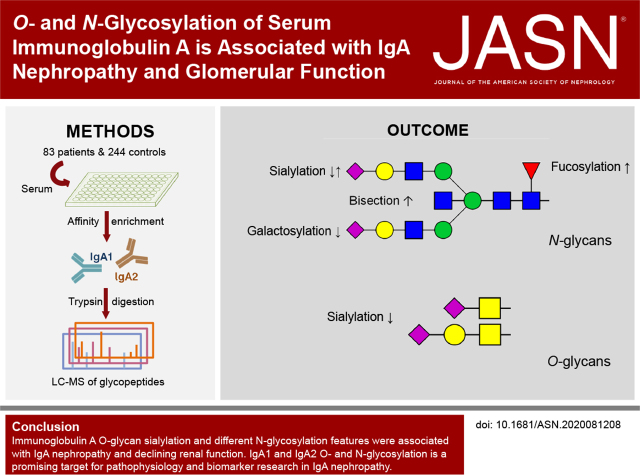
Significance Statement
IgA nephropathy (IgAN) is the most common primary glomerular disease worldwide, with galactose-deficient IgA (gd-IgA) considered to play a key role in its pathogenesis. Although this association is widely reported, it is unclear how IgA glycosylation changes with the disease. A novel mass spectrometry–based approach provided a more complete picture of IgA glycosylation changes in IgAN and of the relationship between IgA glycosylation and kidney function. Multiple structural features of both O- and N-linked glycans were associated with the presence and severity of IgAN and kidney function. Our high-resolution data suggest that IgA O- and N-glycopeptides are promising targets for future studies on the pathophysiology of IgAN and as potential noninvasive biomarkers for disease prediction.
Keywords: IgA nephropathy, glycosylation, immunoglobulin, mass spectrometry, posttranslational modification
Visual Abstract
Abstract
Background
IgA nephropathy (IgAN) is the most common primary glomerular disease worldwide and is a leading cause of renal failure. The disease mechanisms are not completely understood, but a higher abundance of galactose-deficient IgA is recognized to play a crucial role in IgAN pathogenesis. Although both types of human IgA (IgA1 and IgA2) have several N-glycans as post-translational modification, only IgA1 features extensive hinge-region O-glycosylation. IgA1 galactose deficiency on the O-glycans is commonly detected by a lectin-based method. To date, limited detail is known about IgA O- and N-glycosylation in IgAN.
Methods
To gain insights into the complex O- and N-glycosylation of serum IgA1 and IgA2 in IgAN, we used liquid chromatography-mass spectrometry (LC-MS) for the analysis of tryptic glycopeptides of serum IgA from 83 patients with IgAN and 244 age- and sex-matched healthy controls.
Results
Multiple structural features of N-glycosylation of IgA1 and IgA2 were associated with IgAN and glomerular function in our cross-sectional study. These features included differences in galactosylation, sialylation, bisection, fucosylation, and N-glycan complexity. Moreover, IgA1 O-glycan sialylation was associated with both the disease and glomerular function. Finally, glycopeptides were a better predictor of IgAN and glomerular function than galactose-deficient IgA1 levels measured by lectin-based ELISA.
Conclusions
Our high-resolution data suggest that IgA O- and N-glycopeptides are promising targets for future investigations on the pathophysiology of IgAN and as potential noninvasive biomarkers for disease prediction and deteriorating kidney function.
IgA nephropathy (IgAN), or Berger disease, is the most common GN worldwide.1 The disease course is complex, varying from a mild form to a progressive disease leading to renal failure in up to 40% of patients within 20 years.1,2 Clinical presentation also differs greatly with sex, ethnicity, and age.1 IgAN is diagnosed by the presence of IgA dominant or codominant mesangial deposits on renal biopsy.3 Improved noninvasive biomarkers of disease severity and progression to CKD are needed to appropriately stratify patient treatment and develop novel, effective therapies.
IgAN pathogenesis is generally considered to follow the “four-hit hypothesis.”4 In this hypothesis, the pathogenesis is initiated by increased levels of circulating galactose-deficient IgA1 (gd-IgA; hit 1). gd-IgA is then recognized by antiglycan autoantibodies (hit 2), leading to the formation of immune complexes (hit 3) that may deposit in the kidney (hit 4) and cause glomerular inflammation, complement activation, and kidney injury.4
IgA1, unlike IgA2, has a unique hinge region located between conserved regions 1 and 2 of the heavy chain.5 The hinge region has nine potential sites for O-glycosylation, of which three to six are reported to be consistently glycosylated.6–8 O-glycans located in the hinge region of IgA1 are typically core 1 glycans with the structure galactose β1–3N-acetylgalactosamine (GalNAc), which may be extended with up to two sialic acid residues6 (Figure 1). The IgA1 hinge region of patients with IgAN is characterised by an increased presence of aberrantly glycosylated O-glycans, which terminate with GalNAc or sialylated GalNAc rather than galactose.9–13 The levels of degalactosylated IgA (gd-IgA) are elevated in patients with progressive IgAN compared with stable patients, and a negative correlation between gd-IgA level and eGFR has been found.12
Figure 1.
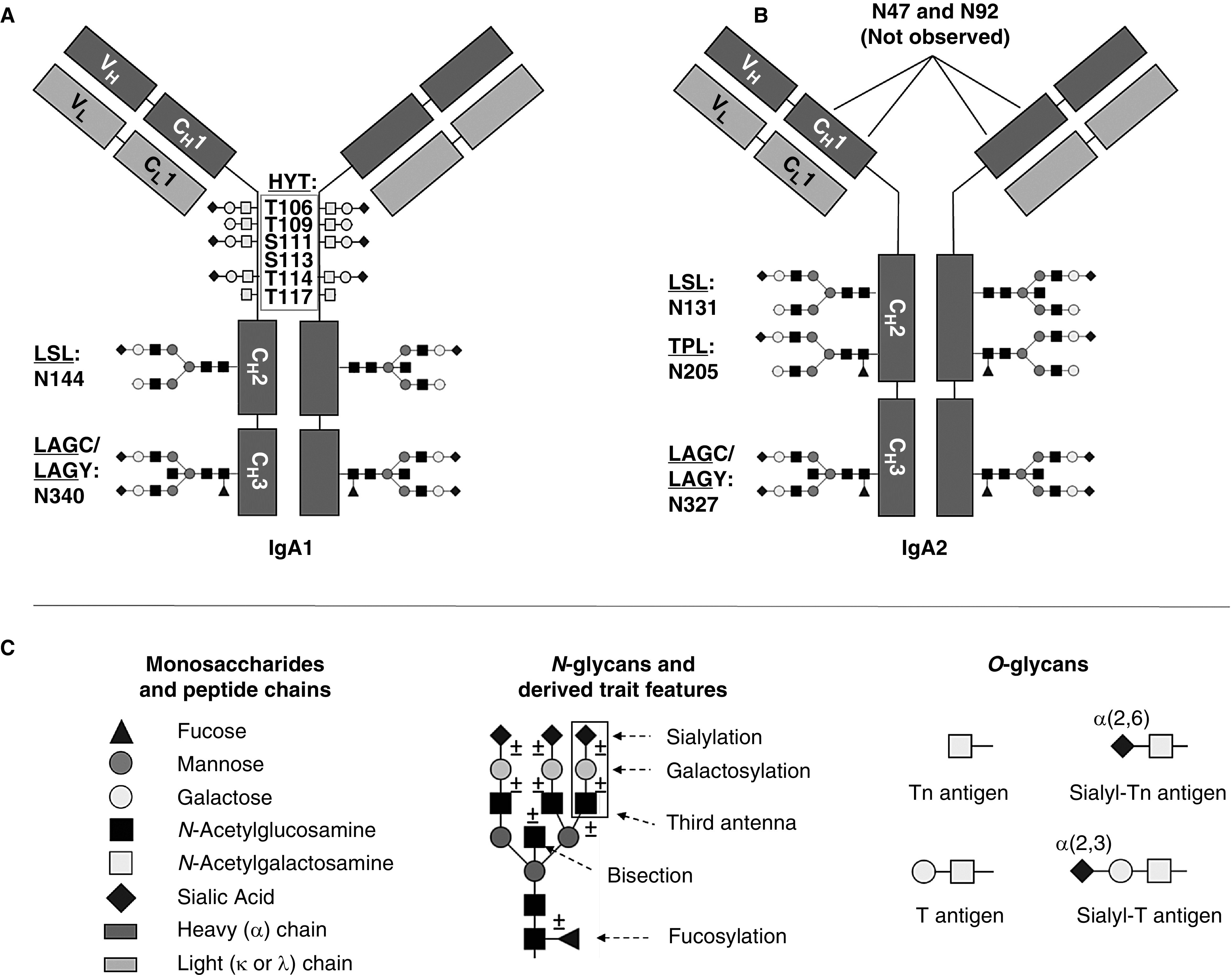
Schematic representation of IgA1 and IgA2 with examples for O- and N-glycan structures. (A) Each IgA1 heavy chain contains two N-glycosylation sites (i.e., at N144 and N340), which are occupied by complex-type N-glycans, next to six O-glycosylation sites (i.e., at T106, T109, S111 , S113, T114, and T117). All six O-glycosylation sites in the hinge region of IgA1 are present on a single tryptic peptide (HYT).6,8 (B) With our MS-based approach, we observed three different N-glycosylation sites on IgA2 (i.e., at N131, N205, and N327), which were occupied with complex-type N-glycans. Glycopeptides indicating glycosylation on the two other potential N-glycosylation sites were not detected in our study. (C) Symbols and example structures of O- and N-glycans. In this work, we refer to the first three letters of the tryptic peptide sequence of the detected glycopeptides: HYT for the multiply O-glycosylated hinge-region peptide; LSL for the glycopeptide with the N-glycosylation site N144 or N131 on IgA1 or IgA2, respectively; TPL for the glycopeptide with the IgA2 N-glycosylation site N205; and LAG for the glycopeptide with the N-glycosylation site N340/N327 on IgA1/IgA2, which was detected with either a terminal tyrosine (LAGY) or as the truncated form (LAGC). Glycosylation site numbering was according to UniProtKB. Modified from reference 55, with permission.
Measurement of gd-IgA in blood samples is typically achieved by ELISA incorporating the use of a lectin, Helix aspersa agglutinin (HAA), which recognizes terminal GalNAc residues on O-glycans. Another lectin, Jacalin, is often used to isolate IgA from samples for further analysis. Although lectin-based approaches are useful tools in this aspect, variability in their specificities and interferences, especially by the copresence of sialic acids, limits their robustness.14–16
Although aberrant O-glycosylation of gd-IgA in IgAN has been widely reported, not much is known about the role of N-glycans. Both IgA1 and IgA2 are N-glycosylated. IgA1 contains two N-linked glycosylation sites on each heavy chain (Asn263/Asn459), and IgA2 contains an additional two or three N-glycans (Figure 1). The N-glycans on IgA are reported to be mainly complex-type, digalactosylated diantennary structures.6,8 Elevated levels of sialylation17 and mannosylation18 of serum IgA1 N-glycans from patients with IgAN have been identified. Moreover, mice with a gene knockout (β4GalT) leading to agalactosylated N-glycans developed IgAN-like glomerular lesions upon IgA deposition.19
Despite the involvement of IgA glycosylation in the pathogenesis of IgAN, it is still largely unclear how IgA glycosylation changes with the disease. The molecular nature of IgA O- and N-glycosylation in IgAN has hitherto been incompletely explored. Here, we used our new mass spectrometry (MS)–based approach for IgA O- and N-glycosylation analysis in a sizable patient-control cohort to obtain a more complete picture on the IgA glycosylation changes in IgAN at an unprecedented level of detail and resolution and to further investigate the relationship of IgA glycosylation and kidney function.
Methods
Study Populations
Samples from patients with IgAN were collected as part of the Causes and Predictors of Outcome in IgA Nephropathy Study, a retrospective cohort study ethically approved by the U.K. National Research Ethics Service Committee. All individuals provided informed written consent (14/LO/0155). Here, we investigated 83 unrelated patients from the United Kingdom with serum samples available and complete clinical follow-up at the time of recruitment (Supplemental Table 1). eGFR, estimated by the Chronic Kidney Disease Epidemiology Collaboration equation, and corrected for body surface area, was used as a biomarker of renal function.12
The control samples were randomly ascertained among healthy British twins from the TwinsUK adult twin registry20 and age- and sex-matched with the patients with IgAN (Supplemental Figure 1). The sample included 244 individuals (49 monozygotic and 64 dizygotic twin pairs and 18 singletons) (Supplemental Table 1). St. Thomas’ Hospital Research Ethics Committee approved this study, and all twins provided informed written consent.
Measurement of Serum IgA and gd-IgA Levels
Serum IgA levels were measured using ELISA as previously described.21 The capture antibody was the F(ab’)2 fragment goat anti-human IgA (Jackson Immuno-Research, West Grove, PA), and the detection antibody was the F(ab’)2 fragment biotinylated goat anti-human IgA1 (invitrogen, CA, USA).
Serum gd-IgA1 levels were measured using a lectin-based ELISA as previously described.21 The capture antibody was a polyclonal rabbit antihuman IgA (Dako, Glostrup, Denmark). The detection involved HAA-biotin (Sigma, Darmstadt, Germany), followed by polystreptavidin horseradish peroxidase (Pierce, Waltham, MA).
The intraclass correlation coefficient for the IgA assay was 0.74 (95% confidence interval, 0.63 to 0.83), and that for the gd-IgA1 assay was 0.89 (95% confidence interval, 0.73 to 0.95).
IgA Glycopeptide Analysis by MS
A detailed description of the material and methods can be found in Supplemental Material. Briefly, serum samples from patients and controls together with 22 pooled plasma standards were pipetted onto 96-well plates in a randomized manner. IgA was captured from 10 µl of serum using CaptureSelectTM IgA affinity beads (ThermoFisher). (Glyco-)peptides were generated by reduction, alkylation, and digestion of the protein with trypsin. Tryptic digests were separated by reversed-phase nanoliquid chromatography (nano-LC) on a C18 column (75 µm × 100 mm, particle size 1.7 µm) and analyzed by MS using an Impact HD quadrupole time-of-flight MS system (Bruker Daltonics, Bremen, Germany) equipped with a nanoBooster, as described previously22 and in the Supplemental Material.
Raw LC-MS data were converted to mzXML using MSConvert. LaCyTools23 (version 1.0.1) was used to align the LC runs, to calibrate (Supplemental Table 2) the mass spectra, and to extract glycopeptide signal intensities. For the extraction step, a previously reported list of potential IgA glycopeptide analytes was used,24–26 in addition to manual identification of glycoforms in the averaged spectra of 20 samples of both healthy individuals and patients.
Quality control was performed on the basis of signal to noise, exact mass deviation, and isotopic pattern as described previously23 and in the Supplemental Material. Sixty-nine glycopeptides were retained and quantified. Their absolute signal intensities were normalized to the intensity sum of all glycopeptide species sharing the same tryptic peptide sequence, resulting in relative intensities. In this manuscript, IgA1 and IgA2 glycopeptide names are composed of the letter codes of the first three amino acids of the peptide sequence: HYT, LSL, TPL, and LAG, the last detected in two variants (i.e., as LAGC and LAGY) (Figure 1). The peptide name is followed by the glycan composition indicating the number of hexoses (H), N-acetylhexosamines (N), fucoses (F), and sialic acids (S) (Supplemental Table 2).
Structurally similar glycopeptides were summarized into 52 derived traits calculated from the relative intensities, as illustrated in Supplemental Table 3. For example, the derived trait TPL_A2FB, bisection of fucosylated diantennary glycans, was calculated as the sum of all bisected diantennary structures within the TPL glycopeptide cluster divided by the sum of the abundances of all structures within the TPL cluster, i.e., A2FB = (H4N5F1S0 + H5N5F1S0 + H4N5F1S1 + H5N5F1S1 + H5N5F1S2)/(H4N5F1S0 + H5N5F1S0 + H5N4F1S1 + H4N5F1S1 + H5N5F1S1 + H5N4F1S2 + H5N5F1S2). Because each measured glycopeptide structure carries different types of monosaccharides, derived traits can give a more composite and robust measure of the different glycosylation features (i.e., for N-glycans,27 complexity/branching [diantennary versus triantennary], bisection, and fucosylation and for both O- and N-glycans, galactosylation and sialylation).
Statistical Analyses
The relative intensities of the detected glycopeptides and the derived trait values were corrected for batch effects (plate, plate row, and column) in R (version 3.3.3) using the function ComBat from the R package sva (release 3.2) on log-transformed data. Outliers, defined as measurements deviating more than three SDs from the mean of each trait, were removed. To ensure the normality of their distribution, the relative intensities of the detected glycopeptides as well as of the derived traits were quantile normalized.
gd-IgA level and IgAN status (patients versus control) were tested for association with glycopeptides and derived traits using a linear mixed model using the function lmer from the R package lmerTest (version 3.1), including age, sex, and their interaction term as fixed effects and family structure as a random effect to correct for the nonindependence of the twin observations. To avoid potential spurious associations due to differences in glycan composition between patients and controls, association with gd-IgA levels was assessed using healthy individuals only. eGFR (assessed in patients with IgAN only) was tested for association using a linear regression model (function lm, from the stats R package, version 3.6.1). Age, sex, and their interaction term were included as covariates.
We considered an association significant when its P value passed a Bonferroni-derived threshold of 0.05/Neff, where Neff is the effective number of independent tests taking into account the strong correlation among glycan relative intensities. Neff was calculated using the approach proposed by Li and Ji29 and multiplied by the number of phenotypes analyzed in this study. Neff was 23(×3) for measured glycopeptides and 16(×3) for derived traits.
Power calculation was performed using the pwr R package (version 1.3) and asking for the power to detect, in a sample of 83 patients and 244 controls, a Cohen conventional medium effect size28 of 0.5 of an SD at α-levels of 0.05/(23 × 3)=7.3 × 10−4 and 0.05/(16 × 3)=1.0 × 10−3 for measured and derived traits, respectively.
We further evaluated, for both IgAN and glomerular function, the predictive power of a model including only the gd-IgA serum levels and a model including either the glycopeptides or derived traits significantly associated with IgAN/glomerular function. In this second model, because of the high correlation among traits, if two traits had a Pearson correlation larger than 0.9, only the most significantly associated was used. Predictive powers were evaluated using the McFadden adjusted pseudo-R230 (evaluated via the function PseudoR2, from the DescTools R package, version 0.99.39) for the binary trait IgAN and adjusted R2 for the continuous trait eGFR (evaluated via the lm function). The adjusted values allow for penalizing for the number of predictors in the model (k=1 when only gd-IgA levels are used and k>1 when the glycopeptides or derived traits are used).
Results
Glycosylation Features Are Associated with the Level of gd-IgA in Healthy Individuals
As a first comparison of the traditional lectin-based method and our MS-based approach for measuring IgA glycosylation, we assessed the cross-sectional associations between gd-IgA values and MS-detected glycosylation traits. Using data from 236 healthy individuals, we found associations between gd-IgA, 26 of 30 detected O-glycopeptides (HYT cluster), and all seven derived O-glycan traits (Supplemental Table 4). The strongest associations were observed with decreased sialylation (HYT_nS, HYT_nS > nG, HYT_SperG) and galactosylation (HYT_GalperGalNAc and HYT_nGal), along with a relative increase of GalNAcylation (HYT_nGalNAc > nG and HYT_nGalNAc) (Figure 2), which showed a similar trend in patients with IgAN (Supplemental Figure 2). N-glycosylation traits from the LAGC cluster were also associated with gd-IgA, although to a lesser extent than O-glycosylation (Supplemental Table 4).
Figure 2.
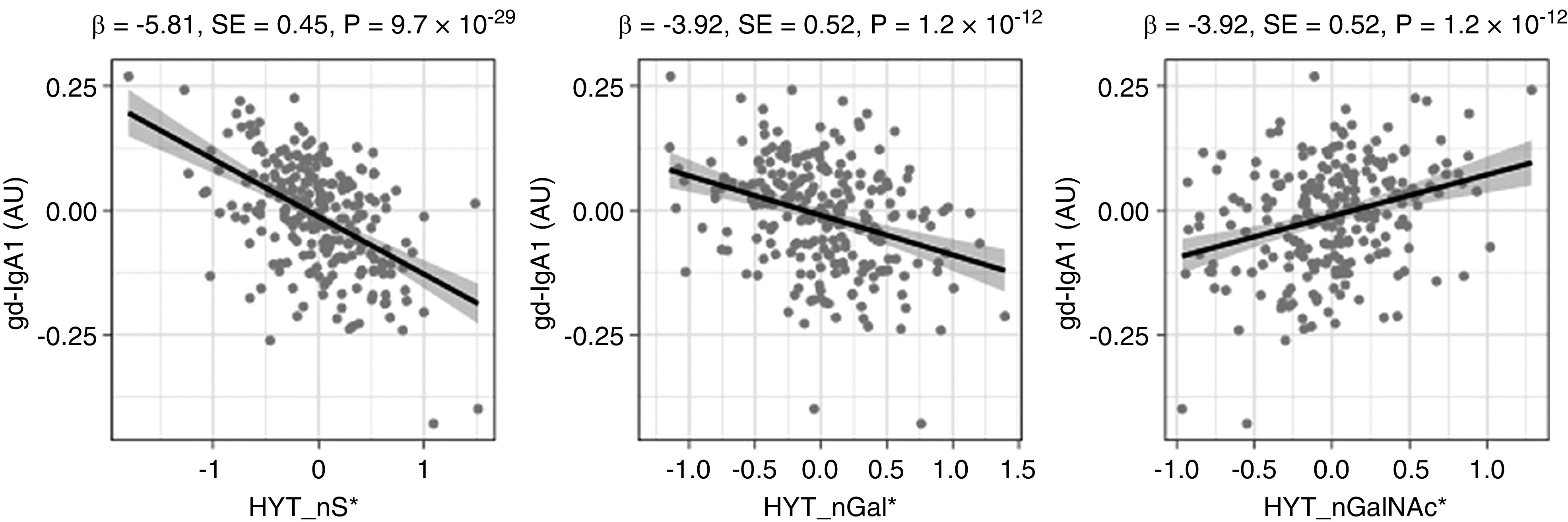
Main associations between derived glycosylation traits and gd-IgA1 levels detected by HAA lectin in 236 healthy individuals. Quantile-normalized age- and sex-corrected values are plotted, and each scatterplot reports effect size (β), SE, and P value (P) of the linear regression analysis. Derived traits HYT_nS, HYT_nGal, and HYT_nGalNAc correspond to average numbers of sialic acids, galactoses, and N-acetylgalactosamines, respectively. Monosaccharide symbols are depicted, in black and white, according with the nomenclature of the Consortium for Functional Glycomics and were generated using GlycoWorkbench.56 AU, arbitrary units; * indicates derived traits.
Moreover, we compared the associations between gd-IgA and glycopeptides with and without correction for IgA1 titer in a subset of 156 healthy individuals for whom IgA1 titer was available. IgA1 titer correction had negligible effects on the associations (Supplemental Table 5).
O- and N-Glycosylation of IgA Is Associated with IgA Nephropathy
We used a patient-control study design, including 83 patients with IgAN and 244 healthy controls, to investigate cross-sectional associations between IgAN and IgA O- and N-glycosylation features detected by MS. This sample has ≥70% power to detect a difference of 0.5 of an SD between groups at Bonferroni-derived P values of 0.05/(23 × 3)=7.3 × 10−4 and 0.05/(16 × 3)=1.0 × 10−3 for measured and derived traits, respectively.
We found that galactosylation of the N-glycopeptides in the TPL and LSL clusters, next to sialylation in TPL and sialylation of the HYT O-glycopeptides, was lower in patients with IgAN compared with controls, whereas bisection and sialylation in LSL; diantennary glycans in LAGCa, TPL, and LAGCb; and fucosylation in LAGCb were higher in patients (Figure 3, Supplemental Table 6).
Figure 3.
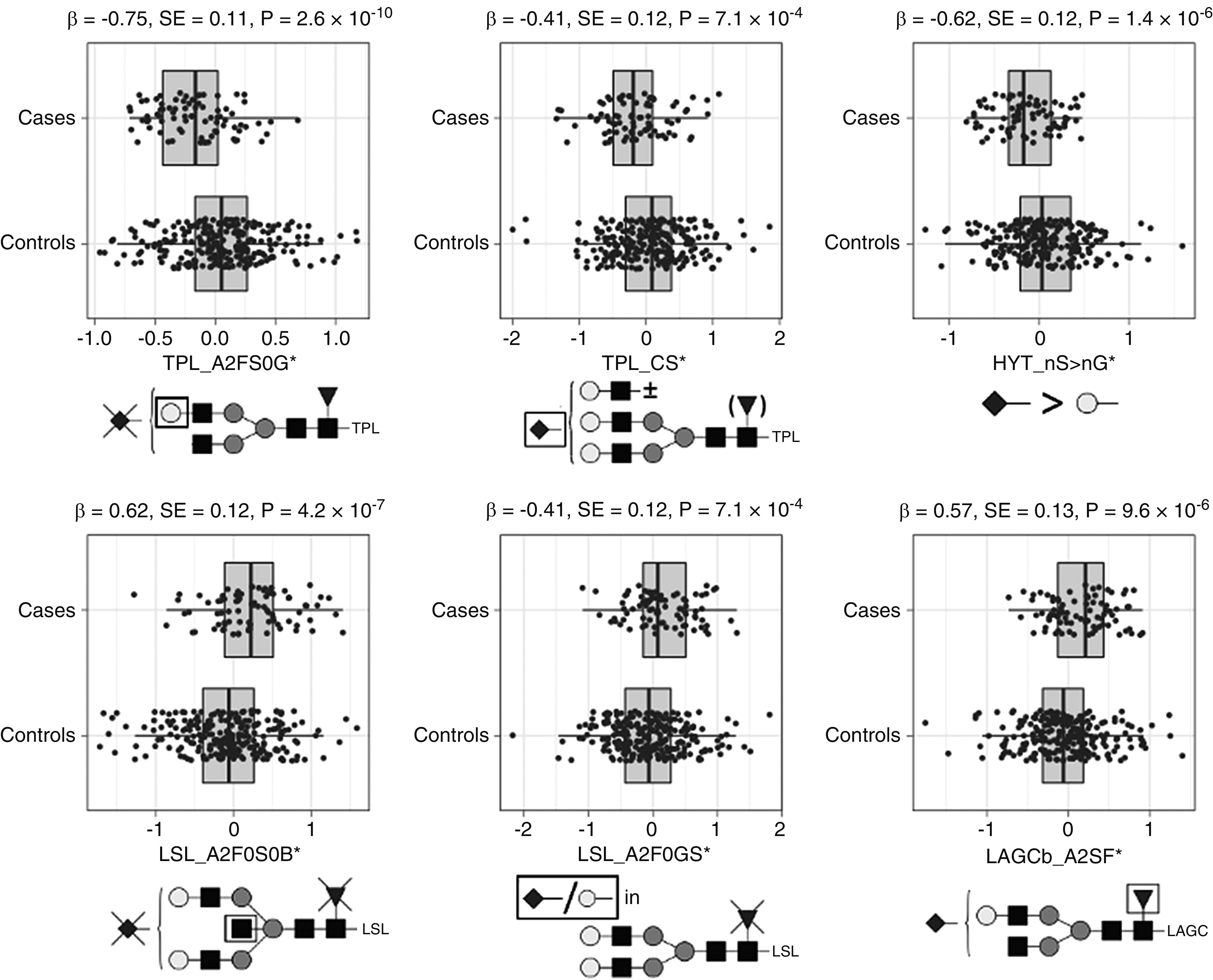
Main associations between derived glycosylation traits in serum IgA from healthy controls and individuals with IgAN (n=327). Quantile-normalized age- and sex-corrected values are plotted, and each box plot reports effect size (β), SE, and P value (P) of the regression analysis. Glycopeptide-derived trait nomenclature refers to the first three letters of the tryptic amino acid sequence followed by the glycosylation features as calculated from detected glycopeptides (Supplemental Table 3). Glycosylation traits: A2FS0G, galactosylation of nonsialylated fucosylated diantennary glycans; CS, sialylation within complex glycans; nS > nG, relative intensity sum of structures in which the number of sialic acids exceeds the number of galactoses; A2F0S0B, bisection of nonfucosylated nonsialylated diantennary; A2F0GS, sialic acid per galactose in nonfucosylated diantennary; and A2SF, fucosylation of sialylated diantennary. Monosaccharide symbols are depicted, in black and white, according with the nomenclature of the Consortium for Functional Glycomics and were generated using GlycoWorkbench.56
* indicates derived traits.
O- and N-Glycosylation of IgA Is Associated with Renal Function
Using data from patients with IgAN, we sought association between glycan traits and eGFR, a marker of renal function. N-glycosylation features from all detected IgA glycopeptide clusters were associated with eGFR: bisection of LAGY, LSL, LAGC, and TPL was lower, whereas galactosylation and sialylation of TPL and LSL were higher in patients with higher eGFR (Figure 4, Supplemental Table 7). Regarding O-glycosylation, only sialylation showed significant, positive associations with eGFR (HYT_nS, HYT_SperG, and HYT_nS > nG), reflected in low levels of mono- or disialylated glycopeptides (e.g., HYT_H4N4F0S1) and high levels of multisialylated ones (e.g., HYT_H4N4F0S4) (Figure 4, Supplemental Table 7).
Figure 4.
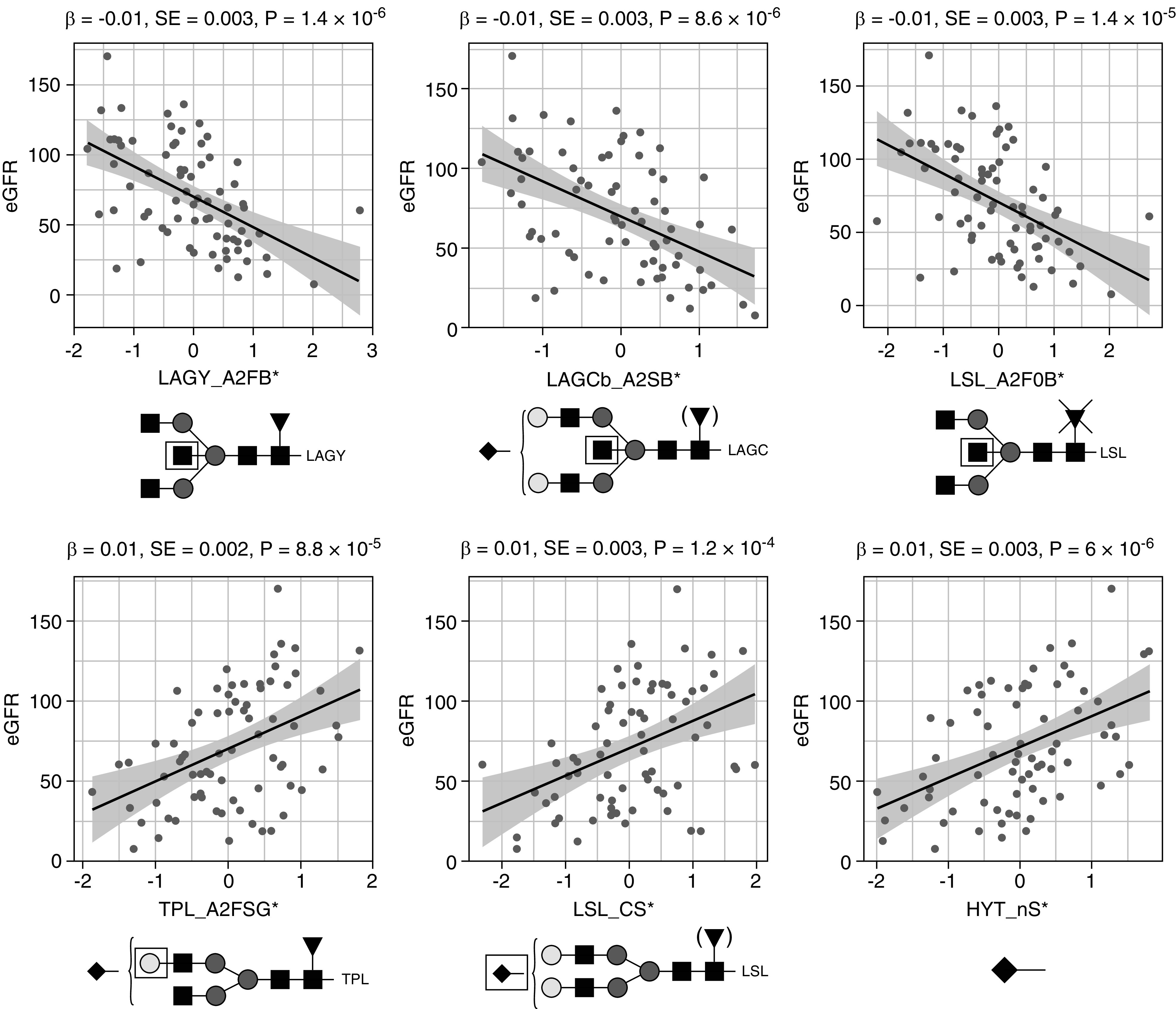
Main associations between IgA glycosylation traits and eGFR in 75 patients with IgAN. Quantile-normalized age- and sex-corrected values are plotted, and each scatterplot reports effect size (β), SE, and P value (P) for the linear regression analysis. Glycopeptide-derived trait nomenclature refers to the first three letters of the tryptic amino acid sequence followed by the glycosylation features as calculated from detected glycopeptides (Supplemental Table 3). Glycosylation traits: A2FB, bisection of fucosylated diantennary glycans; A2SB, bisection of sialylated diantennary; A2F0B, bisection of nonfucosylated diantennary; A2FSG, galactosylation of sialylated fucosylated diantennary; CS, sialylation within complex glycans; and nS, average number of sialic acids. Glycan structures are reported below each panel. Monosaccharide symbols are depicted, in black and white, according to the nomenclature of the Consortium for Functional Glycomics and were generated using GlycoWorkbench.56 * indicates derived traits.
Glycopeptides and Derived Traits Are Better Predictors of IgAN Status and Renal Function Than gd-IgA Levels
Using McFadden adjusted pseudo-R2,30 we found that glycopeptides and derived traits that were associated with IgAN from our analyses were better predictors of the disease than gd-IgA levels, with pseudo-R2 values of 0.14, 0.12 and 0.02 for glycopeptides, derived traits, and gd-IgA levels, respectively. Analogously, glycopeptides and derived traits associated with eGFR showed pseudo-R2 values of 0.23 and 0.22, respectively, versus 0.07 of gd-IgA levels. These results suggest that MS glycosylation data may not only give insights into the pathophysiology of IgAN but can also provide leads for noninvasive biomarkers for disease and deteriorating kidney function.
A summary of the major associations with gd-IgA, IgAN, and glomerular function is visualized in Figure 5 for derived traits and Supplemental Figure 3 for measured glycopeptides.
Figure 5.
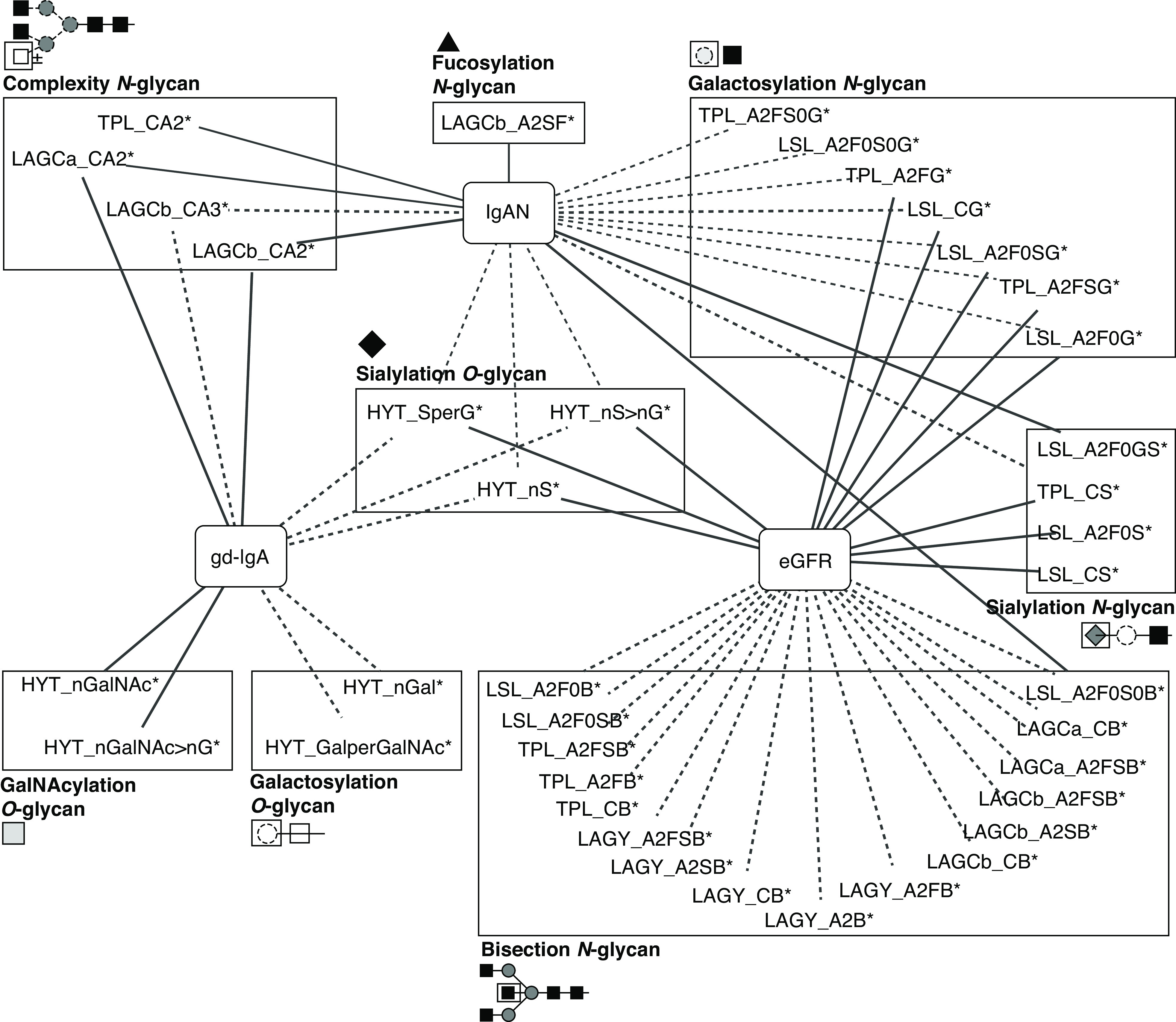
Summary of the associations of derived IgA glycan traits with gd-IgA levels, IgAN, and glomerular function. Line thickness represents effect size (the thicker the line, the stronger the effect’s absolute value). Solid lines indicate positive associations; dotted lines indicate negative associations. Derived traits are grouped by glycosylation features along with their structural representations. Monosaccharide symbols are depicted, in black and white, according to the nomenclature of the Consortium for Functional Glycomics and were generated using GlycoWorkbench.56 * indicates derived traits. Complete summary statistics are reported in Supplemental Tables 4, 6, and 7.
Discussion
This study is the first detailed report on site-specific O- and N-glycosylation signatures of serum IgA1 and IgA2 in IgAN. We analyzed a reasonably large cohort of 83 patients and 244 age- and sex-matched controls. Our high-resolution MS-based method features the relative quantitation of, in total, 69 O- and N-glycopeptide species, further summarized in 52 derived traits. Our data revealed disease associations with O-glycan sialylation and with all main N-glycosylation features, which include complexity, bisection, galactosylation, fucosylation, and sialylation.
Altered glycosylation of IgA1 O-glycans in IgAN is widely reported. Our MS data reflect relative shifts of different glycosylation features due to total area normalization within a glycopeptide cluster. This is different to terminal GalNAc abundance detected by the lectin-based gd-IgA assay. Nevertheless, our MS data on relative O-glycan galactosylation and on the relative abundance of GalNAc on O-glycopeptides can be related to the traditional lectin-based detection of truncated O-glycans with terminal GalNAc (GalNAcα1-Ser/Thr; Tn antigen). Both gd-IgA and IgA titers represent absolute concentrations but become more comparable with our relative quantitation of MS data when gd-IgA is adjusted for IgA titers. Accordingly, we found a strong positive association of HAA lectin binding with the glycosylation trait HYT_nGalNAc > nG, which reflects the presence of terminal GalNAc, and consequently, a negative association with hinge-region galactosylation (HYT_GalperGalNAc, HYT_nGal) that is supposed to cap the HAA binding motif. Similarly, sialylation (HYT_nS > nG) was also inversely associated with the gd-IgA level. This can partly be explained by the fact that terminal α2,3-linked sialic acids can only be present when a galactose has been attached to the core GalNAc. This could also be explained by virtue of the lectin-based assay simply detecting gd-IgA as exposed GalNAc. In 2017, the first GWAS for aberrant O-glycosylation of IgA1 identified variants in the C1GALT1 and C1GALT1C1 genes that had large effects on gd-IgA1 levels31 These genes encode, respectively, the enzyme core 1 β1–3-galactosyltransferase (C1GalT1) and COSMC, its molecular chaperone—molecular partners that are essential for the galactosylation of IgA1 O-glycans.32 Decreased expression and activity of C1GalT1 have been demonstrated in the B cells of patients with IgAN.33–35 Further studies have also demonstrated that genetic variation at CIGALT1 influences gd-IgA levels.36,37 A recent study has also shown that decreased expression of Golgi matrix protein GM130, which is involved in glycosyltransferase tethering, is associated with reduced C1GalT1 protein level and increased galactose deficiency of IgA1.38 It is therefore likely that downregulated expression of CIGALT1 in patients with IgAN leads to reduced levels of galactosylation and subsequent reduced levels of sialylation and increased levels of exposed GalNAc, as detected in the lectin ELISA and reflected in our results.
The relative abundance of sialic acids was the only derived O-glycosylation trait that associated with IgAN and renal decline in patients with IgAN. Our data suggest that decreased sialylation may also lead to increased presentation of terminal GalNAc, as lower sialylation significantly correlates with higher gd-IgA levels. It is unclear from our study if decreased sialylation is an alteration of IgA glycosylation in itself or a product of reduced galactosylation. Data on O-linked sialylation in IgAN are conflicting. In agreement with our findings two small-scale MS-based studies without quantitation reported decreased numbers of galactose, GalNAc, and especially, sialic acid residues in both glomerular and serum IgA1 in pooled samples from patients with IgAN as compared with control serum pools.39,40 Conversely, increased expression of ST6GALNAC2, a gene encoding an enzyme that mediates sialylation of O-glycans, has been reported to be positively correlated with IgAN.41 Interestingly, increased expression of another enzyme, ST6Gal1, correlates with gd-IgA levels.42 Further studies are required to disentangle the relationship between sialylation and IgAN.
Regarding serum IgA N-glycosylation in IgAN, only a few small studies exist, and the possible implication of N-glycosylation in pathogenesis or renal decline is unknown. Here, we present the first detailed report on associations between serum IgA N-glycan galactosylation, sialylation, bisection, and fucosylation with IgAN and related clinical parameters. Previously, lower IgA1 Fc-region galactosylation and lower IgA2 sialylation in patients with IgAN were detected by lectin-based assays.43 Intriguingly, our findings of decreased N-linked sialylation and galactosylation and increased bisection in IgAN and with worsening renal function are similar to the N-glycosylation differences reported for human salivary versus plasma IgA.25 With this in mind, it is possible that our findings might partly reflect a disease-related increase of IgA molecular species connected to mucosal immune response, which has previously been suggested for IgAN.44
It is also feasible that our results could reflect a causal relationship of IgA N-glycosylation and IgAN pathogenesis via increased formation of polymeric IgA. In comparison with monomeric, polymeric IgA is increased in patients with IgAN, and it has been implicated in higher immune complex formation and glomerular deposition.45 Strikingly, mice with impaired terminal N-glycan galactosylation due to a knockout of the β4-galactosyltransferase I gene were shown to develop human IgAN-like glomerular lesions, with an increased serum IgA, especially the polymeric form.19 Our finding of decreased sialylation in IgAN and with worsening renal function might also reflect a higher abundance of polymeric IgA, which was reported to exhibit a lower degree of sialylation compared with its monomeric form and thereby, enhance binding to mannose-binding lectin as well as to mesangial cells.46–48 Similarly, a higher abundance of fucose and terminal GlcNAc (e.g., bisecting GlcNAc or ungalactosylated antenna GlcNAc) might also be involved in enhanced binding to mannose-binding lectin and subsequent complement activation via the lectin pathway.48 Desialylation and to a lesser extent, additional degalactosylation have been shown to enhance the binding of polymeric IgA1 to human mesangial cells, as compared with untreated IgA1 in vitro.49 Of note, it is unclear to which extent this effect was attributed to O- or N-glycosylation or a combination thereof. Notably, the ST6GAL1 gene, coding for an enzyme responsible for the terminal sialylation of N-glycans on different proteins including IgA, was associated with IgAN in Han Chinese.50
Large-scale O-glycomic studies, other than the one reported here and a site-specific O- and N-glycosylation associations study with rheumatoid arthritis,24 are hitherto lacking due to the technologically challenging nature of these studies. Although a few reports do exist that associate N-glycosylation of IgG with kidney disease or glomerular function,51–53 none are available for IgAN, hampering our ability to compare IgAN glycosylation signatures displayed by different molecules. Analogously, linking phenotypic associations of the total plasma N-glycome with those found here for IgA glycosylation is complicated as many different glycoproteins contribute to the total plasma N-glycome, with mostly overlapping structures.54 An analysis of total serum glycans in kidney disease showed eGFR to be associated with higher absolute levels of biantennary digalactosylated disialylated glycans with and without bisection,53 yet information on the glycoproteins and glycosylation sites contributing to this signature is lacking. In comparison, our novel approach for specific IgA glycosylation analysis presented here provides these extra layers of information by covering all main glycosylation features present on 69 measured glycopeptides of IgA.54
We have made a first attempt to elucidate the complex O- and N-glycosylation of human serum IgA in relation to IgAN in a comprehensive fashion with direct detection using high-resolution MS. Because of the small sample size, we could not build and validate a predictive model for IgAN pathogenesis and renal decline. However, we have shown that directly measured glycopeptide-level IgA glycosylations are better predictors of both IgAN status and renal function than gd-IgA levels alone. Our results widen the current view on the potential role of IgA glycosylation in IgAN pathogenesis and in renal decline and open new opportunities for investigations on glycopeptides as potential biomarkers for disease onset and progression. We envisage that these results, together with the increasing interest in the use of glycomics in clinical settings, will encourage increased inclusion of IgA glycomics in studies, which will promote the development of targeted analysis panels and of absolute quantification approaches, currently hindered by the lack of stable isotope-labeled glycopeptide standards.
In summary, we provide the first evidence of a possible role for IgA N-glycosylation in IgAN pathogenesis, which should be taken forward in mechanistic studies and could result in novel therapeutic and preventive approaches in the future.
Disclosures
H.T. Cook reports consultancy agreements with Alexion Pharmaceuticals, Apellis Pharmaceuticals, and Novartis and honoraria from Alexion Pharmaceuticals. V. Dotz is employed by Janssen Vaccines (a Johnson & Johnson company). M. C. Pickering reports consultancy agreements with Achillion Pharmaceuticals as a scientific advisor, Alexion Pharmaceuticals for scientific advisory board attendance, the ChemoCentryx Sciviaentific Advisory Board (meeting on May 7th, 2016) as an invited speaker, Gyroscope for scientific board membership, and Ra Pharma for scientific advisory board attendance; research funding from Achillion for the C3 Glomerulopathy Natural History Study, Alexion for preclinical studies in animal models, Laboratoires Français de Fractionnement et des Biotechnologies for preclinical studies in animal models, and Ra Pharma for complement C9 in lupus nephritis; honoraria from Gyroscope as advisory board fees; and scientific advisor or membership with Gyroscope Pharma via the advisory board. M. Falchi reports research funding from Sanofi for the identification of multi-disease drug targets through systems-immunology dissection of immune ageing. All remaining authors have nothing to disclose.
Funding
This work was supported by funding from Medical Research Council grant MR/K01353X/2. M. C. Pickering is a Wellcome Trust Senior Fellow in Clinical Science under grant 212252/Z/18/Z.
Acknowledgments
TwinsUK is funded by the Wellcome Trust, the Medical Research Council, the European Union, the Chronic Disease Research Foundation, the National Institute for Health Research–funded BioResource, the Clinical Research Facility, and the Biomedical Research Centre based at Guy’s and St. Thomas’ National Health Service (NHS) Foundation Trust in partnership with King’s College London. We acknowledge support from the National Institute for Health Research Biomedical Research Centre based at Imperial College Healthcare National Health Service Trust and Imperial College London and from the National Institute for Health Research Clinical Research Network.
The views expressed are those of the authors and are not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
M. Falchi and M. Wuhrer designed the study; H. T. Cook, H. J. Lomax-Browne, N. R. Medjeral-Thomas, and M. C. Pickering collected the samples and clinical data in the Causes and Predictors of Outcome in IgA Nephropathy Study; H. J. Lomax-Browne measured serum IgA and gd-IgA levels; A. L. Hipgrave Ederveen optimized the LC-MS method; F. Clerc and A. L. Hipgrave Ederveen performed IgA glycopeptide analysis by MS; F. Clerc processed the raw data and calculated the derived traits; A. Visconti carried out the statistical analyses; V. Dotz, M. Falchi, F. Clerc, H. J. Lomax-Browne, A. Visconti, and M. Wuhrer interpreted the results; F. Clerc generated the figures for the glycopeptides and derived traits; A. Visconti generated the figures for the association study results; M. Falchi and A. Visconti generated the figures summarizing the association results; V. Dotz, M. Falchi, F. Clerc, H. J. Lomax-Browne, and A. Visconti drafted the manuscript; and all authors approved the final version of the manuscript.
Footnotes
V.D., A.V., H.J.L.-B., and F.C. contributed equally to this work. M.W. and M.F. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
Present address: Dr. Viktoria Dotz, BioTherapeutics Analytical Development, Janssen Biologics BV, Einsteinweg 101, 2333 CB Leiden, The Netherlands
Data Sharing Statement
Data on study participants are available to bona fide researchers under managed access due to governance and ethical constraints. The raw data of patients with IgAN should be requested via contact with the corresponding author. Twins’ raw data should be requested via the TwinsUK website (http://twinsuk.ac.uk/resources-for-researchers/access-our-data/), and requests are reviewed by the TwinsUK Resource Executive Committee regularly.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020081208/-/DCSupplemental.
References
- 1.Schena FP, Nistor I: Epidemiology of IgA nephropathy: A global perspective. Semin Nephrol 38: 435–442, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol 28: 4–9, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC: The immunohistology of IgA nephropathy. Am J Kidney Dis 12: 348–352, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, et al.: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr MA: The structure and function of human IgA. Biochem J 271: 285–296, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, et al.: The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem 273: 2260–2272, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Tarelli E, Smith AC, Hendry BM, Challacombe SJ, Pouria S: Human serum IgA1 is substituted with up to six O-glycans as shown by matrix assisted laser desorption ionisation time-of-flight mass spectrometry. Carbohydr Res 339: 2329–2335, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Woof JM, Russell MW: Structure and function relationships in IgA. Mucosal Immunol 4: 590–597, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Mestecky J, Tomana M, Crowley-Nowick PA, Moldoveanu Z, Julian BA, Jackson S: Defective Galactosylation and Clearance of IgA1 Molecules as a Possible Etiopathogenic Factor in IgA Nephropathy. In: Contributions to Nephrology, vol. 104 edited by Béné MC, Faure G, Kessler M, pp 172–182, 1993. Available at: https://www.karger.com/Article/FullText/422410. Accessed February 19, 2021 [DOI] [PubMed]
- 10.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, et al.: Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Sun Q, Zhang Z, Zhang H, Liu X: Aberrant IgA1 glycosylation in IgA nephropathy: A systematic review. PLoS One 11: e0166700, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith H, Willicombe M, McLean AG, Brookes P, et al.: Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int 92: 942–952, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastings MC, Moldoveanu Z, Julian BA, Novak J, Sanders JT, McGlothan KR, et al.: Galactose-deficient IgA1 in African Americans with IgA nephropathy: Serum levels and heritability. Clin J Am Soc Nephrol 5: 2069–2074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tachibana K, Nakamura S, Wang H, Iwasaki H, Tachibana K, Maebara K, et al.: Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: Quantitative analysis by frontal affinity chromatography. Glycobiology 16: 46–53, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, et al.: Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol 44: 2598–2604, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasutake J, Suzuki Y, Suzuki H, Hiura N, Yanagawa H, Makita Y, et al.: Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant 30: 1315–1321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linossier M-T, Palle S, Berthoux F: Different glycosylation profile of serum IgA1 in IgA nephropathy according to the glomerular basement membrane thickness: Normal versus thin. Am J Kidney Dis 41: 558–564, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Amore A, Cirina P, Conti G, Brusa P, Peruzzi L, Coppo R: Glycosylation of circulating IgA in patients with IgA nephropathy modulates proliferation and apoptosis of mesangial cells. J Am Soc Nephrol 12: 1862–1871, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Nishie T, Miyaishi O, Azuma H, Kameyama A, Naruse C, Hashimoto N, et al.: Development of immunoglobulin A nephropathy- like disease in β-1,4-galactosyltransferase-I-deficient mice. Am J Pathol 170: 447–456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrew T, Hart DJ, Snieder H, de Lange M, Spector TD, MacGregor AJ: Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res 4: 464–477, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Lomax-Browne HJ, Visconti A, Pusey CD, Cook HT, Spector TD, Pickering MC, et al.: IgA1 glycosylation is heritable in healthy twins. J Am Soc Nephrol 28: 64–68, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falck D, Jansen BC, de Haan N, Wuhrer M: High-throughput analysis of IgG Fc glycopeptides by LC-MS. Methods Mol Biol 1503: 31–47, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Jansen BC, Falck D, de Haan N, Hipgrave Ederveen AL, Razdorov G, Lauc G, et al.: LaCyTools: A targeted liquid chromatography-mass spectrometry data processing package for relative quantitation of glycopeptides. J Proteome Res 15: 2198–2210, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Bondt A, Nicolardi S, Jansen BC, Kuijper TM, Hazes JMW, van der Burgt YEM, et al.: IgA N- and O-glycosylation profiling reveals no association with the pregnancy-related improvement in rheumatoid arthritis. Arthritis Res Ther 19: 160, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plomp R, de Haan N, Bondt A, Murli J, Dotz V, Wuhrer M: Comparative glycomics of immunoglobulin A and G from saliva and plasma reveals biomarker potential. Front Immunol 9: 2436, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bondt A, Nicolardi S, Jansen BC, Stavenhagen K, Blank D, Kammeijer GSM, et al.: Longitudinal monitoring of immunoglobulin A glycosylation during pregnancy by simultaneous MALDI-FTICR-MS analysis of N- and O-glycopeptides. Sci Rep 6: 27955, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudelj I, Lauc G, Pezer M: Immunoglobulin G glycosylation in aging and diseases. Cell Immunol 333: 65–79, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Cohen J: Statistical Power Analysis for the Behavioral Sciences, New York, Academic Press, 1988 [Google Scholar]
- 29.Li J, Ji L: Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95: 221–227, 2005 [DOI] [PubMed] [Google Scholar]
- 30.McFadden D: Conditional logit analysis of qualitative choice behavior. In: Frontiers in Econometrics, edited by Zarembka P, New York, Academic Press, 1974, pp 105–142 [Google Scholar]
- 31.Kiryluk K, Li Y, Moldoveanu Z, Suzuki H, Reily C, Hou P, et al.: GWAS for serum galactose-deficient IgA1 implicates critical genes of the O-glycosylation pathway. PLoS Genet 13: e1006609, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ju T, Cummings RD: A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A 99: 16613–16618, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen AC, Topham PS, Harper SJ, Feehally J: Leucocyte beta 1,3 galactosyltransferase activity in IgA nephropathy. Nephrol Dial Transplant 12: 701–706, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, et al.: IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118: 629–639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing Y, Li L, Zhang Y, Wang F, He D, Liu Y, et al.: C1GALT1 expression is associated with galactosylation of IgA1 in peripheral B lymphocyte in immunoglobulin a nephropathy. BMC Nephrol 21: 18, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale DP, Molyneux K, Wimbury D, Higgins P, Levine AP, Caplin B, et al.: Galactosylation of IgA1 is associated with common variation in C1GALT1. J Am Soc Nephrol 28: 2158–2166, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y-N, Zhou X-J, Chen P, Yu G-Z, Zhang X, Hou P, Liu L-J, Shi S-F, Lv J-C, Zhang H: Interaction between G ALNT12 and C1GALT1 associates with galactose-deficient IgA1 and IgA nephropathy. J Am Soc Nephrol 32: 545–552, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Ye M, Zhao Q, Xia M, Liu D, He L, et al.: Loss of the Golgi matrix protein 130 cause aberrant IgA1 glycosylation in IgA nephropathy. Am J Nephrol 49: 307–316, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, et al.: Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 59: 1077–1085, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Odani H, Hiki Y, Takahashi M, Nishimoto A, Yasuda Y, Iwase H, et al.: Direct evidence for decreased sialylation and galactosylation of human serum IgA1 Fc O-glycosylated hinge peptides in IgA nephropathy by mass spectrometry. Biochem Biophys Res Commun 271: 268–274, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Moldoveanu Z, Julian BA, Wyatt RJ, Novak J: Autoantibodies specific for galactose-deficient IgA1 in IgA vasculitis with nephritis. Kidney Int Rep 4: 1717–1724, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Wang F, Zhang Y, Jia J, Yan T: ST6Gal1 is up-regulated and associated with aberrant IgA1 glycosylation in IgA nephropathy: An integrated analysis of the transcriptome. J Cell Mol Med 24: 10493–10500, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baharaki D, Dueymes M, Perrichot R, Basset C, Le Corre R, Clèdes J, et al.: Aberrant glycosylation of IgA from patients with IgA nephropathy. Glycoconj J 13: 505–511, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, et al.: The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 7: 117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung JCK, Tang SCW, Lam MF, Chan TM, Lai KN: Charge-dependent binding of polymeric IgA1 to human mesangial cells in IgA nephropathy. Kidney Int 59: 277–285, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Lombana TN, Rajan S, Zorn JA, Mandikian D, Chen EC, Estevez A, et al.: Production, characterization, and in vivo half-life extension of polymeric IgA molecules in mice. MAbs 11: 1122–1138, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oortwijn BD, Roos A, Royle L, van Gijlswijk-Janssen DJ, Faber-Krol MC, Eijgenraam J-W, et al.: Differential glycosylation of polymeric and monomeric IgA: A possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol 17: 3529–3539, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR: Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 167: 2861–2868, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Gao Y-H, Xu L-X, Zhang J-J, Zhang Y, Zhao M-H, Wang H-Y: Differential binding characteristics of native monomeric and polymeric immunoglobulin A1 (IgA1) on human mesangial cells and the influence of in vitro deglycosylation of IgA1 molecules. Clin Exp Immunol 148: 507–514, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, Foo J-N, Wang J-Q, Low H-Q, Tang X-Q, Toh K-Y, et al.: Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun 6: 7270, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrios C, Zierer J, Gudelj I, Štambuk J, Ugrina I, Rodríguez E, et al.: Glycosylation profile of IgG in moderate kidney dysfunction. J Am Soc Nephrol 27: 933–941, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bermingham ML, Colombo M, McGurnaghan SJ, Blackbourn LAK, Vučković F, Pučić Baković M, et al.; SDRN Type 1 Bioresource Investigators : N-glycan profile and kidney disease in type 1 diabetes. Diabetes Care 41: 79–87, 2018 [DOI] [PubMed] [Google Scholar]
- 53.Colombo M, Asadi Shehni A, Thoma I, McGurnaghan SJ, Blackbourn LAK, Wilkinson H, Collier A, Patrick AW, Petrie JR, McKeigue PM, Saldova R, Colhoun HM; the Scottish Diabetes Research Network (SDRN) Type 1 Bioresource Investigators: Quantitative levels of serum N -glycans in type 1 diabetes and their association with kidney disease. Glycobiology 31: 613–623, 2021 [DOI] [PubMed] [Google Scholar]
- 54.Clerc F, Reiding KR, Jansen BC, Kammeijer GSM, Bondt A, Wuhrer M: Human plasma protein N-glycosylation. Glycoconj J 33: 309–343, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Haan N, Falck D, Wuhrer M: Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology 30: 226–240, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM: GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res 7: 1650–1659, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.