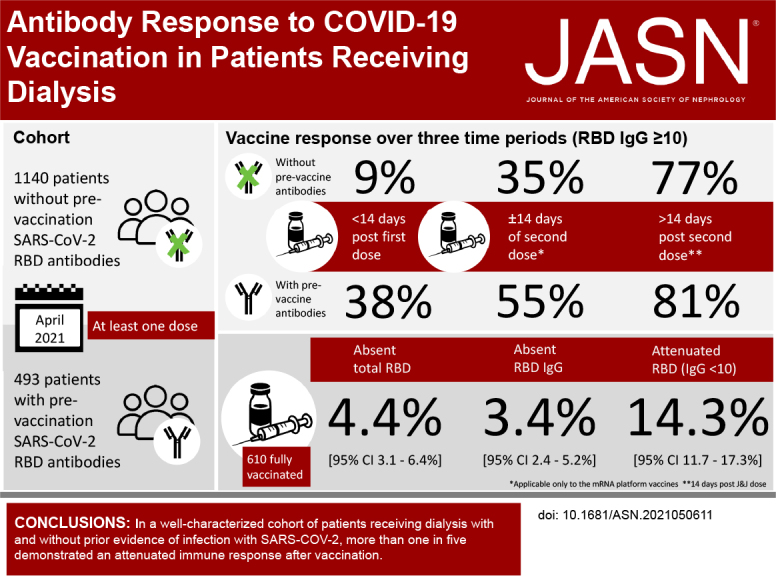
Visual Abstract
Keywords: immunology, chronic dialysis, clinical nephrology, immunosuppression, COVID-19, antibody formation, renal dialysis, vaccination
Data from hepatitis B1 and influenza vaccination2 studies indicate blunted and foreshortened response to immunization in patients receiving dialysis, raising the worrisome possibility that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine may also yield lower efficacy in this population. We report early data on receptor binding domain (RBD) seroconversion and semiquantitative IgG values post-vaccination in 1633 patients. Among the subset who completed two doses of vaccination, we assess rates of, and risk factors for, absent or attenuated response.
Our study was conducted in partnership with the dialysis network US Renal Care and Ascend Clinical Laboratory. In the first two weeks of January 2021—before widespread vaccine rollout—we tested the SARS-CoV-2 antibody status of 21,570 patients receiving dialysis. From among the 17,390 patients who were seronegative in January 2021, we used systematic sampling with fraction intervals stratified by age to randomly select 4346 patients to follow with monthly SARS-CoV-2 serology assays, in association with type and date of vaccination(s) (see Supplemental Methods for assay characteristics and sample size details). We also followed 540 patients seropositive as of January 2021, and any additional patients who seroconverted before vaccination (see Supplemental Figure 1 for “seropositive before vaccination” and “seronegative before vaccination” cohorts). We tested remainder samples using the Siemens total RBD Ig assay, which measures IgG and IgM antibodies, in January 2021 and monthly thereafter in the seronegative before vaccination cohort. Subsequent to a positive total RBD Ig result in the patients who were seronegative and among all patients in the seropositive before vaccination cohorts, we tested samples using a semiquantitative Siemens RBD IgG assay monthly.
We evaluated response over three time periods: <14 days after the first dose of vaccine (early post-vaccine), between 14 days after the first dose and 14 days after the second dose (partially vaccinated, applicable only to the mRNA platform vaccines), and >14 days after the Johnson and Johnson vaccine or the second dose of the mRNA platform vaccines (fully vaccinated). We classified responses as absent total RBD Ig antibody, absent semiquantitative IgG antibody (index value less than one), or attenuated (semiquantitative IgG index value of less than ten)3 (see Supplemental Methods for cut points and statistical analysis details).
As of April 2021, 1140 patients on dialysis without prior SARS-CoV-2 antibodies and 493 patients with extant antibodies received a dose of vaccine (Supplemental Table 1). In the seronegative before vaccination cohort, 9%, 35%, and 77% of patients had RBD IgG values of ten or more in the early postvaccine, partially vaccinated, and fully vaccinated groups, respectively. In the seropositive cohort, the proportions were higher in the earlier periods (38%, 55%, and 81%, respectively). Median IgG levels were lower in the seronegative compared with the seropositive cohorts (Figure 1). Among the seronegative before vaccine cohort, median IgG index values post vaccination were 0.5 (25th, 75th percentile 0.5, 0.5), 3.2 (0.5, 27.8), and 41.6 (11.3, 150.0) in the early, partially and fully vaccinated periods respectively. Among the seropositive prior to vaccine cohorts, median IgG index values post vaccination were 0.5 (0.5, 7.6), 1.1 (0.5, 102.3), 15.3 (1.2, 150), and 150 (23.2, 150) in the prior to, early, partially and fully vaccinated periods respectively.
Figure 1.
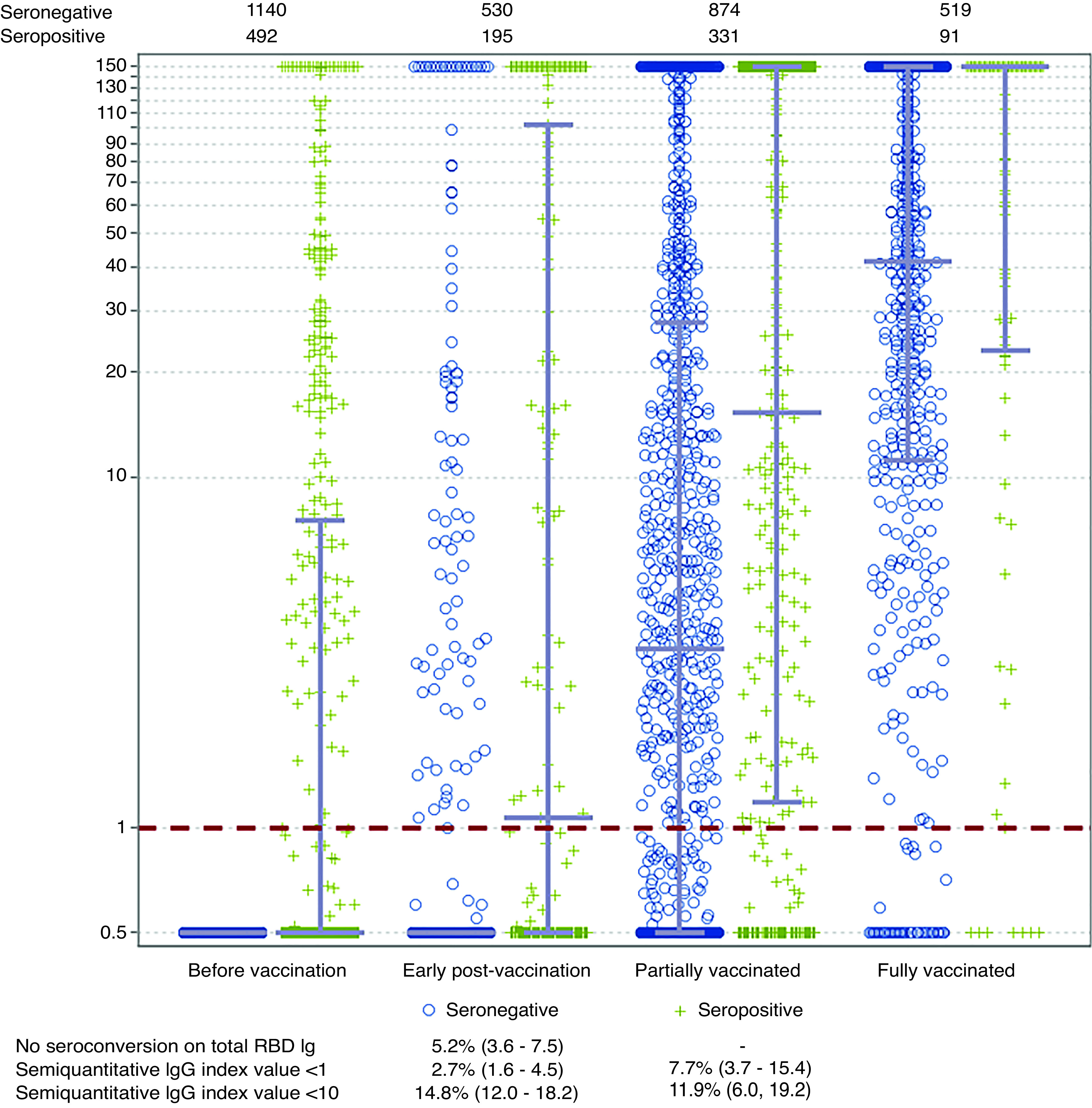
Substantial proportion of patients on dialysis have absent or attenuated RBD antibody response to COVID-19 vaccination. In this figure, each marker represents an individual semiquantitative RBD IgG index value in the time period related to vaccination. Blue circles represent persons who did not have evidence of SARS-CoV-2 infection before vaccination (seronegative prior to vaccination cohort); green markers represent persons who did have evidence of SARS-CoV-2 infection before vaccination (seropositive prior to vaccination cohort). The overlying gray lines represent median with IQR. The IgG index values assay range is from 0.5 to 150, but index values greater than one are considered reactive. In the seropositive before vaccination cohort, the RBD IgG value closest to vaccination is graphed; otherwise, for both cohorts, the value most proximal to the start of each time period is graphed. Although 1140 and 493 patients were included in the two cohorts (because they received at least one vaccine dose at the time of the study), all patients do not have values available for each time period, because these depend on the timing of the vaccination and the routine monthly blood draw. The numbers above the figure show the numbers of persons with a result available in the seronegative and seropositive cohorts for each time period. The table below provides estimates for prevalence of no seroconversion on the total RBD Ig assay, and absent (index value less than one) and attenuated (index value greater than one but less than ten) semiquantitative RBD IgG, respectively.
We assessed vaccine response among patients who were fully vaccinated at a median (IQR) of 29 (22–39) days post–vaccine completion. Of the 610 patients who were fully vaccinated, 27 (prevalence, 4.4%; 95% CI, 3.1% to 6.4%), 21 (prevalence, 3.4%; 95% CI, 2.4% to 5.2%), and 87 (prevalence, 14.3%; 95% CI, 11.7% to 17.3%) had absent total RBD, absent semiquantitive IgG, and attenuated IgG response, respectively. The prevalence of absent or attenuated response was similar in seronegative and seropositive cohorts (Figure 1). Supplemental Tables 2 and 3 show results stratified by age, prevaccination seropositive status, and sensitivity analysis assessing responses at least 28 or more days post vaccine completion.
Non-White race and Hispanic ethnicity were associated with a lower risk of absent or attenuated response; longer dialysis vintage and lower serum albumin were associated with a higher risk (Supplemental Table 4). Median RBD IgG levels were modestly lower for Pfizer than for the Moderna vaccine (Supplemental Figure 2, A and B), and, correspondingly, there was a modestly higher prevalence of absent or attenuated response in the subgroup receiving Pfizer compared with Moderna (Supplemental Table 5). Data on the Johnson and Johnson vaccination were sparse (n=18), but suggested higher prevalence of absent response among patients who were fully vaccinated (prevalence of 83.3% [95% CI, 59.1% to 94.5%] without detectable response on total RBD or RBD IgG, and prevalence of 5.6% [95% CI, 0.8% to 30.7%] for attenuated IgG).
Limitations of the study include the modest sample size. Our assessment was performed during the early phase of vaccine rollout, a time period during which elderly or persons with comorbidities were prioritized. Estimates for vaccine response may improve over time as a broader patient population receives vaccination, although 40% of our cohort was <65 years of age, indicating reasonable representativeness by age of patients receiving dialysis. Antibody titers are only one way to assess immunologic response to vaccination. We do not yet know whether a measurable antibody response correlates with protection from infection.
In summary, in a well-characterized cohort of patients receiving dialysis with and without prior evidence of infection with SARS-COV-2, more than one in five demonstrated an attenuated immune response after vaccination with one of three vaccines granted emergency use authorization by the Food and Drug Administration. There were differences in responses by vaccine type that require further study. Although median IgG titers are higher among patients with evidence of prior SARS-CoV-2 infection compared with those without, rates of absent or attenuated response to vaccination were similar between the two groups. These data are in line with some recently published reports,4,5 and portend the critical need for studies evaluating real-world efficacy of vaccination in the ESKD population and other vulnerable populations with chronic diseases, and for trials evaluating modified schedules of vaccination.
Disclosures
S. Anand reports receiving honoraria from the American Kidney Fund; receiving research funding from a Satellite Healthcare Applied Pragmatic Research Grant; and serving as a scientific advisor for, or member of, International Society of Nephrology and Consortium for the Epidemic of Nephropathy in Central America and Mexico. P. Beyer, L. Cadden, P. Hunsader, and R. Kerschmann report being employed by Ascend Clinical Laboratories. G.A. Block reports receiving research funding from Akebia, Ardelyx, and GlaxoSmithKline; having consultancy agreements with Akebia, Keryx, Kirin, and Reata; receiving honoraria from Amgen and Kirin; serving as a scientific advisor for, or member of, Ardelyx, CJASN, Kirin, and Reata; having ownership interest in Ardelyx and Reata; having other interests in/relationships with Davita (prior medical director), Kidney Disease Improving Global Outcomes (prior member of European Society of Cardiology), and Reata (prior employment); and current employment with US Renal Care. S.D. Boyd reports consultancy agreements with Regeneron, Sanofi, and Novartis; ownership interest in AbCellera and CareDx; honoraria from NIH, and Karolinska Institutet; patents and inventions from U.S. Patent No. 9,068,224, licensed to Adaptive Biotechnologies; and scientific advisor or membership with Food Allergy Fund. R. Kerschmann reports consultancy agreements with Ascend Clinical, Grail, Inc., Notable Labs, and Octave Bioscience. M. Dittrich is employed by US Renal Care; and has ownership interest in US Renal Care, Signify Health, and Multiple dialysis units. G.M. Chertow reports having consultancy agreements with Akebia, Amgen, Ardelyx, AstraZeneca, Baxter, Cricket, DiaMedica, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex; serving on data and safety monitoring boards for Angion, Bayer, NIDDK, and ReCor; having ownership interest in Ardelyx, CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset, Physiowave, and PuraCath; serving as a coeditor of Brenner and Rector’s The Kidney (Elsevier); receiving research funding from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of Allergy and Infectious Diseases; and being on the board of Satellite Healthcare, a not-for-profit dialysis organization. M. Dittrich reports having ownership interest in Signify Health, US Renal Care, and multiple dialysis units; and being employed by US Renal Care. R. Kerschmann reports having consultancy agreements with Ascend Clinical, Grail Inc., Notable Labs, and Octave Bioscience. M. E. Montez-Rath reports receiving research funding from Sanofi. All remaining authors have nothing to disclose.
Funding
S. Anand was supported by NIH grant R01DK127138. G.M. Chertow was supported by NIH grant K24DK085446. Ascend Clinical Laboratory supported the remainder plasma testing for SARS-CoV-2 antibodies.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021050611/-/DCSupplemental.
Supplemental Methods.
Supplemental Table 1. Participant characteristics according to SARS-CoV-2 spike protein receptor binding domain antibody status prior to vaccination.
Supplemental Table 2. Prevalence of absent or attenuated response among fully vaccinated individuals overall and by age group, at least 14 days after completion of vaccine.
Supplemental Table 3. Prevalence of absent or attenuated response among fully vaccinated individuals overall and by age group, at least 28 days after completion of vaccine.
Supplemental Table 4. Risk factors for absent or attenuated response to SARS-CoV-2 vaccination in fully vaccinated patients receiving dialysis.
Supplemental Table 5. Prevalence of absent or attenuated response among fully vaccinated individuals by vaccine type, at least 14 days after completion of vaccine.
Supplemental Figure 1. Study flowchart of participants.
Supplemental Figure 2. Semiquantitative IgG values in patients receiving Moderna (a) or Pfizer (b) vaccines.
References
- 1.Edey M, Barraclough K, Johnson DW: Review article: Hepatitis B and dialysis. Nephrology (Carlton) 15: 137–145, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Broeders NE, Hombrouck A, Lemy A, Wissing KM, Racapé J, Gastaldello K, et al.: Influenza A/H1N1 vaccine in patients treated by kidney transplant or dialysis: a cohort study. Clin J Am Soc Nephrol 6: 2573–2578, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al.: Serial SARS-CoV-2 receptor-binding domain antibody responses in patients receiving dialysis [published online ahead of print May 18, 2021]. Ann Intern Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al.: Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis [published online ahead of print April 6, 2021]. Clin J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attias P, Sakhi H, Rieu P, Soorkia A, Assayag D, Bouhroum S, et al.: Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int 99: 1490–1492, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


