ABSTRACT
Objectives:
To estimate the in vivo effect of nano-hydroxyapatite (HA) modification of banding glass-ionomer cement on microleakage under orthodontic bands.
Materials and Methods:
Eighty noncarious premolars scheduled for extraction in 20 orthodontic patients were randomly divided into four groups. Grouping was based on the ratio of nano-HA (0%, 5%, 10%, 15% by weight) added to the luting glass-ionomer cement (GIC) Ketac-Cem, which was used for cementation of prefabricated micro-etched orthodontic bands. Dye penetration method was used for microleakage evaluation at the cement-band and cement-enamel interfaces. Statistical evaluation was performed with a Kruskal-Wallis test and a Mann-Whitney U-test, and a Bonferroni-adjusted significance level was calculated.
Results:
Bands cemented with conventional GIC showed the highest microleakage scores in comparison to those cemented with nano-HA-modified GIC. No significant difference was found between teeth banded with 10% and 15% modified GIC.
Conclusions:
Modification of the banding GIC with 15% nano-HA revealed a positive effect on reducing microleakage around orthodontic bands.
Keywords: Microleakage, Nano-HA, Orthodontic bands, Glass-ionomer
INTRODUCTION
Orthodontic treatment requires anchorage from the posterior teeth to hold and guide other teeth in the arch; this necessitates a firm attachment on the anchor teeth. Such an attachment can be practically obtained by orthodontic bands cemented on premolars and molars.1 Enamel demineralization adjacent to fixed orthodontic appliances such as bands and brackets is a major complication in orthodontic patients, especially those with poor oral hygiene.2–4 Different studies have reported the prevalence of these lesions to be up to 95% during fixed orthodontic treatment.5,6
Glass-ionomer cement (GIC) has been widely used for cementing orthodontic bands because of its anticariogenic property, which is attributed to release of fluoride.7–10 In comparison with other banding cements, such as zinc phosphate or zinc polycarboxylate, GIC offers considerable advantages. Its capacity for adhesion to enamel and metal, provided by its content of polyacrylic acid, combined with its higher strength result in superior clinical performance and reduced band failure.11,12
HA is one of the most biocompatible and bioactive materials, and its nano-sized particles are similar to the apatite crystal of tooth enamel in morphology, crystal structure, and crystallinity.13 The effect of nano-structuring on the properties of HA was described by Kaehler,14 who attributed the enhanced biocompatibility of nano-HA with the increased surface area and proportion of atomicity to decreasing its particle size.
Remineralization in enamel was previously defined as “the deposition of mineral in demineralized defects at a molecular level.”15 Thus, an increasing number of reports have shown that nano-HA has the potential to remineralize artificial carious lesions following its addition to toothpastes, mouthwashes, etc.16–18 Several studies19–21 sought to determine the most effective concentration of HA for enamel remineralization. However, a study by Huang et al.22 reported that a concentration of 10% nano-HA may be optimal for remineralization of early enamel lesions.
Addition of HA to conventional GIC was tried by some researchers in an attempt to enhance its chemical and mechanical properties.23–25 However, there are no reported in vivo studies regarding modification of GIC by HA nanoparticles for orthodontic banding. Accordingly, the aims of this in vivo investigation were to determine and compare microleakage patterns of conventional GIC and GIC with different concentrations of nano-HA for band cementation. Our null hypothesis assumed that there were no statistically significant differences in microleakage between GIC groups with different ratios of nano-HA (HA, 0%, 5%, 10%, 15% by weight).
MATERIALS AND METHODS
The study was designed as a split-mouth, within-subject clinical trial. It was scientifically and ethically approved by the Research Committee of Mansoura University. Informed consent was obtained from patients older than 18 and from parents of the younger subjects. The subjects included 12 females and eight males who were scheduled for extraction of four premolars as part of their orthodontic treatment. The inclusion criteria were:
Age less than 20 years, because the highest incidence of caries occurs during the teenage years;
Premolars fully erupted and intact without visible defects on their buccal and lingual surfaces; and
Regular tooth-brushing habit.
For each patient, the four first premolars were randomly assigned to one of the following treatment groups: conventional GIC (group 1; Ketac-Cem; 3M ESPE, Gmbh, Seefeld, Germany); GIC with 5% nano-hydoxyapatite (group 2); GIC with 10% nano-hydoxyapatite (group 3), and GIC with 15% nano-hydoxyapatite (group 4). This distribution allowed for the same environment for all the tested teeth. For groups 2, 3, and 4, nano-HA powder was prepared from aqueous solutions using the wet precipitation method. This method depended basically on the addition of phosphate-containing aqueous solutions to calcium-containing aqueous solutions, or the reverse, to obtain precipitates of calcium phosphates.26 Nano-HA crystals were of nanometer grade and had a crystal size of 5–26.7 nm in diameter by 30–84 nm in length. These nano-HA crystals are similar in crystallinity to apatite in bone and enamel, as revealed by x-ray diffraction. The addition of HA to glass ionomer cement powder was done by weight: 5%, 10%, and 15% (for example, in the 10% group, each 10 mg of HA was mixed with 90 mg of GIC to get GIC reinforced by 10% of HA). The weight of elements was measured using an electronic balance and admixed in a period of about 30 minutes to get homogenous mixtures and to reduce the amount of microbubbles during mixing. After cleaning teeth with nonfluoride pumice, stainless steel, micro-etched orthodontic bands (3M Unitek, Monrovia, Calif) with attachments were fitted, and margins were adapted by a band pusher. Bands were approximately seated at the same position of each tooth on the middle third of the crowns. Then, bands were tightly fitted to decrease the possibility of enamel dissolution, and excess cement was removed to prevent affecting test results.27
For the testing procedure, 20 bands were cemented randomly in each group (10 upper and 10 lower first premolars in each of the four groups). After 60 days, teeth were extracted and stored in distilled water for 24 hours. Before methylene blue dye penetration, teeth apices were sealed with sticky wax. After that, teeth were rinsed in tap water and air dried, and nail varnish was applied to the entire surface of the tooth except for approximately 1 mm from the bands. To minimize dehydration of the specimens, teeth were placed in water as soon as the nail polish dried. Then teeth were immersed in 0.5% solution of methylene blue for 24 hours at room temperature. After removal from the solution, teeth were rinsed in tap water, the superficial dye was removed with a brush, and then teeth were dried and embedded in self-curing acrylic up to the occlusal surface of the band.28 Each premolar was separated into two parts in the mesiodistal direction. Four parallel, longitudinal sections from the middle part of each premolar were made at the occlusobuccal and occlusolingual surfaces with a low-speed diamond saw (Isomet; Buehler, Lake Bluff, Ill) in the bucco-lingual direction according to the method of Arhun et al.29 The specimens were evaluated with a stereomicroscope (20× magnification; SZ 40; Olympus, Tokyo, Japan) for dye penetration along the cement-band interface. Then the band materials were gently removed from the cement, and dye penetration at the cement-enamel interface was also evaluated with the stereomicroscope. Each section was scored from both the buccal and lingual sides of the bands between the cement-band and cement-enamel interfaces. Microleakage was measured directly by an electronic digital caliper (Digimess; Shiko Precision Gaging Ltd, Shandong, China). The depth of dye penetration in specimens was evaluated by two examiners up to 0.1 mm, and the average of the examiners' measurements for each sample was recorded.27
Statistical Analysis
Data were analyzed with the statistical software SPSS (version 11.0J; SPSS Japan, Tokyo, Japan). Five randomly selected specimens from each group were reexamined, and intraexaminer and interexaminer method errors were evaluated by kappa test. Microleakage scores of the cement-band and cement-enamel interfaces were obtained by calculating the buccal and lingual microleakage scores. After statistical evaluation of leakage for each specimen, the score for each group was obtained by calculating the means of the buccal and lingual microleakage scores. The Shapiro-Wilks normality test and Levene variance homogeneity test were applied to the microleakage data. Data showed nonnormal distribution, and there was no homogeneity of variances among the groups. Thus, the statistical comparison between groups was performed with nonparametric tests (Kruskal-Wallis and Mann-Whitney U-tests). The overall difference between the studied groups in every interface was evaluated by a Kruskal-Wallis test. Each pair in the four studied groups in the two interfaces was compared by Mann-Whitney U-test, and the resulting P value was corrected according to the total number of comparisons done in every interface (six comparisons) using the Bonferroni method to overcome the possibility of a type I error. P < 0.05 was considered significant (<0.008 in Bonferoni-corrected Mann-Whitney U-test).
RESULTS
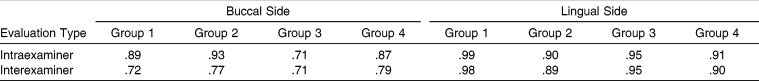
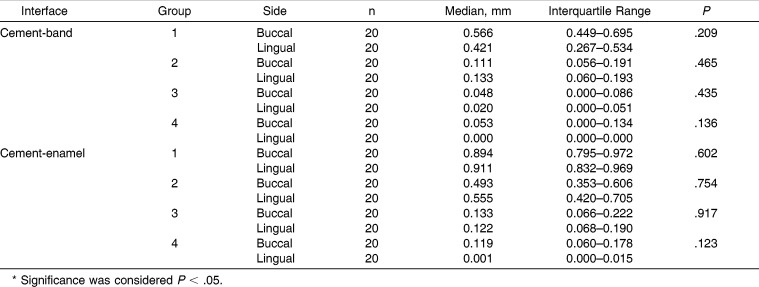
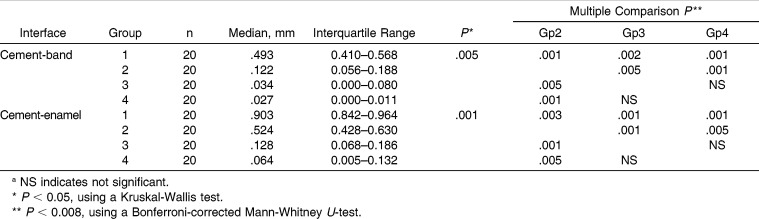
The intraexaminer and interexaminer kappa scores for assessment of microleakage were high, with all values greater than .70 (Table 1). Descriptive statistical values and buccal and lingual microleakage comparisons between the cement-band and cement-enamel interfaces are shown in (Table 2). Comparisons of the buccal and lingual microleakage scores for all specimens had no statistically significant differences for the side (P > .05). Thus, the buccal and lingual microleakage scores for each specimen were pooled, and the microleakage values for each band-cement and cement-enamel interface were obtained by calculating the mean of the buccal and lingual microleakage scores. Descriptive statistics and results of the statistical tests for microleakage between the cement-band and cement-enamel interfaces are shown in (Table 3). Statistical comparisons showed significant differences among the four groups interfaces (P = .005 and P = .001 for cement-band and cement-enamel, respectively). Therefore, the null hypothesis of no statistically significant differences in microleakage between different groups was rejected. In both studied interfaces, significant microleakage differences were found between group 1 and groups 2, 3, and 4 (P < .008). Significant differences were also found between group 2 and groups 3 and 4 (P < .008). However, no significant difference was found between groups 3 and 4. Group 1 had the highest microleakage scores between the cement-band (median, 0.49 mm) and cement-enamel (median, 0.90 mm) interfaces.
Table 1.
Intraexaminer and Interexaminer Kappa Scores for Assessment of Microleakage
Table 2.
Buccal and Lingual Microleakage Comparisons Between Cement-Band and Cement-Enamel Interfaces
Table 3.
Total Microleakage Comparisons Between Cement-Band and Cement-Enamel Interfaces of the Test Groupsa
DISCUSSION
Clinical practice showed that orthodontic banding increases susceptibility to enamel decalcification, because the orthodontic band with its welded attachment provides a shelter for plaque accumulation.30
Recently, nano-HA has been widely suggested to rebuild tooth enamel that is prone to mineral loss due to its unique capability of remineralization.31,32 Most research has suggested that nano-HA could repair damaged enamel through adsorption onto its surface32,33 or even by filling up defects and micropores on demineralized teeth.16,17 In the current study, nano-HA was selected as a demineralizing agent, as it was easily added to conventional GIC, used for band cementation, and able to reduce enamel decalcification, and thus microleakage, around orthodontic bands.
Dye penetration is a common method to assess microleakage in restorative dentistry34 and orthodontics28,29 because it is simple, relatively cheap, and quantitative.34 Because of the range of bacteria sizes, dyes such as methylene blue and fuchsine are realistic agents to determine the sealing ability of the tested material.35
In our study, micro-etched bands were used because they showed higher bond strength and lower clinical failure rate than untreated bands.11,36 Our assessments were made from four parallel longitudinal sections through the buccal and lingual surfaces according to the methods of Arhun et al.29 and Arikan et al.37
Different failure sides for bands were reported.38–40 Evaluations in this study were performed for all groups from two interfaces: cement-band and cement-enamel. Microleakage at the cement-band interface might play a role in band failure caused by adhesion degradation. However, the cement-enamel interface is more critical because it can also cause white spot lesions.
Results of this study showed significant microleakage differences among the four groups. Conventional Ketac-Cem GIC (group 1) had the highest microleakage scores for both interfaces, whereas groups 3 and 4 had the lowest values. Dye penetration was correlated with the degree of enamel demineralization caused by acid incubation.29 Larger demineralization areas are likely with bands cemented with conventional GIC compared to those of bands modified with nanoparticles. These findings can be related to the demineralizing effect of nano-HA, which was incorporated in the banding GIC. As a result of enamel remineralization, deposition of nano-HA would probably block surface pores and inhibit diffusion of the dye into the lesion. The ability of nano-HA to promote remineralization was explained by Huang et al.,22 who applied an in vitro pH-cycling model to evaluate the effect of nano-HA concentration on initial enamel lesion. It was assumed that nano-HA particles penetrate the enamel pores, where they precipitate and attract a large amount of Ca2+ and PO43− from the remineralization solution to fill the vacant positions of enamel calcium crystals. This would enhance crystal integrity and growth.22
Regarding the effect of HA concentration, the present results indicated a direct proportionality between HA concentration and the rate of enamel remineralization, up to a certain limit. Kim et al.17 mentioned that higher concentration increases the rate and amount of nano-HA precipitation, along with the deposition of extensive amounts of Ca2+ and PO43−, thus significantly promoting the remineralization effect. However, this deposition would eventually come to a stable level even though the concentration increased, which would result in no significant difference between the 10% and 15% nano-HA groups.
The demineralization-inhibition results of the present study indicate that GIC with 15% nano-HA is a promising material for orthodontic banding that may guarantee a minimal amount of demineralization around the bands at the end of orthodontic treatment. However, further long-term studies may be needed to evaluate the correlation between microleakage and shear bond strength of the bands.
CONCLUSIONS
Dye penetration records of the tested specimens indicated a significant positive effect of nano-HA on reducing microleakage around orthodontic bands.
The buccal and lingual sides in all groups had similar microleakage scores for both the cement-enamel and cement-band interfaces.
Teeth banded with 15% modified GIC had lower microleakage scores.
Additional studies are required for evaluating the physical properties of this modified GIC.
ACKNOWLEDGMENT
The authors are most grateful to Dr Reda Rashad Sheha, Professor and Head of the Nuclear Chemistry Department, Hot Laboratory Center, Atomic Energy Authority, Egypt, for preparation of nano-HA powder.
REFERENCES
- 1.Goje SK, Sangolgi VK, Neela P, Lalita CH. A comparison of resistance to enamel demineralization after banding with four orthodontic cements: an in vitro Study. J Indian Orthod Soc. 2012;46:141–147. [Google Scholar]
- 2.Behnan SM, Arruda AO, González-Cabezas C, Sohn W, Peters MC. In-vitro evaluation of various treatments to prevent demineralization next to orthodontic brackets. Am J Orthod Dentofacial Orthop. 2010;138:712–713. doi: 10.1016/j.ajodo.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Atack NE, Sandy JR, Addy M. Periodontal and microbiological changes associated with the placement of orthodontic appliances. A review. J Periodontol. 1996;67:78–85. doi: 10.1902/jop.1996.67.2.78. [DOI] [PubMed] [Google Scholar]
- 4.Brown WE, Gregory TM, Chow LC. Effect of fluoride on enamel solubility and cariostasis. Caries Res. 1977;11:118–141. doi: 10.1159/000260298. [DOI] [PubMed] [Google Scholar]
- 5.Lovrov S, Hertrich K, Hirschfelder U. Enamel demineralization during fixed orthodontic treatment—incidence and correlation to various oral-hygiene parameters. J Orofac Orthop. 2007;68:353–363. doi: 10.1007/s00056-007-0714-1. [DOI] [PubMed] [Google Scholar]
- 6.Richter AE, Arruda AO, Peters MC, Sohn W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am J Orthod Dentofacial Orthop. 2011;139:657–664. doi: 10.1016/j.ajodo.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Hallgren A, Oliveby A, Twetman S. Fluoride concentration in plaque adjacent to orthodontic appliances retained with glass ionomer cement. Caries Res. 1993;27:51–54. doi: 10.1159/000261515. [DOI] [PubMed] [Google Scholar]
- 8.Wilson AD, Kent BE. The glass ionomer cement. A new translucent dental filling material. J Appl Chem Biotechnol. 1971;313:21. [Google Scholar]
- 9.Burke F, Lynch E. Glass polyalkenoate bond strength to dentine after chemomechanical caries removal. J Dent. 1994;22:283–291. doi: 10.1016/0300-5712(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 10.Olivia A, Della Ragione F, Salerno A. Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials. 2000;17:1351–1356. [PubMed] [Google Scholar]
- 11.Mennemeyer VA, Neuman P, Powers JM. Bonding of hybrid ionomers and resin cements to modified orthodontic band materials. Am J Orthod Dentofacial Orthop. 1999;115:143–147. doi: 10.1016/s0889-5406(99)70341-0. [DOI] [PubMed] [Google Scholar]
- 12.Keim RG, Gottlieb EL, Nelson AH, Vogels DS. JCO study of orthodontic diagnosis and treatment procedures, part 1: results and trends. J Clin Orthod. 2002;36:553–568. [PubMed] [Google Scholar]
- 13.Pendrys DG. Dental fluorosis in perspective. J Am Dent Assoc. 1991;122:63–66. doi: 10.14219/jada.archive.1991.0271. [DOI] [PubMed] [Google Scholar]
- 14.Kaehler T. Nanotechnology—basic concepts and definitions. Clin Chem. 1994;40:1797–1799. [PubMed] [Google Scholar]
- 15.Arends J, Ten Bosch JJ. In vivo de- and remineralization of dental enamel. In: Leach SA, editor. Factors Relating to Demineralization and Remineralization of the Teeth. Oxford, UK: IRL Press; 1985. pp. 1–11. [Google Scholar]
- 16.Lu KL, Zhang JX, Meng XC, Li XY. Remineralization effect of the nano-HA toothpaste on artificial caries. Key Eng Mater. 2007;330–332:267–270. [Google Scholar]
- 17.Kim MY, Kwon HK, Choi CH, Kim BI. Combined effects of nano-HA and NaF on remineralization of early caries lesion. Key Eng Mater. 2007;330–332:1347–1350. [Google Scholar]
- 18.Yamagishi Y, Onuma K, Suzuki T, et al. A synthetic enamel for rapid tooth repair. Nature. 2005;433:819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]
- 19.Mielnik BM, Krawczyk D, Pels E. The application of synthetic HA in children and adolescents in various clinical cases. Ann Univ Mariae Curie Sklodowska [Med] 2001;56:95–98. [PubMed] [Google Scholar]
- 20.Li BG, Wang JP, Zhao ZY, Sui YF, Zhang YX. Mineralizing of nano-HA powders on artificial caries. Rare Metal Mater Eng. 2007;36(suppl 2):128–130. [Google Scholar]
- 21.Gao W. The Remineralization Research of Nanohydroxypatite [MsD thesis] Chengdu, Sichuan: West China College of Stomatology, Sichuan University; 2004. pp. 4–11. [Google Scholar]
- 22.Huang SB, Gao SS, Yu HY. Effect of nano-HA concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4(3):034104. doi: 10.1088/1748-6041/4/3/034104. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- 23.Yap AUJ, Pek YS, Kumar RA, Cheang P, Khor KA. Experimental studies on a new bioactive material: HA ionomer cements. Biomaterials. 2002;23:955–962. doi: 10.1016/s0142-9612(01)00208-3. [DOI] [PubMed] [Google Scholar]
- 24.Arita K, Lucas ME, Nishino M. The effect of adding HA on the flexural strength of glass ionomer cement. Dent Mater J. 2003;22:126–136. doi: 10.4012/dmj.22.126. [DOI] [PubMed] [Google Scholar]
- 25.Arita K, Yamamoto A, Shinonaga Y, et al. HA particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glass ionomer cement. Dent Mater J. 2011;30:672–683. doi: 10.4012/dmj.2011-029. [DOI] [PubMed] [Google Scholar]
- 26.Markovic M, Fowler BO, Tung MS. Preparation and comprehensive characterization of a calcium HA reference material. J Res Natl Inst Stand Technol. 2004;109:553–568. doi: 10.6028/jres.109.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashani M, Farhadi S, Rastegarfard N. Comparison of the effect of three cements on prevention of enamel demineralization adjacent to orthodontic bands. J Dent Res Dent Clin Dent Prospect. 2012;6(3):89–93. doi: 10.5681/joddd.2012.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillgrass TJ, Millett DT, Creanor SL, et al. Fluoride release, microbial inhibition and microleakage pattern of two orthodontic band cements. J Dent. 1999;27:455–461. doi: 10.1016/s0300-5712(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 29.Arhun N, Arman A, Cehreli SB, Arikan S, Karabulut E, Gulsahi K. Microleakage beneath ceramic and metal brackets bonded with a conventional and an antibacterial adhesive system. Angle Orthod. 2006;76:1028–1034. doi: 10.2319/101805-368. [DOI] [PubMed] [Google Scholar]
- 30.Radlanski RJ, Renz H, Reulen A. Distribution of the cement film beneath the orthodontic band: a morphometric in vitro study. J Orofac Orthop. 2003;64:284–292. doi: 10.1007/s00056-003-0311-x. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi Y, Onuma K, Suzuki T, et al. A synthetic enamel for rapid tooth repair. Nature. 2005;433:819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Pan HH, Tao JH, et al. Repair of enamel by using HA nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–4084. [Google Scholar]
- 33.Roveri N, Battistella E, Bianchi CL, et al. Surface enamel remineralization: biomimetic apatite nanocrystals and fluoride ions different effects. J Nanomater. 2009 Article ID 746383, 9 pages doi:10.1155/2009/746383. Available at www.hindawi.com/journals/jnm/2009/746383/. Accessed Aug. 28, 2012. [Google Scholar]
- 34.Ozturk NA, Usumez A, Ozturk B, Usumez S. Influence of different light sources on microleakage of class V composite resin restorations. J Oral Rehabil. 2004;31:500–504. doi: 10.1111/j.1365-2842.2004.01273.x. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari M, Garcia-Godoy F. Sealing ability of new generation adhesive restorative materials placed on vital teeth. Am J Dent. 2002;15:117–128. [PubMed] [Google Scholar]
- 36.Aggarwal M, Foley TF, Rix D. A comparison of shear-peel band strengths of 5 orthodontic cements. Angle Orthod. 2000;70:308–316. doi: 10.1043/0003-3219(2000)070<0308:ACOSPB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Arikan S, Arhun N, Arman A, Cehreli SB. Microleakage beneath ceramic and metal brackets photopolymerized with LED or conventional light curing units. Angle Orthod. 2006;76:1035–1040. doi: 10.2319/110905-392. [DOI] [PubMed] [Google Scholar]
- 38.Fricker JP. A 12-month clinical comparison of resin-modified light-activated adhesives for the cementation of orthodontic molar bands. Am J Orthod Dentofacial Orthop. 1997;112:239–243. doi: 10.1016/S0889-5406(97)70250-6. [DOI] [PubMed] [Google Scholar]
- 39.Millett DT, Kamahli K, McColl J. Comparative laboratory investigation of dual-cured vs conventional glass ionomer cements for band cementation. Angle Orthod. 1998;68:345–350. doi: 10.1043/0003-3219(1998)068<0345:CLIODC>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Millett DT, Cummings A, Letters S, Roger E, Love J. Resin-modified glass ionomer, modified composite or conventional glass ionomer for band cementation?—an in vitro evaluation. Eur J Orthod. 2003;25:609–614. doi: 10.1093/ejo/25.6.609. [DOI] [PubMed] [Google Scholar]