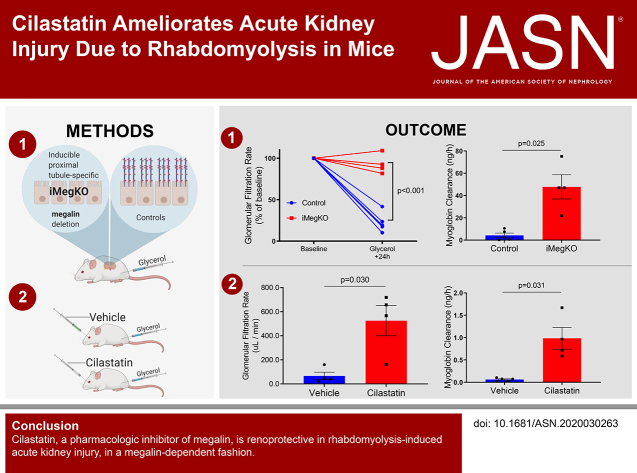
Significance Statement
Rhabdomyolysis causes severe AKI and death in settings such as earthquakes and armed conflict. Specific treatment is not available and care is difficult to provide in these austere environments. Skeletal muscle myoglobin is a renal toxin that causes AKI in this syndrome. Proximal tubular megalin participates in myoglobin endocytosis and may be an AKI mediator. The authors demonstrate in a mouse model that proximal tubular megalin plays a critical role in rhabdomyolysis-induced AKI. In this model, proximal tubule–specific megalin deletion ameliorated AKI, and this effect was recapitulated by administration of cilastatin, a megalin inhibitor. This translational study thus identifies megalin as a mediator of rhabdomyolysis-induced AKI and suggests a novel mechanism by which it may be possible to ameliorate this condition.
Keywords: rhabdomyolysis, endocytosis, acute renal failure, chronic kidney disease, renal protection
Visual Abstract
Abstract
Background
Rhabdomyolysis, the destruction of skeletal muscle, is a significant cause of AKI and death in the context of natural disaster and armed conflict. Rhabdomyolysis may also initiate CKD. Development of specific pharmacologic therapy is desirable because supportive care is nearly impossible in austere environments. Myoglobin, the principal cause of rhabdomyolysis-related AKI, undergoes megalin-mediated endocytosis in proximal tubule cells, a process that specifically injures these cells.
Methods
To investigate whether megalin is protective in a mouse model of rhabdomyolysis-induced AKI, we used male C57BL/6 mice and mice (14–32 weeks old) with proximal tubule–specific deletion of megalin. We used a well-characterized rhabdomyolysis model, injection of 50% glycerol in normal saline preceded by water deprivation.
Results
Inducible proximal tubule–specific deletion of megalin was highly protective in this mouse model of rhabdomyolysis-induced AKI. The megalin knockout mice demonstrated preserved GFR, reduced proximal tubule injury (as indicated by kidney injury molecule-1), and reduced renal apoptosis 24 hours after injury. These effects were accompanied by increased urinary myoglobin clearance. Unlike littermate controls, the megalin-deficient mice also did not develop progressive GFR decline and persistent new proteinuria. Administration of the pharmacologic megalin inhibitor cilastatin to wild-type mice recapitulated the renoprotective effects of megalin deletion. This cilastatin-mediated renoprotective effect was dependent on megalin. Cilastatin administration caused selective proteinuria and inhibition of tubular myoglobin uptake similar to that caused by megalin deletion.
Conclusions
We conclude that megalin plays a critical role in rhabdomyolysis-induced AKI, and megalin interference and inhibition ameliorate rhabdomyolysis-induced AKI. Further investigation of megalin inhibition may inform translational investigation of a novel potential therapy.
Rhabdomyolysis, the destruction of striated skeletal muscle, causes systemic circulation of the principal protein in muscle, myoglobin. Each year >25,000 patients with rhabdomyolysis are reported in the United States, caused by crush injury, physical training, or medication. Rhabdomyolysis-induced myoglobinemia causes AKI; this form of AKI, termed “crush syndrome,” is a significant cause of mortality in the settings of earthquakes and armed conflict.1–3 Clinical and translational studies suggest crush syndrome can lead to CKD, which itself is characterized by excess morbidity and mortality.4,5 Mortality-reducing treatment is limited to intravenous fluid administration6 and dialysis; the difficulty and expense of such treatments prevent their effective use in austere environments. Specific, simple therapy that is deliverable in such settings might therefore have clinical importance.
Myoglobin, a small protein (19 kD), is abundantly filtered in the glomerulus and reaches the renal proximal tubule, where it injures renal proximal tubular cells, causing rhabdomyolysis-induced AKI.7 Proximal tubular epithelial cells avidly take in filtered proteins, primarily through a proximal tubule–specific endocytic complex composed of megalin and cubilin,8 which has >40 known ligands, including myoglobin.9 Once within the endosomal system, megalin ligands undergo endosomal sorting and can undergo subsequent lysosomal degradation or transcytosis. In particular, transcytosis increases the serum t1/2 of albumin and likely conserves systemic resources by reducing the need for de novo synthesis of important small proteins that would otherwise be excreted in the urine.10 Because proximal tubule cells are extensively injured in rhabdomyolysis-induced AKI, it is possible that megalin-mediated myoglobin retrieval functions in a maladaptive way when excess myoglobin is present. Therefore, we hypothesized that interference with proximal tubule megalin would ameliorate rhabdomyolysis-induced AKI. We further hypothesized that cilastatin, recently identified as a megalin inhibitor, would also function in this manner.
Methods
Animals
Animal procedures were performed on male C57BL/6 mice (obtained from the Jackson Laboratory) at the age of 8–12 weeks, and on proximal tubule–specific inducible megalin-deleted (iMegKO) mice at the age of 14–32 weeks; these procedures were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee (IP0001188) or the Portland Veterans Affairs Medical Center (4374–18). The generation and breeding of iMegKO mice were as previously described.11 Briefly, Lrp2fl/fl mice (a generous gift of Professor T. Willnow, Max Delbrück Center for Molecular Medicine) were bred to mice expressing tamoxifen-dependent Cre recombinase in cells that express Ndrg1 (Ndrg1-CreERT2), which is abundantly expressed in the renal proximal tubule.12 Mice were genotyped using quantitative PCR by Transnetyx (Cordova, TN) before experimentation. Deletion of megalin in the proximal tubule was induced by injection of tamoxifen (150 mg/kg body wt, intraperitoneally, daily for 5 days). Mice were used for experiments 15 days after the first tamoxifen injection. In experiments involving iMegKO mice, Cre-negative littermates served as controls, and received tamoxifen identically to experimental mice.
Rhabdomyolysis Model
We modeled rhabdomyolysis with a well-characterized model, glycerol intramuscular injection of 50% glycerol in normal saline, preceded by a period of water deprivation.13–15 On the basis of preliminary experiments in wild-type mice that demonstrated consistent reduction in 24-hour urine output and GFR after 4 hours of water deprivation and 8.0 ml/kg glycerol, this regimen was used for all experiments except for the 60-day experiment. In this experiment, 6.5 ml/kg of glycerol was used to ensure 100% survival, because long-term survival is only translationally relevant in the setting of 100% short-term survival. Glycerol was injected into the anterior thigh muscle (half the dose to each side).
Cilastatin and Vehicle Injection
Cilastatin, 200 mg/kg or equivalent volume of vehicle (<150 µl per mouse) was administered by retroorbital injection contemporaneously with glycerol injection in the rhabdomyolysis experiments, or alone in experiments testing the mechanism of cilastatin.
Measurement of Urine Volume and Proteins
Urine was collected in urine collection cups pretreated with protease inhibitor for 24 hours, starting immediately after an animal’s recovery from experimental anesthesia. Total urine protein was measured using bicinchoninic acid. Equal volumes of urine were loaded for urine gel electrophoresis, and gels were stained with Coomassie blue. Urine myoglobin and retinol binding protein 4 (RBP4) were measured by ELISA (see Supplemental Table 1 for specific kits).
Measurement of GFR
The GFR was measured in mice under isoflurane anesthesia by determining elimination of FITC-sinistrin transcutaneously as previously described16 after a FITC-sinistrin (75 mg/kg body wt) bolus injection into the retroorbital plexus.
Measurement of Plasma Urea Nitrogen
Whole blood drawn from the cardiac left ventricle at the time of euthanasia was stored in sodium EDTA–containing tubes (BD, Franklin Lakes, NJ), and plasma was separated by centrifugation. Plasma urea nitrogen was determined using a commercially available colorimetric assay (Invitrogen, Carlsbad, CA).
Measurement of Plasma Myoglobin and Determination of Myoglobin Clearance
A separate cohort of mice underwent glycerol injection as described above and left ventricular transcardiac puncture 6 hours later for plasma myoglobin studies. Myoglobin was quantified in plasma samples using a commercially available ELISA (Mybiosource.com, San Diego, CA). Myoglobin clearance was calculated using the group (strain) mean 24-hour plasma myoglobin and 24 urine collections from the same groups according to the following formula:
Immunofluorescence, Immunohistochemistry, and Immunoblotting
A list of antibodies, probes, kits, buffers, and reagents is in Supplemental Table 1. Kidneys were perfusion-fixed via the left ventricular apex with 4% paraformaldehyde. Paraffin-embedded sections 5 µm thick were stained using periodic acid–Schiff stain and an α-smooth muscle actin (αSMA)–FITC conjugate. Immunofluorescence staining and immunoblotting were performed as described in Supplemental Methods.
Imaging and Semiautomated Unbiased Stereology
Fluorescence images were captured an epifluorescence microscope (Axio Imager M2; Zeiss, Jena, Germany, and Keyence BZ-X800; Itasca, IL). To ensure unbiased quantification of the extracellular matrix component αSMA, kidney injury molecule-1 (KIM-1), cleaved caspase-3, and myoglobin, slides containing five sagittal kidney sections (cut at 160 µm intervals starting at random distance from the caudal renal pole) were scanned using a slide scanner (Axioscan; Zeiss, Jena, Germany); semiautomated unbiased stereology was performed using a custom macro and the Fiji ImageJ distribution, as previously described.17,18
Quantification of Myoglobin Endocytosis
We characterized in vivo endocytosis of myoglobin first in control and iMegKO mice and then in wild-type mice treated with vehicle or cilastatin. We injected FITC-myoglobin (0.5 mg) retroobitally alone or 1 hour after injection of cilastatin (200 mg/kg, also retroorbital); mice were killed 15 or 30 minutes after injection and perfused. Kidney sections were prepared and scanned. Using ImageJ, FITC-positive puncta were identified and segmented by thresholding (identically across all images and treatments), and area and mean fluorescence for each puncta extracted. The number of puncta per unit tissue area, puncta mean area, and puncta mean fluorescence were compared. To test the hypothesis that cilastatin altered overall renal uptake of endogenous myoglobin, we administered vehicle or cilastatin, followed 1 hour later by intramuscular glycerol, to wild-type mice as described above, preparing kidney sections 2 hours after glycerol injection. To compare overall renal myoglobin content, we performed unbiased stereology, as previously described.
Statistical Analysis
Statistical analysis was primarily performed using Prism 7.0 (GraphPad, LaJolla, CA); analysis of endocytic punctae was performed using R (v4.0.5). Two-group comparisons were performed using Student’s t test, with Welch’s correction as appropriate. Multiple-group comparisons were performed with ANOVA (or two-way ANOVA in the case of before-after comparisons in the same mice), with Holm–Sidak’s test as appropriate. For the experiment testing the effect of cilastatin in iMegKO mice (depicted in Figure 6), a priori power analysis (performed using R 4.05, package pwr) determined the experimental number. Statistical significance was inferred from P<0.05. Mean and SEM are shown in the figures and text.
Figure 6.
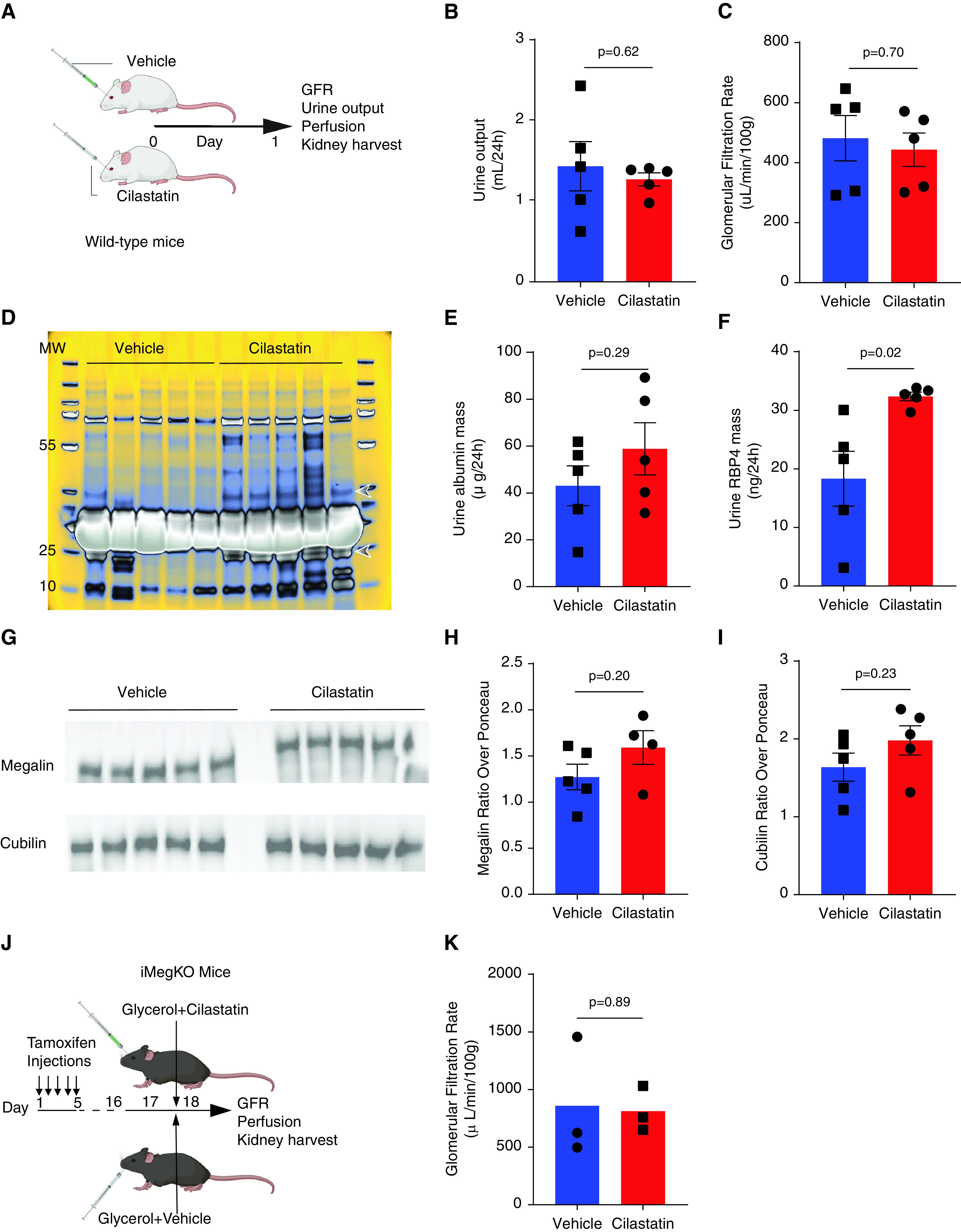
Cilastatin and megalin deletion have similar effects on renal function. (A) Experimental design for results (B–I). Wild-type mice received cilastatin (200 mg/kg) or vehicle injection as in prior experiments, without injection of glycerol. The 24 hours later, outcomes were assessed. (B and C) Urine output and GFR were not altered by cilastatin administration. (D) Coomassie-stained urine electrophoresis (loaded with identical volumes of urine from each animal). Bands at approximately 23 kD and approximately 40 kD marked by arrowheads appear differentially expressed in cilastatin versus vehicle samples. The same bands may be seen in the urine of iMegKO mice in Figure 1. Unaltered gel image shown in pseudo-color to better visualize peak protein density. (E) Urine albumin was not significantly increased. (F) RBP4, a plasma protein that is a known megalin ligand, and is greatly increased in the urine of megalin-deleted mice, was nearly doubled by cilastatin administration. (G–I) Immunoblots performed on kidney lysate 24 hour after vehicle or cilastatin injection. Megalin expression was reduced after cilastatin administration, whereas cubilin was not significantly altered. (J) Experimental design. iMegKO mice received cilastatin or vehicle with injection of glycerol. Then 24 hours later, mean GFR was identical between groups (K), indicating that cilastatin-dependent protection from rhabdomyolysis-induced AKI is absent in mice without proximal tubule megalin. Statistical analysis presented is derived from the t test.
Results
Renal Effects of Proximal Tubule–specific Megalin Deletion
On day 15 after initiating tamoxifen induction of Cre recombinase, megalin was absent in LRP2fl/fl-Ndrg1CreERT2+ (iMegKO) mice; it remained abundantly expressed in cortex but was not expressed in the medulla of LRP2fl/fl-Ndrg1CreERT2- (control) mice (Figure 1, A and B, Supplemental Figures 1 and 2). Deletion of proximal tubule megalin did not alter renal abundance of cubilin, as determined by immunoblot (OD ratio, 1.1±0.3 in control versus 0.9±0.2 in iMegKO; P=0.52) (Figure 1B). Electrophoresis demonstrated low molecular weight bands in urine from iMegKO mice and not that of controls (Figure 1C), consistent with prior reports in mice with megalin interference.11,19 To further evaluate this selective proteinuria, we quantified RBP4, a megalin ligand20 that is specifically upregulated in the urine of megalin-deleted mice21; it was dramatically increased in the urine of iMegKO mice compared with controls (184.6±10.9 ng/ml in iMegKO versus 25.7±6.4 ng/ml in controls (Figure 1D). Body weight and urine output did not differ between iMegKO and controls (urine output 4.6±0.4 ml/24 hours in iMegKO versus 4.8±0.4 ml/24 hours in controls (Figure 1E). Surprisingly, iMegKO status conferred a baseline GFR that was reduced 21% compared with that of controls (700.3±34.0 µl/min per 100 g in iMegKO mice versus 882.8±32.7 µl/min per 100 g in controls; P<0.001; n=11–12 (Figure 1F). In accordance with prior reports,11 total urine protein and urine albumin were significantly increased by proximal tubule–specific megalin deletion (for total protein, 0.4±0.1 mg/24 hours in controls versus 4.0±0.4 mg/24 hours in iMegKO mice, P<0.001; for albumin, 30.45±5.4 µg/24 hours in controls versus 522.2±57.7 µg/24 hours in iMegKO, P<0.0001); n=11–12 per group (Figure 1G).
Figure 1.
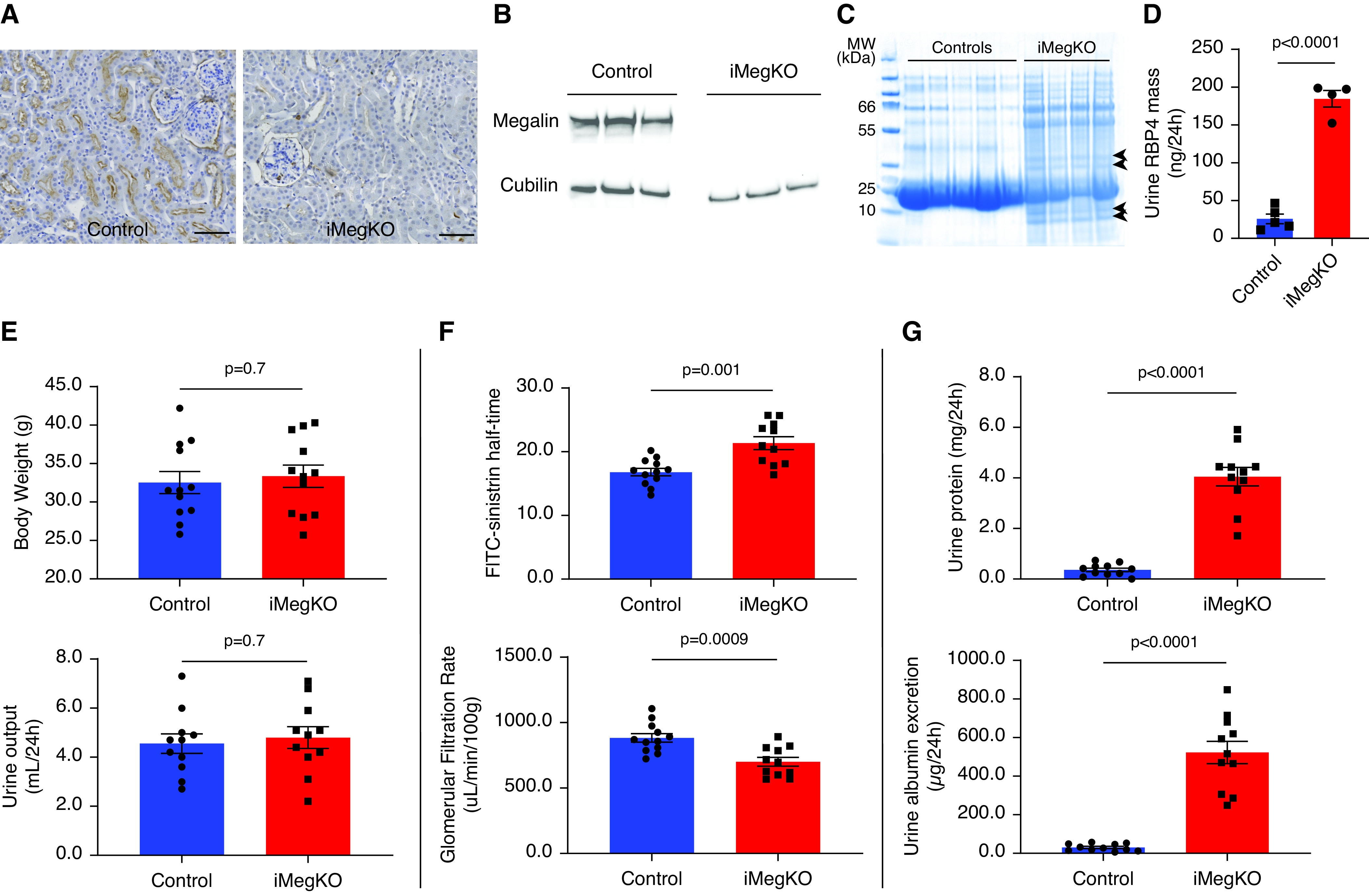
Animal and experimental models. (A–G) Results of tamoxifen treatment in Lrp2fl/fl; Ndrg1CreERT2+ (iMegKO) and cre- littermates (control). (A) High-power micrographs obtained 14 days after the first of five daily tamoxifen injections demonstrate that control mice exhibit robust immunostaining for megalin at proximal tubule brush borders, whereas iMegKO mice demonstrate near-complete absence of megalin. (B) Megalin is absent in immunoblots performed on renal homogenate of iMegKO mice, whereas cubilin is not affected by iMegKO status. (C) The urine of iMegKO mice contains low molecular-weight proteins (arrowheads), which are not present in the urine of controls as demonstrated by Coomassie-stained electrophoresis of equal volumes of urine obtained from 24 hours collection. (D) Urine RBP4, a megalin ligand, is greatly upregulated by iMegKO. (E) Body weight and 24 hours urine output are not altered by megalin deletion. (F) Clearance of FITC-conjugated sinistrin (FITC-sinistrin) is reduced by megalin deletion, indicating a reduction in GFR. (G) Urine protein and urine albumin excretion are greatly increased by megalin deletion. Scale bars: 100 µm. Statistical test used for all comparisons: t test.
Proximal Tubule–specific Megalin Deletion Abrogates Rhabdomyolysis-induced AKI
Then 6 hours after glycerol injection, all mice demonstrated discolored urine, and serum myoglobin was markedly elevated (81.9±6.2 ng/ml at 6 hours compared with 6.9±1.1 ng/ml 24 hours after injection; P<0.0001; n=9–10 per group). All mice survived to the planned endpoint. Then 24 hours after glycerol injection (experimental design shown in Figure 2A), there was a noticeable difference in the urine color and quantity between control and iMegKO mice; with iMegKO demonstrating increased quantity (6.0±0.7 ml/24 hours versus 2.4±0.7 ml/24 hours; P=0.008) (Figure 2B) and clearer urine compared with controls. The GFR was completely preserved in iMegKO mice (621.1±27.2 µl/min per 100 g at baseline versus 572.4±17.1 µl/min per 100 g in 24 hours after glycerol injection; P=0.7, n=4), but dramatically reduced in controls (864.5±62.7 µl/min per 100 g at baseline versus 192.5±43.4 µl/min per 100 g in 24 hours after glycerol injection; P<0.001, n=5) (Figure 2C, Supplemental Figure 3). This sharp distinction in GFR was reflected in dramatic differences in serum urea nitrogen (146.5±41.6 mg/dl in controls versus 37.4±3.5 mg/dl in iMegKO mice, P=0.03) (Figure 2D). Preserved renal function was accompanied by preservation of renal architecture and reduced immunofluorescence signal for injury-specific molecules. Sections from control mice stained with periodic acid–Schiff demonstrated extensive necrosis, inflammatory cell infiltrate, and protein casts; these were present but attenuated in iMegKO mice, resulting in reduced blinded injury score (430±43 in controls versus 247±28 in iMegKO; n=4 per group, P=0.03) (Figure 2E). Similarly, acute tubular injury as demonstrated by KIM-1 and tubular apoptosis (determined by cleaved caspase-3 immunofluorescence) were attenuated by iMegKO status (Figure 2, F and G). There was disruption of normal spatial organization of megalin in control mice, which was not observed in iMegKO because of their lack of megalin (Supplemental Figure 4). Overall, these data demonstrate that proximal tubule–specific megalin deficiency abrogates AKI due to rhabdomyolysis.
Figure 2.
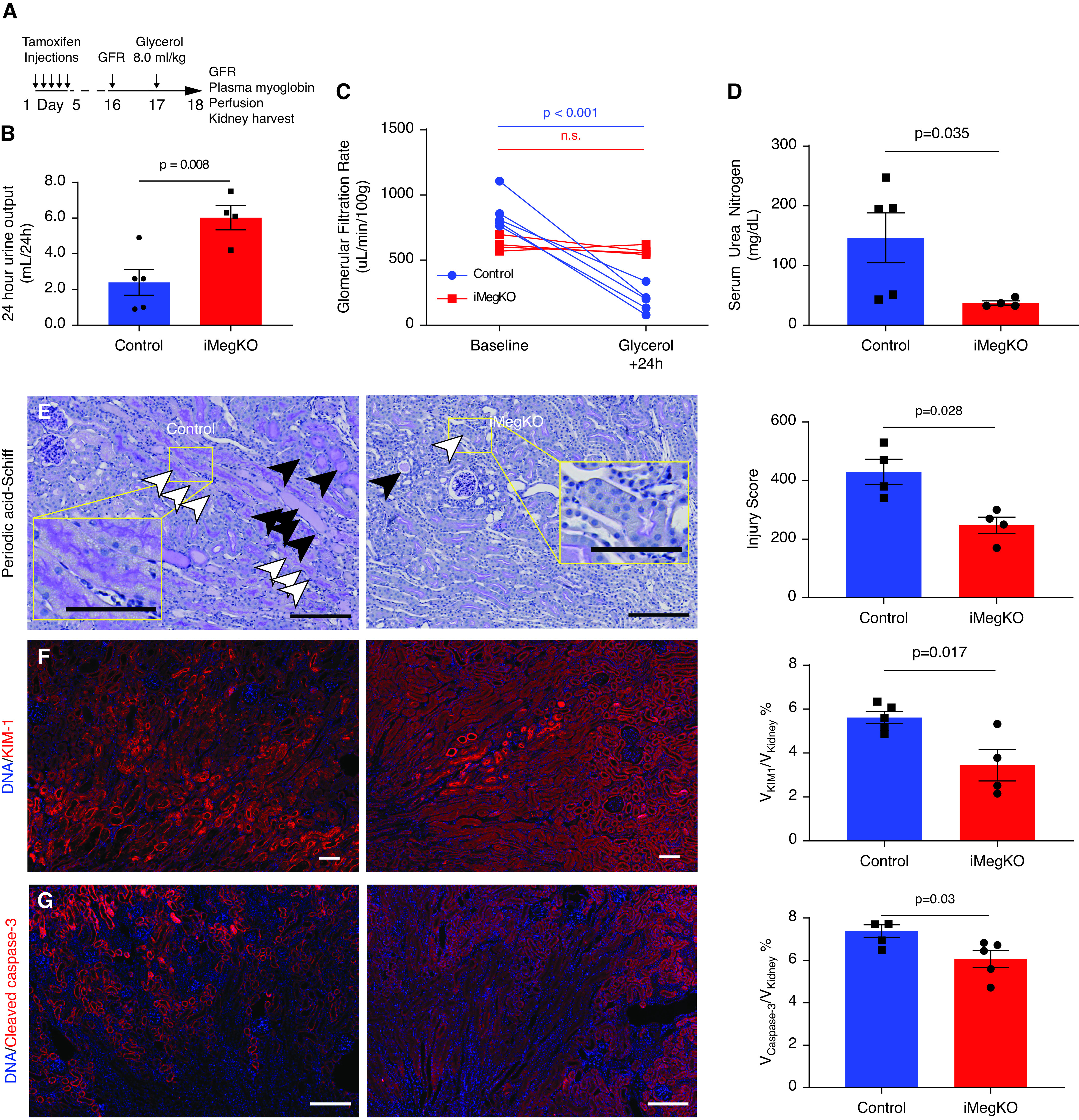
Deletion of proximal tubular megalin mediates rhabdomyolysis-induced AKI 24 hours after glycerol injection. (A) Experimental design. Tamoxifen induction started 15 days before glycerol injection; mice with inducible proximal tubule–specific megalin deletion (iMegKO) and littermate control mice received identical tamoxifen regimens. (B–G) At 24 hours after glycerol injection, control mice demonstrated AKI, which was attenuated in iMegKO mice. (B) Control mice were oliguric, whereas iMegKO mice demonstrated urine output that was greater than baseline. (C) Control mice demonstrate severe loss of GFR, whereas in iMegKO, GFR is not different from baseline value. (D) Serum urea nitrogen is much greater in control mice than iMegKO mice. (E) Photomicrographs of periodic acid–Schiff stained sections in control and iMegKO mice are distinguished by extensive proteinaceous material in distal tubule and collecting ducts (black arrowheads) and cell swelling and luminal effacement (white arrowheads) in control, with more normal architecture in iMegKO. Composite injury score, right, is greater in control mice. (F) KIM-1 stain is greatly attenuated in iMegKO mice, and accordingly, apoptosis, indicated by cleaved caspase-3 staining (G), is also reduced by megalin interference. Scale bars are 100 µM. Statistical test used for all comparisons: t test.
Proximal Tubule–specific Megalin Deletion Ameliorates Rhabdomyolysis-induced Progressive GFR Loss And Proteinuria
Experimental rhabdomyolysis leads to CKD,4 and there may be an association between crush syndrome and late development of CKD and hypertension.3,5 Therefore, to assess whether megalin mediates development of CKD, we induced mild experimental rhabdomyolysis and followed iMegKO and controls for 60 days (experimental design shown in Figure 3A). Survival and body weight did not differ between controls and iMegKO mice (Figure 3, B and C). In controls, urine output (Figure 3D) and GFR (Figure 3E) declined steadily after rhabdomyolysis induction (GFR, 864.5±62.7 µl/min per 100 g body wt at baseline, 660.1±32.0 µl/min per 100 g 30 days after glycerol injection, and 659.1±53.3 µl/min per 100 g 60 days after glycerol injection; corresponding to percentages of baseline of 100.0%, 93.8%±5.3%, 75.9%±3.4%, and 76.2%±6.9%, respectively). In contrast, iMegKO mice demonstrated stable urine output and GFR (with urine output at 60 days the same as baseline and GFR at 60 days 106.3%±9.5% of baseline; P=0.003, compared with controls). Controls also developed early proteinuria and albuminuria on day 2 after glycerol injection (approximately 1 mg/d of total protein and 35–75 µg additional albumin over baseline) (Figure 3, F and G, Supplemental Figure 5), which persisted for 60 days. The iMegKO mice also developed acute protein and albuminuria, however, this excess proteinuria resolved to baseline levels by 30 days. At the 60-day endpoint, consistent with the relatively small difference in GFR (22.1% between groups), serum urea nitrogen did not differ between groups (Figure 3H). Unblinded assessment of injury score on periodic acid–Schiff stained histologic sections did not differ, and immunostaining for the fibrosis marker α-smooth muscle actin was sparse and did not differ between groups (Figure 3, H–J, Supplemental Figure 6). Collectively, these data support progression of acute injury with loss of GFR, progressive oliguria, and persistent proteinuria in control mice; this progressive injury did not occur in mice with proximal tubule–specific megalin deletion. Mice had similar indices of histologic injury at 60 days and little α-smooth muscle proliferation. Therefore, we conclude that proximal tubule megalin mediates mild chronic kidney injury after rhabdomyolysis-induced AKI and may contribute to long-term change in kidney function.
Figure 3.
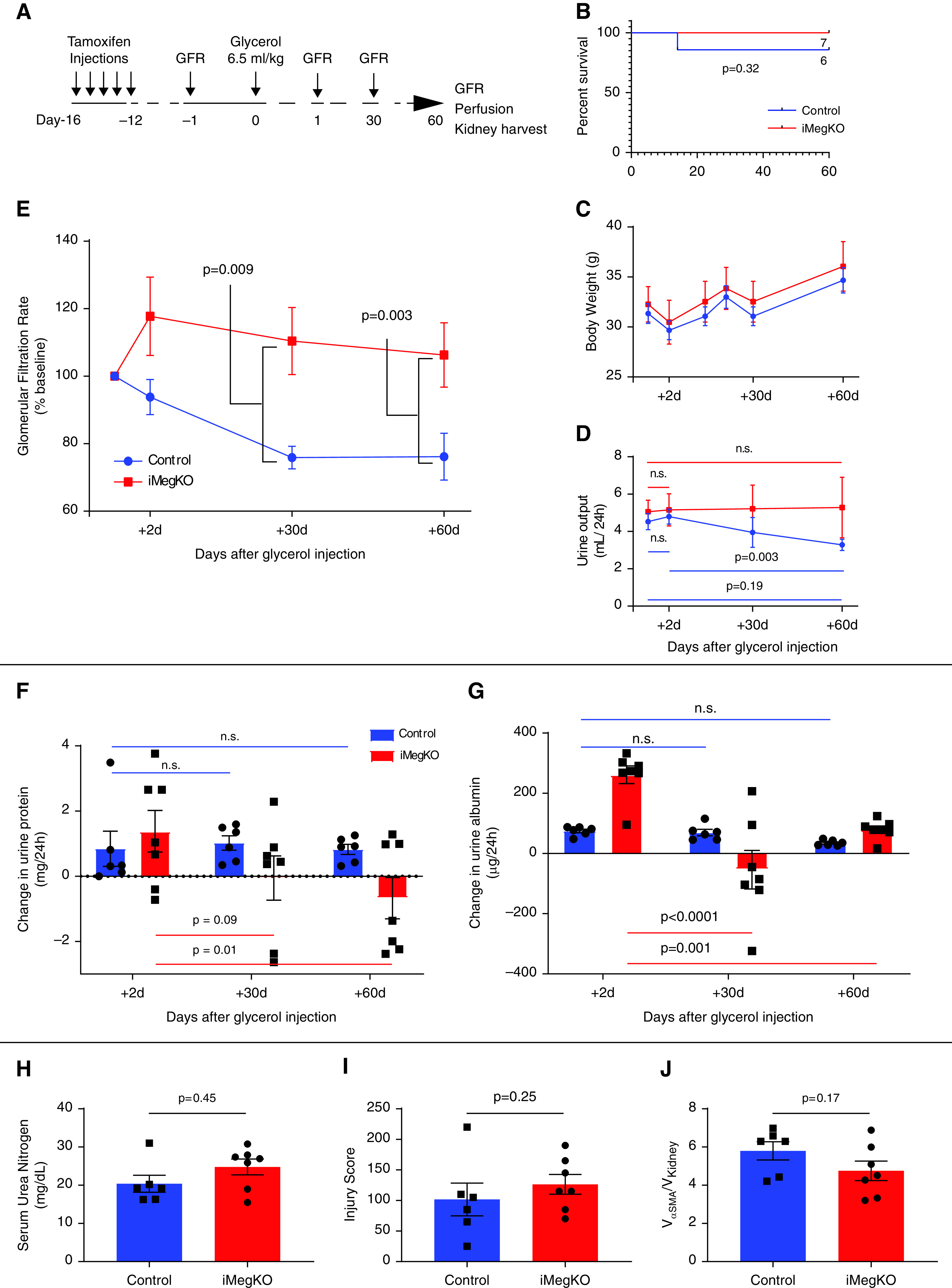
Megalin mediates progressive kidney disease due to rhabdomyolysis. (A) Experimental design. (B) Survival plot. One control mouse died during the 60-day experiment. (C) Body weight after glycerol injection was not mediated by induced proximal tubule-specific megalin deletion (iMegKO). (D) Control mice developed relative oliguria at 60 days when compared with 2 days after glycerol injection, whereas iMegKO mice demonstrated unchanged urine output throughout the experiment. (E) GFR progressively declined in control mice, whereas in iMegKO mice, GFR did not change from baseline. (F and G) Because megalin interference causes proteinuria (see Figure 1), urine protein (F) and urine albumin (G) are displayed as change from baseline. Control mice demonstrated increased proteinuria and albuminuria, compared with baseline, which persisted through the full 60-day experimental course, whereas in iMegKO mice, both proteinuria and albuminuria were reduced at 60 days compared with 2 days after glycerol injection. iMegKO mice did not have significantly increased proteinuria at day 60, whereas controls did. (H) Serum urea nitrogen, pathologic injury score (I), and α smooth muscle actin deposition (αSMA, an indicator of renal fibrosis) were not mediated by proximal tubule megalin status 60 days after glycerol injection. Statistical analysis presented is Mantell-Cox logrank test (B), repeated measures ANOVA (C–G), and t test (H–J).
Pharmacologic Inhibition of Megalin with Cilastatin Ameliorates Rhabdomyolysis-induced AKI
Recapitulation of the protective effect of megalin interference by using drug therapy could be clinically important. Therefore, we administered cilastatin, recently identified as a megalin inhibitor,22 to wild-type mice. When administered at the time of induction of rhabdomyolysis (Figure 4A), cilastatin was well tolerated; survival at 24 hours was 100% in the cilastatin-treated group and 80% in the vehicle-treated group. Vehicle-treated mice were oliguric, whereas cilastatin-treated mice demonstrated greater urine output (0.4±0.1 ml/24 hours with vehicle versus 1.8±0.3 ml/24 hours with cilastatin, P=0.01) (Figure 4B). Cilastatin administration increased both overall proteinuria (Figure 4C) and albuminuria (Figure 4D), in parallel with findings in iMegKO mice. Mice treated with cilastatin demonstrated eight times greater GFR 24 hours after glycerol injection compared with vehicle-treated mice (525.9±125.6 µl/min per 100 g versus 66.7±31 µl/min per 100 g, respectively; n=4–5 per group, P=0.03) (Figure 4E). Accordingly, plasma urea nitrogen was also lower in cilastatin-treated mice than in vehicle-treated mice (37.4±3.5 mg/dl versus 146.5±41.6 mg/dl, P=0.03) (Figure 4F). Both groups demonstrated histologic injury scores similar to those observed in prior experiments, trending lower in cilastatin-treated mice (378±30 in vehicle-treated mice versus 276±46 in cilastatin-treated mice; P=0.12) (Figure 4G). Renal KIM-1 immunopositivity was modestly reduced by cilastatin treatment, indicating reduced cellular injury (Figure 4H); however, renal apoptosis as indicated by cleaved caspase-3 immunopositivity, although similar to prior experiments, was not different (not shown). Overall, these results indicate that administration of the renal megalin inhibitor cilastatin mitigated rhabdomyolysis-induced renal functional decline, with modest effects on overall renal acute tubular injury, in a manner similar to that seen in mice with proximal tubule–specific megalin interference.
Figure 4.
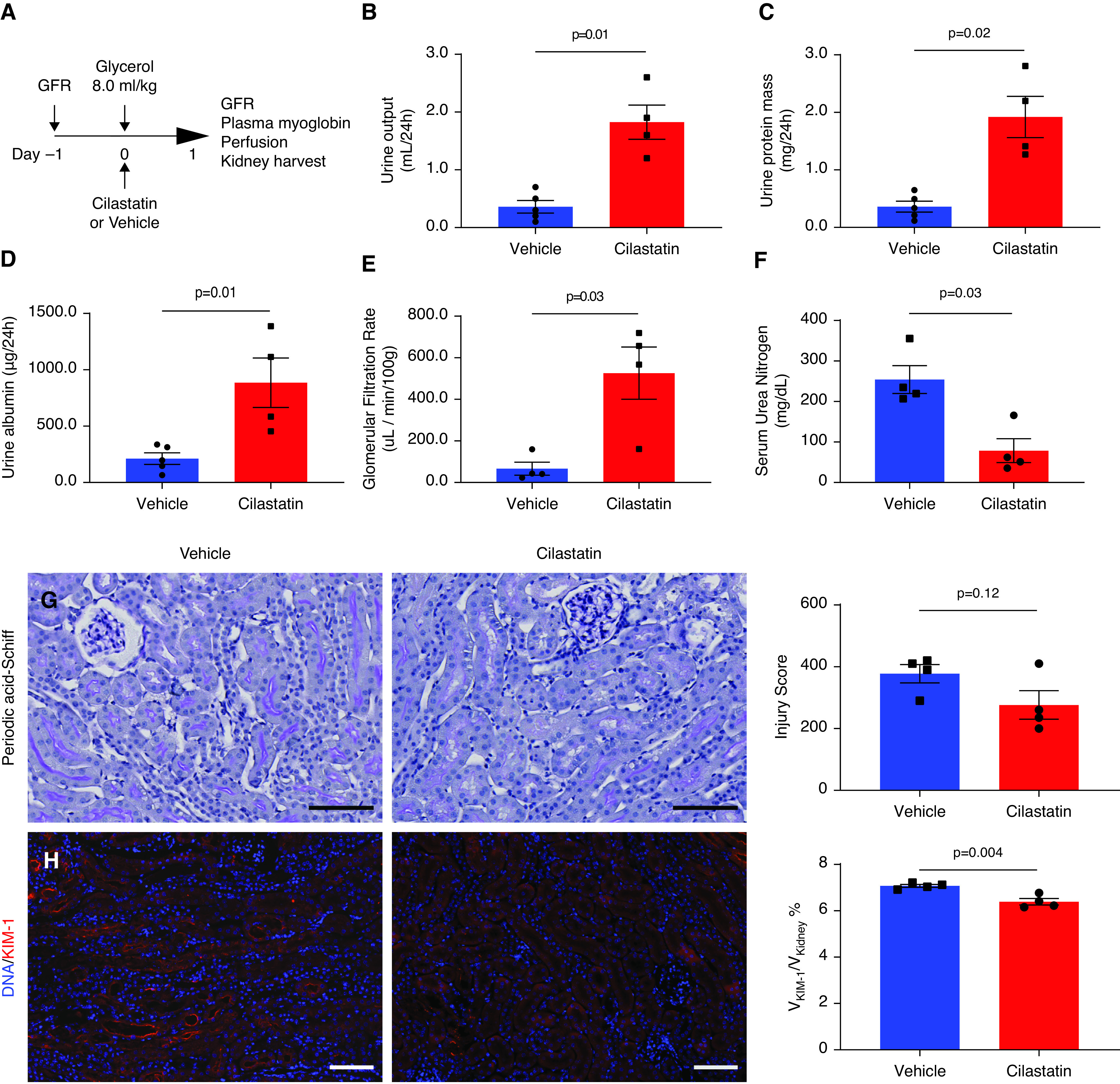
Pharmacologic treatment with megalin inhibitor cilastatin recapitulates AKI amelioration by proximal tubule-specific megalin deletion. (A) Experimental design. Cilastatin was administered immediately after glycerol. (B–D) In accordance with findings in iMegKO mice, cilastatin administration at the time of rhabdomyolysis induction resulted in increased urine output (B), increased acute proteinuria (C), and increased urine albumin (D), compared with vehicle. Cilastatin administration also prevented severe loss of GFR observed in vehicle-treated mice (E), and prevented the highly elevated serum urea nitrogen seen in vehicle-treated mice (F). Periodic acid–Schiff stained sections were also generally in accordance with findings in control and iMegKO mice (G), although histopathologic injury scoring was not different between cilastatin- and vehicle-treated mice. KIM-1 was elevated in both groups (H), but significantly reduced in cilastatin-treated mice compared with vehicle-treated mice. Scale bars are 100 µm. Statistical analysis presented is derived from the t test.
Myoglobin Clearance after Glycerol Injection Is Similarly Mediated by Megalin and Cilastatin
To test the hypothesis that megalin interference altered myoglobin clearance, we assessed urine and blood myoglobin concentration, myoglobin clearance, and renal myoglobin retention. In iMegKO mice and controls, urine myoglobin was not detectable before glycerol injection, but was greatly elevated in 24-hour urine collections begun after glycerol injection and dramatically increased in iMegKO mice compared with control mice (Figure 5A). Figure 5B illustrates that 6 hours after glycerol injection, plasma myoglobin was elevated (95.1±6.7 ng/ml and 68.8±6.2 ng/ml in control and iMegKO mice, respectively; P=0.02; n=5 per group). By 24 hours, plasma myoglobin had declined in both groups (7.5±1.2 ng/ml versus 6.2±1.9 ng/ml in control and iMegKO mice, respectively; P=0.6; n=4–5 per group). Thus, myoglobin clearance was >11 times greater than that of controls in mice with proximal tubule–specific megalin interference (47.8±10.9 ng/h versus 4.2±2.1 ng/h; P=0.03) (Figure 5C). Similarly, cilastatin greatly increased urine myoglobin excretion (16.6±4.23 ng/24 hours in vehicle versus 127.7±30.4 ng/24 hours in cilastatin-treated mice; P=0.04) (Figure 5D), resulting in reduced plasma myoglobin (5.2±2.0 ng/ml in cilastatin-treated mice and 11.3±1.0 ng/ml in vehicle-treated mice; P=0.04) (Figure 5E), and a 16-fold increase in myoglobin clearance (0.06±0.02 ng/h in vehicle versus 0.98±0.24 ng/h in cilastatin-treated mice; P=0.03) (Figure 5F). Myoglobin clearance was similar in cilastatin-treated mice and iMegKO mice (P=0.38). Renal myoglobin retention at 24 hours after glycerol injection was not mediated by megalin interference (Supplemental Figure 7). Overall, these data demonstrate that megalin interference and cilastatin administration similarly increase urinary clearance of myoglobin in experimental rhabdomyolysis.
Figure 5.
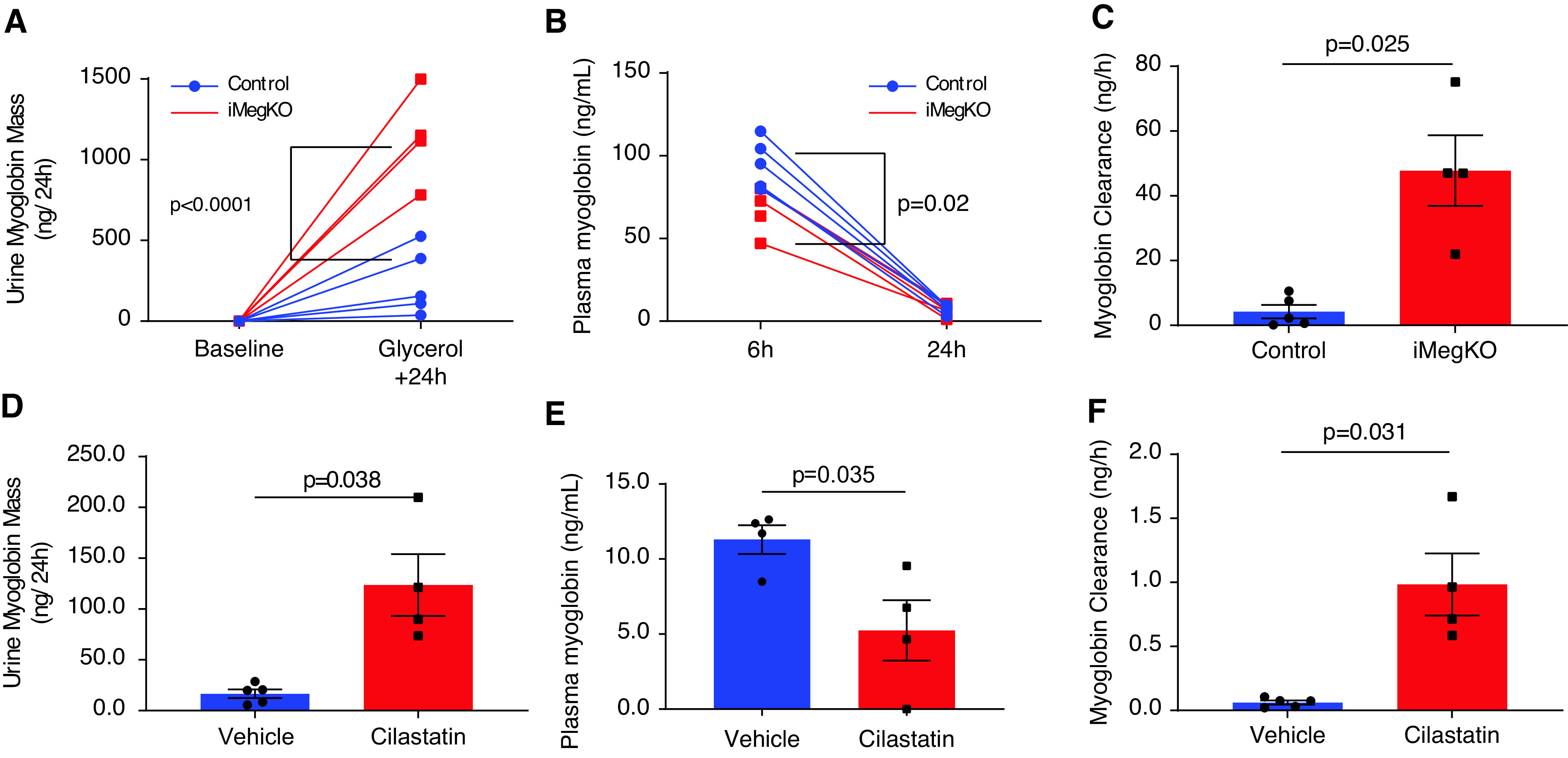
Myoglobin clearance is similarly altered by proximal tubule megalin interference and cilastatin administration. (A) Induced proximal tubule–specific megalin deletion (iMegKO) status conferred much greater excretion of myoglobin in the urine. (B) Plasma myoglobin concentration was elevated in controls relative to iMegKO mice with 6 hours, but not 24 hours, after glycerol injection, with rapid decline in plasma myoglobin occurring in both groups. (C) Myoglobin clearance was significantly greater in iMegKO mice than controls. (D–F) Treatment with the pharmacologic inhibitor of renal megalin, cilastatin, had similar actions to proximal tubule–specific deletion of megalin. (D) Urine myoglobin was increased by cilastatin treatment. (E) After 24 hours plasma myoglobin was reduced by cilastatin treatment. (F) Myoglobin clearance was increased approximately 16× by cilastatin treatment. Statistical analysis presented is derived from (A, B) repeated measures ANOVA (C–F) and t test.
Cilastatin and Megalin Deletion Have Similar Effects on Renal Function
To investigate the effects of cilastatin on renal function, we administered cilastatin or vehicle (identically to previous experiments) to surgically naïve wild-type mice and assessed renal function 24 hours later (Figure 6A). Cilastatin administration did not alter urine output or GFR (Figure 6, B and C). Urine electrophoresis revealed a pattern in cilastatin-treated mice similar to that of iMegKO mice, with similar novel bands (Figure 6D), although urine albumin was not significantly increased (Figure 6E). Therefore, to determine whether cilastatin inhibited megalin function, we quantified the megalin ligand RBP4,20 which is specifically increased in the urine of megalin-deleted mice,21 and performed immunoblotting for megalin and cubilin in kidney lysate. In a striking similarity to iMegKO mice, cilastatin administration to wild-type mice greatly increased urinary excretion of RBP4 (18.3±4.7 ng/24 hours in vehicle-treated mice versus 32.4±0.7 ng/24 hours in cilastatin-treated mice, n=5 per group; P=0.02) (Figure 6F); this strongly suggests cilastatin inhibits renal uptake of this megalin ligand. Cilastatin administration did not alter renal expression of megalin or cubilin (Figure 6, G–I and Supplemental Figure 8). Taken together, these data indicate that cilastatin does not by itself alter urine output or GFR; rather, cilastatin increases the urinary excretion of megalin ligands, likely by acting directly on megalin. Next, to determine whether the protective effect of cilastatin administration was megalin-dependent, we administered cilastatin or vehicle to male and female iMegKO mice subjected to experimental rhabdomyolysis (Figure 6J). By 24 hours after glycerol injection, the GFR in both groups was similar to that of iMegKo mice after glycerol injection. The mean GFR of vehicle-treated iMegKO mice was not different than that of cilastatin-treated iMegKO mice (859.3±301.3 μl/min per 100 g versus 813.7±113.4 μl/min per 100 g, respectively; P=0.89) (Figure 6K), indicating that cilastatin-mediated renoprotection from AKI resulting from experimental rhabdomyolysis is megalin dependent.
Cilastatin Reduces Myoglobin Endocytosis In Vivo
To evaluate a mechanism by which cilastatin might increase myoglobin clearance, we characterized and compared the uptake of injected FITC-myoglobin in vivo. Then, 15 minutes after injection, healthy wild-type mice exhibited abundant FITC-myoglobin punctae at the apical brush border and within the cytoplasm of proximal tubular epithelial cells, but by 30 minutes, punctae were absent, suggesting rapid clearance (not shown). Therefore, to characterize the role of megalin in the formation of myoglobin endocytic punctae, we administered FITC-myoglobin to iMegKO mice and controls. Histologic sections from iMegKO mice demonstrated considerable attenuation of puncta signal compared with controls (Figure 7, A and B). By 15 minutes after injection, control mice exhibited a greater number of endocytic puncta compared with iMegKO mice (0.33±0.01 puncta/µm2 versus 0.19±0.01 puncta/µm2; n=3–5 per group; P=0.0002); this result was identical when not normalized to tissue area. A comparison of puncta from control mice (n=29,1330) and from iMegKO mice (n=79,826) revealed that those from control mice were larger (0.97±1.12 µm2 versus 0.88±0.95 µm2; P<2.2 × 10−16) and demonstrated greater FITC-fluorescence (19,611±3385 arbitrary fluorescence units versus 12,603±4481 arbitrary fluorescence units; P<2.2 × 10−16) (Figure 7C). Thus, megalin deletion is characterized by reduction in myoglobin endocytic puncta number, size, and area.
Figure 7.
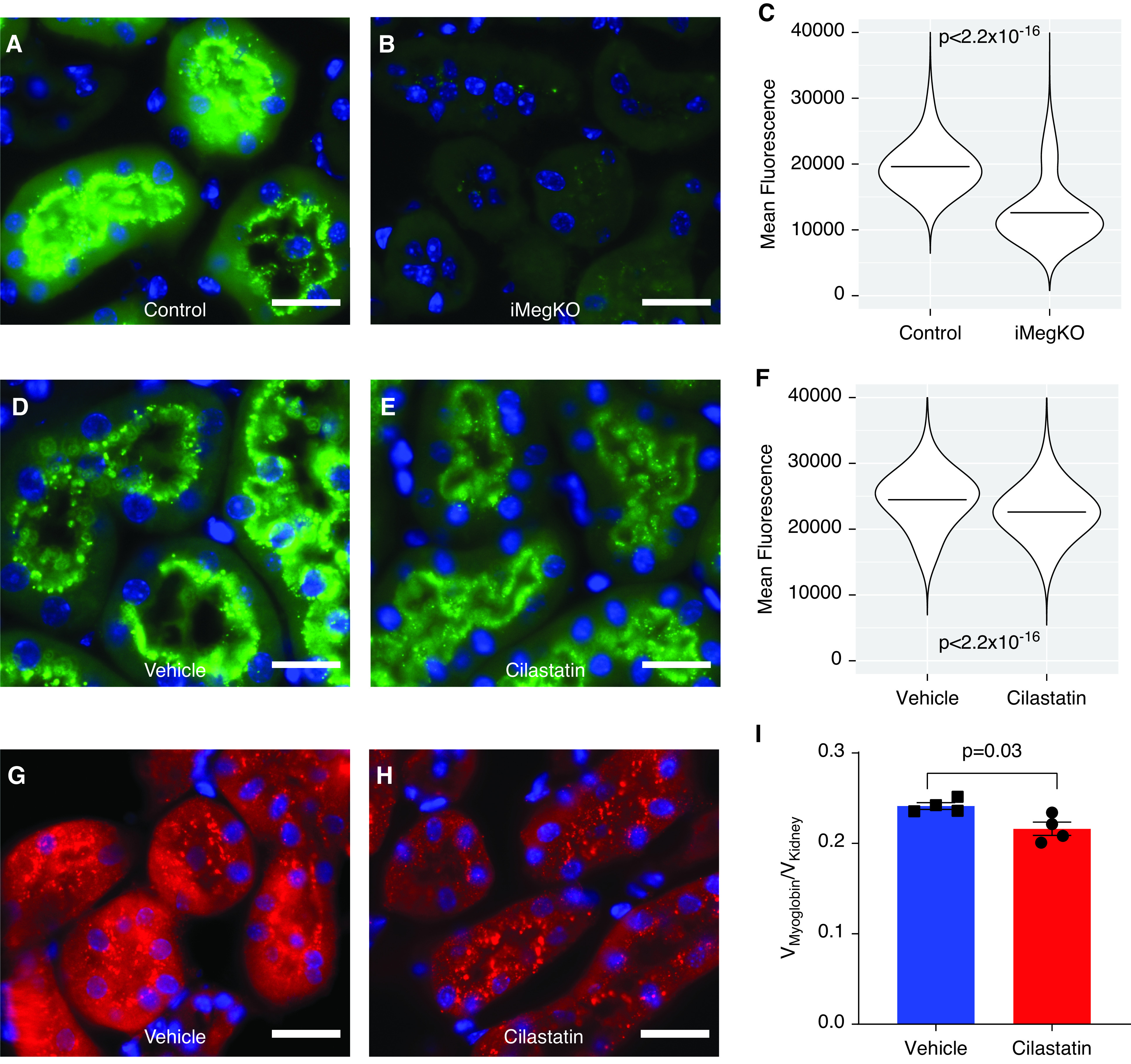
Similar interference in myoglobin uptake in iMegKO and cilastatin-treated mice. To characterize the role of megalin in myoglobin endocytosis, iMegKO mice and controls were injected with FITC-myoglobin (0.5 mg). Then 15 minutes later, mice were killed and kidneys prepared for histologic examination. (A) Controls demonstrated abundant tubular epithelial FITC signal, primarily organized in punctae within the apical brush border and the adjacent cytoplasm, whereas (B), iMegKO mice demonstrated attenuated FITC-myoglobin signal, and far fewer punctae. (C) Mean fluorescence of all puncta (n=371,156) quantified by strain demonstrates that puncta from iMegKO mice exhibit reduced fluorescence compared with those from controls. Horizontal lines depict the mean. (D–F) To characterize the effect of cilastatin on myoglobin endocytosis, wild-type mice were injected with FITC-myoglobin (0.5 mg). Then 15 minutes later, mice were killed and kidneys prepared for histologic examination. Vehicle-treated mice exhibited abundant FITC-myoglobin punctae, whereas (D) cilastatin-treated mice exhibited fewer punctae and overall reduced FITC-fluorescence. (H) Mean fluorescence of all puncta (n=2,298,499) compared by drug treatment demonstrates that puncta from cilastatin-treated mice exhibit reduced fluorescence compared with those from vehicle-treated mice. Horizontal lines depict the mean. (G–I) To determine whether cilastatin interfered with overall renal myoglobin uptake, wild-type mice received vehicle or cilastatin injection 1 hour before glycerol intramuscular injection. (G, H) Then 2 hours after glycerol injection, myoglobin-directed immunofluorescence demonstrates abundant myoglobin-positive punctae at the brush border and within the cytoplasm of proximal tubules of vehicle injected mice, this signal appeared attenuated in tubules from cilastatin-injected mice. (I) Unbiased stereology demonstrates reduced total intrarenal myoglobin content in cilastatin-treated mice. Scale bars are 20 µm. Statistical analysis presented is derived from the t test.
To characterize alterations in endocytic punctae induced by cilastatin administration, we administered cilastatin or vehicle to wild-type mice, followed 10 minutes later by FITC-myoglobin. Then 15 minutes after the FITC-myoglobin injection, the mice were killed and their kidneys were prepared for histologic examination; the histologic sections demonstrated attenuated fluorescence in cilastatin-treated mice (Figure 7, D and E). Although the number of puncta was not significantly different in cilastatin- and vehicle-treated mice (0.11±0.011 for cilastatin versus 0.14±0.04 for vehicle; P=0.33), puncta from cilastatin-treated mice were smaller (0.92±0.98 µm2 versus 0.95±0.99 µm2; P<2.2 × 10−16, not shown) and demonstrated reduced FITC fluorescence (22,603±3775 arbitrary fluorescence units versus 24,480±4066 arbitrary fluorescence units; P<2.2 × 10−16) (Figure 7F). Therefore, cilastatin reduced the area and FITC-myoglobin content of endocytic puncta.
Lastly, to confirm that overall renal myoglobin uptake is altered by cilastatin use, we administered cilastatin or vehicle at the time of glycerol intramuscular injection to wild-type mice; 2 hours after glycerol administration, we assessed renal myoglobin immunofluorescence using unbiased stereology. Endocytic punctae were widely present in the kidneys of mice in both groups (Figure 7, G and H), and myoglobin-positive puncta did not differ between groups (0.18±0.07 puncta/µm2 in vehicle versus 0.11±0.14 puncta/µm2 in cilastatin; n=3–5 per group, P=0.41). However, unbiased stereology demonstrated that overall renal myoglobin immunofluorescence was reduced by cilastatin treatment (0.216±0.007 Vmyoglobin/Vkidney versus 0.241±0.003 Vmyoglobin/Vkidney; P=0.03) (Figure 7I). Taken together, these data demonstrate alterations in myoglobin endocytic puncta by cilastatin and further implicate altered endocytosis of myoglobin as a mechanism of cilastatin’s action in glycerol-induced rhabdomyolysis.
Discussion
This study has two important findings. The first is that the renal endocytic transporter megalin plays a critical role in AKI resulting from rhabdomyolysis. The second important finding is that cilastatin, a pharmacologic inhibitor of megalin, is renoprotective in rhabdomyolysis-induced AKI in a megalin-dependent fashion.
Proximal tubule–specific deletion of megalin prevented rhabdomyolysis-induced GFR loss almost completely, and reduced other measures of renal injury, including histopathologic injury score, renal apoptosis, and renal KIM-1. Myoglobin clearance was greatly increased in the absence of proximal tubule megalin. We conclude that this dramatic increase in myoglobin clearance preserved renal function.
Increased myoglobin clearance may underlie three renoprotective mechanisms. First, myoglobin is present in the kidney for less time, resulting in reduced renal exposure to a toxic molecule. Second, plasma myoglobin is more rapidly depleted, resulting in more rapid reduction of tubular concentration of myoglobin, also potentially reducing renal injury. Third, preservation of renal function, including urine output, due to the first two mechanisms, likely further reduces plasma and urine concentrations, thereby reducing renal exposure to myoglobin.
Myoglobin was first identified in 2003 by Gburek et al.9 as a megalin ligand in mice with incomplete but renal-specific megalin deletion. Investigators in this seminal study speculated that megalin interference might have a beneficial effect in rhabdomyolytic renal failure. Megalin functions as part of an endocytic complex, including the proteins cubilin and amnionless23 and possibly other components.24 In all, the complex is promiscuous, with >40 known ligands. Extensive and elegant investigations have elucidated the contributions of specific components of the complex to ligand uptake and disposition in tubular epithelium; some ligands, such as albumin, may have multiple paths to endocytosis. On the basis of molecular weight and similarity to hemoglobin, which undergoes both megalin- and cubilin-mediated uptake,25 myoglobin uptake could also be mediated by cubilin. In fact, myoglobin binds both megalin and cubilin, with lower rate constant for megalin than for cubilin.9 Further, receptor specificity for tubular endocytic complex ligands is concentration dependent. For example, albumin uptake is megalin dependent in diabetic mice,11 but less so at nephrotic-range concentrations.26 A recent study suggests megalin and cubilin provide complementary pathways with differential affinities for albumin uptake.27 This concentration-dependent pathway might be invoked when tubular concentration of myoglobin is high in rhabdomyolysis. Because the model we used specifically depleted proximal tubule megalin but not cubilin, we cannot eliminate the possibility that myoglobin clearance could be further increased by interference with cubilin. Together, these data and the increasing understanding of this critical proximal tubule–endocytosis complex suggest tantalizing potential for specific therapy in rhabdomyolysis-induced AKI.
We also found that megalin interference prevents progressive rhabdomyolysis-induced proteinuria and GFR loss. Our findings in control mice, which progressively developed approximately 20% GFR loss and increased protein/albuminuria, are in accordance with those of Belliere et al.,4 which identified rhabdomyolysis as an initiator of AKI-CKD transition. Our study is distinct from this prior study in evaluating a lower dose of glycerol (6.5 ml/kg versus 7.5 ml/kg). We observed discordance between functional measures (GFR and albuminuria, which demonstrated impairment in controls) and structural evaluation (histology and αSMA quantification, which were similar and mild). We suggest these observations are consistent with early CKD, in which mild proteinuria and GFR loss occurs but structural injury may not be evident.28 Our finding may be contrasted with studies suggesting that partial megalin deletion does not alter tubulointerstitial fibrosis in severe GN.29,30 Our distinct result may stem from the acute nature of rhabdomyolysis, contrasted with the unremitting injury of GN, from more complete reduction in megalin function in our mouse model, or both.
The clinical literature supports the possibility that rhabdomyolysis-induced AKI may lead to long-term kidney disease, although the relationship appears complex and has only been studied in the context of armed conflict. Rhabdomyolysis occurs in 31% of US combat casualties admitted to intensive care.2 These patients have elevated risk of developing CKD and hypertension, and in this context, AKI independently quadruples the risk of subsequent CKD.5 However, a subsequent study by the same investigators in a similar population did not support a strong independent relationship between rhabdomyolysis alone and subsequent CKD.31 We found progressive decline in GFR after rhabdomyolysis, accompanied by persistent proteinuria, both mediated by megalin. These findings further support rhabdomyolysis-induced AKI-CKD transition and provide data that we hope may assist in future clinical studies. For example, we speculate that megalin polymorphisms may modify risk of CKD after rhabdomyolysis, as they do other disease,32,33 and exploring such polymorphisms in clinical cohorts may help elucidate the relationship between rhabdomyolysis, AKI, and subsequent CKD. Because rhabdomyolysis commonly occurs in young people (such as athletes and soldiers), such studies hold promise for addressing the decades-long burden of early-onset CKD.
Finally, an important finding in this study is that the megalin inhibitor cilastatin preserves renal function in rhabdomyolysis in a megalin-dependent fashion. As with megalin deletion, cilastatin administration preserved GFR. Cilastatin administration to healthy mice did not alter GFR or urine output, but caused selective proteinuria similar to that of iMegKO mice, including that of a specific marker of megalin function, RBP4. These findings further support the megalin-inhibiting role of cilastatin. In wild-type mice, cilastatin increased myoglobin clearance after glycerol injection, decreased the size and fluorescence of renal endocytic puncta when administered with FITC-myoglobin, and reduced the overall renal myoglobin signal 2 hours after glycerol injection (although not 24 hours after glycerol injection). In cultured proximal tubular epithelial cells, low concentrations of cilastatin modestly inhibited uptake of myoglobin (Supplemental Figure 9). Therefore, although reduced myoglobin uptake is an action of cilastatin, this mechanism may not account for the entirety of the increased myoglobin clearance induced by cilastatin, or all of the protective effect observed. Further investigation should include evaluation of other possible protective mechanisms induced by cilastatin.
Cilastatin was originally identified as a renal dipeptidase inhibitor in the 1970s and received Food and Drug Administration approval for coadministration with the antibiotic imipenem, which is metabolized rapidly by renal dipeptidase. Therefore there is considerable clinical experience with use of cilastatin, which has low toxicity (cilastatin LD50 estimates are >30 times the dose we used in this study).34 Recent investigation demonstrated that cilastatin also inhibits megalin.22 Because rhabdomyolysis is commonly lethal in austere environments such as natural disasters and armed conflict, specific and effective therapy with low burden of administration is highly desirable and has great potential to transform care. Further study in clinically relevant models and preparation for clinical study are therefore imperative.
Our study has limitations. We performed experiments entirely in a mouse model and results may not extend to humans. We did not evaluate the role of myoglobin casts in injury. In addition, myoglobin is not the only cause of renal injury in rhabdomyolysis. Others have demonstrated that hemolysis and renal injury due to free hemoglobin also occur and are ameliorated by heme oxygenase13,14,35,36; we did not evaluate the role of either of these proteins in our studies. Also, the proximal tubule may not be the only site of renal injury in rhabdomyolysis. We note that glycerol-treated iMegKO controls developed lasting albuminuria, possibly a sign of a damaged glomerular filtration barrier, but we did not rigorously evaluate for signs of glomerular damage. Lastly, cilastatin has known off-target effects; we did not exclude a beneficial effect of dipeptidase inhibition in rhabdomyolysis, and we cannot exclude other, unknown off-target effects. Given the translational importance of the finding that cilastatin is renoprotective in rhabdomyolysis-induced AKI, we are pursuing further studies to clarify these questions.
In conclusion, the renal proximal tubule endocytic receptor megalin plays a critical role in the development of rhabdomyolysis-induced AKI. Administration of the megalin inhibitor cilastatin recapitulates effects of megalin interference, and ameliorates AKI resulting from rhabdomyolysis.
Disclosures
J.A. McCormick reports being a scientific advisor or member via the Editorial boards of American Journal of Physiology: Renal Physiology, Frontiers in Physiology: Renal and Epithelial Physiology, and Kidney360. J.F. Hebert reports being a scientific advisor or membership as Chair of In-Training Committee, Society for Reproductive Investigation. M.P. Hutchens reports having an ownership interest and patents and inventions with ProjectLite. M. Yanagita reports receiving research funding from Baxter, FUSO Pharmaceutical Industries, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceuticals, and the Termo Company; reports receiving honoraria from Astellas, Baxter, Chugai, Kyowa Hakko Kirin, Takeda, and others for lecture honoraria; and other interests/relationships with International Society of Nephrology and Japanese Society of Nephrology. All remaining authors have nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK098141 to J.A. McCormick, DFG German Research Foundation (332853055) Else Kröner-Fresenius Stiftung 2015_A197, and Returner and START-Program of the Faculty of Medicine, RWTH Aachen to T. Saritas; the Japan Agency for Medical Research and Development under grants 19gm1210009, JP19gm5010002, and JP19gm0610011 to M. Yanagita; the United States Department of Defense (W81XWH2010196 to M.P. Hutchens), the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (VA Merit Award 1I01BX004288 to M.P. Hutchens), and is the result of work M.P. Hutchens that was supported with resources and the use of facilities at the Portland Veterans Affairs Medical Center.
Acknowledgments
The contents of this study do not represent the views of the US Defense Department, the Department of Veterans Affairs, or the US Government. M.B. Eiwaz performed experiments and edited the manuscript. Y. Funahashi designed and performed cell culture experiments, and wrote and critiqued the manuscript. J.F. Hebert developed critical methods, performed immunoblot experiments, and wrote and critiqued the manuscript. M.P. Hutchens conceived, designed, and performed experiments, analyzed data, wrote and edited the manuscript, and assembled and led the research team. K. Matsushita designed and performed experiments, analyzed data, and wrote and edited the manuscript. J.A. McCormick critiqued and designed experiments, and edited the manuscript. K. Mori provided critical materials, critiqued experimental design, analyzed results, and edited the manuscript; A.C. Munhall designed experiments, contributed figures, performed critical experiments, and critiqued the manuscript. M.N. Nickerson designed experiments, contributed figures, performed critical experiments, and critiqued the manuscript. T. Saritas analyzed data, and wrote and edited the manuscript; M. Yanagita provided critical materials, critiqued experimental design, analyzed results, and edited the manuscript. Parts of some figures in this work were created with BioRender.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020030263/-/DCSupplemental.
Supplemental Table 1. List of reagents and resources used in this study.
Supplemental Figure 1. Immunohistochemistry performed using antibody directed at megalin in kidneys of control and iMegKO mice after induction of cre recombinase.
Supplemental Figure 2. iMegKO mice do not express megalin in the kidney.
Supplemental Figure 3. GFR expressed as percent of baseline, measured before and 24 hours after glycerol injection.
Supplemental Figure 4. Immunofluorescence with antibody directed at megalin 24 hours after glycerol injection in a control mouse and one with inducible, proximal tubule-specific megalin deletion (iMegKO).
Supplemental Figure 5. Quantitation of urine protein and albumin in control and iMegKO mice over 60 days after glycerol injection (relative change from baseline is presented in Figure 3).
Supplemental Figure 6. Representative renal sections from control and iMegKO mice 60 days after glycerol injection, stained with fluorescent antibody to α-smooth muscle actin (αSMA).
Supplemental Figure 7. Uncropped immunoblot performed on renal homogenate from control and iMegKO mice 24 hours after glycerol injection.
Supplemental Figure 8. Full blots for megalin and cubilin obtained from renal homogenate of wild-type mice treated with vehicle or cilastatin.
Supplemental Figure 9. Results of cell culture experiment testing uptake of FITC-myoglobin in human kidney cells (HK2 cell line).
References
- 1.Stewart IJ, Snow BD, Clemens MS, Sosnov JA, Ross JD, Howard JT, et al. : Hyperkalemia in combat casualties: Implications for delayed evacuation. Mil Med 182: e2046–e2051, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Stewart IJ, Faulk TI, Sosnov JA, Clemens MS, Elterman J, Ross JD, et al. : Rhabdomyolysis among critically ill combat casualties: Associations with acute kidney injury and mortality. J Trauma Acute Care Surg 80: 492–498, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Stewart IJ, Sosnov JA, Howard JT, Chung KK: Acute kidney injury in critically injured combat veterans: A retrospective cohort study. Am J Kidney Dis 68: 564–570, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Belliere J, Casemayou A, Ducasse L, Zakaroff-Girard A, Martins F, Iacovoni JS, et al. : Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J Am Soc Nephrol 26: 1363–1377, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart IJ, Sosnov JA, Howard JT, Orman JA, Fang R, Morrow BD, et al. : Retrospective analysis of long-rerm outcomes after combat injury: A hidden cost of war. Circulation 132: 2126–2133, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen JS, Sally M, Mullins RJ, Slater M, Groat T, Gao X, et al. : Bicarbonate and mannitol treatment for traumatic rhabdomyolysis revisited. Am J Surg 213: 73–79, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Zager RA: Studies of mechanisms and protective maneuvers in myoglobinuric acute renal injury. Lab Invest 60: 619–629, 1989 [PubMed] [Google Scholar]
- 8.Nielsen R, Christensen EI, Birn H: Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int 89: 58–67, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Gburek J, Birn H, Verroust PJ, Goj B, Jacobsen C, Moestrup SK, et al. : Renal uptake of myoglobin is mediated by the endocytic receptors megalin and cubilin. Am J Physiol Renal Physiol 285: F451–F458, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, et al. : Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol 24: 1966–1980, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori KP, Yokoi H, Kasahara M, Imamaki H, Ishii A, Kuwabara T, et al. : Increase of total nephron albumin filtration and reabsorption in diabetic nephropathy. J Am Soc Nephrol 28: 278–289, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo T, Nakamura J, Sato Y, Asada M, Yamada R, Takase M, et al. : Exploring the origin and limitations of kidney regeneration. J Pathol 236: 251–263, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Wei Q, Hill WD, Su Y, Huang S, Dong Z: Heme oxygenase-1 induction contributes to renoprotection by G-CSF during rhabdomyolysis-associated acute kidney injury. Am J Physiol Renal Physiol 301: F162–F170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zager RA, Burkhart KM, Conrad DS, Gmur DJ: Iron, heme oxygenase, and glutathione: Effects on myohemoglobinuric proximal tubular injury. Kidney Int 48: 1624–1634, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Thiel G, Wilson DR, Arce ML, Oken DE: Glycerol induced hemoglobinuric acute renal failure in the rat. II. The experimental model, predisposing factors, and pathophysiologic features. Nephron 4: 276–297, 1967 [DOI] [PubMed] [Google Scholar]
- 16.Matsushita K, Saritas T, Eiwaz MB, McClellan N, Coe I, Zhu W, et al. : The acute kidney injury to chronic kidney disease transition in a mouse model of acute cardiorenal syndrome emphasizes the role of inflammation. Kidney Int 97: 95–105, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakasaki R, Eiwaz M, McClellan N, Matsushita K, Golgotiu K, Hutchens MP: Automated systematic random sampling and Cavalieri stereology of histologic sections demonstrating acute tubular necrosis after cardiac arrest and cardiopulmonary resuscitation in the mouse. Histol Histopathol 33: 1227–1234, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. : Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, et al. : Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361–1370, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjaer A, et al. : Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol 10: 685–695, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Wakasaki R, Matsushita K, Golgotiu K, Anderson S, Eiwaz MB, Orton DJ, et al. : Glomerular filtrate proteins in acute cardiorenal syndrome. JCI Insight 4: e122130, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori Y, Aoki N, Kuwahara S, Hosojima M, Kaseda R, Goto S, et al. : Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol 28: 1783–1791, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R: Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 27: 223–236, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Morace I, Pilz R, Federico G, Jennemann R, Krunic D, Nordström V, et al. : Renal globotriaosylceramide facilitates tubular albumin absorption and its inhibition protects against acute kidney injury. Kidney Int 96: 327–341, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, et al. : Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol 13: 423–430, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Weyer K, Andersen PK, Schmidt K, Mollet G, Antignac C, Birn H, et al. : Abolishment of proximal tubule albumin endocytosis does not affect plasma albumin during nephrotic syndrome in mice. Kidney Int 93: 335–342, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Ren Q, Weyer K, Rbaibi Y, Long KR, Tan RJ, Nielsen R, et al. : Distinct functions of megalin and cubilin receptors in recovery of normal and nephrotic levels of filtered albumin. Am J Physiol Renal Physiol 318: F1284–F1294, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. : Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Available at: https://kdigo.org/wpcontent/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed December 10, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Theilig F, Kriz W, Jerichow T, Schrade P, Hähnel B, Willnow T, et al. : Abrogation of protein uptake through megalin-deficient proximal tubules does not safeguard against tubulointerstitial injury. J Am Soc Nephrol 18: 1824–1834, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Motoyoshi Y, Matsusaka T, Saito A, Pastan I, Willnow TE, Mizutani S, et al. : Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int 74: 1262–1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faulk T, Walker LE, Howard JT, Janak JC, Sosnov JA, Stewart IJ: Rhabdomyolysis among critically ill combat casualties: Long-term outcomes. Am J Nephrol 48: 399–405, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Beydoun MA, Tajuddin SM, Dore GA, Canas JA, Beydoun HA, Evans MK, et al. : Vitamin D receptor and megalin gene polymorphisms are associated with longitudinal cognitive change among African-American urban adults. J Nutr 147: 1048–1062, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riedemann L, Lanvers C, Deuster D, Peters U, Boos J, Jürgens H, et al. : Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J 8: 23–28, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Lin JH, Chen IW, Ulm EH: Dose-dependent kinetics of cilastatin in laboratory animals. Drug Metab Dispos 17: 426–432, 1989 [PubMed] [Google Scholar]
- 35.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, et al. : Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Zarjou A, Traylor AM, Bolisetty S, Jaimes EA, Hull TD, et al. : In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int 82: 278–291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]