects what is, to our knowledge, the largest and most comprehensive sample and evaluation of a remote monitoring service for COVID-19 in the United States. There is careful adjustment for differences in patient characteristics that have a conservative bias. The effect size is large, and the results are accompanied by plausible mechanisms whose specific elements are suggested by secondary analyses.
In conclusion, enrollment in an automated text messaging service among community-dwelling adults newly diagnosed with COVID-19 in outpatient settings was associated with reduced mortality, potentially explained by increased and earlier presentation to the ED by those benefiting from early interventions. These results reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.
Supplementary Material
Footnotes
This article was published at Annals.org on 16 November 2021.
References
- 1. Centers for Disease Control and Prevention. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Accessed at https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases on 23 April 2021.
- 2. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458-464. [PMID: ] doi: 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. COVID-19: what to do if you are sick. Accessed at www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html on 16 May 2020.
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [PMID: ] doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757-1766. [PMID: ] doi: 10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 6. Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181:471-478. [PMID: ] doi: 10.1001/jamainternmed.2020.8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg S, Patel K, Pham H, et al. Clinical trends among US adults hospitalized with COVID-19, March to December 2020. A cross-sectional study. Ann Intern Med. 2021;174:1409-1419. [PMID: ] doi: 10.7326/M21-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catal Innov Care Deliv. 2020. doi:10.1056/CAT.20.0193
- 9. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits - United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:699-704. [PMID: ] doi: 10.15585/mmwr.mm6923e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324:96-99. [PMID: ] doi: 10.1001/jama.2020.9972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watson AR, Wah R, Thamman R. The value of remote monitoring for the COVID-19 pandemic. Telemed J E Health. 2020;26:1110-1112. [PMID: ] doi: 10.1089/tmj.2020.0134 [DOI] [PubMed] [Google Scholar]
- 12. Morgan AU, Balachandran M, Do D, et al. Remote monitoring of patients with Covid-19: design, implementation, and outcomes of the first 3,000 patients in COVID Watch. NEJM Catal Innov Care Deliv. 2020. doi:10.1056/CAT.20.0342
- 13. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382:1679-1681. [PMID: ] doi: 10.1056/NEJMp2003539 [DOI] [PubMed] [Google Scholar]
- 14. HealthShare Exchange. Current participants. Accessed at www.healthshareexchange.org/current-participants on 30 March 2021.
- 15. Moonesinghe SR, Jackson AIR, Boney O, et al; Standardised Endpoints in Perioperative Medicine-Core Outcome Measures in Perioperative and Anaesthetic Care (StEP-COMPAC) Group. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine initiative: patient-centred outcomes. Br J Anaesth. 2019;123:664-670. [PMID: ] doi: 10.1016/j.bja.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 16. Jerath A, Austin PC, Wijeysundera DN. Days alive and out of hospital: validation of a patient-centered outcome for perioperative medicine. Anesthesiology. 2019;131:84-93. [PMID: ] doi: 10.1097/ALN.0000000000002701 [DOI] [PubMed] [Google Scholar]
- 17. Szarek M, Steg PG, DiCenso D, et al. Alirocumab reduces total hospitalizations and increases days alive and out of hospital in the ODYSSEY OUTCOMES trial. Circ Cardiovasc Qual Outcomes. 2019;12:e005858. [PMID: ] doi: 10.1161/CIRCOUTCOMES.119.005858 [DOI] [PubMed] [Google Scholar]
- 18. Fanaroff AC, Cyr D, Neely ML, et al. Days alive and out of hospital: exploring a patient-centered, pragmatic outcome in a clinical trial of patients with acute coronary syndromes. Circ Cardiovasc Qual Outcomes. 2018;11:e004755. [PMID: ] doi: 10.1161/CIRCOUTCOMES.118.004755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang W, Liang H, Ou L, et al; China Medical Treatment Expert Group for COVID-19. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081-1089. [PMID: ] doi: 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PMID: ] doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kilaru AS, Lee K, Snider CK, et al. Return hospital admissions among 1419 COVID-19 patients discharged from five U.S. emergency departments. Acad Emerg Med. 2020;27:1039-1042. [PMID: ] doi: 10.1111/acem.14117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolff D, Nee S, Hickey NS, et al. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49:15-28. [PMID: ] doi: 10.1007/s15010-020-01509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haimovich AD, Ravindra NG, Stoytchev S, et al. Development and validation of the quick COVID-19 severity index: a prognostic tool for early clinical decompensation. Ann Emerg Med. 2020;76:442-453. [PMID: ] doi: 10.1016/j.annemergmed.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta S, Hayek SS, Wang W, et al; STOP-COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1436-1447. [PMID: ] doi: 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-79. [PMID: ] doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kricke G, Roemer PE, Barnard C, et al. Rapid implementation of an outpatient Covid-19 monitoring program. NEJM Catal Innov Care Deliv. 2020. doi:10.1056/CAT.20.0214
- 27. Hutchings OR, Dearing C, Jagers D, et al. Virtual health care for community management of patients with COVID-19 in Australia: observational cohort study. J Med Internet Res. 2021;23:e21064. [PMID: ] doi: 10.2196/21064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaw JG, Sankineni S, Olaleye CA, et al. A novel large scale integrated telemonitoring program for COVID-19. Telemed J E Health. 2021. [PMID: ] doi: 10.1089/tmj.2020.0384 [DOI] [PubMed] [Google Scholar]
- 29. Moreland A, Herlihy C, Tynan MA, et al; CDC Public Health Law Program. Timing of state and territorial COVID-19 stay-at-home orders and changes in population movement - United States, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1198-1203. [PMID: ] doi: 10.15585/mmwr.mm6935a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sen S, Karaca-Mandic P, Georgiou A. Association of stay-at-home orders with COVID-19 hospitalizations in 4 states. JAMA. 2020;323:2522-2524. [PMID: ] doi: 10.1001/jama.2020.9176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedman AB, Barfield D, David G, et al. Delayed emergencies: the composition and magnitude of non-respiratory emergency department visits during the COVID-19 pandemic. J Am Coll Emerg Physicians Open. 2021;2:e12349. [PMID: ] doi: 10.1002/emp2.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deerberg-Wittram J, Knothe C. Do not stay at home: we are ready for you. NEJM Catal Innov Care Deliv. 2020. doi:10.1056/CAT.20.0146
- 33. Jeffery MM, D’Onofrio G, Paek H, et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med. 2020;180:1328-1333. [PMID: ] doi: 10.1001/jamainternmed.2020.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhambhvani HP, Rodrigues AJ, Yu JS, et al. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181:272-274. [PMID: ] doi: 10.1001/jamainternmed.2020.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic [Letter]. J Am Coll Cardiol. 2020;75:2871-2872. [PMID: ] doi: 10.1016/j.jacc.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holland M, Burke J, Hulac S, et al. Excess cardiac arrest in the community during the COVID-19 pandemic [Letter]. JACC Cardiovasc Interv. 2020;13:1968-1969. [PMID: ] doi: 10.1016/j.jcin.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benzakoun J, Hmeydia G, Delabarde T, et al. Excess out-of-hospital deaths during the COVID-19 outbreak: evidence of pulmonary embolism as a main determinant [Letter]. Eur J Heart Fail. 2020;22:1046-1047. [PMID: ] doi: 10.1002/ejhf.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marijon E, Karam N, Jost D, et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health. 2020;5:e437-e443. [PMID: ] doi: 10.1016/S2468-2667(20)30117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mountantonakis SE, Epstein LM, Coleman K, et al; Northwell COVID-19 Research Consortium. The association of structural inequities and race with out-of-hospital sudden death during the COVID-19 pandemic. Circ Arrhythm Electrophysiol. 2021;14:e009646. [PMID: ] doi: 10.1161/CIRCEP.120.009646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. [PMID: ] doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boglione L, Olivieri C, Rostagno R, et al. Role of the early short-course corticosteroids treatment in ARDS caused by COVID-19: a single-center, retrospective analysis. Adv Med Sci. 2021;66:262-268. [PMID: ] doi: 10.1016/j.advms.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fadel R, Morrison AR, Vahia A, et al; Henry Ford COVID-19 Management Task Force. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71:2114-2120. [PMID: ] doi: 10.1093/cid/ciaa601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee HW, Park J, Lee J, et al. The effect of the timing of dexamethasone administration in patients with COVID-19 pneumonia. Tuberc Respir Dis (Seoul). 2021. [PMID: ] doi: 10.4046/trd.2021.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Monedero P, Gea A, Castro P, et al; COVID-19 Spanish ICU Network. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care. 2021;25:2. [PMID: ] doi: 10.1186/s13054-020-03422-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goyal DK, Mansab F, Iqbal A, et al. Early intervention likely improves mortality in COVID-19 infection. Clin Med (Lond). 2020;20:248-250. [PMID: ] doi: 10.7861/clinmed.2020-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Asch DA, Muller RW, Volpp KG. Automated hovering in health care—watching over the 5000 hours. N Engl J Med. 2012;367:1-3. [PMID: ] doi: 10.1056/NEJMp1203869 [DOI] [PubMed] [Google Scholar]
- 47. Iqbal FM, Joshi M, Davies G, et al. Design of the pilot, proof of concept REMOTE-COVID trial: remote monitoring use in suspected cases of COVID-19 (SARS-CoV-2). Pilot Feasibility Stud. 2021;7:62. [PMID: ] doi: 10.1186/s40814-021-00804-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med. 2020;27:681-692. [PMID: ] doi: 10.1111/acem.14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bell LC, Norris-Grey C, Luintel A, et al; University College London Hospitals COVID response team. Implementation and evaluation of a COVID-19 rapid follow-up service for patients discharged from the emergency department. Clin Med (Lond). 2021;21:e57-e62. [PMID: ] doi: 10.7861/clinmed.2020-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bokolo AJ. Application of telemedicine and eHealth technology for clinical services in response to COVID-19 pandemic. Health Technol (Berl). 2021;11:359-366. [PMID: ] doi: 10.1007/s12553-020-00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3:e2026881. [PMID: ] doi: 10.1001/jamanetworkopen.2020.26881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Accessed at www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html on 19 April 2021.
- 53. Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382:2534-2543. [PMID: ] doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anesi GL, Jablonski J, Harhay MO, et al. Characteristics, outcomes, and trends of patients with COVID-19-related critical illness at a learning health system in the United States. Ann Intern Med. 2021;174:613-621. [PMID: ] doi: 10.7326/M20-5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16:90-92. [PMID: ] doi: 10.12788/jhm.3552 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.