Significance Statement
In patients with steroid-dependent and calcineurin inhibitor–depe ndent nephrotic syndrome, rituximab, a chimeric monoclonal anti body directed against CD20+ B cells, helps maintain remission, but relapse within a year is common. This randomized trial investigated wheth er ofatumumab, a fully human anti-CD20 monoclonal antibody, is superior to rituximab in maintaining oral drug–free remission in patients with this condition. The findings show ofatumumab is not superior to rituximab in achieving oral drug–free remission at 1 year of follow-up, and had similar adverse effects. Although ofatumumab treatment resulted in a more prolonged depletion of B cells compared with rituximab, this did not translate into clinical effects. These findings suggest human or humanized anti-CD20 antibodies may not offer advantages over the chimeric anti-CD20 rituximab for treatment of idiopathic nephrotic syndrome.
Keywords: steroid dependent nephrotic syndrome, calcineurin inhibitor-dependent nephrotic syndrome, human anti-CD20 monoclonal antibodies, ofatumumab, rituximab, memory B cells, prediction of response, antirituximab antibodies, idiopathic nephrotic syndrome, glomerular disease
Visual Abstract
Abstract
Background
The chimeric anti-CD20 monoclonal antibody rituximab is effective in steroid-dependent and calcineurin inhibitor–dependent forms of nephrotic syndrome, but many patients relapse at 1 year. Because ofatumumab, a fully human anti-CD20 monoclonal antibody, has a more extended binding site and higher affinity to CD20 compared with rituximab, it might offer superior efficacy in these patients.
Methods
We designed a single-center randomized clinical trial to compare the long-term efficacy of ofatumumab versus rituximab in children and young adults with nephrotic syndrome maintained in remission with prednisone and calcineurin inhibitors. We randomized 140 children and young adults (aged 2–24 years) to receive intravenous ofatumumab (1.50 mg/1.73 m2) or rituximab (375 mg/m2). After infusions, oral drugs were tapered and withdrawn within 60 days. The primary outcome was relapse at 1 year, which was analyzed following the intent-to-treat principle. The secondary endpoint was relapse within 24 months from infusion, on the basis of urine dipstick and confirmed by a urine protein-to-creatinine ratio <200.
Results
At 12 months, 37 of 70 (53%) participants who received ofatumumab experienced relapse versus 36 of 70 (51%) who received rituximab (odds ratio [OR], 1.06; 95% confidence interval [95% CI], 0.55 to 2.06). At 24 months, 53 of 70 (76%) participants who received ofatumumab experienced relapse, versus 46 of 70 (66%) who received rituximab (OR, 1.6; 95% CI, 0.8 to 3.3). The two groups exhibited comparable B cell subpopulation reconstitution and did not differ in adverse events.
Conclusions
A single dose of ofatumumab was not superior to a single dose of rituximab in maintaining remission in children with steroid-dependent and calcineurin inhibitor–dependent nephrotic syndrome.
Clinical Trial registration numbers:
ClinicalTrials.gov (NCT02394119) and https://www.clinicaltrialsregister.eu/ctr-search/search (2015–000624–28).
Idiopathic nephrotic syndrome, a disease characterized by severe proteinuria, hypoalbuminemia, and dyslipidemia, affects 2–3 new children per 100,000 per year in Western countries and has a prevalence of 16 cases per 100,000.1 Oral corticosteroids, the first-line treatment, induce remission in approximately 90% of patients.2 However, <85% of these patients relapse3 and many become steroid dependent.4 To minimize steroid-related adverse effects, children with steroid-dependent nephrotic syndrome (SDNS) receive steroid-sparing agents, including alkylating agents, calcineurin inhibitors, and mycophenolate-mofetil.5 In most of these patients, steroids are still required in combination with any of these steroid-sparing agents to maintain remission. Clinical trials6–9have shown that rituximab, a chimeric monoclonal antibody directed against CD20+ B cells,10 can be successfully utilized in these forms of SDNS to maintain remission.11,12
Response to rituximab seems to depend on both disease duration and severity. Relapse-free survival after rituximab infusion was shorter in children previously maintained in remission with both steroids and calcineurin inhibitors, compared with those on steroids alone or mycophenolate mofetil.6–8,13–15 These limitations, and the possibility that rituximab infusion may elicit antirituximab antibodies, have led to the development of novel, less immunogenic molecules.16–18 Ofatumumab, a fully human anti-CD20 monoclonal IgG1(k) antibody, has a more extended binding site than rituximab, a higher affinity to the CD20 antigen and a more efficient complement-dependent cytotoxicity in vitro.19 Whether these cellular effects may translate into superior clinical benefits is unknown.20–22 The mechanisms responsible for the clinical efficacy of these monoclonal antibodies are unclear.23,24 Memory B cells have been associated with disease activity, which may explain the effects of B cell depletion.25 Other immune cells may be involved, including regulatory T cells (Tregs).26
We compared the efficacy and safety of ofatumumab versus rituximab in children and young adults with SDNS maintained in remission with steroids and calcineurin inhibitors (NCT02394119). We evaluated the risk of relapse after steroid and calcineurin inhibitor tapering and withdrawal. We also investigated the changes in different lymphocyte subpopulations that can be directly or indirectly modified by anti-CD20 antibodies.25,26
Materials and Methods
Design
In this open-label, two-parallel-arm, randomized controlled trial, we tested the superiority of ofatumumab over rituximab in maintaining steroid- and calcineurin inhibitor-free remission in children and young adults (aged ≤24 years) with calcineurin inhibitor and SDNS (Figure 1, Table 1).27
Figure 1.

Schematic view of trial design. CNI, calcineurin inhibitors; ITT, intention-to-treat.
Table 1.
Demographic and clinical characteristics of study participants
| Characteristic | Total | Ofatumumab | Rituximab | P value |
|---|---|---|---|---|
| n=140 | n=70 | n=70 | ||
| Male/female, n | 96/44 | 44/26 | 52/18 | 0.15 |
| Age at onset, yrs | 3 (2–4) | 3 (2–4) | 3 (2–5) | 0.74 |
| Age at enrolment, yrs | 11 (6–16) | 10 (6–16) | 11 (5–15) | |
| Body weight, kg | 41 (21) | 40 (20) | 42 (21) | 0.50 |
| Height, m | 136 (25) | 135 (23) | 137 (26) | 0.69 |
| Body mass index, kg/m2 | 20 (17–24) | 19 (16–24) | 20 (17–23) | 0.61 |
| Body surface area, m2 | 1.2 (0.8–1.5) | 1.2 (0.8–1.5) | 1.3 (0.9–1.6) | 0.65 |
| Systolic blood pressure, mmHg | 110 (12) | 109 (11) | 111 (12) | 0.36 |
| Diastolic blood pressure, mmHg | 68 (10) | 67 (10) | 69 (11) | 0.25 |
| Hemoglobin, g/dl | 14 (13–14) | 13 (12–14) | 14 (13–15) | 0.17 |
| Platelets × 1000, per mcl | 302(254–384) | 310(262–385) | 291(250–382) | 0.49 |
| White blood cells × 1000, per mcl | 10 (8–13) | 11 (8–13) | 10 (8–13) | 0.90 |
| Polymorphonuclear cells × 1000, per mcl | 5 (3–7) | 5 (3–7) | 5 (3–7) | 0.65 |
| Lymphocytes × 1000, per mcl | 4 (3–5) | 4 (3–5) | 4 (2–5) | 0.09 |
| Serum creatinine, mg/dl | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) | 0.33 |
| Estimated GFR, ml/min per 1.73 m2 | 146 (33) | 147 (31) | 144 (34) | 0.65 |
| Proteinuria, mg/day | 80 (60–120) | 80 (60–130) | 75 (50–120) | 0.15 |
| Urine protein/creatinine, mg/mg | 0.12 (0.08–0.18) | 0.13 (0.09–0.18) | 0.11 (0.07–0.17) | 0.16 |
| Serum albumin, g/dl | 4 (1) | 4 (1) | 4 (1) | 0.67 |
| Total cholesterol, mg/dl | 214 (77) | 220 (85) | 208 (70) | 0.37 |
| Previous RTX n (%) | 64(46%) | 31(44%) | 33(47%) | 0.50 |
| Infusions, n | 0(0–2) | 0(0–2) | 0(0–2) | 0.69 |
| Previous MMF, n (%) | 29(21) | 14(20) | 15(21) | 0.26 |
| Prednisone, n (%) | 140 (100) | 70 (100) | 70 (100) | 0.90 |
| mg/kg | 0.55 (0.31–0.94) | 0.54 (0.35–0.94) | 0.56 (0.26–0.85) | 0.071 |
| Cyclosporine, n (%) | 96 (69) | 49 (35) | 47 (34) | 0.65 |
| Tacrolimus, n (%) | 43 (31) | 19 (14) | 24 (17) | 0.29 |
Data are presented as mean (SD) or median (interquartile range) for continuous measures, and n (%) for categorical measures. Prednisone, cyclosporin, and tacrolimus indicate the number (% of total) receiving oral treatment to maintain remission; mg/kg indicates the limit of steroid dependence that is the mi nimum dose of prednisone that allowed remission during the run-in month. RTX, rituximab; MMF, mycofenolatemofetil.
Eligible participants entered a 1-month run-in, during which prednisone and calcineurin inhibitor doses were reduced to the minimum amounts required to maintain complete remission (usually the minimum doses utilized in the previous 6 months). After run-in, children were randomized to either the intervention arm (ofatumumab) or the active comparator arm (rituximab; Supplemental Materials and Methods). After relapse, children were treated as detailed below. Follow-up lasted 24 months in nonrelapsing patients or until relapse in those who did. We expected a high acceptance rate in our national referral center for pediatric disorders, as in previous studies.7,9,15
Participants
We planned to enroll 140 patients aged 2–24 years with a proven clinical history of SDNS requiring prednisone and a calcineurin inhibitor (Table 1). SDNS was defined by two consecutive relapses during corticosteroid therapy tapering, or within 14 days of steroid withdrawal, that responded to the association of prednisone with cyclosporine or tacrolimus. The need of maintaining prednisone was defined by a relapse of nephrotic syndrome after its withdrawal in patients who were treated with a calcineurin inhibitor. The doses of cyclosporine and tacrolimus were tailored to achieve morning serum trough levels between 50 and 100 ng/ml and between 5 and 10 ng/ml, respectively.
Exclusion criteria included positivity to autoimmunity tests (Anti-nuclear antibody-ANA, anti-dsDNA, ANCA), reduction of C3 levels, eGFR <90/ml per min/1.73 m2 (revised Bedside Schwartz Formula for patients 2–17 years of age; CKD Epidemiology Collaboration equation for those who were aged 18–24 years), pregnancy, neoplasm, previous or actual Hepatitis B Virus (HBV) (with HBcAb positivity) or Hepatitis C Virus (HCV) infection, and treatment with rituximab or cyclophosphamide in the last 6 months.
The trial recruited patients across Italy and was carried out at the Gaslini Institute, Genoa. Interventions and follow-up visits were administered on an in-patient or day-medicine setting (Supplemental Table 1). In a preliminary interview, the study coordinator illustrated the project, delivered the information material and collected written informed consent from parents and child assent. Participants or their families could withdraw the consent at any time during the study.
Treatment Arms
Children randomized to the intervention arm (one dose of ofatumumab) or to the comparator (one dose of rituximab) received a premedication with methyl-prednisolone (2 mg/kg infused in 30’ intravenous diluted in 100 ml of normal saline), oral cetirizine (0.2 mg/kg), and oral paracetamol (15 mg/kg), to reduce common reactions. Ofatumumab was infused intravenously, at a dose of 1500 mg/1.73 m2, diluted in 1000 ml of normal saline, at a speed of 12 ml/hour in the first 30’. The dose was established on the basis of existing studies.21,22 The infusion rate was doubled every 30 minutes up to a maximum of 200 ml/hour. Rituximab was administered at a dose of 375 mg/m2 diluted in the required volume of saline to achieve the desired concentrations (Supplemental Material).7,9
After infusion of the intervention drug or the comparator, steroids were maintained at the baseline dose (post–run-in) for 30 days, then tapered off by 0.3 mg/kg per dose, every week, until complete withdrawal. Then 1 week after the steroid withdrawal, calcineurin inhibitors were tapered to 50% of the initial dose if in use, and stopped after 2 additional weeks. All children were followed for 24 months. Relapses were treated with oral prednisone (60 mg/m2) until remission, which was obtained in all patients using prednisone alone, followed by a further infusion of rituximab or ofatumumab according to the original treatment arms. Study enrolment started in June 2015 and was completed at the end of October 2018.
Outcomes
The primary endpoint was the occurrence of disease relapse within 12 months of randomization. Complete remission was defined by negative/trace protein on urine dipstick for 3 consecutive days, and confirmed by urine PCR <200 mg/g (<20 mg/mmol).
The secondary endpoints included the occurrence of a relapse within 24 months of randomization.
Safety endpoints included frequency and severity of adverse events and abnormal values in biochemical tests and hematology assessments.
Ancillary studies included biochemical or lymphocyte subpopulation analysis. Antirituximab antibodies were measured in participants enrolled in the rituximab arm who already received rituximab. The analysis was done at the enrolment and after 6 months (Supplemental Material). We planned to include approximately half of the study sample in the cell population study, for which we requested an additional consent. Peripheral blood mononuclear cells were isolated by Ficoll-Paque Plus (Amersham Biosciences) density gradient centrifugation and cryopreserved. Peripheral blood mononuclear cells were then stained and analyzed by multicolor flow cytometry (Supplemental Figure 1).
Sample Size
Sample size was calculated assuming a baseline risk of relapse at 1 year of 0.65 among children assigned to rituximab.7 On the basis of previous studies, we anticipated that we would recruit 140 participants (70 per arm) in this study.7,9 We estimated that this sample size would have allowed to detect as significant at the two-sided P value of 0.01 with a power >0.8 a reduction in the risk of 1-year relapse of at least 0.3 (i.e., from 0.65 to 0.35; relative risk reduction, 0.46, odds ratio [OR], 0.3). In power analysis, we accounted for a total proportion of drop-out (lost to follow-up for any reason) and drop-in (i.e., effect dilution due to use of the treatment of the other arm) <10%.
Randomization
Participants were randomized 1:1 to the intervention or active comparator arm. A distant site with no clinical involvement in the trial (Cumming School of Medicine, University of Calgary) generated two randomization lists (for age >9 years and ≤9 years) using permuted blocks of variable size. Stratification by age was motivated by the need to maximize the likelihood of balancing factors potentially affecting the effects of the intervention on outcomes, which are associated with age (disease duration, age at onset, relapse history, and disease severity).13 We did not stratify by previous use of rituximab because our registry data suggested that potential participants had a probability of previous rituximab use close to 0.5.
Blinding
Different infusion requirements motivated the open-label design (Supplemental Material).
Data Collection, Management, and Analysis
Time schedule of enrolment, interventions assessment, and visits for participants are shown in Supplemental Table 1. Investigators were responsible for data management and processing, in accordance with G. Gaslini’s data management procedures (see Supplemental Material).
Statistical Methods
We used standard statistical methods for quantitative and qualitative data to summarize the sample characteristics overall and by arm, and in analyses of cellular data. Continuous data were expressed as mean±SD or medians and interquartile ranges otherwise. Variables were compared by unpaired t test or nonparametric Mann–Whitney U test, as appropriate; a chi-squared test was used for categorical variables.
For the analysis of the primary outcome, we used binary logistic regression to compare the risk of relapse at 12 months with an intention-to-treat approach. The comparison was repeated at 24 months (secondary outcome). We used survival methods to plot time to relapse by treatment group and the association between lymphocyte subpopulations and relapse. We planned to replace missing data in a way that reflects the worst-case scenario: missing data in the active comparator group were considered as successes, and missing data in the active intervention were considered failures; P values ≤0.05 were considered significant.
Monitoring of levels of each circulating cell subpopulation at different time points was analyzed using a nonparametric Kruskal–Wallis test and Dunn’s multiple comparison test as appropriate. The association of previous rituximab administration with risk of relapse was evaluated by Cox proportional hazards regression model.
Statistical analyses were performed using R, version 4.0.3 (www.R-project.org), SPSS 20.0, and GraphPad Prism 9.0. Complete statistical analysis is provided in the Supplemental Material.
Study Safety and Monitoring Board, Registration, and Ethics Approval
Adverse events were defined on the basis of the time of their occurrence and on clinical severity. Study safety and monitoring procedures are described in the Supplemental Material.27 We obtained study approval from the local Independent Ethics Committee (ComitatoEticoRegione Liguria), and the Italian Drug Agency (AgenziaItaliana del Farmaco). We registered the study at https://clinicaltrials.gov (study number NCT02394119) and https://eudract.ema.europa.eu (Eudract study number 2015–000624–28).
Results
Study Participants
From June 2015 through October 2018, 152 potential participants were screened and 140 were randomized, 70 to each arm (Figure 1; Table 1). None was lost during the study and all were analyzed as randomized. The mean age of the patients was 11 years (interquartile range, 6–16), 49% were aged ≤9 years, 69% were boys. At the enrolment, all participants were on prednisone, 69% were treated with cyclosporine, and 31% with tacrolimus with comparable cumulative doses of each drug (Table 1). Overall, 46% of patients (45% and 47% in the ofatumumab and rituximab arm respectively) have been already treated with rituximab 36 months on average before enrolment. All participants were in stable remission when randomized and received the assigned intervention and contributed to the main outcome data.
Outcomes
A total of 73 participants relapsed at 12 months (primary outcome), 37 (52.8%) in the ofatumumab arm, and 36 (51.4%) in the rituximab arm (OR, 1.06; 95% confidence interval [95% CI], 0.55 to 2.06). At 24 months (secondary outcomes), 53 participants relapsed in the ofatumumab arm (75.7%) and 46 in the rituximab arm (65.7%) (OR, 1.6; 95% CI, 0.8 to 3.3). Time to relapse is reported in Supplemental Figure 2. Analyses stratified by age (≤9 years and >9 years) confirmed the same results although children aged ≤9 years seemed to benefit less from ofatumumab: 84% of children under 9 years were in relapse at 24 months versus 63% of children aged >9 years (Figures 2 and 3). In total, 64 patients (31 in the ofatumumab arm and 33 in the rituximab arm) received rituximab before enrollment in this study (median 36 months, interquartile range, 12–48). Previous treatment with rituximab was not associated with time to relapse (hazard ratio, 0.98; 95% CI, 0.7 to 1.4; P=0.92).
Figure 2.
Relapse-free survival by treatment arm. Ofatumumab (OFA; intervention; blue line) and rituximab (RTX; control; red line) (OR, 1.06; 95% CI, 0.55 to 2.06).
Figure 3.
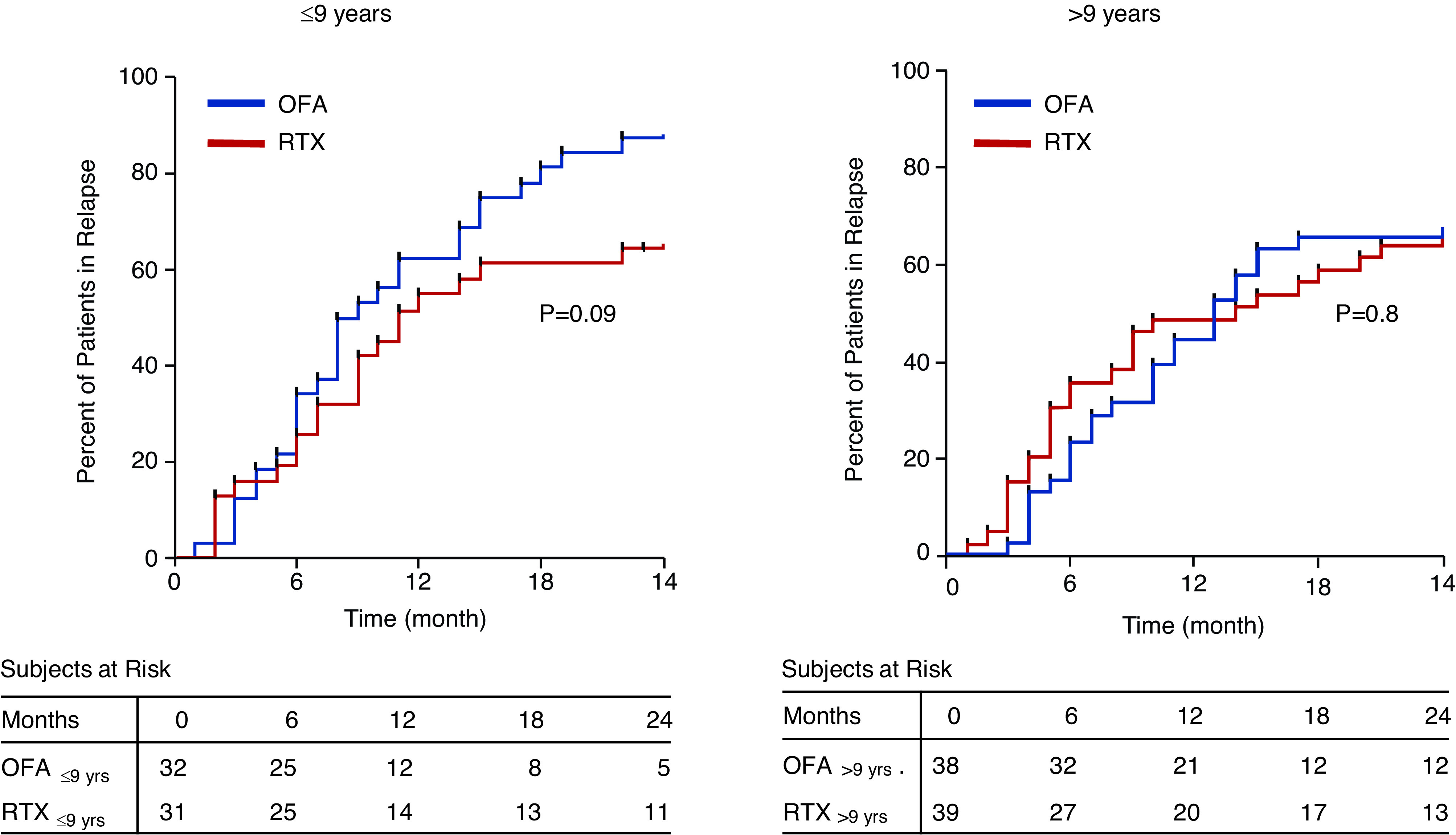
Relapse-free survival by treatment arm in patients stratified for age. Patients were stratified as aged <9 or >9 years.
Safety
No acute or clinically relevant side effects were observed in either the ofatumumab or rituximab arms during infusion. Follow-up tests included monthly biochemical and hematologic tests that did not reveal relevant modifications (Supplemental Table 1). There were no serious side effects during infusion of both drugs, most of which were limited to pruritus or erythema (Table 2). Respiratory symptoms were very limited.28 Participants remained free from severe complication during the entire duration of the study, with the exception of acute articular pain mimicking serum sickness. This complication was observed only in the rituximab arm (Table 2) and resolved within 72 hours with the use of paracetamol. Neutropenia occurred in one and three children in the ofatumumab and rituximab, respectively. All patients were resolved with a low dose of prednisone (0.5 mg/kg).
Table 2.
Adverse events
| Ofatumumab | Rituximab | P value | |
|---|---|---|---|
| (n=70), n (%) | (n=70), n (%) | ||
| Adverse events infusion related | |||
| SAEs (Grade ≥3) | 0 | 0 | 1.00 |
| Not SAEs (Grade <3) | 4 (6) | 7 (10) | 0.34 |
| Pruritus | 1 (1) | 3 (4) | 0.37 |
| Erythema | 2 (3) | 2 (3) | 1.00 |
| Glottis edema | 0 | 0 | 1.00 |
| Dyspnea | 0 | 1 (1) | 0.68 |
| Fever | 0 | 0 | 1.00 |
| Cough | 1 (1) | 1 (1) | 1.00 |
| Adverse events within 6 months | |||
| SAEs (Grade ≥3) | 0 | 0 | 1.00 |
| Not SAEs (Grade <3) | 1 (1) | 4 (6) | 0.31 |
| Neutropenia | 1 (1) | 2 (3) | 0.68 |
| Duration, days | 20 | 30/50 | |
| Articular pain | 0 | 2 (3) | 0.58 |
| Duration, days | 3 | ||
| Therapy | paracetamol |
Adverse events were reported on the basis of the time of their occurrence and on clinical severity. Articular pain is due to the chimeric structure of rituximab and mimics a serum sickness effect. SAEs, serious adverse events.
Ancillary Studies
Serum Immunoglobulin Levels.
Serum levels of IgA and IgG remained stable and were, on average, at the lower limit of the normal range for the 24-month study duration (Supplemental Figure 2). In contrast, median levels of serum IgM decreased within 3 months after infusion and showed a complete recovery only at 24 months (Supplemental Figure 3).
Serum Antirituximab IgG.
Serum antirituximab antibody levels were undetectable at baseline in 64 participants who had previously received rituximab. Then 6 months after rituximab infusion, antirituximab antibody levels increased in 14 of the 33 patients who were randomized in the rituximab arm (42%) with levels of 1–5 ng/ml in eight participants and 5–10 ng/ml in six participants. The incidence of relapse was not affected by the presence of antirituximab antibodies (Supplemental Figure 4).
Circulating B Cell Subpopulations.
In 69 patients consented to participate in this ancillary study (36 from the ofatumumab arm and 33 from the rituximab arm). Circulating B and T cell subpopulations were evaluated before and at prespecified time intervals, after either ofatumumab or rituximab treatments up to 15 months (Supplemental Table 2). Levels of the analyzed B cell subsets were not significantly different at baseline (before randomization) in the two arms (Figures 4 and 5). At 1 month after either treatment, there was a complete depletion of total CD19+ B cells and of all of the other B cell subpopulations including transitional, mature-naïve, total memory, switched memory, IgM memory, and plasmablasts (P<0.001 versus baseline). Total CD19+ B cells started to reappear after rituximab treatment at 3 months (P<0.05 versus month 1), whereas they were still depleted in the ofatumumab arm (P<0.01 versus rituximab at 3 months). These differences were observed in all of the analyzed B cell subsets at 3 months, but disappeared from 6 months onward. An initial rise of transitional B cells followed by mature-naïve B cells was observed, with a complete repletion of total CD19+, transitional, and mature-naïve B cells at 12–15 months from treatment; circulating plasmablasts started to reappear at 3 months, with a complete reconstitution at 6 months in both arms (Figure 4). In contrast, total memory, IgM memory, and switched memory B cells started to reappear after 6 months, but were still significantly reduced in both arms after 12–15 months (Figure 5).
Figure 4.
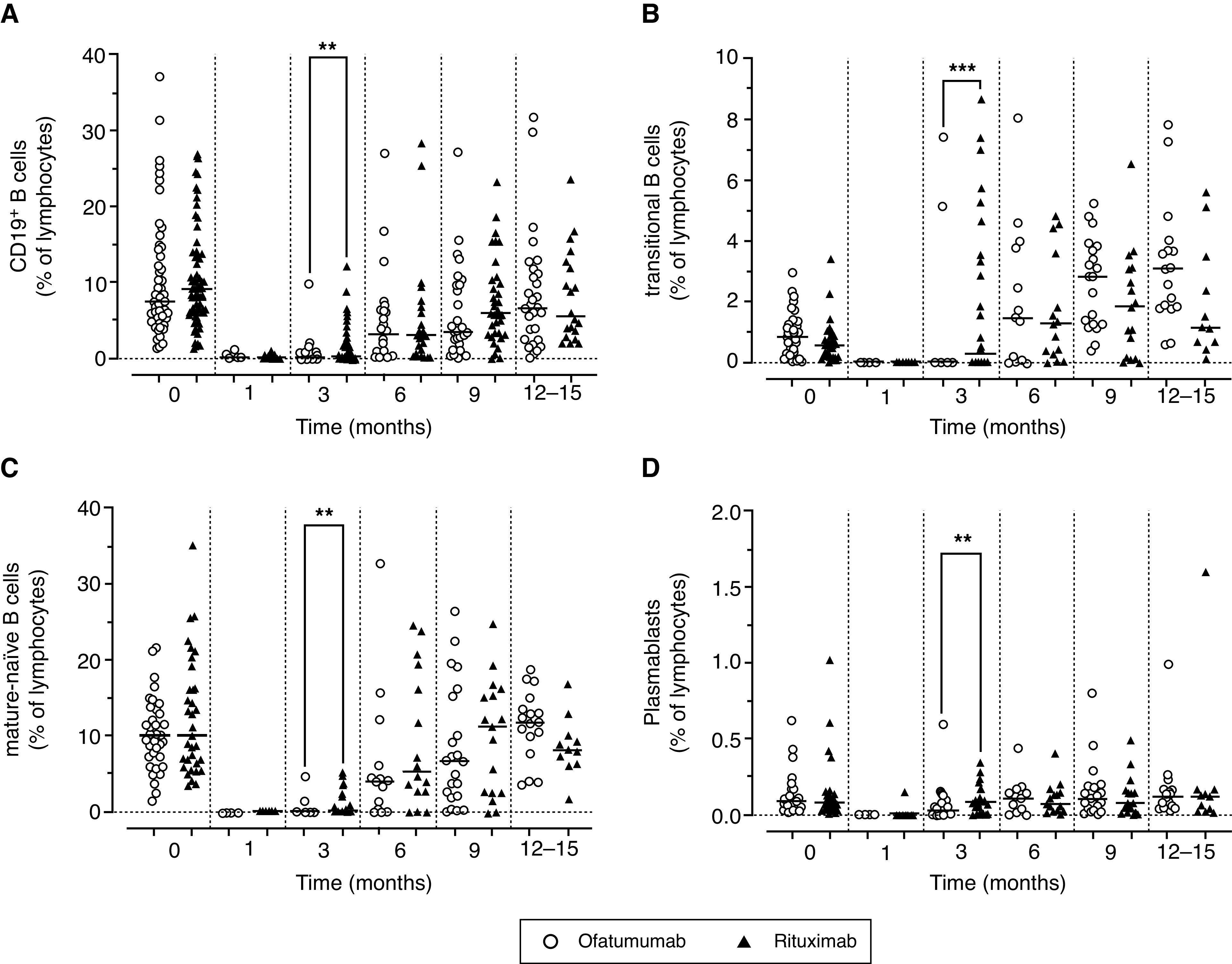
Circulating levels of B cells. Circulating levels of (A) total CD19+, (B) transitional, (C) mature-naïve B cells, and (D) plasmablasts. Values are expressed as % of lymphocytes as determined by flow cytometry in patients enrolled in the two arms of the study and treated with rituximab (red triangles) and ofatumumab (blue circles). Data were evaluated before infusion of drugs and at 1–15 months. Gating strategy was described in Supplemental Figure 1. Horizontal lines indicate the medians. Levels of the B cell subpopulations at different time points were analyzed using a nonparametric Kruskal–Wallis test followed by a Dunn’s multiple comparison. **P<0.01;***P<0.001.
Figure 5.
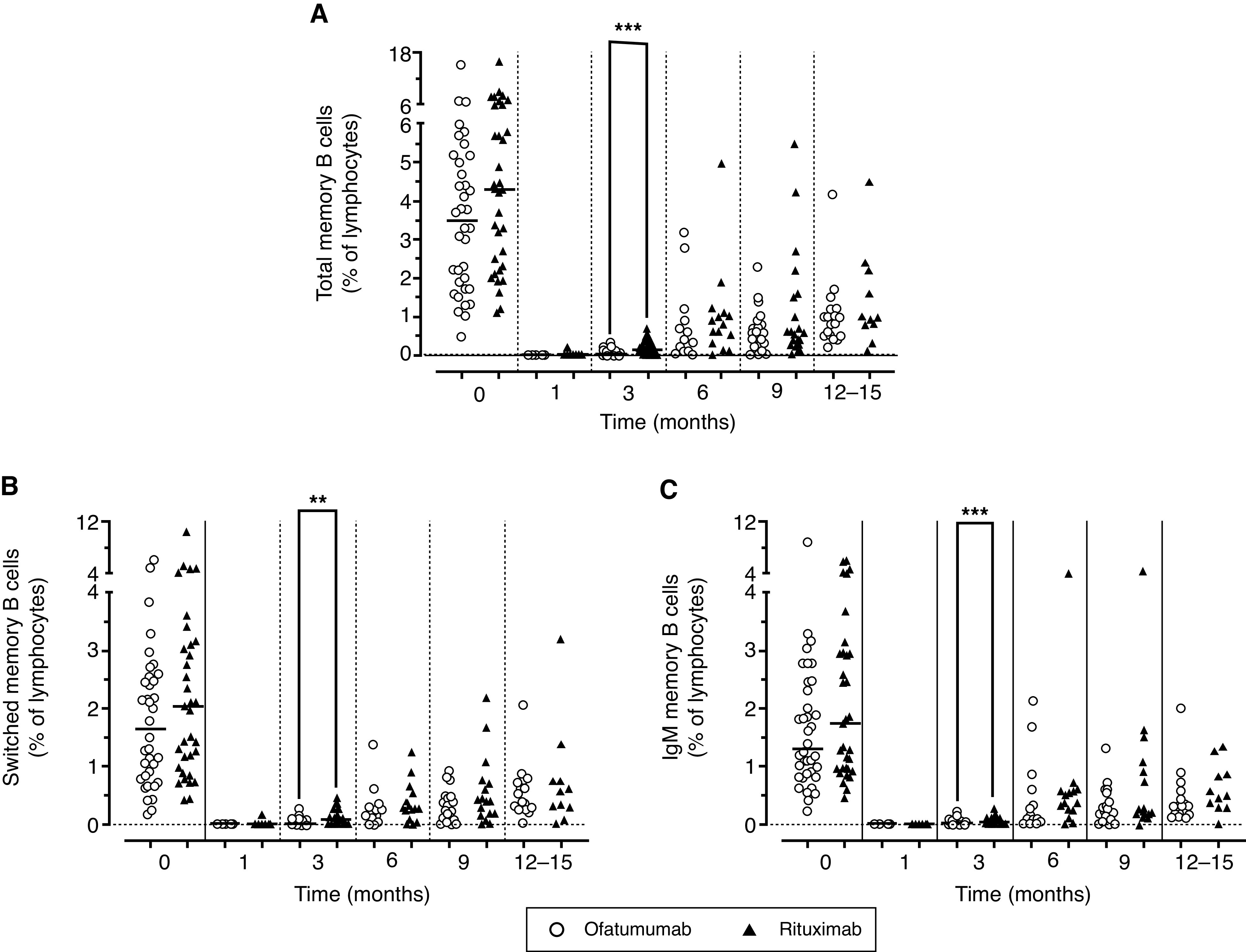
Circulating levels of memory B cells. Circulating levels of (A) total memory, (B) switched memory, (C) and IgM memory B cells. Values are expressed as % of lymphocytes as determined by flow cytometry in patients enrolled in the two arms of the study and treated with rituximab (red triangles) and ofatumumab (blue circles). Data were evaluated before infusion of drugs and at 1–15 months. Gating strategy was described in Supplemental Figure 1. Horizontal lines indicate the medians. Levels of the B cell subpopulations at different time points were analyzed using a nonparametric Kruskal–Wallis test followed by a Dunn’s multiple comparison. **P<0.01;***P<0.001.
Circulating T and Natural Killer Subpopulations.
A transient increase of total CD3+ T cells and CD56+ natural killer cells was observed 1 and 3 months after treatment, which returned to basal levels within 12–15 months (Figure 6). Tregs also significantly increased 3 months after anti-CD20 therapies and were still significantly higher at 12–15 mo nths. The same increment was observed in the total number of each cell subset. The CD4+/CD8+ T cell ratio was not significantly altered after either treatment.
Figure 6.

Circulating levels of T cells. (A) CD3+ T cells, (B) CD56+ natural killer (NK) cells, (C) CD4+/CD8+ T cells, and (D) Treg cells expressed as % of lymphocytes as determined by flow cytometry in patients enrolled in the two arms of the study and treated with rituximab (red triangles) and ofatumumab (blue circles). Data were evaluated before infusion of drugs and at 1–15 months. Gating strategy was described in Supplemental Figure 1. Horizontal lines indicate the medians. Levels of the B cell subpopulations at different time points were analyzed using a nonparametric Kruskal-Wallis test followed by a Dunn’s multiple comparison. *P<0.05.
Discussion
This is the first large clinical trial that compared the efficacy of a human anti-CD20 monoclonal antibody ofatumumab to the chimeric analog rituximab in children and young adults with SDNS who were maintained in remission with steroids and calcineurin inhibitors. We found that ofatumumab was not superior to rituximab in maintaining oral drug-free disease remission: approximately 50% of patients remained in remission 1 year after the infusion of either anti-CD20 antibody treatment and 25% were still in remission at 2 years. Response to ofatumumab tended to be worse in patients younger than 9 years. Both treatments were safe.28 Overall, both treatments affected the course of nephrotic syndrome, maintaining patients in remission (out of drug) for long periods, and avoiding any further calcineurin inhibitors treatment at the recurrence of the disease. Infusion-associated arthritis requires prompt recognition and treatment with paracetamol. Although retreatment with rituximab has shown positive effects in maintaining a prolonged remission,29 previous treatment with rituximab did not modify the risk of relapse in our cohort.
Our results are in agreement with a recent clinical trial (Single Agent Ofatumumab vs. Single Agent Rituximab in Indolent B-Cell Non Hodgkin Lymphoma Relapsed After Rituximab-Containing Therapy [HOMER] randomized study)30 that compared the effects of ofatumumab and rituximab in patients with non-Hodgkin lymphoma. The HOMER study failed to demonstrate a superiority of ofatumumab treatment in preventing disease relapse and was stopped early because of futility at the planned interim analysis.30 This finding and our own results are consistent with the notion that the stronger receptor affinity and lytic effects of ofatumumab observed in vitro may lead to a longer B cell depletion from peripheral blood, but do not necessarily translate into superior clinical outcomes.31
From an immunologic point of view, ofatumumab and rituximab had a similar effect on serum immunoglobulin and circulating immune cell levels in our cohort. Serum IgG and IgA levels were not significantly modified, whereas median levels of serum IgM waned during the study after both treatments and tended to normalize only after 24 months, as already reported after rituximab treatment in several autoimmune diseases.32
Previous exposure to rituximab may result in a rise of antirituximab antibodies and may modify the composition of lymphocyte population that maintain the memory for antibody production. However, we found undetectable levels of antirituximab antibodies at baseline in those patients who previously received rituximab treatment and the rise of these antibodies in the rituximab arm during the study period was not associated with relapse. All B cell subpopulations, including memory B cells, had similar distributions at baseline suggesting no effects of previous infusions in our study populations.
Both ofatumumab and rituximab were effective in completely depleting circulating levels of all of the analyzed B cell subsets. Ofatumumab treatment induced a more prolonged depletion of B cells compared with rituximab, likely due to its stronger affinity to CD20 and its lytic ability.19 However, this effect was transient and the reappearance of the B cell subpopulations was comparable between the two treatments from 6 months onward. This probably explains why the incidence of relapse at 12 and at 24 months was similar in the two arms. As previously observed following rituximab treatment, an initial recovery of transitional and mature-naïve B cells and a delayed recovery of the memory B cell compartment was observed.25,31,32 Plasmablasts, which lack CD20 expression and have a short lifespan, were also indirectly depleted by either antibodies, but promptly reappeared and remained stable during time. The role of this B cell subpopulation in the disease and whether its rapid recovery after anti-CD20 treatment requires a cell-specific treatment is still being evaluated.33 Total CD3+ T cells and CD56+ natural killer cells transiently increased after treatment, but returned to basal levels within 12–15 months. Of note, in agreement with previous reports in either idiopathic nephrotic syndrome and membranous nephropathy26 Tregs significantly increased after anti-CD20 treatment and were still high at 12–15 months in both arms.26,34–38
The strengths of our study include its randomized design, the stratification for age, the long follow-up for the assessment of both potential benefits and harms, and the inclusion of cellular studies that helps to support clinical evidence with biologic observations. The relatively large study cohort, considering that idiopathic nephrotic syndrome is a rare disease,1 was also important to definitely evaluate the superiority of ofatumumab compared with rituximab therapy, which was suggested by previously reported evidence on a limited number of patients.20,23
Our study has limitations. We were unable to mask the intervention to participants and staff because the two antibodies require different methods for infusion. However, we followed a rigorous protocol to minimize bias due to the use of cointerventions potentially affecting outcomes. We estimated the power of this study on the basis of a fixed sample size of 140 participants to ensure feasibility. Our study had the power to detect large effects (>50% risk reduction) and we could not account for additional testing (i.e., subgroup analyses). Although we found no signal of effect favoring ofatumumab, an effect of smaller size (e.g., relative risk reduction of 10%–20%) cannot be excluded. Similarly, an age-modification effect of ofatumumab is possible. Further studies are needed of such effect is considered relevant. We included patients who previously received rituximab to study antirituximab antibodies and memory B cells composition. Although these participants were evenly distributed by treatment arm, we could not exclude that previous treatment with rituximab may have enhanced the response to the intervention in the rituximab arm, thus reducing the difference between the two interventions. Yet, relapse rates were higher in the ofatumumab arm than in the rituximab arm. Finally, we used an intermediate measure of outcome (disease relapse) instead of a hard endpoint (kidney failure). The laboratory-based measures we adopted to define disease relapse are objective, clinically relevant, and valid predictors of more distant outcomes, including cardiovascular or infectious complications of nephrotic syndrome, or kidney failure, which are rare in children and young adults.
In summary, this trial in children and young adults with SDNS maintained in remission with steroids and calcineurin inhibitors does not support the superiority of the fully human molecule ofatumumab versus the chimeric anti-CD20 antibody rituximab. Stronger receptor affinity and lytic effects of fully humanized anti-CD20 antibodies observed in vitro may lead to a longer B cell depletion from peripheral blood but do not seem to translate into superior clinical outcomes in children and young adults with SDNS.
Disclosures
F. Emma reports consultancy agreements with Kyowa Kirin and Otsuka Pharmaceutical; reports receiving honoraria from Avrobio, Chiesi Pharmaceuticals, Kyowa Kirin, Orphan Europe, and Recordati Rare Diseases; and reports being a scientific advisor or members of Otsuka Pharmaceutical’s safety board for pediatric trials. M. Colucci reports having interests/relationships with Associazione per la Cura del Bambino Nefropatico ONLUS, Italian Ministry of Health, Fondazione Bambino Gesù, and Ricerca Corrente. M. Prunotto reports current employment with Galapagos Ltd. M. Vivarelli reports consultancy agreements with Achillion Pharmaceuticals, Alexion Pharmaceuticals, Apellis Pharmaceuticals, Novartis Pharmaceuticals, Roche Pharmaceuticals, and Travere Pharmaceuticals; reports receiving research funding from Alexion Pharmaceuticals for participation in clinical studies and from Chemocentrix for participation in clinical studies; reports being a scientific advisor or member of the Editorial Board of Pediatric Nephrology, Editorial Board of Kidney International, Editorial Board of Frontiers in Pediatrics, Pediatric Nephrology; and reports having other interests/relationships as Chair of Scientific Committee for the 2022 European Society of Pediatric Nephrology meeting, Chair of European Rare Kidney Diseases Network Working Group on Immune Glomerulopathies, Chair of European Society of Pediatric Nephrology Working Group on Glomerular Disease, and Member of atypical hemolytic uremic syndrome International Task Force. All remaining authors have nothing to disclose.
Funding
P. Ravani, F. Lugani, and G. M. Ghiggeri were supported by the Italian Ministry of Health, RicercaFinalizzata (Grant WFR:PE/Number:). 2016-02361576
Supplementary Material
Acknowledgments
The Institute Giannina Gaslini (trial sponsor) provided logistic and financial support to the trial through grants from the Ministry of Health (Cinque per mille of Imposta sul reddito delle persone fisiche-Finanziamentodellaricerca sanitaria). The funder had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication. We thank the Associazione per la Cura del Bambino Nefropatico ONLUS for supporting Drs. M. Colucci and M. Vivarelli and the Fondazione MalattieRenali del Bambino non-profit organization (ONLUS) for supporting Drs. G. M. Ghiggeri, M. Bruschi, and M. Cioni. The following co-authors were involved in data collection and we acknowledge their role; they were involved in clinical settings and data collection, and we obtained written permission to include their names in this section of the paper: Drs. Maria Ludovica Degl’innocenti, Giorgio Piaggio, and Enrico Verrina. We thank the members of the Data and Safety Monitoring Board (Drs. Giovanni Candiano, Gianluca Caridi, and Antonella Trivelli) and endpoint adjudication committee (Drs. Fabrizio Ginevri and Enrico Verrina).
F. Lugani and G.M. Ghiggeri were responsible for following the clinical part of the study; G.M. Ghiggeri and P. Ravani designed the study; M. Bruschi, M. Cioni, P. Cravedi, M. Colucci, A. DiDonato, M. Prunotto, and M. Vivarelli were responsible for the laboratory part; M. Bruschi, F. Emma, G. M. Ghiggeri, P. Ravani, A. Angeletti and M. Vivarelli analyzed the data; F. Antonini made the figures; G.M. Ghiggeri, P. Ravani, and M. Vivarelli drafted and revised the paper; all authors approved the final version of the manuscript.
Footnotes
P.R., M.C., and A. A. contributed equally to the study
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040561/-/DCSupplemental.
Supplemental Materials and Methods.
Supplemental Figure 1. Gating strategy for flow cytometry analysis.
Supplemental Figure 2. Time (months) to relapse in patients treated with ofatumumab and rituximab.
Supplemental Figure 3. Serum levels of immunoglobulins A, G and M and circulating counts.
Supplemental Figure 4. Circulating levels of antirituximab antibodies.
Supplemental Table 1. Participant timeline.
Supplemental Table 2. Circulating B and T cell subpopulations expressed as a percentage of total lymphocytes at baseline and after ofatumumab or rituximab treatment.
References
- 1.Cameron JS, Turner DR, Ogg CS, Sharpstone P, Brown CB: The nephrotic syndrome in adults with ‘minimal change’ glomerular lesions. Q J Med 43: 461–488, 1974 [PubMed] [Google Scholar]
- 2.McEnery PT, Strife CF: Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr Clin North Am 29: 875–894, 1982 [PubMed] [Google Scholar]
- 3.Kyrieleis HA, Löwik MM, Pronk I, Cruysberg HR, Kremer JA, Oyen WJ, et al.: Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 4: 1593–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noone DG, Iijima K, Parekh R: Idiopathic nephrotic syndrome in children. Lancet 392: 61–74, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Eckardt K-U, Kasiske BL.: Chapter 5: Minimal-change disease in adults. Kidney Int Suppl 2: 177–180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al.; Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group : Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384: 1273–1281, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, et al.: Rituximab in children with steroid-dependent nephrotic syndrome: A multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 26: 2259–2266, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravani P, Lugani F, Pisani I, Bodria M, Piaggio G, Bartolomeo D, et al.: Rituximab for very low dose steroid-dependent nephrotic syndrome in children: A randomized controlled study. Pediatr Nephrol 35: 1437–1444, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM: Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 11: 710–720, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rougé L, Chiang N, Steffek M, Kugel C, Croll TI, Tam C, et al.: Structure of CD20 in complex with the therapeutic monoclonal antibody rituximab. Science 367: 1224–1230, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Rovin BH, Caster DJ, Cattran DC, Gibson KL, Hogan JJ, Moeller MJ, et al.; Conference Participants : Management and treatment of glomerular diseases (part 2): Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf. Accessed August 13, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Chan EY, Webb H, Yu E, Ghiggeri GM, Kemper MJ, Ma AL, et al.: Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int 97: 393–401, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, et al.: Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: A randomized controlled trial. Clin J Am Soc Nephrol 6: 1308–1315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, et al.: Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84: 1025–1033, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravani P, Lugani F, Drovandi S, Caridi G, Angeletti A, Ghiggeri GM: Rituximab vs low-dose mycophenolate mofetil in recurrence of steroid-dependent nephrotic syndrome in children and young adults: A randomized clinical trial. JAMA Pediatr 175: 631–632, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, et al.; Mayo Nephrology Collaborative Group : Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wincup C, Menon M, Smith E, Schwartz A, Isenberg D, Jury EC, et al.; ABIRISK Consortium : Presence of anti-rituximab antibodies predicts infusion-related reactions in patients with systemic lupus erythematosus. Ann Rheum Dis 78: 1140–1142, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer-Suavet S, Andreani M, Lateb M, Savenkoff B, Brglez V, Benzaken S, et al.: Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol 10: 3069, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Planchais C, Fronzes R, Mouquet H, Reyes N: Binding mechanisms of therapeutic antibodies to human CD20. Science 369: 793–799, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Basu B: Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med 370: 1268–1270, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Bonanni A, Rossi R, Murtas C, Ghiggeri GM.. Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep vol. 2015 bcr2015210208. 2015-210208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravani P, Pisani I, Bodria M, Caridi G, Degl’Innocenti ML, Ghiggeri GM: Low-dose ofatumumab for multidrug-resistant nephrotic syndrome in children: A randomized placebo-controlled trial. Pediatr Nephrol 35: 997–1003, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Vivarelli M, Colucci M, Bonanni A, Verzani M, Serafinelli J, Emma F, et al.: Ofatumumab in two pediatric nephrotic syndrome patients allergic to rituximab. Pediatr Nephrol 32: 181–184, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Wang CS, Liverman RS, Garro R, George RP, Glumova A, Karp A, et al.: Ofatumumab for the treatment of childhood nephrotic syndrome. Pediatr Nephrol 32: 835–841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Ravà L, et al.: B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 27: 1811–1822, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia D, Sinha A, Hari P, Sopory S, Saini S, Puraswani M, et al.: Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr Res 84: 520–526, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Ravani P, Bonanni A, Ghiggeri GM: Randomised controlled trial comparing ofatumumab to rituximab in children with steroid-dependent and calcineurin inhibitor-dependent idiopathic nephrotic syndrome: Study protocol. BMJ Open 7: e013319, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonanni A, Calatroni M, D’Alessandro M, Signa S, Bertelli E, Cioni M, et al.: Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol 84: 1238–1249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi T, Okamoto T, Sato Y, Yamazaki T, Hayashi A, Aoyagi H, et al.: Periodically repeated rituximab administrations in children with refractory nephrotic syndrome: 2-year multicenter observational study. Pediatr Nephrol 34: 87–96, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Maloney DG, Ogura M, Fukuhara N, Davis J, Lasher J, Izquierdo M, et al.: A phase 3 randomized study (HOMER) of ofatumumab vs rituximab in iNHL relapsed after rituximab-containing therapy. Blood Adv 4: 3886–3893, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, et al.: Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104: 1793–1800, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kridin K, Ahmed AR: Post-rituximab immunoglobulin M (IgM) hypogammaglobulinemia. Autoimmun Rev 19: 102466, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Dossier C, Prim B, Moreau C, Kwon T, Maisin A, Nathanson S, et al.: A global antiB cell strategy combining obinutuzumab and daratumumab in severe pediatric nephrotic syndrome. Pediatr Nephrol 36: 1175–1182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al.: Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum 56: 3044–3056, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC: Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54: 613–620, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, et al.: A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 6: 256ra136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamin A, Berthelot L, Couderc A, Chemouny JM, Boedec E, Dehoux L, et al.: Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun 89: 149–161, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Colucci M, Carsetti R, Rosado MM, Cascioli S, Bruschi M, Candiano G, et al.: Atypical IgM on T cells predict relapse and steroid dependence in idiopathic nephrotic syndrome. Kidney Int 96: 971–982, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




